Microplastics in Freshwater: A Focus on the Russian Inland Waters
Abstract
:1. Introduction
2. Microplastics as Pollutants of Aquatic Ecosystems: State of Research for Freshwater Bodies in the Russian Federation
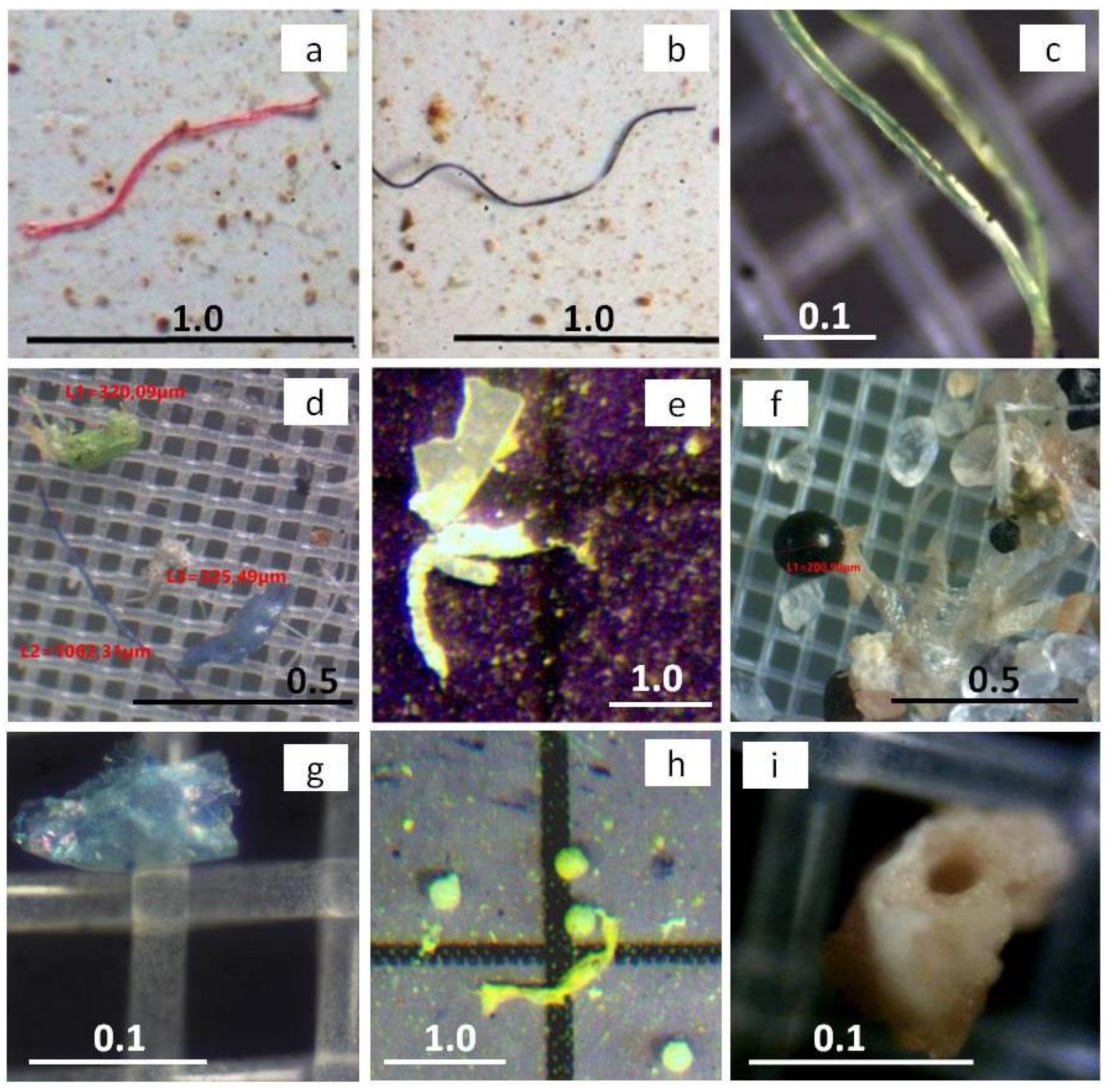
2.1. Overview of the Sources, Sampling, and Analysis of Microplastics in Aquatic Ecosystems
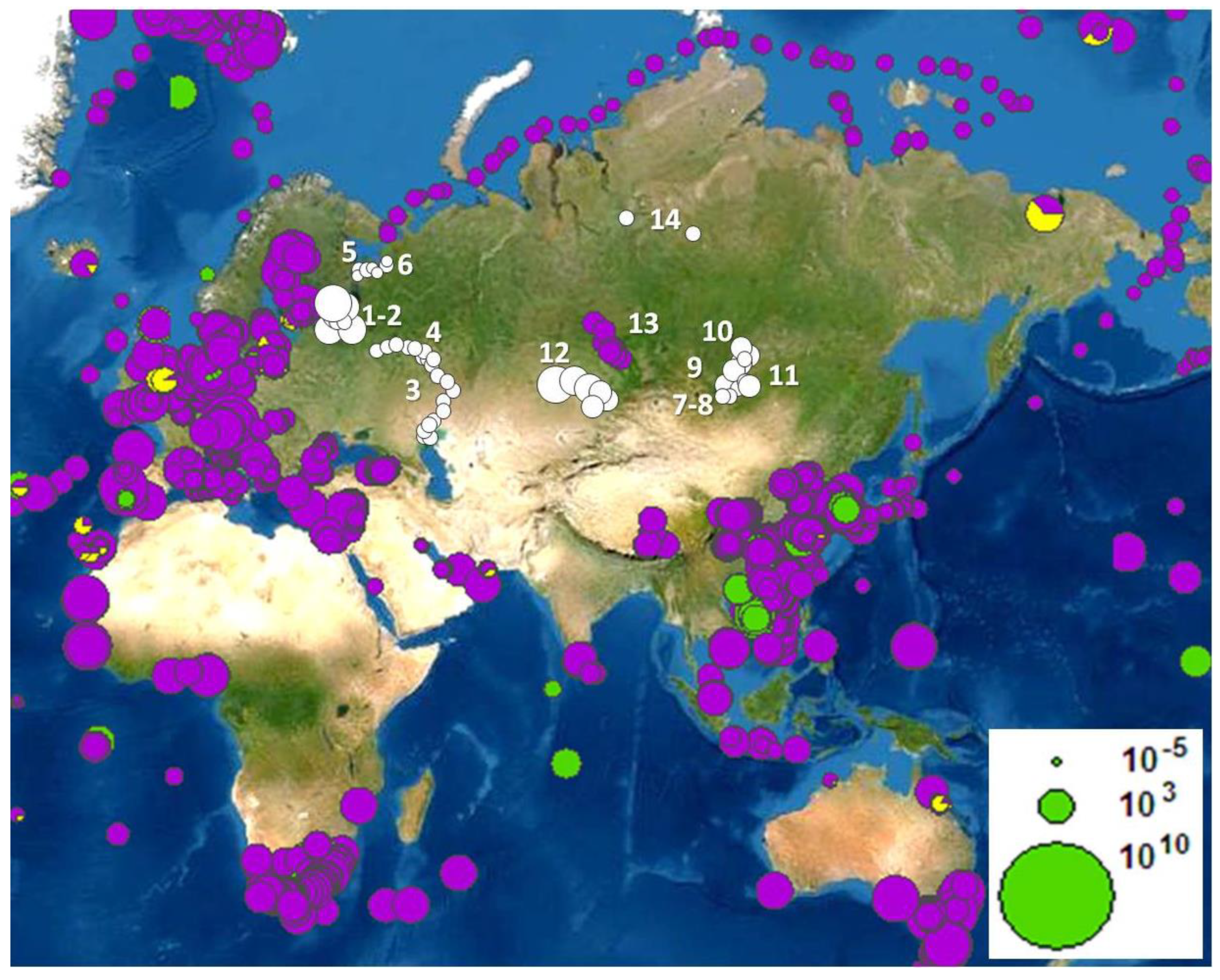
2.2. Abundance and Distribution of Microplastics in Freshwater Ecosystems
2.3. Detection and Quantification of Microplastic Content in Freshwater Lakes and Rivers in the Russian Federation
2.3.1. Microplastics in the Freshwater Bodies of the European Part of the Russian Federation
