Sustainable Use of Nano-Assisted Remediation for Mitigation of Heavy Metals and Mine Spills
Abstract
:1. Introduction
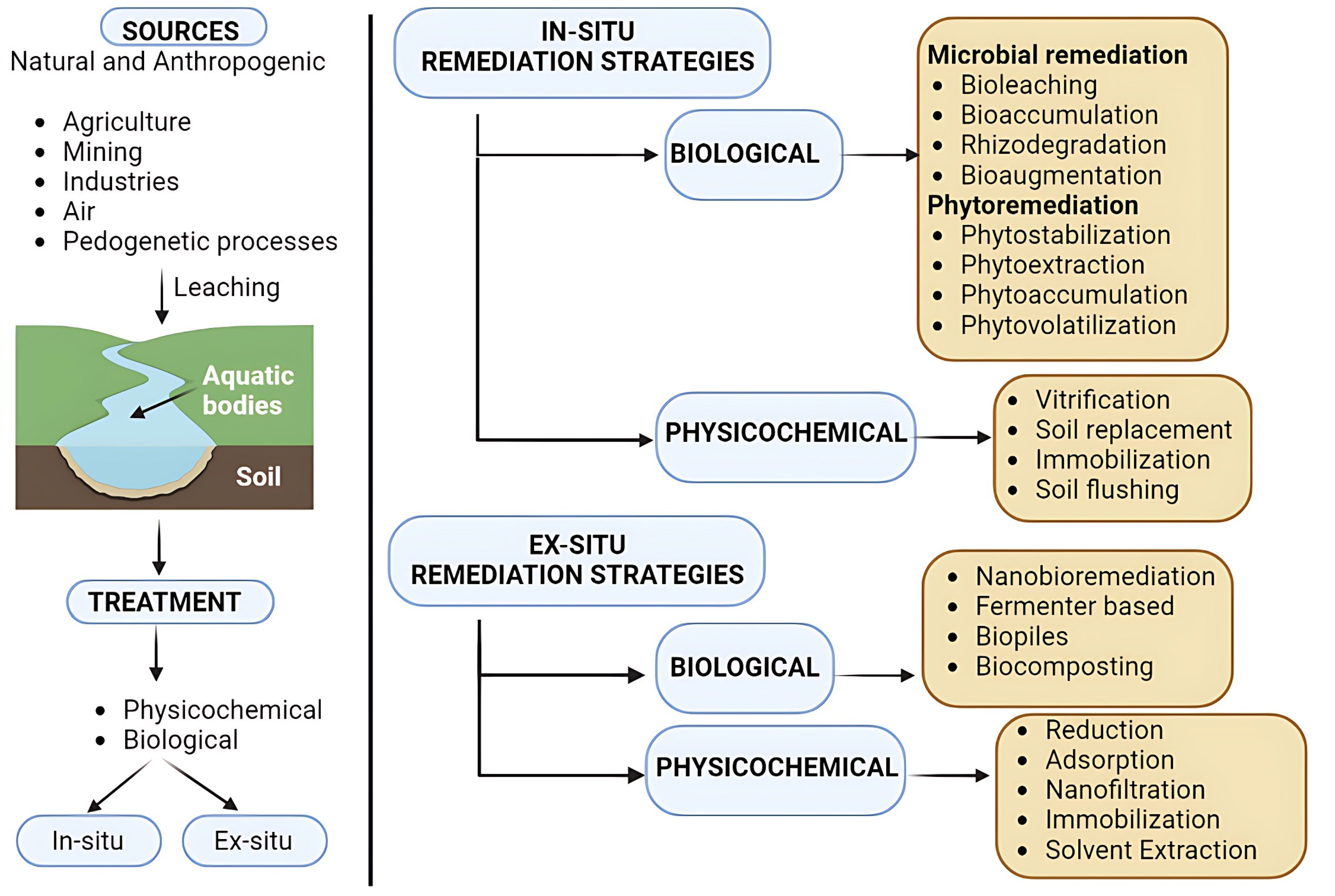
2. Mechanism of Action of Nanoparticles
2.1. Remediation Techniques
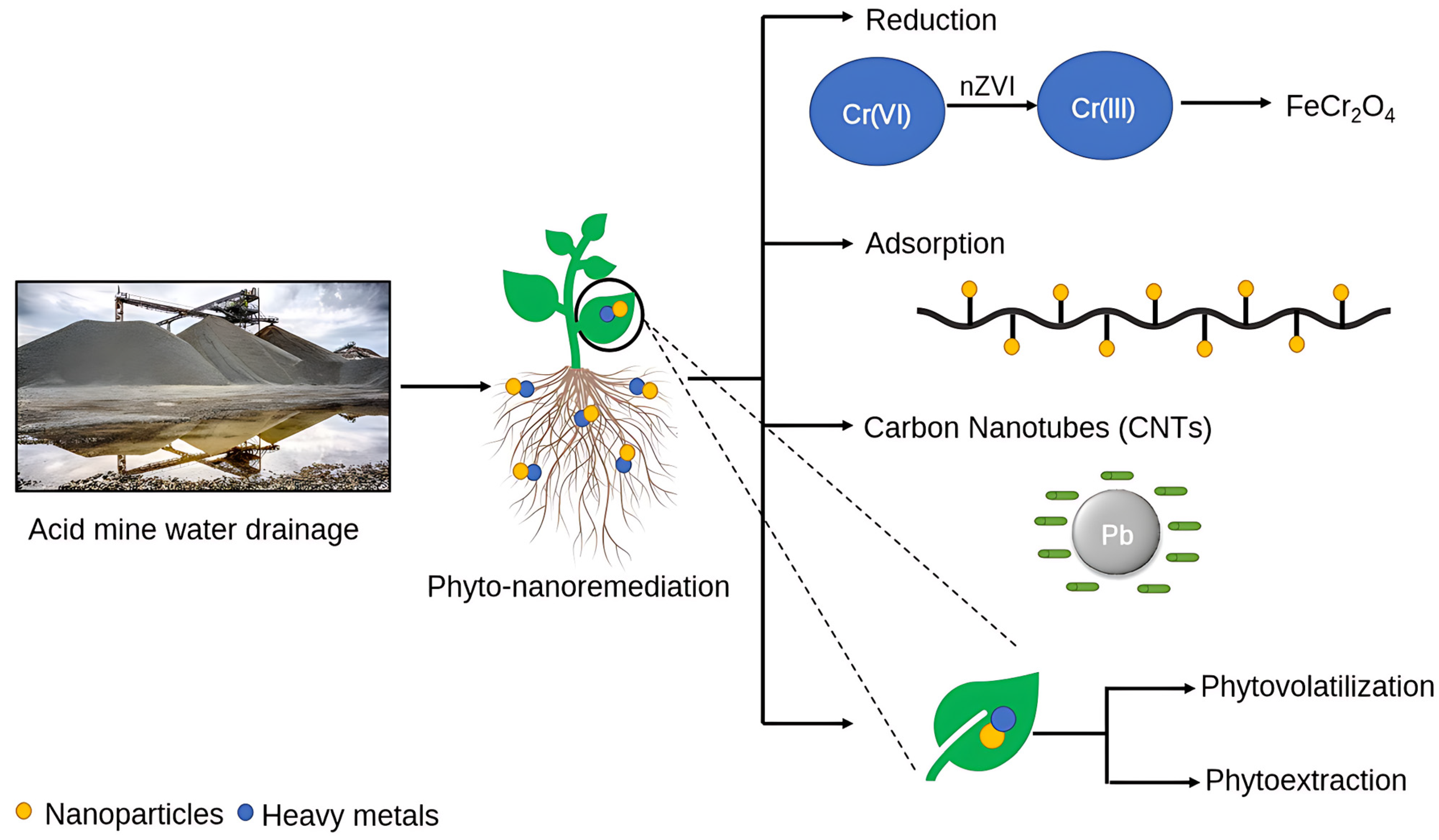
2.2. Reduction
2.3. Phytoremediation
2.4. Rhizodegradation of Heavy Metals
3. Types of Nanomaterials Used in the Removal of Heavy Metals
3.1. Nano Zero-Valent-Iron-Based Nanomaterials
3.2. Magnetic Nanomaterials
3.3. Carbon Nanotubes (CNTs)
3.4. Metal Oxide Nanomaterials
4. Impact of Environmental Factors
4.1. Temperature
4.2. pH
4.3. Contact Time
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilardi, G.; Ochando-Pulido, J.M.; Verdone, N.; Stoller, M.; Di Palma, L. On the removal of hexavalent chromium by olive stones coated by iron-based nanoparticles: Equilibrium study and chromium recovery. J. Clean. Prod. 2018, 190, 200–210. [Google Scholar] [CrossRef]
- Yu, G.; Liu, J.; Long, Y.; Chen, Z.; Sunahara, G.I.; Jiang, P.; You, S.; Lin, H.; Xiao, H. Phytoextraction of cadmium-contaminated soils: Comparison of plant species and low molecular weight organic acids. Int. J. Phytoremediation 2019, 22, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Khan, M.A.; Kameda, T.; Xu, H.; Wang, F.; Xia, M.; Yoshioka, T. New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material. J. Hazard. Mater. 2022, 426, 128062. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb(II) on l-Lysine Modified Montmorillonite and the simulation of Interlayer Structure. Appl. Clay Sci. 2018, 169, 40–47. [Google Scholar] [CrossRef]
- Volesky, B.; Holan, Z.R. Biosorption of Heavy Metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments-A Review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Gaikwad, R. REVIEW ON REMOVAL OF HEAVY METALS FROM ACID MINE DRAINAGE. Appl. Ecol. Environ. Res. 2008, 6, 81–98. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Southam, G. Geomicrobiology of sulfide mineral oxidation. Rev. Mineral. Geochem. 1997, 35, 361–390. [Google Scholar]
- Das, P.K. Phytoremediation and Nanoremediation: Emerging Techniques for Treatment of Acid Mine Drainage Water. Def. Life Sci. J. 2018, 3, 190–196. [Google Scholar] [CrossRef]
- Singh, G. Mine water quality deterioration due to acid mine drainage. Mine Water Environ. 1987, 6, 49–61. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; BlakeII, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genom. 2008, 9, 597. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, G.; González-González, R.B.; Iqbal, H.M. Bioremediation and decontamination potentials of metallic nanoparticles loaded nanohybrid matrices—A review. Environ. Res. 2021, 204, 112407. [Google Scholar] [CrossRef]
- Sharma, T.; Sharma, N. Nanoparticles: Uptake, Translocation, Physiological, Biochemical Effects in Plants and their Molecular Aspects; Springer: Cham, Switzerland, 2022; pp. 103–116. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Nogueira, S.S.; de Araujo-Nobre, A.R.; Mafud, A.C.; Guimarães, M.A.; Alves, M.M.M.; Plácido, A.; Carvalho, F.A.A.; Arcanjo, D.D.R.; Mascarenhas, Y.; Costa, F.G.; et al. Silver nanoparticle stabilized by hydrolyzed collagen and natural polymers: Synthesis, characterization and antibacterial-antifungal evaluation. Int. J. Biol. Macromol. 2019, 135, 808–814. [Google Scholar] [CrossRef]
