Different Adsorption Behaviors and Mechanisms of Anionic Azo Dyes on Polydopamine–Polyethyleneimine Modified Thermoplastic Polyurethane Nanofiber Membranes
Abstract
:1. Introduction
2. Experimental Sections
2.1. Chemicals and Materials
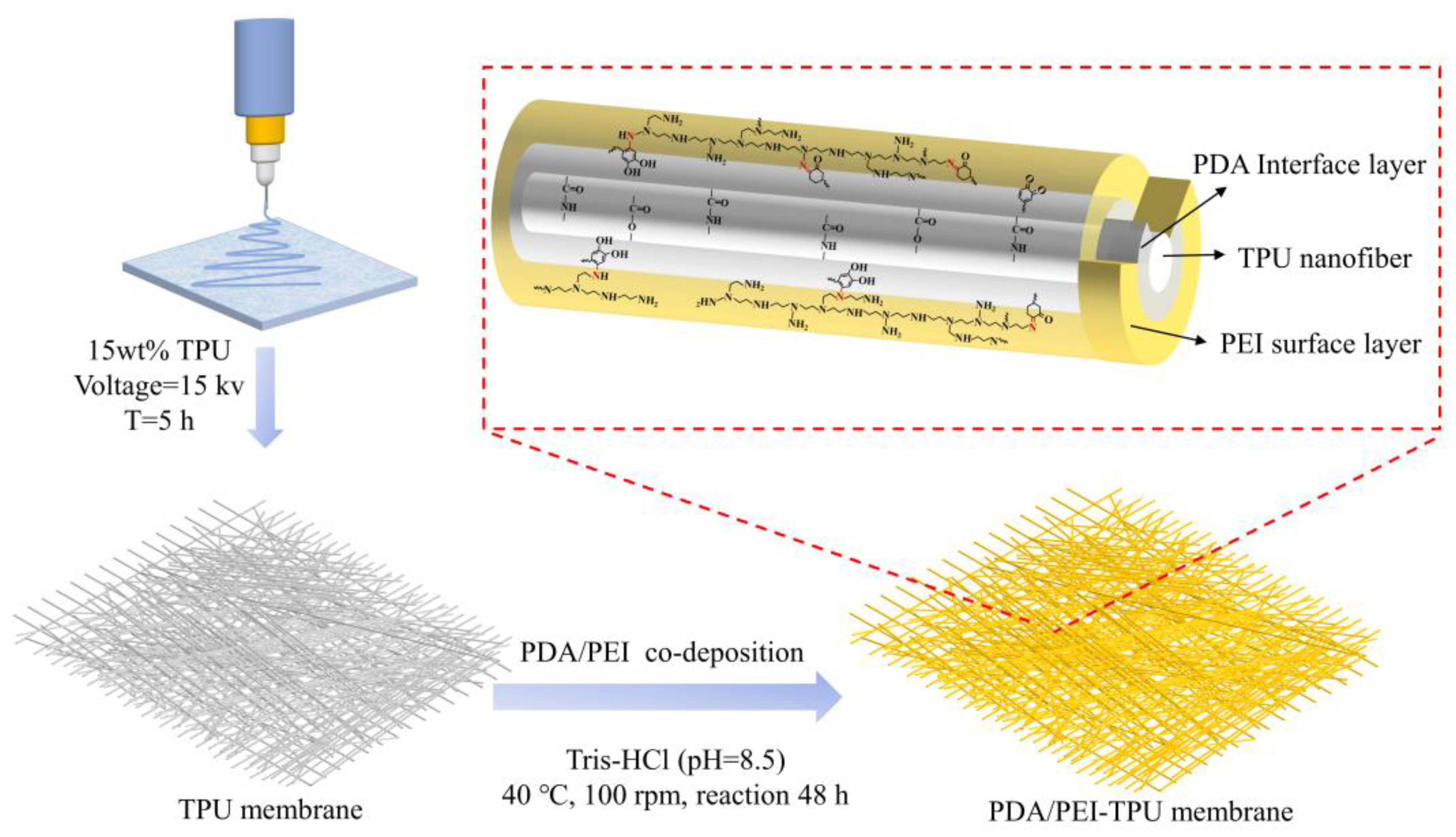
2.2. Synthesis of TPU and PDA/PEI-TPU NFMs
2.3. Characterization of Adsorbents
2.4. Batch Adsorption Experiments
2.4.1. The Impact of Initial Solution pH
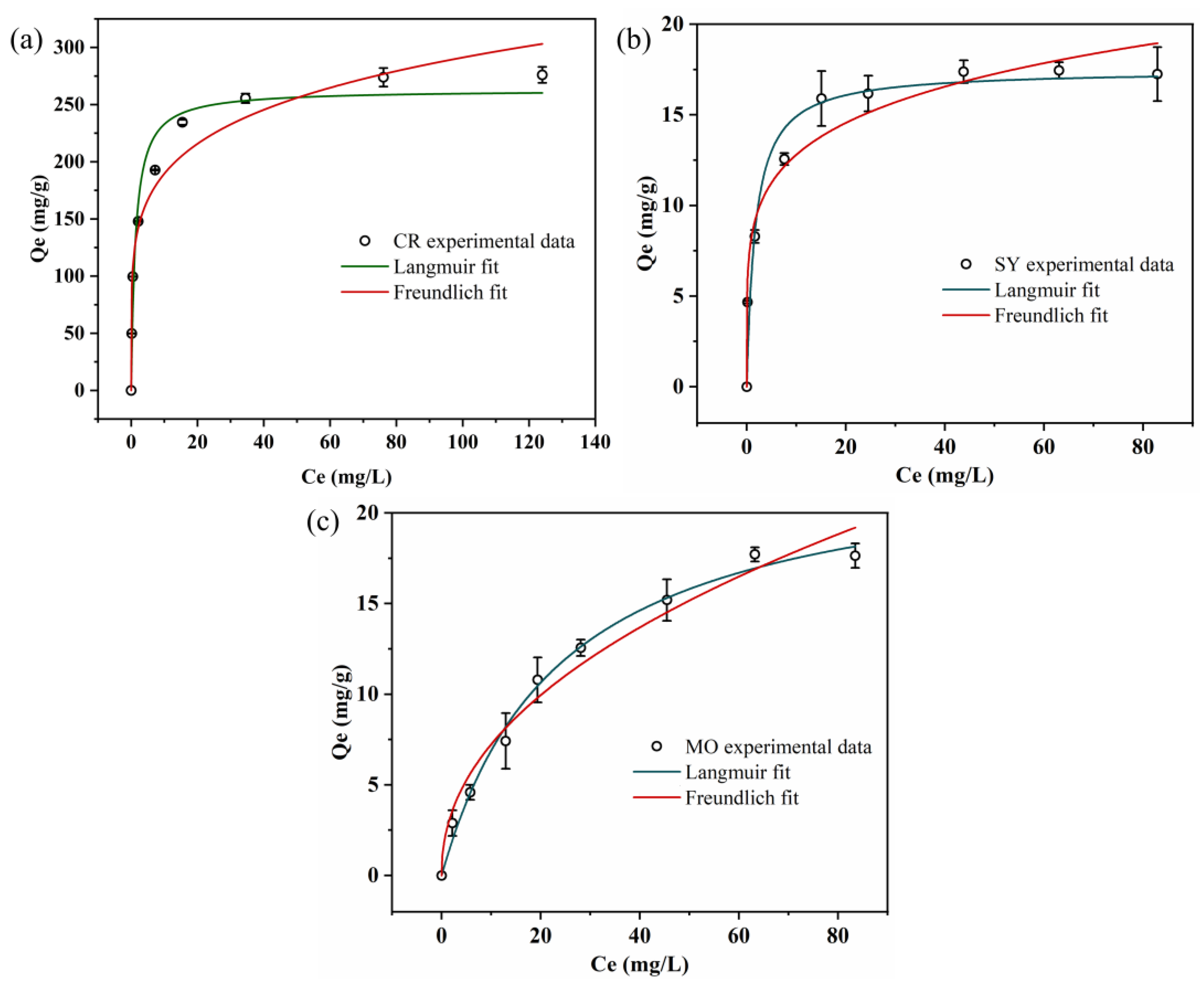
2.4.2. Equilibrium Isotherms
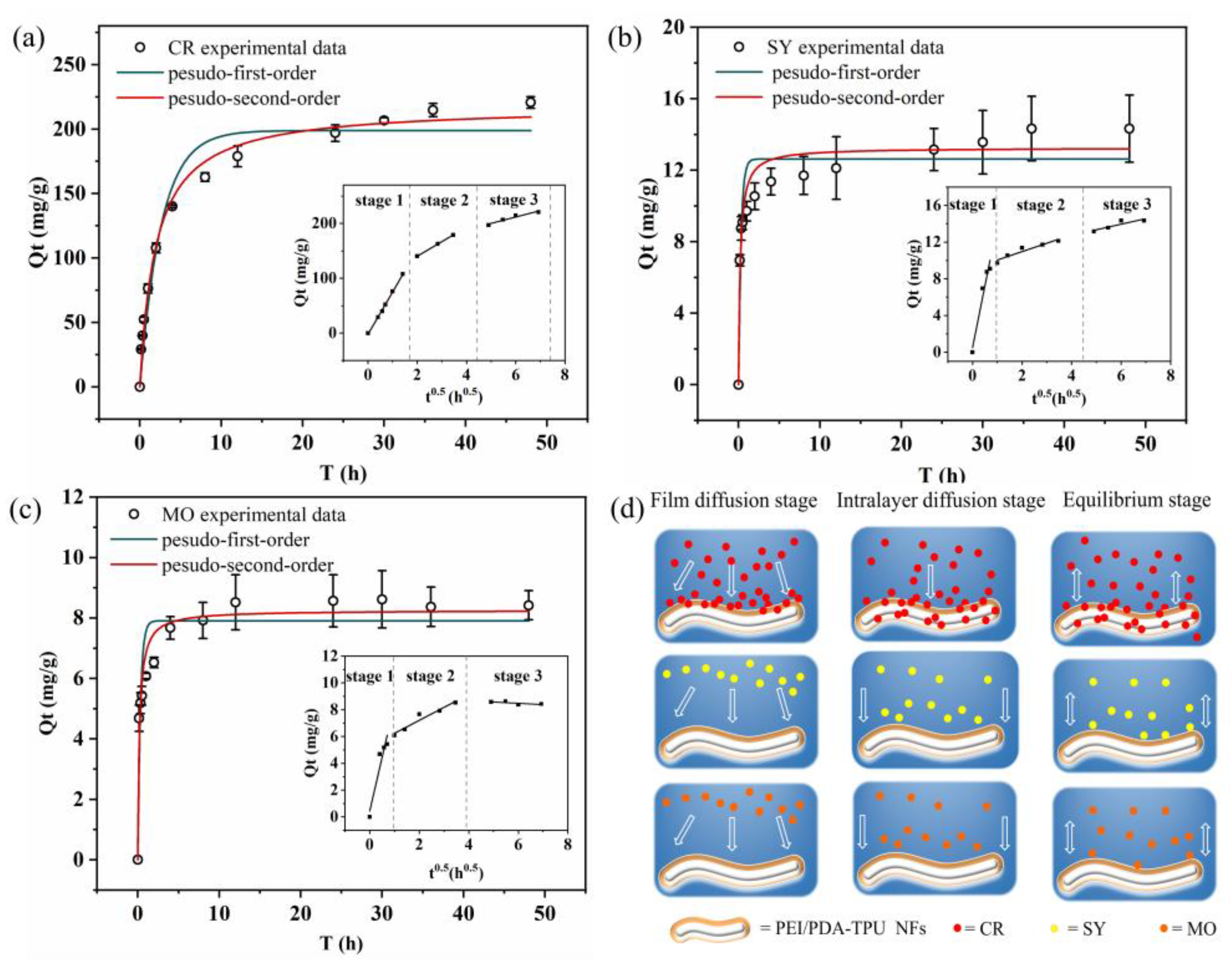
2.4.3. Kinetic Adsorption
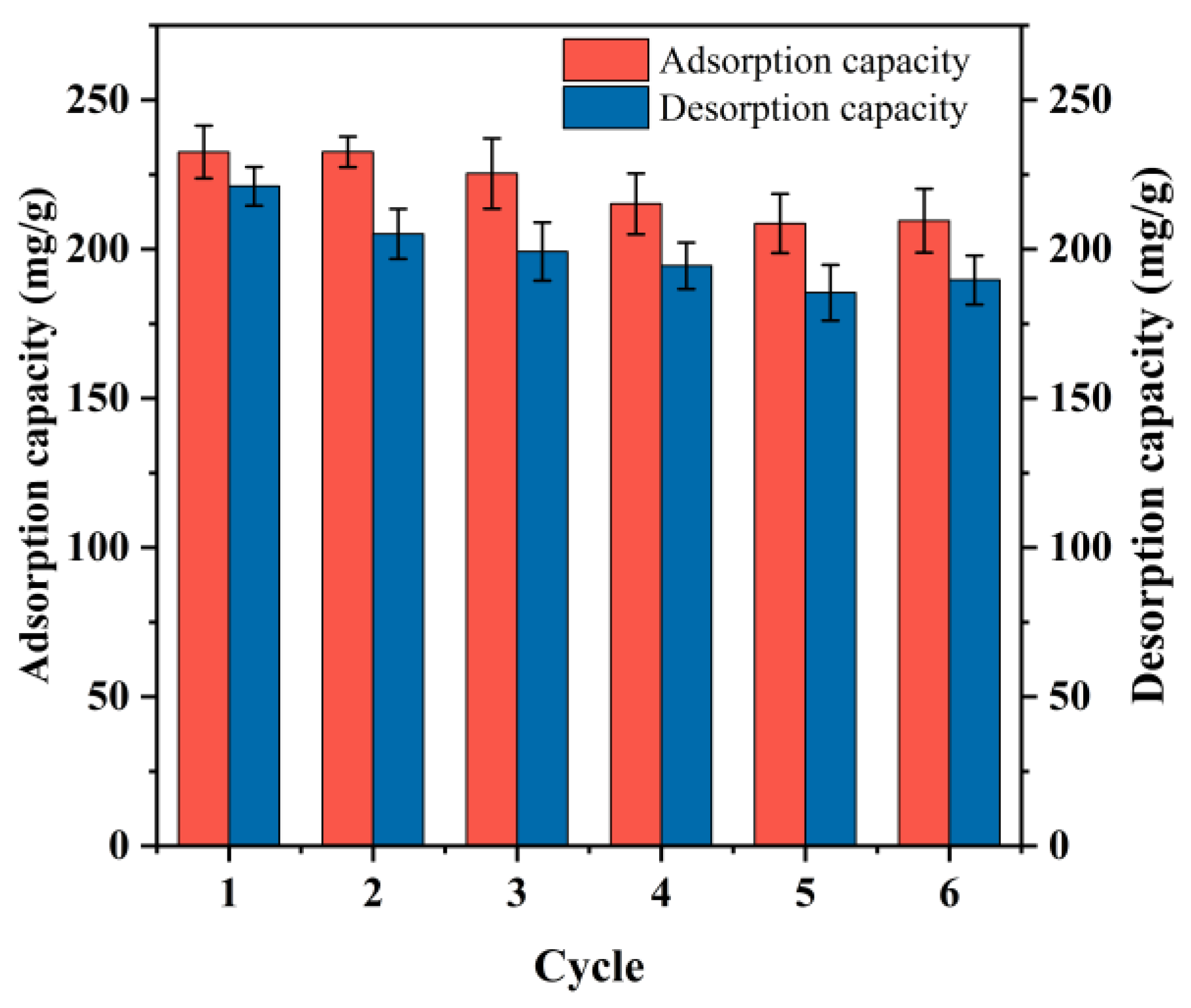
2.4.4. Desorption Experiments
3. Results and Discussion
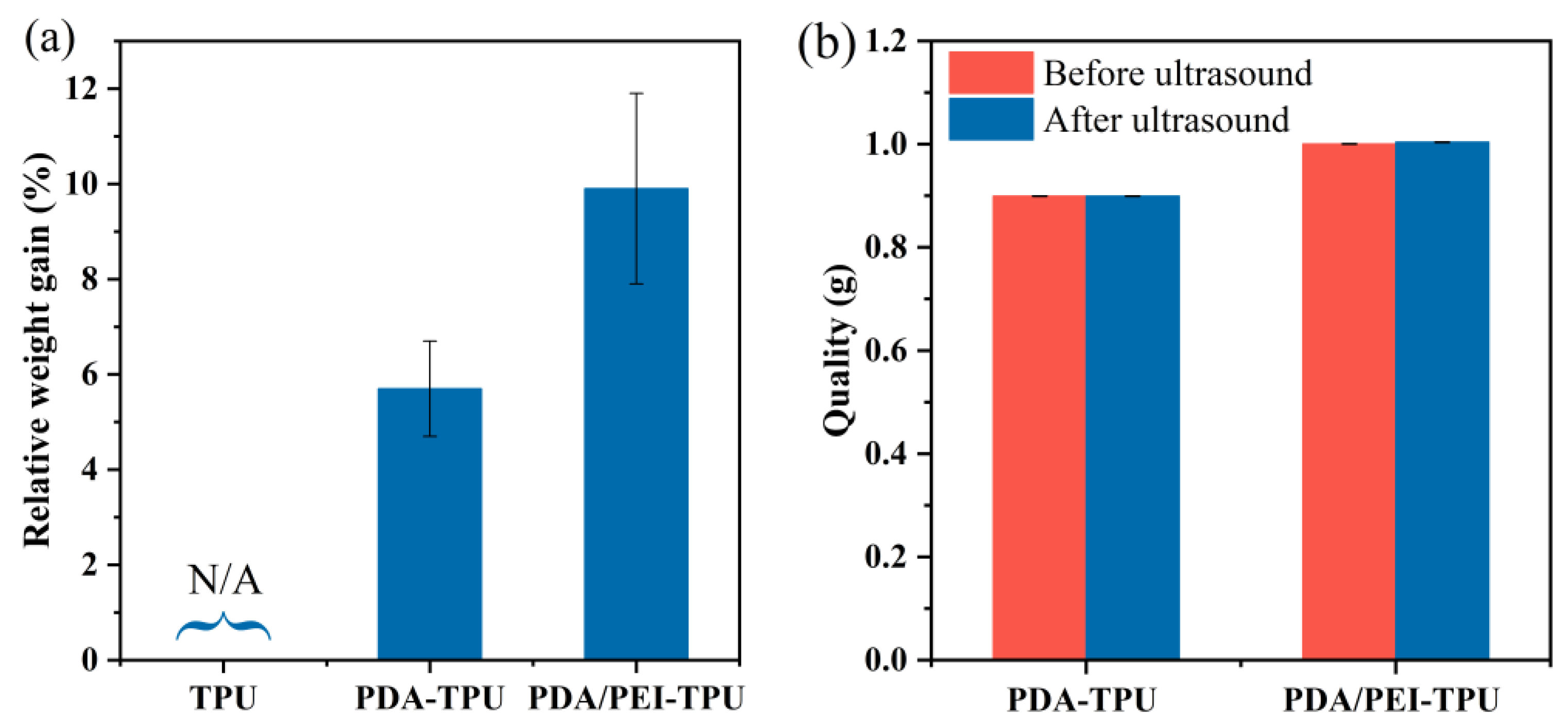
3.1. The Grafted Yield and Stability of PDA/PEI-TPU NFMs
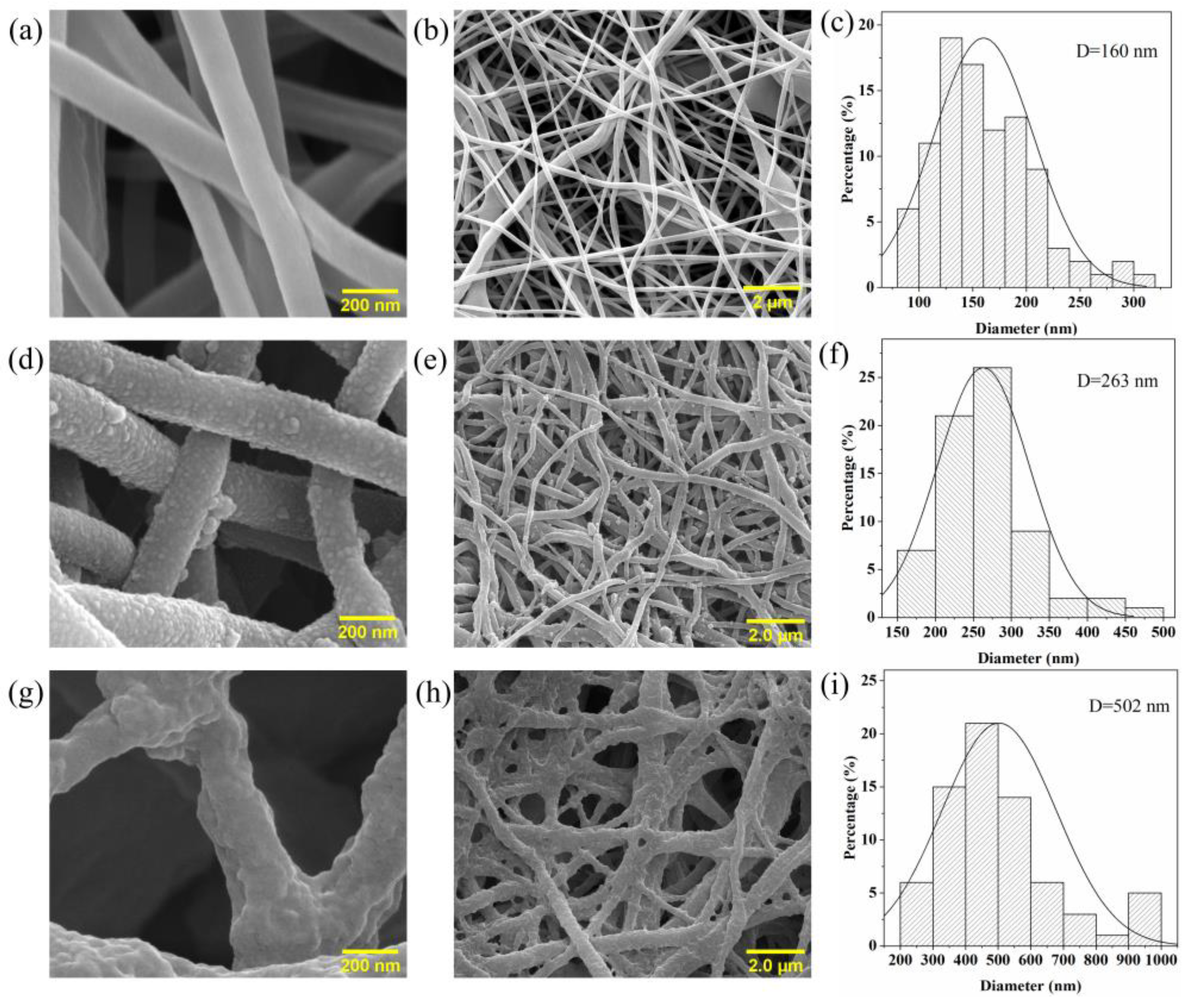
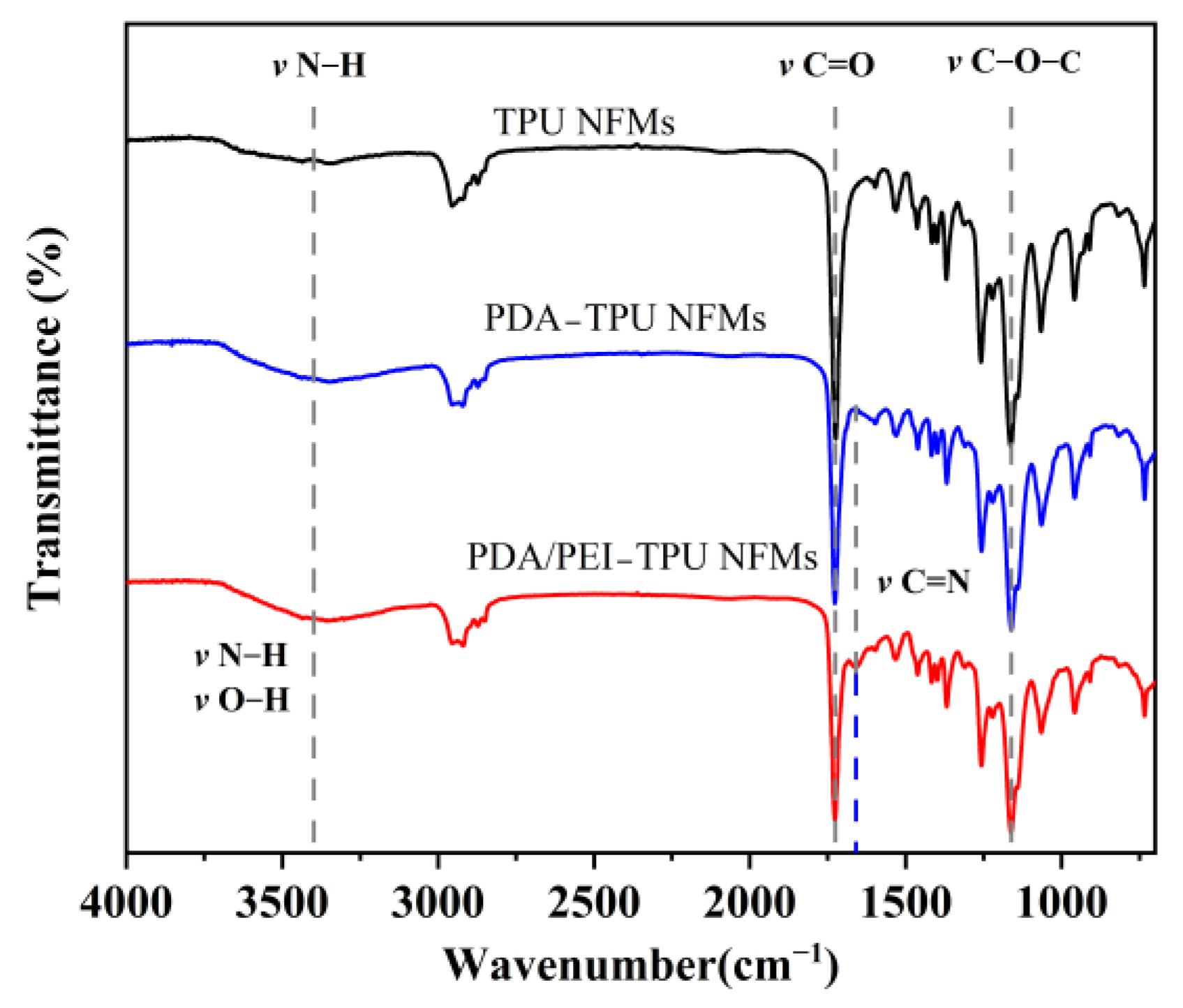
3.2. Morphology and Microstructure of the Nanofiber Membranes
3.3. Dye Adsorption Ability
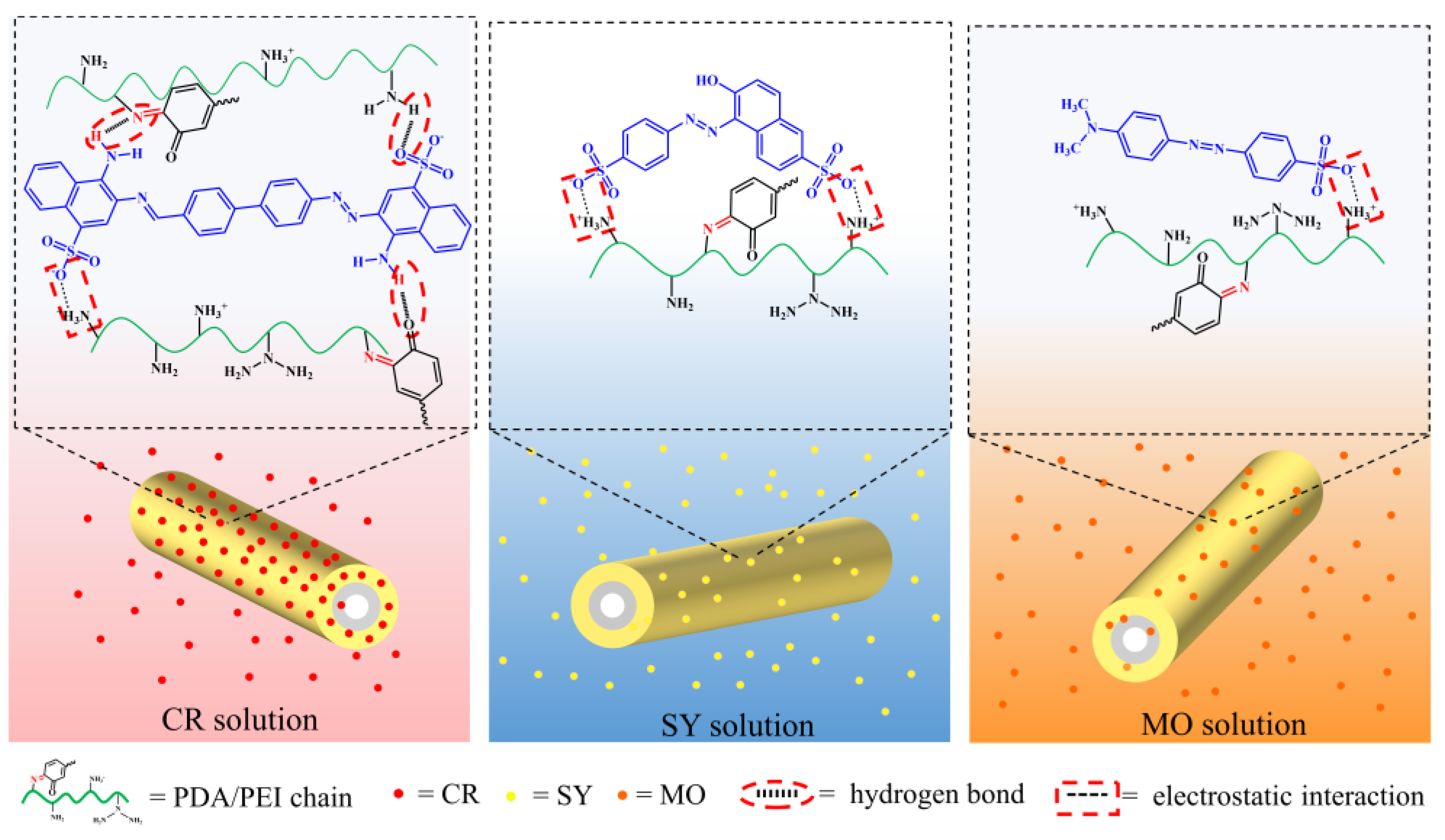
3.4. Possible Adsorption Mechanism
3.5. Adsorption Kinetics and Adsorption Isotherm for Three Dyes
3.6. Regeneration of the PDA/PEI-TPU NFMs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Usman, M.; Ahmed, A.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Simultaneous Adsorption of Heavy Metals and Organic Dyes by β-Cyclodextrin-Chitosan Based Cross-Linked Adsorbent. Carbohydr. Polym. 2021, 255, 117486. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, P.; Yin, Y.; Zhou, X.; Fu, L.; Wang, L.; Chen, S.; Tang, X. Preparation of Pyridine-Modified Cotton Fibers for Anionic Dye Treatment. React. Funct. Polym. 2022, 172, 105155. [Google Scholar] [CrossRef]
- Chung, K.-T. Azo Dyes and Human Health: A Review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Puvaneswari, N.; Muthukrishnan, J.; Gunasekaran, P. Toxicity Assessment and Microbial Degradation of Azo Dyes. Indian J. Exp. Biol. 2006, 44, 618–626. [Google Scholar] [PubMed]
