Abstract
In the present study, two temperate sandy beaches, which were located on the coast of different seas with different hydrological states, were selected to investigate the variations and drivers of the taxonomic structure and functional traits of the free-living marine nematode. According to the present study, Xyalidae and Chromadoridae were widely observed in both locations, but the species composition and functional traits were not completely the same. In fine sands, non-selective deposit feeders or colonizers (nematodes with c-p = 2) were far more quantitatively than other functional traits, suggesting a relatively restricted range of functional traits. The increased microhabitat heterogeneity in coarse sands can support species with more diverse functional traits. Chl-a was the most prominent variable that significantly related to nematode species composition and functional traits at XB sites. Nematode data were closely related to temperature or temperature-related environmental factors, such as DO and salinity, at GB sites.
1. Introduction
Sandy ocean beaches are the most extensive intertidal systems worldwide and make up two-third of the world’s ice-free coastlines [1]. Sandy beaches dominate a majority of temperate and tropical coastlines where they represent both important recreational assets and functions as a buffer zone against the sea [2]. Although the variety of faunal species at sandy beaches is less than that in rocky shores or tidal flats, individual numbers are often abundant at sandy beaches [3]. A large number of microscopic organisms, including bacteria, microalgae, protozoans, and metazoans, occupy the interstices [1].
Meiofauna, namely classified as protists and metazoans between 31 μm and 500 μm [4], are usually diverse in sandy beaches where they live in the interstices between sand grains. The relatively large open pores of the sands provide space for foraging and shelter, while biofilms and microalgae growing on the grain surfaces represent a prolific sedimentary food source [5]. Free-living marine nematodes are the most dominant metazoan meiofauna both in abundance and biomass [6]. They are a key component of the sandy beach ecosystem, not only are they important in the remineralization of detritus, but they are also an important prey resource for many shrimps, shellfishes, other invertebrates’ larvae, and surf fishes. Studies on sandy beach nematodes have focused on their distribution patterns as well as their underlying causes, discovering the determinants of their diversity and distribution across scales of centimeters to kilometers [3,5,7,8,9,10,11,12,13,14]. A number of studies suggested that the physical and chemical features of the interstitial environment were responsible for structuring nematode assemblages on large scales [5,8,15], whereas biotic interactions, such as competition and predation, have been shown to be important determinants of fine-scale distribution [5,13]. However, hydrographical and sedimentological heterogeneity, as defined by waves, currents, and sands, varied among sandy beaches, to some extent hindering researchers from discovering the regular patterns and causes of nematode distribution along sandy coastlines. For example, although the sand particle size has a narrow range of change in sandy beaches, each sandy beach has its own granulometric properties of the sediment [16]. Available knowledge suggests that coarse and fine particles will present very different physical and microbiological environments, mainly due to their differences in surface area available for microbial activity and their capacity to retain water at low tide. Nematode assemblage composition would vary according to the granulometric properties of the sediment [17,18]. These findings call for paying more attention to the hydrographical and sedimentological heterogeneity of sandy beach habitats when investigating nematode distribution and its underlying drivers over different scales.
In addition, nematodes are ecologically heterogeneous and several of their traits, such as feeding type, life strategy, and morphological features, were thought to be related to important ecological functions [19]. In order to understand how environmental disturbance affects the functional role of nematodes, functional traits analyses of nematodes have received extensive attention in recent decades [20,21,22]. However, the functional traits and their variations over seasons have rarely been studied, and the variation pattern of the functional traits of nematodes across scales of centimeters to kilometers is still not fully established.
Dalian city, located at the southernmost tip of the Liaodong Peninsula in China is surrounded by the sea on three sides with a 2211 km long coastline. Several studies on meiofauna, including the free-living marine nematode, were conducted at sandy beaches in Dalian [12,23]. According to the previous studies, nematode abundance and dominance were high, indicating an important ecological role in sandy beaches. Additionally, the nematode community structure differed among different sandy beaches in Dalian due to natural [24] or anthropogenic disturbance [23]. However, the functional traits of nematodes have rarely been studied here. More importantly, Dalian city borders two different seas in the east and the west, namely the Yellow Sea and the Bohai Sea. The Bohai Sea is a shallow semi-enclosed continental shelf sea, and surrounded by land to the north, west, and south. By comparison, the Yellow Sea situated between the Chinese mainland and the Korean Peninsula, has a larger area and a deeper water depth. The hydrological state of the two seas differed with respect to water depth, waves, currents, etc. Accordingly, the abiotic and biotic environmental variables varied among the sandy beaches facing different seas.
The present study chose two sandy beaches in Dalian, which were located on the coast of the Bohai Sea and the Yellow Sea, respectively. The different hydrological states of the two seas make it possible to compare the variations and drivers of the free-living marine nematode’s taxonomic structure and functional traits in different sandy beach habitats. The aim of the present study was to investigate and describe how the taxonomic structure and functional traits of a nematode community vary in relation to environmental variables over time at these two contrasting sandy beaches.
2. Material and Methods
2.1. Study Area
Dalian city is located in the warm temperate zone of the Northern Hemisphere. It has a warm temperate continental monsoon climate with maritime characteristics. There are no severely cold days in winter and no severely hot days in summer. The average temperature of the city is 10.5 °C, and the annual precipitation is from 550–950 mm. Dalian city sits along the Yellow Sea on the east and the Bohai Sea on the west. The studied beaches, namely Golden Beach and the Xiajiahezi Beach, are located on the coast of the North Yellow Sea and the Bohai Sea, respectively (Figure 1). Since both of them were open to public access, human impact can be considered as relatively high. The two studied beaches are only approximately 20 km apart, but hydrographical and sedimentological heterogeneity existed between the two beaches (Table 1).
