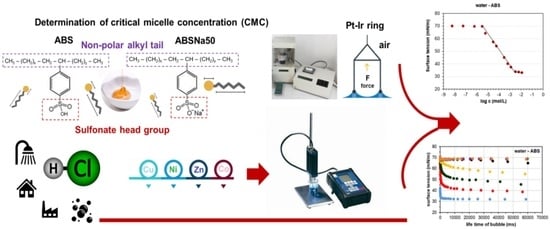
A Comprehensive Analysis of Selected Anionic Surfactants Behaviour in Aqueous Systems Containing Metal Ions and Inorganic Acid
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
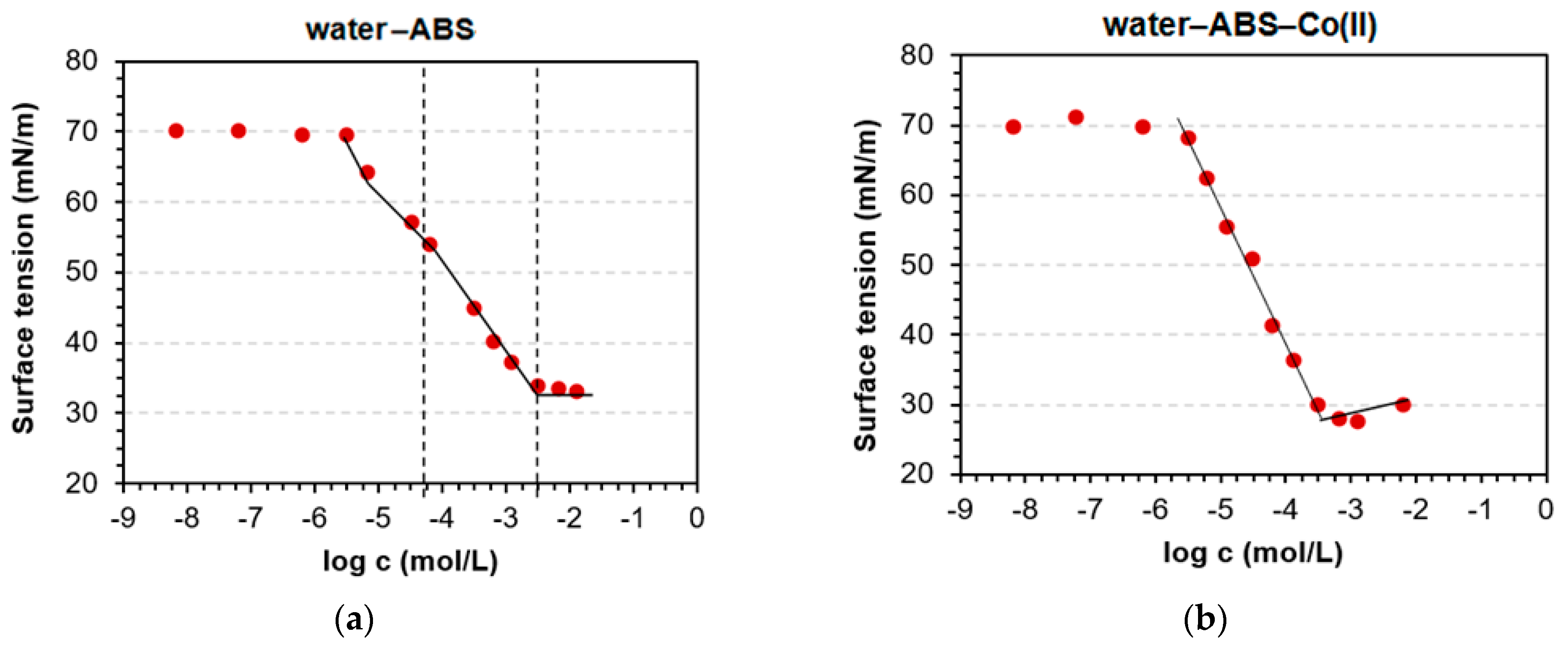
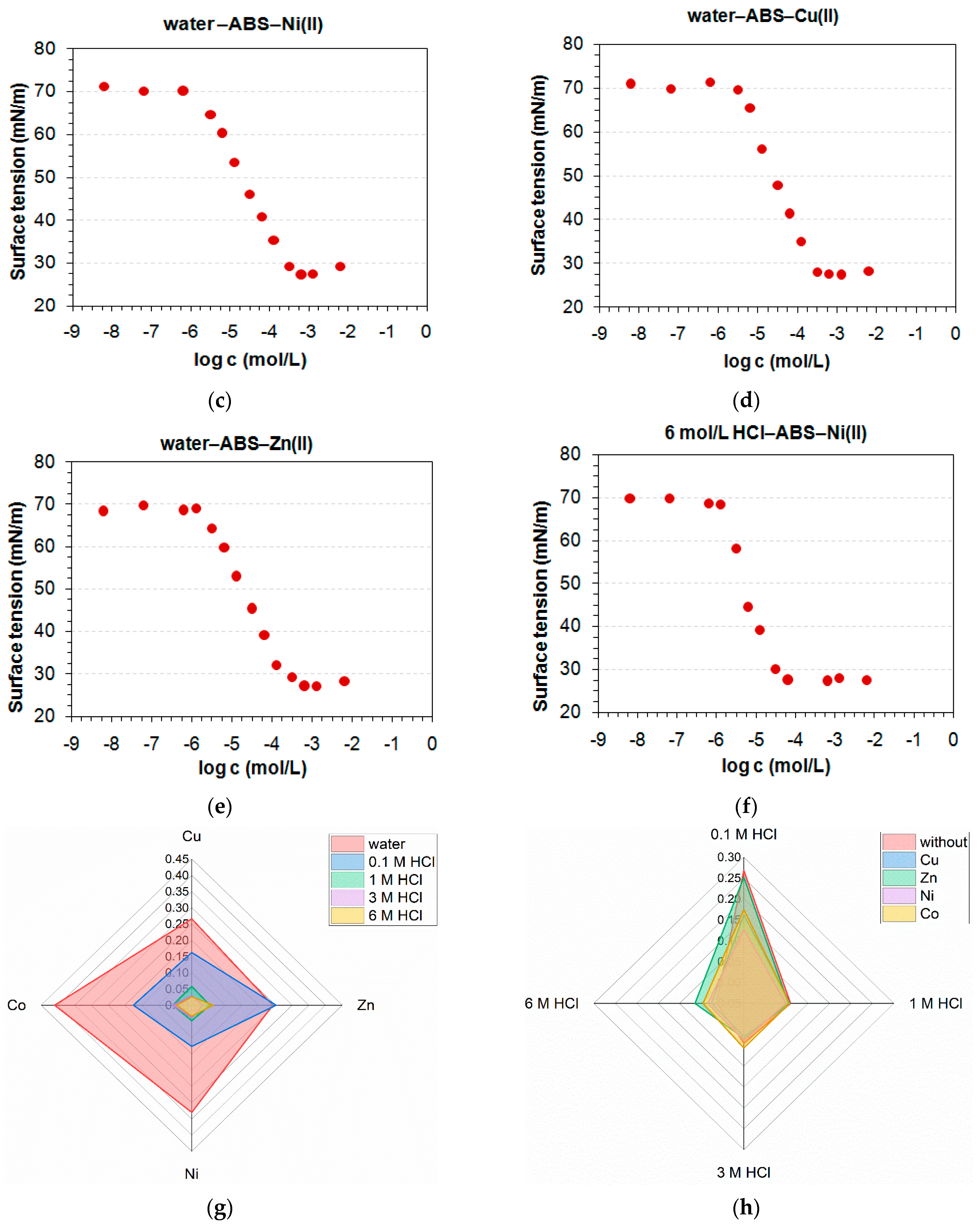
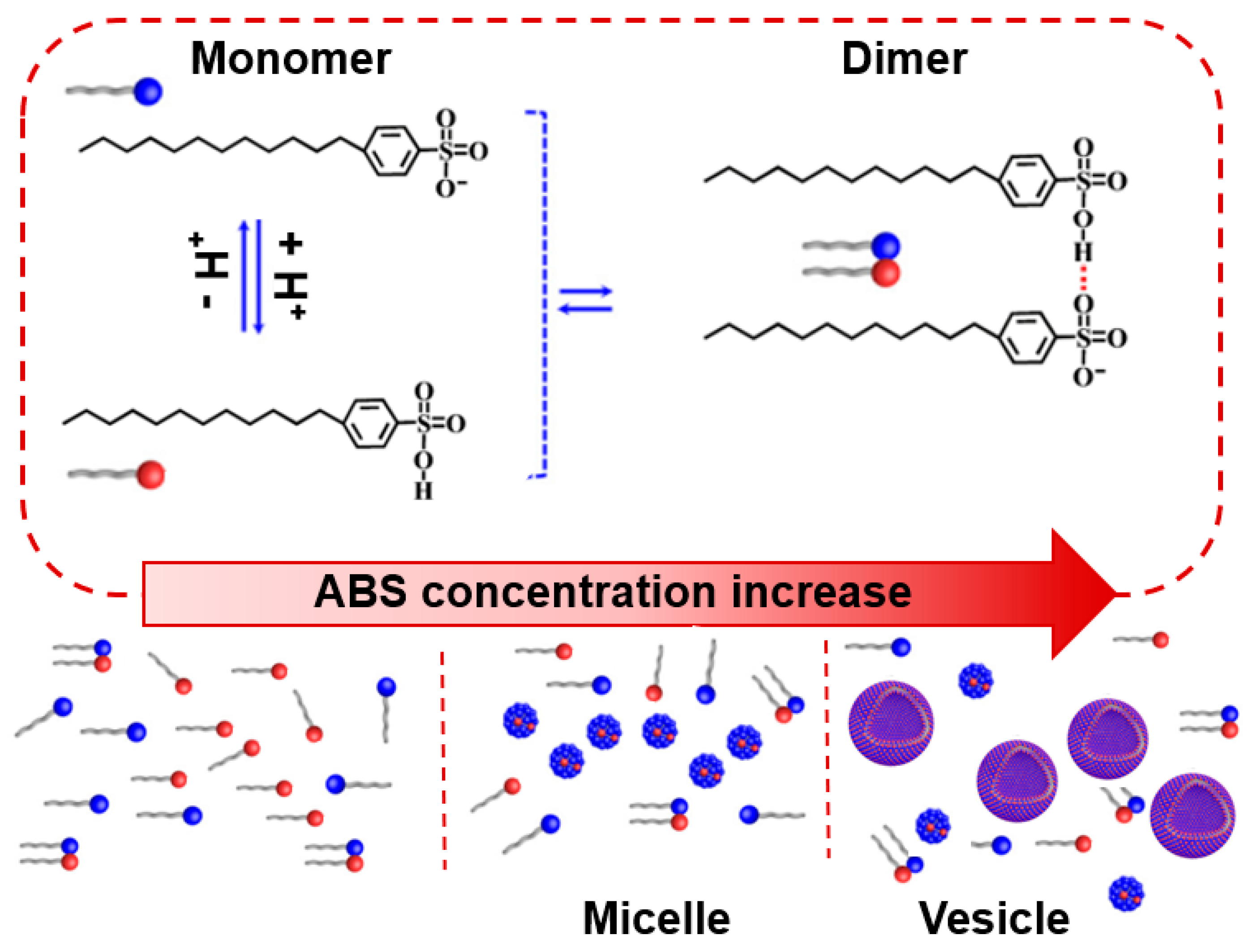
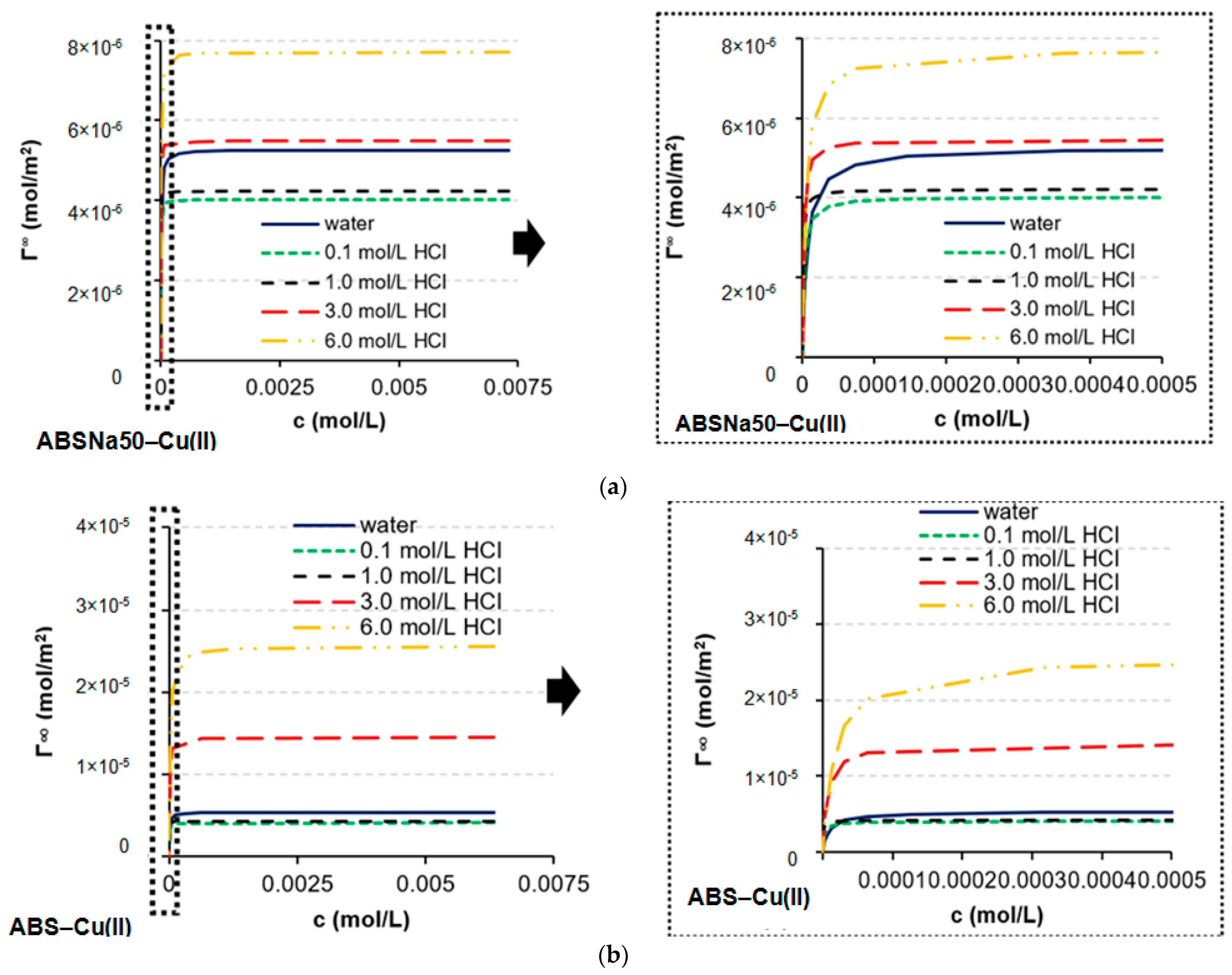
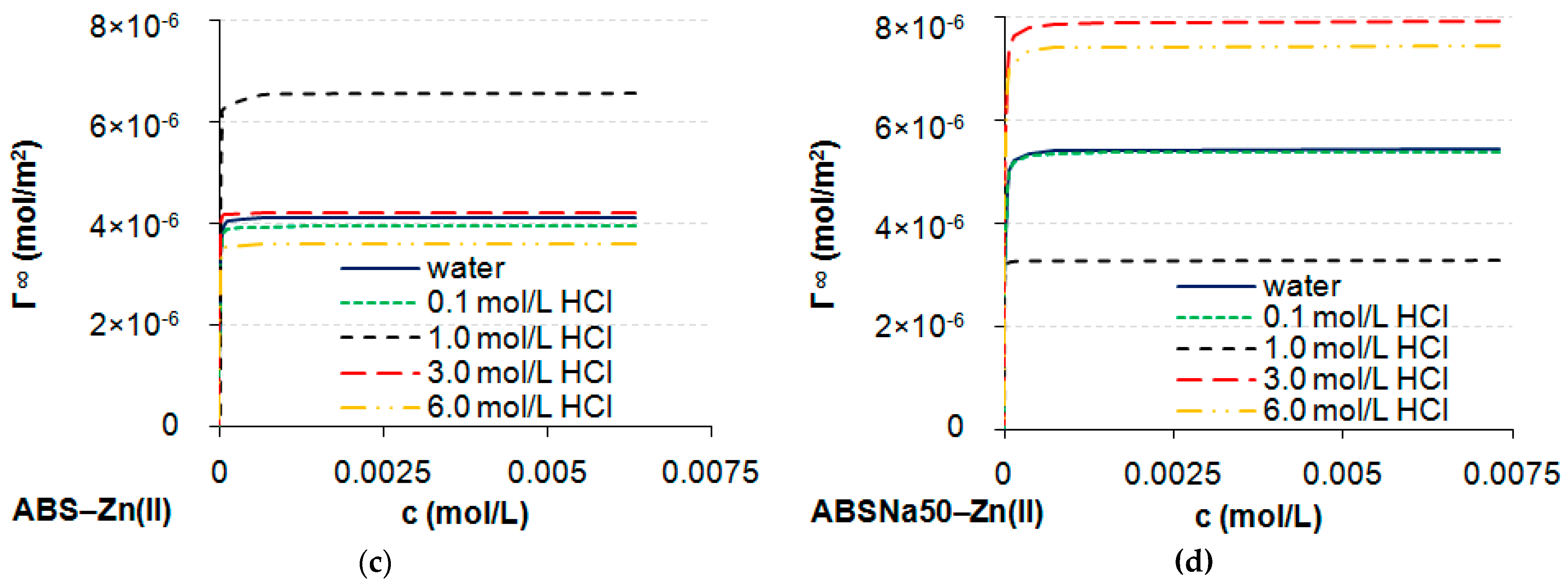
3.1. Determination of CMC and Adsorption Parameters
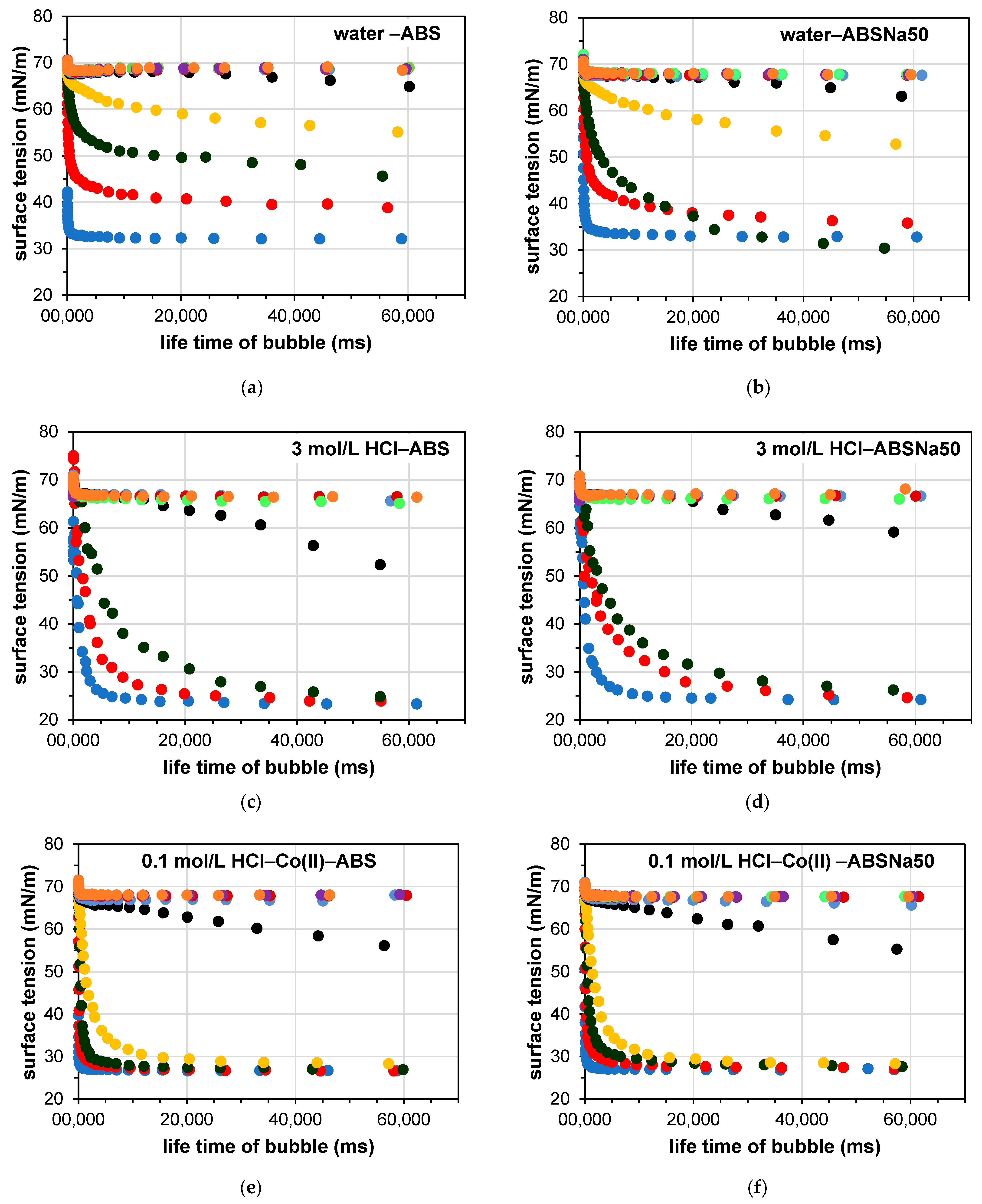
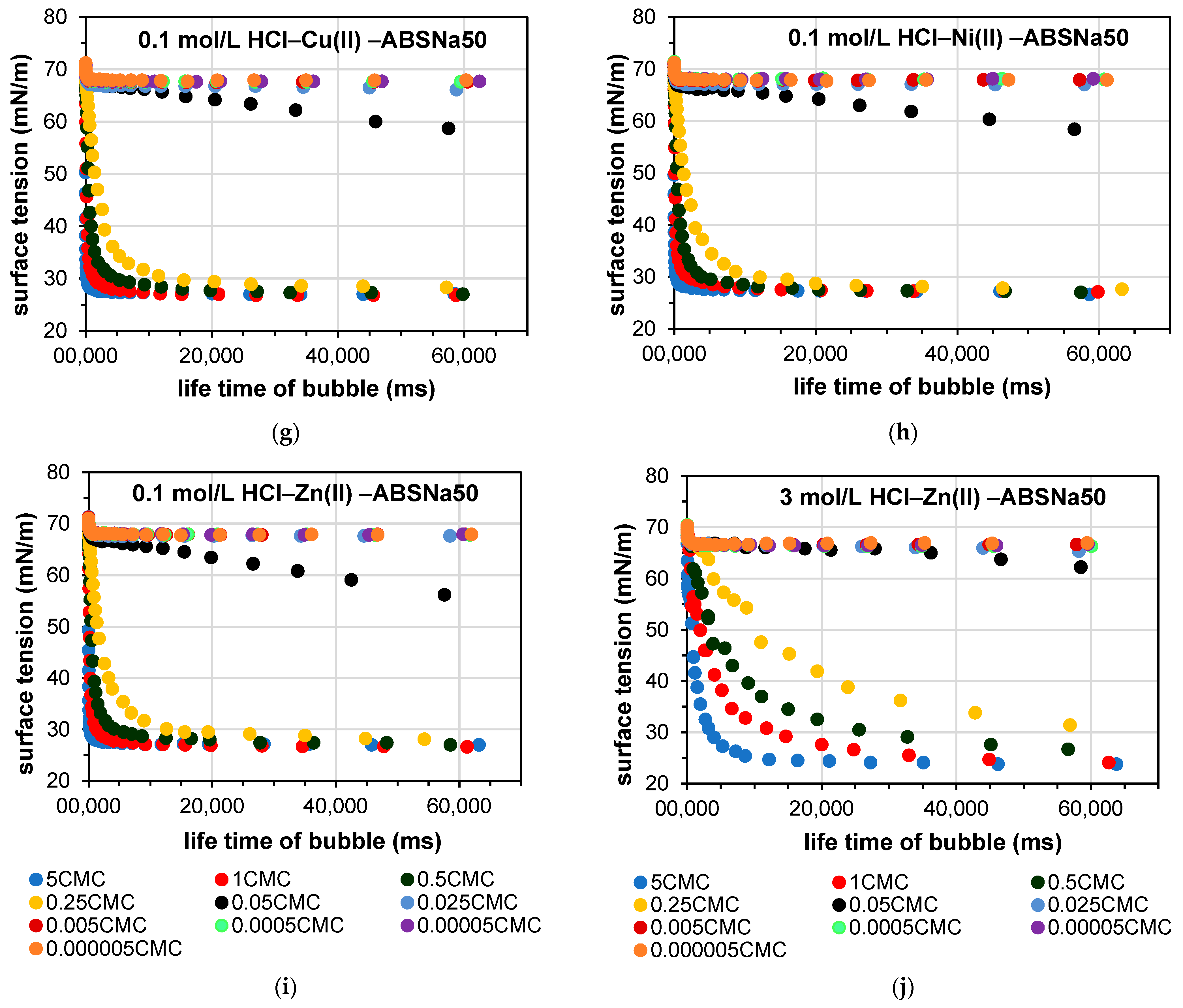
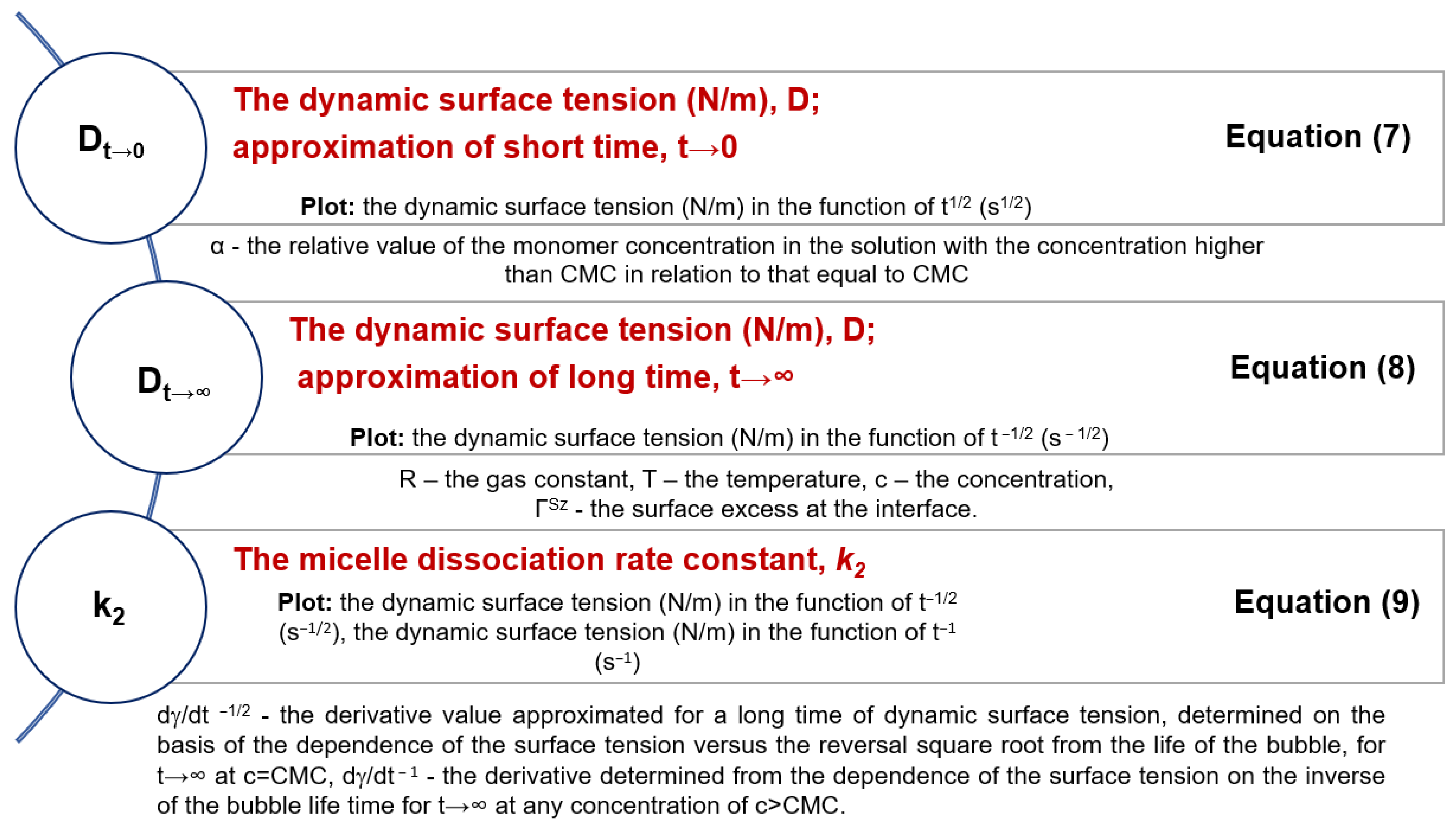
3.2. Determination of the Dynamic Surface Tension of the Anionic Surfactants in the Aqueous Solution and Role of Diffusion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jusufi, A.; Lebard, D.N.; Levine, B.G.; Klein, M.L. Surfactant Concentration Effects on Micellar Properties. J. Phys. Chem. B 2012, 116, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile Nanoarchitectonics: From Basic Physical Chemistry to Advanced Applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, Y.; Wang, Y. Selective Separation of Heavy Metal Ions from Dilute Aqueous Solutions by Foams and Micelles of Surfactants. Soft Matter 2018, 14, 9830–9837. [Google Scholar] [CrossRef] [PubMed]
- Wołowicz, A.; Staszak, K. Study of Surface Properties of Aqueous Solutions of Sodium Dodecyl Sulfate in the Presence of Hydrochloric Acid and Heavy Metal Ions. J. Mol. Liq. 2020, 299, 112170. [Google Scholar] [CrossRef]
- Du, J.; Jiang, B.; Xie, J.; Zeng, X. Effects of Metal Ions on the Micellization of Ionic Surfactants. J. Dispers. Sci. Technol. 2001, 22, 529–533. [Google Scholar] [CrossRef]
- Horvath, L.; Mihaljević, B.; Tomašić, V.; Risović, D.; Filipović-Vinceković, N. Counterion Binding to Ionic Micelles: Effects of Counterior Specificity. J. Dispers. Sci. Technol. 2001, 22, 221–229. [Google Scholar] [CrossRef]
- Hąc-Wydro, K.; Mateja, A.; Ożóg, A.; Miśkowiec, P. Influence of Metal Ions on the Aggregation of Anionic Surfactants. Studies on the Interactions between Environmental Pollutants in Aqueous Solutions. J. Mol. Liq. 2017, 240, 514–521. [Google Scholar] [CrossRef]
- Gamboa, C.; Olea, A.F. Association of Cationic Surfactants to Humic Acid: Effect on the Surface Activity. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 241–245. [Google Scholar] [CrossRef]
- Staszak, K.; Wieczorek, D.; Michocka, K. Effect of Sodium Chloride on the Surface and Wetting Properties of Aqueous Solutions of Cocamidopropyl Betaine. J. Surfactants Deterg. 2015, 18, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Jiang, Y.; Liu, R.; Liu, L.; He, Y. Experimental Study on the Improvement of Wetting Performance of OP-10 Solution by Inorganic Salt Additives. Atmos. Pollut. Res. 2020, 11, 153–161. [Google Scholar] [CrossRef]
