Host Range and Phylogenetic Position of Acipenserobdella volgensis (Zykoff, 1904) (Hirudinea: Piscicolidae) with a Global Checklist of Bivalve-Associated Fish Leeches
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sampling
2.2. DNA Sequences and Sequence Alignment of Leeches
2.3. Phylogenetic Analyses, Divergence Dating, and Ancestral Environment Reconstruction
3. Results
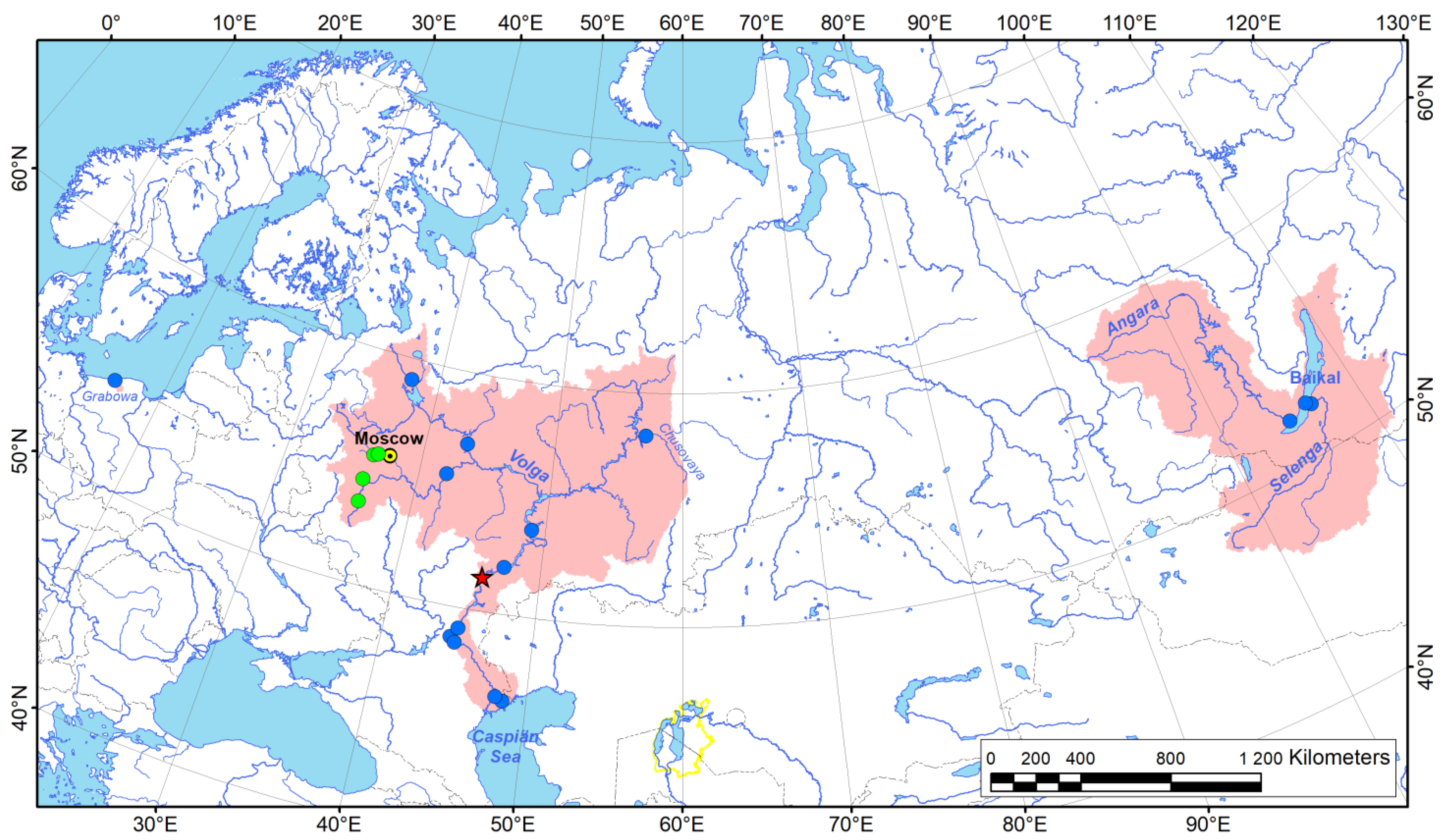
3.1. New Geographic Occurrences and Distribution Summary of Acipenserobdella volgensis
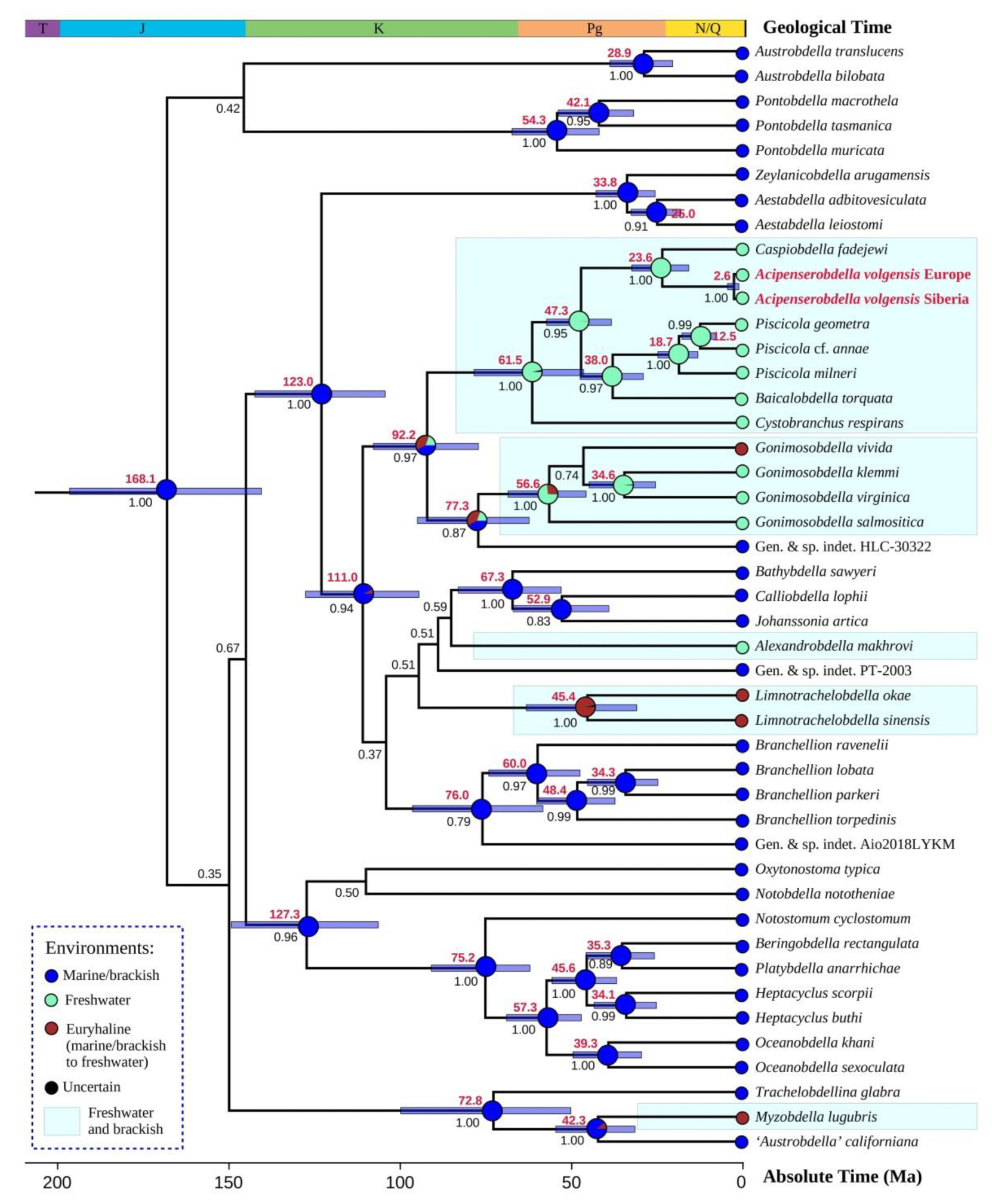
3.2. Phylogenetic and Ancestral Environment Reconstructions for Acipenserobdella volgensis and Related Taxa
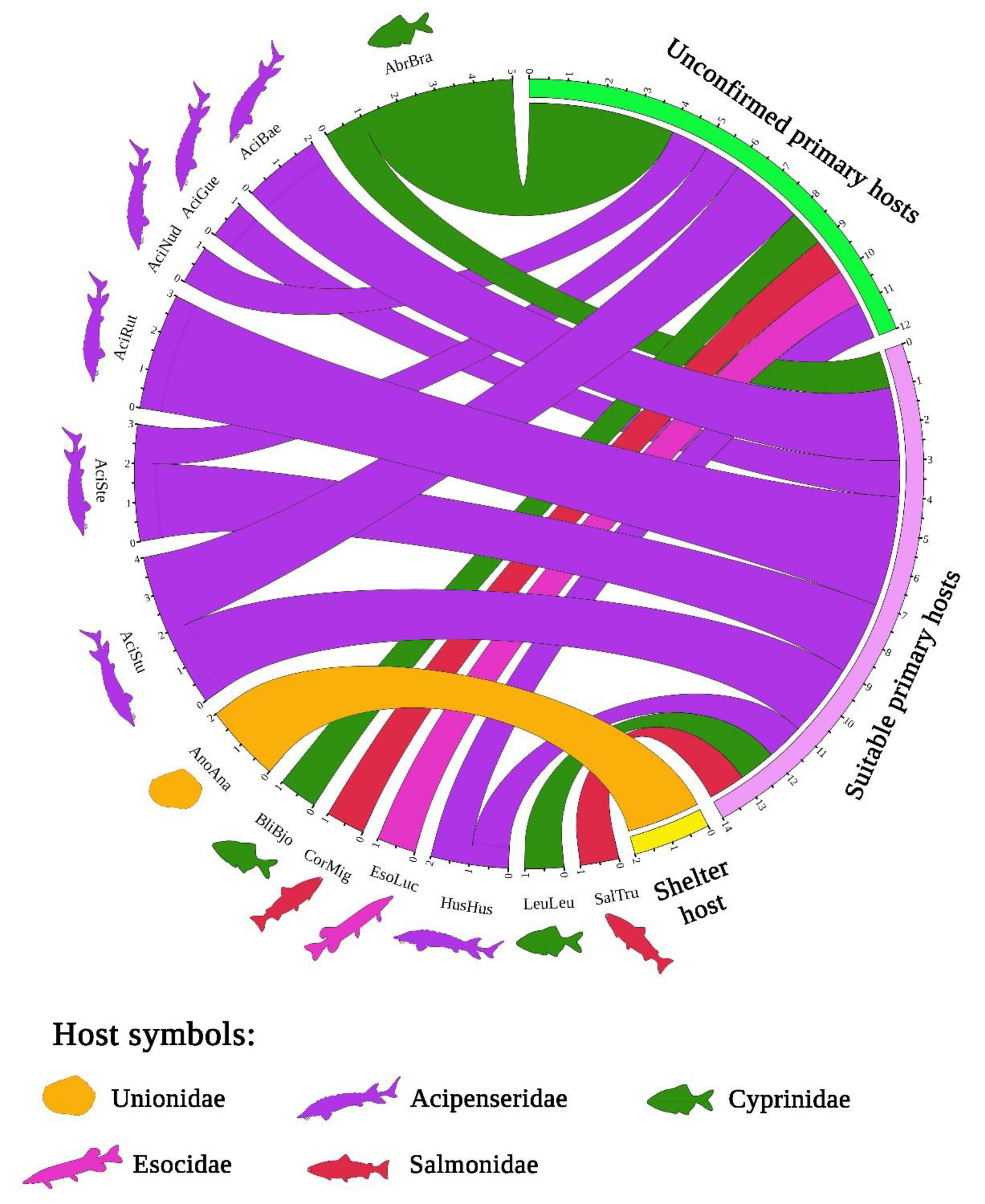
3.3. Host Range of Acipenserobdella volgensis and Its Records from the Mantle Cavity of a Freshwater Mussel
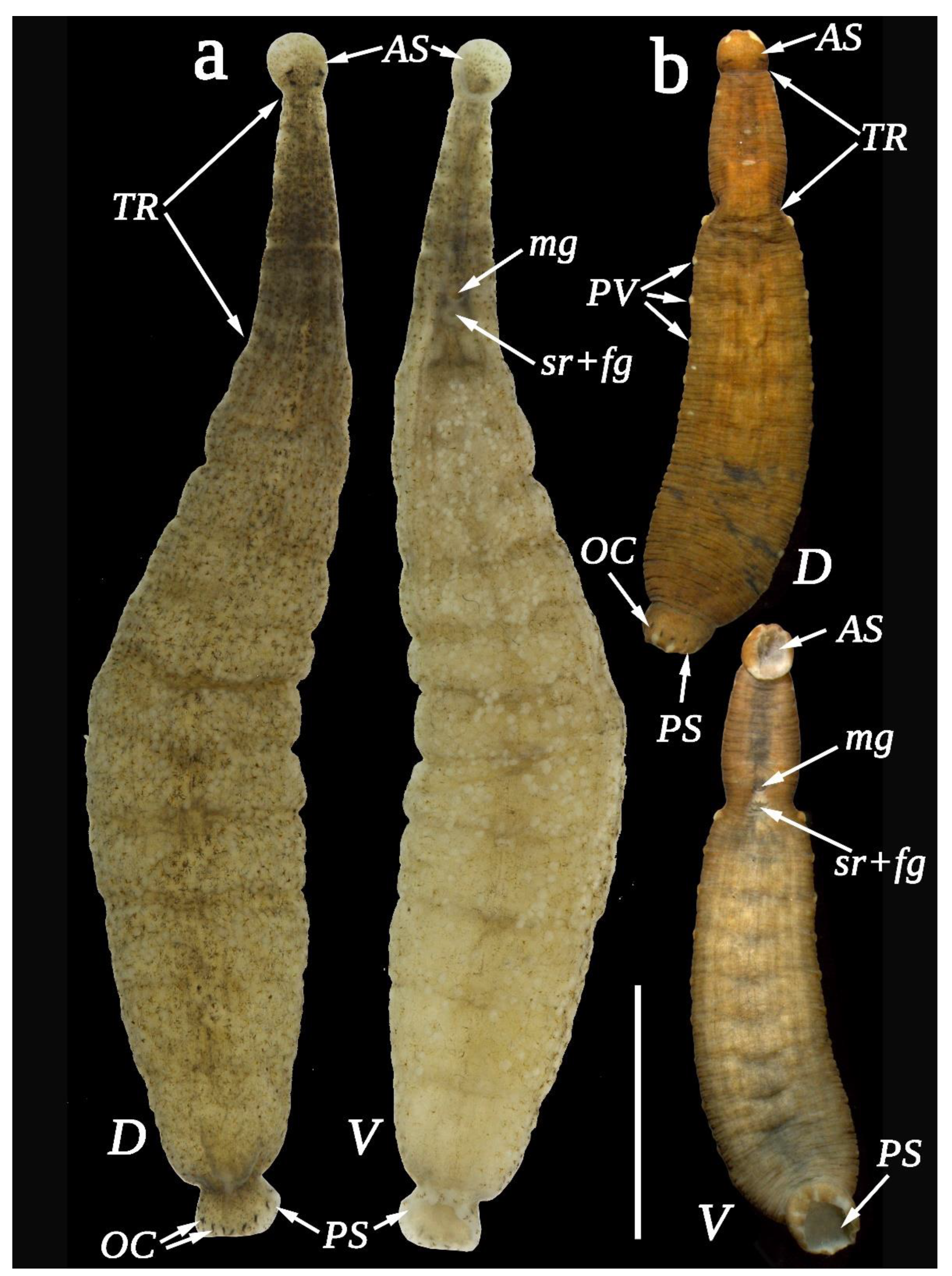
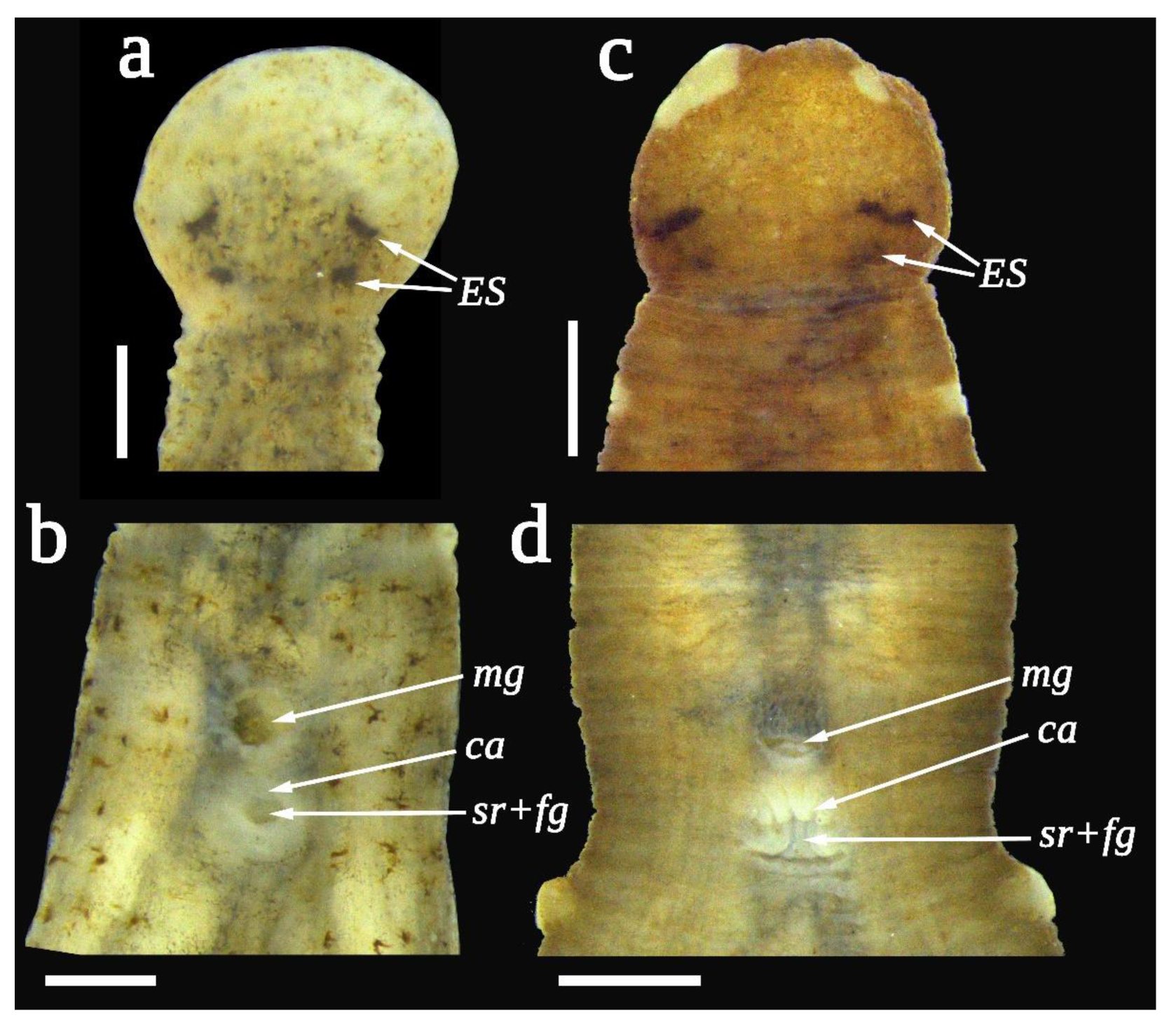
3.4. Taxonomy
4. Discussion
4.1. Phylogenetic Position and Evolutionary Origin of Acipenserobdella volgensis
4.2. A Review of the Host Associations of Acipenserobdella volgensis
4.3. Associations of Fish Leeches with Bivalve Molluscs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utevsky, S.Y.; Trontelj, P. Phylogenetic relationships of fish leeches (Hirudinea, Piscicolidae) based on mitochondrial DNA sequences and morphological data. Zool. Scr. 2004, 33, 375–385. [Google Scholar] [CrossRef]
- Williams, J.I.; Burreson, E.M. Phylogeny of the fish leeches (Oligochaeta, Hirudinida, Piscicolidae) based on nuclear and mitochondrial genes and morphology. Zool. Scr. 2006, 35, 627–639. [Google Scholar] [CrossRef]
- Sket, B.; Trontelj, P. Global diversity of leeches (Hirudinea) in freshwater. Hydrobiologia 2008, 595, 129–137. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Klass, A.L.; Konopleva, E.S.; Bespalaya, Y.V.; Gofarov, M.Y.; Kondakov, A.V.; Vikhrev, I.V. First freshwater mussel-associated piscicolid leech from East Asia. Sci. Rep. 2020, 10, 19854. [Google Scholar] [CrossRef]
- Bielecki, A.; Cichocka, J.M.; Jelen, I.; Swiatek, P.; Adamiak-Brud, Z. A checklist of leech species from Poland. Wiadomości Parazytol. 2011, 57, 11–20. [Google Scholar]
- Cichocka, J.M.; Bielecki, A.; Kulikowski, M.; Jabłońska-Barna, I.; Najda, K. New record of the fish leech Piscicola pojmanskae (Annelida: Hirudinida: Piscicolidae)—DNA barcoding and phylogeny. Biologia 2018, 73, 693–701. [Google Scholar] [CrossRef]
- Zykoff, V.P. Materials to the fauna of Volga and the hydrofauna of Saratov Province. Bull. Soc. Imp. Nat. Moscou 1904, 1903, 1–148. (In Russian) [Google Scholar]