- Cecchin, I.; Reddy, K.R.; Thomé, A.; Tessaro, E.F.; Schnaid, F. Nanobioremediation: Integration of nanoparticles and bioremediation for sustainable remediation of chlorinated organic contaminants in soils. Int. Biodeterior. Biodegrad. 2017, 119, 419–428. [Google Scholar] [CrossRef]
- Raffa, C.; Chiampo, F.; Shanthakumar, S. Remediation of Metal/Metalloid-Polluted Soils: A Short Review. Appl. Sci. 2021, 11, 4134. [Google Scholar] [CrossRef]
- Gong, Z.; Chan, H.; Chen, Q.; Chen, H. Application of Nanotechnology in Analysis and Removal of Heavy Metals in Food and Water Resources. Nanomaterials 2021, 11, 1792. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.C.A.; Amare, E. Summary on Adsorption and Photocatalysis for Pollutant Remediation: Mini Review. J. Encapsul. Adsorpt. Sci. 2018, 08, 225–255. [Google Scholar] [CrossRef] [Green Version]
- Desiante, W.L.; Minas, N.S.; Fenner, K. Micropollutant biotransformation and bioaccumulation in natural stream biofilms. Water Res. 2021, 193, 116846. [Google Scholar] [CrossRef]
- Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable Application of Biosorption and Bioaccumulation of Persistent Pollutants in Wastewater Treatment: Current Practice. Processes 2021, 9, 1696. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of Acid Mine Drainage-Impacted Water. Curr. Pollut. Rep. 2015, 1, 131–141. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Qian, Y.; Qin, C.; Chen, M.; Lin, S. Nanotechnology in soil remediation—Applications vs. implications. Ecotoxicol. Environ. Saf. 2020, 201, 110815. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.M.; Ahmed, I.; Park, S.-J. Application of Nanotechnology to Remediate Contaminated Soils. In Environmental Remediation Technologies for Metal-Contaminated Soils; Springer: Tokyo, Japan, 2016; pp. 219–229. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Johnston, C.P.; Dahal, G. A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysulfide and green-tea nanoscale zero-valent iron. J. Hazard. Mater. 2012, 201–202, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Kumari, A.; Minkina, T.; Barakhov, A.; Singh, S.; Mandzhieva, S.S.; Sushkova, S.; Ranjan, A.; Rajput, P.; Garg, M.C. A practical evaluation on integrated role of biochar and nanomaterials in soil remediation processes. Environ. Geochem. Health 2022, 1–15. [Google Scholar] [CrossRef]
- Lobzenko, I.; Burachevskaya, M.; Zamulina, I.; Barakhov, A.; Bauer, T.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Tereschenko, A.; Kalinichenko, V.; et al. Development of a Unique Technology for the Pyrolysis of Rice Husk Biochar for Promising Heavy Metal Remediation. Agriculture 2022, 12, 1689. [Google Scholar] [CrossRef]