- Hirano, T.; Kitagawa, S.; Ohtani, H.; Kinoshita, T.; Ishigaki, Y.; Shibata, N.; Nii, S. Evaluation of Interactions between Metal Ions and Nonionic Surfactants in High-Concentration HCl Using Low-Pressure High-Performance Liquid Chromatography with Low-Flow-Resistance Polystyrene-Based Monolithic Column. Anal. Bioanal. Chem. 2013, 405, 8319–8326. [Google Scholar] [CrossRef] [PubMed]
- Asada, A.; Kittagawa, S.; Ohtani, H.; Kinoshita, T.; Ishigaki, Y.; Shibata, N.; Nii, S.; Okano, Y. Evaluation of Interaction between Metal Ions and Nonionic Surfactants Containing Polyoxyethylene Chain by Measurement of Streaming Potential. Bunseki Kagaku 2018, 67, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Haddou, B.; Benkhedja, H.; Teixeira Da Silva De La Salles, K.; Canselier, J.P.; Gourdon, C. Prediction of the Cloud Point of Polyethoxylated Surfactants and Their Mixtures by the Thermodynamic Model of Flory-Huggins-Rupert. J. Dispers. Sci. Technol. 2019, 40, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Chen, Q.; Xu, C.; Shen, X. Selective Cloud Point Extraction of Uranium from Thorium and Lanthanides Using Cyanex 301 as Extractant. Sep. Purif. Technol. 2019, 210, 835–842. [Google Scholar] [CrossRef]
- Samaddar, P.; Sen, K. Cloud Point Extraction: A Sustainable Method of Elemental Preconcentration and Speciation. J. Ind. Eng. Chem. 2014, 20, 1209–1219. [Google Scholar] [CrossRef]
- Staszak, K.; Karaś, Z.; Jaworska, K. Comparison of Polymeric and Ceramic Membranes Performance in the Process of Micellar Enhanced Ultrafiltration of Cadmium(II) Ions from Aqueous Solutions. Chem. Pap. 2013, 67, 380–388. [Google Scholar] [CrossRef]
- Tortora, F.; Innocenzi, V.; Prisciandaro, M.; Vegliò, F.; Mazziotti di Celso, G. Heavy Metal Removal from Liquid Wastes by Using Micellar-Enhanced Ultrafiltration. Water. Air. Soil Pollut. 2016, 227, 240. [Google Scholar] [CrossRef]
- Chen, M.; Jafvert, C.T.; Wu, Y.; Cao, X.; Hankins, N.P. Inorganic Anion Removal Using Micellar Enhanced Ultrafiltration (MEUF), Modeling Anion Distribution and Suggested Improvements of MEUF: A Review. Chem. Eng. J. 2020, 398, 125413. [Google Scholar] [CrossRef]
- Wołowicz, A.; Staszak, K.; Hubicki, Z. Static Sorption of Heavy Metal Ions on Ion Exchanger in the Presence of Sodium Dodecylbenzenesulfonate. Adsorption 2019, 25, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Wołowicz, A.; Staszak, K.; Hubicki, Z. Removal of Copper(II) in the Presence of Sodium Dodecylobenzene Sulfonate from Acidic Effluents Using Adsorption on Ion Exchangers and Micellar-Enhanced Ultrafiltration Methods. Molecules 2022, 27, 2430. [Google Scholar] [CrossRef]
- Benderrag, A.; Daaou, M.; Bounaceur, B.; Haddou, B. Influence of PH and Cationic Surfactant on Stability and Interfacial Properties of Algerian Bitumen Emulsion. Chem. Pap. 2016, 70, 1196–1203. [Google Scholar] [CrossRef]
