Kinetic, Thermodynamic and Adsorption Isotherm Studies of Detoxification of Eriochrome Black T Dye from Wastewater by Native and Washed Garlic Peel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Equipment and Instruments Used
2.3. Preparation of Native and Washed Garlic Peel (Adsorbents)
2.4. Dye Stock Solution and Working Solutions
2.5. Characterization of Garlic Peel
2.6. Adsorption Studies
2.7. Kinetic Modelling
2.8. Adsorption Isotherm Studies
2.9. Adsorption and Desorption Studies
3. Results and Discussion
3.1. Structural and Surface Characterization of GP
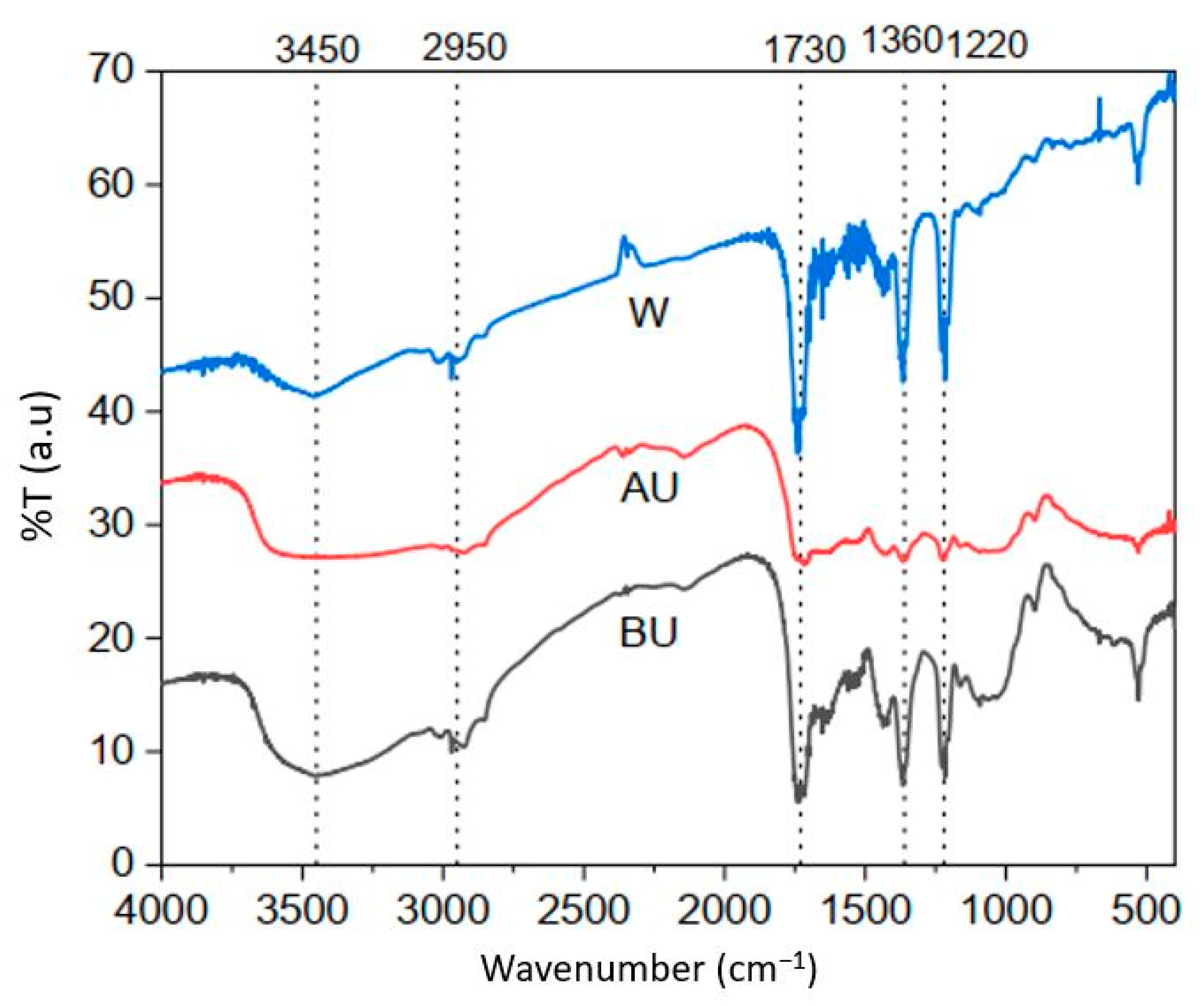
3.1.1. FT-IR Studies
3.1.2. Scanning Electron Microscopy (SEM)
3.2. Effect of pH
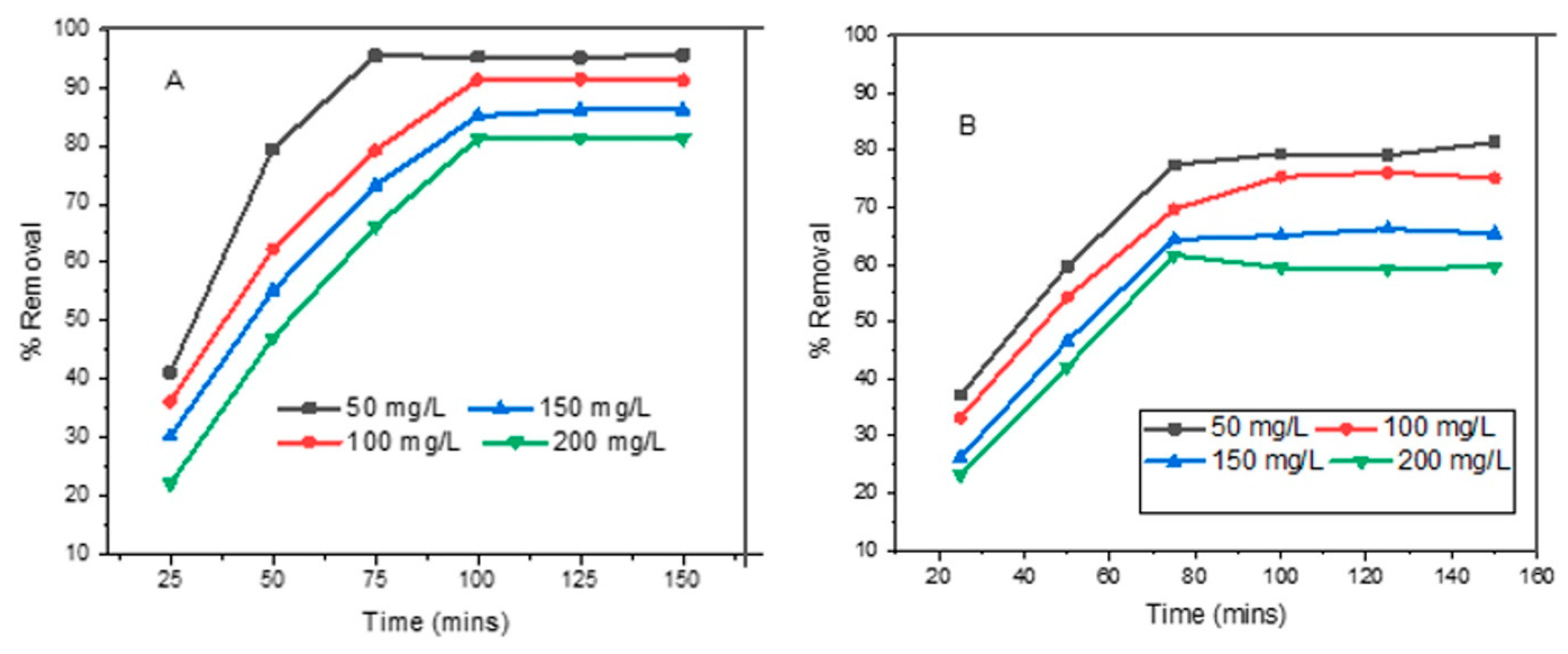
3.3. Effect of Intial Dye Concentration and Contact Time
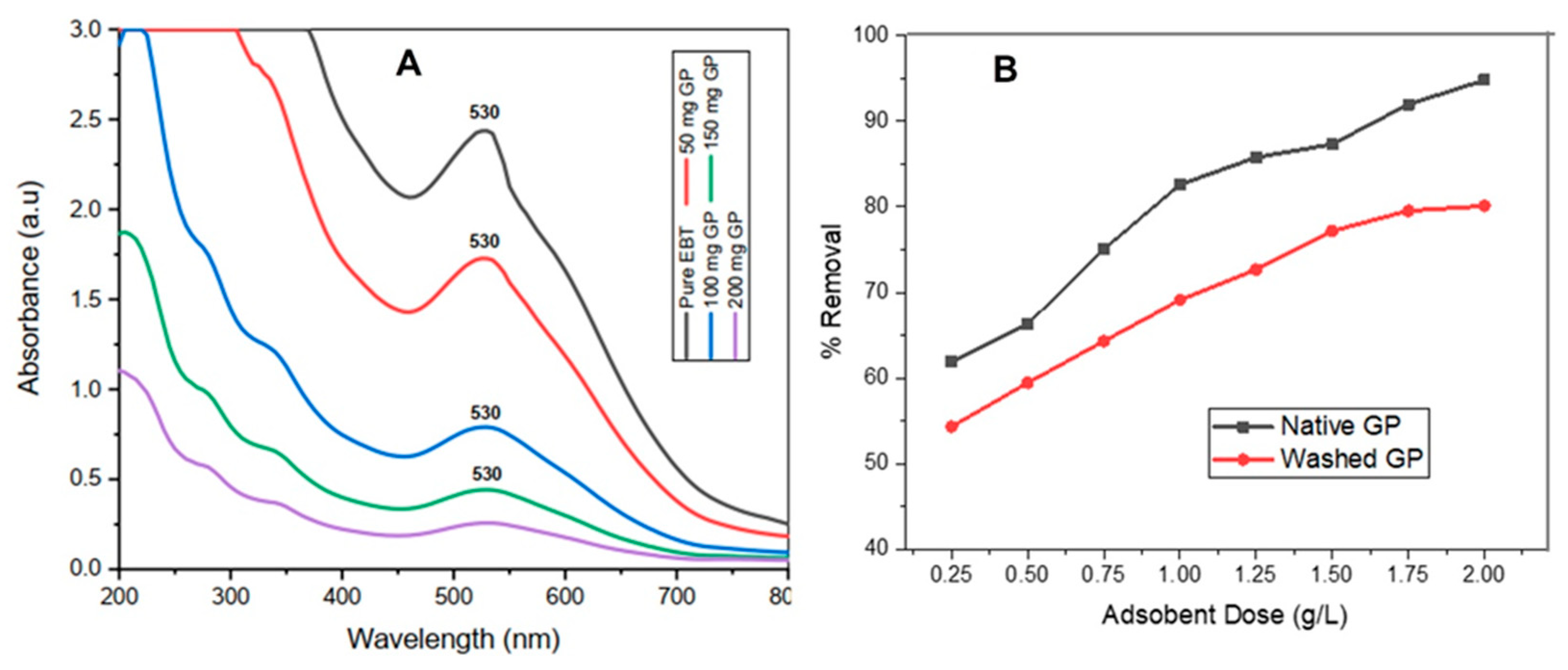
3.4. Effect of Adsorbent Dose
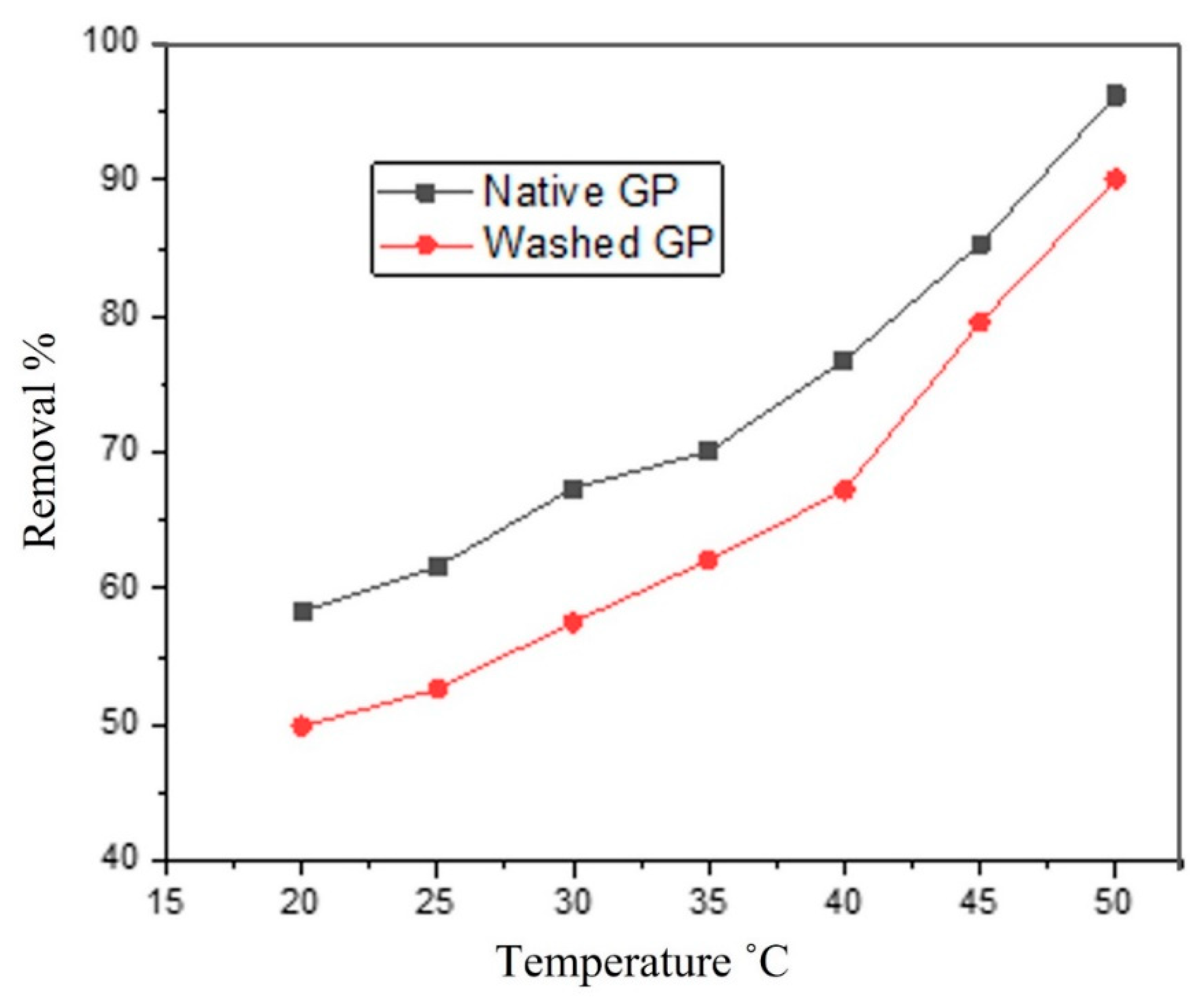
3.5. Effect of Temperature
3.6. Adsorption Kinetics
3.7. Thermodynamic Parameters
3.8. Adsorption Isotherms
3.9. Desorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alprol, A.E.; Heneash, A.M.; Ashour, M.; Abualnaja, K.M.; Alhashmialameer, D.; Mansour, A.T.; Sharawy, Z.Z.; Abu-Saied, M.A.; Abomohra, A.E. Potential Applications of Arthrospira platensis Lipid-Free Biomass in Bioremediation of Organic Dye from Industrial Textile Effluents and Its Influence on Marine Rotifer (Brachionus plicatilis). Materials 2021, 14, 4446. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Li, M.; Yang, C.; Sun, Y.; Chen, J. Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J. Hazard. Mater. 2005, 121, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Barka, N.; Abdennouri, M.; El Makhfouk, M. Removal of Methylene Blue and Eriochrome Black T from aqueous solutions by biosorption on Scolymus hispanicus L.: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng. 2011, 42, 320–326. [Google Scholar] [CrossRef]