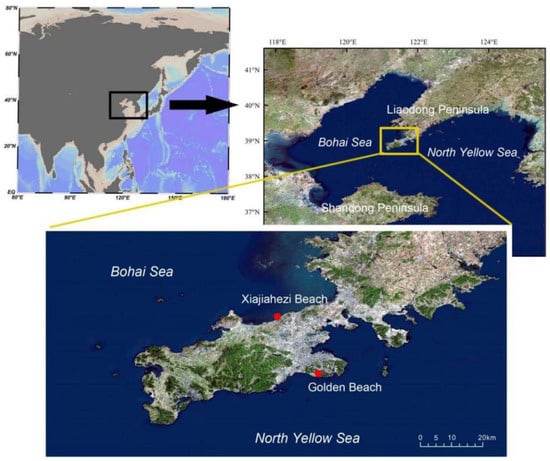
Figure 1.
Map of sampling sandy beaches.

Table 1.
Environmental characteristics of the study locations.
- (1)
- Golden Beach (121°35′ E, 38°52′ N; GB in short) is 1.0 km long, with an average slope of 9.0°. The average height of spring tide and neap tide is 292 cm and 235 cm, respectively [25]. It is located on the coast of the North Yellow Sea, which is situated between the Chinese mainland and the Korean Peninsula, and a line between Chengshanjiao of the Shangdong Peninsula and Changshanchuan of the Korean Peninsula is the boundary between the North Yellow Sea and the South Yellow Sea. The mean water depth is 38 m in the North Yellow Sea [26]. The tides in this area are regular semi-diurnal, with a tidal range from 2–4 m, and a current velocity of from approximately 1.5–2.5 m/s [27]. The wave height is from 2.0–6.0 m in autumn and winter in the North Yellow Sea [26].
- (2)
- Xiajiahezi Beach (121°30′ E, 39°02′ N; XB in short) is 1.2 km long, with an average slope of 1.3°. The average height of spring and neap tide is 233 cm and 194 cm [25], respectively. It is located on the coast of the Bohai Sea, a shallow semi-enclosed continental shelf sea with a mean depth of 18 m. Currents are weak with a maximum surface velocity <1.5 m/s, and the wave height is from 0.3–0.7 m on near shore areas [28]. The tides in this area are irregular semi-diurnal tides, with a tidal range from 2–3 m.
2.2. Sampling Methods
Sampling was conducted in December 2015 and April, July, and October 2016. At each sampling location, two random transects (100 m apart), orientated perpendicular to the waterline, were set up for nematode sampling. Three equally distanced sampling sites from high-water line to low-water line (H, M, L) were established along each transect. At each sampling site, three replicated core samples were collected using Plexiglass cores (inner diameter of 4.8 cm) to a depth of 20 cm for nematode analyses. Two additional core samples were collected at each site for analyses of granulometric composition, Chlorophyll a (Chl-a) concentration, and total organic matter content (TOM). Sea water temperature, salinity, dissolved oxygen (DO), and pH were measured using in situ Smartroll MP.
2.3. Laboratory Process
In the laboratory, samples were stained with Rose Bengal for more than 24 h, and then washed through 0.5 and 0.031 mm sieves. Meiofauna, including marine nematodes, were extracted using the decantation method. All marine nematodes were counted and picked out under a stereoscopic microscope and were then mounted on permanent slides after a transparent treatment to prevent dehydration. Two hundred nematode individuals were randomly extracted if the number was above two hundred. Nematodes were identified to the genus level according to Platt and Warwick [29,30], Warwick et al. [31], and Nemys online identification keys [32] using an Olympus BX51 compound microscope (Olympus, Tokyo, Japan). Additionally, each nematode was identified to species level (indicated by the genus name followed by sp. 1, sp. 2, etc.). The functional traits of nematodes, namely feeding type and life strategy, were analyzed. According to the buccal cavity morphology, nematode specimens were divided into four feeding groups as selective deposit feeders (1A), non-selective deposit feeders (1B), epigrowth feeders (2A), and predators/omnivores (2B) [33]. Based on life strategy characteristics, marine nematodes can be allocated from colonizers to persisters (c–p classes 1 to 5; r- to K-strategists, sensu lato) [34]. Nematodes with short generation times, high egg production rates, and an ability to form dauer larvae are assigned as extreme colonizers (c–p = 1), while nematodes with a long-life span and low reproduction rates are extreme persisters (c–p = 5). Each specimen was classified from 1 to 5 according to the family and genus list compiled by Bongers et al. [34,35]. The family c–p class was assigned if a genus was not present on the Bongers’ list.
Sediment particle size was analyzed using a dry sieve method. Particle size was classified using the Wentworth scale in phi units, where Φ = −log2 diameter (mm). The medium particle diameter (MdΦ) indicated the diameter corresponding to the 50% mark on the cumulative curve (Φ50). Sediment total organic matter (TOM) content was measured using the K2Cr2O7-H2SO4 oxidization method [36]. The sediment Chl-a content in the sediment was determined using the spectrophotofluorometic method of Lorenzen and Jeffrey [37] and Liu et al. [38].
2.4. Statistical Analyses
Environmental and nematode datasets were analyzed using PRIMER v7 and the PERMANOVA add-on package [39,40]. The differences in environmental variables were analyzed using principal coordinates analysis (PCO) on normalized data and the Euclidean resemblance matrix. Biological data (ind. 10 cm−2) were standardized and forth-root transformed prior to calculating Bray–Curtis resemblance. Non-metric Multi-Dimensional Scaling (nMDS) was performed to identify different groups, along with the SIMPROF permutations test. The similarity percentages program (SIMPER) was applied to identify the species, primarily providing the discrimination between groups. A distance-based linear model (DistLM) using a step-wise selection procedure was applied to test the relationships between environmental variables with the nematode taxonomic structure and functional traits. The fitted models from DistLM were visualized in multi-dimensional space, using distance-based redundancy analysis (dbRDA).
