Application of RSM for Bioremoval of Methylene Blue Dye from Industrial Wastewater onto Sustainable Walnut Shell (Juglans regia) Biomass
Abstract
:1. Introduction
- To use a locally available J. regia shell as a precursor to creating a nano-biomass product with a high biosorption capacity and a cheap cost;
- To use experimental design methodologies to optimize the selected parameters to thus explore the possible removal of biomass adsorption for MB.
2. Materials and Methods
2.1. Chemicals
2.2. Collection and Preparation of Adsorbent
2.3. Preparation of the Adsorbate (MB Dye)
2.4. Characterization
2.5. Optimization of Biosorption Experiments for MB Removal by Face-Centred Central Composite Design (FCCCD)
2.6. RSM-Based Experimental Design
3. Result and Discussion
3.1. Statistical Optimization of MB Using RSM
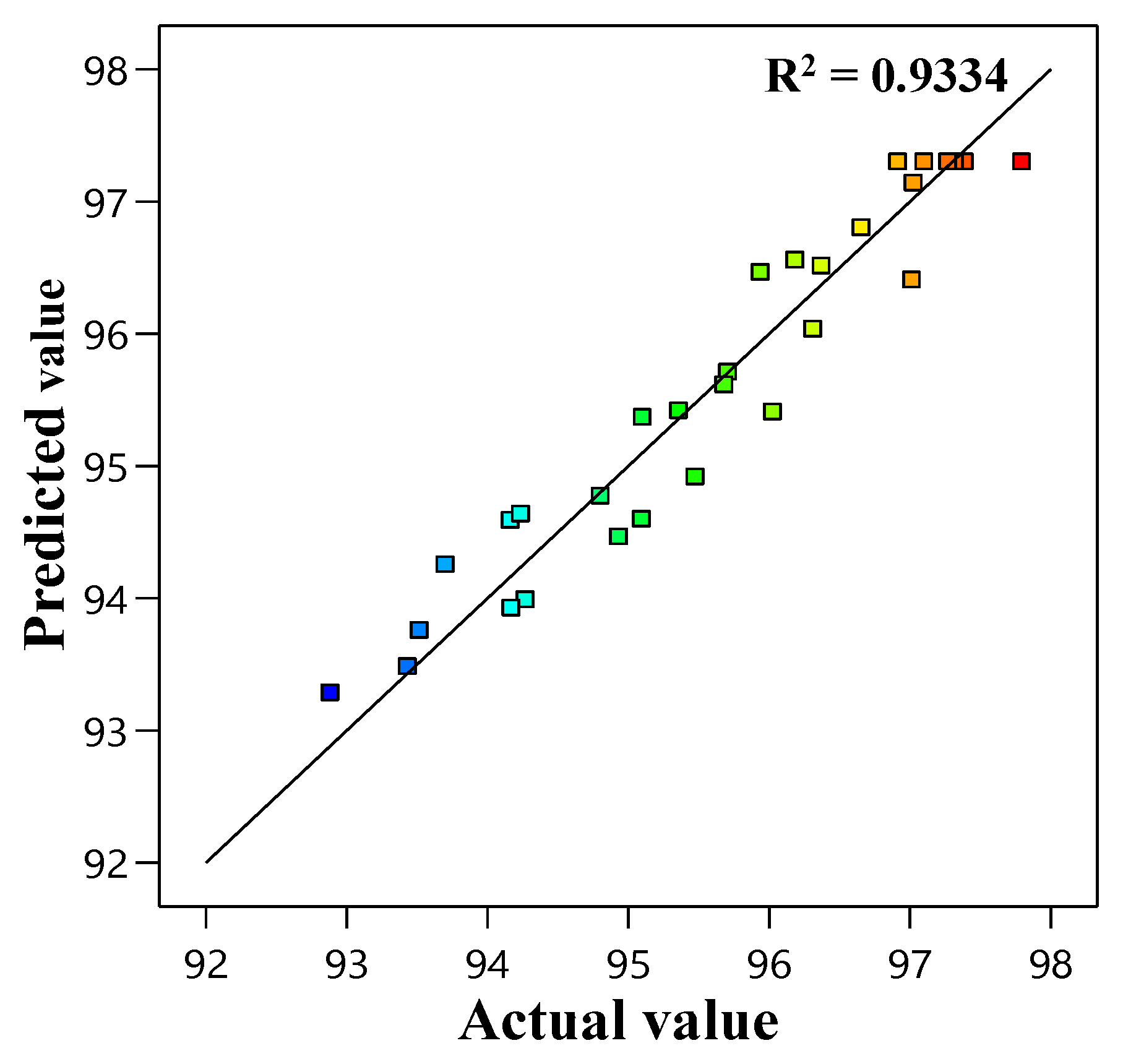
Adequacy of the Model
3.2. ANOVA and Regression Analysis
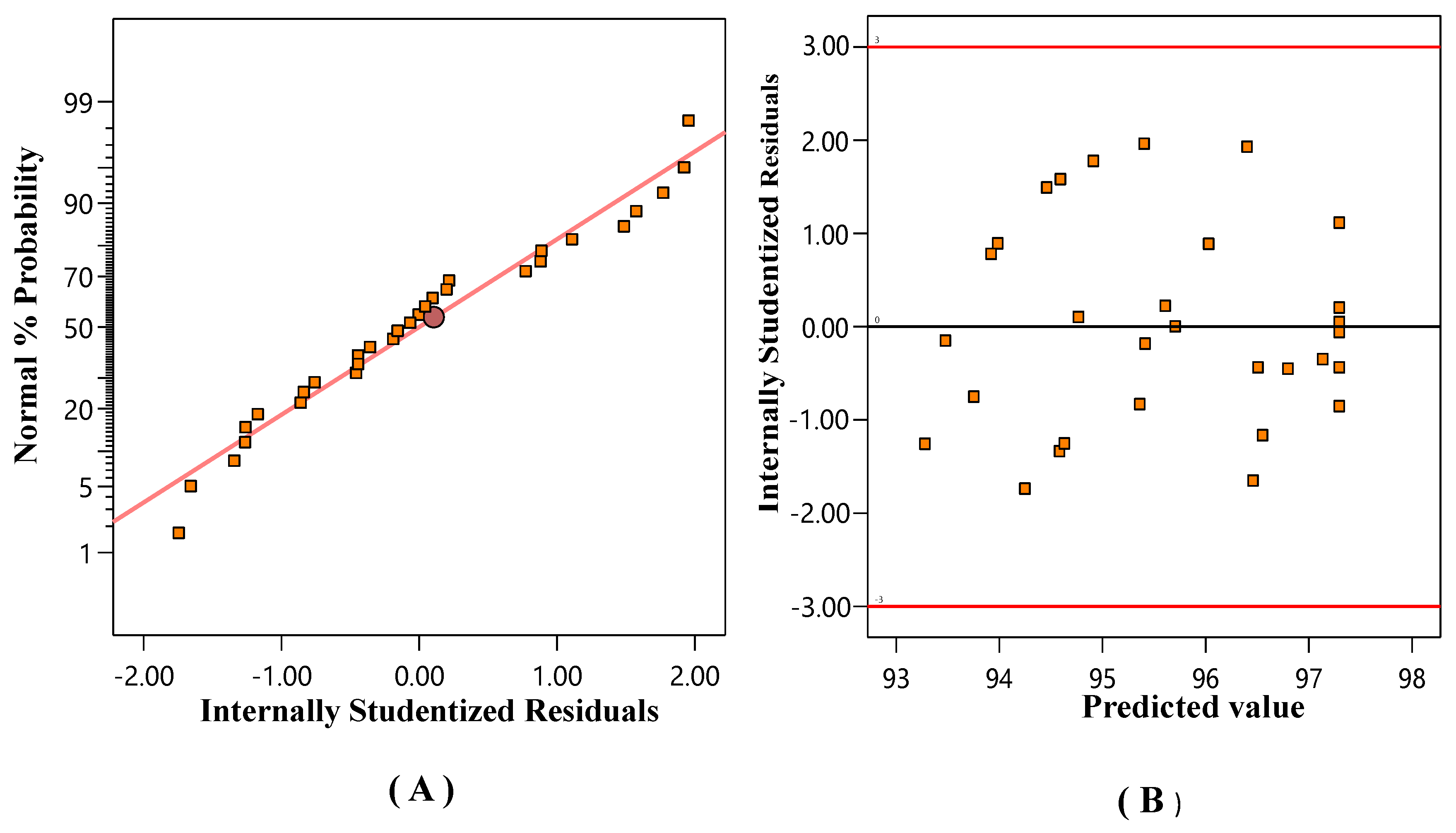
Residuals’ Normal Probability Plot (NPP)
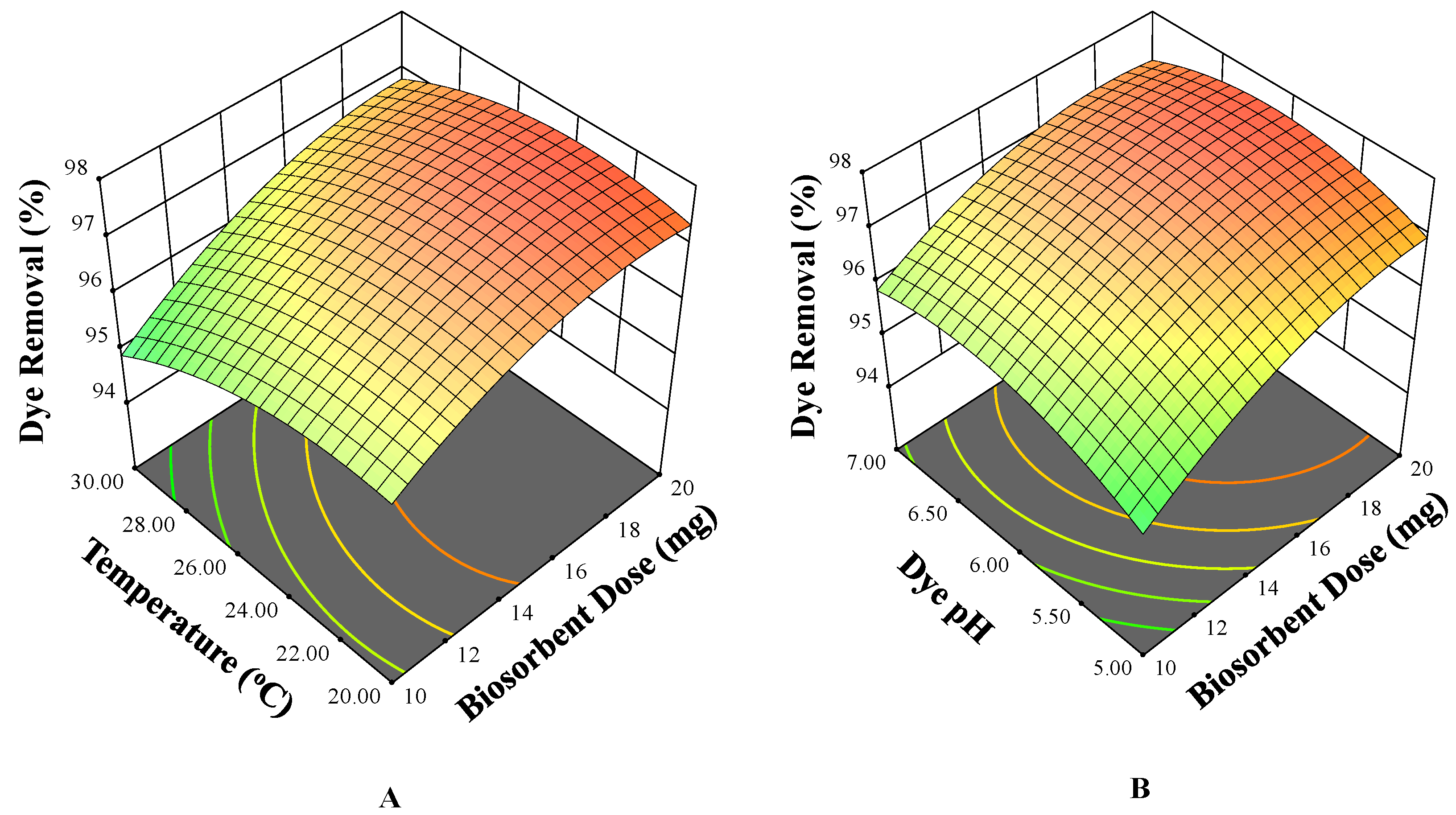
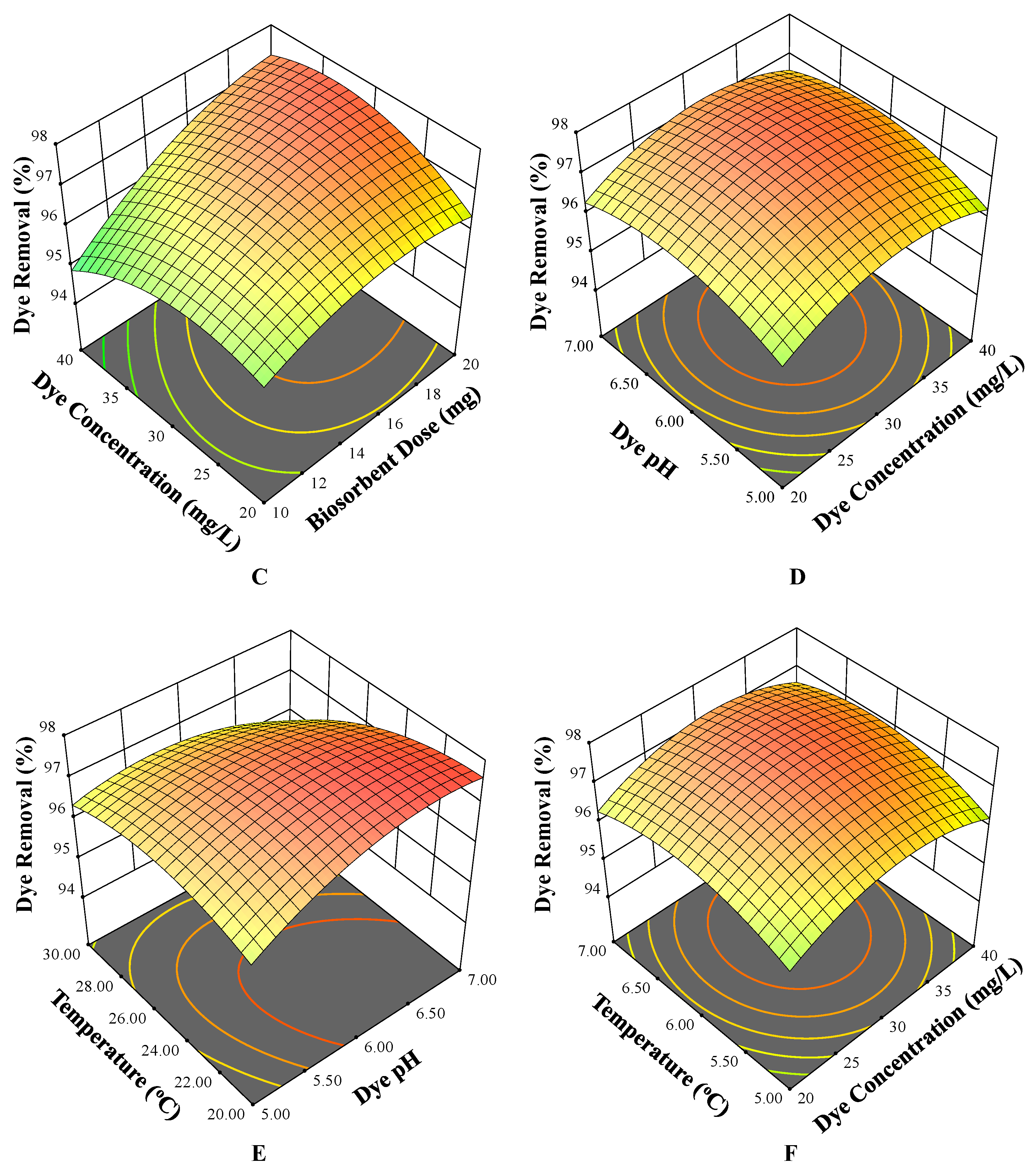
3.3. Three-Dimensional Surface Plots
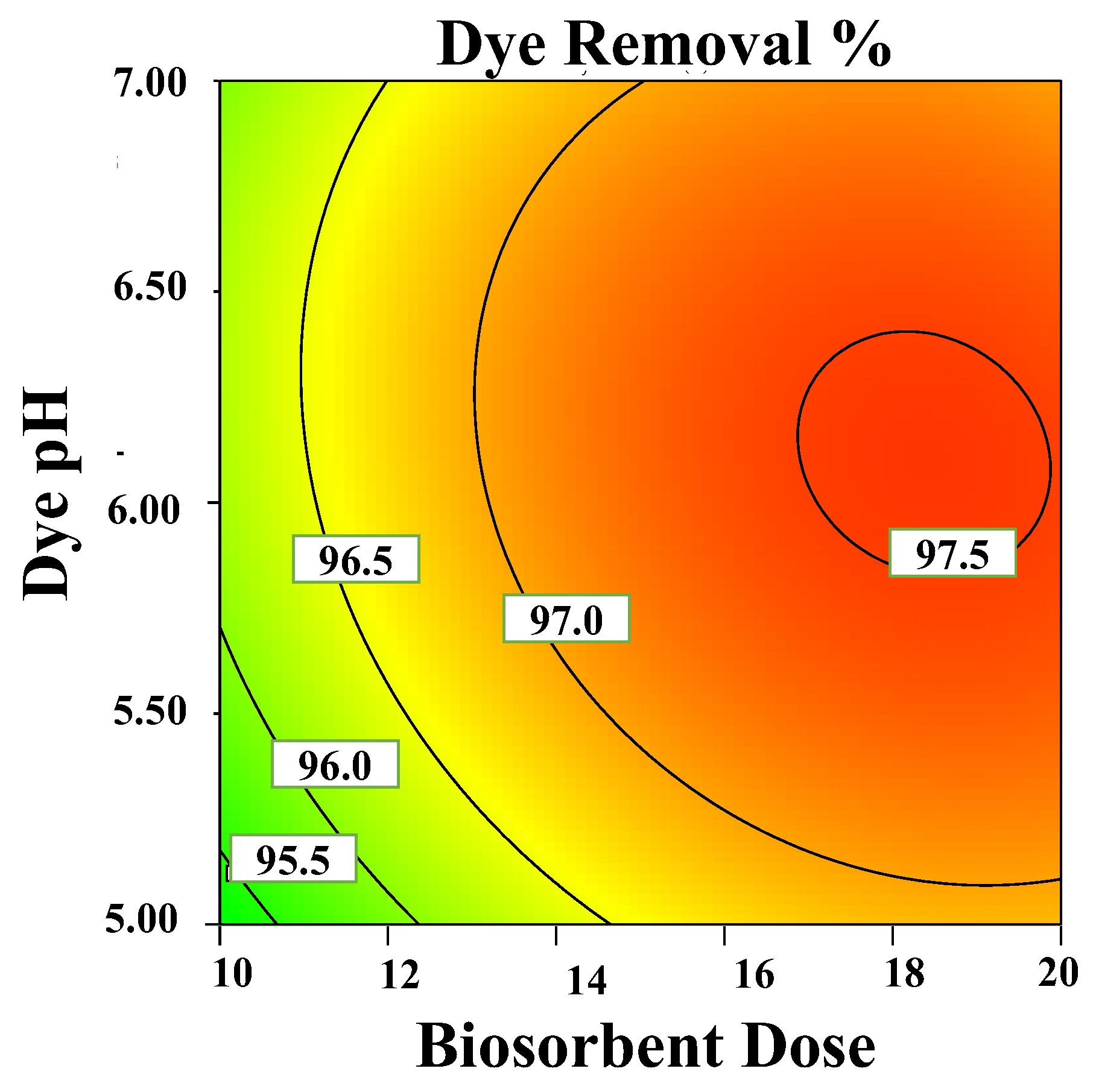
3.3.1. Effects on pH
3.3.2. Effect of Biosorbent Dose
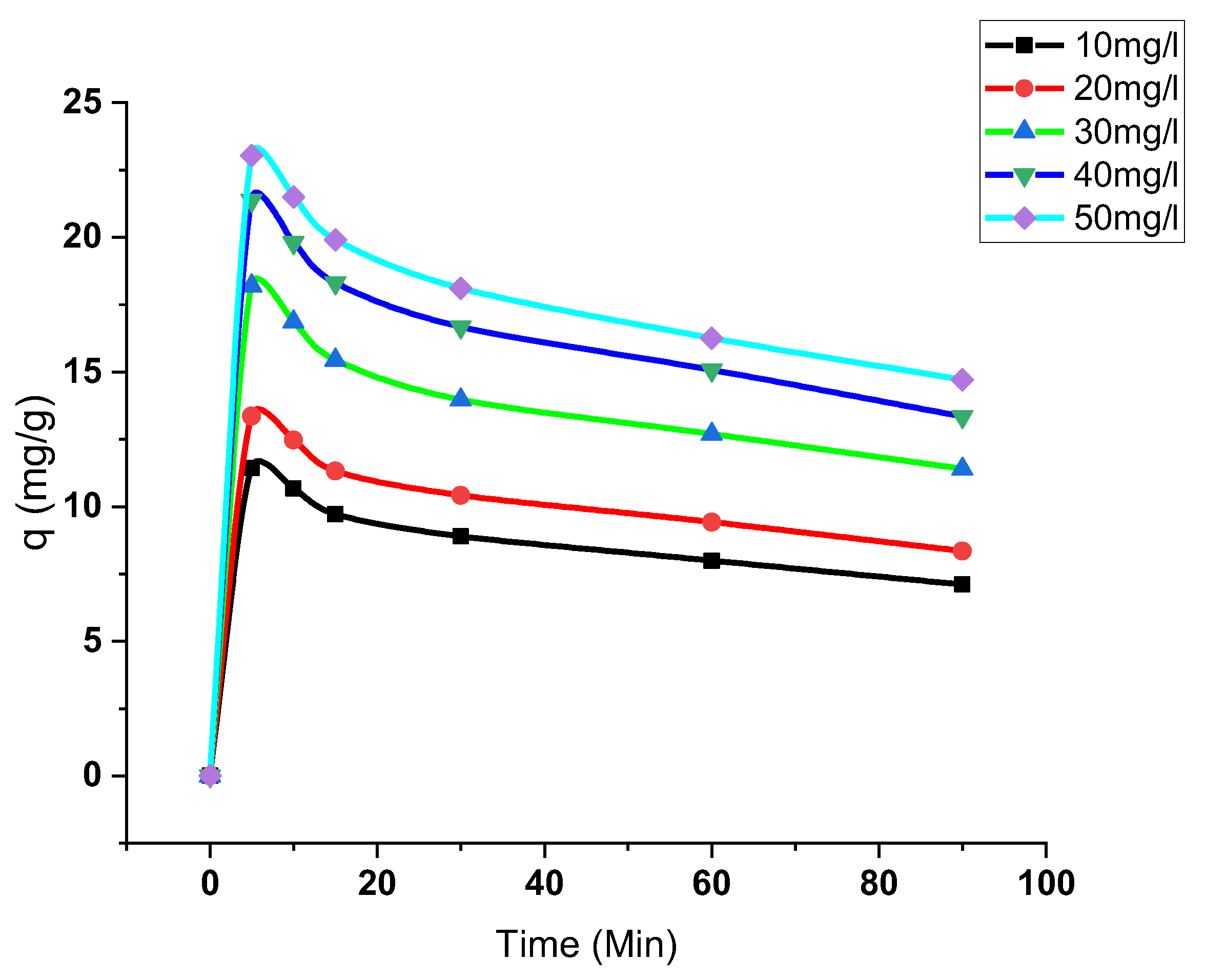
3.3.3. Effect of Initial Concentration and Contact Time
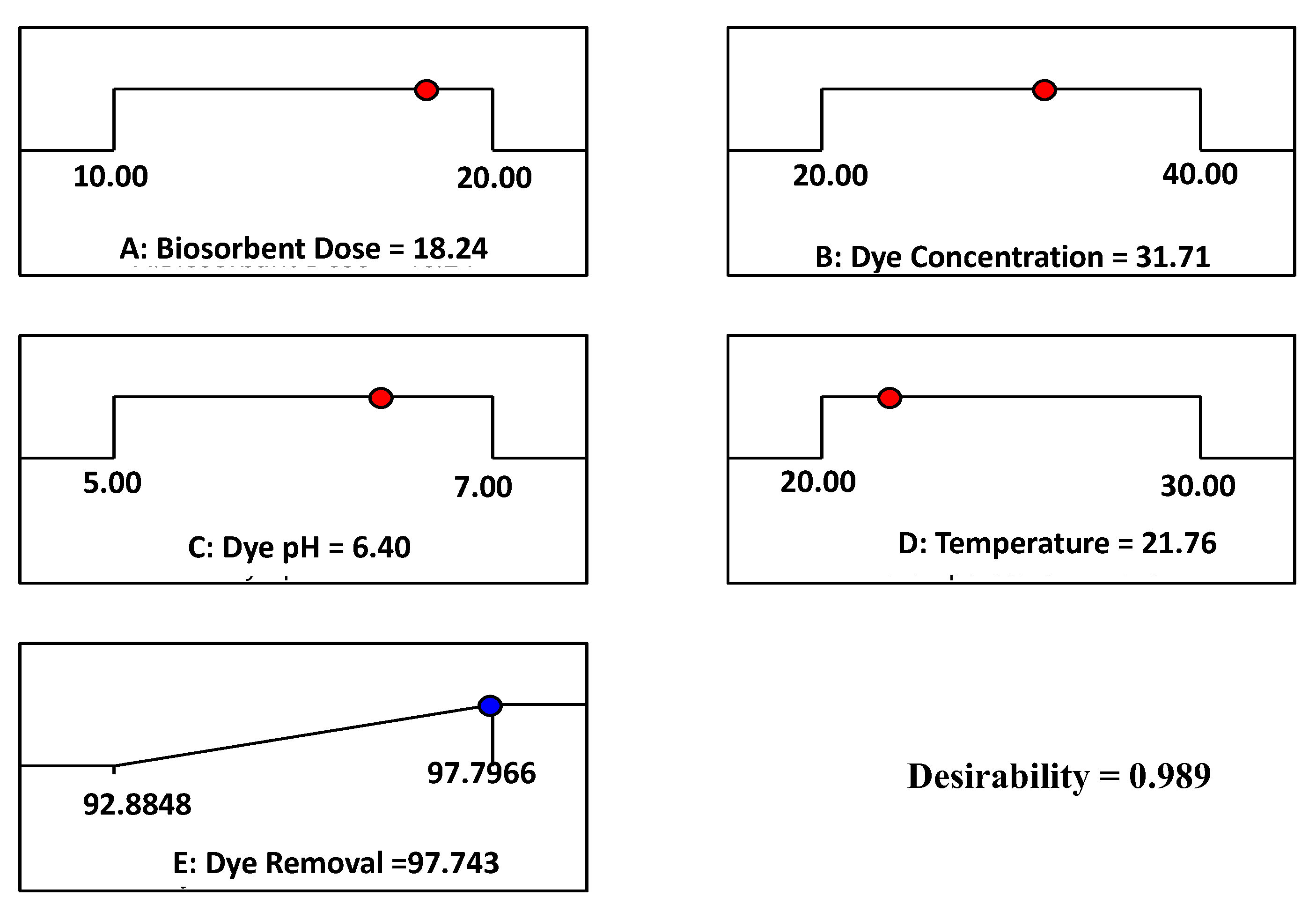
3.4. Desirability Function (DF)
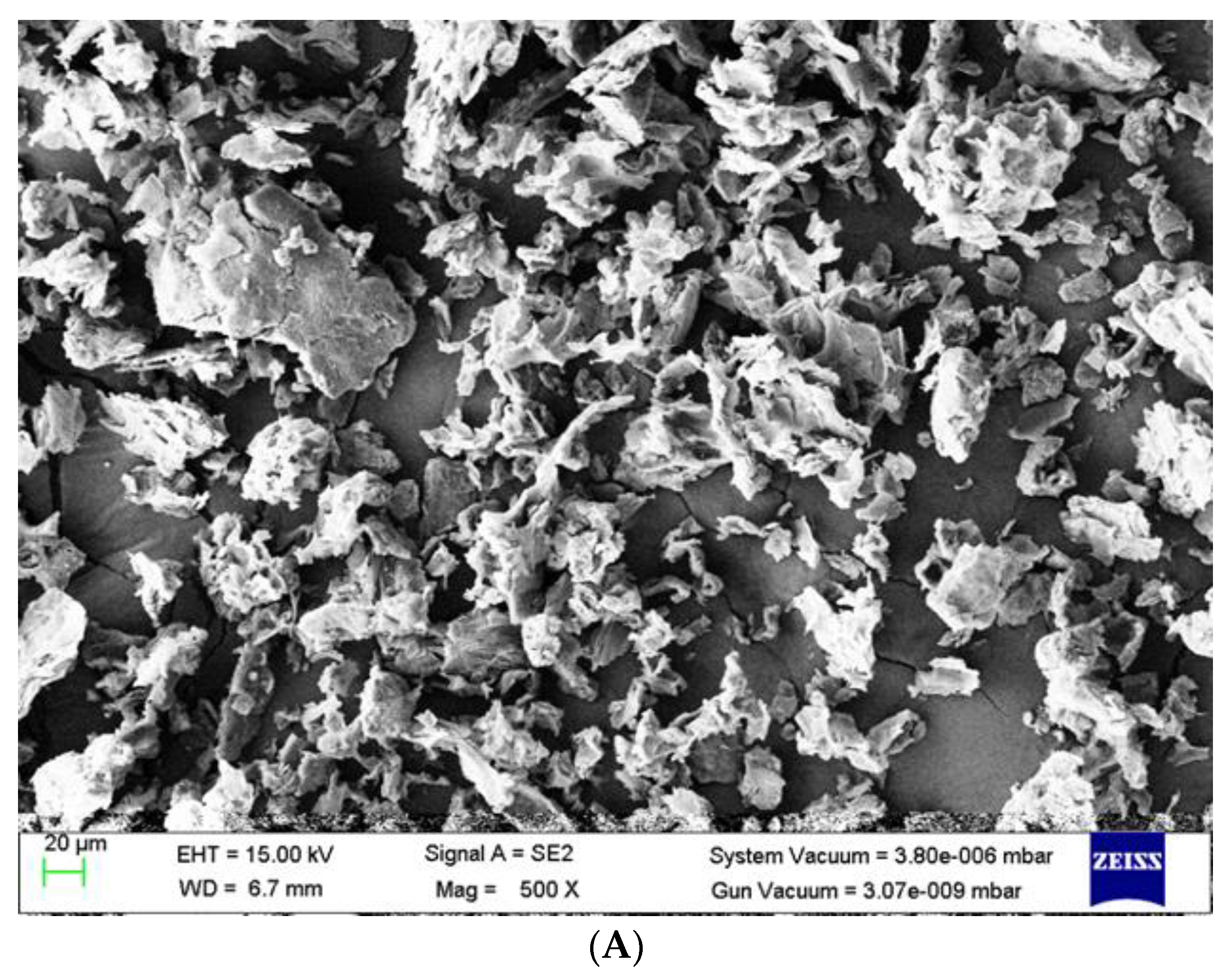
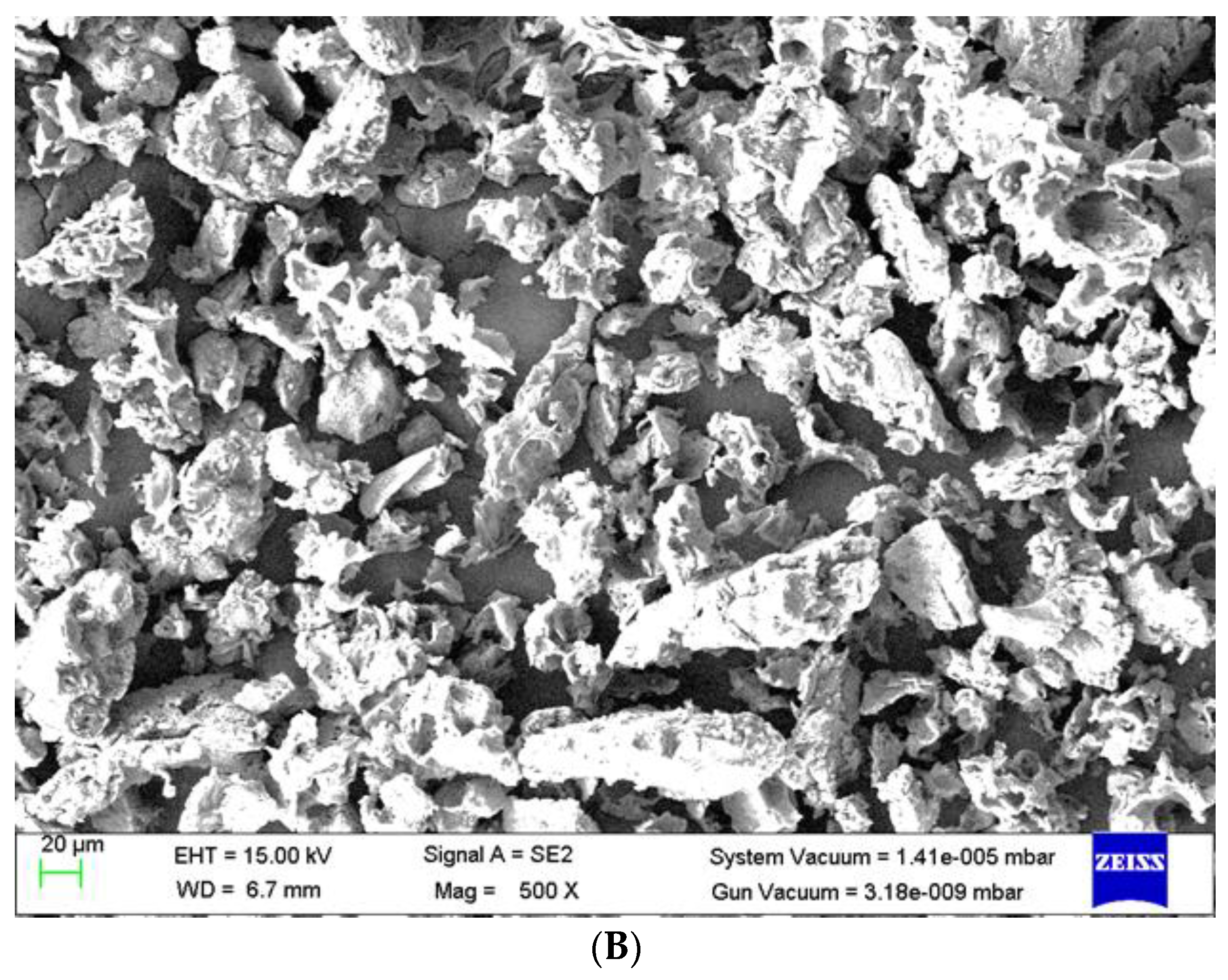
3.5. Surface Morphology of Adsorbent
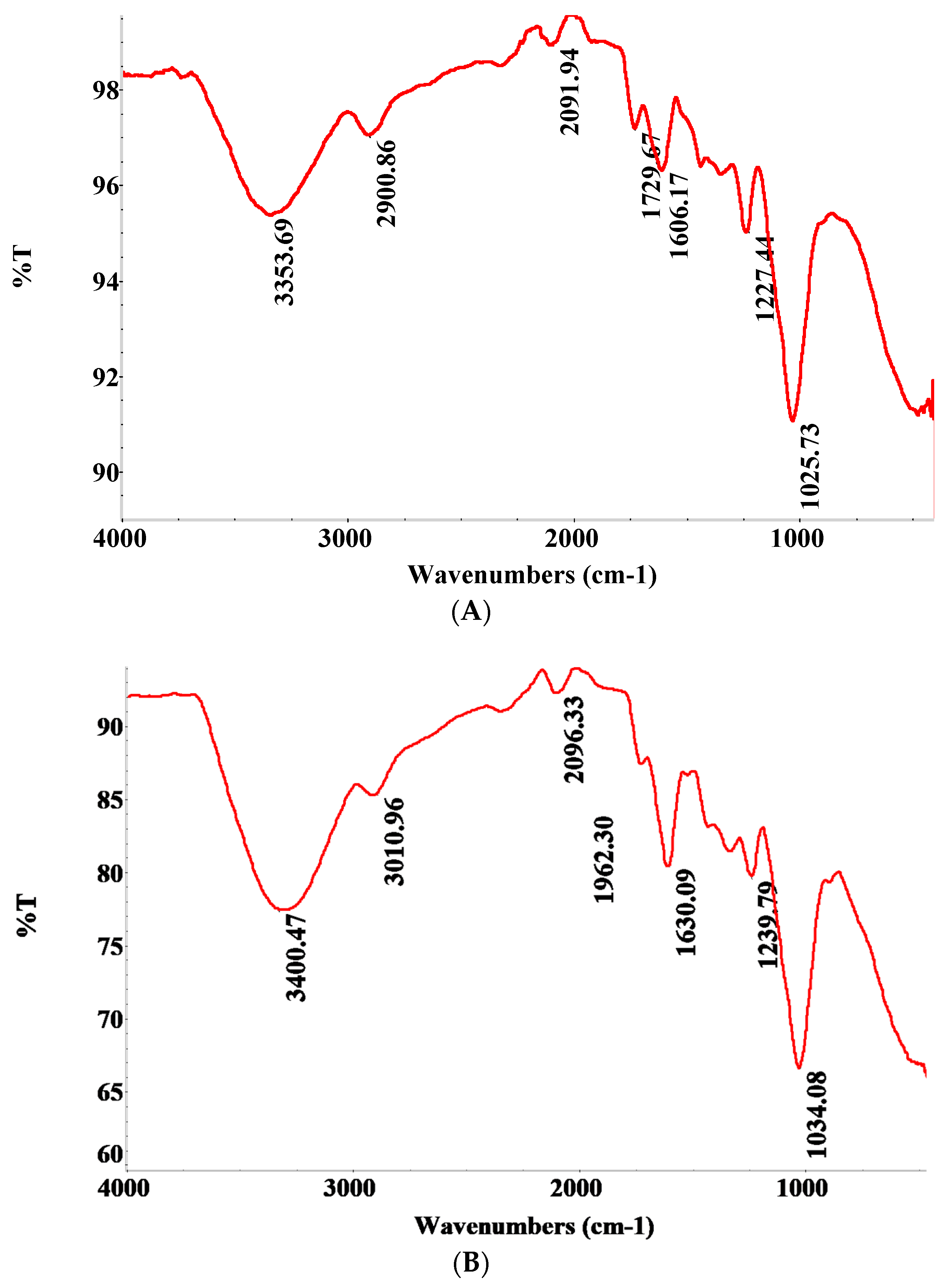
3.6. Fourier Transform Infrared (FTIR) Scan Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousavi, S.A.; Mehralian, M.; Khashij, M.; Parvaneh, S. Methylene Blue Removal from Aqueous Solutions by Activated Carbon Prepared from N. Microphyllum (AC-NM): RSM Analysis, Isotherms and Kinetic Studies. Glob. Nest J. 2017, 19, 697–705. [Google Scholar]
- World Health Organization. Preventing Disease through Healthy Environments: Exposure to Highly Hazardous Pesticides: A Major Public Health Concern; World Health Organization: Genève, Switzerland, 2019. [Google Scholar]
- Dotto, G.L.; McKay, G. Current Scenario and Challenges in Adsorption for Water Treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Aggarwal, S.C.; Kumar, S. Industrial Water Demand in India: Challenges and Implications for Water Pricing. India Infrastruct. Rep. 2011, 274–284. [Google Scholar]
