Copepod Assemblages at the Base of Mangrove Food Webs during a Severe Drought
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Intra-Annual and Spatial Sampling and Analysis
2.3. Intra-Annual Environmental Analysis: Physicochemical Factors and Phytoplankton Pigments
2.4. Data Analysis
3. Results
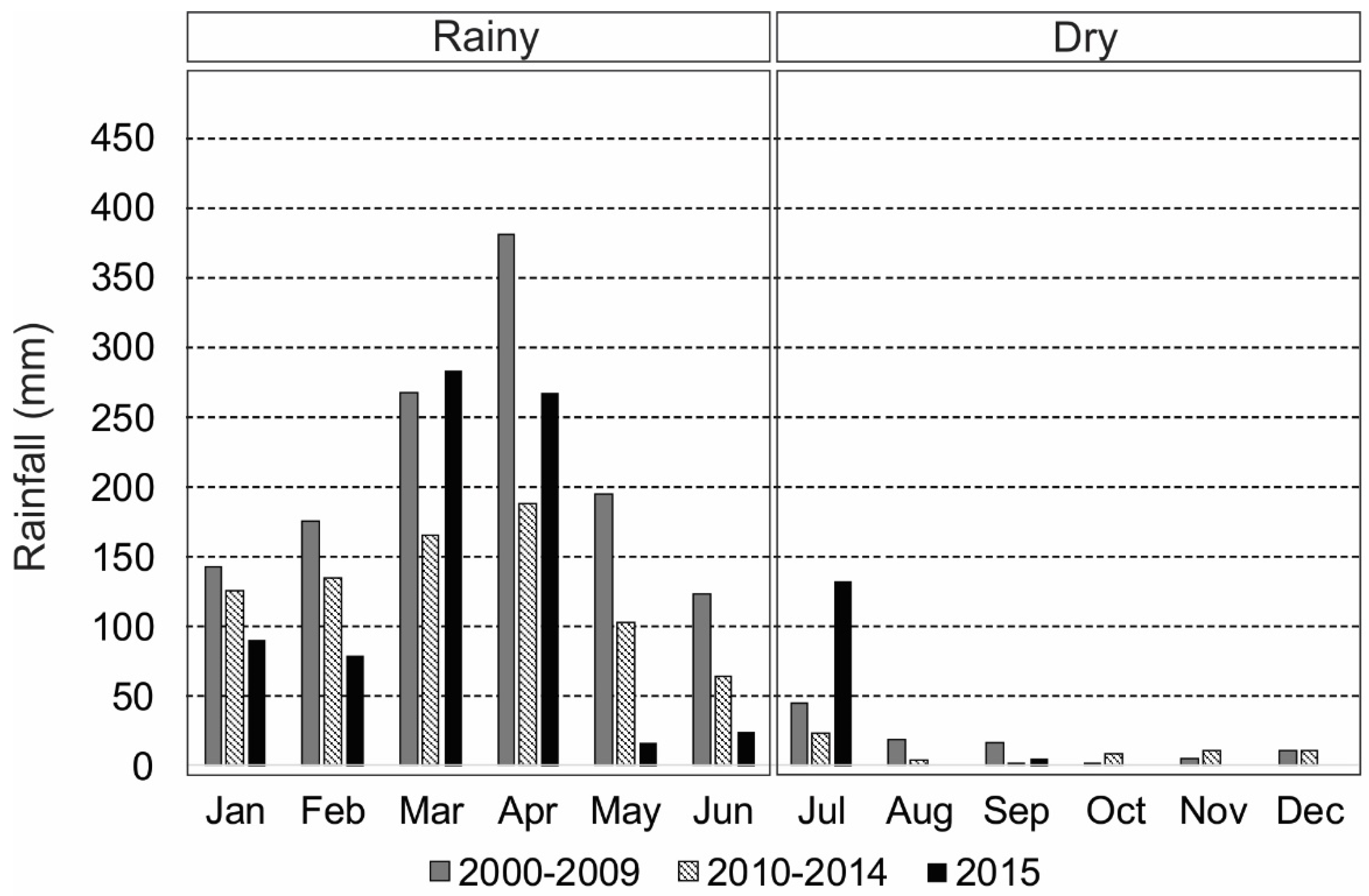
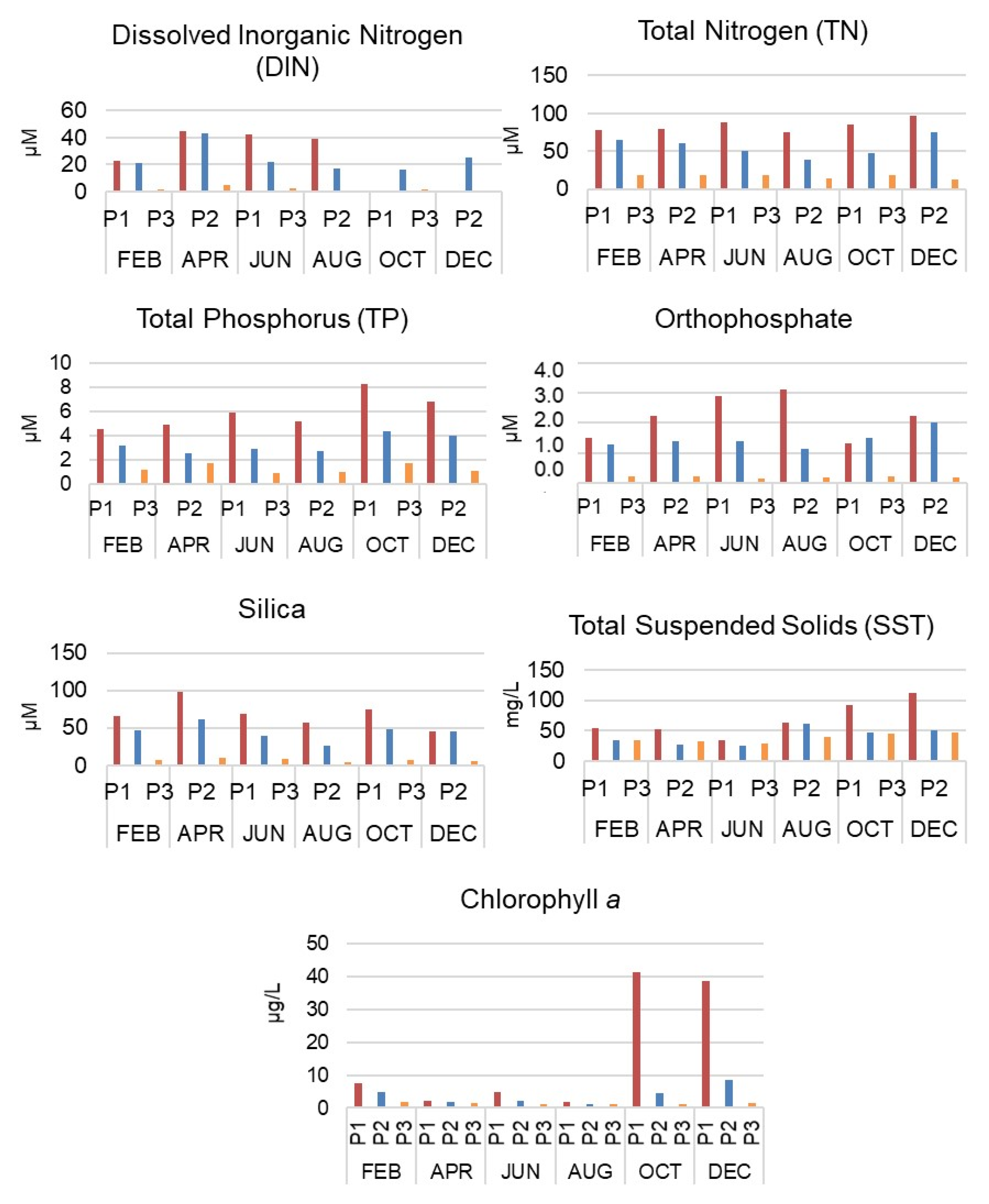
3.1. Intra-Annual and Spatial Variation: Environmental Data
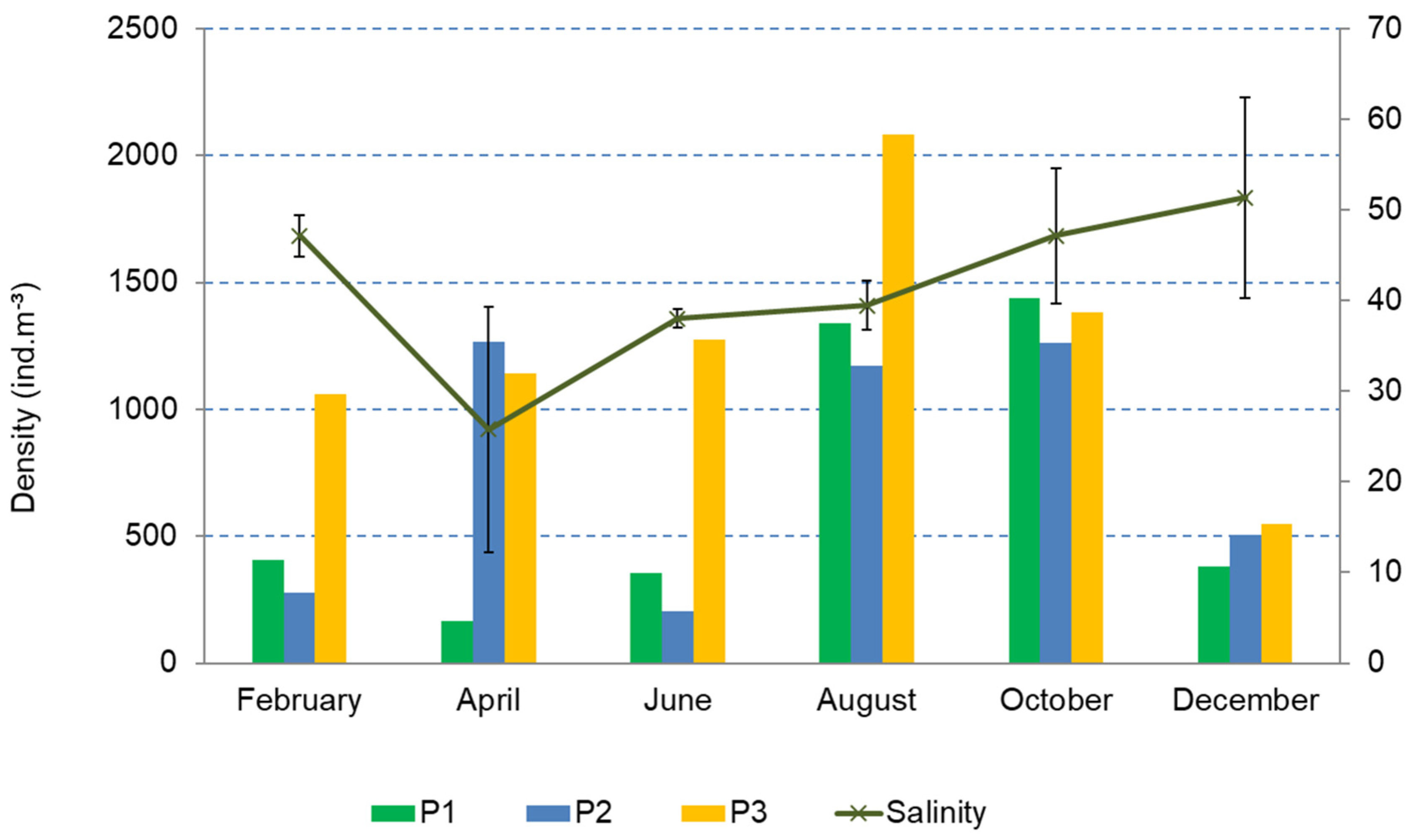
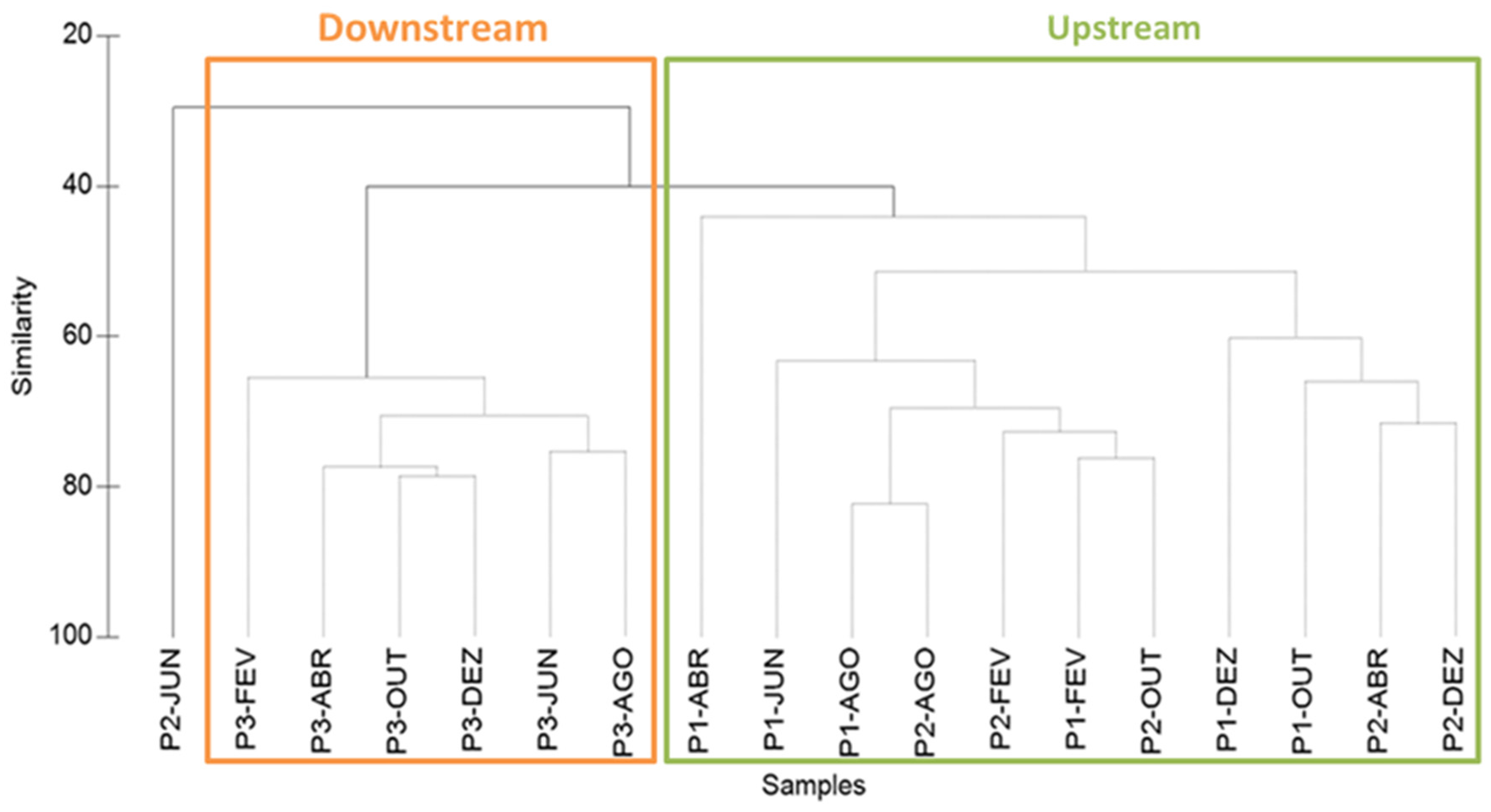
3.2. Structure and Composition of the Copepod Assemblage
3.3. Copepod Assemblage and Environmental Factors