- Rousseau, E. Les Hirudinées d’eau douce d’Europe. Ann. Biol. Lacustre 1912, 5, 259–295. [Google Scholar]
- Stschegolew, G.G. Leeches of River Oka. In Works of the Oka Biological Station in the City of Murom; State Typography No. 10: Murom, USSR, 1922; Volume 2, pp. 20–28. (In Russian) [Google Scholar]
- Behning, A.L. Zur Erforschung der am Flussboden der Wolga lebenden Organismen. Monogr. Biol. Wolga-Stn. Nat. Ges. Saratov 1924, 1, 1–398. (In Russian) [Google Scholar]
- Epshtein, V.M. Class Leeches Hirudinea Lamarck, 1818. In Guide to Parasites of Freshwater Fishes of the USSR; Pavlovsky, E.N., Ed.; Publishing House of the USSR Academy of Sciences: Moscow-Leningrad, USSR, 1962; pp. 617–626. (In Russian) [Google Scholar]
- Epshtein, V.M. A revision of the genera Piscicola and Cystobranchus (Hirudinea, Piscicolidae). In The Problems of Parasitology, Proceedings of the 6th Scientific Conference of Parasitologists of the Ukrainian SSR; Naukova Dumka Publishing House: Kiev, USSR, 1969; Volume 2, pp. 286–287. (In Russian) [Google Scholar]
- Lukin, E.I. Leeches of fresh and brackish water bodies. Fauna USSR 1976, 109, 1–484. (In Russian) [Google Scholar]
- Sawyer, R.T. Leech Biology and Behaviour. Volume 2. Feeding Biology, Ecology, and Systematics. Clarendon Press: Oxford, UK, 1986; pp. 419–793. [Google Scholar]
- Nesemann, H.; Neubert, E. Süßwasserfauna von Mitteleuropa, Bd. 6/2, Annelida: Clitellata: Branchiobdellida, Acanthobdellea, Hirudinea; Spektrum Akademischer: Heidelberg/Berlin, Germany, 1999; pp. 1–178. [Google Scholar]
- Govedich, F.R.; Moser, W.E.; Nakano, T.; Bielecki, A.; Bain, B.A.; Utevsky, S. Subclass Hirudinida. In Thorp and Covich’s Freshwater Invertebrates. Keys to Palaearctic Fauna, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; Volume 4, pp. 491–507. [Google Scholar] [CrossRef]
- Epshtein, V.M. Type Annelida. Class Hirudinea. In Guide to Parasites of Freshwater Fishes of the Fauna of the USSR; Bauer, O.N., Ed.; Zoological Institute of the USSR Academy of Sciences: Leningrad, USSR, 1987; Volume 3, pp. 340–372. (In Russian) [Google Scholar]
- Bielecki, A.; Kapusta, A.; Cichocka, J.M. Atlantic sturgeon, Acipenser oxyrinchus Mitchill, infected by the parasitic leech, Caspiobdella fadejewi (Epshtein) (Hirudinea; Piscicolidae), in the Drwęca River. Fish. Aquat. Life 2011, 19, 87–93. [Google Scholar] [CrossRef]
- Zaika, V.E. Fish parasitofauna of Lake Baikal; Nauka Publishing House: Moscow, USSR, 1965; pp. 1–107. (In Russian) [Google Scholar]