- Cui, M.-H.; Liu, W.-Z.; Tang, Z.-E.; Cui, D. Recent Advancements in Azo Dye Decolorization in Bio-Electrochemical Systems (BESs): Insights into Decolorization Mechanism and Practical Application. Water Res. 2021, 203, 117512. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; Fan, J.; Yu, J.; Ho, W. Review on Nickel-Based Adsorption Materials for Congo Red. J. Hazard. Mater. 2021, 403, 123559. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, M.O.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and Decontamination Strategies of Congo Red Dye. Groundw. Sustain. Dev. 2022, 19, 100844. [Google Scholar] [CrossRef]
- Moradi, O.; Pudineh, A.; Sedaghat, S. Synthesis and Characterization Agar/GO/ZnO NPs Nanocomposite for Removal of Methylene Blue and Methyl Orange as Azo Dyes from Food Industrial Effluents. Food Chem. Toxicol. 2022, 169, 113412. [Google Scholar] [CrossRef]
- Brüschweiler, B.J.; Merlot, C. Azo Dyes in Clothing Textiles Can Be Cleaved into a Series of Mutagenic Aromatic Amines Which Are Not Regulated Yet. Regul. Toxicol. Pharmacol. 2017, 88, 214–226. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon Composite Lignin-Based Adsorbents for the Adsorption of Dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef]
- Bichave, M.S.; Kature, A.Y.; Koranne, S.V.; Shinde, R.S.; Gongle, A.S.; Choudhari, V.P.; Topare, N.S.; Raut-Jadhav, S.; Bokil, S.A. Nano-Metal Oxides-Activated Carbons for Dyes Removal: A Review. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Easwaran, G.; Packialakshmi, J.S.; Syed, A.; Elgorban, A.M.; Vijayan, M.; Sivakumar, K.; Bhuvaneswari, K.; Palanisamy, G.; Lee, J. Silica Nanoparticles Derived from Arundo Donax L. Ash Composite with Titanium Dioxide Nanoparticles as an Efficient Nanocomposite for Photocatalytic Degradation Dye. Chemosphere 2022, 307, 135951. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Feng, T.; Zhou, Z.; Wu, H. Removal of Methylene Blue in Water by Electrospun PAN/β-CD Nanofibre Membrane. e-Polymers 2021, 21, 398–410. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, F.F.; Zhang, N.; Wei, X.; Yang, J.; Zhou, Z.W. Blend-Electrospun Poly(Vinylidene Fluoride)/Polydopamine Membranes: Self-Polymerization of Dopamine and the Excellent Adsorption/Separation Abilities. J. Mater. Chem. A 2017, 5, 14430–14443. [Google Scholar] [CrossRef]