2.3.2. Microplastics in the Freshwater Bodies of the Asian Part of the Russian Federation
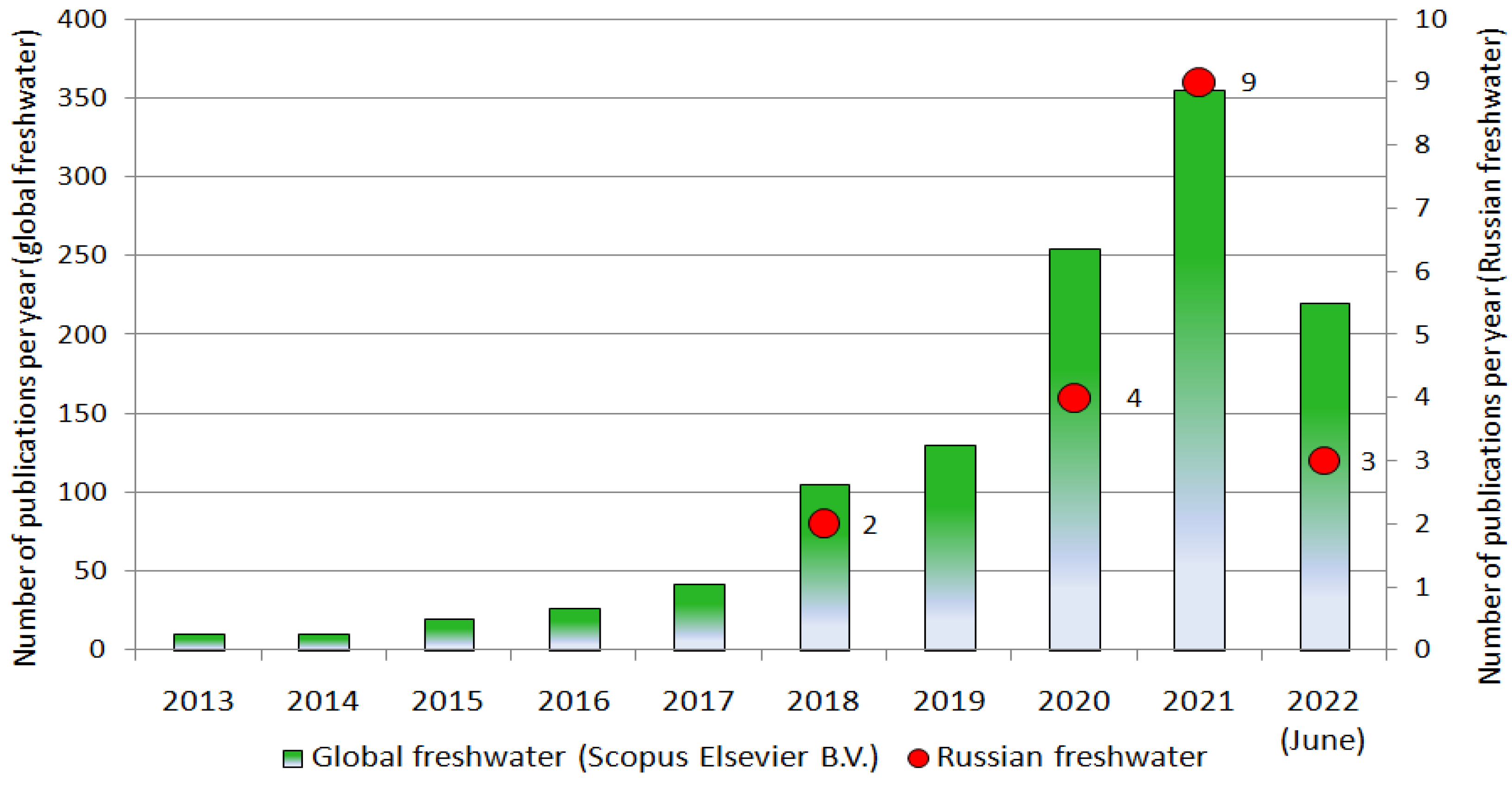
2.3.3. Actual Research Trajectories of Microplastic Pollution of Continental Waters in the Russian Federation and Other Countries
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shashoua, Y. Conservation of Plastics: Materials Science, Degradation and Preservation; Elsevier/Butterworth-Heinemann: Amsterdam, The Netherlands, 2008; pp. 19–38. [Google Scholar]
- Plastics—The Facts 2021. Available online: https://plasticseurope.org/wp-content/uploads/2021/12/AF-Plastics-the-facts-2021_250122.pdf (accessed on 21 October 2022).
