Design and Modeling of a Biotechnological Nanofiltration Module Using Bacterial Cellulose Membranes for the Separation of Oily Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Characterization of Bacterial Cellulose Membranes
2.1.1. Microorganisms and Maintenance Medium
2.1.2. Culture Conditions and Purification
2.1.3. Production Yield of Bacterial Cellulose and Water Retention Capacity
2.1.4. Characterization of Membranes
Determination of Water Contact Angle and Sorption Index
Swelling Ratio
Fourier Transform Infrared (FTIR) Spectroscopy
Thermogravimetry
X-ray Diffractometry
Scanning Electron Microopy and Energy-Dispersive Spectroscopy
Porosity
Mechanical Tests
Compression
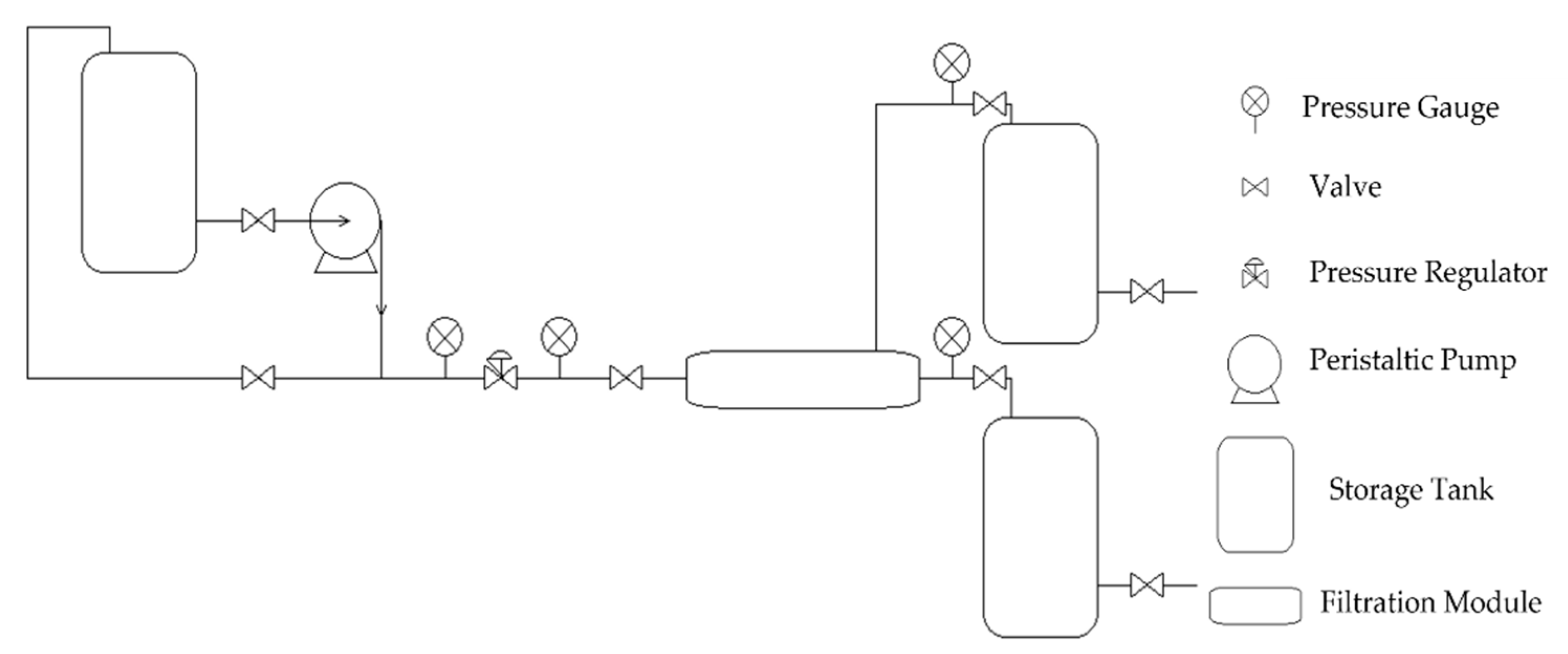
2.2. Modeling and Simulation of Filtering Module
2.2.1. Conceptual Project
2.2.2. Study of Constructive Forms
2.2.3. Optimization Process
2.2.4. Material Selection
2.2.5. Basic Dimensional Calculations
2.2.6. Adaptation of Optimized Solution
2.2.7. Refining the Final Solution (CFD, FEA, Setup, Adjustments)
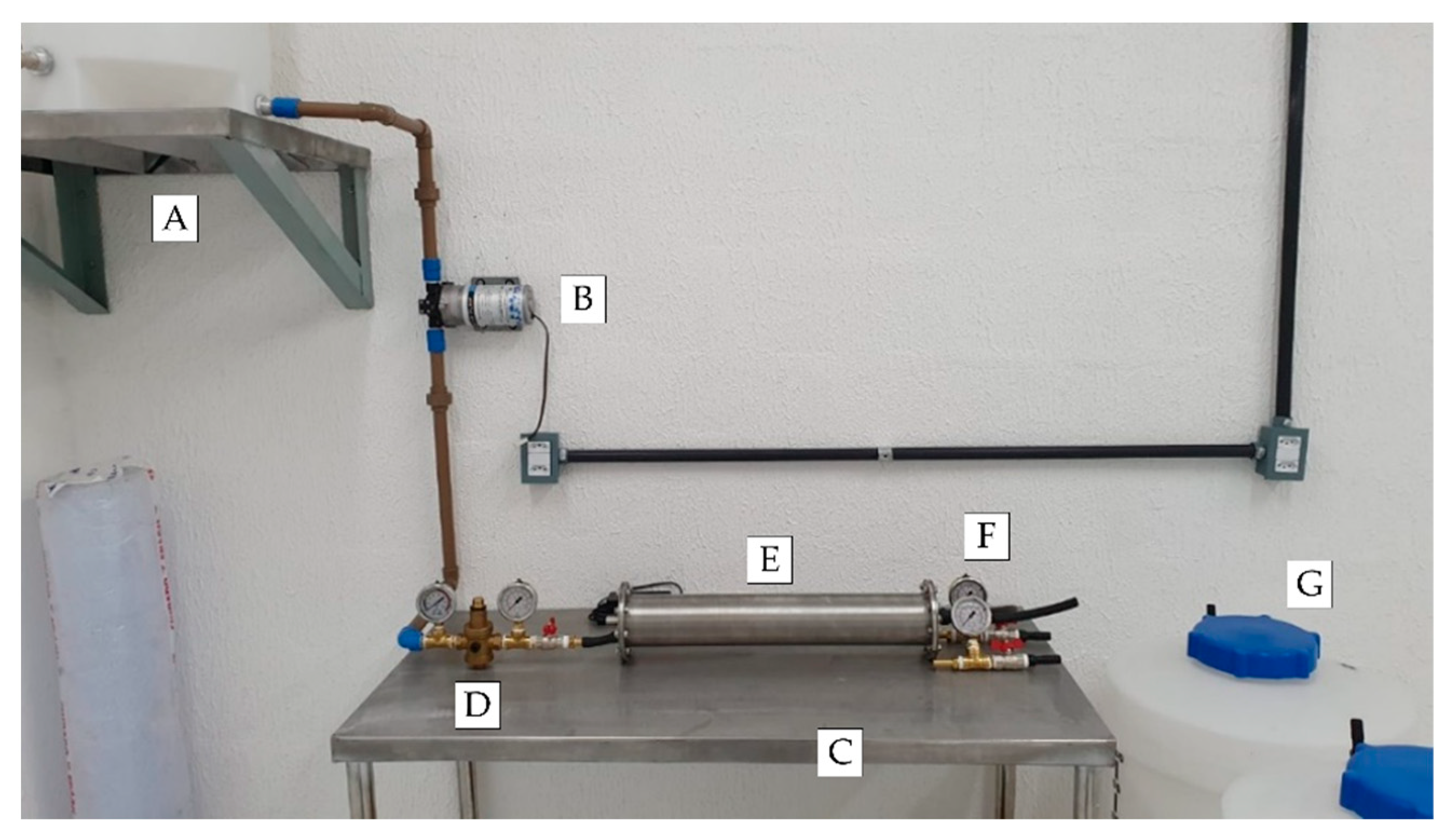
2.2.8. Summary of Final Configuration (Fabrication)
2.2.9. Adaptation of Physical Space and Assembly of Subsystems
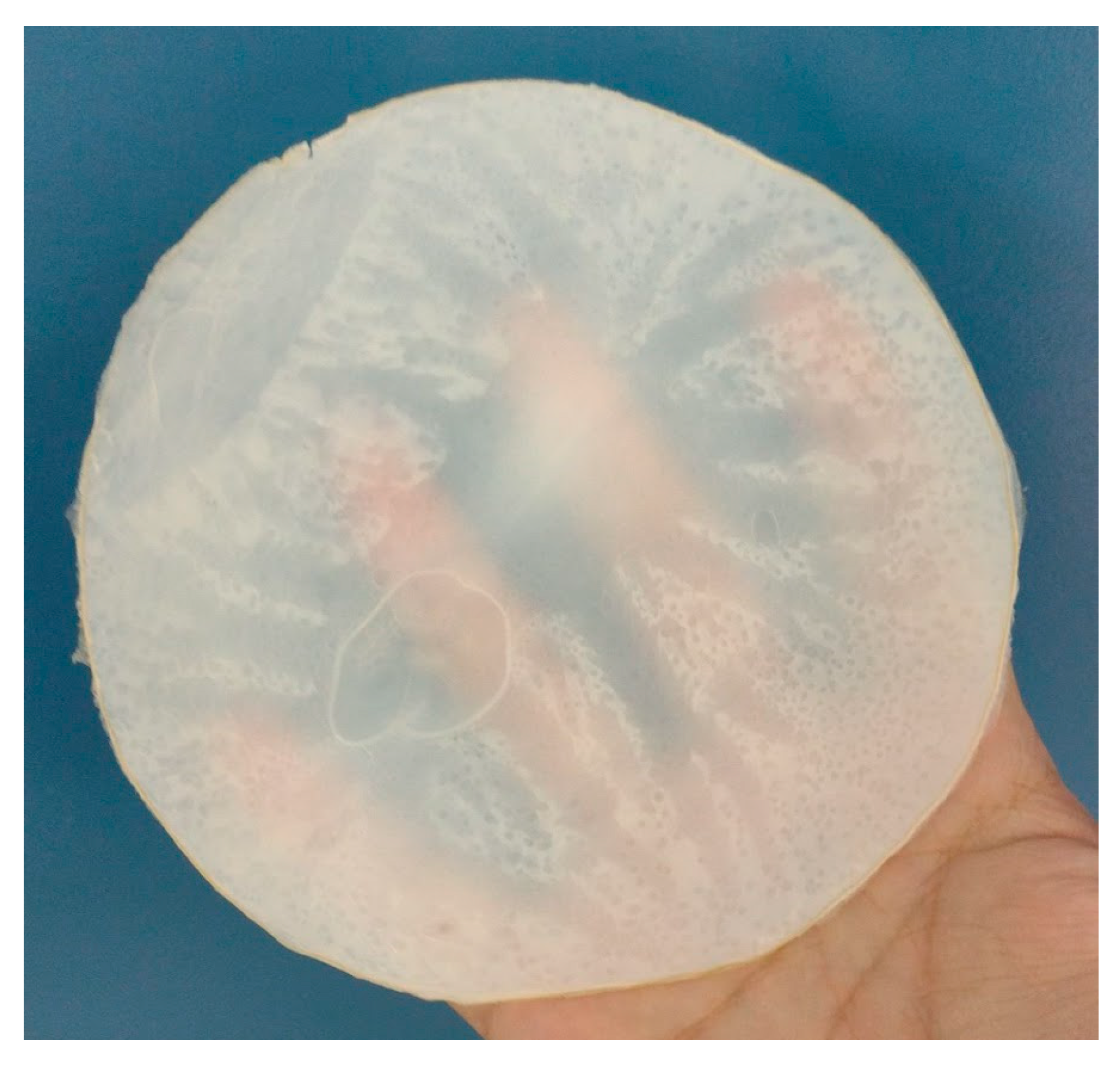
2.2.10. Production of Cellulose Membranes following Model Proposed in Computational Simulation
3. Results and Discussion (Bacterial Cellulose Membrane)
3.1. Bacterial Cellulose Yield and Water Retention Capacity (WRC)
3.2. Water Contact Angle, Swelling Ratio, Sorption Index and Porosity
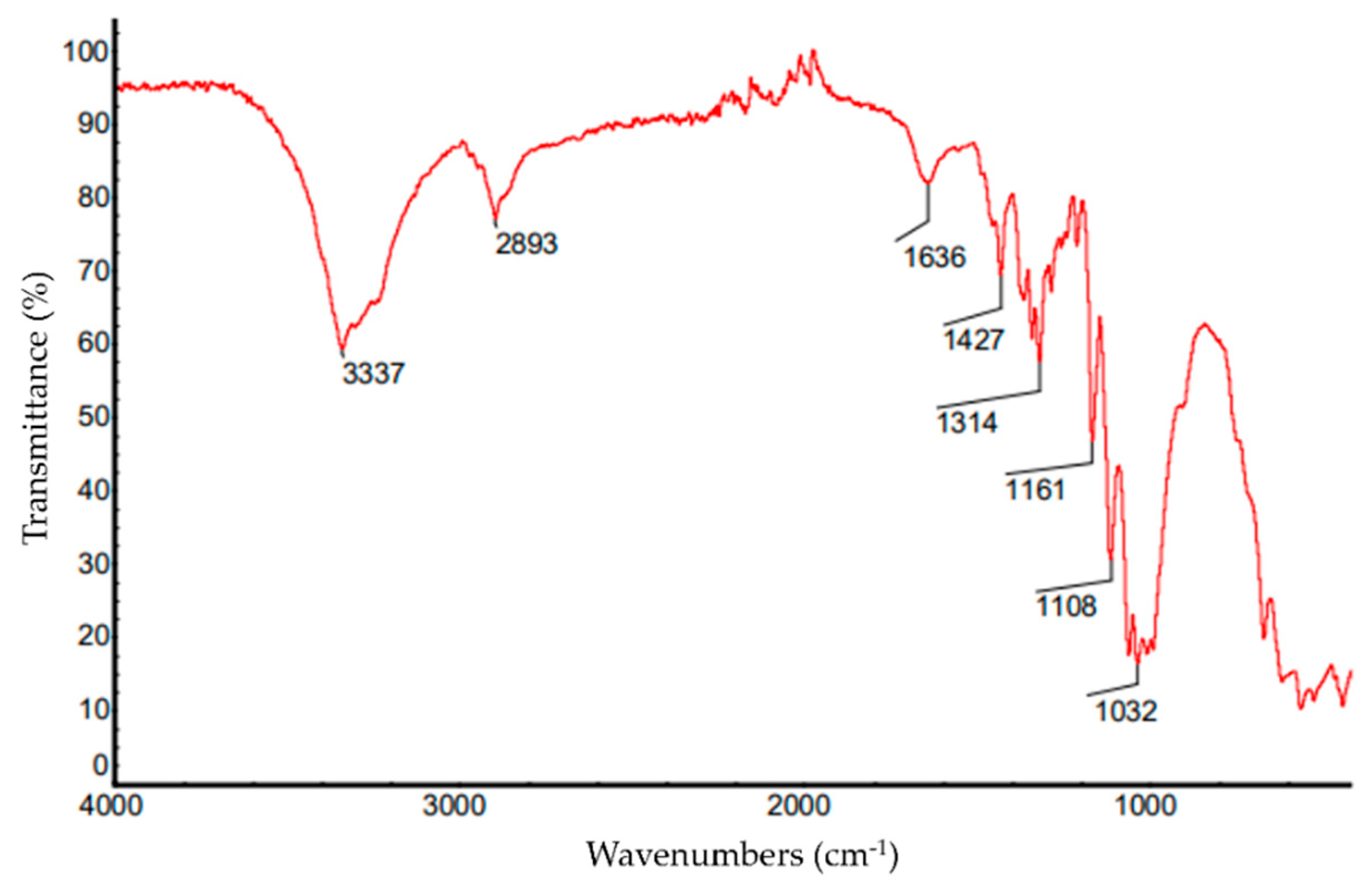
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
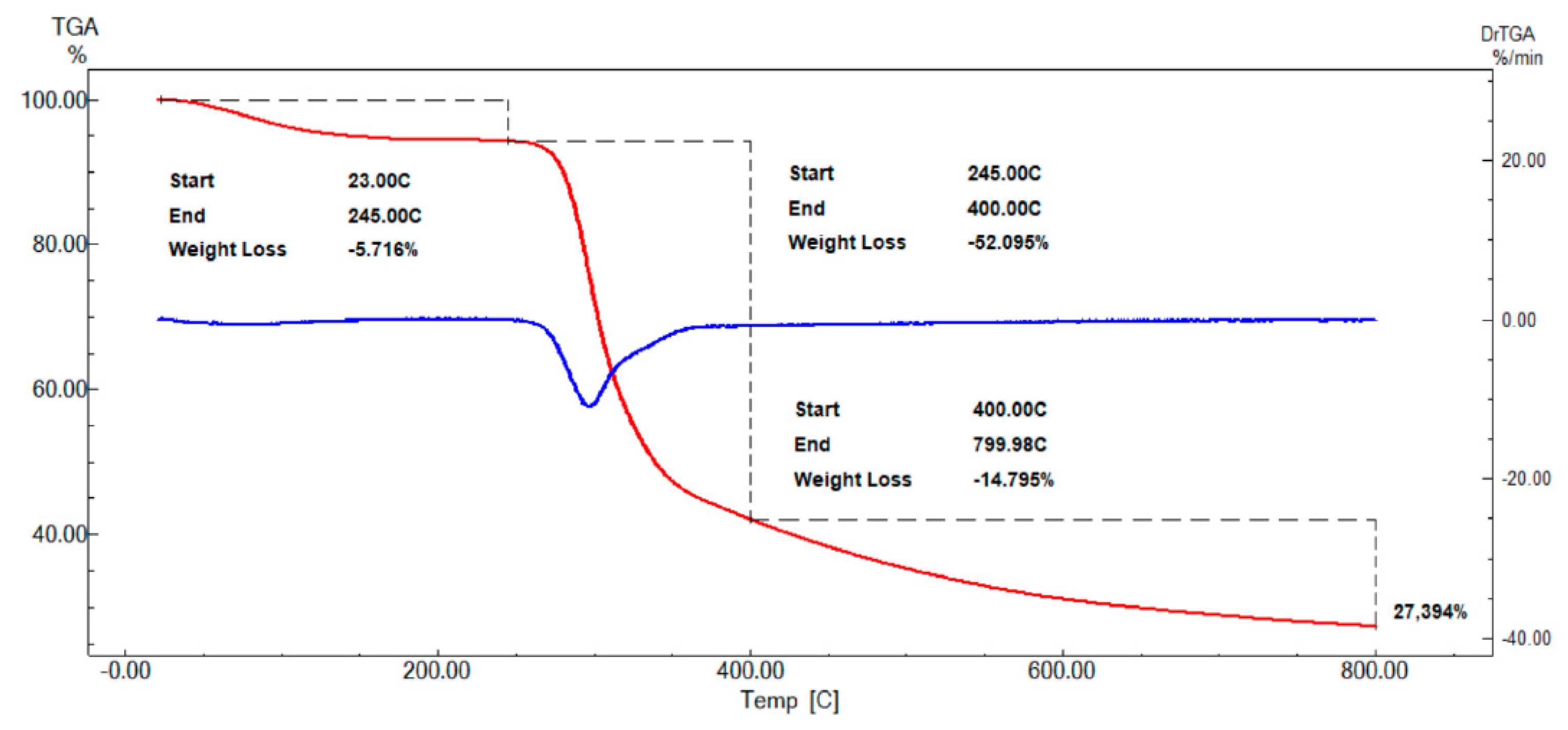
3.4. Thermogravimetry
3.5. X-ray Diffractometry
3.6. Scanning Electron Microscopy (SEM) and EDS
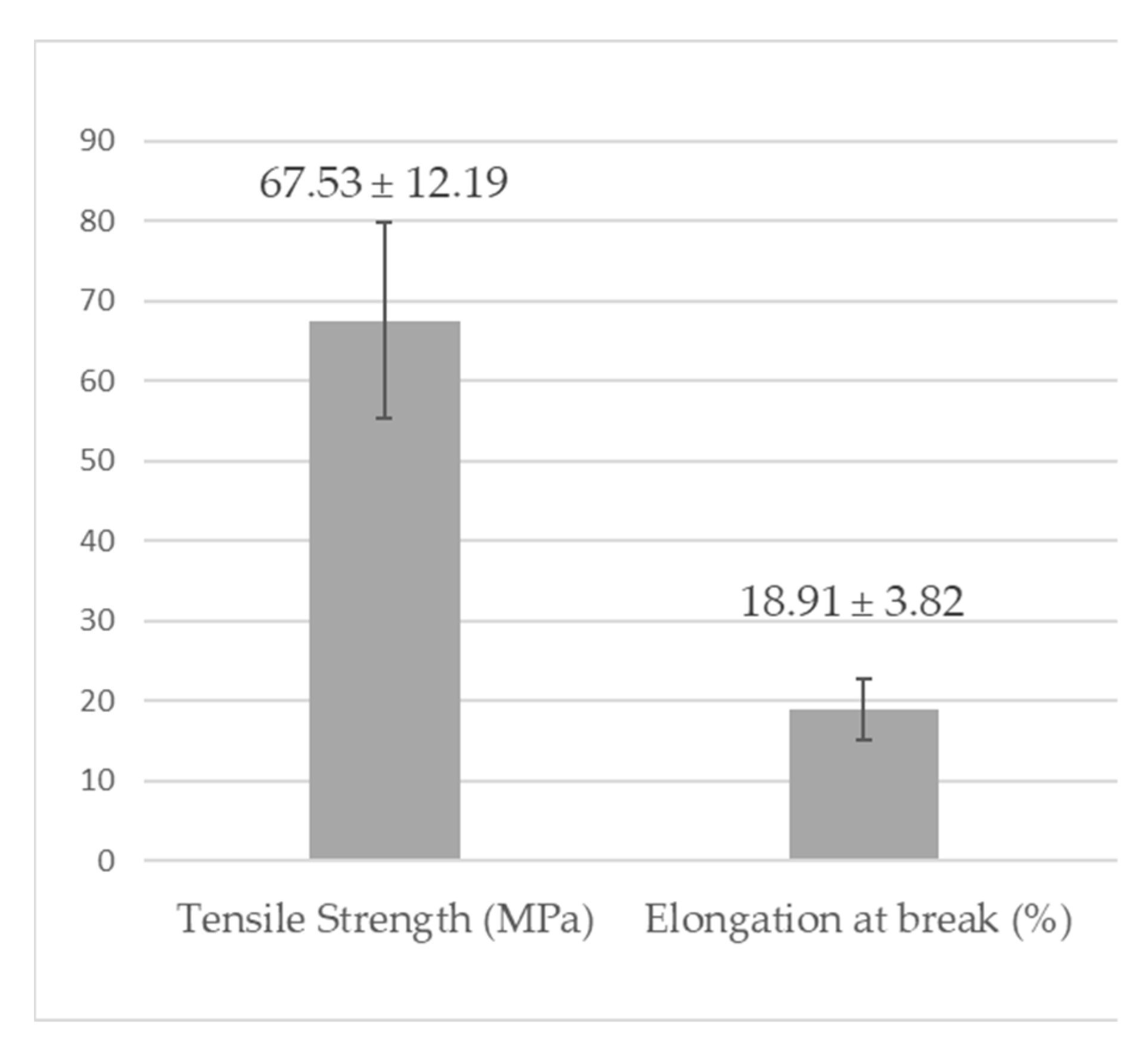
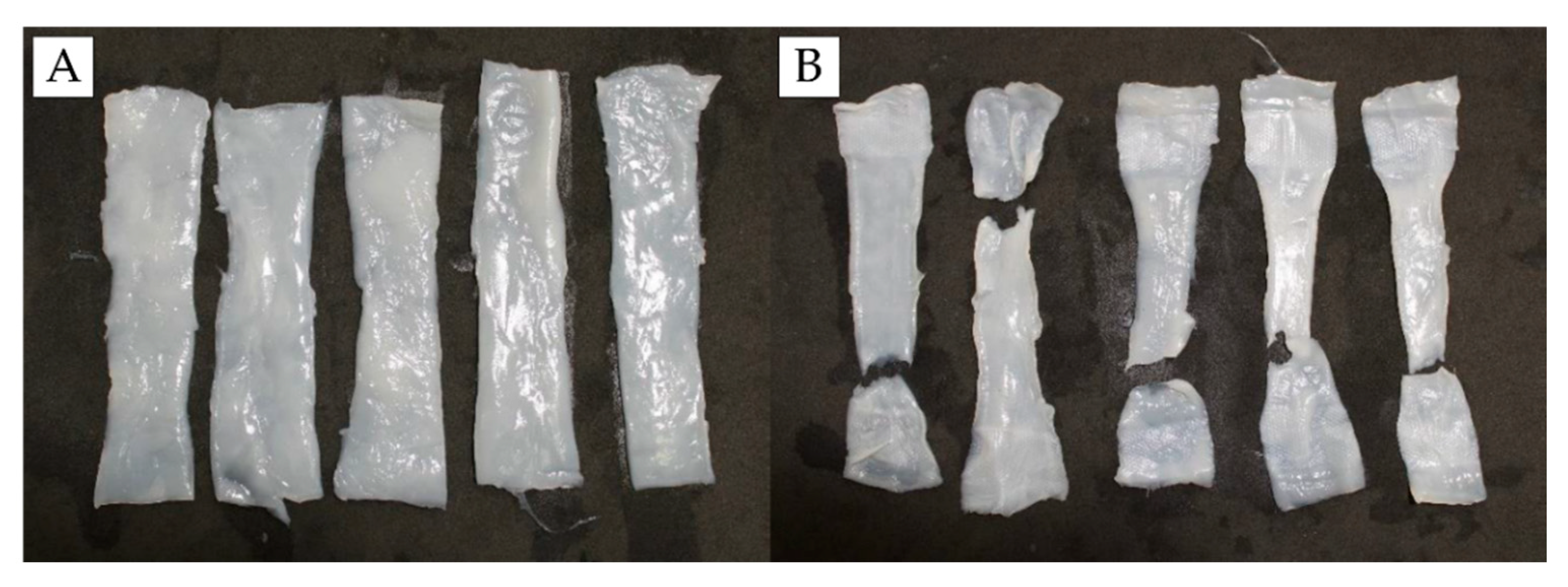
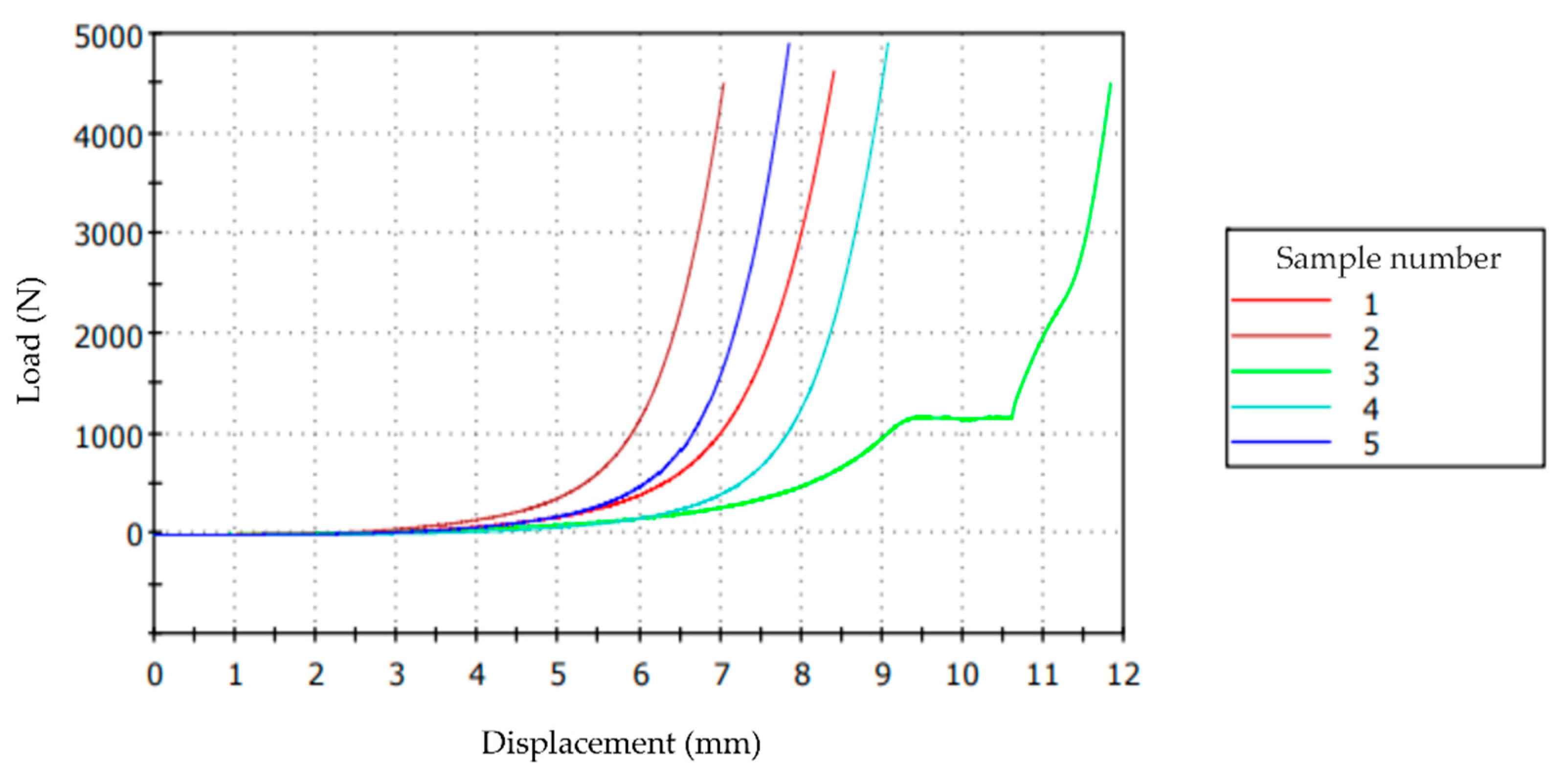
3.7. Mechanical Tests
3.8. Compression
4. Results and Discussion (Filtering Module)
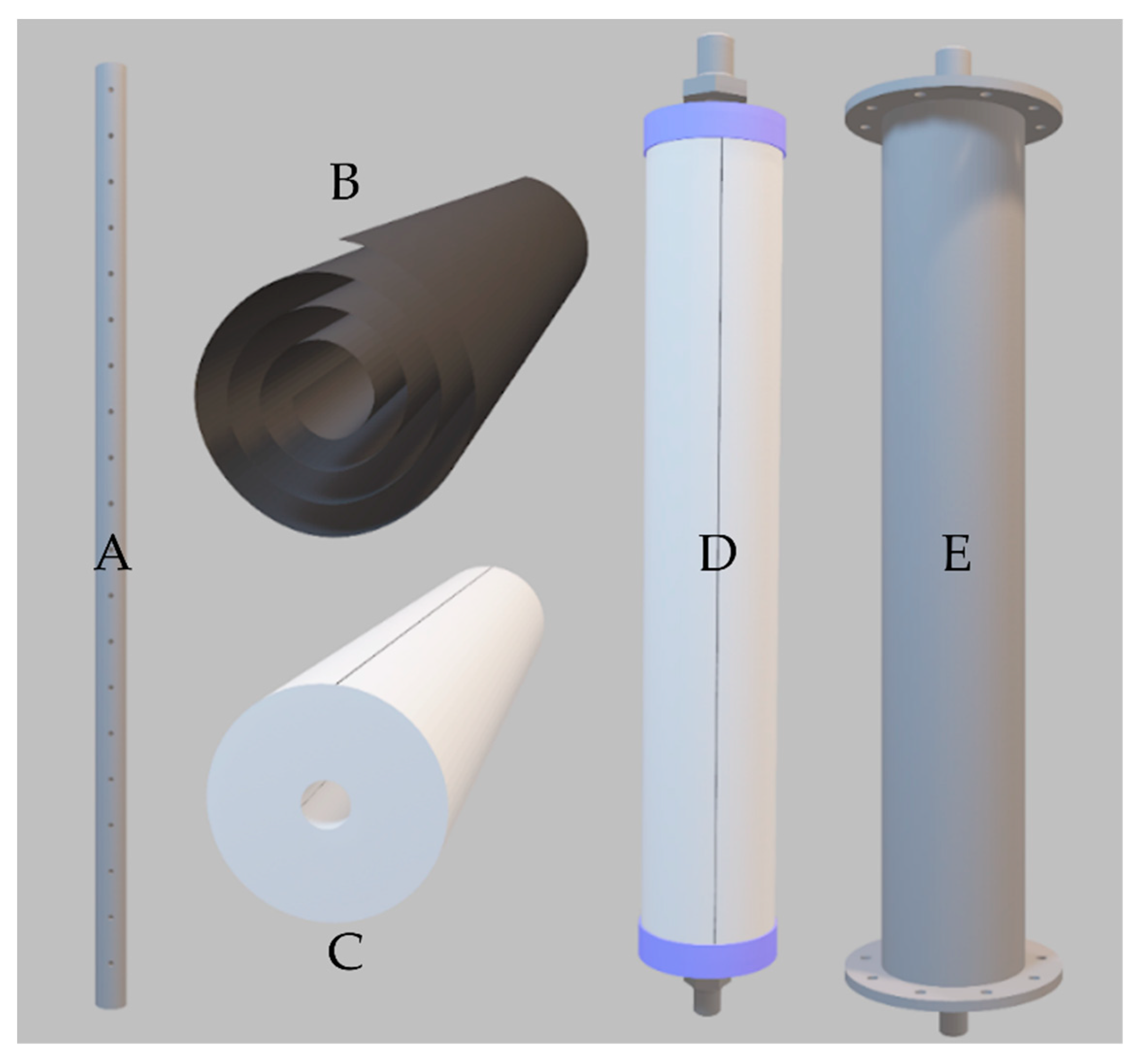
4.1. Three-Dimensional Module
4.2. Summary Final Configuration (Fabrication and Adaptation of Physical Space)
4.3. Membrane Production