- Abd Halim, N.S.; Wirzal, M.D.H.; Hizam, S.M.; Bilad, M.R.; Nordin, N.A.H.M.; Sambudi, N.S.; Putra, Z.A.; Yusoff, A.R.M. Recent Development on Electrospun Nanofiber Membrane for Produced Water Treatment: A Review. J. Environ. Chem. Eng. 2021, 9, 104613. [Google Scholar] [CrossRef]
- Chen, H.; Huang, M.; Liu, Y.; Meng, L.; Ma, M. Functionalized Electrospun Nanofiber Membranes for Water Treatment: A Review. Sci. Total Environ. 2020, 739, 139944. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.-F.; Al Yousif, E.; Xia, F.; Wang, Y.; Guo, L.; Tu, Y.; Zhang, X.; Shao, J.; He, Y. Novel Electrospun TPU/PDMS/PMMA Membrane for Phenol Separation from Saline Wastewater via Membrane Aromatic Recovery System. Sep. Purif. Technol. 2019, 212, 21–29. [Google Scholar] [CrossRef]
- Selvasembian, R.; Gwenzi, W.; Chaukura, N.; Mthembu, S. Recent Advances in the Polyurethane-Based Adsorbents for the Decontamination of Hazardous Wastewater Pollutants. J. Hazard. Mater. 2021, 417, 125960. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, Z.; Liu, T.; Wang, Q.; Li, C.; Li, X. NH2-Grafting on Micro/Nano Architecture Designed PS/TPU@SiO2 Electrospun Microfiber Membrane for Adsorption of Cr(VI). Desalin. Water Treat. 2019, 154, 82–91. [Google Scholar] [CrossRef]
- Anju, M.; Renuka, N.K. Magnetically Actuated Graphene Coated Polyurethane Foam as Potential Sorbent for Oils and Organics. Arab. J. Chem. 2020, 13, 1752–1762. [Google Scholar] [CrossRef]
- Santos, O.S.H.; Coelho da Silva, M.; Silva, V.R.; Mussel, W.N.; Yoshida, M.I. Polyurethane Foam Impregnated with Lignin as a Filler for the Removal of Crude Oil from Contaminated Water. J. Hazard. Mater. 2017, 324, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Vieira Amorim, F.; José Ribeiro Padilha, R.; Maria Vinhas, G.; Ramos Luiz, M.; Costa de Souza, N.; Medeiros Bastos de Almeida, Y. Development of Hydrophobic Polyurethane/Castor Oil Biocomposites with Agroindustrial Residues for Sorption of Oils and Organic Solvents. J. Colloid Interface Sci. 2021, 581, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-J.; Lim, J.S.; Hwang, J.Y.; Kim, M.; Jeong, H.S.; Park, M.S. Carboxymethlyated Cellulose Nanofibrils(CMCNFs) Embedded in Polyurethane Foam as a Modular Adsorbent of Heavy Metal Ions. Carbohydr. Polym. 2018, 195, 136–142. [Google Scholar] [CrossRef]
