Spatial Distribution and Ecological Risk Assessment of Heavy Metals in the Sediment of a Tropical Mangrove Wetland on Hainan Island, China
Abstract
:1. Introduction
2. Materials and Methods
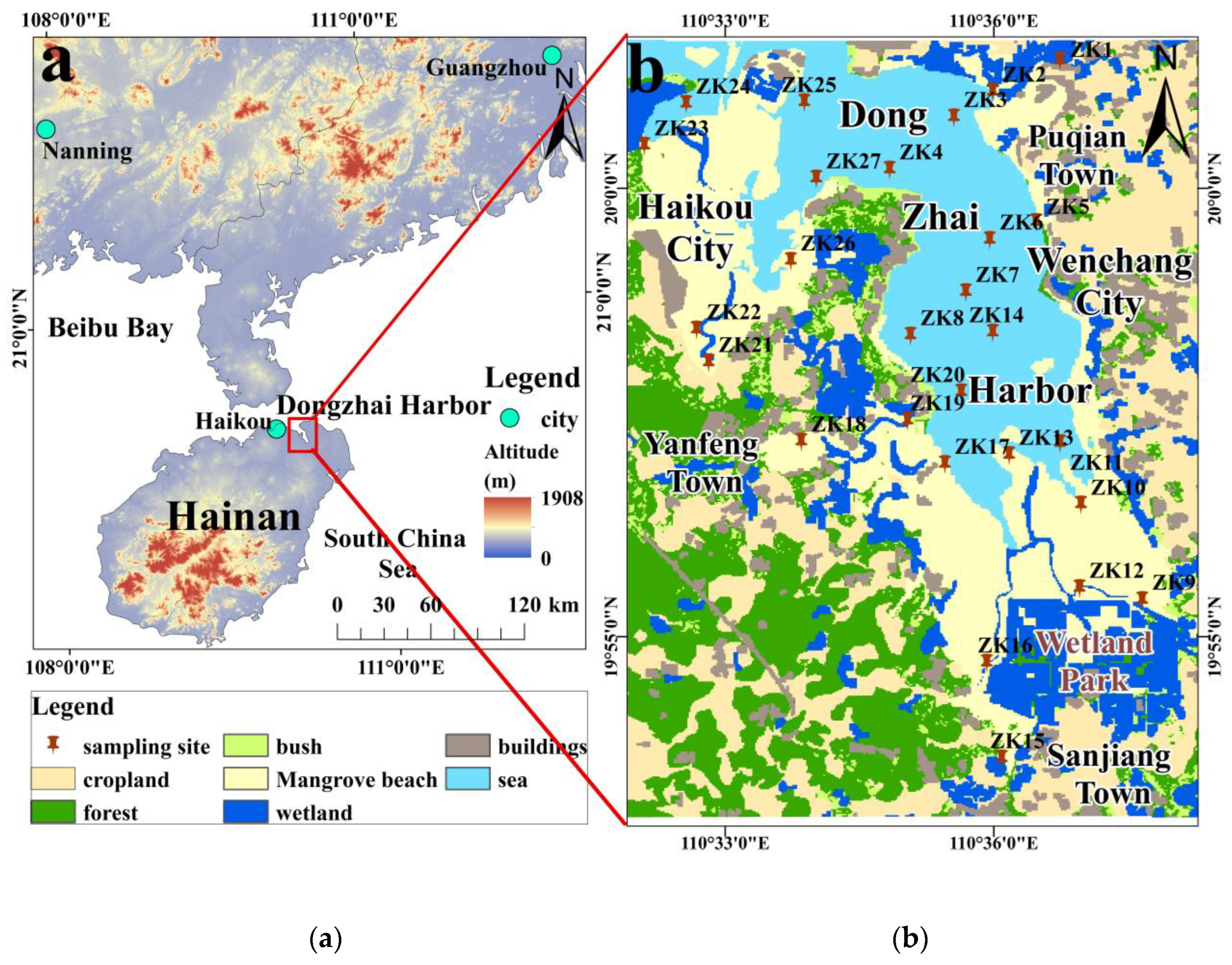
2.1. Study Area
2.2. Sample Collection and Analysis
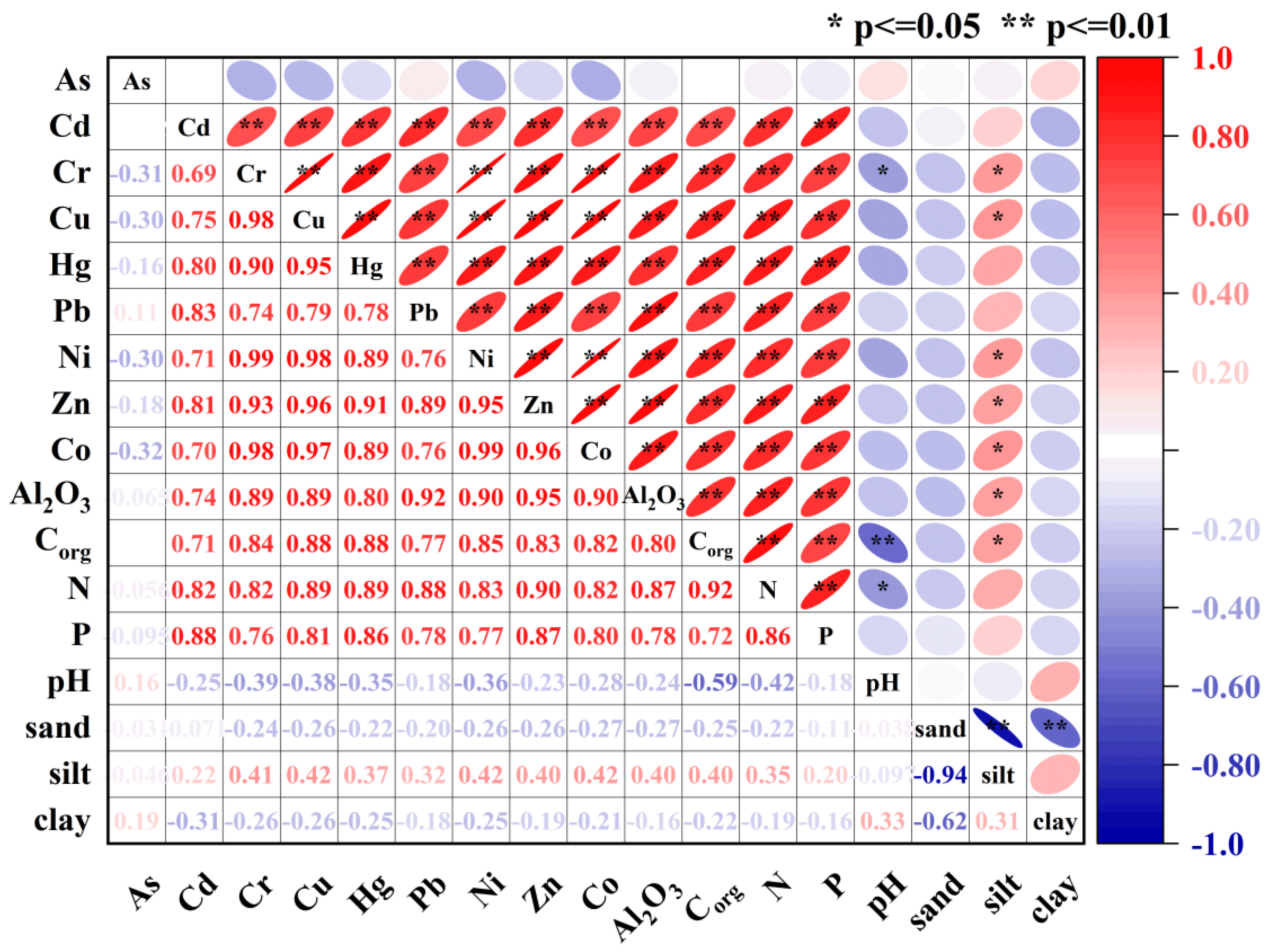
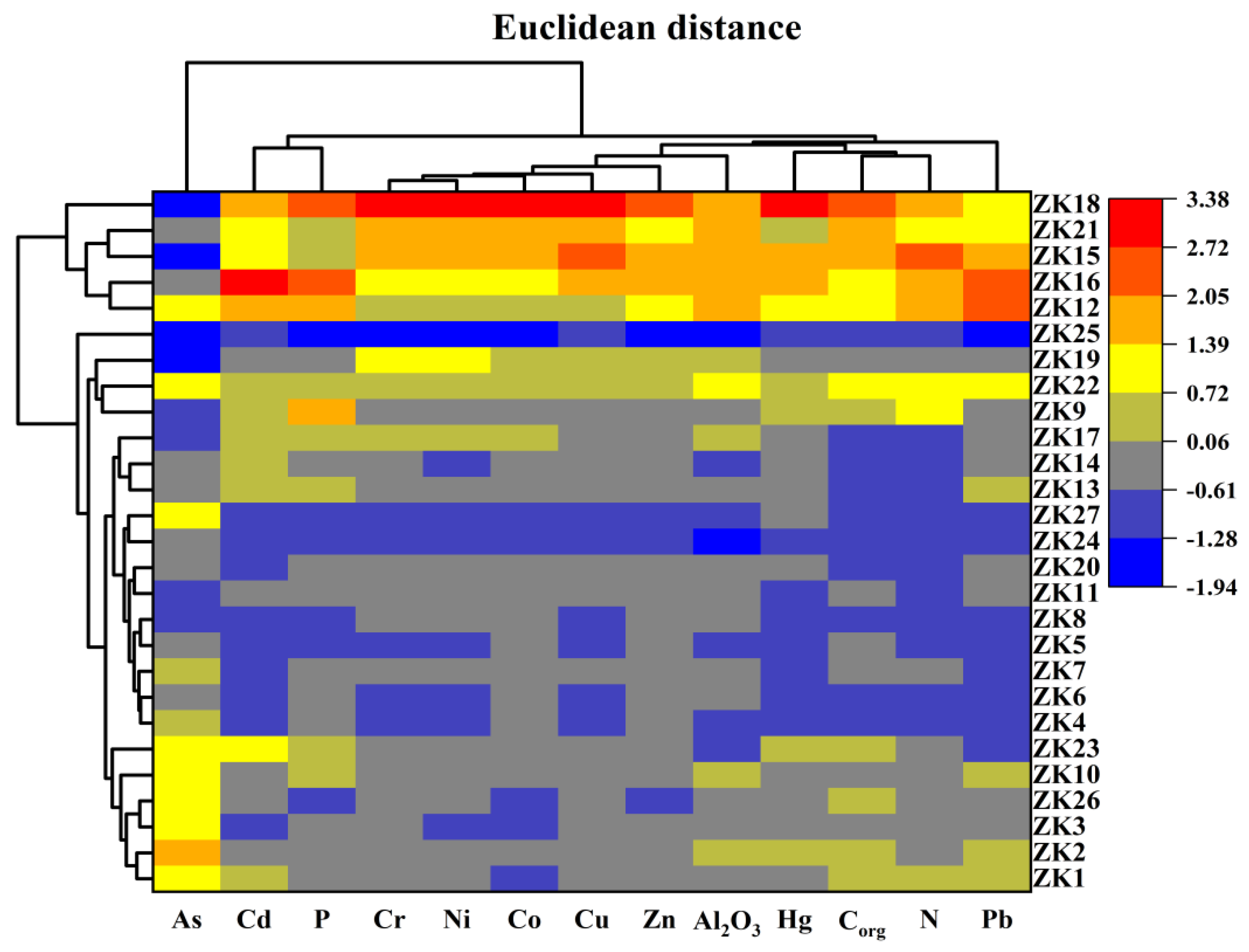
2.3. Statistical Analysis
2.4. Risk Assessment Methods
3. Results
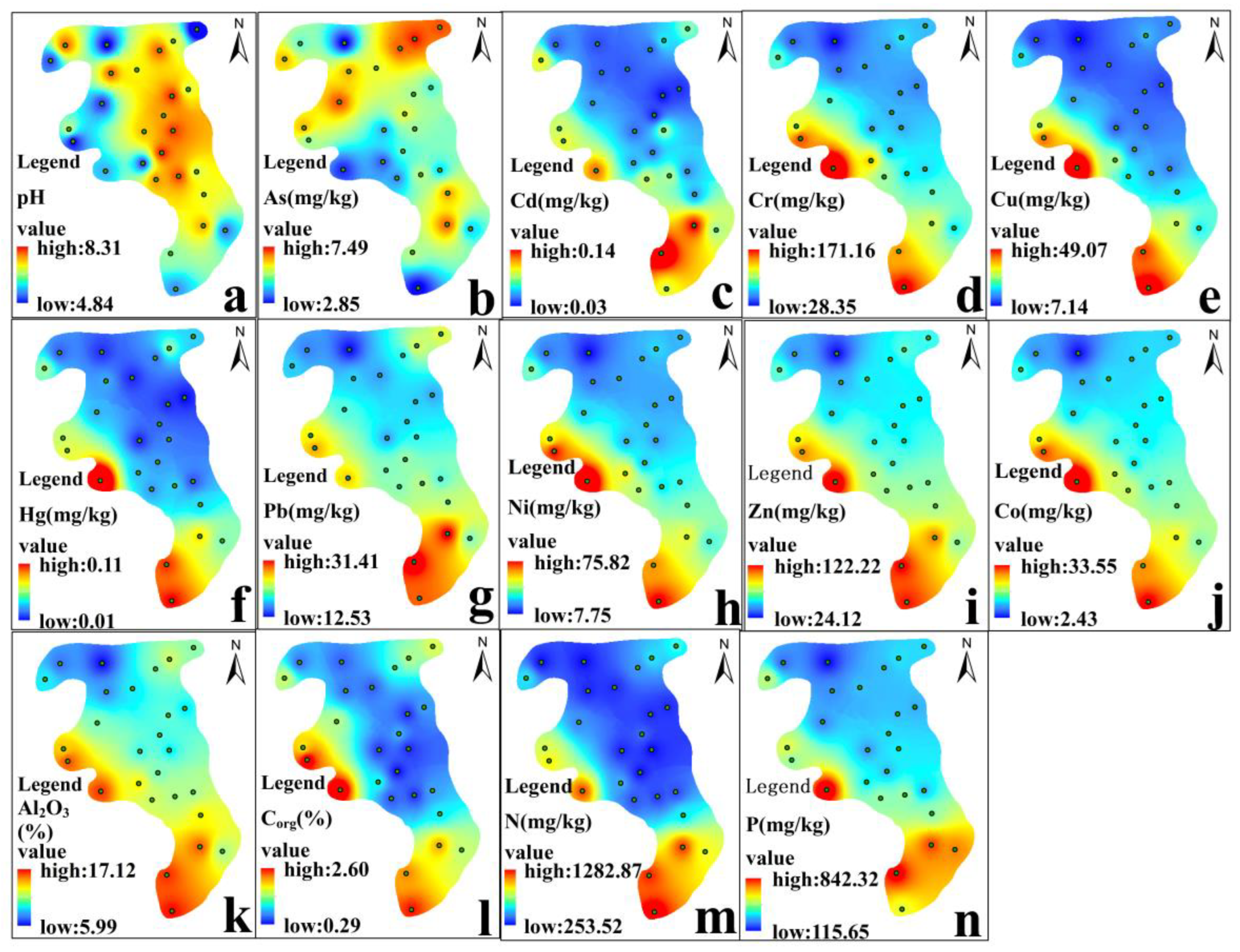
3.1. Spatial Distribution of Physico-Chemical Properties
3.2. Spatial Distributions of Heavy Metals
4. Discussion
4.1. Source Apportionment of Metals in Sediment
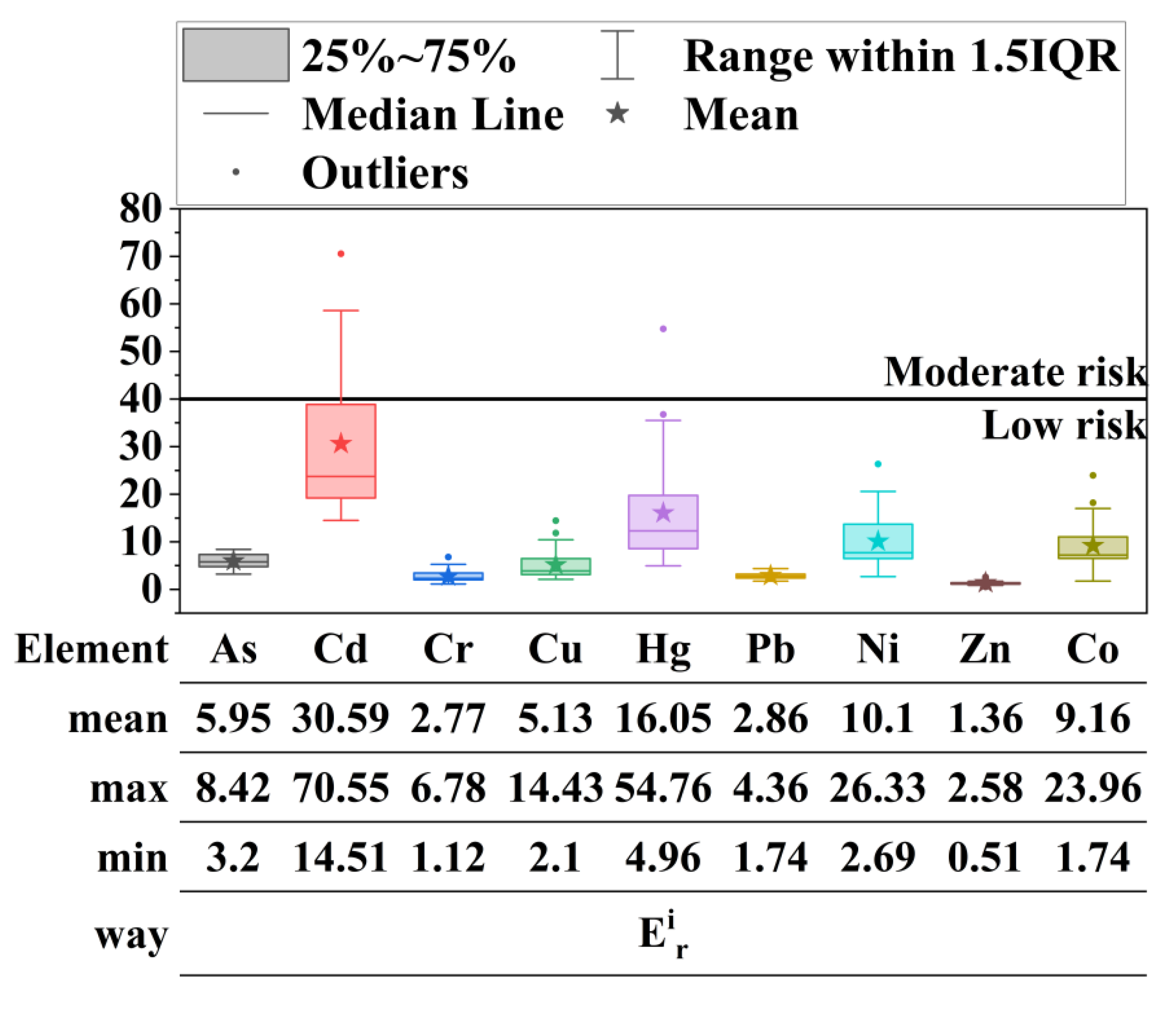
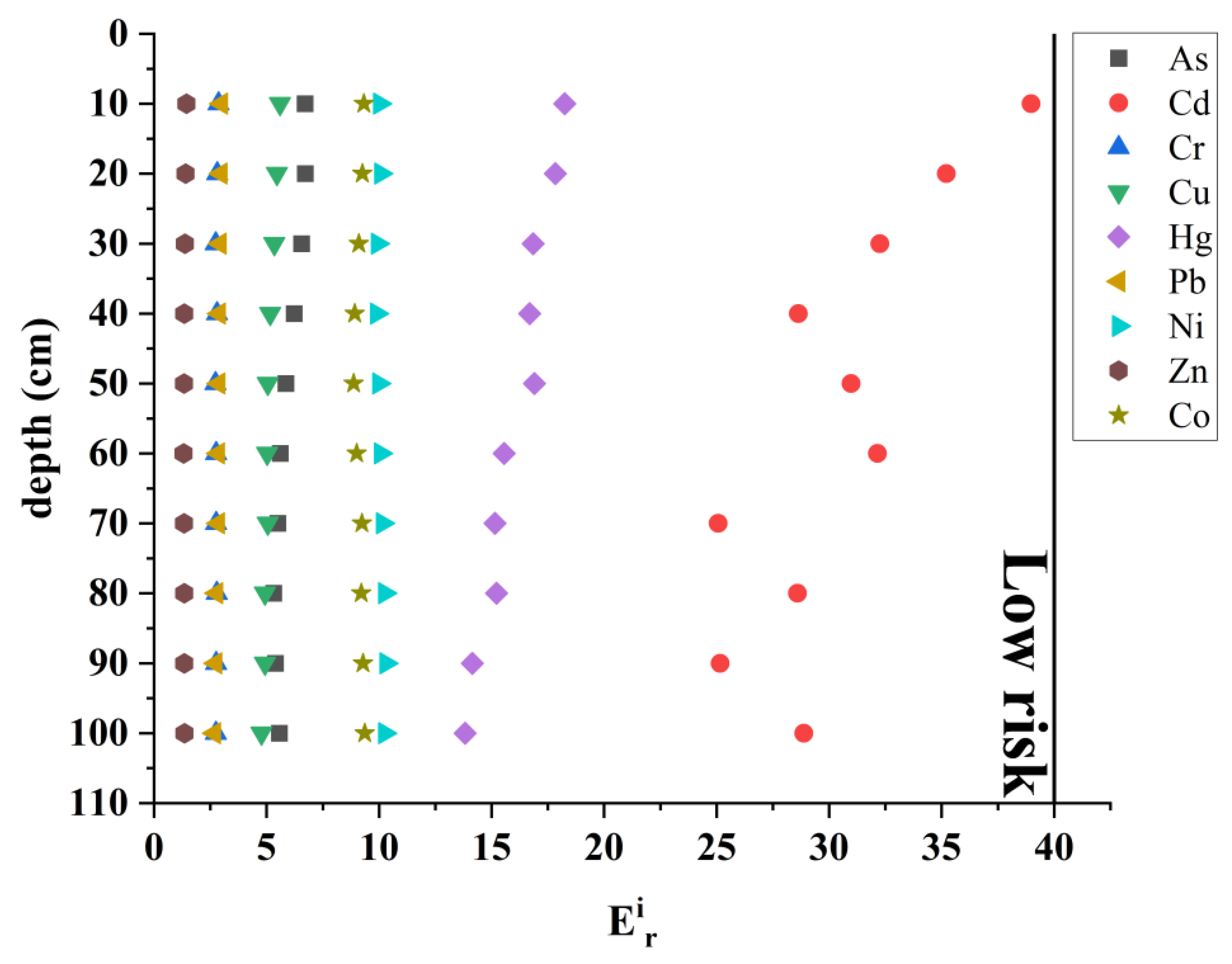
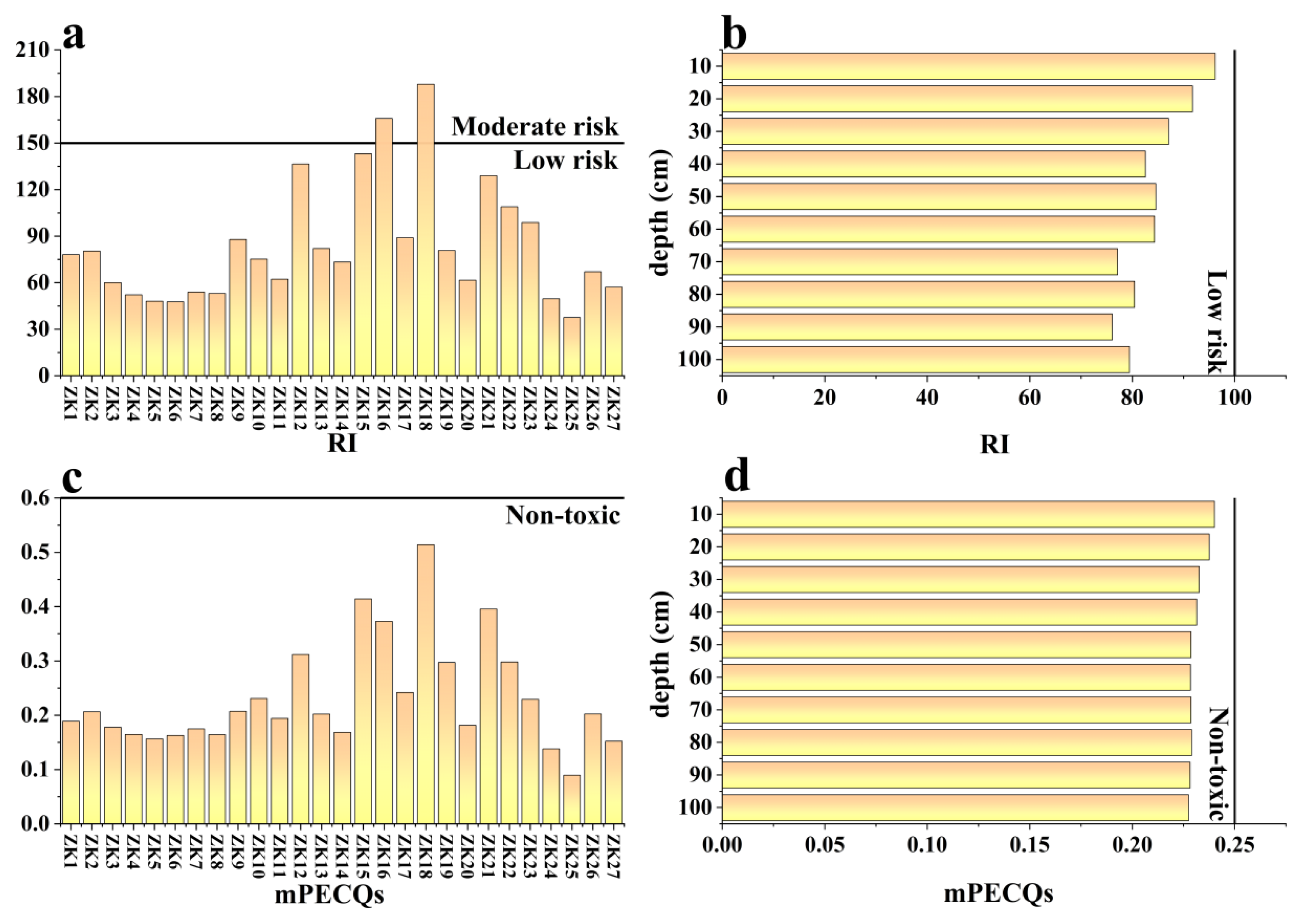
4.2. Contamination Status and Potential Ecological Risk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; Mckee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C. Ecological