Rapid AOP Method for Estrogens Removal via Persulfate Activated by Hydrodynamic Cavitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experiment Design
2.3. Analytical Method
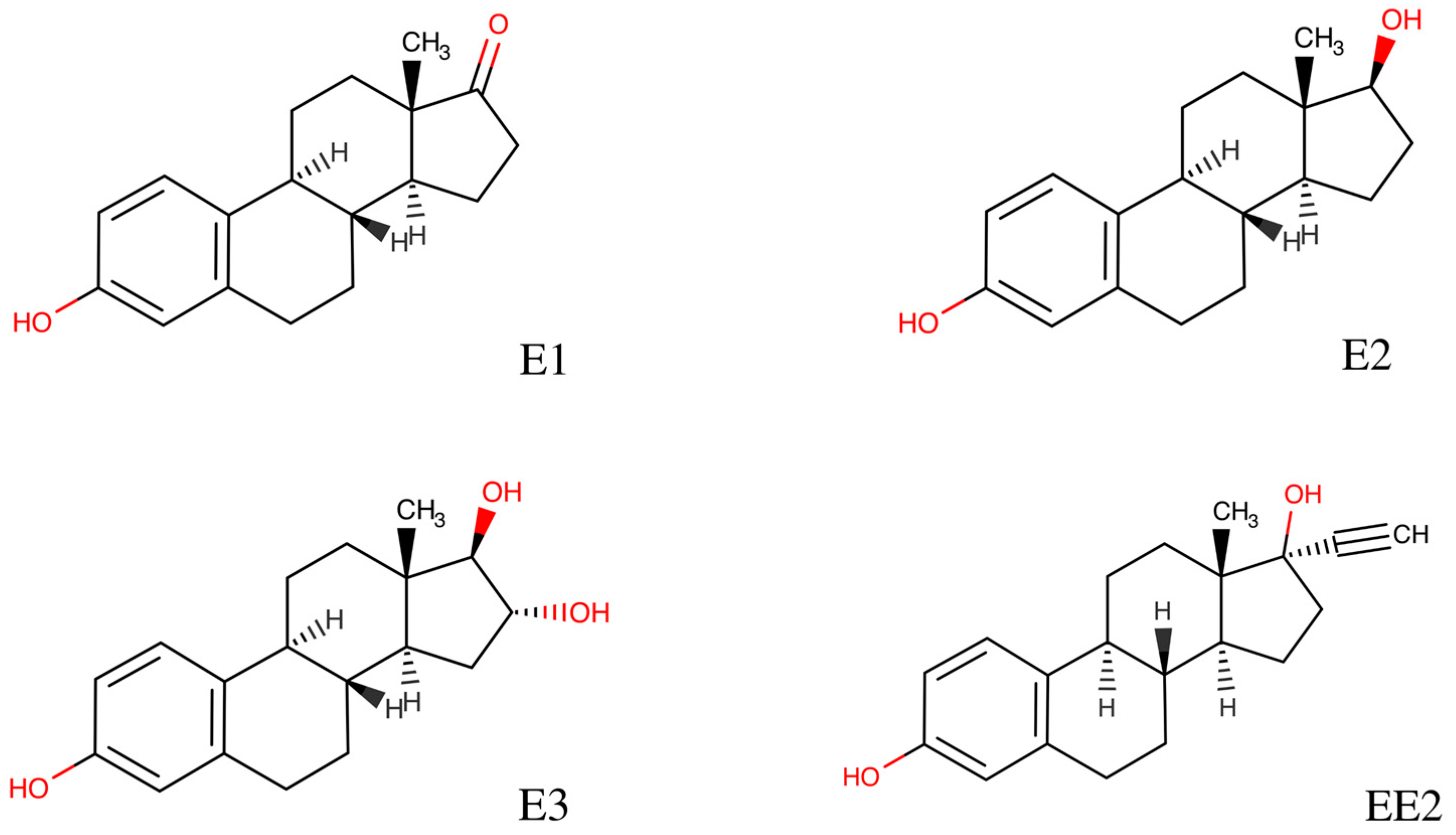
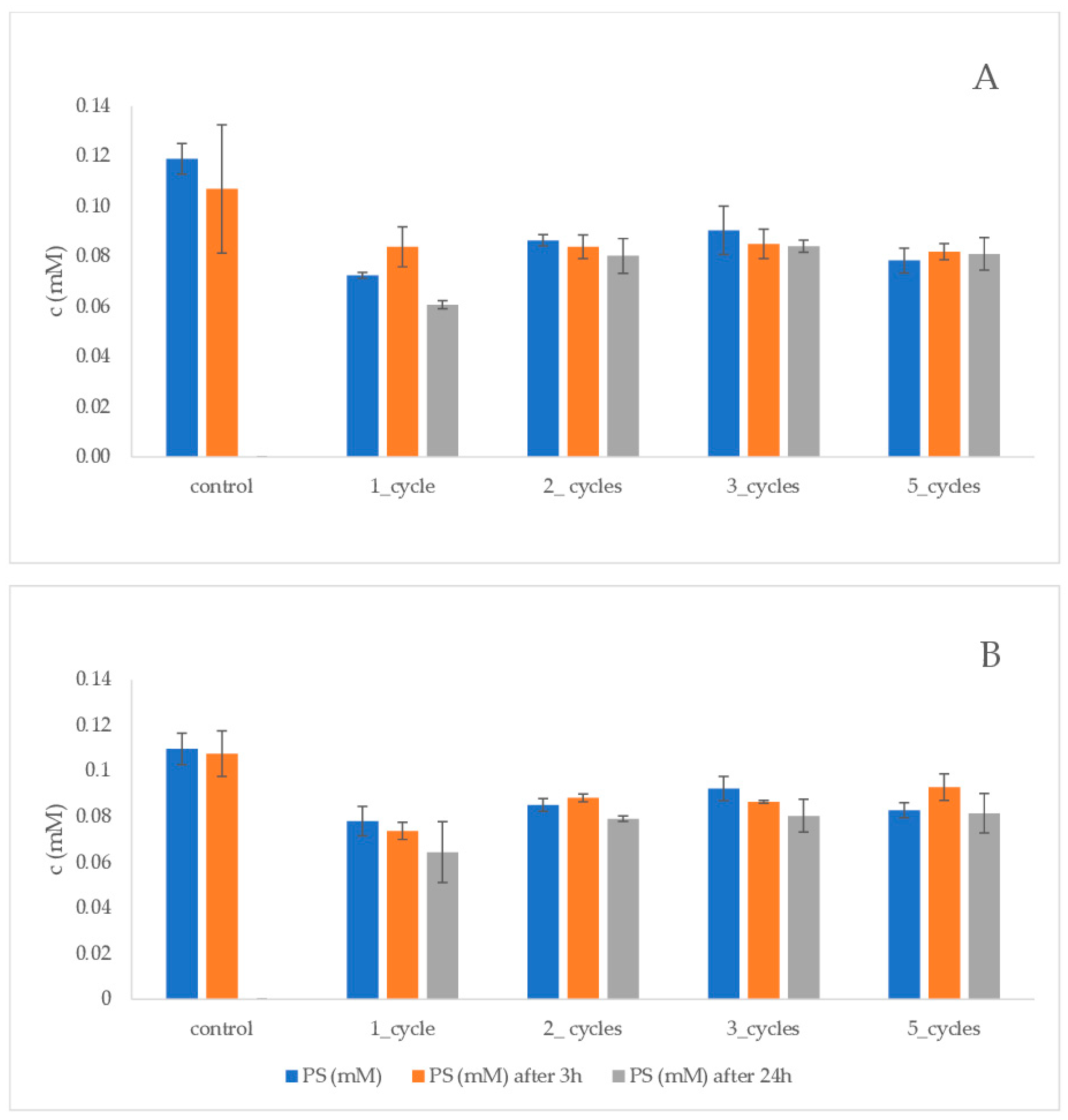
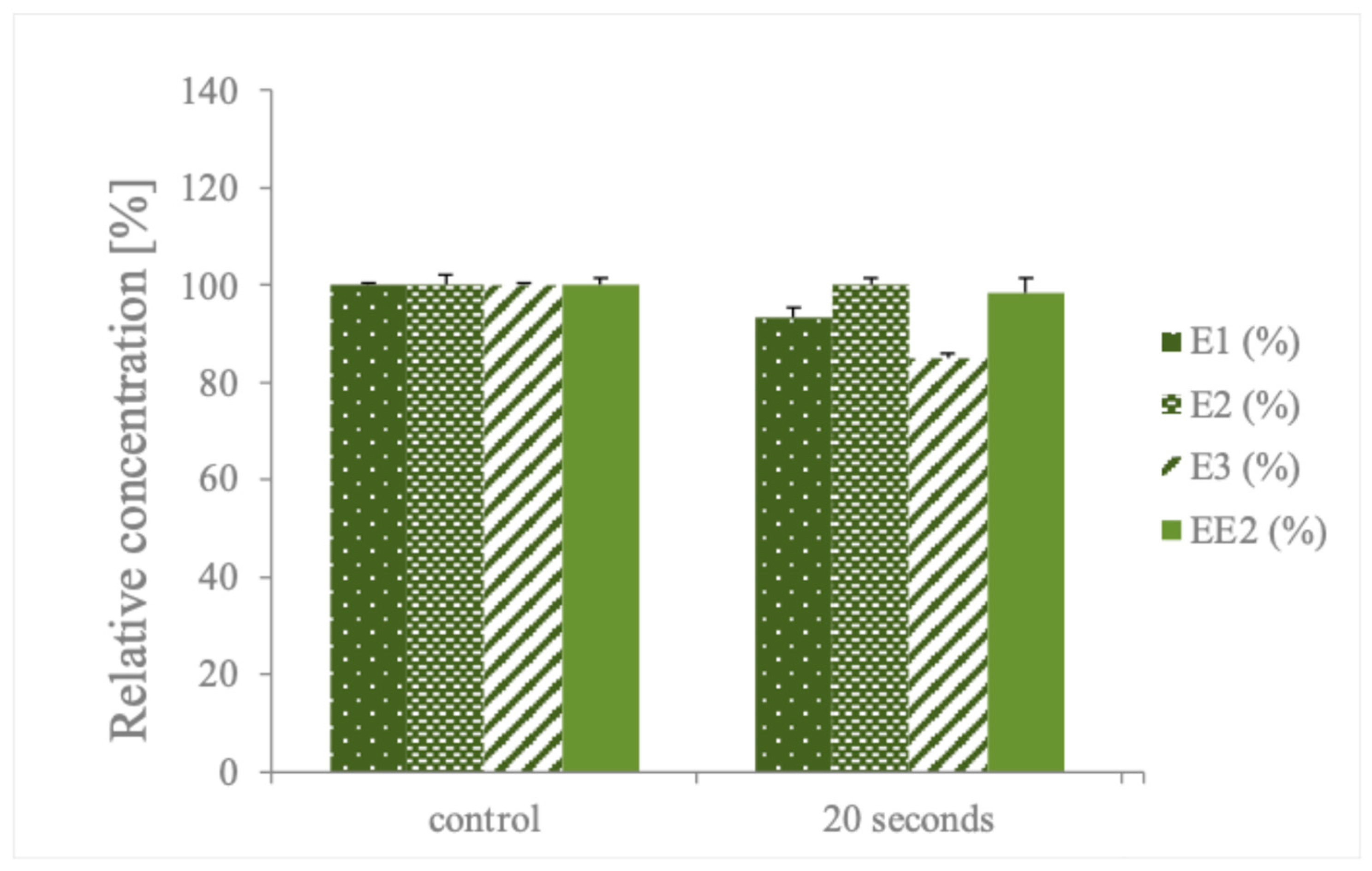
3. Results
4. Discussion
| Method | Estrogen | Efficiency | Reference |
|---|---|---|---|
| Fenton oxidation | EE2 (200 µg L−1) | 100% in 10 min | [27] |
| Photo-Fenton | E2 (272 µg L−1) | 86.4% in 8 h | [28] |
| Photo-Fenton | E2 (1 mg L−1) | 98% in 60 min | [29] |
| PS/modified Fenton-like process | E2 (6 mg L−1) | 100% in 90 min | [30] |
| PS/UV | E1, E2 and EE2 (5 µM) | over 95% in 5 min | [31] |
| UVC/PS/TiO2 (on ceramic membrane) | E2 and EE2 (100 µg L−1) | uder 45% (radiation time 4.6 s) | [40] |