- Xue, D.; Li, T.; Liu, Y.; Yang, Y.; Zhang, Y.; Cui, J.; Guo, D. Selective Adsorption and Recovery of Precious Metal Ions from Water and Metallurgical Slag by Polymer Brush Graphene–Polyurethane Composite. React. Funct. Polym. 2019, 136, 138–152. [Google Scholar] [CrossRef]
- Jin, L.; Gao, Y.; Yin, J.; Zhang, X.; He, C.; Wei, Q.; Liu, X.; Liang, F.; Zhao, W.; Zhao, C. Functionalized Polyurethane Sponge Based on Dopamine Derivative for Facile and Instantaneous Clean-up of Cationic Dyes in a Large Scale. J. Hazard. Mater. 2020, 400, 123203. [Google Scholar] [CrossRef]
- Li, Z.; Chen, K.; Chen, Z.; Li, W.; Biney, B.W.; Guo, A.; Liu, D. Removal of Malachite Green Dye from Aqueous Solution by Adsorbents Derived from Polyurethane Plastic Waste. J. Environ. Chem. Eng. 2021, 9, 104704. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Z.; Long, R.; Sun, Y.; Wang, M.; Li, X.; Zeng, G. Polydopamine Intimate Contacted Two-Dimensional/Two-Dimensional Ultrathin Nylon Basement Membrane Supported RGO/PDA/MXene Composite Material for Oil-Water Separation and Dye Removal. Sep. Purif. Technol. 2020, 247, 116945. [Google Scholar] [CrossRef]
- Lefebvre, L.; Agusti, G.; Bouzeggane, A.; Edouard, D. Adsorption of Dye with Carbon Media Supported on Polyurethane Open Cell Foam. Catal. Today 2018, 301, 98–103. [Google Scholar] [CrossRef]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Schadeck Netto, M.; Oliveira, M.L.S.; Seliem, M.K.; Luiz Dotto, G.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of Congo Red and Methylene Blue Dyes on an Ashitaba Waste and a Walnut Shell-Based Activated Carbon from Aqueous Solutions: Experiments, Characterization and Physical Interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Guo, R.; Guo, W.; Pei, H.; Wang, B.; Guo, X.; Liu, N.; Mo, Z. Polypyrrole Deposited Electrospun PAN/PEI Nanofiber Membrane Designed for High Efficient Adsorption of Chromium Ions (VI) in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127183. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal Violet Dye Sorption over Acrylamide/Graphene Oxide Bonded Sodium Alginate Nanocomposite Hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Liao, K.-J.; Huang, H.; Wu, Q.-Y.; Wan, L.-S.; Xu, Z.-K. Mussel-Inspired Modification of a Polymer Membrane for Ultra-High Water Permeability and Oil-in-Water Emulsion Separation. J. Mater. Chem. A 2014, 2, 10225–10230. [Google Scholar] [CrossRef]