- Elbehiry, F.; Darweesh, M.; Al-Anany, F.S.; Khalifa, A.M.; Almashad, A.A.; El-Ramady, H.; El-Banna, A.; Rajput, V.D.; Jatav, H.S.; Elbasiouny, H. Using Biochar and Nanobiochar of Water Hyacinth and Black Tea Waste in Metals Removal from Aqueous Solutions. Sustainability 2022, 14, 10118. [Google Scholar] [CrossRef]
- Awasthi, G.; Nagar, V.; Mandzhieva, S.; Minkina, T.; Sankhla, M.S.; Pandit, P.P.; Aseri, V.; Awasthi, K.K.; Rajput, V.D.; Bauer, T.; et al. Sustainable Amelioration of Heavy Metals in Soil Ecosystem: Existing Developments to Emerging Trends. Minerals 2022, 12, 85. [Google Scholar] [CrossRef]
- Burachevskaya, M.; Mandzhieva, S.; Bauer, T.; Minkina, T.; Rajput, V.; Chaplygin, V.; Fedorenko, A.; Chernikova, N.; Zamulina, I.; Kolesnikov, S.; et al. The Effect of Granular Activated Carbon and Biochar on the Availability of Cu and Zn to Hordeum sativum Distichum in Contaminated Soil. Plants 2021, 10, 841. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, N.; Fang, Z. In situ remediation of hexavalent chromium contaminated soil by CMC-stabilized nanoscale zero-valent iron composited with biochar. Water Sci. Technol. 2018, 77, 1622–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Temsah, Y.S.; Sevcu, A.; Bobcikova, K.; Cernik, M.; Joner, E.J. DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil. Chemosphere 2016, 144, 2221–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabeen, R.; Ahmad, A.; Iqbal, M.F. Phytoremediation of Heavy Metals: Physiological and Molecular Mechanisms. Bot. Rev. 2009, 75, 339–364. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Ahmad, M.; Srivastava, A.K.; Abhilash, P.C.; Sharma, B. Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in chickpea (Cicer arietinum L.) plant. Chemosphere 2021, 267, 129216. [Google Scholar] [CrossRef]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef] [Green Version]
- Kisku, G.C.; Kumar, V.; Sahu, P.; Kumar, P.; Kumar, N. Characterization of coal fly ash and use of plants growing in ash pond for phytoremediation of metals from contaminated agricultural land. Int. J. Phytoremediation 2018, 20, 330–337. [Google Scholar] [CrossRef]
- Das, S.; Chou, M.-L.; Jean, J.-S.; Yang, H.-J.; Kim, P.J. Arsenic-enrichment enhanced root exudates and altered rhizosphere microbial communities and activities in hyperaccumulator Pteris vittata. J. Hazard. Mater. 2017, 325, 279–287. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Grenni, P.; Garbini, G.L.; Rolando, L.; Campanale, C.; Aimola, G.; Fernandez-Lopez, M.; Fernandez-Gonzalez, A.J.; Villadas, P.J.; Ancona, V. Characterization of the Belowground Microbial Community in a Poplar-Phytoremediation Strategy of a Multi-Contaminated Soil. Front. Microbiol. 2020, 11, 2073. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, G.K.; Bhat, B.A.; Mushtaq, M.; Tariq, L.; Rai, P.K.; Basu, U.; Dar, A.A.; Islam, S.T.; Dar, T.U.H.; Bhat, J.A. Insights into decontamination of soils by phytoremediation: A detailed account on heavy metal toxicity and mitigation strategies. Physiol. Plant. 2021, 137, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Rajput, V.D.; Kumari, A.; Espinosa-Saiz, D.; Menendez, E.; Minkina, T.; Dwivedi, P.; Mandzhieva, S. Plant growth-promoting rhizobacteria: A potential bio-asset for restoration of degraded soil and crop productivity with sustainable emerging techniques. Environ. Geochem. Health 2022, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Benavides, L.C.L.; Pinilla, L.A.C.; Serrezuela, R.R.; Serrezuela, W.F.R. Extraction in laboratory of heavy metals through rhizofiltration using the plant Zea mays (maize). Int. J. Appl. Environ. Sci. 2018, 13, 9–26. [Google Scholar]