- Nielsen, T.D.; Hasselbalch, J.; Holmberg, K.; Stripple, J. Politics and the plastic crisis: A review throughout the plastic life cycle. WIReS Energy Environ. 2019, 9, e360. [Google Scholar] [CrossRef] [Green Version]
- Andrady, A.L. Persistence of plastic litter in the oceans. In Marine Anthropogenic Litter; Bergman, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 57–72. [Google Scholar]
- Galgani, F.; Hanke, G.; Maes, T. Global distribution, composition and abundance of marine litter. In Marine Anthropogenic Litter; Bergman, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 29–56. [Google Scholar]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eerkes-Medrano, D.; Thompson, R. Occurrence, fate, and effect of microplastics in freshwater systems. In Microplastic Contamination in Aquatic Environments: An Emerging Matter of Environmental Urgency; Zeng, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–132. [Google Scholar]
- Rochman, C.M.; Hoellein, T. The global odyssey of plastic pollution. Science 2020, 368, 317–325. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, e3263. [Google Scholar] [CrossRef] [Green Version]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; 46p. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P. Lost at sea: Where is all the plastic? Science 2004, 304, e5672. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Lehel, J.; Murphy, S. Microplastics in the food chain: Food safety and environmental aspects. Environ. Contam. Toxicol. 2021, 259, 1–49. [Google Scholar] [CrossRef]
- Reisser, J.; Shaw, J.; Hallegraeff, G.; Proietti, M.; Barnes, D.K.A.; Thums, M.; Wilcox, C.; Hardesty, B.D.; Pattiaratchi, C. Millimeter-sized marine plastics: A new pelagic habitat for microorganisms and invertebrates. PLoS ONE 2014, 9, e100289. [Google Scholar] [CrossRef] [Green Version]
- Lusher, A.; McHugh, M.; Thompson, R.S. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Fossi, M.C.; Panti, C.; Coppola, D.; Matteo, M.; Minutoli, R.; Guerranti, C. Are baleen whales exposed to microplastics threat? The case study of the Mediterranean Fin whale. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, 25–26. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.G.; Van Franeker, J.A.; Moloney, C. Monitoring the abundance of plastic debris in the marine environment. Philos. Trans. R. Soc. B 2009, 364, 1999–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cózar, A.; Martí, E.; Duarte, C.M.; García-de-Lomas, J.; van Sebille, E.; Ballatore, T.J.; Eguíluz, V.M.; González-Gordillo, J.I.; Pedrotti, M.L.; Echevarría, F.; et al. The Arctic Ocean as a dead end for floating plastics in the North Atlantic branch of the Thermohaline Circulation. Sci. Adv. 2017, 3, e1600582. [Google Scholar] [CrossRef] [Green Version]
- van Sebille, E.; England, M.H.; Froyland, G. Origin, dynamics and evolution of ocean garbage patches from observed surface drifters. Environ. Res. Lett. 2012, 7, e044040. [Google Scholar] [CrossRef]
- Yakushev, E.; Gebruk, A.; Osadchiev, A.; Pakhomova, S.; Lusher, A.; Berezina, A.; van Bavel, B.; Vorozheikina, E.; Chernukh, D.; Kolbasova, G.; et al. Microplastics distribution in the Eurasian Arctic is affected by Atlantic waters and Siberian Rivers. Commun. Earth Environ. 2021, 2, 23. [Google Scholar] [CrossRef]
- Bagaev, A.; Esiukova, E.; Litvinyuk, D. Investigations of plastic contamination of seawater, marine and coastal sediments in the Russian seas: A review. Environ. Sci. Pollut. Res. 2021, 28, 32264–32281. [Google Scholar] [CrossRef]
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On some physical and dynamical properties of microplastic particles in marine environment. Mar. Pollut. Bull. 2016, 1–2, 105–112. [Google Scholar] [CrossRef]
- Chubarenko, I.; Esiukova, E.; Isachenko, I.; Demchenko, N.; Efimova, I.; Bagaeva, M.; Khatmullina, L.; Bagaev, A.; Zobkov, M. Behavior of microplastics in coastal zones. In Microplastic Contamination in Aquatic Environments: An Emerging Matter of Environmental Urgency; Zeng, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–223. [Google Scholar]
- Bagaev, A.; Khatmullina, L.; Chubarenko, I. Anthropogenic microlitter in the Baltic Sea water column. Mar. Pollut. Bull. 2018, 2, 918–923. [Google Scholar] [CrossRef]
- Chubarenko, I.P.; Esiukova, E.E.; Bagaev, A.V.; Bagaeva, M.A.; Grave, A.N. Three-dimensional distribution of anthropogenic microparticles in the body of sandy beaches. Sci. Total Environ. 2018, 628–629, 1340–1351. [Google Scholar] [CrossRef]
- Esiukova, E. Plastic contamination on the Baltic beaches of Kaliningrad region, Russia. Mar. Pollut. Bull. 2017, 2, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Chubarenko, I.; Stepanova, N. MPs in sea coastal zone: Lessons learned from the Baltic amber. Environ. Pollut. 2017, 224, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Eremina, T.; Ershova, A.; Martin, G.; Shilin, M. Marine litter monitoring: Review for the Gulf of Finland coast. In Proceedings of the 2018 IEEE/OES Baltic International Symposium (BALTIC), Klaipeda, Lithuania, 12–15 June 2018; pp. 1–8. [Google Scholar] [CrossRef]
- Haseler, M.; Balciunas, A.; Hauk, R.; Sabaliauskaite, V.; Chubarenko, I.; Ershova, A.; Schernewski, G. Marine litter pollution in Baltic Sea beaches: Application of the Sand Rake method. Front. Environ. Sci. 2020, 8, e599978. [Google Scholar] [CrossRef]
- Ershova, A.A.; Eremina, T.R.; Chubarenko, I.P.; Esiukova, E.E. Marine litter in the Russian Gulf of Finland and South-East Baltic: Application of different methods of beach sand sampling, In Plastics in the Aquatic Environment—Part I. The Handbook of Environmental Chemistry; Stock, F., Reifferscheid, G., Brennholt, N., Kostianaia, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 111, pp. 461–485. [Google Scholar]
- Martyanov, S.D.; Ryabchenko, V.A.; Ershova, A.; Eremina, T.R. On the assessment of the spread of microplastics in the eastern part of the Gulf of Finland. Fundam. Appl. Hydrophys. 2019, 12, 32–41. (In Russian) [Google Scholar] [CrossRef]
- Ershova, A.; Makeeva, I.; Malgina, E.; Sobolev, N.; Smolokurov, A. Combining citizen and conventional science for microplastics monitoring in the White Sea basin (Russian Arctic). Mar. Pollut. Bull. 2021, 173, e112955. [Google Scholar] [CrossRef] [PubMed]
- Pakhomova, S.; Berezina, A.; Lusher, A.L.; Zhdanov, I.; Silvestrova, K.; Zavialov, P.; van Bavel, B.; Yakushev, E. Microplastic variability in subsurface water from the Arctic to Antarctica. Environ. Pollut. 2022, 298, 118808. [Google Scholar] [CrossRef]
- Tošic, T.; Vruggink, M.; Vesman, A. Microplastics quantification in surface waters of the Barents, Kara and White Seas. Mar. Pollut. Bull. 2020, 161 Pt A, 111745. [Google Scholar] [CrossRef]
- Arthur, C.; Baker, J.E.; Bamford, H.A. (Eds.) NOAA Technical Memorandum NOS-OR&R-30. In Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, University of Washington Tacoma, Tacoma, WA, USA, 9–11 September 2008; Available online: https://repository.library.noaa.gov/view/noaa/2509 (accessed on 15 October 2022).