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily Wastewater Treatment: Overview of conventional and modern methods, challenges, and future opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Dell’anno, F.; Van Zyl, L.J.; Trindade, M.; Buschi, E.; Cannavacciuolo, A.; Pepi, M.; Sansone, C.; Brunet, C.; Ianora, A.; Pascale, D.D. Microbiome enrichment from contaminated marine sediments unveils novel bacterial strains for petroleum hydrocarbon and heavy metal bioremediation. Environ. Pollut. 2023, 317, 120772. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-mass, S.; Gómez-Cornelio, S.; Castillo-Vidal, M.; Alvarez-Villagomez, C.S.; Quintana, P.; Larosa-García, S.D. Biodegradation of hydrocarbons from contaminated soils by microbial consortia: A laboratory microcosm study. Electron. J. Biotechnol. 2023, 61, 24–32. [Google Scholar] [CrossRef]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation combined to biosurfactants: A clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Biotechnol. 2018, 17, 591–602. [Google Scholar] [CrossRef]
- Galdino, C.J.S.; Maia, A.D.M.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Medeiros, A.D.L.M.; Silva Junior, C.J.G.; de Amorim, J.D.P.; do Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Bacterial cellulose for Treatment of Wastewaters Generated by Energy Consuming Industries: A Review. Energies 2021, 14, 5066. [Google Scholar] [CrossRef]
- Medeiros, A.D.M.; Silva Junior, C.J.G.; Amorim, J.D.P.; Durval, I.J.B.; Costa, A.F.S.; Sarubbo, L.A. Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes 2022, 10, 743. [Google Scholar] [CrossRef]
- Njoku, K.L. Responses of accessions of Zea mays to crude oil pollution using growth indices and enzyme activities as markers. Pollution 2017, 4, 183–193. [Google Scholar] [CrossRef]
- Pothula, G.K.; Vij, R.K.; Bera, A. An overview of chemical enhanced oil recovery and its status in India. Pet. Sci. 2023, in press. [Google Scholar] [CrossRef]
- El-Gawad, H.S.A. Oil and grease removal from industrial wastewater using new utility approach. Adv. Environ. Chem. 2014, 2014, 916878. [Google Scholar] [CrossRef]
- Xia, X.; Ma, J.; Geng, S.; Liu, F.; Yao, M. A review of Oil–Solid Separation and Oil–Water Separation in Unconventional Heavy Oil Production Process. Int. J. Mol. Sci. 2022, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.; Chen, X.; Beaumont, M.; Hassinen, J.; Orelma, H.; Dumée, L.F.; Tardy, B.L.; Rojas, O.J. Impact of incubation conditions and post-treatment on the properties of bacterial cellulose membranes for pressure-driven filtration. Carbohydr. Polym. 2021, 251, 117073–117082. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Cannon, R.; Anderson, S.M. Biogenesis of bacterial cellulose. Crit. Rev. Microbiol. 