- Pugachev, O.N. Checklist of the Freshwater Fish Parasites of the Northern Asia. Nematoda, Acanthocephala, Hirudinea, Mollusca, Crustacea, Acari. In Proceedings of the Zoological Institute of the Russian Academy of Sciences; 2004; Volume 304, pp. 1–250. (In Russian). [Google Scholar]
- Mhaisen, F.T.; Al-Jawda, J.M.; Asmar, K.M.; Ali, M.H. Checklists of fish parasites of Al-Anbar Province, Iraq. Biol. Appl. Environ. Res. 2017, 1, 17–56. [Google Scholar]
- Mhaisen, F.T. Checklists of blood parasites of fishes of Iraq. Aalb. Acad. J. Pure Sci. 2020, 1, 1–12. [Google Scholar]
- Salimi, B.; Mobedi, I.; Khiabanian, H.A.; Soltani, M. On the diversity of leeches (Annelida: Hirudina) in the fresh waters of Kurdistan province, Iran. Arch. Biol. Sci. 2011, 63, 837–840. [Google Scholar] [CrossRef]
- Bauer, O.N.; Pugachev, O.N.; Voronin, V.N. Study of parasites and diseases of sturgeons in Russia: A review. J. Appl. Ichthyol. 2002, 18, 420–429. [Google Scholar] [CrossRef]
- Lapkina, L.N.; Zharikova, T.I.; Svirskiĭ, A.M. Invasion of fish with leeches (Fam. piscicolidae) in reservoirs of the Volga River. Parazitologiya 2002, 36, 132–139. (In Russian) [Google Scholar]
- Molodozhnikova, N.M.; Zhokhov, A.E. Taxonomic diversity of parasites in agnathans and fishes from the Volga River basin. VI. Acanthocephala, Hirudinea and Bivalvia. Parazitologiya 2008, 42, 179–190. (In Russian) [Google Scholar]
- Lapkina, L.N. Notes on bio-ecological features of ectoparasites of sturgeon fishes—the leech Acipenserobdella volgensis Zykoff, 1903. In Parasitological Studies in Siberia and on the Far East; Gulyaev, V.D., Ed.; Lada Publishing House: Novosibirsk, Russia, 2002; pp. 110–113. (In Russian) [Google Scholar]
- Bolotov, I.N.; Klass, A.L.; Kondakov, A.V.; Vikhrev, I.V.; Bespalaya, Y.V.; Gofarov, M.Y.; Filippov, B.Y.; Bogan, A.E.; Lopes-Lima, M.; Zau, L.; et al. Freshwater mussels house a diverse mussel-associated leech assemblage. Sci. Rep. 2019, 9, 16449. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, W.; Zhang, Y.; Xu, Y. High speed BLASTN: An accelerated MegaBLAST search tool. Nucleic Acids Res. 2015, 43, 7762–7768. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Krzywinski, M.I.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Apakupakul, K.; Siddall, M.E.; Burreson, E.M. Higher level relationships of leeches (Annelida: Clitellata: Euhirudinea) based on morphology and gene sequences. Mol. Phylogenet. Evol. 1999, 12, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Bottari, T.; Profeta, A.; Rinelli, P.; Gaglio, G.; La Spada, G.; Smedile, F.; Giordano, D. On the presence of Pontobdella muricata (Hirudinea: Piscicolidae) on some elasmobranchs of the Tyrrhenian Sea (Central Mediterranean). Acta Adriat. Int. J. Mar. Sci. 2017, 58, 225–233. [Google Scholar] [CrossRef]