| PS activated on nanoscale zero-valent iron loaded porous graphitized biochar | E2 (3 mg L−1) | 100% in 45 min | [41] |
| PS/visible light/Bi2WO6/Fe3O4 | E2 (5 mg L−1) | ~70% in 60 min | [42] |
| PS activated by reduced graphene oxide–elemental silver/magnetite nanohybrids | EE2 (10 μM) | ~90% in 15 min | [43] |
| PS/ultrasound | E2 (5 mg L−1) | over 90% in 90 min | [44] |
| PS/ultrasound | E1, E2, E3 and EE2 (17–239 ng L−1), real wastewater sample | over 95% in 10 min | [45] |
| PS/HC | E1, E2, E3 and EE2 (300 µg L−1) | 99% in 4 s treatment | This study |
- <1 kWh m−3 order−1 for representing a realistic range for full-scale application,
- 1–100 kWh m−3 order−1 for a group that is possibly too energy intensive for most practical applications, but that can still be recommended for further full-scale-application investigation,
- >100 kWh m−3 order−1, which is considered as not (yet) energy efficient [55].
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lecomte, S.; Habauzit, D.; Charlier, T.D.; Pakdel, F. Emerging Estrogenic Pollutants in the Aquatic Environment and Breast Cancer. Genes 2017, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Adeel, M.; Yang, Y.; Wang, Y.; Song, X.; Ahmad, M.A.; Rogers, H.J. Uptake and transformation of steroid estrogens as emerging contaminants influence plant development. Environ. Pollut. 2018, 243, 1487–1497. [Google Scholar] [CrossRef] [Green Version]
- Hamid, H.; Eskicioglu, C. Fate of estrogenic hormones in wastewater and sludge treatment. A review of properties and analytical detection techniques in sludge matrix. Water Res. 2012, 46, 5813–5833. [Google Scholar] [CrossRef] [PubMed]
- Jones-Lepp, T.L.; Stevens, R. Pharmaceuticals and personal care products in biosolids/sewage sludge. the interface between analytical chemistry and regulation. Anal. Bioanal. Chem. 2007, 387, 1173–1183. [Google Scholar] [CrossRef]
- Munter, R. Advanced oxidation processes—Current status and prospects, in: Proceedings of the Estonian Academy of Science. Chemistry 2001, 50, 59–80. [Google Scholar]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Wang, Y.; Le Roux, J.; Yang, Y.; Croué, J.-P. Efficient Peroxydisulfate Activation Process Not Relying on Sulfate Radical Generation for Water Pollutant Degradation. Environ. Sci. Technol. 2014, 48, 5868–5875. [Google Scholar] [CrossRef] [Green Version]
- FMC. Persulfates Technical Information, in, FMC Corporation. 2001. Available online: http://www.peroxychem.com/media/90826/aod_brochure_persulfate.pdf (accessed on 3 March 2022).