3. Results
3.1. Habitat Heterogeneity
The most prominent environmental difference between the two beaches studied was sediment particle size (Figure 2, Table 2). High fractions of fine sand (FS, 125–250 μm) and very fine sand (VFS, 63–125 μm) were recorded at XB, accounting for 66.3% and 29.5% on average, respectively. The fractions of very coarse sand (VCS, 1000–2000 μm) and coarse sand (CS, 500–1000 μm) were high at GB, accounting for 55.5% and 18.5%, respectively. Ordination by PCO of environmental variables revealed that the sampling sites were aggregated into the two groups along the second principal coordinate (PCO2) (Figure 2), indicating a habitat heterogeneity between the study locations. MdΦ, pH, and sea water salinity were highly related to PCO2 (Figure 2). Additionally, the sampling sites were more or less aggregated into four months at each location, a difference among sampling months was noted along the first principal coordinate (PCO1). Seawater temperature, dissolved oxygen, pH, and sediment Chl-a showed high correlations with PCO1 (Figure 2).
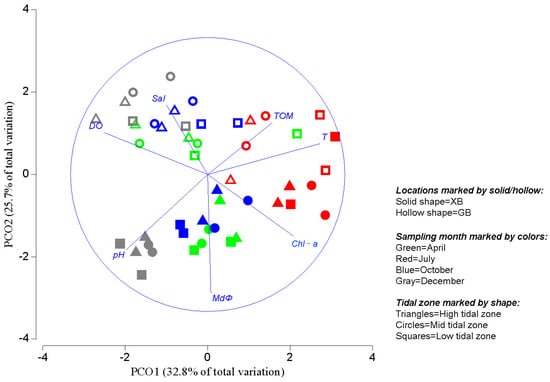
Figure 2.
Principal coordinates analysis (PCO) output based on environmental variables. Vectors are the Pearson correlations of variables with the PCO axes. Abbreviations: XB, Xiajiahezi Beach; GB, Golden Beach; T, sea water temperature; Sal, sea water salinity; DO, sea water dissolved oxygen; Chl-a, Chlorophyll-a; TOM, total organic matter; MdΦ, medium particle diameter.

Table 2.
Environmental variables of the two studied beaches in four sampling months. Abbreviations: XB, Xiajiahezi Beach; GB, Golden Beach; T, sea water temperature; Sal, sea water salinity; DO, sea water dissolved oxygen; Chl-a, Chlorophyll-a; TOM, total organic matter; MdΦ, medium particle diameter. H, high tidal zone; M, mid-tidal zone; L, low tidal zone.
3.2. Species Composition of Nematode Community
A total of 84 putative species of marine nematodes (70 and 41 putative species on XB and GB, respectively) were identified, belonging to 60 genera and 23 families. Xyalidae, Chromadoridae, Tripyloididae, Cyatholaimidae, Comesomatidae prevailed at XB, contributing 87.8% to the whole nematode community. Chromadoridae, Oncholaimidae and Enoplidae were the dominant families at GB, accounting for 67.8% of the whole community. The dominant species (≥5%) at XB were Daptonema sp. 2, Paracyatholaimus sp. 1, Daptonema sp. 1, Sabatieria sp. 2, Daptonema sp. 3, Setosabatieria sp. 1, Prochromadorella sp. 1, and Bathylaimus sp. 1, while the dominant species at GB were Oncholaimus sp. 1, Chromadorita sp. 1, Enopolaimus sp. 1, Promonhystera sp. 1, Oncholaimus sp. 2, Enoplus sp. 1, and Dichromadora sp. 1. The result of SIMPER analyses showed a 93.4% dissimilarity in nematode species composition between the two locations (Table 3). The major species providing the discrimination among tidal zones and sampling months also varied at each location (Table 3).

Table 3.
The species primarily providing the discrimination between locations, tidal zones, and sampling months (SIMPER, cumulative contribution >90%).
Cluster and SIMPROF analyses (at 5% significance level) of nematode community revealed that the similarity of nematode communities at the two locations was low (8.1%), indicating two distinct groups (Figure 3). Differences in the relative abundance of several dominant families (e.g., Cyatholaimidae, Comesomatidae, Oncholaimidae, and Thoracostomopsidae) were conspicuous between the two locations (Figure 4). In addition, clear differences in the community structure among sampling months were also observed at both locations (Figure 4).
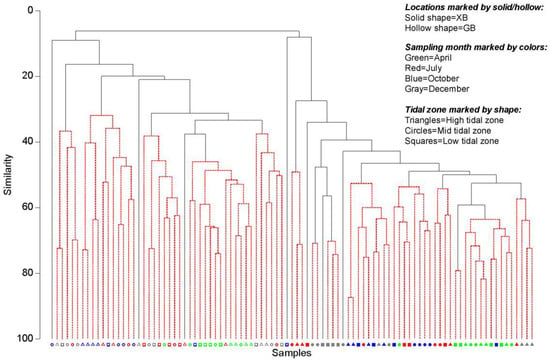
Figure 3.
Cluster analysis of the nematode community. Nematode species data were standardized and fourth root transformed, SIMPROF analysis at 5% significance level.
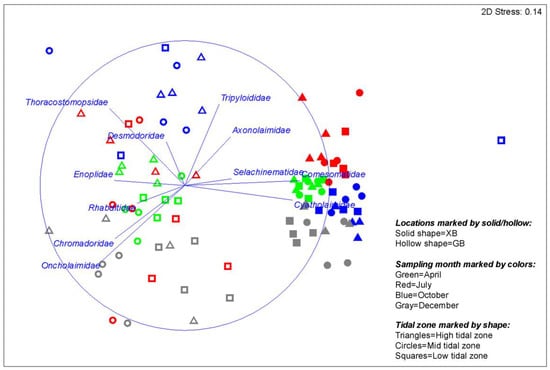
Figure 4.
Non-metric multidimensional scaling (nMDS) of nematode community. Nematode species data were standardized and fourth root transformed. Vectors overlain on plot show correlations of families (>0.3) with nMDS axes, indicating important families that differentiate the communities.