- Kaygorodova, I.; Matveenko, E.; Dzyuba, E. Unexpected discovery of an ectoparasitic invasion first detected in the Baikal coregonid fish population. Fishes 2022, 7, 298. [Google Scholar] [CrossRef]
- Siddall, M.E.; Burreson, E.M. Phylogeny of leeches (Hirudinea) based on mitochondrial cytochrome c oxidase subunit I. Mol. Phylogenetics Evol. 1998, 9, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.M. Investigating DNA Barcoding Potentials and Genetic Structure in Ozobranchus spp. from Atlantic and Pacific Ocean Sea Turtles. Master’s Thesis, Wright State University, Dayton, OH, USA, 2014. Available online: http://rave.ohiolink.edu/etdc/view?acc_num=wright1392769367 (accessed on 19 August 2022).
- Tseng, C.; Leu, J.; Cheng, I. On the genetic diversity of two species of the genus Ozobranchus (Hirudinida; Ozobranchidae) from the Atlantic and Pacific oceans. J. Mar. Biol. Assoc. UK 2018, 98, 955–960. [Google Scholar] [CrossRef]
- Utevsky, S.Y.; Utevsky, A.Y.; Schiaparelli, S.; Trontelj, P. Molecular phylogeny of pontobdelline leeches and their place in the descent of fish leeches (Hirudinea, Piscicolidae). Zool. Scr. 2007, 36, 271–280. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Villesen, P. FaBox: An online toolbox for fasta sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Gateway Computing Environments Workshop (GCE); IEEE: New Orleans, LA, USA, 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Yu, Y.; Pfosser, M.; Wetschnig, W. Inferences of biogeographical histories within subfamily Hyacinthoideae using S-DIVA and Bayesian binary MCMC analysis implemented in RASP (Reconstruct Ancestral State in Phylogenies). Ann. Bot. 2012, 109, 95–107. [Google Scholar] [CrossRef]
- Yu, Y.; Blair, C.; He, X. RASP 4: Ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 2020, 37, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Rees, T.; Vandepitte, L.; Decock, W.; Vanhoorne, B. IRMNG 2006–2016: 10 years of a global Taxonomic Database. Biodivers. Inform. 2017, 12, 1–44. [Google Scholar] [CrossRef]
- Smith, D.G.; Taubert, B.D. New records of leeches (Annelida: Hirudinea) from the shortnose sturgeon (Acipenser brevirostrum) in the Connecticut River. Proc. Helminthol. Soc. Wash. 1980, 47, 147–148. [Google Scholar]
- Appy, R.G.; Dadswell, M.J. Marine and estuarine piscicolid leeches (Hirudinea) of the Bay of Fundy and adjacent waters with a key to species. Can. J. Zool. 1981, 59, 183–192. [Google Scholar] [CrossRef]