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B Environ. 2004, 54, 155–163. [Google Scholar] [CrossRef]
- Al-Shamsi, M.A.; Thomson, N.R.; Forsey, S.P. Iron based bimetallic nanoparticles to activate peroxygens. Chem. Eng. J. 2013, 232, 555–563. [Google Scholar] [CrossRef]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e− transfer mechanisms. Appl. Catal. B Environ. 2010, 96, 290–298. [Google Scholar] [CrossRef]
- Ding, D.; Liu, C.; Ji, Y.; Yang, Q.; Chen, L.; Jiang, C.; Cai, T. Mechanism insight of degradation of norfloxacin by magnetite nanoparticles activated persulfate: Identification of radicals and degradation pathway. Chem. Eng. J. 2017, 308, 330–339. [Google Scholar] [CrossRef]
- Lin, J.C.-T.; de Luna, M.D.G.; Aranzamendez, G.L.; Lu, M.-C. Degradations of acetaminophen via a K2S2O8 -doped TiO2 photocatalyst under visible light irradiation. Chemosphere 2016, 155, 388–394. [Google Scholar] [CrossRef]
- Ahmad, M.; Teel, A.L.; Watts, R.J. Mechanism of Persulfate Activation by Phenols. Environ. Sci. Technol. 2013, 47, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Gao, J.; Dionysiou, D.D.; Liu, C.; Zhou, D. Activation of Persulfate by Quinones: Free Radical Reactions and Implication for the Degradation of PCBs. Environ. Sci. Technol. 2013, 47, 4605–4611. [Google Scholar] [CrossRef]
- Rodriguez, S.; Santos, A.; Romero, A. Oxidation of priority and emerging pollutants with persulfate activated by iron: Effect of iron valence and particle size. Chem. Eng. J. 2017, 318, 197–205. [Google Scholar] [CrossRef]
- Sun, H.; Kwan, C.; Suvorova, A.; Ang, H.M.; Tadé, M.O.; Wang, S. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals. Appl. Catal. B Environ. 2014, 154–155, 134–141. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef]
- Huang, K.-C.; Zhao, Z.; Hoag, G.E.; Dahmani, A.; Block, P.A. Degradation of volatile organic compounds with thermally activated persulfate oxidation. Chemosphere 2005, 61, 551–560. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Rong, W.; Zhou, S.; Lu, N. Degradation of antipyrine by heat activated persulfate. Sep. Purif. Technol. 2013, 109, 122–128. [Google Scholar] [CrossRef]
- Gao, Y.-Q.; Gao, N.-Y.; Deng, Y.; Yang, Y.-Q.; Ma, Y. Ultraviolet (UV) light-activated persulfate oxidation of sulfamethazine in water. Chem. Eng. J. 2012, 195–196, 248–253. [Google Scholar] [CrossRef]
- He, X.; de la Cruz, A.A.; Dionysiou, D.D. Destruction of cyanobacterial toxin cylindrospermopsin by hydroxyl radicals and sulfate radicals using UV-254nm activation of hydrogen peroxide, persulfate and peroxymonosulfate. J. Photochem. Photobiol. A Chem. 2013, 251, 160–166. [Google Scholar] [CrossRef]
- Luo, C.; Jiang, J.; Ma, J.; Pang, S.; Liu, Y.; Song, Y.; Guan, C.; Li, J.; Jin, Y.; Wu, D. Oxidation of the odorous compound 2,4,6-trichloroanisole by UV activated persulfate: Kinetics, products, and pathways. Water Res. 2016, 96, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, N. Removal of carbamazepine from aqueous solution using sono-activated persulfate process. Ultrason. Sonochem. 2016, 29, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Darsinou, B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Sono-activated persulfate oxidation of bisphenol A: Kinetics, pathways and the controversial role of temperature. Chem. Eng. J. 2015, 280, 623–633. [Google Scholar] [CrossRef]
- Frontistis, Z.; Xekoukoulotakis, N.P.; Hapeshi, E.; Venieri, D.; Fatta-Kassinos, D.; Mantzavinos, D. Fast degradation of estrogen hormones in environmental matrices by photo-Fenton oxidation under simulated solar radiation. Chem. Eng. J. 2011, 178, 175–182. [Google Scholar] [CrossRef]