3.3. Feeding Types of Nematode Community
Non-selective deposit feeders (1B) dominated at XB sites (67.9 ± 12.8%), followed by epistrate feeders (2A, 28.8 ± 12.7%). The relative abundances of omnivores/predators (2B, 2.9 ± 5.5%) and selective deposit feeders (1A, 0.3 ± 0.3%) were low (Figure 5A). In contrast, the relative abundance of 2B nematodes was the highest (44.1 ± 16.5%) at GB sites. The mean relative abundance of non-selective deposit feeders was 27.2 ± 17.6%, and that of epistrate feeders was 26.3 ± 13.0%. The mean relative abundance of selective deposit feeders was the lowest (2.5 ± 5.2%) (Figure 5A). nMDS results showed a clear differentiation between the two locations, and the differences in the abundance of 1B and 2B were conspicuous between the two locations (Figure 6A).
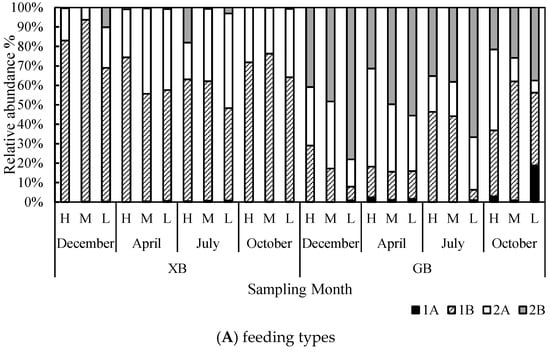
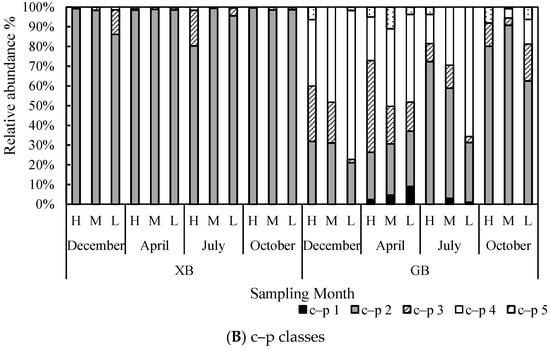
Figure 5.
Relative abundance of feeding types (A) and c–p classes (B) of nematodes at study locations over different sampling months.
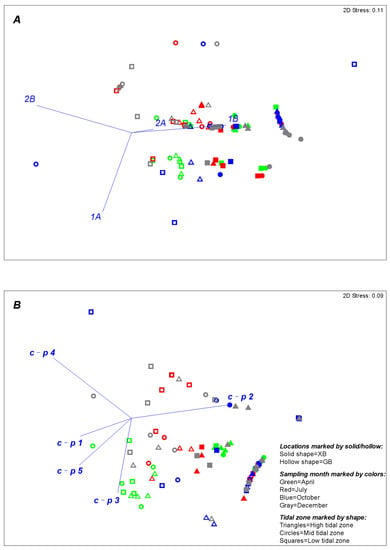
Figure 6.
nMDS plots of feeding types (A) and c–p classes (B) data of nematodes at study locations over different sampling months. Vectors overlain on plot show correlations of traits, indicating important traits that differentiate the assemblages.
3.4. Life Strategies of Nematode Community
At XB sites, nematodes with a c–p class of 2 dominated (96.0 ± 6.1%), followed by nematodes with c–p classes of 3 (3.5 ± 5.7%) and 4 (0.5 ± 0.5%). No extreme colonizers (c–p = 1) and extreme persisters (c–p = 5) were observed. At GB sites, nematodes with c–p classes of 2 were also dominant (46.1 ± 24.7%), followed by nematodes with c–p classes of 4 (32.5 ± 23.4%) and 3 (15.8 ± 12.5%). Additionally, extreme colonizers (e.g., Rhabditis) and extreme persisters (e.g., Enoplus) were observed at GB sites, and their relative abundances were 1.7 ± 2.8% and 3.9 ± 3.6%, respectively. The proportions of c–p classes were observed as being relatively consistent among the sampling months at XB sites (Figure 5B). In contrast, a fluctuation in the proportions of nematodes with different c–p classes was observed at GB (Figure 5B). nMDS results based on the c–p classes data also showed a clear differentiation between the two locations, and the differences in the abundance of nematodes with c–p = 2 and c–p = 4 were conspicuous between the two locations (Figure 6B).
3.5. Relationships between Nematode Datasets and Environmental Variables
The results of DistLM analyses revealed that investigated environmental variables could explain between 42.2% and 61.7% of the variations in nematode species composition, feeding type, and life history (Table 4). Among them, MdΦ was the most prominent variable explaining >30% of the observed variances (Table 4).

Table 4.
Results of the DistLM sequential test for nematode datasets. SS, sum of square; F, F statistic; Prop, the proportion of the variability explained; T, sea water temperature; Sal, sea water salinity; DO, sea water dissolved oxygen; Chl-a, Chlorophyll-a; TOM, total organic matter; MdΦ, median particle diameter. **, p < 0.01; *, p < 0.05. Statistically significant correlations are indicated in bold.
Further DistLM analyses, breaking down the locations separately, were performed to test the relationships between environmental variables with the nematode taxonomic structure and functional traits at each location. The results of DistLM analyses revealed that the investigated environmental variables could explain between 43.6% and 48.2% of the variations in nematode species composition, feeding type, and life history at XB sites (Table 5). Temperature, MdΦ, and sediment Chl-a concentration were significantly (p < 0.05) related to nematodes species composition, explaining 34.1% of the observed variance in total. Chl-a was significantly related to the nematode composition based on c–p groups (p < 0.05), explaining 16.3% of the observed variance. The dbRDA routine was used to perform an ordination of fitted values from the given DistLM model (Figure 7). The first two dbRDA axes captured >67% of the variability in the fitted model and >29% of the total variation in the datasets (Figure 7).

Table 5.
Results of the DistLM sequential test for nematode datasets of XB. SS, sum of square; F, F statistic; Prop, the proportion of the variability explained; T, sea water temperature; Sal, sea water salinity; DO, sea water dissolved oxygen; Chl-a, Chlorophyll-a; TOM, total organic matter; MdΦ, median particle diameter. **, p < 0.01; *, p < 0.05. Statistically significant correlations are indicated in bold.