role and services of tropical mangrove ecosystems: A reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Bayen, S. Occurrence bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ. Int. 2012, 48, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.S.; Aich, A.; Sengupta, P.; Chakraborty, A.; Sudarshan, M. Assessment of trace metal contamination of wetland sediments from eastern and western coastal region of India dominated with mangrove forest. Chemosphere 2018, 211, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.; Chaudhuri, P.; Birch, G. Assessment of biotic response to heavy metal contamination in Avicennia marina mangrove ecosystems in Sydney Estuary Australia. Ecotoxicol. Environ. Saf. 2014, 107, 284–290. [Google Scholar] [CrossRef]
- Sultana, S.; Huang, R.Q.; Zhu, Y.J.; Thura, K.; Htwe, H.Z.; Song, L.; Jin, R.J.; Gu, J.L.; Christakos, G.; Wu, J.P. Enrichment of trace elements by blue carbon habitats in Maoyan Island of Yueqing Bay China. Stoch. Environ. Res. Risk Assess. 2022, 36, 3753–3767. [Google Scholar] [CrossRef]
- Yan, Y.; Wan, R.A.; Yu, R.L.; Hu, G.R.; Lin, C.Q.; Huang, H.B. A comprehensive analysis on source-specific ecological risk of metal(loid)s in surface sediments of mangrove wetlands in Jiulong River Estuary China. Catena 2022, 209, 105817. [Google Scholar] [CrossRef]
- Wu, H.; Liu, J.; Bi, X.; Lin, G.; Feng, C.C.; Li, Z.; Xie, L. Trace metals in sediments and benthic animals from aquaculture ponds near a mangrove wetland in Southern China. Mar. Pollut. Bull. 2017, 117, 486–491. [Google Scholar] [CrossRef]
- Qiu, Y.W.; Yu, K.F.; Zhang, G.; Wang, W.X. Accumulation and partitioning of seven trace metals in mangroves and sediment cores from three estuarine wetlands of Hainan Island China. J. Hazard. Mater. 2011, 190, 631–638. [Google Scholar] [CrossRef]