- Islam, A.; Ahmad, A.; Laskar, M.A. Characterization of a Chelating Resin Functionalized via Azo Spacer and Its Analytical Applicability for the Determination of Trace Metal Ions in Real Matrices. J. Appl. Polymer Sci. 2011, 123, 3448–3458. [Google Scholar] [CrossRef]
- Ejhieh, A.N.; Khorsandi, M. Photodecolorization of Eriochrome Black T using NiS-P zeolite as a heterogeneous catalyst. J. Hazard. Mater. 2010, 176, 629–637. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Noor, N.H.M.; Serrà, A.; Mohamad Ibrahim, M.N. Advances and challenges in developing efficient graphene oxide-based ZnO photocatalysts for dye photo-oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef]
- Badawi, A.K.; Ismail, B.; Baaloudj, O.; Abdalla, K.Z. Advanced wastewater treatment process using algal photo-bioreactor associated with dissolved-air flotation system: A pilot-scale demonstration. J. Water Process. Eng. 2022, 46, 102565. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B. Fixed-bed Adsorption of Reactive Azo Dye onto Granular Activated Carbon Prepared from Waste. J. Hazard. Mater. 2009, 175, 298–303. [Google Scholar] [CrossRef]
- Idris, M.O.; Kim, H.C. Exploring the effectiveness of microbial fuel cell for the degradation of organic pollutants coupled with bio-energy generation. Sustain. Energy Technol. Assess. 2022, 52, 102183. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, A.A.; Noor, N.H.M.; Umar, K.; Adnan, R.; Rashid, M. Graphene oxide–ZnO nanocomposite: An efficient visible light photocatalyst for degradation of rhodamine B. Appl. Nanosci. 2021, 11, 1291–1302. [Google Scholar] [CrossRef]
- García-Montaño, J.; Ruiz, N.; Muñoz, I.; Domènech, X.; García-Hortal, J.A.; Torrades, F.; Peral, J. Environmental assessment of different photo-Fenton approaches for commercial reactive dye removal. J. Hazard. Mater. 2006, 138, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Abualnaja, K.M.; Alprol, A.E.; Abu-Saied, M.A.; Ashour, M.; Mansour, A.T. Removing of anionic dye from aqueous solutions by adsorption using of multiwalled carbon nanotubes and poly (Acrylonitrile-styrene) impregnated with activated carbon. Sustainability 2021, 13, 7077. [Google Scholar] [CrossRef]