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment (Part 1). Available online: http://www.gesamp.org/publications/reports-and-studies-no-90/ (accessed on 13 October 2022).
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment (Part 2). Available online: http://www.gesamp.org/publications/microplastics-in-the-marine-environment-part-2/ (accessed on 29 October 2022).
- Cowger, W.; Gray, A.B.; Eriksen, M.; Moore, C.; Thiel, M. Evaluating wastewater effluent as a source of microplastics in environmental samples. In Microplastics in Water and Wastewater; Karapanagioti, H.K., Kalavrouziotis, I.K., Eds.; IWA Publishing: London, UK, 2019; pp. 109–131. [Google Scholar]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hasselov, M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, R.C.; Seely, M.E.; Guardia, M.J.; Mai, L.; Zeng, E.Y.A. Global perspective on microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.T.; Baudrimont, N.; Pascal, P.Y. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Brandon, J.A.; Freibott, A.; Sala, L.M. Patterns of suspended and salp-ingested microplastic debris in the North Pacific investigated with epifluorescence microscopy. Limnol. Oceanogr. Lett. 2020, 5, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, G.L.; Gallardo, J.D.; Jones, E.W.; Holliman, P.J.; Watson, T.M.; Sarp, S. Detection of trace sub-micron (nano) plastics in water samples using pyrolysis-gas chromatography time of flight mass spectrometry (PY-GCToF). Chemosphere 2020, 249, 126179. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Dixon, S.J. Microplastics: An introduction to environmental transport processes. Wiley Interdiscip. Rev. 2017, 5, e1268. [Google Scholar] [CrossRef] [Green Version]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, V.P.; Goel, S. Degradation of low-density polyethylene film exposed to UV radiation in four environments. J. Hazard. Toxic. Radioact. Waste 2019, 23, 04019015. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Crocker, B.K.; Gray, A.D. From macroplastic to microplastic: Degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ. Toxicol. Chem. 2016, 35, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- ter Halle, A.; Ladirat, L.; Gendre, X.; Goudouneche, D.; Pusineri, C.; Routaboul, C.; Tenailleau, C.; Duployer, B.; Perez, E. Understanding the fragmentation pattern of marine plastic debris. Environ. Sci. Technol. 2016, 50, 5668–5675. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Andrady, A.L.; Pandey, K.K.; Heikkilä, A.M. Interactive effects of solar UV radiation and climate change on material damage. Photochem. Photobiol. Sci. 2019, 18, 804–825. [Google Scholar] [CrossRef]
- de Falco, F.; di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, e6633. [Google Scholar] [CrossRef] [Green Version]
- Vassilenko, E.; Watkins, M.; Chastain, S.; Mertens, J.; Posacka, A.M.; Patankar, S.; Ross, P.S. Domestic laundry and microfiber pollution: Exploring fiber shedding from consumer apparel textiles. PLoS ONE 2021, 16, e0250346. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.S.; Chastain, S.; Vassilenko, E.; Etemadifar, A.; Zimmermann, S.; Quesnel, S.A.; Eert, J.; Solomon, E.; Patankar, S.; Posacka, A.M.; et al. Pervasive distribution of polyester fibers in the Arctic Ocean is driven by Atlantic inputs. Nat. Commun. 2021, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, e14947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ershova, A.A.; Eremina, T.R.; Dunayev, A.L.; Makeeva, I.N.; Tatarenko, Y.A. Study of microplastic pollution in the seas of the Russian Arctic and the Far East. Arct. Ecol. Econ. 2021, 11, 164–177. (In Russian) [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritization. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Wang, J.; Peng, J.; Tan, Z.; Gao, Y.; Zhan, Z.; Chen, Q.; Cai, L. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar] [CrossRef]
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and their possible sources: The example of Ofanto river in Southeast Italy. Environ. Pollut. 2019, 258, e113284. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Int-Veena, I.; Löderac, M.G.J.; Primpkea, S.; Gerdtsa, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [Green Version]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Lahens, L.; Strady, E.; Kieu-Le, T.C.; Dris, R.; Boukerma, K.; Rinnert, E.; Gasperi, J.; Tassin, B. Macroplastic and microplastic contamination assessment of a tropical river (Saigon River, Vietnam) transversed by a developing megacity. Environ. Pollut. 2018, 236, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svedsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017, 127, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, F. A detailed review study on potential effects of microplastics and additives of concern on human health. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, T.M.; Kärrman, A.; Rotander, A.; Hassellöv, M. Comparison between manta trawl and in situ pump filtration methods, and guidance for visual identification of microplastics in surface waters. Environ. Sci. Pollut. Res. 2020, 27, 5559–5571. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, T.G.; Almeda, R.; Torres, R.; Vollertsen, J.; Winding, M.H.; Sichlau, V.A.; Rist, S. Quantification of plankton-sized microplastics in a productive coastal Arctic marine ecosystem. Environ. Pollut. 2020, 266, e115248. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Zobkov, M.; Zobkova, M.; Galakhina, N.; Efremova, T. Microplastic abundance and accumulation behavior in Lake Onego sediments: A journey from the river mouth to pelagic waters of the large boreal lake. J. Environ. Chem. Eng. 2020, 8, 104–367. [Google Scholar] [CrossRef]