- Zykoff, V.P. Compte-Rendu des Travaux des Vacances 1900 de la Station Biologique du Volga Organisée par la Société des Naturalistes à Saratow; Imprimerie de la Régence du Gouvernement: Saratow, Russian Empire, 1900; pp. 1–25. (In Russian) [Google Scholar]
- Plotnikov, V. Leeches from the vicinities of the city of Saratov. In Works of the Volga Biological Station; The Volga Biological Station: Saratov, USSR, 1909; Volume 3, pp. 11–17. (In Russian) [Google Scholar]
- Bogdanova, A.E.; Nikolskaya, N.P. Parasite fauna of Volga River before the regulation. In Proceedings of the State Scientific-Research Institute on Lake and River Fisheries; Lenizdat: Leningrad, USSR, 1965; Volume 60, pp. 5–110. (In Russian). [Google Scholar]
- Reshetnikova, A.V. Parasites of fishes of the downstream of the Volga HPS named after the XXII congress of CPSU. In Proceedings of the Volgograd Department of the State Scientific-Research Institute on Lake and River Fisheries; The Volgograd Department of the State Scientific-Research Institute on Lake and River Fisheries: Volgograd, USSR, 1967; Volume 3, pp. 299–320. (In Russian). [Google Scholar]
- Ivanov, V.P. Parasitofauna of sturgeons of Volgo-Caspian Basin. In Parasitic Animals of Volgograd Oblast: Proceedings of Department of Zoology; Markov, G.S., Ed.; Volgograd Pedagogical Institute: Volgograd, USSR, 1969; pp. 306–314. (In Russian) [Google Scholar]
- Dontzov, Y.S. Influence of regulation of the Volga River runoff on the helminth fauna of fish from the reservoirs of the Volga cascade. In Fauna, Systematics, Biology and Ecology of Helminths and Their Intermediate Hosts; Shaldybin, L.S., Ed.; Gorky State Pedagogical Institute: Gorky, USSR, 1979; pp. 13–40. (In Russian) [Google Scholar]
- Epshtein, V.M. On the origin of the Hirudinea fauna, especially Piscicolidae, in ancient lakes. Lauterbornia 2004, 52, 181–193. [Google Scholar]
- Kaygorodova, I.A. A revised checklist of the Lake Baikal Hirudinida fauna. Lauterbornia 2012, 75, 49–62. [Google Scholar]
- Kaygorodova, I.A.; Dzyuba, E.V.; Sorokovikova, N.V. First records of potamic leech fauna of Eastern Siberia, Russia. Dataset Pap. Biol. 2012, 2013, 362683. [Google Scholar] [CrossRef]
- Minelli, A.; Sket, B.; de Jong, Y. Fauna Europaea: Annelida—Hirudinea, incl. Acanthobdellea and Branchiobdellea. Biodivers. Data J. 2014, 2, e4015. [Google Scholar] [CrossRef]
- Grube, E. Beschreibungen einiger Egel-Arten. Arch. Für Nat. 1871, 37, 87–121. [Google Scholar]