- Kansal, S.; Sood, S.; Umar, A.; Mehta, S. Photocatalytic degradation of Eriochrome Black T dye using well-crystalline anatase TiO2 nanoparticles. J. Alloys Compd. 2013, 581, 392–397. [Google Scholar] [CrossRef]
- Moeinpour, F.; Alimoradi, A.; Kazemi, M. Efficient removal of Eriochrome black-T from aqueous solution using NiFe2O4 magnetic nanoparticles. J. Environ. Health Sci. Eng. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Zhang, L.; Guo, X.; Jiang, X.; Li, T. Adsorption behavior of direct red 80 and congo red onto activated carbon/surfactant: Process optimization, kinetics and equilibrium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.; Kaur, S.; Khosla, E. Removal of Eriochrome black-T by adsorption on to eucalyptus bark using green technology. Indian J. Chem. Technol. 2011, 18, 53–60. [Google Scholar]
- Kallel, F.; Chaabouni Ellouz, S. Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: A review. Environ. Prog. Sustain. Energy 2017, 36, 1765–1777. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Tao, Y.; Yu, Y.; Jiang, H.; Lian, H. Comparative study of adsorption of Pb(II) on native garlic peel and mercerized garlic peel. Environ. Sci. Pollut. Res. Int. 2014, 21, 2054–2063. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Saif Ur Rehman, M.; Kim, I.; Rashid, N.; Adeel Umer, M.; Sajid, M.; Han, J.-I. Adsorption of Brilliant Green Dye on Biochar Prepared From Lignocellulosic Bioethanol Plant Waste. CLEAN Soil Air Water 2016, 44, 55–62. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Removal of Brilliant Green dye from water by modified Bambusa Tulda: Adsorption isotherm, kinetics and thermodynamics study. Int. J. Environ. Sci. Technol. 2018, 16, 1649–1662. [Google Scholar] [CrossRef]