- Kumar, P.; Rehab, H.; Hegde, K.; Brar, S.K.; Cledon, M.; Kermanshahi-Pour, A.; Duy, S.V.; Sauvé, S.; Surampalli, R.Y. Physical and biological removal of Microcystin-LR and other water contaminants in a biofilter using Manganese Dioxide coated sand and Graphene sand composites. Sci. Total Environ. 2020, 703, 135052. [Google Scholar] [CrossRef]
- Prasad, A.S.; Rao, K.V. Aerobic Biodegradation of Azo Dye by Bacillus Cohnii MTCC 3616; an Obligately Alkaliphilic Bacterium and Toxicity Evaluation of Metabolites by Different Bioassay Systems. Appl. Microbiol. Biotechnol. 2013, 97, 7469–7481. [Google Scholar] [CrossRef]
- Gedam, V.V.; Raut, P.; Chahande, A.; Pathak, P. Kinetic, thermodynamics and equilibrium studies on the removal of Congo red dye using activated teak leaf powder. Appl. Water Sci. 2019, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Gao, B.; Zhao, P.; Li, R.; Yue, Q.; Shon, H.K. Exploration of Polyepoxysuccinic Acid as a Novel Draw Solution in the Forward Osmosis Process. RSC Adv. 2017, 7, 30687–30698. [Google Scholar] [CrossRef] [Green Version]
- Lade, H.; Govindwar, S.; Paul, D. Mineralization and Detoxification of the Carcinogenic Azo Dye Congo Red and Real Textile Effluent by a Polyurethane Foam Immobilized Microbial Consortium in an Upflow Column Bioreactor. Int. J. Environ. Res. Public Health. 2015, 12, 6894–6918. [Google Scholar] [CrossRef] [Green Version]
- Jain, H.; Yadav, V.; Rajput, V.D.; Minkina, T.; Agarwal, S.; Garg, M.C. An Eco-Sustainable Green Approach for Biosorption of Methylene Blue Dye from Textile Industry Wastewater by Sugarcane Bagasse, Peanut Hull, and Orange Peel: A Comparative Study Through Response Surface Methodology, Isotherms, Kinetic, and Thermodynamics. Water Air Soil Pollut. 2022, 233, 187. [Google Scholar] [CrossRef]
- Khedmati, M.; Khodaii, A.; Haghshenas, H.F. A Study on Moisture Susceptibility of Stone Matrix Warm Mix Asphalt. Constr. Build. Mater. 2017, 144, 42–49. [Google Scholar] [CrossRef]
- Behbahani, M.; Moghaddam, M.R.A.; Arami, M. Techno-Economical Evaluation of Fluoride Removal by Electrocoagulation Process: Optimization through Response Surface Methodology. Desalination 2011, 271, 209–218. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The Uptake and Bioaccumulation of Heavy Metals by Food Plants, Their Effects on Plants Nutrients, and Associated Health Risk: A Review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- El-Ahmady El-Naggar, N.; Rabei, N.H.; El-Malkey, S.E. Eco-Friendly Approach for Biosorption of Pb2+ and Carcinogenic Congo Red Dye from Binary Solution onto Sustainable Ulva Lactuca Biomass. Sci. Rep. 2020, 10, 16021. [Google Scholar] [CrossRef] [PubMed]