- Feng, X.; Ding, S.; Tu, J.; Wu, F.; Deng, N. Degradation of estrone in aqueous solution by photo-Fenton system. Sci. Total Environ. 2005, 345, 229–237. [Google Scholar] [CrossRef]
- Mboula, V.M.; Héquet, V.; Andrès, Y.; Gru, Y.; Colin, R.; Doña-Rodríguez, J.; Pastrana-Martínez, L.; Silva, A.; Leleu, M.; Tindall, A.; et al. Photocatalytic degradation of estradiol under simulated solar light and assessment of estrogenic activity. Appl. Catal. B Environ. 2015, 162, 437–444. [Google Scholar] [CrossRef]
- Yaping, Z.; Jiangyong, H. Photo-Fenton degradation of 17β-estradiol in presence of α-FeOOHR and H2O2. Appl. Catal. B Environ. 2008, 78, 250–258. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, X.; Liu, S.; Liu, Y.; Zeng, G.; Ye, S.; Yin, Z.; Hu, X.; Liu, N. Catalytic degradation of estrogen by persulfate activated with iron-doped graphitic biochar: Process variables effects and matrix effects. Chem. Eng. J. 2019, 378, 122141. [Google Scholar] [CrossRef]
- Gabet, A.; Métivier, H.; de Brauer, C.; Mailhot, G.; Brigante, M. Hydrogen peroxide and persulfate activation using UVA-UVB radiation: Degradation of estrogenic compounds and application in sewage treatment plant waters. J. Hazard. Mater. 2021, 405, 124693. [Google Scholar] [CrossRef] [PubMed]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation—A review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Yi, L.; Li, B.; Sun, Y.; Li, S.; Qi, Q.; Qin, J.; Sun, H.; Wang, X.; Wang, J.; Fang, D. Degradation of norfloxacin in aqueous solution using hydrodynamic cavitation: Optimization of geometric and operation parameters and investigations on mechanism. Sep. Purif. Technol. 2021, 259, 118166. [Google Scholar] [CrossRef]
- Sadílek, J.; Spálovská, P.; Vrana, B.; Vávrová, M.; Maršálek, B.; Šimek, Z. Comparison of extraction techniques for isolation of steroid oestrogens in environmentally relevant concentrations from sediment. Int. J. Environ. Anal. Chem. 2016, 96, 1022–1037. [Google Scholar] [CrossRef]
- Wacławek, S.; Grübel, K.; Černík, M. Simple spectrophotometric determination of monopersulfate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 928–933. [Google Scholar] [CrossRef]
- Perondi, T.; Michelon, W.; Junior, P.R.; Knoblauch, P.M.; Chiareloto, M.; Moreira, R.D.F.P.M.; Peralta, R.A.; Düsman, E.; Pokrywiecki, T.S. Advanced oxidative processes in the degradation of 17β-estradiol present on surface waters: Kinetics, byproducts and ecotoxicity. Environ. Sci. Pollut. Res. 2020, 27, 21032–21039. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Hung, C.-M.; Huang, C.-P.; Chen, C.-W.; Dong, C.-D. Hydrodynamic cavitation activation of persulfate for the degradation of polycyclic aromatic hydrocarbons in marine sediments. Environ. Pollut. 2021, 286, 117245. [Google Scholar] [CrossRef]
- Castellanos, R.M.; Presumido, P.H.; Dezotti, M.; Vilar, V.J. Ultrafiltration ceramic membrane as oxidant-catalyst/water contactor to promote sulfate radical AOPs: A case study on 17β-estradiol and 17α-ethinylestradiol removal. Environ. Sci. Pollut. Res. 2022, 29, 42157–42167. [Google Scholar] [CrossRef]
- Ding, J.; Xu, W.; Liu, S.; Liu, Y.; Tan, X.; Li, X.; Li, Z.; Zhang, P.; Du, L.; Li, M. Activation of persulfate by nanoscale zero-valent iron loaded porous graphitized biochar for the removal of 17β-estradiol: Synthesis, performance and mechanism. J. Colloid Interface Sci. 2021, 588, 776–786. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Tang, W. Feasible oxidation of 17β-estradiol using persulfate activated by Bi 2 WO 6 /Fe 3 O 4 under visible light irradiation. RSC Adv. 2016, 6, 79910–79919. [Google Scholar] [CrossRef]