- Fayoud, N.; Tahiri, S.; Alami Younssi, S.; Albizane, A.; Gallart-Mateu, D.; Cervera, M.L.; de la Guardia, M. Kinetic, isotherm and thermodynamic studies of the adsorption of methylene blue dye onto agro-based cellulosic materials. Desalination Water Treat. 2016, 57, 16611–16625. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Flores, E.D.; Genuino, D.A.D.; Futalan, C.M.; Wan, M.-W. Adsorption of Eriochrome Black T (EBT) dye using activated carbon prepared from waste rice hulls—Optimization, isotherm and kinetic studies. J. Taiwan Inst. Chem. Eng. 2013, 44, 646–653. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, Q.; Dong, Y.; Li, J.; Zhang, X. The Adsorption Capacity, Pore Structure, and Thermal Behavior of the Modified Clay Containing SSA. Adv. Mater. Sci. Eng. 2016, 2016, 9894657. [Google Scholar] [CrossRef] [Green Version]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Khalid, A.; Zubair, M.; Ihsanullah. A Comparative Study on the Adsorption of Eriochrome Black T Dye from Aqueous Solution on Graphene and Acid-Modified Graphene. Arab. J. Sci. Eng. 2018, 43, 2167–2179. [Google Scholar] [CrossRef]
- Ahmad, A.; Setapar, S.H.M.; Yaqoob, A.A.; Ibrahim, M.N.M. Synthesis and characterization of GO-Ag nanocomposite for removal of malachite dye from aqueous solution. Mater. Today Proc. 2021, 47, 1359–1365. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Parameswaranpillai, J.; Siengchin, S. Adsorption Study of Anionic Dye, Eriochrome Black T from Aqueous Medium Using Polyvinyl Alcohol/Starch/ZSM-5 Zeolite Membrane. J. Polym. Environ. 2020, 28, 2631–2643. [Google Scholar] [CrossRef]
- Sukla Baidya, K.; Kumar, U. Adsorption of brilliant green dye from aqueous solution onto chemically modified areca nut husk. S. Afr. J. Chem. Eng. 2021, 35, 33–43. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef] [Green Version]