- Ayele, A.; Getachew, D.; Kamaraj, M.; Suresh, A. Phycoremediation of Synthetic Dyes: An Effective and Eco-Friendly Algal Technology for the Dye Abatement. J. Chem. 2021, 2021, 9923643. [Google Scholar] [CrossRef]
- Almasi, A.; Omidi, M.; Khodadadian, M.; Khamutian, S.; Gholivand, M.B. Lead(II) and cadmium(II) removal from aqueous solution using processed walnut shell: Kinetic and equilibrium study. Toxicol. Environ. Chem. 2012, 94, 660–671. [Google Scholar] [CrossRef]
- Pollard, S.; Fowler, G.; Sollars, C.; Perry, R. Low-cost adsorbents for waste and wastewater treatment: A review. Sci. Total Environ. 1992, 116, 31–52. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S. Mesoporous Iraqi Red Kaolin Clay as an Efficient Adsorbent for Methylene Blue Dye: Adsorption Kinetic, Isotherm and Mechanism Study. Surf. Interfaces 2020, 18, 100422. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an Efficient Method for Removing Heavy Metals from Industrial Effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Maksoud, M.I.A.A.; Elgarahy, A.M.; Farrell, C.; Al-Muhtaseb, A.H.; Rooney, D.W.; Osman, A.I. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord. Chem. Rev. 2020, 403, 213096. [Google Scholar] [CrossRef]
- Idalina, D.; José, F.; Bruno, E. Liquefaction and Chemical Composition of Walnut Shells. Open Agric. 2022, 7, 249–256. [Google Scholar]
- El-Naggar, N.E.-A.; Hamouda, R.A.; Saddiq, A.A.; Alkinani, M.H. Simultaneous Bioremediation of Cationic Copper Ions and Anionic Methyl Orange Azo Dye by Brown Marine Alga Fucus Vesiculosus. Sci. Rep. 2021, 11, 3555. [Google Scholar] [CrossRef] [PubMed]
- Pulicharla, R.; Brar, S.K.; Rouissi, T.; Auger, S.; Drogui, P.; Verma, M.; Surampalli, R.Y. Degradation of Chlortetracycline in Wastewater Sludge by Ultrasonication, Fenton Oxidation, and Ferro-Sonication. Ultrason. Sonochem. 2017, 34, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Igwegbe, C.A.; Mohmmadi, L.; Ahmadi, S.; Rahdar, A.; Khadkhodaiy, D.; Dehghani, R.; Rahdar, S. Modeling of Adsorption of Methylene Blue Dye on Ho-CaWO4 Nanoparticles Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Techniques. MethodsX 2019, 6, 1779–1797. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, H.; Ghaemi, A.; Nasiri, M.; Karami, B. Experimental Modeling and Optimization of CO2 Absorption into Piperazine Solutions Using RSM-CCD Methodology. ACS Omega 2020, 5, 8432–8448. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, R.; Ghaedi, M.; Asfaram, A.; Dil, E.A.; Javadian, H. RSM-CCD Design of Malachite Green Adsorption onto Activated Carbon with Multimodal Pore Size Distribution Prepared from Amygdalus Scoparia: Kinetic and Isotherm Studies. Polyhedron 2019, 171, 464–472. [Google Scholar] [CrossRef]