- Park, C.M.; Heo, J.; Wang, D.; Su, C.; Yoon, Y. Heterogeneous activation of persulfate by reduced graphene oxide–elemental silver/magnetite nanohybrids for the oxidative degradation of pharmaceuticals and endocrine disrupting compounds in water. Appl. Catal. B Environ. 2018, 225, 91–99. [Google Scholar] [CrossRef]
- Alvarez Corena, J.R.; Bergendahl, J.A. Effect of pH, temperature, and use of synergistic oxidative agents on the ultrasonic degradation of tris-2-chloroethyl phosphate, gemfibrozil, and 17β estradiol in water. J. Environ. Chem. Eng. 2021, 9, 105005. [Google Scholar] [CrossRef]
- Choi, J.; Cui, M.; Lee, Y.; Ma, J.; Kim, J.; Son, Y.; Khim, J. Hybrid reactor based on hydrodynamic cavitation, ozonation, and persulfate oxidation for oxalic acid decomposition during rare-earth extraction processes. Ultrason. Sonochem. 2019, 52, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Domingues, E.; Silva, M.J.; Vaz, T.; Gomes, J.; Martins, R.C. Sulfate radical based advanced oxidation processes for agro-industrial effluents treatment: A comparative review with Fenton’s peroxidation. Sci. Total Environ. 2022, 832, 155029. [Google Scholar] [CrossRef]
- Angkaew, A.; Sakulthaew, C.; Satapanajaru, T.; Poapolathep, A.; Chokejaroenrat, C. UV-activated persulfate oxidation of 17β-estradiol: Implications for discharge water remediation. J. Environ. Chem. Eng. 2019, 7, 102858. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.M.; Kibbi, N. Ibuprofen removal by heated persulfate in aqueous solution: A kinetics study. Chem. Eng. J. 2012, 197, 483–492. [Google Scholar] [CrossRef]
- Ji, Y.; Fan, Y.; Liu, K.; Kong, D.; Lu, J. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res. 2016, 87, 1–9. [Google Scholar] [CrossRef]
- Ghauch, A.; Baalbaki, A.; Amasha, M.; El Asmar, R.; Tantawi, O. Contribution of persulfate in UV-254 nm activated systems for complete degradation of chloramphenicol antibiotic in water. Chem. Eng. J. 2017, 317, 1012–1025. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.M. Oxidation of bisoprolol in heated persulfate/H2O systems: Kinetics and products. Chem. Eng. J. 2012, 183, 162–171. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Chokejaroenrat, C.; Satapanajaru, T.; Chirasatienpon, T.; Angkaew, A. Removal of 17β-Estradiol Using Persulfate Synergistically Activated Using Heat and Ultraviolet Light. Water Air Soil Pollut. 2020, 231. [Google Scholar] [CrossRef]
- Joshi, S.M.; Gogate, P.R. Intensification of industrial wastewater treatment using hydrodynamic cavitation combined with advanced oxidation at operating capacity of 70 L. Ultrason. Sonochem. 2019, 52, 375–381. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, H.; Dong, Y.; Zhu, X.; Liu, X.; Li, H. A novel ternary MQDs/NCDs/TiO2 nanocomposite that collaborates with activated persulfate for efficient RhB degradation under visible light irradiation. New J. Chem. 2021, 45, 1327–1338. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R. Cavitational reactors for process intensification of chemical processing applications: A critical review. Chem. Eng. Process. Process Intensif. 2008, 47, 515–527. [Google Scholar] [CrossRef]



| Method | Pollutant | Efficiency | Commentary | Reference |
|---|---|---|---|---|
| UV and/or transition metals: Fe(II), Fe(III), Co(II), Ag(I) | 2,4-dichlorophenol | 99.9% within 4 h | The high scavenging effect, possible inhibition by dissolved oxygen, secondhand water contamination with high concentrations of metal ions, prolonged reaction time | [9] |
| Iron-based nanoparticles (bimetallic zero valent nanoparticles) Fe/PS process | trichloroethylene | >99% in 20 s reaction time | High cost and potential environmental risk caused by nanoparticles | [10] |