- Javanbakht, V.; Shafiei, R. Preparation and performance of alginate/basil seed mucilage biocomposite for removal of eriochrome black T dye from aqueous solution. Int. J. Biol. Macromol. 2020, 152, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman Oyekanmi, A.; Abd Latiff, A.A.; Daud, Z.; Saphira Radin Mohamed, R.M.; Ismail, N.; Ab Aziz, A.; Rafatullah, M.; Hossain, K.; Ahmad, A.; Kamoldeen Abiodun, A. Adsorption of cadmium and lead from palm oil mill effluent using bone-composite: Optimisation and isotherm studies. Int. J. Environ. Anal. Chem. 2019, 99, 707–725. [Google Scholar] [CrossRef]
- Singh, R.K.; Patil, T.; Sawarkar, A.N. Pyrolysis of garlic husk biomass: Physico-chemical characterization, thermodynamic and kinetic analyses. Bioresour. Technol. Rep. 2020, 12, 100558. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, X.; Wei, B.; Zhao, Y.; Wang, J. Evaluation of adsorption potential of bamboo biochar for metal-complex dye: Equilibrium, kinetics and artificial neural network modeling. Int. J. Environ. Sci. Technol. 2014, 11, 1093–1100. [Google Scholar] [CrossRef]
- Salman, S.M.; Ali, A.; Khan, B.; Iqbal, M.; Alamzeb, M. Thermodynamic and kinetic insights into plant-mediated detoxification of lead, cadmium, and chromium from aqueous solutions by chemically modified Salvia moorcroftiana leaves. Environ. Sci. Pollut. Res. Int. 2019, 26, 14339–14349. [Google Scholar] [CrossRef]
- Mane, V.S.; Babu, P.V.V. Studies on the adsorption of Brilliant Green dye from aqueous solution onto low-cost NaOH treated saw dust. Desalination 2011, 273, 321–329. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198–199, 219–227. [Google Scholar] [CrossRef]
- Mattson, J.S.M.H.B. Activated Carbon: Surface Chemistry and Adsorption from Solution; M. Dekker: New York, NY, USA, 1971. [Google Scholar]
- Rashidi, R.; Omidi Khaniabadi, Y.; Ghaderpoori, M. Adsorption of Eriochrome black-T from aqueous environment by raw Montmorillonite. Int. J. Environ. Anal. Chem. 2021, 1–15. [Google Scholar] [CrossRef]