1991, 17, 435–447. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S. Carboxymethyl bacterial cellulose from nata de coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Souza, K.C.; Duarte, C.R.; Duarte, I.S.; Ribeiro, F.A.S.; Silva, G.S.; Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Silva Junior, C.J.G.; Medeiros, A.D.M.; Nascimento, H.A.; Sarubbo, M.; Medeiros, T.P.M.; Costa, A.F.S.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef]
- Silva Junior, C.J.G.; Medeiros, A.D.M.; Amorim, J.D.P.; Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Bacterial cellulose biotextiles for the future of sustainable fashion: A review. Environ. Chem. Lett. 2021, 19, 2967–2980. [Google Scholar] [CrossRef]
- Carpenter, A.W.; Lannoy, S.-F.; Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces. 2015, 7, 7373–7738. [Google Scholar] [CrossRef]
- Kim, D.; Livazovic, S.; Falca, G.; Nunes, S.P. Oil–Water Separation using Membranes Manufactured from Cellulose/Ionic Liquid Solutions. ACS Sustain. Chem. Eng. 2019, 7, 5649–5659. [Google Scholar] [CrossRef]
- Yang, C.; Topuz, F.; Park, S.-H.; Szekely, G. Biobased thin-film composite membranes comprising priamine–genipin selective layer on nanofibrous biodegradable polylactic acid support for oil and solvent-resistant nanofiltration. Green Chem. 2022, 24, 5291–5303. [Google Scholar] [CrossRef]
- Alammar, A.; Hardian, R.; Szekely, G. Upcycling agricultural waste into membranes: From date seed biomass to oil and solvent-resistant nanofiltration. Green Chem. 2022, 24, 365–374. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Xu, Q.; Wua, S.; Chen, Y. Regenerated cellulose strongly adhered by a supramolecular adhesive onto the PVDF membrane for a highly efficient oil/water separation. Green Chem. 2021, 23, 5633–5646. [Google Scholar] [CrossRef]
- Silva Junior, C.J.G.; Amorim, J.D.P.; Medeiros, A.D.M.; Cavalcanti, A.K.L.H.; Nascimento, H.A.; Henrique, M.A.; Maranhão, L.J.C.N.; Vinhas, G.M.; Souto Silva, K.K.O.; Costa, A.F.S.; et al. Design of a Naturally Dyed and Waterproof Biotechnological Leather from Reconstituted Cellulose. J. Funct. Biomater. 2021, 13, 49. [Google Scholar] [CrossRef]
- Marin, E.; Rojas, J. Preparation and characterization of crosslinked poly (vinyl) alcohol films with waterproof properties. Int. J. Pharm. Pharm. Sci. 2015, 7, 242–248. [Google Scholar]