- Sundaran, S.P.; Reshmi, C.R.; Sagitha, P.; Manaf, O.; Sujith, A. Multifunctional Graphene Oxide Loaded Nanofibrous Membrane for Removal of Dyes and Coliform from Water. J. Environ. Manag. 2019, 240, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, H.; Zhu, L.; Wang, G.; Zeng, Z.; Li, X. High Flux and High Selectivity Thin-Film Composite Membranes Based on Ultrathin Polyethylene Porous Substrates for Continuous Removal of Anionic Dyes. J. Environ. Chem. Eng. 2022, 10, 107202. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm Models for Adsorption of Heavy Metals from Water—A Review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Peng, Y.; Feng, L.; Li, X.; Zhao, C.; Sarfaraz, K. Novel Cationic Polymer Modified Magnetic Chitosan Beads for Efficient Adsorption of Heavy Metals and Dyes over a Wide PH Range. Int. J. Biol. Macromol. 2020, 156, 289–301. [Google Scholar] [CrossRef]
- Almasian, A.; Jalali, M.L.; Fard, G.C.; Maleknia, L. Surfactant Grafted PDA-PAN Nanofiber: Optimization of Synthesis, Characterization and Oil Absorption Property. Chem. Eng. J. 2017, 326, 1232–1241. [Google Scholar] [CrossRef]
- Fang, J.; Chen, Y.; Fang, C.; Zhu, L. Regenerable Adsorptive Membranes Prepared by Mussel-Inspired Co-Deposition for Aqueous Dye Removal. Sep. Purif. Technol. 2022, 281, 119876. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Ibrahim, Y.; Almarzooqi, F.; Naddeo, V.; Karanikolos, G.N.; Alhseinat, E.; Banat, F.; Hasan, S.W. Synthesis of Polydopamine Coated Tungsten Oxide@ Poly(Vinylidene Fluoride-Co-Hexafluoropropylene) Electrospun Nanofibers as Multifunctional Membranes for Water Applications. Chem. Eng. J. 2022, 427, 131021. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of Membrane Chemistry and Coating Layer on Physiochemical Properties of Thin Film Composite Polyamide RO and NF Membranes: I. FTIR and XPS Characterization of Polyamide and Coating Layer Chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, W.; Song, X.; Duan, Q.; Zhu, E. A Novel