- Malygina, N.; Mitrofanova, E.; Kuryatnikova, N.; Biryukov, R.; Zolotov, D.; Pershin, D.; Chernykh, D. Microplastic pollution in the surface waters from plain and mountainous lakes in Siberia, Russia. Water 2021, 13, 2287. [Google Scholar] [CrossRef]
- Skalska, K.; Ockelford, A.; Ebdon, J.E.; Cundy, A.B. Riverine microplastics: Behaviour, spatio-temporal variability, and recommendations for standardised sampling and monitoring. J. Water Process Eng. 2020, 38, e101600. [Google Scholar] [CrossRef]
- Moore, C.J.; Lattin, G.; Zellers, A.F. Quantity and type of plastic debris flowing from two urban rivers to coastal waters and beaches of Southern California. J. Integr. Coast. Zone Manag. 2011, 11, 65–73. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Microplastics are contaminants of emerging concern in freshwater environments: An overview. In Freshwater Microplastics. The Handbook of Environmental Chemistry, 58; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–23. [Google Scholar] [CrossRef] [Green Version]
- Bellasi, A.; Binda, G.; Pozzi, A.; Galafassi, S.; Volta, P.; Bettinetti, R. Microplastic contamination in freshwater environments: A review, focusing on interactions with sediments and benthic organisms. Environments 2020, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- van Emmerik, T.; Mellink, Y.; Hauk, R.; Waldschläger, K.; Schreyers, L. Rivers as plastic reservoirs. Front. Water 2022, 3, 786936. [Google Scholar] [CrossRef]
- Lebreton, L.; van der Zwet, J.; Damsteeg, J.W. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, e15611. [Google Scholar] [CrossRef] [Green Version]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, M.; van der Meulen, M.; Tweehuijsen, G.; Peterlin, M.; Palatinus, A.; Kovač Viršek, M.; Coscia, L.; Kržan, A. Identification and Assessment of Riverine Input of (Marine) Litter; IFREMER: Brest, France, 2015; 186p. [Google Scholar]
- Zhdanov, I.; Lokhov, A.; Belesov, A.; Kozhevnikov, A.; Pakhomova, S.; Berezina, A.; Frolova, N.; Kotova, E.; Leshchev, A.; Wang, X.; et al. Assessment of seasonal variability of input of microplastics from the Northern Dvina River to the Arctic Ocean. Mar. Pollut. Bull. 2022, 175, 113370. [Google Scholar] [CrossRef]
- van Emmerik, T.; Schwarz, A. Plastic debris in rivers. WIREs Water 2020, 7, e1398. [Google Scholar] [CrossRef] [Green Version]
- Tramoy, R.; Gasperi, J.; Colasse, L.; Tassin, B. Transfer dynamic of macroplastics in estuaries—New insights from the seine estuary: Part 1. Long term dynamic based on date-prints on stranded debris. Mar. Pollut. Bull. 2020, 152, e110894. [Google Scholar] [CrossRef] [PubMed]
- Newbould, R.A.; Powell, D.M.; Whelan, M.J. Macroplastic debris transfer in rivers: A travel distance approach. Front. Water 2021, 3, e724596. [Google Scholar] [CrossRef]
- Ryan, P.G.; Weideman, E.A.; Perold, V.; Hofmeyr, G.; Connan, M. Message in a bottle: Assessing the sources and origins of beach litter to tackle marine pollution. Environ. Pollut. 2021, 288, e117729. [Google Scholar] [CrossRef]
- Zobkov, M.B.; Chubarenko, I.P.; Esiukova, E.E.; Belkina, N.A.; Kovalevski, V.V.; Zobkova, M.V.; Efremova, T.A.; Galakhina, N.E. Lakes as accumulators of microplastics on the way from land to the world ocean: Review. Proc. Russ. Geogr. Soc. 2021, 153, 68–86, (In Russian, English summary). [Google Scholar] [CrossRef]
- Faure, F.A.; Demars, C.A.; Wieser, O.A.; Kunz, M.B.; de Alencastro, L.F. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ. Chem. 2015, 12, 582–591. [Google Scholar] [CrossRef]
- Zbyszewski, M.; Corcoran, P.L.; Hockin, A. Comparison of the distribution and degradation of plastic debris along shorelines of the Great Lakes, North America. J. Great Lakes Res. 2014, 40, 288–299. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Y.; Huang, H.; Xu, W. A Regional difference analysis of microplastic pollution in global freshwater bodies based on a regression model. Water 2020, 12, 1889. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic debris in 29 Great Lakes tributaries: Relations to watershed attributes and hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics profile along the Rhine River. Sci. Rep. 2015, 5, e17988. [Google Scholar] [CrossRef] [Green Version]
- Scherer, C.; Weber, A.; Stock, F.; Vurusic, S.; Egerci, H.; Kochleus, C.; Arendt, A.; Foeldi, C.; Dierkes, G.; Wagner, M.; et al. Comparative assessment of microplastics in water and sediment of a large European river. Sci. Total Environ. 2020, 738, e139866. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Tassin, B. Sources and fate of microplastics in urban areas: A Focus on Paris Megacity. In Freshwater Microplastics. The Handbook of Environmental Chemistry; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 58, pp. 69–83. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Yin, L.; Wen, X.; Du, C.; Wu, L.; Long, Y.; Liu, Y.; Ma, Y.; Yin, Q.; Zhou, Z.; et al. Microplastics in sediment and surface water of west Dongting Lake and south Dongting Lake: Abundance, source and composition. Int. J. Environ. Res. Public Health 2018, 15, 2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Jiang, C.; Wen, X.; Du, C.; Zhong, W.; Feng, Z.; Long, Y.; Ma, Y. Microplastic pollution in surface water of urban lakes in Changsha, China. Int. J. Environ. Res. Public Health 2019, 16, 1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eo, S.; Hee, S.; Kyoung, Y.; Myung, G. Spatiotemporal distribution and annual load of microplastics in the Nakdong River, South Korea. Water Res. 2019, 160, 228–237. [Google Scholar] [CrossRef]