- Epshtein, V.M. On the systematic position, distribution and origin of the Caspian endemic leech—Piscicola caspica Selensky (Hirudinea, Piscicolidae). Zool. Zhurnal 1965, 44, 1858–1861. (In Russian) [Google Scholar]
- Sawyer, R.T.; Lawler, A.R.; Oversrteet, R.M. Marine leeches of the eastern United States and the Gulf of Mexico with a key to the species. J. Nat. Hist. 1975, 9, 633–667. [Google Scholar] [CrossRef]
- Appy, R.G.; Cone, D.K. Attachment of Myzobdella lugubris (Hirudinea: Piscicolidae) to logperch, Percina caprodes, and brown bullhead, Ictalurus nebulosus. Trans. Am. Microsc. Soc. 1982, 101, 135–141. [Google Scholar] [CrossRef]
- Sherbakov, D.Y. Molecular phylogenetic studies on the origin of biodiversity in Lake Baikal. Trends Ecol. Evol. 1999, 14, 92–95. [Google Scholar] [CrossRef]
- Dumont, H.J. The Caspian Lake: History, biota, structure, and function. Limnol. Oceanogr. 1998, 43, 44–52. [Google Scholar] [CrossRef]
- Sokolova, A.M.; Palatov, D.M.; Itskovich, V.B. Genetic analysis confirms the freshwater origin of the endemic Caspian sponges (Demospongiae, Spongillida, Metschnikowiidae). ZooKeys 2020, 915, 1–16. [Google Scholar] [CrossRef]
- Vinarski, M.V.; Aksenova, O.V.; Bespalaya, Y.V.; Bolotov, I.N.; Schniebs, K.; Gofarov, M.Y.; Kondakov, A.V. Radix dolgini: The integrative taxonomic approach supports the species status of a Siberian endemic snail (Mollusca, Gastropoda, Lymnaeidae). Comptes Rendus Biol. 2016, 339, 24–36. [Google Scholar] [CrossRef]
- Mérő, T.O.; Málnás, K. The first record of Piscicola fasciata Kollar, 1842 (Hirudinea: Piscicolidae) from Serbia, with recommendations for sampling. Acta Zool. Bulg. 2019, 71, 129–132. [Google Scholar]
- Jueg, U.; Grosser, C.; Bielecki, A. Zur Kenntnis der Fischegelfauna (Hirudinea: Piscicolidae) in Deutschland. Lauterbornia 2004, 52, 39–73. [Google Scholar]
- Lapkina, L.N.; Komov, V.T. New data on the finding of the leech Caspiobdella fadejevi in water reservoirs of the Volga. Parazitologiya 1983, 17, 70–72. (In Russian) [Google Scholar]
- Bolotov, I.N.; Eliseeva, T.A.; Kondakov, A.V.; Gofarov, M.Y.; Konopleva, E.S.; Lyubas, A.A.; Vikhrev, I.V. Helobdella stagnalis (Hirudinea: Glossiphoniidae), the first facultative mussel-associated leech in Europe. Ecol. Montenegrina 2022, 54, 32–43. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Eliseeva, T.A.; Kondakov, A.V.; Konopleva, E.S.; Palatov, D.M.; Sokolova, A.M.; Vikhrev, I.V.; Gofarov, M.Y.; Bovykina, G.V.; Chan, N.; et al. Hidden shelter-like associations of minute Alboglossiphonia leeches (Hirudinea: Glossiphoniidae) with sedentary animals and molluscs. Limnologica 2022, 97, 126028. [Google Scholar] [CrossRef]