- He, S.Y.; Yang, X.E.; He, Z.L.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.Y.; Xu, R.K. Distribution of manganese (II) chemical forms on soybean roots and manganese (II) toxicity. Pedosphere 2019, 29, 656–664. [Google Scholar] [CrossRef]
- Chiappetta, J.M.M.; Machado, W.; Santos, J.M.; Lessa, J.A. Trace metal bioavailability in sediments from a reference site Ribeira Bay Brazil. Mar. Pollut. Bull. 2016, 106, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ustaoğlu, F.; Islam, M.S. Potential toxic elements in sediment of some rivers at Giresun Northeast Turkey: A preliminary assessment for ecotoxicological status and health risk. Ecol. Indic. 2020, 113, 106237. [Google Scholar] [CrossRef]
- Dung, T.T.T.; Linh, T.M.; Chau, T.B.; Hoang, T.M.; Swennen, R.; Cappuyns, V. Contamination status and potential release of trace metals in a mangrove forest sediment in Ho Chi Minh City, Vietnam. Environ. Sci. Pollut. Res. 2020, 26, 9536–9551. [Google Scholar] [CrossRef] [PubMed]
- Herbeck, L.S.; Krumme, U.; Andersen, T.J.; Jennerjahn, T.C. Decadal trends in mangrove and pond aquaculture cover on Hainan (China) since 1966: Mangrove loss fragmentation and associated biogeochemical changes. Estuarine Coast. Shelf Sci. 2020, 233, 106531. [Google Scholar] [CrossRef]
- Zhang, D.L.; Liu, N.; Yin, P.; Zhu, Z.G.; Lu, J.F.; Lin, X.H.; Jiang, X.J.; Meng, X.W. Characterization sources and ecological risk assessment of polycyclic aromatic hydrocarbons in surface sediments from the mangroves of China. Wetl. Ecol. Manag. 2017, 25, 105–117. [Google Scholar] [CrossRef]
- Herbeck, L.S. Ecological Impact of Land-Derived Anthropogenic Nutrients and Organic Matter on Tropical Estuarine and Coastal Systems of Hainan China; University of Bremen: Bremen, Germany, 2011; Available online: https://d-nb.info/1071992678/34 (accessed on 5 September 2022).