- Nel, H.A.; Hean, J.W.; Noundou, X.S.; Froneman, P.W. Do microplastic loads reflect the population demographics along the southern African coastline? Mar. Pollut. Bull. 2018, 115, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mondal, A.; Bagri, A.; Tiwari, E.; Khandelwal, N.; Monikh, F.A.; Darbha, G.K. Characteristics and spatial distribution of microplastics in the lower Ganga River water and sediment. Mar. Pollut. Bull. 2021, 163, 111960. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bruge, A.; Barreau, C.; Carlot, J.; Collin, H.; Moreno, C.; Maison, P. Monitoring litter inputs from the Adour River (Southwest France) to the marine environment. J. Mar. Sci. Eng. 2018, 6, 24. [Google Scholar] [CrossRef]
- Roebroek, C.T.J.; Harrigan, S.; van Emmerik, T.H.M.; Baugh, C.; Eilander, D.; Prudhomme, C.; Pappenberger, F. Plastic in global rivers: Are floods making it worse? Environ. Res. Lett. 2021, 16, e025003. [Google Scholar] [CrossRef]
- Haberstroh, C.J.; Arias, M.A.; Yin, Z.; Wang, M.C. Effects of hydrodynamics on the cross-sectional distribution and transport of plastic in an urban coastal river. Water Environ. Res. 2021, 93, 186–200. [Google Scholar] [CrossRef]
- Treilles, R.; Gasperi, J.; Gallard, A.; Mohamed, S.A.A.D.; Rachid, D.R.I.S.; Partibane, C. Microplastics and microfibers in urban runoff from a suburban catchment of Greater Paris. Environ. Pollut. 2021, 287, e117352. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, e135578. [Google Scholar] [CrossRef] [PubMed]
- Khatmullina, L.; Isachenko, I. Settling velocity of microplastic particles of regular shapes. Mar. Pollut. Bull. 2017, 114, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Khatmullina, L.; Chubarenko, I. Transport of marine microplastic particles: Why is it so difficult to predict? Anthr. Coasts 2019, 2, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Häbel, H.; Olsson, A.; Sandhagen, S.; von Corswant, C.; Hjärtstam, J.; Persson, M.; Stading, M.; Larsson, A. The influence of the molecular weight of the water-soluble polymer on phase-separated films for controlled release. Int. J. Pharm. 2016, 511, 223–235. [Google Scholar] [CrossRef]
- Corcoran, P.L.; Norris, T.; Ceccanese, T.; Walzak, M.J.; Helm, P.A.; Marvin, C.H. Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record. Env. Pollut. 2015, 204, 17–25. [Google Scholar] [CrossRef]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.R.; Narayanaswamy, B.E.; Thompson, R.S. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 17, 140317. [Google Scholar] [CrossRef]
- Seltenrich, N. New link in the food chain? Marine plastic pollution and seafood safety. Environ. Health Perspect. 2015, 123, A34–A41. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in freshwater sediment: A review on methods, occurrence, and sources. Sci. Total Environ. 2021, 754, 141948. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Drapper, D.; Hornbuckle, A.; Leusch, F.D.L. Microplastic pollution in a stormwater floating treatment wetland: Detection of tyre particles in sediment Shima. Sci. Total Environ. 2019, 713, e136356. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Driedger, A.G.; Dürr, H.H.; Mitchell, K.; Van Cappellen, P. Plastic debris in the Laurentian great lakes: A review. J. Great Lakes Res. 2015, 41, 9–19. [Google Scholar] [CrossRef]
- Frank, Y.A.; Vorobiev, E.D.; Vorobiev, D.S.; Trifonov, A.A.; Antsiferov, D.V.; Soliman, H.T.; Wilson, S.P.; Strezov, V. Preliminary screening for microplastic concentrations in the surface water of the Ob and Tom rivers in Siberia, Russia. Sustainability 2021, 13, 80. [Google Scholar] [CrossRef]
- United Nations Educational, Scientific, Cultural Organization. The United Nations World Water Development Report. 2018. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000261424 (accessed on 3 October 2022).
- Tekman, M.B.; Gutow, L.; Macario, A.; Haas, A.; Walter, A.; Bergmann, M. Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research. Literbase. Available online: https://litterbase.awi.de/litter (accessed on 11 November 2022).
- Frank, Y.A.; Vorobiev, D.S.; Kayler, O.A.; Vorobiev, E.D.; Kulinicheva, K.S.; Trifonov, A.A.; Hunter, T.S. Evidence for microplastics contamination of the remote tributary of the Yenisei River, Siberia. The pilot study results. Water 2021, 13, 3248. [Google Scholar] [CrossRef]
- Pozdnyakov, S.R.; Ivanova, E.V. Estimation of the microplastics concentrations in the water column and bottom sediments of Ladoga Lake. Reg. Ecol. 2018, 4, 48–52. [Google Scholar] [CrossRef]
- Pozdnyakov, S.R.; Ivanova, E.V.; Guzeva, A.V.; Shalunova, E.P.; Martinson, K.D.; Tikhonova, D.A. Studying the concentration of microplastic particles in water, bottom sediments and subsoils in the coastal area of the Neva Bay, the Gulf of Finland. Water Resour. 2020, 47, 599–607. (In Russian) [Google Scholar] [CrossRef]
- Lisina, A.A.; Platonov, M.M.; Lomakov, O.I.; Sazonov, A.A.; Shishova, T.V.; Berkovich, A.K.; Frolova, N.L. Microplastic abundance in Volga River: Results of a pilot study in summer 2020. Geogr. Environ. Sustain. 2021, 14, 82–93. [Google Scholar] [CrossRef]
- Yasinskii, S.V.; Venitsianov, E.V.; Kashutina, E.A.; Sidorova, M.V.; Ershova, A.A.; Makeeva, I.N. Contribution of microparticles to the transport of pollution by rivers and groundwaters in a large city. IOP Conf. Ser. Earth Environ. Sci. 2021, 834, e012047. [Google Scholar] [CrossRef]
- Karnaukhov, D.Y.; Biritskaya, S.A.; Dolinskaya, E.M.; Teplykh, M.A.; Silenko, N.N.; Ermolaeva, Y.K.; Silow, E.A. Pollution by macro- and microplastic of large lacustrine ecosystems in Eastern Asia. Pollut. Res. 2020, 36, 440–442. [Google Scholar]