- De Farias, V.; Maranho, L.T.; De Vasconcelos, E.C.; Filho, M.A.D.S.C.; Lacerda, L.G.; Azevedo, J.A.M.; Pandey, A.; Soccol, C.R. Phytodegradation Potential of Erythrina crista-galli L., Fabaceae, in Petroleum-Contaminated Soil. Appl. Biochem. Biotechnol. 2009, 157, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.L.; Klironomos, J.N.; Lee, H.; Trevors, J.T. The effects of perennial ryegrass and alfalfa on microbial abundance and diversity in petroleum contaminated soil. Environ. Pollut. 2005, 133, 455–465. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Zalidis, G. The Use of Typha Latifolia L. in Constructed Wetland Microcosms for the Remediation of Herbicide Terbuthylazine. Environ. Process. 2019, 6, 985–1003. [Google Scholar] [CrossRef]
- Sampaio, C.J.S.; de Souza, J.R.B.; Damião, A.O.; Bahiense, T.C.; Roque, M.R.A. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) in a diesel oil-contaminated mangrove by plant growth-promoting rhizobacteria. 3 Biotech 2019, 9, 155. [Google Scholar] [CrossRef]
- Vítková, M.; Puschenreiter, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal(loid) contaminated soils. Chemosphere 2018, 200, 217–226. [Google Scholar] [CrossRef]
- Khan, A.; Kuek, C.; Chaudhry, T.; Khoo, C.; Hayes, W. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000, 41, 197–207. [Google Scholar] [CrossRef]
- Khan, A.G. In Situ Phytoremediation of Uranium Contaminated Soils. In Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–151. [Google Scholar] [CrossRef]
- Stone, V.; Nowack, B.; Baun, A.; Brink, N.V.D.; von der Kammer, F.; Dusinska, M.; Handy, R.; Hankin, S.; Hassellöv, M.; Joner, E.; et al. Nanomaterials for environmental studies: Classification, reference material issues, and strategies for physico-chemical characterisation. Sci. Total Environ. 2010, 408, 1745–1754. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Guerrón, D.B.; Capa, J.; Flores, L.C. Retention of heavy metals from mine tailings using Technosols prepared with native soils and nanoparticles. Heliyon 2021, 7, e07631. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, D. Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Res. 2007, 41, 2101–2108. [Google Scholar] [CrossRef]
- Ajala, M.A.; Abdulkareem, A.S.; Tijani, J.O.; Kovo, A.S. Adsorptive behaviour of rutile phased titania nanoparticles supported on acid-modified kaolinite clay for the removal of selected heavy metal ions from mining wastewater. Appl. Water Sci. 2022, 12, 19. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, S.; Zhong, Q.; Wang, G.; Xu, X.; Li, T.; Wang, L.; Jia, Y.; Li, Y. Feasibility of nanoscale zero-valent iron to enhance the removal efficiencies of heavy metals from polluted soils by organic acids. Ecotoxicol. Environ. Saf. 2018, 162, 464–473. [Google Scholar] [CrossRef]