- Ai, C.; Sun, P.; Wu, A.; Chen, X.; Liu, C. Accelerating Leaching of Copper Ore with Surfactant and the Analysis of Reaction Kinetics. Int. J. Miner. Metall. Mater. 2019, 26, 274–281. [Google Scholar] [CrossRef]
- Pattanaik, A.; Venugopal, R. Role of Surfactants in Mineral Processing: An Overview. In Surfactants and Detergents; IntechOpen: London, UK, 2019. [Google Scholar]
- Taseidifar, M.; Makavipour, F.; Pashley, R.M.; Rahman, A.F.M.M. Removal of Heavy Metal Ions from Water Using Ion Flotation. Environ. Technol. Innov. 2017, 8, 182–190. [Google Scholar] [CrossRef]
- Shetty, S.; Chernyshova, I.V.; Ponnurangam, S. Foam Flotation of Rare Earth Elements by Conventional and Green Surfactants. Miner. Eng. 2020, 158, 106585. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A. Surfactant-Modified Chitosan Beads for Cadmium Ion Adsorption. Int. J. Biol. Macromol. 2017, 104, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hankins, N.P. Interaction among Branched Polyethylenimine (PEI), Sodium Dodecyl Sulfate (SDS) and Metal Cations during Copper Recovery from Water Using Polymer-Surfactant Aggregates. J. Water Process Eng. 2020, 34, 101170. [Google Scholar] [CrossRef]
- Cai, Y.; Ma, L.; Xi, X.; Nie, Z.; Nie, Z. Separation of Tungsten and Molybdenum Using Selective Precipitation with Manganese Sulfate Assisted by Cetyltrimethyl Ammonium Bromide (CTAB). Hydrometallurgy 2020, 198, 105494. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Surfactants—A Global Market Overview—Research and Markets. Available online: https://www.researchandmarkets.com/reports/4856171/surfactants-a-global-market-overview (accessed on 23 November 2020).
- Geng, Y.; Huang, J.; Tan, B.; Xu, Y.; Li, P.; Xu, J. Efficient Synthesis of Dodecylbenzene Sulfonic Acid in Microreaction Systems. Chem. Eng. Process. Process Intensif. 2020, 149, 107858. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Lai, T.; Liu, D.; Pan, J.; Lin, L.; Guan, H.; Luo, C.; Song, H.; Xin, Y.; et al. Sodium Dodecylbenzene Sulfonate Film Absorbed on Magnesium Alloy Surface: An Electrochemical, SKPFM, and Molecular Dynamics Study. J. Mol. Liq. 2022, 357, 119095. [Google Scholar] [CrossRef]
- Halit, S.; Benazzouz-Touami, A.; Makhloufi-Chebli, M.; Bouaziz, S.T.; Ahriz, K.I. Sodium Dodecyl Benzene Sulfonate-Catalyzed Reaction for Green Synthesis of Biologically Active Benzylpyrazolyl-Coumarin Derivatives, Mechanism Studies, Theoretical Calculations. J. Mol. Struct. 2022, 1261, 132908. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, C.; Wang, S.; Zeng, N.; Lei, S. Comparison of Anionic Surfactants Dodecylbenzene Sulfonic Acid and 1,2,4-Triazole for Inhibition of Co Corrosion and Study of the Mechanism for Passivation of the Co Surface by Dodecylbenzene Sulfonic Acid. J. Mol. Liq. 2022, 353, 118792. [Google Scholar] [CrossRef]