- Outa, J.O.; Hörweg, C.; Avenant-Oldewage, A.; Jirsa, F. Neglected symbionts and other metazoan invertebrates associated with molluscs from Africa’s largest lake: Diversity, biotic interactions and bioindication. Freshw. Biol. 2022, 67, 2089–2099. [Google Scholar] [CrossRef]
- Kuperman, B.I.; Zhochov, A.E.; Popova, L.B. Parasites of Dreissena polymorpha (Pallas) molluscs of the Volga basin. Parazitologiya 1994, 28, 396–402. (In Russian) [Google Scholar]
- Williams, J.I.; Urrutia, P.M.; Burreson, E.M. Two new species of Austrobdella (Hirudinida: Piscicolidae) from Chile. J. Parasitol. 2007, 93, 184–189. [Google Scholar] [CrossRef]
| Voucher No. | N | Sampling Locality, Date, and Collector | Latitude | Longitude | Host (Number of Examined Host Specimens, If Available) | Comments on Leech Samples |
|---|---|---|---|---|---|---|
| RMBH Hir_0189 | 2 | European Russia: Moscow River, Volga River basin, Moscow Region, 12 June 2018, V. Maryinsky leg. | 55.7038 | 36.7288 | Anodonta anatina (Linnaeus, 1758); Unionidae (N = 25) | Adult leeches from the mantle cavity of freshwater mussels; formalin-preserved sample |
| N/A | 1 | European Russia: Moscow River, Volga River basin, Moscow Region, 30 June 2019, V. Maryinsky leg. | 55.7038 | 36.7288 | Anodonta anatina (Linnaeus, 1758); Unionidae (N = 20) | Adult leech from the mantle cavity of a freshwater mussel; not preserved (examined in the field) |
| RMBH Hir_0461_1 | 5 | European Russia: Moscow River, Volga River basin, Moscow Region, 21 June 2021, D. Palatov leg. | 55.6245 | 36.4033 | Leuciscus leuciscus (Linnaeus, 1758); Cyprinidae (host uncovered by crop content sequencing: GenBank acc. No. of the COI sequence OP585664) | Free-living sub-adult leeches collected from stones; ethanol-preserved sample; sequenced (COI and 18S rRNA) |
| DZAE KHNU | 15 | European Russia: Oka River, Volga River basin, Kaluga Region, 12 June 2015, D. Palatov leg. | 54.5078 | 36.1084 | Acipenser ruthenus Linnaeus, 1758 (Acipenseridae); Blicca bjoerkna (Linnaeus, 1758) (Cyprinidae) | Adult leeches collected from fish hosts; ethanol-preserved sample |
| DZAE KHNU | 2 | European Russia: Oka River, Volga River basin, Orel Region, 09 June 2015, D. Palatov leg. | 53.5452 | 36.2289 | Acipenser ruthenus Linnaeus, 1758 (Acipenseridae) | Adult leeches collected from fish hosts; ethanol-preserved sample |
| Leech Species | Bivalve Host | Type of Association with Bivalves | Primary Fish Host | Environment | Region |
|---|---|---|---|---|---|