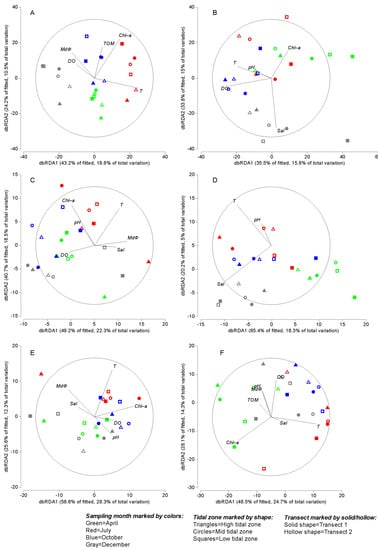
Figure 7.
dbRDA ordination for the fitted model of nematode data (based on Bray–Curtis after fourth root transformation of abundances) versus environmental variables at XB (A,C,E) and GB (B,D,F) separately. (A–B) species composition; (C–D) feeding types; (E–F) c–p classes. When categorical variables are included in a DistLM analysis, then the length of each categorical vector is a measure of the strength of the relationship between that category and the dbRDA axes.
According to the results of DistLM analyses, the investigated environmental variables could explain between 24.9% and 50.8% of the variations in nematode species composition, feeding type, and life history at GB sites (Table 6). Salinity, DO, and temperature were significantly (p < 0.05) related to nematodes species composition, explaining 35.8% of the observed variance in total. Temperature was also significantly related to the nematode composition based on feeding types and c–p classes (p < 0.05), explaining 13.2% and 12.7% of the observed variances, respectively. Furthermore, sediment Chl-a concentration was significantly related to nematode composition based on c–p classes, explaining 13.3% of the observed variance in total. The first two dbRDA axes captured >69% of the variability in the fitted model and >21% of the total variation in the datasets (Figure 7).

Table 6.
Results of the DistLM sequential test for nematodes datasets of GB. SS, sum of square; F, F statistic; Prop, the proportion of the variability explained; T, sea water temperature; Sal, sea water salinity; DO, sea water dissolved oxygen; Chl-a, Chlorophyll-a; TOM, total organic matter; MdΦ, median particle diameter. **, p < 0.01; *, p < 0.05. Statistically significant correlations are indicated in bold.
4. Discussion
Sediment particle size was the most prominent environmental difference between the two locations. In addition, a relatively close relationship among sediment MdΦ and nematode species composition confirmed that sediment particle size has an important effect on structuring nematode assemblage on sandy beaches. Sediment particle size often interacts with beach slope, waves, and tides, reflecting the hydrodynamic state of the coast. Actually, the hydrodynamic state of the Bohai Sea and the North Yellow Sea differed with respect to waves, tides, coastal currents, etc. [26,27,28], leading to the prominent differentiation of sediment particle size between the two study locations. Sediment particle size has an important effect on structuring nematode assemblage and was considered to be an important environmental factor of nematode distribution and diversity [16]. Different particles will present very different physical and biological environments, mainly due to their differences in surface area available for microbial activity and their capacity to retain water at low tide. It has been demonstrated that nematode species composition varied according to the granulometric properties of the sediment [6,17,18]. Nematodes of the families Xyalidae, Chromadoridae, Cyatholaimidae, Desmodoridae, and Oncholaimidae are widely distributed on sandy beaches around the world [41,42]. Among them, some nematode families, e.g., Xyalidae, Cyatholaimidae, and Chromadoridae, predominated in coarser sediments, while other families such as Desmodoridae and Linhomoeidae were dominant in finer sediments [3,6]. In the present study, Xyalidae and Chromadoridae were widely observed at both locations. However, the dominant genera or species were not completely the same. Nematode functional traits (feeding type and life strategy) were also revealed to differ between the two locations due to the sediment particle size in the present study. A higher proportion of 1B or nematodes with c–p = 2 was observed in fine sands, while a higher proportion of 2B or c–p = 4 was found in coarse sands. Fonseca et al. [43] also found a similar pattern in that the abundance of 1B nematodes peaked in fine sands, while the abundance of 2B nematodes peaked in coarse sands. It was generally believed that sediments with a particle size from 125~250 μm (fine sand) were most conducive to the growth of meiobenthos [44]. Therefore, the taxonomic structure and functional traits of the nematode should be expected to vary in these two contrasting habitats.
4.1. Variation in Nematode Taxonomic Structure and Functional Traits in Fine Sand Habitat
The sediment at XB sites, mainly composed of fine sand and very fine sand, was a fine sand habitat. Xyalidae, Chromadoridae, Tripyloididae, Cyatholaimidae, and Comesomatidae prevailed at XB sites. Fine sediments were usually associated with greater concentrations of nutrients contained between the fine grains and may promote coexistence of nematode genera and species [45]. Chl-a is commonly used to denote the number of live microalgae reaching the sea floor and indicates food availability for nematodes. Thus, nematode abundance and diversity often increase with increasing Chl-a concentration [46]. At XB sites, sediment Chl-a was closely related to nematode species composition and the composition based on c–p groups, suggesting that Chl-a has an important effect on structuring nematode assemblage at XB. Additionally, the relatively high Chl-a concentration could also explain the high proportion of the 1B group in fine sand. These nematodes have large buccal cavities to swallow whole organic particles (e.g., microalgae and bacteria), and have been reported to feed predominantly on microalgae in intertidal sediments [15,47]. Furthermore, Chl-a concentration varied over time in the present study, with an increased value in April and July and a low value in December. Therefore, the nematodes, which predominantly feed on microalgae, should be expected to respond to changes in Chl-a concentration. On the one hand, the increasing microalgae is conducive to the growth and reproduction of nematodes, bringing about an increase in 2A nematodes, which predominantly feed on microalgae, in April and the following months, e.g., July and October. On the other hand, this could explain the reduction in the proportion of 2A nematodes in December.