- Gao, G.; Xie, S.; Zheng, S.; Xu, Y.; Sun, Y. Two-Step Modification (Sodium Dodecylbenzene Sulfonate Composites Acid-Base) of Sepiolite (SDBS/ABsep) and Its Performance for Remediation of Cd Contaminated Water and Soil. J. Hazard. Mater. 2022, 433, 128760. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Z.; Han, X.; Hong, J.; Wang, Y. Impact of Sodium Dodecyl Benzene Sulfonate Concentration on the Stability of the Crude Oil-Mineral Water Interfacial Film: A Molecular Dynamics Simulation Study. Energy Fuels 2022, 36, 4358–4369. [Google Scholar] [CrossRef]
- Meng, J.; Wang, C.; Chen, T. Effect of Sodium Dodecylbenzene Sulfonate on the Wetting Mechanism of Tunliu Coal. J. Surfactants Deterg. 2022, 25, 113–123. [Google Scholar] [CrossRef]
- Lin, C.L.; Lin, W.; Huang, S.; Edwards, G.; Lu, M.; Huang, H. Tribological Performance of Zeolite/Sodium Dodecylbenzenesulfonate Hybrid Water-Based Lubricants. Appl. Surf. Sci. 2022, 598, 153764. [Google Scholar] [CrossRef]
- Gao, J.Q.; Guo, Q.Z.; Huang, Z.Z.; Ren, P.; Hu, Z.Z.; Kong, C.G. Performance and Mechanisms of Sodium Dodecyl Benzene Sulfonate-Modified Maifanite for Cr(VI) and Cd(II) Removal from Aqueous Solution. Int. J. Environ. Sci. Technol. 2022, 1–18. [Google Scholar] [CrossRef]
- Imanivarnosfaderani, M.R.; Gomari, S.R.; dos Santos, R.G. Effects of Rhamnolipid Bio-Surfactant and Sodium Dodecylbenzene Sulfonate (SDBS) Surfactant on Enhanced Oil Recovery from Carbonate Reservoirs. Braz. J. Chem. Eng. 2022, 39, 825–833. [Google Scholar] [CrossRef]
- Shiri, M.; Zolfigol, M.A. Surfactant-Type Catalysts in Organic Reactions. Tetrahedron 2009, 65, 587–598. [Google Scholar] [CrossRef]
- Artykulnyi, O.P.; Shibaev, A.V.; Avdeev, M.M.; Ivankov, O.I.; Bulavin, L.A.; Petrenko, V.I.; Philippova, O.E. Structural Investigations of Poly(Ethylene Glycol)-Dodecylbenzenesulfonic Acid Complexes in Aqueous Solutions. J. Mol. Liq. 2020, 308, 113045. [Google Scholar] [CrossRef]
- Petrenko, V.I.; Avdeev, M.V.; Garamus, V.M.; Bulavin, L.A.; Aksenov, V.L.; Rosta, L. Micelle Formation in Aqueous Solutions of Dodecylbenzene Sulfonic Acid Studied by Small-Angle Neutron Scattering. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Yang, X.; Zhang, J.; Zhu, L.; Lv, Y. Adsorption Mechanisms of Dodecylbenzene Sulfonic Acid by Corn Straw and Poplar Leaf Biochars. Materials 2017, 10, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, R.; Quan, X.F.; Ren, Z.H.; Huang, J.; Li, D.N.; Wang, J.R.; Qian, Z.B.; Zhang, Y.X.; Cai, L.L.; Li, B.B.; et al. Molecular Interaction between Sodium Dodecylbenzene Sulfonate and Octylphenol Polyoxyethylene Ether and Effect of Hydrophilic Chain. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127048. [Google Scholar] [CrossRef]
- Amani, P.; Miller, R.; Ata, S.; Hurter, S.; Rudolph, V.; Firouzi, M. Dynamics of Interfacial Layers for Sodium Dodecylbenzene Sulfonate Solutions at Different Salinities. J. Ind. Eng. Chem. 2020, 92, 174–183. [Google Scholar] [CrossRef]
- Sood, A.K.; Aggarwal, M. Evaluation of Micellar Properties of Sodium Dodecylbenzene Sulphonate in the Presence of Some Salts. J. Chem. Sci. 2018, 130, 39. [Google Scholar] [CrossRef] [Green Version]