- Xin, K.; Huang, X.; Hu, J.; Li, C.; Yang, X.B.; Arndt, S.K. Land use change impacts on heavy metal sedimentation in mangrove wetlands—A case study in Dongzhai Harbor of Hainan China. Wetlands 2014, 34, 1–8. [Google Scholar] [CrossRef]
- Li, P.; Li, X.J.; Bai, J.K.; Meng, Y.C.; Diao, X.P.; Pan, K.; Zhu, X.S.; Lin, G.H. Effects of land use on the heavy metal pollution in mangrove sediments: Study on a whole island scale in Hainan China. Sci. Total Environ. 2022, 824, 153856. [Google Scholar] [CrossRef]
- Xu, F.J.; Hu, B.Q.; Li, J.; Cui, R.Y.; Liu, Z.Q.; Jiang, Z.Z.; Yin, X.B. Reassessment of heavy metal pollution in riverine sediments of Hainan Island China: Sources and risks. Environ. Sci. Pollut. Res. 2018, 25, 1766–1772. [Google Scholar] [CrossRef]
- Jiang, W.; Hou, Q.Y.; Yang, Z.F.; Yu, T.; Zhong, C.; Yang, Y.; Fu, Y.R. Annual input fluxes of heavy metals in agricultural soil of Hainan Island China. Environ. Sci. Pollut. Res. 2014, 21, 7876–7885. [Google Scholar] [CrossRef]
- Zhao, D.B.; Wan, S.M.; Yu, Z.J.; Huang, J. Distribution enrichment and sources of heavy metals in surface sediments of Hainan Island rivers China. Environ. Earth Sci. 2015, 74, 5097–5110. [Google Scholar] [CrossRef]
- Wang, J.G.; Wang, P.; Zhao, Z.Z.; Huo, Y.R. Uptake and concentration of heavy metals in dominant mangrove species from Hainan Island South China. Environ. Geochem. Health 2021, 43, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, G.; Luo, Z.; Sun, X.; Xu, J. Spatial distribution source identification and risk assessment of heavy metals in seawater and sediments from Meishan Bay Zhejiang coast China. Mar. Pollut. Bull. 2020, 156, 111217–111225. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, S.; Naito, R.; Nakamura, Y. Spatial patterns of concentrations of Cu Zn Cd and Pb in marine sediments from Japanese port areas. Reg. Stud. Mar. Sci. 2020, 35, 101135–101146. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Ji, C.Y.; Fu, B.; He, L.S.; Fu, Q.Q.; Shen, M.C.; Zhao, Z.Z. Factors influencing the accumulation of Pd in mangrove wetland sediments in Dongzhai Harbor Hainan China. J. Coast. Conserv. 2019, 23, 1039–1045. [Google Scholar] [CrossRef]
- Hu, B.Q.; Li, J.; Cui, R.Y.; Wei, H.L.; Zhao, J.T.; Li, G.G.; Fang, X.S.; Ding, X.; Zou, L.A.; Bai, F.L. Clay mineralogy of the riverine sediments of Hainan Island South China Sea: Implications for weathering and provenance. J. Asian Earth Sci. 2014, 96, 84–92. [Google Scholar] [CrossRef]
- Li, M.; Xi, X.; Xiao, G.; Cheng, H.; Yang, Z.; Zhou, G.; Li, Z. National multi-purpose regional geochemical survey in China. J. Geochem. Explor. 2014, 139, 21–30. [Google Scholar] [CrossRef]
- Maanan, M.; Saddik, M.; Maanan, M.; Chaibi, M.; Assobhei, O.; Zourarah, B. Environmental and ecological risk assessment of heavy metals in sediments of Nador lagoon Morocco. Ecol. Indic. 2015, 48, 616–626. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. a sedimentological approach. Pergamon 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Li, H.; Chai, L.; Yang, Z.; Liao, Q.; Liu, Y.; Ouyang, B. Seasonal and spatial contamination statuses and ecological risk of sediment cores highly contaminated by heavy metals and metalloids in the Xiangjiang River. Environ. Geochem. Health 2019, 41, 1617–1633. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.N.; Li, W.H.; Lv, X. Responses of Tamarix ramosissima ABA Accumulation to groundwater level and soil salt changes in the lower reaches of Tarim River. Acta Ecol. Sin. 2007, 27, 4247–4251. [Google Scholar] [CrossRef]
- Cai, P.J.; Cai, G.Q.; Chen, X.; Li, S.; Zhao, L. The concentration distribution and biohazard assessment of heavy metal elements in surface sediments from the continental shelf of Hainan Island. Mar. Pollut. Bull. 2021, 166, 112254. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, J.; Xu, G.; Chen, B. Distribution and transport of heavy metals in surface sediments of the Zhejiang nearshore area East China Sea: Sedimentary environmental effects. Mar. Pollut. Bull. 2019, 146, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry biomass and productivity of mangrove forests: A review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Y.; Li, Y.Q.; Wang, Q.F.; Hou, S.; Zeng, J.Y. Dynamic characteristics of heavy metals in a eutrophic reservoir in subtropical China. J. Geochem. Explor. 2020, 208, 106391. [Google Scholar] [CrossRef]
- Liu, J.J.; Diao, Z.H.; Xu, X.R.; Xie, Q. Effects of dissolved oxygen salinity nitrogen and phosphorus on the release of heavy metals from coastal sediments. Sci. Total Environ. 2019, 666, 894–901. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Uppadit, B. Accumulation of heavy metals in mangrove sediments of chumphon province Thailand. Appl. Environ. Res. 2012, 34, 21–38. Available online: https://ph01.tci-thaijo.org/index.php/aer/index (accessed on 12 November 2022).