- Nandi, B.; Goswami, A.; Purkait, M. Adsorption Characteristics of Brilliant Green Dye on Kaolin. J. Hazard. Mater. 2008, 161, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Wahab, M.; Zahoor, M.; Shahwar, D.; Sultana, S.; Alamzeb, M.; Ahmed, S. Green mediated biosorption of Pb (II) from aqueous solution using chemically modified low cost Grewia optiva leaves. Desalination Water Treat. 2020, 195, 413–420. [Google Scholar] [CrossRef]
- Jahagirdar, S.S.; Shrihari, S.; Manu, B. Reuse of incinerated textile mill sludge as adsorbent for dye removal. KSCE J. Civ. Eng. 2015, 19, 1982–1986. [Google Scholar] [CrossRef]
- Çelekli, A.; Bozkurt, H. Sorption and desorption studies of a reactive azo dye on effective disposal of redundant material. Environ. Sci. Pollut. Res. 2013, 20, 4647–4658. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Hayati, B.; Arami, M.; Lan, C. Adsorption of textile dyes on Pine Cone from colored wastewater: Kinetic, equilibrium and thermodynamic studies. Desalination 2011, 268, 117–125. [Google Scholar] [CrossRef]
- Çelekli, A.; Tanrıverdi, B.; Bozkurt, H. Predictive modeling of removal of Lanaset Red G on Chara contraria; kinetic, equilibrium, and thermodynamic studies. Chem. Eng. J. 2011, 169, 166–172. [Google Scholar] [CrossRef]


| Parameter | Adsorbent | ||
|---|---|---|---|
| Native GP | After Use GP | Washed GP | |
| Surface Area (m2/g) | 14.001 | 13.025 | 13.020 |
| Pore Volume (cm3/g) | 0.015 | 0.014 | 0.013 |
| Pore size (Based on BJH) (A°) | 15.076 | 16.037 | 15.011 |
| Adsorbent | Pseudo First Order | Pseudo-Second Order | ||||||
|---|---|---|---|---|---|---|---|---|
| C0 | Qe exp | qe cal | K1 | R2 | qe cal | K2 | R2 | |
| (mg/L) [23] | (mg/g) [23] | (mg/g) [23] | ||||||
| Native GP3 | 50 | 79.84 | 2.29 | −0.0003 | 0.812 | 79.57 | 0.0392 | 0.999 |
| Washed GP | 50 | 65.72 | 3.40 | −0.0004 | 0.719 | 65.10 | 0.0231 | 0.999 |
| Adsorbent | Temp (K) [37] | KL | ΔG° (KJ mol−1) [37] | ΔH° (KJ mol−1) [37] | ΔS° (JK−1 mol−1) [37] | R2 |