- Qazi, M.J.; Schlegel, S.J.; Backus, E.H.G.; Bonn, M.; Bonn, D.; Shahidzadeh, N. Dynamic Surface Tension of Surfactants in the Presence of High Salt Concentrations. Langmuir 2020, 36, 7956–7964. [Google Scholar] [CrossRef]
- Tiwari, S.; Namsani, S.; Singh, J.K. Effect of Salt on the Adsorption of Ionic Surfactants at the Air-Water Interface. J. Mol. Liq. 2022, 360, 119498. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of Solvation of Ions. Part 5.—Gibbs Free Energy of Hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Radzio, K.; Prochaska, K. Interfacial Activity of Trioctyloamine in Hydrocarbon/Water Systems with Nonorganic Electrolytes. J. Colloid Interface Sci. 2001, 233, 211–218. [Google Scholar] [CrossRef]
- Mousli, R.; Tazerouti, A. Synthesis and Some Surface Properties of Glycine-Based Surfactants. J. Surfactants Deterg. 2010, 14, 65–72. [Google Scholar] [CrossRef]
- Eastoe, J.; Dalton, J.S. Dynamic Surface Tension and Adsorption Mechanisms of Surfactants at the Air–Water Interface. Adv. Colloid Interface Sci. 2000, 85, 103–144. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Cao, C.; Cao, L.D.; Zheng, L.; Xu, J.; Li, F.M.; Huang, Q.L. Evaporation Kinetics of Surfactant Solution Droplets on Rice (Oryza Sativa) Leaves. PLoS ONE 2017, 12, e0176870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhmud, B.V.; Tiberg, F.; Kizling, J. Dynamic Surface Tension in Concentrated Solutions of CnEm Surfactants: A Comparison between the Theory and Experiment. Langmuir 2000, 16, 2557–2565. [Google Scholar] [CrossRef]
- Li, H.-t.; Cui, C.-z.; Guo, L.-l.; Yuan, F.-q.; Xu, Z.-c.; Gong, Q.-t.; Jin, Z.-q.; Zhang, L.; Zhang, L. Dynamic Interfacial Tensions of Sulfobetaine and Polymers Solutions: Effect of Structures. J. Mol. Liq. 2022, 356, 119018. [Google Scholar] [CrossRef]
- Le, T.T.Y.; Tsay, R.Y.; Lin, S.Y. A Study on the Dynamic Surface Tension of Surfactant Solutions at Dilute Concentrations. J. Mol. Liq. 2021, 324, 115112. [Google Scholar] [CrossRef]
- Song, Q.; Yuan, M. Visualization of an Adsorption Model for Surfactant Transport from Micelle Solutions to a Clean Air/Water Interface Using Fluorescence Microscopy. J. Colloid Interface Sci. 2011, 357, 179–188. [Google Scholar] [CrossRef]
- Paul, B.C.; Islam, S.S.; Ismail, K. Effect of Acetate and Propionate Co-Ions on the Micellization of Sodium Dodecyl Sulfate in Water. J. Phys. Chem. B 1998, 102, 7807–7812. [Google Scholar] [CrossRef] [Green Version]
- Fainerman, V.B. Adsorption Kinetics from Concentrated Micellar Solutions of Ionic Surfactants at the Water—Air Interface. Colloids Surf. 1992, 62, 333–347. [Google Scholar] [CrossRef]
- Casandra, A.; Tsay, R.Y.; Chung, M.C.; Ismadji, S.; Lin, S.Y. A Study on the Method of Short-Time Approximation–Curvature Effect. J. Taiwan Inst. Chem. Eng. 2017, 74, 73–78. [Google Scholar] [CrossRef]