At the same time, the high predominance of 1B and nematodes with c–p = 2 suggested a relatively restricted range of functional traits in fine sands. Strong environmental filters could limit species composition to a relatively restricted range of functional traits [48,49]. Since the species with the same functional traits have the most similar resource requirements, a high niche overlap existed among nematodes in fine sand sediments. As a result, they have to segregate by inhabiting different sediment layers [50] or different tidal zones, leading to a finer division of the available niche space. In the present study, a relatively low proportion of 1B at the low tidal zone and low abundance of nematodes with c-p = 2 at the high tidal zone were observed. At the same time, a spatial variation of the genus Daptonema is noteworthy. Daptonema is a deposit feeder, but also a general opportunist at the same time [33,34]. It can utilize a wide variety of food resources, including the unicellular eukaryote [51]. In the present study, a total of six nematode species of this genus were found in fine sand. As expected, the flexible feeding behavior and short generation time benefited their dominance in fine sand. However, the dominance of these species varied among tidal zones, implying a differentiation by inhabiting different spaces.
4.2. Variation in Nematode Taxonomic Structure and Functional Traits in Coarse Sand Habitat
At the GB sites, where the sediment was mainly composed of very coarse sand and coarse sand, Chromadoridae, Oncholaimidae and Enoplidae were the dominant families. Nematodes displayed little differences in spatial structure at different tidal zones in coarse sediments, indicating that the nematode community is consistent in structure and function. Omnivores and predators (e.g., Oncholaimidae and Enoplidae) were very abundant in coarse sand. Actually, nematodes of these families are facultative predators, with diverse feeding habits and candidate food sources [47]. Indeed, some of them may shift diet with development stage [52]. Therefore, these facultative predators exhibit generalist or flexible feeding behavior, which is beneficial in unstable and fluctuation environments [47]. At the same time, the nematode community appeared more diverse in functional traits, including feeding types and life strategies, at GB sites. It has been demonstrated that the increased microhabitat heterogeneity in coarse sands can support more diverse communities [3,6]. According to the results of the present study, we would like to emphasize that coarse sands are likely to support communities with more diverse functional traits. However, due to a lower niche overlap in coarse sediments, the disappearance or replacement of any species may influence the stability of the nematode community, becoming a potential risk for the coarse sand community.
Temperature was the most prominent variable that significantly related to nematode data at GB sites. Temperature is one of the main factors that controls the structure of meiofauna communities on the beach by promoting fish reproduction [53] or affecting the growth and availability of their food resources, such as bacteria and diatoms [54]. At the same time, nematode data were also closely related to temperature-related environmental factors, such as DO and salinity. In the present study, an abrupt increase in opportunists (nematodes with c–p = 2) and a sharp decrease in persisters (c–p = 4) were observed in October. Actually, the increase in the relative abundance of nematodes with c–p = 2 began from July. With the increasing temperature, DO and salinity declined and reached the lowest values in July. At the same time, the studied beach is a popular visiting place and received a large number of tourists during the period between July and October every year. Intensive human trampling would increase the density of sand and compressed space, which was believed to have a strong influence on marine nematodes [55]. This suggested that marine nematodes faced more stressful habitat in July and October due to natural and anthropogenic disturbance. Nematodes with c–p = 2 have short generation times and are considered as opportunistic or tolerant to stressful habitats, e.g., hypoxia [56,57]. Therefore, the relative abundance of nematodes with c–p = 2, e.g., Axonolaimus, Bathylaimus, Dichromadora and Enoplolaimus, increased quickly in July and October at GB sites.
5. Conclusions
In general, a relatively restricted range of functional traits were observed in fine sands, suggesting that a high niche overlap existed among species. Nematode species composition and functional traits varied, closely related to environmental factors. Chl-a was the most prominent variable that significantly related to nematode data at XB sites. Instead, the increased microhabitat heterogeneity in coarse sands can support species with more diverse functional traits but with lower niche overlap. Nematode species composition and functional traits were closely related to temperature or temperature-related environmental factors, such as DO and salinity at GB sites. The results of this study seem to suggest that nematode taxonomic structure and functional traits should be different between the habitats and may be closely related to the sediment particle size, which deserves further comparisons and tests in future studies.
Author Contributions
Conceptualization, F.M. and E.H.; methodology, H.S. and E.H.; investigation, H.S. and Y.S.; writing—original draft preparation, H.S.; writing—review and editing, E.H. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC, No. 41976100, 41576153).