|---|---|---|---|---|---|---|
| Native GP | 293 | 2.380 | −2.112 | 2.433 | 15.480 | 0.989 |
| 298 | 2.412 | −2.180 | ||||
| 303 | 2.445 | −2.247 | ||||
| 308 | 2.483 | −2.326 | ||||
| 313 | 2.536 | −2.415 | ||||
| 318 | 2.565 | −2.485 | ||||
| 323 | 2.617 | −2.576 | ||||
| Washed GP | 293 | 2.031 | −1.726 | 2.272 | 13.665 | 0.944 |
| 298 | 2.052 | −1.780 | ||||
| 303 | 2.130 | −1.904 | ||||
| 308 | 2.130 | −1.936 | ||||
| 313 | 2.155 | −1.998 | ||||
| 318 | 2.187 | −2.068 | ||||
| 323 | 2.218 | −2.139 |
| Isotherm Type | Parameters | Adsorbent | |
|---|---|---|---|
| Native GP | Washed GP | ||
| Langmuir | qmax (mg/g) [37] | 99.522 | 89.401 |
| KL (L/mg) [37] | 2.382 | 0.1309 | |
| RL | 0.0083 | 0.1326 | |
| R2 | 0.998 | 0.970 | |
| Freundlich | Kf | 5.850 | 4.439 |
| R2 | 0.758 | 0.731 | |
| 1/n | 0.131 | 0.241 | |
| Sr. No. | Bio-Adsorbent | Name of Dye | Qmax (mg/g) |
|---|---|---|---|
| 1 | Na2CO3-Treated Bambusa | BG | 41.67 |
| 2 | HCL-Treated Bambusa | BG | 32.15 |
| 3 | DBT-Treated Bambusa | BG | 33.32 |
| 4 | Coir pith | CR | 3.0 |
| 5 | Banana Peel | BB 9 | 20.8 |
| 6 | Orange peel | AV | 20.0 |
| 7 | Straw | BB 9 | 20.0 |
| 8 | Sugarcane bagasse | AO 10 | 6.10 |
| 9 | Date pit | BB 9 | 17.0 |
| 10 | Coir pith | Direct red 28 | 7.0 |
| 11 | Watermelon | CV | 12 |
| 12 | Charfines | Direct brown 1 | 6.1 |
| 13 | Rice husk | BB 9 | 20.0 |
| 15 | Hazelnut shell | BB 9 | 9.0 |
| 16 | Carbonized acorn seed waste | BG | 2.11 |
| 17 | Rice husk ash | BG | 25.13 |
| 18 | Oxidized cactus fruit peel | BG | 142.85 |
| 19 | Ashoka leaf powder | BG | 125 |
| 20 | Rice straw biochar | BG | 111.11 |
| 21 | Bagasse fly ash | BG | 116.28 |
| 22 | Native Garlic Peel (This Study) | Eriochrome Black T | 99.52 |
| 23 | Washed Garlic Peel (This Study) | Eriochrome Black T | 89.40 |
| S. No. | Adsorbent Detail | Qmax (mg/g) |
|---|---|---|
| 1 | Maize stem | 167 |
| 2 | Graphene | 102.04 |
| 3 | Acid-modified Graphene | 70.42 |