- Moosa, A.; Ridha, A.; Moosa, A.A.; Ridha, A.M.; Hussien, N.A. Removal of Zinc Ions from Aqueous Solution by Bioadsorbents and CNTs Removal of Zinc Ions from Aqueous Solution by Bioadsorbents and CNTs. Am. J. Mater. Sci. 2016, 6, 105–114. [Google Scholar] [CrossRef]
- Alimohammady, M.; Jahangiri, M.; Kiani, F.; Tahermansouri, H. Design and evaluation of functionalized multi-walled carbon nanotubes by 3-aminopyrazole for the removal of Hg(II) and As(III) ions from aqueous solution. Res. Chem. Intermed. 2017, 44, 69–92. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Elsner, M.; Chartrand, M.; VanStone, N.; Couloume, G.L.; Lollar, B.S. Identifying Abiotic Chlorinated Ethene Degradation: Characteristic Isotope Patterns in Reaction Products with Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2008, 42, 5963–5970. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Lu, T.; Peng, L.; Shao, J.; Zeng, Q.; Gu, J.-D. Synthesis of nanoscale zero-valent iron immobilized in alginate microcapsules for removal of Pb(ii) from aqueous solution. J. Mater. Chem. A 2014, 2, 15463–15472. [Google Scholar] [CrossRef]
- Mueller, N.C.; Braun, J.; Bruns, J.; Černík, M.; Rissing, P.; Rickerby, D.; Nowack, B. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ. Sci. Pollut. Res. 2011, 19, 550–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimkova, S.; Cernik, M.; Lacinova, L.; Filip, J.; Jancik, D.; Zboril, R. Zero-valent iron nanoparticles in treatment of acid mine water from in situ uranium leaching. Chemosphere 2011, 82, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Metzler, D.R.; Dwyer, B.P. Removal of As, Mn, Mo, Se, U, V and Zn from groundwater by zero-valent iron in a passive treatment cell: Reaction progress modeling. J. Contam. Hydrol. 2001, 56, 99–116. [Google Scholar] [CrossRef]
- Wilkin, R.T.; McNeil, M.S. Laboratory evaluation of zero-valent iron to treat water impacted by acid mine drainage. Chemosphere 2003, 53, 715–725. [Google Scholar] [CrossRef]
- Reyhanitabar, A.; Alidokht, L.; Khataee, A.R.; Oustan, S. Application of stabilized Fe0nanoparticles for remediation of Cr(VI)-spiked soil. Eur. J. Soil Sci. 2012, 63, 724–732. [Google Scholar] [CrossRef]
- Fang, Z.; Qiu, X.; Chen, J.; Qiu, X. Degradation of the polybrominated diphenyl ethers by nanoscale zero-valent metallic particles prepared from steel pickling waste liquor. Desalination 2011, 267, 34–41. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Warner, C.L.; Chouyyok, W.; Mackie, K.E.; Neiner, D.; Saraf, L.V.; Droubay, T.; Warner, M.G.; Addleman, R.S. Manganese Doping of Magnetic Iron Oxide Nanoparticles: Tailoring Surface Reactivity for a Regenerable Heavy Metal Sorbent. Langmuir 2012, 28, 3931–3937. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-M.; Man, C.; Hu, Z.-B. Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Liang, Q.; Zhao, D. Removal of arsenic(V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles. Water Res. 2011, 45, 1961–1972. [Google Scholar] [CrossRef]