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McLachlan, A.; Defeo, O. The Ecology of Sandy Shores, 3rd ed.; Academic Press: London, UK, 2018; ISBN 978-0-12-809467-9. [Google Scholar]
- McLachlan, A.; Turner, I. The interstitial environment of sandy beaches. Mar. Ecol. 1994, 15, 177–211. [Google Scholar] [CrossRef]
- Gheskiere, T.; Vincx, M.; Urban-Malinga, B.; Rossano, C.; Scapini, F.; Degraer, S. Nematodes from wave-dominated sandy beaches: Diversity, zonation patterns and testing of the isocommunities concept. Estuar. Coast. Shelf Sci. 2005, 62, 365–375. [Google Scholar] [CrossRef]
- Giere, O. Meiobenthology: The Microscopic Motile Fauna of Aquatic Sediments, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-68657-6. [Google Scholar]
- Urban-Malinga, B.; Hedtkamp, S.I.C.; van Beusekom, J.E.E.; Wiktor, J.; Węslawski, J.M. Comparison of nematode communities in baltic and North Sea sublittoral, permeable sands–diversity and environmental control. Estuar. Coast. Shelf Sci. 2006, 70, 224–238. [Google Scholar] [CrossRef]
- Heip, C.; Vinx, M.; Vranken, G. The ecology of marine nematodes. Oceanogr. Mar. Biol. 1985, 23, 399–489. [Google Scholar]
- Nicholas, W.L.; Hodda, M. The free-living nematodes of a temperate, high energy, sandy beach: Faunal composition and variation over space and time. Hydrobiologia 1999, 394, 113–127. [Google Scholar] [CrossRef]
- Nicholas, W.L. Seasonal variations in nematode assemblages on an Australian temperate ocean beach; the effect of heavy seas and unusually high tides. Hydrobiologia 2001, 464, 17–26. [Google Scholar] [CrossRef]
- Gheskiere, T.; Hoste, E.; Vanaverbeke, J.; Vincx, M.; Degraer, S. Horizontal zonation patterns and feeding structure of marine nematode assemblages on a macrotidal, ultra-dissipative sandy beach (De Panne, Belgium). J. Sea Res. 2004, 52, 211–226. [Google Scholar] [CrossRef]
- Lee, M.R.; Riveros, M. Latitudinal trends in the species richness of free-living marine nematode assemblages from exposed sandy beaches along the coast of Chile (18–42° S). Mar. Ecol. 2012, 33, 317–325. [Google Scholar] [CrossRef]
- Cooke, B.C.; Goodwin, I.D.; Bishop, M.J. Small-scale spatial structuring of interstitial invertebrates on three embayed beaches, Sydney, Australia. Estuar. Coast. Shelf Sci. 2014, 150, 92–101. [Google Scholar] [CrossRef]
- Hua, E.; Zhang, Z.N.; Zhou, H.; Mu, F.H.; Li, J.; Zhang, T.; Cong, B.Q.; Liu, X.S. Meiofauna Distribution in Intertidal Sandy Beaches Along China Shoreline(18–40° N). J. Ocean Univ. China 2016, 15, 19–27. [Google Scholar] [CrossRef]
- Maria, T.F.; Silva Filho, M.G.; Souza, T.P.; Vanaverbeke, J.; Vanreusel, A.; Esteves, A.M. Is the vertical distribution of meiofauna similar in two contrasting microhabitats? A case study of a macrotidal sandy beach. J. Exp. Mar. Biol. Ecol. 2018, 502, 39–51. [Google Scholar] [CrossRef]
- Sahraeian, N.; Sahafi, H.H.; Mosallanejad, H.; Ingels, J.; Semprucci, F. Temporal and spatial variability of free-living nematodes in a beach system characterized by domestic and industrial impacts (Bandar Abbas, Persian Gulf, Iran). Ecol. Indic. 2020, 118, 106697. [Google Scholar] [CrossRef]
- Hua, E.; Mu, F.H.; Zhang, Z.N.; Yang, S.C.; Zhang, T.; Li, J. Nematode community structure and diversity pattern in sandy beaches of Qingdao, China. J. Ocean Univ. China 2016, 15, 33–40. [Google Scholar] [CrossRef]
- Wieser, W. The effect of grain size on the distribution of small invertebrates inhabiting the beaches of Puget Sound. Limnol. Oceanogr. 1959, 4, 181–194. [Google Scholar] [CrossRef]
- Vanaverbeke, J.; Gheskiere, T.; Steyaert, M.; Vincx, M. Nematode assemblages from subtidal sandbanks in the Southern Bight of the North Sea: Effect of small sedimentological differences. J. Sea Res. 2002, 48, 197–207. [Google Scholar] [CrossRef]
- Vanaverbeke, J.; Merckx, B.; Degraer, S.; Vincx, M. Sediment-related distribution patterns of nematodes and macrofauna: Two sides of the benthic coin? Mar. Environ. Res. 2011, 71, 31–40. [Google Scholar] [CrossRef]
- Schratzberger, M.; Warr, K.; Rogers, S.I. Functional diversity of nematode communities in the southwestern North Sea. Mar. Environ. Res. 2007, 63, 368–389. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q.; Zhang, Y.; Hua, E.; Zhang, Z. Effects of Yellow Sea Cold Water Mass on marine nematodes based on biological trait analysis. Mar. Environ. Res. 2018, 141, 167–185. [Google Scholar] [CrossRef]
- Liao, J.-X.; Wei, C.-L.; Yasuhara, M. Species and Functional Diversity of Deep-Sea Nematodes in a High Energy Submarine Canyon. Front. Mar. Sci. 2020, 7, 591. [Google Scholar] [CrossRef]
- Sroczynska, K.; Chainho, P.; Vieira, S.; Adão, H. What makes a better indicator? Taxonomic vs functional response of nematodes to estuarine gradient. Ecol. Indic. 2021, 121, 107113. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, W.D.; Gao, Y.; Ning, S.X.; Yang, B. Preliminary study on responses of marine nematode community to crude oil contamination in intertidal zone of Bathing Beach, Dalian. Mar. Pollut. Bull. 2011, 62, 2700–2706. [Google Scholar] [CrossRef]
- Song, H.L.; Mu, F.H.; Sun, Y.; Hua, E. Comparison of community structure and diversity of free-living marine nematodes in the sandy intertidal zone of Dalian in winter. Haiyang Xuebao 2021, 43, 139–151. [Google Scholar]
- National Marine Data and Information Service. Tide Tables; China Ocean Press: Beijing, China, 2016; Volume 1, ISBN 9787502791278. [Google Scholar]
- Su, J.L.; Yuan, L.Y. Hydrology in China Offshore; China Ocean Press: Beijing, China, 2005; ISBN 9787502762926. [Google Scholar]
- Xu, F.X. Lectures on ocean wave forecast part 5: Geographical distribution and seasonal variation of ocean waves. Mar. Forecasts 2002, 19, 74–79. [Google Scholar]