Strategy of Lock-in Effect between Conjugated Polymer and TiO2 towards Dramatic Enhancement of Photocatalytic Activity under Visible Light. Sci. Rep. 2020, 10, 6513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Xu, W.Z.; Charpentier, P.A. Synthesis and Photocatalytic Antibacterial Properties of Poly [2,11′-Thiopheneethylenethiophene-Alt-2,5-(3-Carboxyl)Thiophene]. ACS Appl. Polym. Mater. 2020, 2, 1886–1896. [Google Scholar] [CrossRef]
- Zúñiga-Zamora, A.; García-Mena, J.; Cervantes-González, E. Removal of Congo Red from the Aqueous Phase by Chitin and Chitosan from Waste Shrimp. Desalin. Water Treat. 2016, 57, 14674–14685. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, Z.; Wu, D. Synthesis of Graphene Oxide/Polyethyleneimine Sponge and Its Performance in the Sustainable Removal of Cu(II) from Water. Sci. Total Environ. 2022, 806, 151258. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kimura, S.; Wada, M. Highly Enhanced Adsorption of Congo Red onto Dialdehyde Cellulose-Crosslinked Cellulose-Chitosan Foam. Carbohydr. Polym. 2019, 214, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wei, J.; Liu, K.; Liu, N.; Zhou, B. Adsorption of Bisphenol A Based on Synergy between Hydrogen Bonding and Hydrophobic Interaction. Langmuir 2014, 30, 13861–13868. [Google Scholar] [CrossRef]
- Ahmad, Z.U.; Yao, L.; Wang, J.; Gang, D.D.; Islam, F.; Lian, Q.; Zappi, M.E. Neodymium Embedded Ordered Mesoporous Carbon (OMC) for Enhanced Adsorption of Sunset Yellow: Characterizations, Adsorption Study and Adsorption Mechanism. Chem. Eng. J. 2019, 359, 814–826. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.M.; Tadé, M.O. Adsorptive Remediation of Environmental Pollutants Using Novel Graphene-Based Nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Hu, Y.; Chen, H.; Liu, X.; Dang, X. Adsorption Mechanism Study of Multinuclear Metal Coordination Cluster Zn5 for Anionic Dyes Congo Red and Methyl Orange: Experiment and Molecular Simulation. Appl. Surf. Sci. 2022, 586, 152745. [Google Scholar] [CrossRef]