- Dave, P.N.; Chopda, L.V. Application of Iron Oxide Nanomaterials for the Removal of Heavy Metals. J. Nanotechnol. 2014, 2014, 398569. [Google Scholar] [CrossRef] [Green Version]
- Xin, X.; Wei, Q.; Yang, J.; Yan, L.; Feng, R.; Chen, G.; Du, B.; Li, H. Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticles. Chem. Eng. J. 2012, 184, 132–140. [Google Scholar] [CrossRef]
- Fan, L.; Song, J.; Bai, W.; Wang, S.; Zeng, M.; Li, X.; Zhou, Y.; Li, H.; Lu, H. Chelating capture and magnetic removal of non-magnetic heavy metal substances from soil. Sci. Rep. 2016, 6, 21027. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhang, S.; Wang, X. Surface functional groups and defects on carbon nanotubes affect adsorption–desorption hysteresis of metal cations and oxoanions in water. Environ. Sci. Nano 2014, 1, 488–495. [Google Scholar] [CrossRef]
- Trojanowicz, M. Analytical applications of carbon nanotubes: A review. TrAC Trends Anal. Chem. 2006, 25, 480–489. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S. Nanomaterials and Water Purification: Opportunities and Challenges. J. Nanoparticle Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Lv, X.; Xu, J.; Jiang, G.; Xu, X. Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 2011, 85, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Helland, A.; Wick, P.; Koehler, A.; Schmid, K.; Som, C. Reviewing the Environmental and Human Health Knowledge Base of Carbon Nanotubes. Environ. Health Perspect. 2007, 115, 1125–1131. [Google Scholar] [CrossRef] [Green Version]
- Sheng, G.; Alsaedi, A.; Shammakh, W.; Monaquel, S.; Sheng, J.; Wang, X.; Li, H.; Huang, Y. Enhanced sequestration of selenite in water by nanoscale zero valent iron immobilization on carbon nanotubes by a combined batch, XPS and XAFS investigation. Carbon 2015, 99, 123–130. [Google Scholar] [CrossRef]
- Rodríguez, C.; Leiva, E. Enhanced Heavy Metal Removal from Acid Mine Drainage Wastewater Using Double-Oxidized Multiwalled Carbon Nanotubes. Molecules 2019, 25, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakes, K.; Shan, Z.; Kaliaperumal, R.; Zhang, S.X.; Mkandawire, M. Nanotechnology in Contemporary Mine Water Issues; Springer: Cham, Switzerland, 2014; pp. 307–361. [Google Scholar] [CrossRef]
- Al-Hobaib, A.S.; Al-Sheetan, K.M.; Shaik, M.R.; Al-Suhybani, M.S. Modification of thin-film polyamide membrane with multi-walled carbon nanotubes by interfacial polymerization. Appl. Water Sci. 2017, 7, 4341–4350. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Amal, R.; Beydoun, D. Effect of formate and methanol on photoreduction/removal of toxic cadmium ions using TiO2 semiconductor as photocatalyst. Chem. Eng. Sci. 2003, 58, 4429–4439. [Google Scholar] [CrossRef]
- Singh, J.; Lee, B.-K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96. [Google Scholar] [CrossRef]