- Kamiński, K.; Jarosz, M.; Grudzień, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A.M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef]
- Constantino, M.G. Espectroscopia no Infravermelho, 3rd ed.; LTC: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Canevarolo, S.J. Técnicas de Caracterização de Polímeros; Artliber: Sao Paulo, Brazil, 2003. [Google Scholar]
- Mothé, C.G.; Azevedo, A.D.D. Análise Térmica de Materiais, 3rd ed.; Artliber: Sao Paulo, Brazil, 2009. [Google Scholar]
- Rethwisch, D.G.J.; William, D.C. Ciência e Engenharia de Materiais: Uma Introdução, 9th ed.; LTC: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Dutta, P.; Arun Kumar, G.; Ram, G.; Suneel Varma, D. Spatiotemporal Nonreciprocal Filters: Theoretical Concepts and Literature Review. IEEE Microw. 2022, 23, 85–101. [Google Scholar] [CrossRef]
- Altaee, A. Computational model for estimating reverse osmosis system design and performance: Part-one binary feed solution. Desalination 2012, 291, 101–105. [Google Scholar] [CrossRef]
- Martínez-Rocamora, A.; Solís-Guzmán, J.; Marrero, M. LCA databases focused on construction materials: A review. Renew. Sust. Energ. Rev. 2016, 58, 565–573. [Google Scholar] [CrossRef]
- Augiseau, V.; Barles, S. Studying construction materials flows and stock: A review. Resour. Conserv. Recycl. 2017, 123, 153–164. [Google Scholar] [CrossRef]
- Boyko, E.; Stone, H.A. Reciprocal theorem for calculating the flow rate–pressure drop relation for complex fluids in narrow geometries. Phys. Rev. Fluids 2021, 6, L081301. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Garofalo, M.L.; Lu, Z. Water Management in A PEMFC: Water transport mechanism and material degradation in gas diffusion layers. Fuel Cells 2011, 11, 814–823. [Google Scholar] [CrossRef]
- Kaboudan, A.; Naderi, M.; Afshar, M.A. The efficiency of Darcy and two-dimensional diffusion flow models to estimate water penetration into concrete. J. Build. Eng. 2021, 34, 102012. [Google Scholar] [CrossRef]
- Matsson, J.E. An Introduction to ANSYS Fluent 2022, 1st ed.; SDC Publications: Mission, KS, USA, 2022. [Google Scholar]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Comparative study of plant and bacterial cellulose pellicles regenerated from dissolved states. Int. J. Biol. Macromol. 2020, 137, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 2017. [Google Scholar] [CrossRef] [PubMed]