- Ritacco, H.; Langevin, D.; Diamant, H.; Andelman, D. Dynamic Surface Tension of Aqueous Solutions of Ionic Surfactants: Role of Electrostatics. Langmuir 2011, 27, 1009–1014. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Makievski, A.V. Micelle Dissociation Kinetics Study by Dynamic Surface Tension of Micellar Solutions. Colloids Surf. 1993, 69, 249–263. [Google Scholar] [CrossRef]


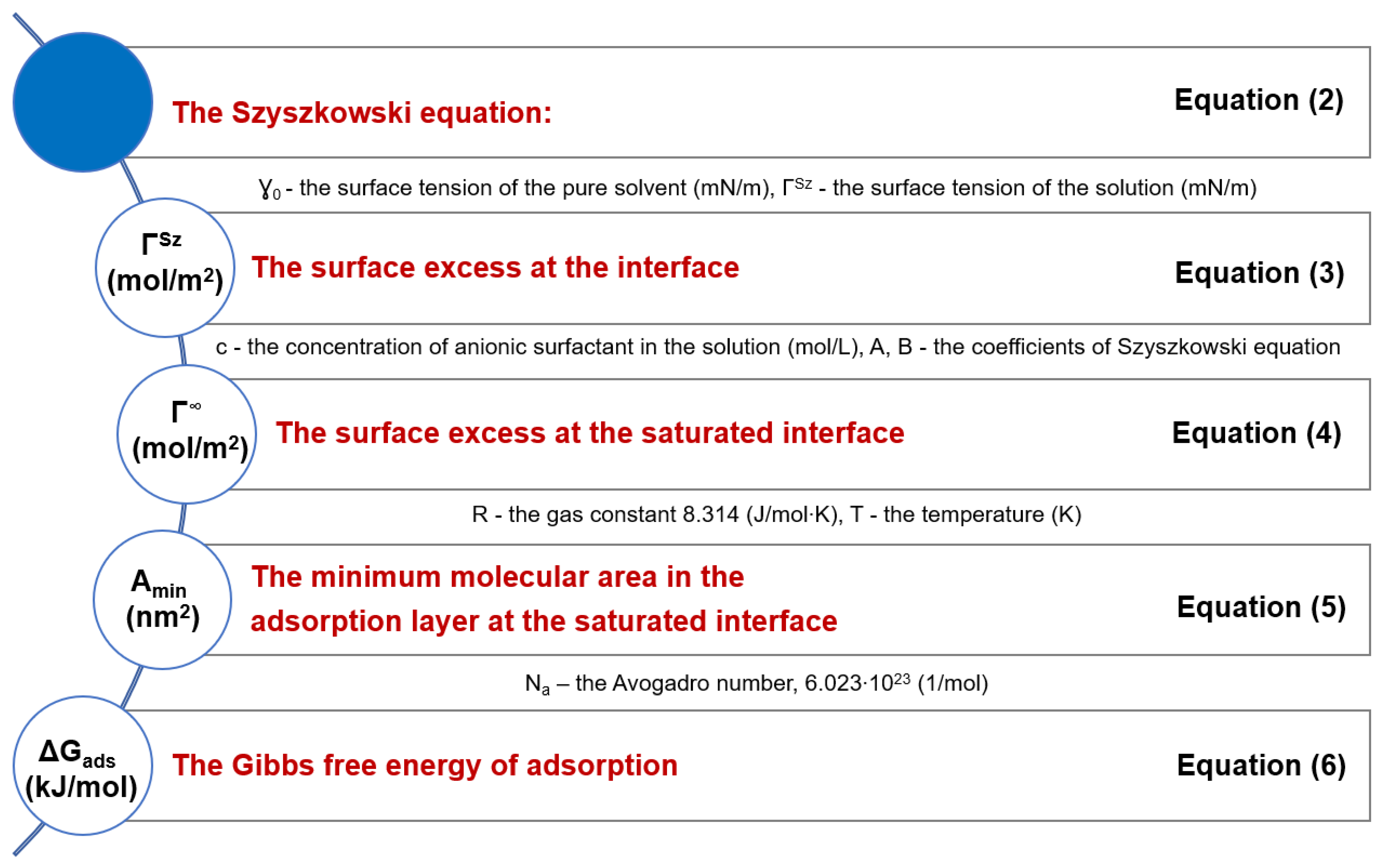
| Surfactant | Dodecylbenzene Sulfonic Acid | Sodium Dodecylbenzene Sulfonate |
|---|---|---|
| Alternative names | dodecylbenzenesulfonic or benzenosufonic acid, 4-C10-13-sec-alkyl derive, linear alkyl benzene sulfonic acid (LABSA) | sodium salts, C10-13 alkyl derivatives, benzenesulfonic acids |
| Abbreviation | ABS | ABSNa50 |
| Type | anionic | anionic |
| Structure | 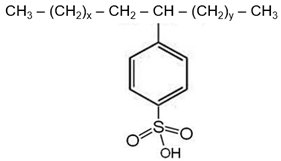 | 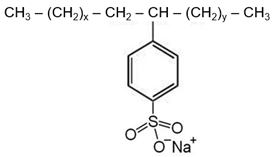 |
| Molecular weight (g/mol) | approx. 320 | 340–348 |
| Density (20 °C) (g/mL) | 1.06 | 1.07 |
| Form of occurrence | light brown to dark brown liquid | liquid |
| Odour | characteristic | weak, characteristic |
| Properties | dispersing, emulsifying, wetting, and foaming properties | dispersing, emulsifying, and wetting properties |
| Application | metal cleaning, laundry, cleaning detergents for the household and professional use, production of hydraulic fluids (fire resistant), production of textiles, plant protection products | agrochemicals, pesticides, fertilizers, industrial cleaning and washing, detergents, paints and varnishes, lubricants and functional fluids, construction industry, drilling and tunneling, metallurgical industry, machining, mining industry, oil and gas extraction, textiles |
| Parameters | System without Heavy Metal Ions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ABS | ABSNa50 | |||||||||
| Water | 0.1 mol/L HCl | 1 mol/L HCl | 3 mol/L HCl | 6 mol/L HCl | Water | 0.1 mol/L HCl | 1 mol/L HCl | 3 mol/L HCl | 6 mol/L HCl | |
| A × 10−6 | 4.64 | 29.5 | 2.72 | 0.044 | 0.15 | 6.96 | 7.58 | 1.13 | 1.84 | 4.54 |
| B | 0.085 | 0.091 | 0.233 | 0.091 | 0.103 | 0.11 | 0.228 | 0.171 | 0.164 | 0.265 |
| R2 | 0.997 | 0.980 | 0.989 | 0.994 | 0.934 | 0.993 | 0.993 | 0.934 | 0.979 | 0.976 |
| Γ∞ × 10−6 (mol/m2) | 2.41 | 2.51 | 6.58 | 2.60 | 2.89 | 3.12 | 6.28 | 4.83 | 4.69 | 7.43 |
| ∆Gads (kJ/mol) | −30.4 | −37.3 | −31.7 | −42 | −38.9 | −29.4 | −29.2 | −33.9 | −32.7 | −30.5 |
| Amin (nm2) | 0.689 | 0.662 | 0.252 | 0.638 | 0.575 | 0.533 | 0.264 | 0.344 | 0.354 | 0.224 |
| System with heavy metal ions | ||||||||||
| Co(II) | ||||||||||
| A × 10−6 | 3.67 | 0.83 | 2.30 | 1.60 | 3.04 | 9.05 | 3.03 | 6.21 | 1.87 | 4.88 |
| B | 0.135 | 0.114 | 0.2 | 0.138 | 0.327 | 0.205 | 0.155 | 0.237 | 0.17 | 0.283 |
| R2 | 0.995 | 0.995 | 0.981 | 0.994 | 0.987 | 0.992 | 0.992 | 0.99 | 0.97 | 0.979 |