- Tan, X.; Wang, X.; Chen, C.; Sun, A. Effect of soil humic and fulvic acids, pH and ionic strength on Th(IV) sorption to TiO2 nanoparticles. Appl. Radiat. Isot. 2007, 65, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Decome, L.; Rose, J.; Orsiere, T.; De Meo, M.; Briois, V.; Chaneac, C.; Olivi, L.; Berge-Lefranc, J.-L.; Botta, A.; et al. In Vitro Interactions between DMSA-Coated Maghemite Nanoparticles and Human Fibroblasts: A Physicochemical and Cyto-Genotoxical Study. Environ. Sci. Technol. 2006, 40, 4367–4373. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Chandra, R.; Tyagi, I.; Verma, M. Removal of hexavalent chromium ions using CuO nanoparticles for water purification applications. J. Colloid Interface Sci. 2016, 478, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Gervas, C.; Mubofu, E.B.; Mdoe, J.E.G.; Revaprasadu, N. Functionalized mesoporous organo-silica nanosorbents for removal of chromium (III) ions from tanneries wastewater. J. Porous Mater. 2015, 23, 83–93. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Nanoenhanced Materials for Reclamation of Mine Lands and Other Degraded Soils: A Review. J. Nanotechnol. 2012, 2012, 461468. [Google Scholar] [CrossRef] [Green Version]
- Dubey, R.; Bajpai, J.; Bajpai, A. Chitosan-alginate nanoparticles (CANPs) as potential nanosorbent for removal of Hg (II) ions. Environ. Nanotechnology, Monit. Manag. 2016, 6, 32–44. [Google Scholar] [CrossRef]
- Velardi, L.; Scrimieri, L.; Serra, A.; Manno, D.; Calcagnile, L. Effect of temperature on the physical, optical and photocatalytic properties of TiO2 nanoparticles. SN Appl. Sci. 2020, 2, 707. [Google Scholar] [CrossRef] [Green Version]
- Kefeni, K.K.; Kefeni, K.K.; Mamba, B.; Mamba, B.; Msagati, T.A.; Msagati, T.A. Magnetite and cobalt ferrite nanoparticles used as seeds for acid mine drainage treatment. J. Hazard. Mater. 2017, 333, 308–318. [Google Scholar] [CrossRef]
- Feng, Y.; Gong, J.-L.; Zeng, G.-M.; Niu, Q.-Y.; Zhang, H.-Y.; Niu, C.-G.; Deng, J.-H.; Yan, M. Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem. Eng. J. 2010, 162, 487–494. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, X.; Fang, Z.; Yang, M.; Pokeung, T.; Gu, F.; Cheng, W.; Lan, B. Removal mechanism of antibiotic metronidazole from aquatic solutions by using nanoscale zero-valent iron particles. Chem. Eng. J. 2011, 181–182, 113–119. [Google Scholar] [CrossRef]
- Su, Y.; Adeleye, A.S.; Keller, A.A.; Huang, Y.; Dai, C.; Zhou, X.; Zhang, Y. Magnetic sulfide-modified nanoscale zerovalent iron (S-nZVI) for dissolved metal ion removal. Water Res. 2015, 74, 47–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, D.; Zhou, J.; Cao, Z.; Xu, J.; Liu, Y.; Li, Y.; Yang, K.; Lou, Z.; Lou, L.; Xu, X. Mechanism and influence factors of chromium(VI) removal by sulfide-modified nanoscale zerovalent iron. Chemosphere 2019, 224, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Z.-L.; Yan, X.; Zhang, B. Removal of mercury (II) and chromium (VI) from wastewater using a new and effective composite: Pumice-supported nanoscale zero-valent iron. Chem. Eng. J. 2014, 245, 34–40. [Google Scholar] [CrossRef]
- Rodríguez, C.; Tapia, C.; Leiva-Aravena, E.; Leiva, E. Graphene Oxide–ZnO Nanocomposites for Removal of Aluminum and Copper Ions from Acid Mine Drainage Wastewater. Int. J. Environ. Res. Public Health 2020, 17, 6911. [Google Scholar] [CrossRef] [PubMed]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef]