- Classen, E. Comfort testing of textiles. Adv. Charact. Test. Text. 2018, 59–69. [Google Scholar] [CrossRef]
- Nascimento, H.A.; Amorim, J.D.P.; Filho, L.E.P.T.M.; Costa, A.F.S.; Sarubbo, L.A.; Napoleão, D.C.; Vinhas, G.M. Production of bacterial cellulose with antioxidant additive from grape residue with promising cosmetic applications. Polym. Eng. Sci. 2022, 62, 2826–2839. [Google Scholar] [CrossRef]
- Tang, W.; Jia, S.; Jia, Y.; Yang, H. The influence of fermentation conditions and post-treatment methods on porosity of bacterial cellulose membrane. World J. Microbiol. Biotechnol. 2010, 26, 125–131. [Google Scholar] [CrossRef]
- Indrarti, L.; Indriyati, I.; Syampurwadi, A.; Pujiastuti, S. Physical and mechanical properties of modified bacterial cellulose composite films. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2016; Volume 1711, p. 050007. [Google Scholar] [CrossRef]
- Revin, V.V.; Dolganov, A.V.; Liyaskina, E.V.; Nazarova, N.B.; Balandina, A.V.; Devyataeva, A.A.; Revin, V.D. Characterizing Bacterial Cellulose Produced by Komagataeibacter sucrofermentans H-110 on Molasses Medium and Obtaining a Biocomposite Based on It for the Adsorption of Fluoride. Polymers 2021, 13, 1422. [Google Scholar] [CrossRef]
- Gayathry, G.; Gopalaswamy, G. Production and characterisation of microbial cellulosic fibre from Acetobacter xylinum. Indian J. Fibre Text. Res. 2014, 39, 93–96. [Google Scholar]
- Shi, Z.; Xu, G.; Deng, J.; Dong, M.; Murugadoss, V.; Liu, C.; Shao, Q.; Wu, S.; Guo, Z. Structural characterization of lignin from D. sinicus by FTIR and NMR techniques. Green Chem. Lett. Rev. 2019, 12, 235–243. [Google Scholar] [CrossRef]
- Ashori, A.; Sheykhnazari, S.; Tabarsa, T.; Shakeri, A.; Golalipour, M. Bacterial cellulose/silica nanocomposites: Preparation and characterization. Carbohydr. Polym. 2012, 90, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Redjala, S.; Ferhoum, R.; Aït Hocine, N.; Azem, S. Degradation of Polycarbonate Properties Under Thermal Aging. J Fail. Anal. Preven. 2019, 19, 536–542. [Google Scholar] [CrossRef]
- Zhu, P.; Sui, S.; Wang, B.; Sun, K.; Sun, G. A study of pyrolysis and pyrolysis products of flame-retardant cotton fabrics by DSC, TGA, and PY–GC–MS. J. Anal. Appl. Pyrolysis. 2004, 71, 645–655. [Google Scholar] [CrossRef]
- Zhao, M.; Qi, Z.; Tao, X.; Newkirk, C.; Hu, X.; Lu, S. Chemical, Thermal, Time, and Enzymatic Stability of Silk Materials with Silk I Structure. Int. J. Mol. Sci. 2021, 22, 4136. [Google Scholar] [CrossRef]