| Γ∞ × 10−6 (mol/m2) | 3.90 | 3.28 | 5.73 | 3.89 | 9.25 | 5.93 | 4.46 | 6.79 | 4.84 | 7.86 |
| ∆Gads (kJ/mol) | −31.0 | −34.7 | −32.2 | −33.1 | −31.5 | −28.8 | −31.5 | −29.7 | −32.7 | −30.3 |
| Amin (nm2) | 0.425 | 0.507 | 0.29 | 0.427 | 0.179 | 0.28 | 0.373 | 0.245 | 0.343 | 0.211 |
| Ni(II) | ||||||||||
| A × 10−6 | 1.77 | 0.47 | 2.12 | 24.5 | 2.19 | 3.28 | 5.43 | 2.90 | 3.57 | 2.61 |
| B | 0.118 | 0.103 | 0.216 | 0.242 | 0.221 | 0.15 | 0.201 | 0.196 | 0.25 | 0.222 |
| R2 | 0.998 | 0.988 | 0.957 | 0.997 | 0.983 | 0.993 | 0.996 | 0.97 | 0.979 | 0.979 |
| Γ∞ × 10−6 (mol/m2) | 3.44 | 2.92 | 6.25 | 6.96 | 6.31 | 4.38 | 5.82 | 5.67 | 7.19 | 6.34 |
| ∆Gads (kJ/mol) | −32.8 | −36.1 | −32.4 | −26.3 | −32.3 | −31.3 | −30 | −31.6 | −31.1 | −31.9 |
| Amin (nm2) | 0.482 | 0.569 | 0.266 | 0.238 | 0.263 | 0.379 | 0.285 | 0.293 | 0.231 | 0.262 |
| Zn(II) | ||||||||||
| A × 10−6 | 2.56 | 2.07 | 2.87 | 0.55 | 0.87 | 6.21 | 5.46 | 0.76 | 5.16 | 4.21 |
| B | 0.142 | 0.137 | 0.229 | 0.153 | 0.126 | 0.187 | 0.187 | 0.114 | 0.277 | 0.267 |
| R2 | 0.996 | 0.997 | 0.981 | 0.988 | 0.974 | 0.996 | 0.988 | 0.951 | 0.957 | 0.968 |
| Γ∞ × 10−6 (mol/m2) | 4.14 | 3.95 | 6.58 | 4.21 | 3.60 | 5.45 | 5.40 | 3.28 | 7.92 | 7.45 |
| ∆Gads (kJ/mol) | −31.9 | −32.4 | −31.6 | −35.7 | −34.6 | −29.7 | −30 | −34.9 | −30.2 | −30.7 |
| Amin (nm2) | 0.401 | 0.420 | 0.252 | 0.395 | 0.461 | 0.305 | 0.308 | 0.507 | 0.21 | 0.223 |
| Cu(II) | ||||||||||
| A × 10−6 | 8.85 | 2.34 | 0.85 | 7.08 | 17.2 | 6.52 | 2.37 | 0.85 | 1.59 | 4.70 |
| B | 0.19 | 0.144 | 0.152 | 0.515 | 0.908 | 0.186 | 0.142 | 0.152 | 0.192 | 0.274 |
| R2 | 0.991 | 0.999 | 0.993 | 0.956 | 0.931 | 0.985 | 0.998 | 0.993 | 0.959 | 0.99 |
| Γ∞ × 10−6 (mol/m2) | 5.37 | 4.08 | 4.23 | 14.5 | 25.6 | 5.26 | 4.02 | 4.22 | 5.48 | 7.72 |
| ∆Gads (kJ/mol) | −28.8 | −32.1 | −34.6 | −29.4 | −27.2 | −29.6 | −32.1 | −34.6 | −33.1 | −30.4 |
| Amin (nm2) | 0.309 | 0.407 | 0.393 | 0.114 | 0.0649 | 0.316 | 0.413 | 0.394 | 0.303 | 0.215 |
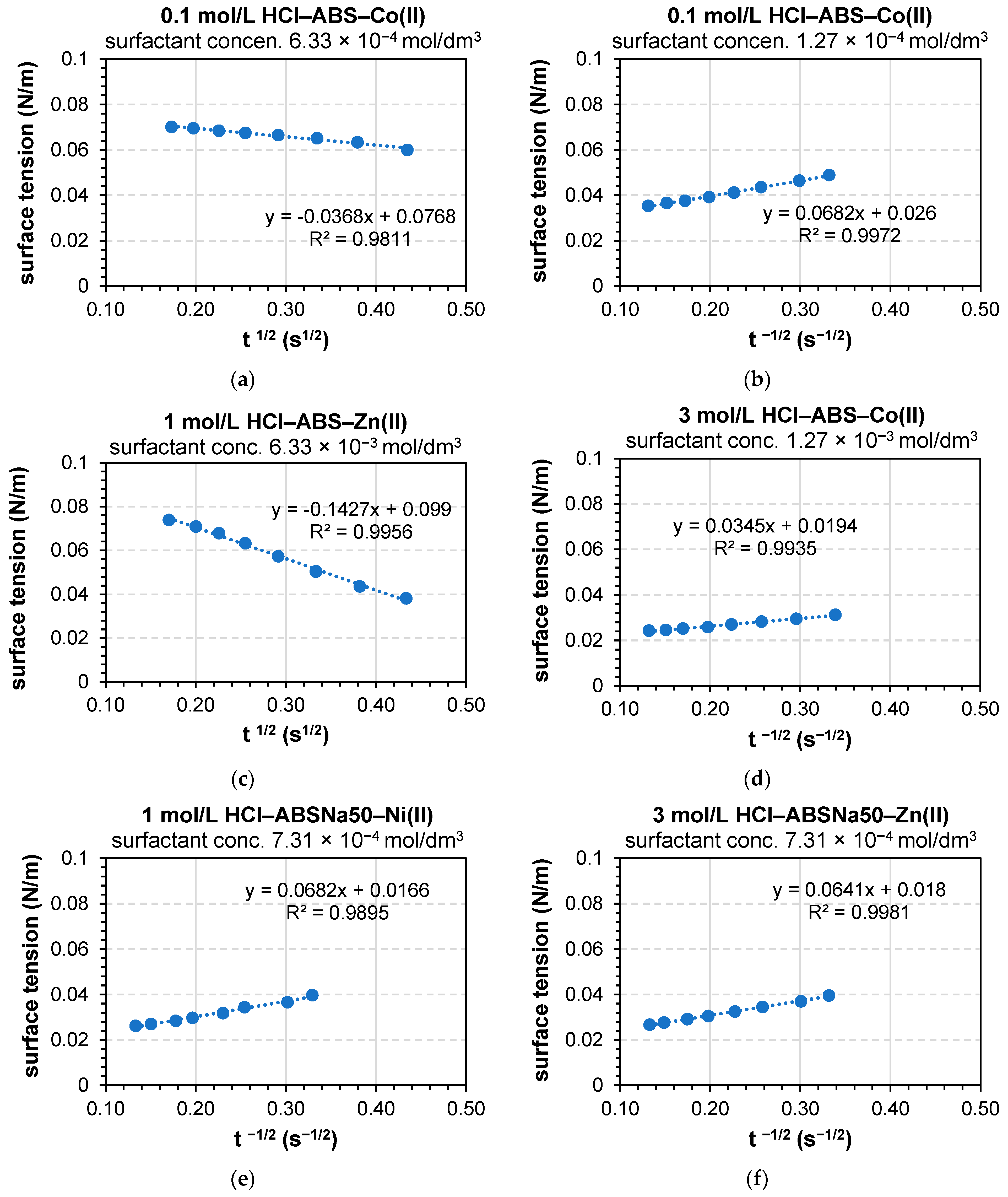
| 0.1 mol/L HCl–ABS–Co(II) | 0.1 mol/L HCl–ABS–Cu(II) | |||||
|---|---|---|---|---|---|---|
| c (mol/L) | Dt→0 (m2/s) | Dt→∞ (m2/s) | k2 (s−1) | Dt→0 (m2/s) | Dt→∞ (m2/s) | k2 (s−1) |
| 6.33 × 10−3 | 5.33 × 10−12 | 2.94 × 10−11 | 35,200 | 4.10 × 10−12 | 2.48 × 10−10 | 64,640 |
| 1.27 × 10−3 | 1.46 × 10−9 | 2.68 × 10−11 | 3930 | 1.55 × 10−9 | 1.14 × 10−10 | 5210 |
| 6.33 × 10−4 | 4.33 × 10−10 | 6.20 × 10−11 | 62,900 | 6.32 × 10−10 | 6.27 × 10−11 | 93,272 |
| 3.17 × 10−4 | 2.65 × 10−10 | 2.08 × 10−11 | 348,000 | 2.90 × 10−10 | 5.59 × 10−11 | 464,108 |
| 1.27 × 10−4 | 6.17 × 10−10 | 7.27 × 10−12 | 6.11 × 10−10 | 1.12 × 10−11 | ||
| 6.33 × 10−5 | 2.93 × 10−9 | 6.67 × 10−11 | 2.53 × 10−9 | 8.76 × 10−11 | ||
| 3.17 × 10−5 | 1.16 × 10−8 | 1.26 × 10−7 | 9.34 × 10−9 | 6.03 × 10−9 | ||
| 0.1 mol/L HCl–ABS–Zn(II) | 0.1 mol/L HCl–ABS–Ni(II) | |||||
| 6.33 × 10−3 | 5.35 × 10−12 | 3.82 × 10−11 | 35,355 | 5.35 × 10−12 | 3.82 × 10−11 | 35,355 |