- Sivaranjanee, R.; Saravanan, A. Carbon sphere: Synthesis, characterization and elimination of toxic Cr(VI) ions from aquatic system. J. Ind. Eng. Chem. 2018, 60, 307–320. [Google Scholar] [CrossRef]
- Khoso, W.A.; Haleem, N.; Baig, M.A.; Jamal, Y. Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s). Sci. Rep. 2021, 11, 3790. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Xu, J.; Jiang, G.; Tang, J.; Xu, X. Highly active nanoscale zero-valent iron (nZVI)–Fe3O4 nanocomposites for the removal of chromium(VI) from aqueous solutions. J. Colloid Interface Sci. 2012, 369, 460–469. [Google Scholar] [CrossRef]
| Sr. No. | Adsorbent | Target Heavy Metals/Pollutants | Percentage Removal | Source | pH | References |
|---|---|---|---|---|---|---|
| 1 | nZVI | Cr (VI) | 98 | Soil | 5 | [27] |
| 2 | nZVI + Carboxymethyl cellulose | DDT | 25 | Soil | [34] | |
| 3 | Technosol | Cu, Cd, Zn, Pb, As | 75 | Soil | [58] | |
| 4 | nZVI + Carboxymethyl cellulose | TCE | 44 | Soil | [34] | |
| 5 | nZVI + Cellulose | Cr(VI) | 30 | Soil | 5 | [59] |
| 6 | TiO2 | Fe(III) | 91.99 | Mining waste water | - | [60] |
| Mn(II) | 89.37 | Mining waste water | - | [60] | ||
| Pb(II) | 32.39 | Mining waste water | - | |||
| Cu(II) | 81.95 | Mining waste water | - | [45] | ||
| Cd(II) | 96 | Wastewater | 7 | [46] | ||
| Pb(II) | 99.1 | Wastewater | 7 | [46] | ||
| Cr(II) | 94 | Wastewater | 7 | [46] | ||
| Th(IV) | 94 | Soil | 4 | [21] | ||
| 7 | Biochar + nZVI | Cr (VI) | 66 | Soil | [21] | |
| 8 | Biochar | Phenol | Mining waste water | 5.8 | [47] | |
| Cd | Mining waste water | 7 | [47] | |||
| 9 | Graphite oxide | Cd | 88.33 | Soil | 3 | [48] |
| Pb | 85 | Soil | 3 | [48] | ||
| Cr | 63 | Soil | 3 | [48] | ||
| Ni | 89.9 | Soil | 3 | [48] | ||
| Zn | 85.6 | Soil | 3 | [48] | ||
| 10 | Silver-iron oxide NPs | Cr (VI) | 97 | Soil | 4 | [49] |
| 11 | Magnetite | Pb2+, Cd2+, Cu2+, Ni2+ | ≈90 | Soil | 6 | [50] |
| 12 | Carbon nanotubes | Cu | 79 | Acid Mine drainage | 5.5 | [51] |
| Mn | 78 | Acid Mine drainage | 5.5 | [51] | ||
| Zn | 48 | Acid Mine drainage | [51] | |||
| Mn | 100 | - | 3 | [52] | ||
| Cr(VI) | - | Soil | 5 | [53] | ||
| DDT | 59 | Soil | - | [54] | ||
| HCH | 75 | Soil | - | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Singh, G.; Sharma, M.; Mandzhieva, S.; Minkina, T.; Rajput, V.D. Sustainable Use of Nano-Assisted Remediation for Mitigation of Heavy Metals and Mine Spills. Water 2022, 14, 3972. https://doi.org/10.3390/w14233972
Sharma N, Singh G, Sharma M, Mandzhieva S, Minkina T, Rajput VD. Sustainable Use of Nano-Assisted Remediation for Mitigation of Heavy Metals and Mine Spills. Water. 2022; 14(23):3972. https://doi.org/10.3390/w14233972
Chicago/Turabian StyleSharma, Neetu, Gurpreet Singh, Monika Sharma, Saglara Mandzhieva, Tatiana Minkina, and Vishnu D. Rajput. 2022. "Sustainable Use of Nano-Assisted Remediation for Mitigation of Heavy Metals and Mine Spills" Water 14, no. 23: 3972. https://doi.org/10.3390/w14233972