- Zhao, S.; Zhan, Y.; Feng, Q.; Yang, W.; Dong, H.; Sun, A.; Wen, X.; Chiao, Y.-H.; Zhang, S. Easy-Handling Carbon Nanotubes Decorated Poly(Arylene Ether Nitrile)@tannic Acid/Carboxylated Chitosan Nanofibrous Composite Absorbent for Efficient Removal of Methylene Blue and Congo Red. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127069. [Google Scholar] [CrossRef]
- Lu, M.; Wu, Q.; Guan, X.-H.; Zheng, Q.-Y.; Wang, G.-S. Preparation of C/CoFe2O4 Nanocomposites Based on Membrane Dispersion-Hydrothermal Carbonization and Their Application for Dyeing Removal. Desalin. Water Treat. 2019, 148, 285–295. [Google Scholar] [CrossRef]
- Wang, J.; Cai, C.; Zhang, Z.; Li, C.; Liu, R. Electrospun Metal-Organic Frameworks with Polyacrylonitrile as Precursors to Hierarchical Porous Carbon and Composite Nanofibers for Adsorption and Catalysis. Chemosphere 2020, 239, 124833. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, C.; Hou, T.; Cai, Y.; Liang, L.; Chen, L.; Li, M. Polyhexamethylene Guanidine Functionalized Chitosan Nanofiber Membrane with Superior Adsorption and Antibacterial Performances. React. Funct. Polym. 2019, 145, 104379. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, M.; Guo, X.; Zeng, L.; Luo, J. Fabrication and Adsorption Behavior for Congo Red of Chitosan and Alginate Sponge. Integr. Ferroelectr. 2014, 151, 61–75. [Google Scholar] [CrossRef]
- Pi, Y.; Duan, C.; Zhou, Y.; Sun, S.; Yin, Z.; Zhang, H.; Liu, C.; Zhao, Y. The Effective Removal of Congo Red Using a Bio-Nanocluster: Fe3O4 Nanoclusters Modified Bacteria. J. Hazard. Mater. 2022, 424, 127577. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Noreen, S.; Tahir, M.A.; Park, S.-J. Chemically Modified Sugarcane Bagasse-Based Biocomposites for Efficient Removal of Acid Red 1 Dye: Kinetics, Isotherms, Thermodynamics, and Desorption Studies. Chemosphere 2022, 291, 132796. [Google Scholar] [CrossRef]
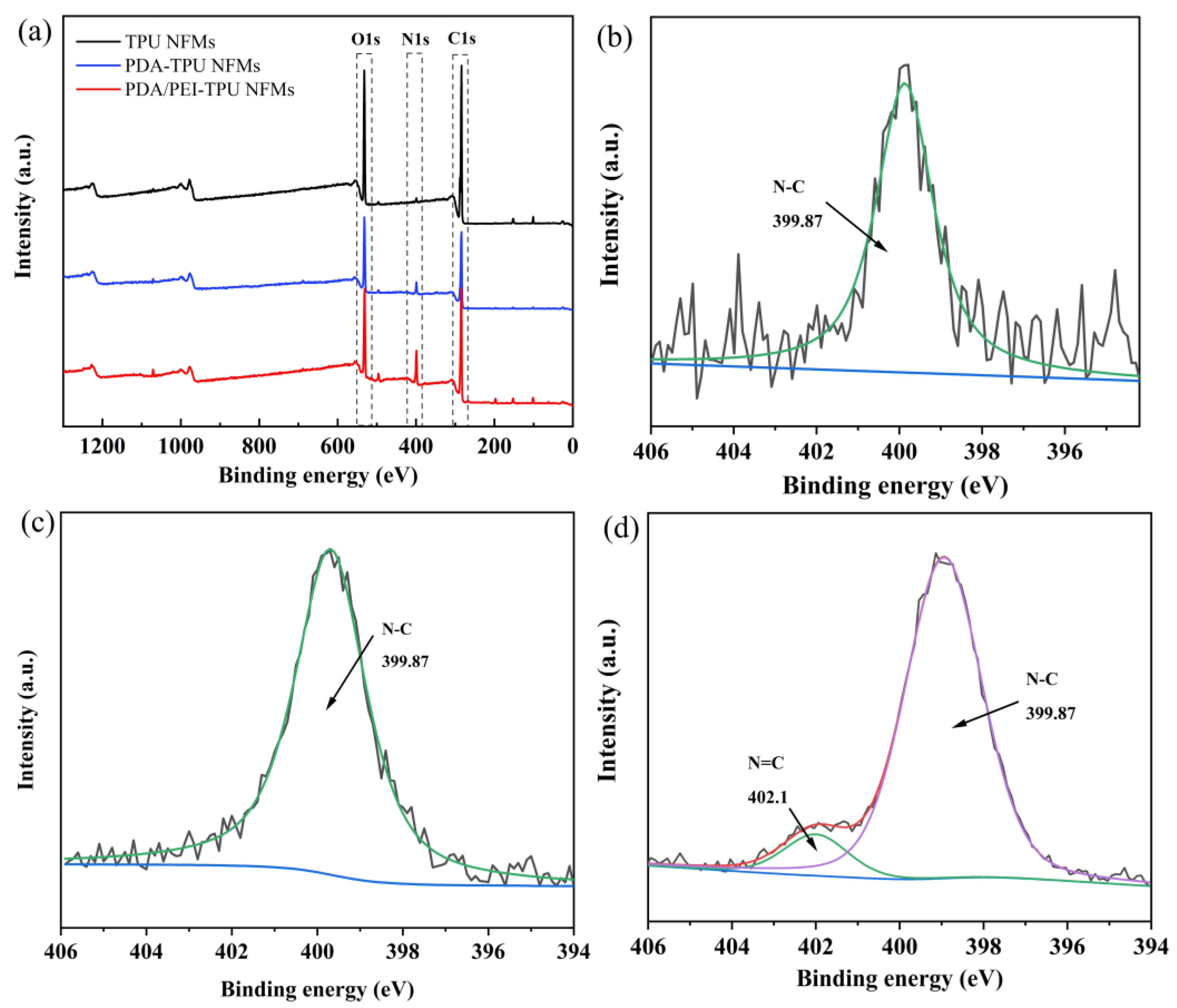
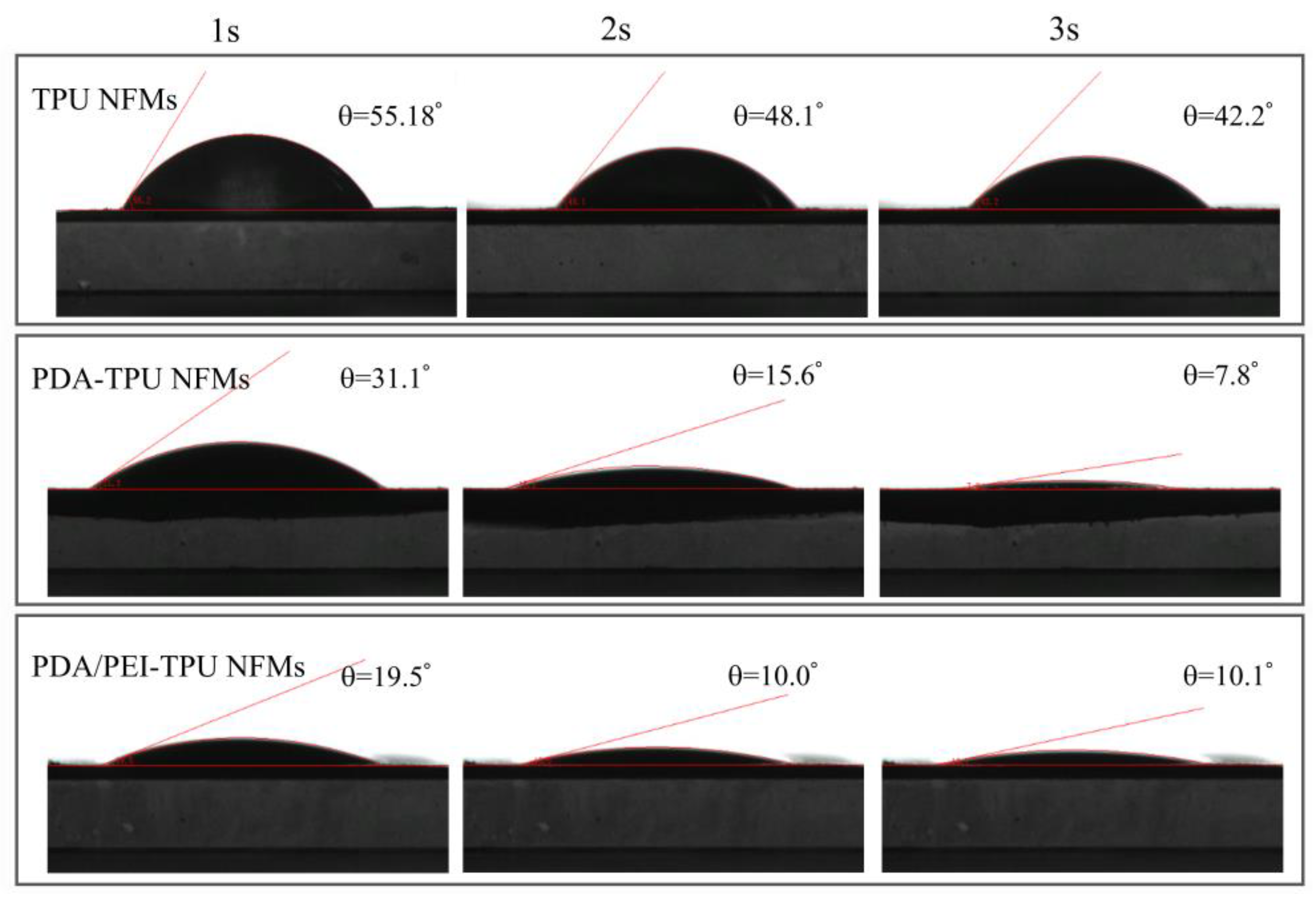
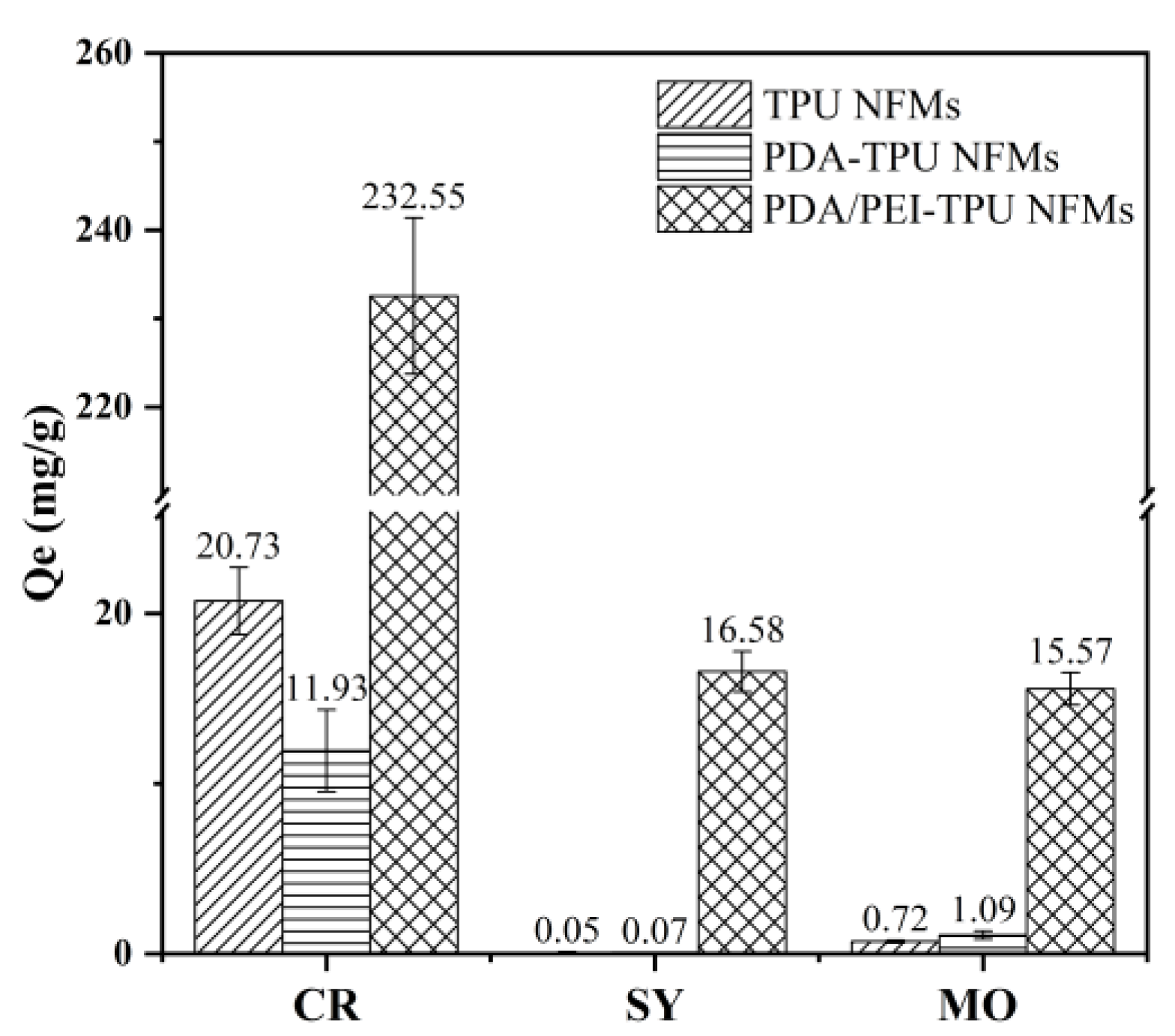

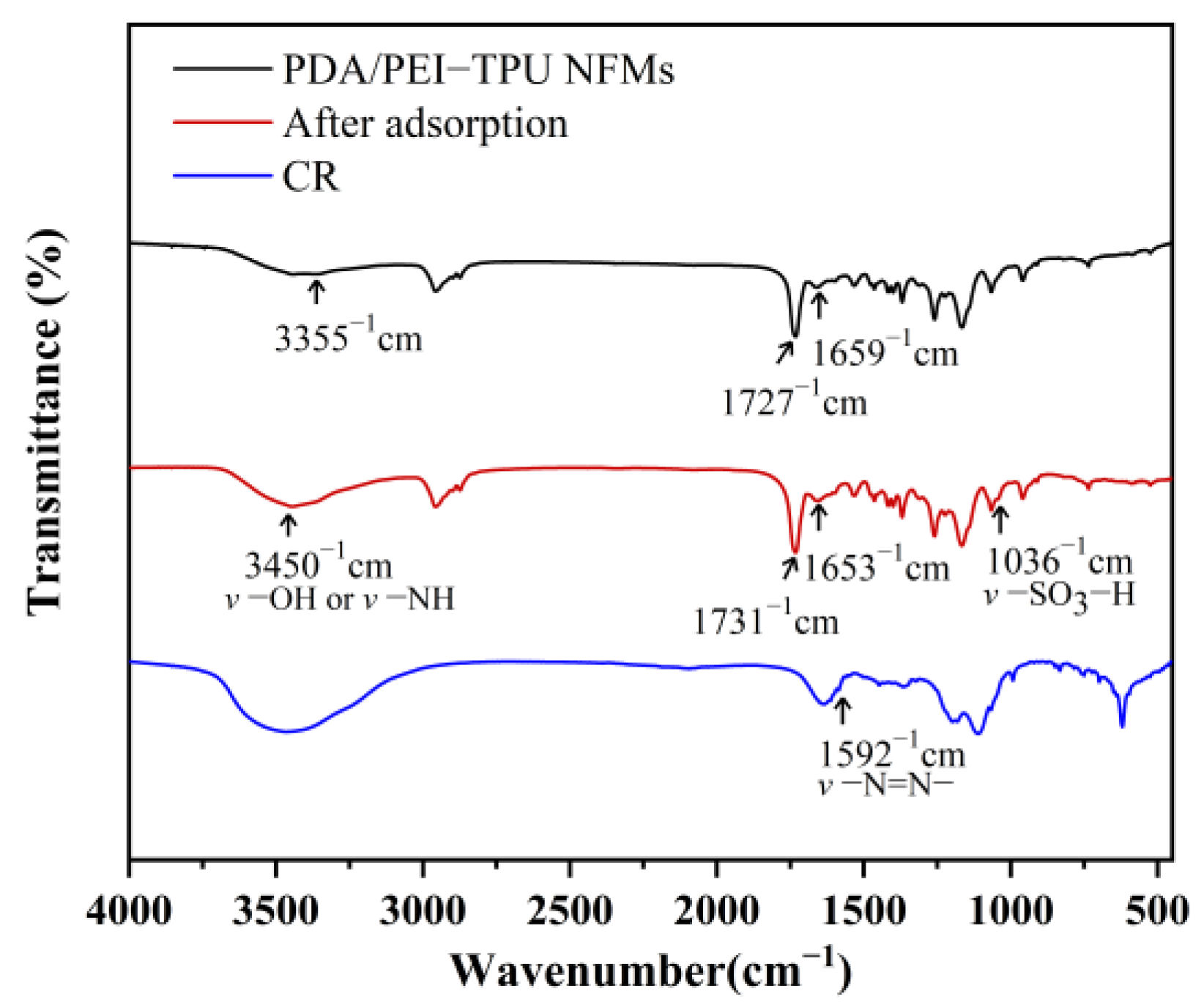
| Membranes | C1s | O1s | N1s | N/C Ratio | N/O Ratio |
|---|---|---|---|---|---|
| TPU NFMs | 71.77 | 26.81 | 1.42 | 0.02 | 0.052 |
| PDA-TPU NFMs | 68.78 | 25.71 | 5.51 | 0.08 | 0.21 |
| PDA/PEI-TPU NFMs | 66.46 | 22.35 | 11.19 | 0.168 | 0.500 |
| Kinetic Models | Parameters | CR | SY | MO |
|---|---|---|---|---|