- Xu, K.; Li, Q.; Xie, L.; Shi, Z.; Su, G.; Harper, D.; Tang, Z.; Zhou, J.; Du, G.; Wang, S. Novel flexible, strong, thermal-stable, and high-barrier switchgrass-based lignin-containing cellulose nanofibrils/chitosan biocomposites for food packaging. Ind. Crop. Prod. 2022, 179, 114661. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, D.K.; Bansal, V.; Mehta, D.; Sangwan, R.S.; Yadav, S.K. Efficient and economic process for the production of bacterial cellulose from isolated strain of Acetobacter pasteurianus of RSV-4 bacterium. Bioresour. Technol. 2019, 275, 430–433. [Google Scholar] [CrossRef]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef]
- Souza, E.F.; Furtado, M.R.; Carvalho, C.W.P.; Freitas-Silva, O.; Gottschalk, L.M.F. Production and characterization of Gluconacetobacter xylinus bacterial cellulose using cashew apple juice and soybean molasses. Int. J. Biol. Macromol. 2020, 146, 285–289. [Google Scholar] [CrossRef]
- Leonarski, E.; Cesca, K.; Zanella, E.; Stambuk, B.U.; Oliveira, D.; Poletto, P. Production of kombucha-like beverage and bacterial cellulose by acerola byproduct as raw material. Lwt 2021, 135, 110075. [Google Scholar] [CrossRef]
- Avcioglu, N.H.; Birben, M.; Bilkay, I.S. Optimization and physicochemical characterization of enhanced microbial cellulose production with a new Kombucha consortium. Process Biochem. 2021, 108, 60–68. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Zhang, R.; Wu, H.; Zeng, W.; Liang, Z. Production of high crystallinity type-I cellulose from Komagataeibacter hansenii JR-02 isolated from Kombucha tea. Biotechnol. Appl. Biochem. 2019, 66, 108–118. [Google Scholar] [CrossRef]
- Adepu, S.; Khandelwal, M. Ex-situ modification of bacterial cellulose for immediate and sustained drug release with insights into release mechanism. Carb. Polym. 2020, 249, 116816. [Google Scholar] [CrossRef]
- Brazel, C. Dimensionless analysis of swelling of hydrophilic glassy polymers with subsequent drug release from relaxing structures. Biomaterials 1999, 20, 721–732. [Google Scholar] [CrossRef]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial Cellulose Production from Industrial Waste and by-Product Streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef] [PubMed]
- Hamed, D.A.; Maghrawy, H.H.; Kareem, H.A. Biosynthesis of bacterial cellulose nanofibrils in black tea media by a symbiotic culture of bacteria and yeast isolated from commercial kombucha beverage. World J. Microbiol. Biotechnol. 2022, 39, 48. [Google Scholar] [CrossRef]