| 4 | Native almond shells | 6.0 |
| 5 | Almond shells (cold plasma treated) | 18.18 |
| 6 | Almond shells (Microwave treated) | 29.41 |
| 7 | Rice hulls based activated carbon | 161.0 |
| 8 | Magnetite/silica/pectin nanoparticles | 65.35 |
| 9 | Bottom ash | 94.96 |
| 10 | H3PO4-modified berry leaves | 133.33 |
| 11 | NiFe2O4 nanoparticles | 81.52 |
| 12 | NiFe2O4 magnetic nanoparticles | 47.0 |
| 13 | Polyzwitterionic acid (Hydrophobic cross-linked) | 15.9 |
| 14 | β-Cyclodextrins | 20.0 |
| 15 | NiFe-hydroxides | 133.0 |
| 16 | Mosambi peel Activated carbon | 46.50 |
| 17 | Bentonite carbon composite | 2.89 |
| 18 | Polymer/Kaolinite | 0.32 |
| 19 | Talc | 1.09 |
| 20 | HCl modified Clay | 16.20 |
| 21 | H2SO4 modified Clay | 16.50 |
| 22 | Montmorillonite | 99.0 |
| 23 | Eucalyptus bark | 52.37 |
| 24 | Native Garlic Peel (This study) | 99.52 |
| 25 | Washed Garlic Peel (This study) | 89.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamzeb, M.; Tullah, M.; Ali, S.; Ihsanullah; Khan, B.; Setzer, W.N.; Al-Zaqri, N.; Ibrahim, M.N.M. Kinetic, Thermodynamic and Adsorption Isotherm Studies of Detoxification of Eriochrome Black T Dye from Wastewater by Native and Washed Garlic Peel. Water 2022, 14, 3713. https://doi.org/10.3390/w14223713
Alamzeb M, Tullah M, Ali S, Ihsanullah, Khan B, Setzer WN, Al-Zaqri N, Ibrahim MNM. Kinetic, Thermodynamic and Adsorption Isotherm Studies of Detoxification of Eriochrome Black T Dye from Wastewater by Native and Washed Garlic Peel. Water. 2022; 14(22):3713. https://doi.org/10.3390/w14223713
Chicago/Turabian StyleAlamzeb, Muhammad, Madiha Tullah, Saqib Ali, Ihsanullah, Behramand Khan, William N. Setzer, Nabil Al-Zaqri, and Mohamad Nasir Mohamad Ibrahim. 2022. "Kinetic, Thermodynamic and Adsorption Isotherm Studies of Detoxification of Eriochrome Black T Dye from Wastewater by Native and Washed Garlic Peel" Water 14, no. 22: 3713. https://doi.org/10.3390/w14223713