- Sen, S.; Nandi, S.; Dutta, S. Application of RSM and ANN for Optimization and Modeling of Biosorption of Chromium (VI) Using Cyanobacterial Biomass. Appl. Water Sci. 2018, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Ali, J.; Garg, M.C. Biosorption of Methylene Blue Dye from Textile-Industry Wastewater onto Sugarcane Bagasse: Response Surface Modeling, Isotherms, Kinetic and Thermodynamic Modeling. J. Hazard. Toxic Radioact. Waste 2021, 25, 04020067. [Google Scholar] [CrossRef]
- Dbik, A.; El Messaoudi, N.; Bentahar, S.; el Khomri, M.; Lacherai, A.; Faska, N. Optimization of Methylene Blue Adsorption on Agricultural Solid Waste Using Box–Behnken Design (BBD) Combined with Response Surface Methodology (RSM) Modeling. Biointerface Res. Appl. Chem. 2022, 12, 4567–4583. [Google Scholar]
- Gupta, A.; Balomajumder, C. Simultaneous Adsorption of Cr (VI) and Phenol onto Tea Waste Biomass from Binary Mixture: Multicomponent Adsorption, Thermodynamic and Kinetic Study. J. Environ. Chem. Eng. 2015, 3, 785–796. [Google Scholar] [CrossRef]
- Repo, E.; Malinen, L.; Koivula, R.; Harjula, R.; Sillanpää, M. Capture of Co (II) from Its Aqueous EDTA-Chelate by DTPA-Modified Silica Gel and Chitosan. J. Hazard. Mater. 2011, 187, 122–132. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and Adsorption Application of Succinyl-Grafted Chitosan for the Simultaneous Removal of Zinc and Cationic Dye from Binary Hazardous Mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Esmaeili, A.; Beirami, P.; Ghasemi, S. Evaluation of the Marine Algae Gracilaria and Its Activated Carbon for the Adsorption of Ni (II) from Wastewater. E-J. Chem. 2011, 8, 1512–1521. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Bhat, A.H.; Buang, A. Biosorption of Transition Metals by Freely Suspended and Ca-Alginate Immobilised with Chlorella Vulgaris: Kinetic and Equilibrium Modeling. J. Clean. Prod. 2018, 171, 1361–1375. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Rabei, N.H. Bioprocessing Optimization for Efficient Simultaneous Removal of Methylene Blue and Nickel by Gracilaria Seaweed Biomass. Sci. Rep. 2020, 10, 17439. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.A.; Hamouda, R.A. Antimicrobial Potentialities of Streptomyces Lienomycini NEAE-31 against Human Pathogen Multidrug-Resistant Pseudomonas Aeruginosa. Int. J. Pharmacol. 2016, 12, 769–788. [Google Scholar]