- McBride, M.B. Cadmium uptake by crops estimated from soil total Cd and pH. Soil Sci. 2002, 167, 62–67. [Google Scholar] [CrossRef]
- Sparrow, L.A.; Salardini, A.A.; Johnstone, J. Field studies of cadmium in potatoes (Solanum tuberosum L.). III. Response of cv. Russet Burbank to sources of banded potassium. Aust. J. Agric. Res. 1994, 45, 243–249. [Google Scholar] [CrossRef]
- Aknaf, A.; Akodad, M.; Layachi, M.; Baghour, M.; Oudra, B.; Vasconcelos, V. The chemical characterization and its relationship with heavy metals contamination in surface sediment of Marchica Mediterranean Lagoon (North of Morocco). Environ. Sci. Pollut. Res. 2022, 29, 4159–4169. [Google Scholar] [CrossRef]
- Chen, H.P.; Tang, Z.; Wang, P.; Zhao, F.J. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice. Environ. Pollut. 2018, 238, 482–490. [Google Scholar] [CrossRef]
- Muniz, P.; Danulat, E.; Yannicelli, B.; Alonso, J.G.; Medina, G.; Cego, M. Assessment of contamination by heavy metals and petroleum hydrocarbons in sediments of Montevideo Harbour (Uruguay). Environ. Int. 2004, 29, 1019–1028. [Google Scholar] [CrossRef]
- Atafar, Z.; Mesdaghinia, A.; Nouri, J.; Homaee, M.; Yunesian, M.; Ahmadimoghaddam, M.; Mahvi, A.H. Effect of fertilizer application on soil heavy metal concentration. Environ. Monit. Assess. 2010, 160, 83–89. [Google Scholar] [CrossRef]
- Hakim, A.S.; Adila, H.N.A.; Liyana, Z.F.; Mu’izzah, M.; Joon, C.C.; Fatehah, M.O. Assessment of Heavy Metal Contamination in Sediments in Sungai Pinang River Basin. IOP Conf. Ser. Earth Environ. Sci. 2020, 498, 012059. [Google Scholar] [CrossRef]
- Vane, C.H.; Harrison, I.; Kim, A.W.; Hayes, V.M. Organic and metal contamination in surface mangrove sediments of South China. Mar. Pollut. Bull. 2009, 58, 134–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passos, T.; Penny, D.; Barcellos, R.; Nandan, S.B.; Babu, D.S.S.; Santos, I.R.; Sanders, C.J. Increasing carbon nutrient and trace metal accumulation driven by development in a mangrove estuary in south Asia. Sci. Total Environ. 2022, 832, 154900. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Klumpp, D.W. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuarine Coast. Shelf Sci. 2005, 63, 605–618. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Wang, S.; Mo, L.; Zou, J.; Zhou, Y. Distribution determination risk assessment and source identification of heavy metals in mangrove wetland sediments from Qi’ao Island South China. Reg. Stud. Mar. Sci. 2020, 33, 100961. [Google Scholar] [CrossRef]
- Li, R.; Li, R.; Chai, M.; Shen, X.; Xu, H.; Qiu, G. Heavy metal contamination and ecological risk in Futian mangrove forest sediment in Shenzhen Bay South China. Mar. Pollut. Bull. 2015, 101, 448–456. [Google Scholar] [CrossRef]
- Liu, J.; Ma, K.; Qu, L. Ecological risk assessments and context-dependence analysis of heavy metal contamination in the sediments of mangrove swamp in Leizhou Peninsula China. Mar. Pollut. Bull. 2015, 100, 224–230. [Google Scholar] [CrossRef]
- Deng, J.; Guo, P.; Zhang, X.; Shen, X.; Su, H.; Zhang, Y.; Wu, Y.M.; Xu, C. An evaluation on the bioavailability of heavy metals in the sediments from a restored mangrove forest in the Jinjiang Estuary, Fujian, China. Ecotoxicol. Environ. Saf. 2019, 180, 501–508. [Google Scholar] [CrossRef]
- Bastakoti, U.; Robertson, J.; Bourgeois, C.; Marchand, C.; Alfaro, A.C. Temporal variations of trace metals and a metalloid in temperate estuarine mangrove sediments. Environ. Monit. Assess. 2019, 191, 780.1–780.18. [Google Scholar] [CrossRef] [PubMed]
- Background Values of Soil Elements in China; China Environmental Science Press: Beijing, China, 1990; Available online: www.irgrid.ac.cn/handle/1471x/723390 (accessed on 6 September 2022).
- Persaud, D.; Jaagumagi, R.; Hayton, A. Guidelines for the Protection and Management of Aquatic Sediment Quality in Ontario; Environmental Science: Toronto, ON, Canada, 1993; Available online: http://hdl.handle.net/10214/15797 (accessed on 8 September 2022).
- Pe-Piper, G.; Piper, D.J.W.; Wang, Y.; Zhang, Y.Z.; Trottier, C.; Ge, C.D.; Yin, Y. Quaternary evolution of the rivers of northeast Hainan Island China: Tracking the history of avulsion from mineralogy and geochemistry of river and delta sands. Sediment. Geol. 2016, 333, 84–99. [Google Scholar] [CrossRef]


| Name | Expression | Coefficient of Interpretation | Classification and Contamination Degree | Reference |
|---|---|---|---|---|
| Individual potential ecological risk index (Eir) | Tir: Toxicity coefficient, according to previous research results, the toxicity response coefficients of Hg, Cd, As, Cu, Pb, Ni, Cr, Co and Zn are 40, 30, 10, 5, 5, 5, 2, 2, 1, respectively. Ci: The measured content of element i in sediments (mg/kg). Cref: The geochemical background value of element n (mg/kg). | Eir < 40: Low risk 40 ≤ Eir < 80: Moderate risk 80 ≤ Eir < 160: Heavy risk 160 ≤ Eir < 320: Serious risk Eir ≥ 320: Extremely serious risk | [28] | |