- Biritskaya, S.A.; Dolinskaya, E.M.; Teplykh, M.A.; Ermolaeva, Y.K.; Pushnica, V.A.; Bukhaeva, L.B.; Kuznetsova, I.V.; Okholina, A.I.; Karnaukhov, D.Y.; Silow, E.A. Water pollution by microplastics over the littoral zone in the southern basin of Lake Baikal. Baikal Zool. J. 2020, 2, 29–32, (In Russian, English summary). [Google Scholar]
- Il’ina, O.V.; Kolobov, M.Y.; Il’inskii, V.V. Plastic pollution of the coastal surface water in the Middle and Southern Baikal. Water Resour. 2021, 48, 56–64. [Google Scholar] [CrossRef]
- Moore, M.V.; Yamamuro, M.; Timoshkin, O.A.; Shirokaya, A.A.; Kameda, Y. Lake-wide assessment of microplastics in the surface waters of Lake Baikal, Siberia. Limnology 2021, 23, 265–274. [Google Scholar] [CrossRef]
- Meyer, M.F.; Ozersky, T.; Woo, K.H.; Shchapov, K.; Galloway, A.W.E.; Schram, J.B.; Snow, D.D.; Timofeyev, M.A.; Karnaukhov, D.Y.; Brousil, M.R.; et al. A unified dataset of colocated sewage pollution, periphyton, and benthic macroinvertebrate community and food web structure from Lake Baikal (Siberia). Limnol. Oceanogr. Lett. 2022, 7, 62–79. [Google Scholar] [CrossRef]
- Hampton, S.E.; McGowan, S.; Ozersky, T.; Virdis, S.G.; Thuy, V.T.; Spanbauer, T.L.; Kraemer, B.M.; Swann, G.; Mackay, A.W.; Powers, S.M.; et al. Recent ecological change in ancient lakes. Limnol. Oceanogr. 2018, 63, 2277–2304. [Google Scholar] [CrossRef] [Green Version]
- Karnaukhov, D.; Biritskaya, S.; Dolinskaya, E.; Teplykh, M.; Ermolaeva, Y.; Pushnica, V.; Bukhaeva, L.; Kuznetsova, I.; Okholina, A.; Silow, E. Distribution features of microplastic particles in the Bolshiye Koty bay (Lake Baikal, Russia) in winter. Pollution 2022, 8, 435–446. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Obbard, R.W.; Sadri, S.; Wong, Y.Q.; Khitun, A.A.; Baker, I.; Thompson, R.C. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Future 2014, 2, 315–320. [Google Scholar] [CrossRef]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018, 95, 1505. [Google Scholar] [CrossRef] [Green Version]
- Geilfus, N.X.; Munson, K.M.; Sousa, J.; Germanov, Y.; Bhugaloo, S.; Babb, D.; Wang, F. Distribution and impacts of microplastic incorporation within sea ice. Mar. Pollut. Bull. 2019, 145, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Chubarenko, I. Physical processes behind interactions of microplastic particles with natural ice. Environ. Res. Commun. 2022, 4, e012001. [Google Scholar] [CrossRef]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liu, X.; Wang, W.; Di, M.; Wang, J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2018, 170, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Krokhalevsky, V.R.; Babkina, I.B.; Wieser, A.M.; Dorogin, M.A.; Zhirkov, F.N.; Zaytsev, V.F.; Interesova, E.A.; Karpova, L.N. Status of sturgeon stocks in Siberian water bodies. Probl. Fish. 2018, 19, 269–284, (In Russian, English summary). [Google Scholar] [CrossRef]
- Zobkov, M.; Esiukova, E. Microplastics in Baltic bottom sediments: Quantification procedures and first results. Mar. Pollut. Bull. 2017, 114, 724–732. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics research–from sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Weber, S.; Traunspurger, W. The effects of predation by juvenile fish on the meiobenthic community structure in a natural pond. Freshw. Biol. 2015, 60, 2392–2409. [Google Scholar] [CrossRef]
- Guzzella, L.M.; Novati, S.; Casatta, N.; Roscioli, C.; Valsecchi, L.; Binelli, A.; Parolini, M.; Solcà, N.; Bettinetti, R.; Manca, M. Spatial and temporal trends of target organic and inorganic micropollutants in Lake Maggiore and Lake Lugano (Italian-Swiss water bodies): Contamination in sediments and biota. Hydrobiologia 2018, 824, 271–290. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, D.; Wan, X.; Wu, Y.; Liu, X.; Gao, B. Comparative analysis of microplastic organization and pollution risk before and after thawing in an urban river in Beijing, China. Sci. Total Environ. 2022, 828, e154268. [Google Scholar] [CrossRef]
- Vasiliev, Y.S.; Maslikov, V.I.; Shilin, M.B.; Chusov, A.N.; Molodtsov, D.V.; Eremina, T.R.; Ershova, A.A. New challenges and opportunities of hydroelectric power plants in the fight against floating garbage. Gidrotekhnicheskoe Stroit. 2019, 4, 48–52. (In Russian) [Google Scholar]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernández-León, S.; Palma, A.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Lebreton, L.C.; Greer, S.D.; Borrero, J.C. Numerical modelling of floating debris in the world’s oceans. Mar. Pollut. Bull. 2012, 64, 653–661. [Google Scholar] [CrossRef]
- Maximenko, N.; Hafner, J.; Niiler, P. Pathways of marine debris derived from trajectories of Lagrangian drifters. Mar. Pollut. Bull. 2012, 65, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Hardesty, B.D.; Lawson, T.J.; van der Velde, T.; Lansdell, M.; Wilcox, C. Estimating quantities and sources of marine debris at a continental scale. Front. Ecol. Environ. 2017, 15, 18–25. [Google Scholar] [CrossRef]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High quantities of microplastic in Arctic deep-sea sediments from the Hausgarten Observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kako, S.; Isobe, A.; Magome, S.; Hinata, H.; Seino, S.; Kozima, A. Establishment of numerical beach litter hindcast/forecast models: An application to Goto Islands, Japan. Mar. Pollut. Bull. 2011, 62, 293–302. [Google Scholar] [CrossRef]
- Schuyler, Q.; Hardesty, B.D.; Wilcox, C.; Townsend, K. Global analysis of anthropogenic debris ingestion by sea turtles. Conserv. Biol. 2014, 28, 129–139. [Google Scholar] [CrossRef]