| PS and PMS activation by electrophilic transition metal cations (Ag+ and Co2+), UV (300 < λ < 400 nm) and/or heat (T = 30 °C) | microcystin-LR | ∼77% in 10 min | Best results achieved at lower pH (pH = 3) and higher PS concentrations [PMS]/[MC-LR] molar ratio = 100 | [11] |
| Magnetite nanoparticles/PS | norfloxacin | 90% within 60 min | The concentration of PS 1 mM; dose of nanoparticles: 0.3 g L−1; adjusted pH = 4.0 | [12] |
| TiO2/light/PS | acetaminophen | up to 100% in 9 h, pH 9 | High costs and complicated in practice (high dose of PS, pH adjustment, prolonged reaction time) | [13] |
| Phenols/PS | nitrobenzene | over 60% in 8 h, pH 11.5 | Addition of hazardous chemicals and the need for significant pH adjustment, prolonged reaction time | [14] |
| PS activated by quinones | PCBs | over 60% in 1 h, over 80% in 2 h | The mechanism of persulfate activation was primarily elucidated | [15] |
| PS activated by Fe2+ | diuron, ibuprofen and caffeine | >90% in 240 min | pH adjustment needed; kinetics model primarily evaluated | [16] |
| PS activated by nitrogen-modified carbon nanotubes | phenol | >90% under 30 min | Phenol concentration = 20 ppm; catalyst dose 0.2 g L | [17] |
| PS/activated carbon | Azo dye (orange 7) | 80% degradation and 50% mineralization in 5 h | The activation effectiveness decreased by adsorption of the pollutant on the catalyst | [18] |
| Thermally activated PS | 59 volatile organic compounds | >90% in 72 h | The best results were achieved in combination with 5 g l−1 of Na2S2O8 at 40 °C for 72 h | [19] |
| Thermally activated PS | antipyrine | 80% removal within 2 h | Anaerobic conditions favoured degradation (20%) | [20] |
| UV/PS | sulfamethazine | >95% in 45 min | Photolysis (22.0%), persulfate oxidation (15.10%), UV/H2O2 (87.5%) efficiencies were also investigated | [21] |
| UV/PS | cylindrospermopsin | >99% in 20 min | UV (less than 5%) and UV/H2O2 (~20%) efficiencies were also investigated | [22] |
| UV/PS | 2,4,6-trichloroanisole | >80% in 30 min | Mechanism and kinetics were primarily investigated | [23] |
| PS/sonolysis | carbamazepine | 89.4% in 120 min, pH 3.0 | PS and ultrasound efficiencies were also investigated; PS alone with less than 50% and ultrasound with less than 5% | [24] |
| PS/sonolysis | bisphenol A | >90% under 60 min | High temperatures enhanced sulfate radical formation but impeded sonochemical activity.By-products were also investigated | [25] |
| Conditions | kE1 (min−1) | kE2 (min−1) | kE3 (min−1) | kEE2 (min−1) |
|---|---|---|---|---|
| PS 0.1 mM + HC | 1.24 | 1.51 | 0.94 | 1.2 |
| PS 0.1 mM (heat activated 60 °C) + HC | 1.15 | 1.40 | 1.68 | 1.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Přibilová, P.; Odehnalová, K.; Rudolf, P.; Pochylý, F.; Zezulka, Š.; Maršálková, E.; Opatřilová, R.; Maršálek, B. Rapid AOP Method for Estrogens Removal via Persulfate Activated by Hydrodynamic Cavitation. Water 2022, 14, 3816. https://doi.org/10.3390/w14233816
Přibilová P, Odehnalová K, Rudolf P, Pochylý F, Zezulka Š, Maršálková E, Opatřilová R, Maršálek B. Rapid AOP Method for Estrogens Removal via Persulfate Activated by Hydrodynamic Cavitation. Water. 2022; 14(23):3816. https://doi.org/10.3390/w14233816
Chicago/Turabian StylePřibilová, Petra, Klára Odehnalová, Pavel Rudolf, František Pochylý, Štěpán Zezulka, Eliška Maršálková, Radka Opatřilová, and Blahoslav Maršálek. 2022. "Rapid AOP Method for Estrogens Removal via Persulfate Activated by Hydrodynamic Cavitation" Water 14, no. 23: 3816. https://doi.org/10.3390/w14233816
APA StylePřibilová, P., Odehnalová, K., Rudolf, P., Pochylý, F., Zezulka, Š., Maršálková, E., Opatřilová, R., & Maršálek, B. (2022). Rapid AOP Method for Estrogens Removal via Persulfate Activated by Hydrodynamic Cavitation. Water, 14(23), 3816. https://doi.org/10.3390/w14233816