- El-Naggar, N.E.-A.; El-Shweihy, N.M.; El-Ewasy, S.M. Identification and Statistical Optimization of Fermentation Conditions for a Newly Isolated Extracellular Cholesterol Oxidase-Producing Streptomyces Cavourensis Strain NEAE-42. BMC Microbiol. 2016, 16, 217. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, N.E.-A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, Extraction and Characterization of Chlorella Vulgaris Soluble Polysaccharides and Their Applications in AgNPs Biosynthesis and Biostimulation of Plant Growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.-X.; Reddy, A.M.; Matsuda, N.; Takatsu, A.; Kato, K.; Okada, T. Simultaneous Determination of Methylene Blue and New Methylene Blue by Slab Optical Waveguide Spectroscopy and Artificial Neural Networks. Anal. Chim. Acta 2003, 487, 109–116. [Google Scholar] [CrossRef]
- Singh, R.; Bhateria, R. Optimization and Experimental Design of the Pb2+ Adsorption Process on a Nano-Fe3O4-Based Adsorbent Using the Response Surface Methodology. ACS Omega 2020, 5, 28305–28318. [Google Scholar] [CrossRef]
- Ekpenyong, M.; Antai, S.; Asitok, A.; Ekpo, B. Response Surface Modeling and Optimization of Major Medium Variables for Glycolipopeptide Production. Biocatal. Agric. Biotechnol. 2017, 10, 113–121. [Google Scholar] [CrossRef]
- Aliyu, A. Synthesis, Electron Microscopy Properties and Adsorption Studies of Zinc (II) Ions (Zn2+) onto as-Prepared Carbon Nanotubes (CNTs) Using Box-Behnken Design (BBD). Sci. Afr. 2019, 3, e00069. [Google Scholar] [CrossRef]
- Rai, P.; Pandey, A.; Pandey, A. Optimization of Sugar Release from Banana Peel Powder Waste (BPPW) Using Box-Behnken Design (BBD): BPPW to Biohydrogen Conversion. Int. J. Hydrog. Energy 2019, 44, 25505–25513. [Google Scholar] [CrossRef]
- Sharma, S.; Malik, A.; Satya, S. Application of Response Surface Methodology (RSM) for Optimization of Nutrient Supplementation for Cr (VI) Removal by Aspergillus Lentulus AML05. J. Hazard. Mater. 2009, 164, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Haroun, S.A.; Oweis, E.A.; Sherief, A.A. Identification of Newly Isolated Talaromyces Pinophilus and Statistical Optimization of β-Glucosidase Production under Solid-State Fermentation. Prep. Biochem. Biotechnol. 2015, 45, 712–729. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; El-Bindary, A.A.; Nour, N.S. Statistical Optimization of Process Variables for Antimicrobial Metabolites Production by Streptomyces Anulatus NEAE-94 against Some Multidrug-Resistant Strains. Int. J. Pharmacol. 2013, 9, 322–334. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Keshtkar, A.R.; Safdari, J.; Abadi, Z. Biosorption of Nickel (II) from Aqueous Solution by Brown Algae: Equilibrium, Dynamic and Thermodynamic Studies. J. Hazard. Mater. 2010, 175, 304–310. [Google Scholar] [CrossRef]
- El Maghraby, D.M. Evaluation of Non-Viable Biomass of Laurencia Papillosa for Decolorization of Dye Waste Water. Afr. J. Biotechnol. 2013, 12, 2215–2223. [Google Scholar]
- Singh, B.; Bauddh, K.; Bux, F. Algae and Environmental Sustainability; Springer: Ranchi, India, 2015; Volume 7. [Google Scholar]
- Vijayaraghavan, J.; Pushpa, T.B.; Basha, S.S.; Jegan, J. Isotherm, kinetics and mechanistic studies of methylene blue biosorption onto red seaweed Gracilaria corticata. Desalination Water Treat. 2016, 57, 13540–13548. [Google Scholar] [CrossRef]
- Phugare, S.S.; Kalyani, D.C.; Surwase, S.N.; Jadhav, J.P. Ecofriendly Degradation, Decolorization and Detoxification of Textile Effluent by a Developed Bacterial Consortium. Ecotoxicol. Environ. Saf. 2011, 74, 1288–1296. [Google Scholar] [CrossRef]
- El Hassouni, H.; Abdellaoui, D.; El Hani, S.; Bengueddour, R. Biosorption of Cadmium (II) and Copper (II) from Aqueous Solution Using Red Alga (Osmundea Pinnatifida) Biomass. J. Mater. Environ. Sci. 2014, 5, 967–974. [Google Scholar]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Kleinübing, S.J.; Vieira, R.S.; Beppu, M.M.; Guibal, E.; da Silva, M.G.C. Characterization and Evaluation of Copper and Nickel Biosorption on Acidic Algae Sargassum Filipendula. Mater. Res. 2010, 13, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Mashkoor, F.; Nasar, A.; Asiri, A.M. Exploring the Reusability of Synthetically Contaminated Wastewater Containing Crystal Violet Dye Using Tectona Grandis Sawdust as a Very Low-Cost Adsorbent. Sci. Rep. 2018, 8, 8314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovchinnikov, O.V.; Evtukhova, A.V.; Kondratenko, T.S.; Smirnov, M.S.; Khokhlov, V.Y.; Erina, O.V. Manifestation of Intermolecular Interactions in FTIR Spectra of Methylene Blue Molecules. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Suresh, S.; Karthikeyan, S.; Jayamoorthy, K. FTIR and Multivariate Analysis to Study the Effect of Bulk and Nano Copper Oxide on Peanut Plant Leaves. J. Sci. Adv. Mater. Devices 2016, 1, 343–350. [Google Scholar] [CrossRef]