| 1.27 × 10−3 | 1.49 × 10−9 | 2.20 × 10−11 | 3802 | 1.49 × 10−9 | 2.20 × 10−11 | 3803 |
| 6.33 × 10−4 | 5.51 × 10−9 | 1.79 × 10−10 | 3998 | 5.51 × 10−9 | 1.79 × 10−10 | 3999 |
| 3.17 × 10−4 | 2.85 × 10−10 | 1.71 × 10−11 | 304,327 | 2.85 × 10−10 | 1.71 × 10−11 | 304,328 |
| 1.27 × 10−4 | 4.95 × 10−10 | 4.53 × 10−12 | 4.95 × 10−10 | 4.53 × 10−12 | ||
| 6.33 × 10−5 | 2.55 × 10−9 | 1.53 × 10−11 | 2.55 × 10−9 | 1.53 × 10−11 | ||
| 3.17 × 10−5 | 1.05 × 10−8 | 2.48 × 10−9 | 1.05 × 10−8 | 2.48 × 10−9 | ||
| 0.1 mol/L HCl–ABSNa50–Co(II) | 0.1 mol/L HCl–ABSNa50–Cu(II) | |||||
| 7.31 × 10−3 | 1.28 × 10−11 | 2.78 × 10−10 | 633 | 1.21 × 10−11 | 1.24 × 10−11 | 17,588 |
| 1.46 × 10−3 | 5.30 × 10−10 | 4.30 × 10−11 | 525 | 5.49 × 10−10 | 7.37 × 10−11 | 12,475 |
| 7.31 × 10−4 | 1.65 × 10−10 | 3.64 × 10−11 | 6662 | 1.79 × 10−10 | 3.05 × 10−11 | 158,102 |
| 3.66 × 10−4 | 1.44 × 10−10 | 5.21 × 10−11 | 26,935 | 1.02 × 10−10 | 3.49 × 10−11 | 958,251 |
| 1.46 × 10−4 | 5.17 × 10−10 | 1.32 × 10−11 | 4.52 × 10−10 | 8.09 × 10−12 | ||
| 7.31 × 10−5 | 2.05 × 10−9 | 1.40 × 10−10 | 2.31 × 10−9 | 1.52 × 10−10 | ||
| 3.66 × 10−5 | 7.07 × 10−9 | 3.40 × 10−8 | 8.65 × 10−9 | 1.00 × 10−7 | ||
| 0.1 mol/L HCl–ABSNa50–Zn(II) | 0.1 mol/L HCl–ABSNa50–Ni(II) | |||||
| 7.31 × 10−3 | 1.11 × 10−11 | 2.59 × 10−10 | 20,290 | 1.08 × 10−11 | 1.47 × 10−11 | 23,628 |
| 1.46 × 10−3 | 4.34 × 10−10 | 2.68 × 10−10 | 18,772 | 5.90 × 10−10 | 1.75 × 10−10 | 14,406 |
| 7.31 × 10−4 | 1.55 × 10−10 | 1.20 × 10−10 | 209,952 | 1.75 × 10−10 | 1.85 × 10−10 | 184,938 |
| 3.66 × 10−4 | 1.18 × 10−10 | 1.08 × 10−10 | 940,602 | 1.39 × 10−10 | 1.52 × 10−10 | 821,586 |
| 1.46 × 10−4 | 4.70 × 10−10 | 2.18 × 10−11 | 4.02 × 10−10 | 2.73 × 10−11 | 1,653,535 | |
| 7.31 × 10−5 | 1.97 × 10−9 | 2.88 × 10−10 | 1.91 × 10−9 | 6.15× 10−10 | ||
| 3.66 × 10−5 | 7.49 × 10−9 | 3.69 × 10−4 | 8.52 × 10−9 | 1.11 × 10−5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołowicz, A.; Staszak, K.; Hubicki, Z. A Comprehensive Analysis of Selected Anionic Surfactants Behaviour in Aqueous Systems Containing Metal Ions and Inorganic Acid. Water 2022, 14, 3676. https://doi.org/10.3390/w14223676
Wołowicz A, Staszak K, Hubicki Z. A Comprehensive Analysis of Selected Anionic Surfactants Behaviour in Aqueous Systems Containing Metal Ions and Inorganic Acid. Water. 2022; 14(22):3676. https://doi.org/10.3390/w14223676
Chicago/Turabian StyleWołowicz, Anna, Katarzyna Staszak, and Zbigniew Hubicki. 2022. "A Comprehensive Analysis of Selected Anionic Surfactants Behaviour in Aqueous Systems Containing Metal Ions and Inorganic Acid" Water 14, no. 22: 3676. https://doi.org/10.3390/w14223676
APA StyleWołowicz, A., Staszak, K., & Hubicki, Z. (2022). A Comprehensive Analysis of Selected Anionic Surfactants Behaviour in Aqueous Systems Containing Metal Ions and Inorganic Acid. Water, 14(22), 3676. https://doi.org/10.3390/w14223676