| Potential ecological risk index(RI) | Eir: Individual potential ecological risk index. | RI < 150: Low risk 150 < RI ≤ 300: Moderate risk 300 < RI ≤ 600: Serious risk RI > 600: Extremely serious risk | [29] | |
| Multiple probable effect concentrations quality (mPECQs) | Ci: The measured content of element i in sediments (mg/kg). PECi: The consensus-based probable effect concentration of individual metal. The PEC values of Cr, Ni, Cu, As, Cd, Pb, Zn, and Hg were 111, 48.6, 149, 33, 4.8, 129, 459 and 1.06 mg/kg, n: The number of heavy metals. | mPECQs < 1: Non-toxic; the incidence of toxicity is relatively low (<25%) 1 ≤ mPECQs < 5: the incidence of toxicity is 25–75% mPECQs ≥ 5: Toxic; the incidence of toxicity is more than 75% | [30] |
| Horizontal dimension | pH | As | Cd | Cr | Cu | Hg | Pb | Ni | Zn | Co | P | N | Al2O3 (%) | Corg (%) | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 4.84 | 2.85 | 0.03 | 28.34 | 7.14 | 0.01 | 12.53 | 7.74 | 24.11 | 2.43 | 115.61 | 253.50 | 5.99 | 0.29 | 10.2 | 40.1 | 4.3 |
| Max | 8.32 | 7.49 | 0.14 | 171.16 | 49.07 | 0.11 | 31.41 | 75.82 | 122.23 | 33.55 | 842.35 | 1282.90 | 17.12 | 2.60 | 51.2 | 74.5 | 20.3 |
| Median | 7.18 | 5.14 | 0.05 | 58.38 | 13.18 | 0.02 | 19.95 | 22.14 | 56.88 | 10.09 | 304.79 | 429.00 | 11.01 | 0.90 | 32.85 | 56 | 11.3 |
| Mean | 6.95 | 5.29 | 0.06 | 70.03 | 17.45 | 0.03 | 20.58 | 29.08 | 64.42 | 12.82 | 377 | 548.22 | 11.72 | 1.06 | 33.05 | 55.50 | 11.46 |
| Standard deviation | 1.10 | 1.40 | 0.03 | 32.57 | 10.57 | 0.02 | 4.65 | 16.06 | 23.19 | 6.65 | 180.66 | 334.52 | 2.97 | 0.68 | 15.71 | 13.21 | 4.51 |
| Coefficient of variation (%) | 15.86 | 26.54 | 46.35 | 46.52 | 60.6 | 71.55 | 22.58 | 55.23 | 36.00 | 51.90 | 47.92 | 61.02 | 25.35 | 63.63 | 47.53 | 23.80 | 39.35 |
| Vertical Dimension | As | Cd | Cr | Cu | Hg | Pb | Ni | Zn | Co |
|---|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 5.97 | 0.08 | 72.27 | 19.04 | 0.04 | 21.52 | 28.9 | 68.3 | 13.06 |
| 10–20 cm | 5.98 | 0.07 | 70.90 | 18.58 | 0.04 | 21.42 | 29.02 | 66.39 | 12.96 |
| 20–30 cm | 5.83 | 0.06 | 69.16 | 18.16 | 0.03 | 21.12 | 28.62 | 65.06 | 12.75 |
| 30–40 cm | 5.54 | 0.06 | 70.58 | 17.54 | 0.03 | 20.83 | 28.49 | 63.78 | 12.49 |
| 40–50 cm | 5.22 | 0.06 | 68.87 | 17.19 | 0.03 | 20.73 | 28.77 | 63.16 | 12.42 |
| 50–60 cm | 4.99 | 0.06 | 69.57 | 17.08 | 0.03 | 20.55 | 28.99 | 62.36 | 12.61 |
| 60–70 cm | 4.90 | 0.05 | 69.58 | 17.17 | 0.03 | 20.58 | 29.26 | 63.39 | 12.94 |
| 70–80 cm | 4.73 | 0.06 | 70.38 | 16.74 | 0.03 | 19.98 | 29.54 | 63.61 | 12.9 |
| 80–90 cm | 4.80 | 0.05 | 69.58 | 16.77 | 0.03 | 19.76 | 29.71 | 63.85 | 13.00 |
| 90–100 cm | 4.95 | 0.06 | 69.42 | 16.22 | 0.03 | 19.33 | 29.54 | 64.27 | 13.11 |
| Standard deviation | 0.50 | 0.01 | 1.02 | 0.89 | 0.003 | 0.71 | 0.41 | 1.75 | 0.24 |
| Coefficient of variation (%) | 9.36 | 14.11 | 1.45 | 5.08 | 9.32 | 3.46 | 1.42 | 2.72 | 1.88 |
| Location and Year | Average Content of Heavy Metal Elements (mg/kg) | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Hg | Pb | Ni | Zn | Co | ||
| Dongzhai Harbor, Hainan, 2020 | 5.29 | 0.06 | 70.03 | 17.45 | 0.03 | 20.58 | 29.08 | 64.42 | 12.82 | This Study |
| Qi’ao Island, Guangdong, 2015 | - | 9.50 | 389.2 | 81.50 | - | 70.60 | 50.40 | 241.7 | - | [48] |
| Futian, Shenzhen, 2014 | - | 3.00 | 55.40 | 31.70 | - | 47.80 | - | 296.3 | - | [49] |
| Leizhou Peninsula, Hong Kong, 2012 | 40.63 | 0.21 | 67.29 | 18.41 | - | 33.35 | 49.48 | 85.62 | - | [50] |
| Jinjiang Estuary, Fujian,2017 | - | 0.14 | - | 11.07 | - | 41.93 | 14.63 | 72.41 | - | [51] |
| Jiulong River Estuary, Xiamen, 2018 | 12.99 | - | 63.98 | 34.25 | 0.15 | 73.54 | 28.62 | 146.6 | - | [6] |
| East and west coast of India, 2016 | - | - | 160.5 | 63.35 | - | 30.45 | 56.25 | 116.3 | - | [3] |
| Sydney Estuary, Australia, 2012 | 8.10 | 0.59 | 31.00 | 42.00 | - | 95.00 | 9.50 | 156 | 4.30 | [4] |
| Mangefil, New Zealand, 2014 | 36.94 | 0.24 | - | 10.15 | - | 1.11 | - | 14.64 | - | [52] |
| Ho Chi Minh City, Vietnam, 2017 | 17.00 | - | 124.0 | 26.00 | - | 26.00 | 60.00 | 113.0 | - | [13] |
| Background values of soil heavy metals in Hainan Island, 2018 | 8.90 | 0.06 | 50.50 | 17.00 | 0.08 | 36.00 | 14.40 | 47.30 | 7 | [53] |
| Ontario Guidelines for The Protection and Management of Aquatic Sediments (LEL), 1993 | 6 | 0.6 | 26 | 16 | 0.2 | 31 | 16 | 120 | - | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, C.; Du, S.; Zhang, G.; Wang, Y.; Rao, W. Spatial Distribution and Ecological Risk Assessment of Heavy Metals in the Sediment of a Tropical Mangrove Wetland on Hainan Island, China. Water 2022, 14, 3785. https://doi.org/10.3390/w14223785
Mao C, Du S, Zhang G, Wang Y, Rao W. Spatial Distribution and Ecological Risk Assessment of Heavy Metals in the Sediment of a Tropical Mangrove Wetland on Hainan Island, China. Water. 2022; 14(22):3785. https://doi.org/10.3390/w14223785
Chicago/Turabian StyleMao, Changping, Suming Du, Gucheng Zhang, Yao Wang, and Wenbo Rao. 2022. "Spatial Distribution and Ecological Risk Assessment of Heavy Metals in the Sediment of a Tropical Mangrove Wetland on Hainan Island, China" Water 14, no. 22: 3785. https://doi.org/10.3390/w14223785