- Digel, I.; Akimbekov, N.; Rogachev, E.; Pogorelova, N. Bacterial cellulose produced by Medusomyces gisevii on glucose and sucrose: Biosynthesis and structural properties. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Hassan, E.; Hassan, M.; Abou-Zeid, R.; Berglund, L.; Oksman, K. Use of bacterial cellulose and crosslinked cellulose nanofibers membranes for removal of oil from oil-in-water emulsions. Polymers 2017, 9, 388. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Li, Y.; Ren, J.; Pei, C. Effect of cellulose nanocrystals on bacterial cellulose hydrogel for oil-water separation. Sep. Purif. Technol. 2023, 304, 122349. [Google Scholar] [CrossRef]
- Wang, Y.; Yadav, S.; Heinlein, T.; Konjik, V.; Breitzke, H.; Buntkowsky, G.; Schneider, J.J.; Zhang, K. Ultra-light nanocomposite aerogels of bacterial cellulose and reduced graphene oxide for specific absorption and separation of organic liquids. RSC Adv. 2014, 4, 21553–21558. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Doosthoseini, K.; Azin, M. Effect of ethanol and medium on bacterial cellulose (BC) production by Gluconacetobacter xylinus (ptcc 1734). Cellul. Chem. Technol. 2014, 49, 455–462. [Google Scholar]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Bañó, M.C. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater. 2009, 5, 1605–1615. [Google Scholar] [CrossRef]
- Liu, K.; Catchmark, J.M. Enhanced mechanical properties of bacterial cellulose nanocomposites produced by co-culturing Gluconacetobacter hansenii and Escherichia coli under static conditions. Carb. Polym. 2019, 219, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- Cheng, Z.; Duan, C.; Zeng, J.; Wang, B.; Xu, J.; Gao, W.; Li, J.; Chen, K. Bottom-Up Ecofriendly Strategy for Construction of Sustainable Bacterial Cellulose Bioaerogel with Multifunctional Properties. Adv. Mater. Interfaces. 2021, 8, 2002101. [Google Scholar] [CrossRef]








| BC 14 Days | Mean (g/L) ± Standard Deviation | WRC (%) ± Standard Deviation |
|---|---|---|
| Wet weight | 436.12 ± 17.03 | 97.65 ± 0.36 |
| Dry weight | 10.24 ± 1.49 |
| Wave Number (cm−1) | Characteristic Group |
|---|---|
| 3337 | Axial deformation of O-H |
| 2893 | Axial deformation of CH2 |
| 1636 | Deformation of C=C |
| 1427 | Angular deformation of CH2 |