- Wilcox, C.; van Sebille, E.; Hardesty, B.D. Threat of plastic pollution to seabirds is global, pervasive, and increasing. Environ. Sci. 2015, 112, 11899–11904. [Google Scholar] [CrossRef]
- Santillo, D.; Miller, K.; Johnston, P. Microplastics as contaminants in commercially important seafood species. IEAM Integr. Environ. Assess. Manag. 2017, 13, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Vega-Herrera, A.; Schirinzi, G.; Savva, K.; Abad, E.; Farré, M. Screening of suspected micro(nano)plastics in the Ebro Delta (Mediterranean Sea). J. Hazard. Mater. 2021, 404, e124022. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liang, J.; Niu, Q. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, e124187. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, T.I. Bioavailability and ecotoxicity of metals in water systems: Critical pollution levels. Geochemistry 2019, 7, 675–688. [Google Scholar] [CrossRef]
- Puzanov, A.V.; Bezmaternykh, D.M.; Kirillov, V.V.; Mitrofanova, E.Y.; Yanygina, L.V. Ecosystem features and environmental problems of Lake Teletskoye (Republic of Altai). Limnol. Freshw. Biol. 2020, 4, 624–625. [Google Scholar] [CrossRef]
| Water Body | Drainage Basin | Character of the Study | Sampling Method/Determination of the Water Volume | Volume per Sample, L | Detection and/or Identification Methods | Predominant MP Shape/Polymer Types | Average MP Counts, Items/m3 | Reference |
|---|---|---|---|---|---|---|---|---|
| Northern Dvina River, mouth | Arctic | S/T | neuston net, 0.33 mm/C | nd | visual, FT-IR | fragment/PE, PP | 0.007 | [84] |
| Baikal Lake | Arctic | S | drift net, 0.30 mm/C | 352,000–494,000 | visual, FT-IR | film/PE, PP | 0.27 | [130] |
| Freshwater lakes (White Sea basin) | Arctic | S | direct sampling, 0.10 mm filter/DM | 500 | visual, FT-IR | nd/PE | 0.50 | [34] |
| Volga River | internal watershed | S | manta trawl, 0.30 mm/IM | 25,000–130,000 | visual, DSC | fragment/PE, PP | 0.90 | [126] |
| Baikal Lake, southern part | Arctic | S | tugging neuston net, nd/nd | nd | visual | fiber/nd | 1.07 | [128] |
| Kedovka, Koida, Onego, Vaga rivers (White Sea basin) | Arctic | S | direct sampling, 0.10 mm filter/DM | 500 | visual, FT-IR | film, fiber/PE, PVC | 1.67 | [34] |
| Baikal Lake, southern part | Arctic | S | direct sampling, GF/F filter/DM | 1.50 | visual | fiber, fragment/nd | 1.79 | [132] |
| Yenisei River | Arctic | S | manta trawl, 0.33 mm/IM | 8610–12,400 | visual, hot needle | fiber/nd | 2.89–3.01 | [123] |
| Nizhnaya Tunguska River (Yenisei River system) | Arctic | S | manta trawl, 0.33 mm/IM | 13,100–29,200 | visual, hot needle | fiber/nd | 1.20–4.53 | [123] |
| Baikal Lake, southern part | Arctic | S | neuston net, nd/nd | 16,000–52,000 | visual | fiber/nd | 0.51–6.46 | [129] |
| Tom River (Ob River system) | Arctic | S | manta trawl, 0.33 mm/C | 50,100–61,400 | visual, hot needle | fragment/nd | 44.2 | [120] |
| Ob River | Arctic | S | manta trawl, 0.33 mm/C | 17,700–62,500 | visual, hot needle | fragment/nd | 51.2 | [120] |
| Baikal Lake | Arctic | pumping, plankton net, 0.02 mm/DM | 300 | visual, FT-IR microspectroscopy | fragment/PP, PET | 291 | [131] | |
| Smolenka River, mouth | Atlantic | S | filtering device, 0.10 mm/DM | nd | visual, FT-IR, Raman microspectroscopy | fiber/PET | 1100 | [125] |
| Urban water bodies in Nizhny Novgorod (Volga River system) | internal watershed | S/T | direct sampling, 0.13 mm filter/DM | 10.0 | visual, hot needle | fibers/nd | 500–1300 | [127] |
| Ladoga Lake with tributaries | Atlantic | S | filtering device, 0.10 mm/DM | nd | visual, hot needle | nd/nd | 20–2400 | [124] |
| Neva River, mouth | Atlantic | S | filtering device, 0.10 mm/DM | nd | visual, FT-IR, Raman microspectroscopy | fiber/PET | 3000 | [125] |
| Lakes in Altai | internal watershed | S | direct sampling/DM | 5.00 | visual, SEM/EDS | fragment, film/nd | 11,000 | [75] |
| Water Body | Drainage Basin | Character of the Study | Detection and/or Identification Methods | Predominant Shape/Size Range/Polymer Types | Average MP Content, Items/kg Dry Weight | Reference |
|---|---|---|---|---|---|---|
| Malaya Neva River, mouth | Atlantic | S | visual, FT-IR, Raman microspectroscopy | fiber/PET | 30.0 | [125] |
| Smolenka River, mouth | Atlantic | S | visual, FT-IR, Raman microspectroscopy | fiber/PET | 60.0 | [125] |
| Neva River, mouth | Atlantic | S | visual, FT-IR, Raman microspectroscopy | fiber/PET | 120 | [125] |
| Ladoga Lake with tributaries | Atlantic | S | visual, hot needle | nd/nd | 60–2000 | [124] |
| Yenisei River | Arctic | S | visual, hot needle | fiber/nd | 353–489 | [123] |
| Nizhnaya Tunguska River (Yenisei River system) | Arctic | S | visual, hot needle | fiber/nd | 235–543 | [123] |
| Lake Onego | Arctic | S/T | visual, Raman spectroscopy | fiber/PC, PE, cellophane, PAN | 2189 | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frank, Y.; Ershova, A.; Batasheva, S.; Vorobiev, E.; Rakhmatullina, S.; Vorobiev, D.; Fakhrullin, R. Microplastics in Freshwater: A Focus on the Russian Inland Waters. Water 2022, 14, 3909. https://doi.org/10.3390/w14233909
Frank Y, Ershova A, Batasheva S, Vorobiev E, Rakhmatullina S, Vorobiev D, Fakhrullin R. Microplastics in Freshwater: A Focus on the Russian Inland Waters. Water. 2022; 14(23):3909. https://doi.org/10.3390/w14233909
Chicago/Turabian StyleFrank, Yulia, Alexandra Ershova, Svetlana Batasheva, Egor Vorobiev, Svetlana Rakhmatullina, Danil Vorobiev, and Rawil Fakhrullin. 2022. "Microplastics in Freshwater: A Focus on the Russian Inland Waters" Water 14, no. 23: 3909. https://doi.org/10.3390/w14233909