- Song, J.M.; Duan, L.Q. The Bohai Sea. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: London, UK, 2019; Chapter 17; pp. 377–394. ISBN 978-0-08-100853-9. [Google Scholar]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes: Part I British Enoplids; Cambridge University Press: Cambridge, UK, 1983; ISBN 0-521-25422-1. [Google Scholar]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes: Part II British Chromadorids; Brill Academic Publishing: Leiden, The Netherland, 1988; ISBN 90-04-08595-5. [Google Scholar]
- Warwick, R.M.; Platt, H.M.; Somerfield, P.J. Free-Living Marine Nematodes: Part III British Monhysterids; Field Studies Council: Shrewsbury, UK, 1998; ISBN 1-85153-260-9. [Google Scholar]
- Nemys. Nemys: World Database of Nematodes. 2022. Available online: https://nemys.ugent (accessed on 19 July 2022). [CrossRef]
- Wieser, W. Die Beziehung zwischen Mundhöhlengestalt, Ernährungsweise und Vorkommen bei freilebenden marinen Nematoden. Eine skologisen-morphologische studie. Ark. Zool. 1953, 4, 439–484. [Google Scholar]
- Bongers, T.; Alkemade, R.; Yeates, G.W. Interpretation of disturbance induced maturity decrease in marine nematode assemblages by means of the maturity index. Mar. Ecol. Prog. Ser. 1991, 76, 135–142. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Z.; He, X.L.; Zhang, B.; Ning, X. Rapid determination of organic carbon in marine sediment samples by potassium dichromate oxidation ferrous sulphate titrimetry. Rock Miner. Anal. 2007, 26, 205–208. [Google Scholar]
- Lorenzen, C.J.; Jeffrey, S.W. Determination of chlorophyll in seawater. Unesco Technical Papers in Marine Science. SCORUNE-SCO 1980, 35, 1–12. [Google Scholar]
- Liu, H.; Wu, Y.P.; Gao, S.D.; Zhang, Z.N. The variations of chlorophyll-a and phaeophytin in the sediment of Jimo shrimp pond before the outbreak of shrimp disease. Trans. Oceanol. Limnol. 1998, 1, 65–69. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); PRIMER-E: Plymouth, UK, 2015. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Maria, T.F.; Paiva, P.; Vanreusel, A.; Esteves, A.M. The relationship between sandy beach nematodes and environmental characteristics in two Brazilian sandy beaches (Guanabara Bay, Rio de Janeiro). An. Acad. Bras. Ciênc. 2013, 85, 257–270. [Google Scholar] [CrossRef]
- Baldrighi, E.; Grall, J.; Quillien, N.; Carriço, R.; Verdon, V.; Zeppilli, D. Meiofauna communities’ response to an anthropogenic pressure: The case study of green macroalgal bloom on sandy beach in Brittany. Estuar. Coast. Shelf Sci. 2019, 227, 106326. [Google Scholar] [CrossRef]
- Fonseca, G.; Maria, T.F.; Kandratavicius, N.; Venekey, V.; Gheller, P.E.; Gallucci, F. Testing for nematode–granulometry relationships. Mar. Biodivers. 2014, 44, 435–443. [Google Scholar] [CrossRef]
- Fenchel, T.M. The Ecology of Micro-and Meiobenthos. Annu. Rev. Ecol. Syst. 1978, 9, 99–121. [Google Scholar] [CrossRef]
- Ingels, J.; dos Santos, G.; Hicks, N.; Vazquez, Y.V.; Neres, P.F.; Pontes, L.P.; Amorim, M.N.; Román, S.; Du, Y.; Stahl, H.; et al. Short-term CO2 exposure and temperature rise effects on metazoan meiofauna and free-living nematodes in sandy and muddy sediments: Results from aflume experiment. J. Exp. Mar. Biol. Ecol. 2018, 502, 211–226. [Google Scholar] [CrossRef]
- Hua, E.; Zhu, Y.; Huang, D.; Liu, X. Are free-living nematodes effective environmental quality indicators? Insights from Bohai Bay, China. Ecol. Indic. 2021, 127, 107756. [Google Scholar] [CrossRef]
- Moens, T.; Vincx, M. Observations on the feeding ecology of estuarine nematodes. J. Mar. Biol. Assoc. U. K. 1997, 77, 211–227. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S., III.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Fonseca, G.; Muthumbi, A.W.; Vanreusel, A. Species richness of the genus Molgolaimus (Nematoda) from local to ocean scale along continental slopes. Mar. Ecol. 2007, 28, 446–459. [Google Scholar] [CrossRef]
- Moens, T.; Braeckman, U.; Derycke, S.; Fonseca, G.; Gallucci, F.; Gingold, R.; Guilini, K.; Ingels, J.; Leduc, D.; Vanaverbeke, J.; et al. Ecology of free-living marine nematodes. In Handbook of Zoology; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany, 2013; pp. 109–152. ISBN 978-3-11-027425-7. [Google Scholar]
- Hellwig-Armonies, M.; Armonies, W.; Lorenzen, S. The diet of Enoplus brevis (Nematoda) in a supralittoral salt-marsh of the North Sea. Helgol. Mar. Res. 1991, 45, 357–372. [Google Scholar]
- Riera, R.; Núez, J.; Brito, M.D.C.; Tuya, F. Temporal variability of a subtropical intertidal meiofaunal assemblage: Contrasting effects at the species and assemblage-level. Vie Milieu 2011, 61, 129–137. [Google Scholar]
- Harris, R.P. The distribution and ecology of the interstitial meiofauna of a sandy beach at Whitsand Bay, East Cornwall. J. Mar. Biol. Assoc. U. K. 1972, 52, 1–18. [Google Scholar] [CrossRef]
- Santos, T.M.T.; Petracco, M.; Venekey, V. Recreational activities trigger changes in meiofauna and free-living nematodes on Amazonian macrotidal sandy beaches. Mar. Environ. Res. 2021, 167, 105289. [Google Scholar] [CrossRef]
- Moreno, M.; Ferrero, T.J.; Gallizia, I.; Vezzulli, L.; Albertelli, G.; Fabiano, M. An assessment of the spatial heterogeneity of environmental disturbance within an enclosed harbor through the analysis of meiofauna and nematode assemblages. Estuar. Coast. Shelf Sci. 2008, 77, 565–576. [Google Scholar] [CrossRef]
- Alves, A.S.; Adão, H.; Ferrero, T.J.; Marques, J.C.; Costa, M.J.; Patrício, J. Benthic meiofauna as indicator of ecological changes in estuarine ecosystems: The use of nematodes in ecological quality assessment. Ecol. Indic. 2013, 24, 462–475. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).