| Particulars | Methylene Blue (MB) |
|---|---|
| Molecular Structure | 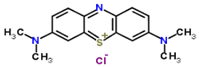 |
| Chemical Formula | C16H18ClN3S |
| Molecular Weight | 319.85 (g/mol) |
| Class | Cationic thiazine dye |
| λ max (nm) | 664 |
| Colour index name | Basic Blue 9 (BG9) |
| CAS number | 61-73-4 |
| Parameters | Content (%) | |
|---|---|---|
| Ashes | 1.32 ± 0.06 | |
| Extractive | Dichloromethane | 2.94 ± 0.41 |
| Ethanol | 2.71 ± 0.08 | |
| Water | 4.56 ± 0.50 | |
| α -Cellulose | 30.36 ± 0.68 | |
| Klason Lignin | 34.98 ± 0.14 | |
| Hemicelluloses | 24.85 ± 0.53 | |
| Code | Factors | Ranges and Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| A | Biosorbent dose (gm) | 0.5 | 1 | 1.5 | 2 | 2.5 |
| B | Dye concentration (mg/L) | 10 | 20 | 30 | 40 | 50 |
| C | Dye solution pH | 4 | 5 | 6 | 7 | 8 |
| D | Temperature (°C) | 15 | 20 | 25 | 30 | 35 |
| Run | A Biosorbent Dose (gm) | B Initial Dye Conc. (mg/L) | C Initial pH | D Temperature (°C) | Dye Removal (%) | |
|---|---|---|---|---|---|---|
| Actual | Predicted | |||||
| 1 | 1.5 | 50 | 6 | 25 | 95.1 | 94.6 |
| 2 | 2 | 20 | 7 | 30 | 94.16 | 94.59 |
| 3 | 1.5 | 30 | 6 | 35 | 94.93 | 94.46 |
| 4 | 1 | 40 | 5 | 20 | 93.52 | 93.76 |
| 5 | 1.5 | 30 | 6 | 25 | 97.8 | 97.3 |
| 6 | 1.5 | 30 | 6 | 25 | 97.27 | 97.3 |
| 7 | 1 | 20 | 5 | 30 | 93.7 | 94.25 |
| 8 | 5 | 30 | 6 | 25 | 94.17 | 93.92 |
| 9 | 1.5 | 30 | 8 | 25 | 95.68 | 95.61 |
| 10 | 1 | 40 | 7 | 30 | 92.88 | 93.28 |
| 11 | 2 | 20 | 5 | 30 | 96.03 | 95.41 |
| 12 | 2 | 40 | 7 | 20 | 97.03 | 97.14 |
| 13 | 1.5 | 30 | 6 | 15 | 96.19 | 96.56 |
| 14 | 1 | 20 | 7 | 30 | 94.27 | 93.99 |
| 15 | 1 | 20 | 5 | 20 | 95.48 | 94.92 |
| 16 | 2 | 40 | 5 | 20 | 96.31 | 96.03 |
| 17 | 1.5 | 30 | 6 | 25 | 97.32 | 97.3 |
| 18 | 1.5 | 30 | 6 | 25 | 97.1 | 97.3 |
| 19 | 1.5 | 30 | 6 | 25 | 96.92 | 97.3 |
| 20 | 2 | 20 | 7 | 20 | 97.01 | 96.41 |
| 21 | 1 | 40 | 7 | 20 | 95.36 | 95.42 |
| 22 | 1 | 40 | 5 | 30 | 93.43 | 93.48 |
| 23 | 1 | 20 | 7 | 20 | 96.37 | 96.51 |
| 24 | 1.5 | 30 | 6 | 25 | 97.39 | 97.3 |
| 25 | 2 | 40 | 5 | 30 | 95.94 | 96.46 |
| 26 | 2 | 20 | 5 | 20 | 95.1 | 95.37 |
| 27 | 1.5 | 30 | 4 | 25 | 94.8 | 94.77 |
| 28 | 2.5 | 30 | 6 | 25 | 96.66 | 96.8 |
| 29 | 2 | 40 | 7 | 30 | 95.71 | 95.71 |
| 30 | 1.5 | 10 | 6 | 25 | 94.24 | 94.64 |
| A (gm) | B (mg/L) | C | D (°C) | Dye Removal (%) | |
|---|---|---|---|---|---|