| Pseudo-first order | qe (mg/g) | 198.84 | 12.63 | 7.90 |
| k1 (1/h) | 0.38 | 3.26 | 3.12 | |
| R2 | 0.954 | 0.834 | 0.867 | |
| Pseudo-second order | qe (mg/g) | 217.99 | 13.24 | 8.25 |
| k2 (g/(mg h)) | 0.0023 | 0.36 | 0.58 | |
| R2 | 0.989 | 0.914 | 0.95 | |
| Intraparticle diffusion | ki 1 (mg/(g h1/2)) | 77.07 | 13.48 | 8.01 |
| b1 | −1.876 | 0.48 | 0.44 | |
| R2 | 0.997 | 0.937 | 0.918 | |
| ki 2 (mg/(g h1/2)) | 26.51 | 0.91 | 0.968 | |
| b2 | 87.33 | 9.13 | 5.27 | |
| R2 | 0.999 | 0.914 | 0.933 | |
| ki 3 (mg/(g h1/2)) | 11.62 | 0.61 | −0.10 | |
| b3 | 142.06 | 10.31 | 9.06 | |
| R2 | 0.917 | 0.806 | 0.250 |
| Dyes | Langmuir Isothermal Model | Freundlich Isothermal Model | ||||
|---|---|---|---|---|---|---|
| KL (L/mg) | qmax (mg/g) | R2 | KF (mg/g)/(mg/L)n | n | R2 | |
| CR | 0.772 | 262.95 | 0.969 | 123.39 | 5.36 | 0.955 |
| SY | 0.583 | 17.46 | 0.962 | 8.345 | 5.383 | 0.958 |
| MO | 0.042 | 23.34 | 0.992 | 2.509 | 2.174 | 0.974 |
| Materials | qm (mg/g) | Refs. |
|---|---|---|
| the coordination cluster Zn5(H2Ln)6(NO3)4 | 166.91 | [50] |
| p-PEN/a-CNTs@TA/CC nanofibrous | 180 | [51] |
| (CEMNPS)-C/CoFe2O4 | 43.07 | [52] |
| (PCNFs) carbon nanofibers | 218 | [53] |
| PHMG-OCS-PVA nanofibers | 76.92 | [54] |
| Chitosan–alginate sponge | 121.95 | [55] |
| Fe3O4@bacteria | 320.1 | [56] |
| PDA/PEI-TPU nanofibers | 262.95 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Zhou, Y.; Jiang, X.; Fan, J. Different Adsorption Behaviors and Mechanisms of Anionic Azo Dyes on Polydopamine–Polyethyleneimine Modified Thermoplastic Polyurethane Nanofiber Membranes. Water 2022, 14, 3865. https://doi.org/10.3390/w14233865
Sun J, Zhou Y, Jiang X, Fan J. Different Adsorption Behaviors and Mechanisms of Anionic Azo Dyes on Polydopamine–Polyethyleneimine Modified Thermoplastic Polyurethane Nanofiber Membranes. Water. 2022; 14(23):3865. https://doi.org/10.3390/w14233865
Chicago/Turabian StyleSun, Jiaoxia, Yao Zhou, Xueting Jiang, and Jianxin Fan. 2022. "Different Adsorption Behaviors and Mechanisms of Anionic Azo Dyes on Polydopamine–Polyethyleneimine Modified Thermoplastic Polyurethane Nanofiber Membranes" Water 14, no. 23: 3865. https://doi.org/10.3390/w14233865