| Acipenserobdella volgensis (Zykoff, 1904) | Anodonta anatina (Linnaeus, 1758) (Unionidae) | Facultative shelter-like | Host generalist: Acipenseridae (preferred hosts), Cyprinidae, Salmonidae; Esocidae (one host record) [18,26] | Freshwater | Russia: Moscow River, Volga River basin |
| Caspiobdella fadejewi (Epshtein, 1961) | Dreissena polymorpha (Pallas, 1771) (Dreissenidae) [81] | Facultative shelter-like [81] | Host generalist: Acipenseridae, Cyprinidae (preferred hosts), Esocidae, Lotidae, Percidae, Salmonidae [18,26] | Freshwater [18,26,81] | Russia: Volga River basin [81] |
| Alexandrobdella makhrovi Bolotov et al., 2020 | Cristaria plicata (Leach, 1814) (Unionidae) [4] | Probably facultative shelter-like [4] | Silurus asotus (Linnaeus, 1758) (Siluridae) [4] | Freshwater [4] | Russia: Lake Khanka, Prymorie Region, Far East [4] |
| Austrobdella coliumicus Williams, Urrutia & Burreson, 2007 | Ensis macha (Molina, 1782) (Pharidae) [82] | Probably obligate shelter-like [82] | Unknown [82] | Marine [82] | Chile: Coliumo Bay, Region VIII [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolotov, I.N.; Maryinsky, V.V.; Palatov, D.M.; Kondakov, A.V.; Eliseeva, T.A.; Konopleva, E.S.; Gofarov, M.Y.; Vikhrev, I.V.; Bespalaya, Y.V. Host Range and Phylogenetic Position of Acipenserobdella volgensis (Zykoff, 1904) (Hirudinea: Piscicolidae) with a Global Checklist of Bivalve-Associated Fish Leeches. Water 2022, 14, 4010. https://doi.org/10.3390/w14244010
Bolotov IN, Maryinsky VV, Palatov DM, Kondakov AV, Eliseeva TA, Konopleva ES, Gofarov MY, Vikhrev IV, Bespalaya YV. Host Range and Phylogenetic Position of Acipenserobdella volgensis (Zykoff, 1904) (Hirudinea: Piscicolidae) with a Global Checklist of Bivalve-Associated Fish Leeches. Water. 2022; 14(24):4010. https://doi.org/10.3390/w14244010
Chicago/Turabian StyleBolotov, Ivan N., Vadim V. Maryinsky, Dmitry M. Palatov, Alexander V. Kondakov, Tatyana A. Eliseeva, Ekaterina S. Konopleva, Mikhail Y. Gofarov, Ilya V. Vikhrev, and Yulia V. Bespalaya. 2022. "Host Range and Phylogenetic Position of Acipenserobdella volgensis (Zykoff, 1904) (Hirudinea: Piscicolidae) with a Global Checklist of Bivalve-Associated Fish Leeches" Water 14, no. 24: 4010. https://doi.org/10.3390/w14244010
APA StyleBolotov, I. N., Maryinsky, V. V., Palatov, D. M., Kondakov, A. V., Eliseeva, T. A., Konopleva, E. S., Gofarov, M. Y., Vikhrev, I. V., & Bespalaya, Y. V. (2022). Host Range and Phylogenetic Position of Acipenserobdella volgensis (Zykoff, 1904) (Hirudinea: Piscicolidae) with a Global Checklist of Bivalve-Associated Fish Leeches. Water, 14(24), 4010. https://doi.org/10.3390/w14244010