| 1314 | Angular deformation of CH |
| 1161 | Stretching of C-O-C |
| 1108 | Stretching of C-O-C |
| 1032 | Deformation of C-O |
| Temperature Range (°C) | Loss of Mass (%) | Residual Stable to 800 °C (%) |
|---|---|---|
| 23 to 245 | 5.72 | |
| 245 to 400 | 52.10 | 27.39 |
| 400 to 800 | 14.80 |
| Element | Mass of Elements (%) | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| C | 52.88 | 64.10 | 52.82 | 52.61 | 53.46 | 52.40 |
| O | 45.48 | 33.34 | 45.91 | 46.09 | 45.16 | 46.44 |
| P | 1.64 | 2.56 | 1.28 | 1.30 | 1.38 | 1.16 |
| Sample | Maximum Load (N) | Displacement under Maximum Load (mm) | Deformation under Maximum Load (%) |
|---|---|---|---|
| 1 | 4619.92 | 8.40 | 91.51 |
| 2 | 4497.26 | 7.04 | 88.55 |
| 3 | 4499.05 | 11.82 | 97.52 |
| 4 | 4898.97 | 9.07 | 92.78 |
| 5 | 4898.25 | 7.84 | 91.63 |
| Mean | 4682.69 | 8.83 | 92.40 |
| Standard deviation | 203.28 | 1.83 | 14.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, A.D.M.d.; Silva Junior, C.J.G.d.; Amorim, J.D.P.d.; Durval, I.J.B.; Damian, R.B.; Cavalcanti, Y.d.F.; Costa, A.F.d.S.; Sarubbo, L.A. Design and Modeling of a Biotechnological Nanofiltration Module Using Bacterial Cellulose Membranes for the Separation of Oily Mixtures. Water 2023, 15, 2025. https://doi.org/10.3390/w15112025
Medeiros ADMd, Silva Junior CJGd, Amorim JDPd, Durval IJB, Damian RB, Cavalcanti YdF, Costa AFdS, Sarubbo LA. Design and Modeling of a Biotechnological Nanofiltration Module Using Bacterial Cellulose Membranes for the Separation of Oily Mixtures. Water. 2023; 15(11):2025. https://doi.org/10.3390/w15112025
Chicago/Turabian StyleMedeiros, Alexandre D’Lamare Maia de, Cláudio José Galdino da Silva Junior, Júlia Didier Pedrosa de Amorim, Italo José Batista Durval, Ricardo Barbosa Damian, Yasmim de Farias Cavalcanti, Andréa Fernanda de Santana Costa, and Leonie Asfora Sarubbo. 2023. "Design and Modeling of a Biotechnological Nanofiltration Module Using Bacterial Cellulose Membranes for the Separation of Oily Mixtures" Water 15, no. 11: 2025. https://doi.org/10.3390/w15112025
APA StyleMedeiros, A. D. M. d., Silva Junior, C. J. G. d., Amorim, J. D. P. d., Durval, I. J. B., Damian, R. B., Cavalcanti, Y. d. F., Costa, A. F. d. S., & Sarubbo, L. A. (2023). Design and Modeling of a Biotechnological Nanofiltration Module Using Bacterial Cellulose Membranes for the Separation of Oily Mixtures. Water, 15(11), 2025. https://doi.org/10.3390/w15112025