| Actual | Predicted | ||||
| 18.24 | 31.71 | 6.4 | 21.7 | 97.74 | 97.3 |
| Source | Degree of Freedom | Mean Square | Sum of Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 14 | 3.61 | 50.48 | 15.03 | <0.0001 |
| A | 1 | 12.41 | 12.41 | 51.71 | |
| B | 1 | 0.0022 | 0.0022 | 0.0093 | |
| C | 1 | 1.06 | 1.06 | 4.42 | |
| D | 1 | 6.57 | 6.57 | 27.38 | |
| AB | 1 | 3.34 | 3.34 | 13.91 | |
| AC | 1 | 0.3093 | 0.3093 | 1.29 | |
| AD | 1 | 0.4973 | 0.4973 | 2.07 | |
| BC | 1 | 0.0042 | 0.0042 | 0.0177 | |
| BD | 1 | 0.1512 | 0.1512 | 0.6299 | |
| CD | 1 | 3.46 | 3.46 | 14.41 | |
| A² | 1 | 6.43 | 6.43 | 26.79 | |
| B² | 1 | 12.34 | 12.34 | 51.44 | |
| C² | 1 | 7.61 | 7.61 | 31.73 | |
| D² | 1 | 5.49 | 5.49 | 22.89 | |
| Residual | 15 | 0.2399 | 3.6 | ||
| Lack of Fit | 10 | 0.3157 | 3.16 | 3.57 | 0.0863 |
| Pure error | 5 | 0.0884 | 0.4421 | ||
| Total | 29 | 54.08 |
| Source | Std. Dev. | R² | Adjusted R² | Predicted R² | PRESS |
|---|---|---|---|---|---|
| Linear | 1.17 | 0.3706 | 0.2699 | 0.179 | 44.39 |
| 2FI | 1.18 | 0.514 | 0.2582 | −0.0049 | 54.34 |
| Quadratic | 0.4898 | 0.9334 | 0.8713 | 0.6519 | 18.82 |
| Cubic | 0.5303 | 0.9636 | 0.8492 | −3.0769 | 220.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Rajput, V.D.; Minkina, T.; Rajput, P.; Sharma, P.; Verma, A.K.; Agarwal, S.; Garg, M.C. Application of RSM for Bioremoval of Methylene Blue Dye from Industrial Wastewater onto Sustainable Walnut Shell (Juglans regia) Biomass. Water 2022, 14, 3651. https://doi.org/10.3390/w14223651
Kumari S, Rajput VD, Minkina T, Rajput P, Sharma P, Verma AK, Agarwal S, Garg MC. Application of RSM for Bioremoval of Methylene Blue Dye from Industrial Wastewater onto Sustainable Walnut Shell (Juglans regia) Biomass. Water. 2022; 14(22):3651. https://doi.org/10.3390/w14223651
Chicago/Turabian StyleKumari, Sheetal, Vishnu D. Rajput, Tatiana Minkina, Priyadarshani Rajput, Pinki Sharma, Anoop Kumar Verma, Smriti Agarwal, and Manoj Chandra Garg. 2022. "Application of RSM for Bioremoval of Methylene Blue Dye from Industrial Wastewater onto Sustainable Walnut Shell (Juglans regia) Biomass" Water 14, no. 22: 3651. https://doi.org/10.3390/w14223651
APA StyleKumari, S., Rajput, V. D., Minkina, T., Rajput, P., Sharma, P., Verma, A. K., Agarwal, S., & Garg, M. C. (2022). Application of RSM for Bioremoval of Methylene Blue Dye from Industrial Wastewater onto Sustainable Walnut Shell (Juglans regia) Biomass. Water, 14(22), 3651. https://doi.org/10.3390/w14223651