3.4. Functional Groups of Copepods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ara, K. Temporal variability and production of the planktonic copepod community in the Cananéia Lagoon estuarine system, São Paulo, Brazil. Zool. Stud. 2004, 43, 179–186. [Google Scholar]
- Hemraj, D.A.; Hossain, M.A.; Ye, Q.; Qin, J.G.; Leterme, S.C. Plankton bioindicators of environmental conditions in coastal lagoons. Estuar. Coast. Shelf Sci. 2017, 184, 102–114. [Google Scholar] [CrossRef]
- Gismervik, I. Top-down impact by copepods on ciliate numbers and persistence depends on copepod and ciliate species composition. J. Plankton Res. 2006, 28, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Bradford-Grieve, J.M. Copepoda. In South Atlantic Zooplankton; Boltovskoy, D., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1999; Volume 2, pp. 869–1098. [Google Scholar]
- Turner, J.T. The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool. Stud. 2004, 43, 255–266. [Google Scholar]
- Calbet, A. The trophic roles of microzooplankton in marine systems. ICES J. Mar. Sci. 2008, 65, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Brun, P.; Payne, M.R.; Kiørboe, T. A trait database for marine copepods. Earth Syst. Sci. Data 2017, 9, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, F.; Vogt, M.; Righetti, D.; Guilhaumon, F.; Ayata, S.D. Do functional groups of planktonic copepods differ in their ecological niches? J. Biogeogr. 2018, 45, 604–616. [Google Scholar] [CrossRef]
- Nagarathinam, A.; Retnamma, J.; Loganathan, J.; Singaram, P.; Arayillath, A.; Jose, A.K. Impact of human-altered hydrographical setting on the Copepod community structure in an extensive tropical estuary along the southwest coast of India. Oceanologia 2021, 63, 115–132. [Google Scholar] [CrossRef]
- Paul, S.; Karan, S.; Bhattacharya, B.D. Daily variability of copepods after successive tropical cyclones in the Ganges River estuary of India. Estuar. Coast. Shelf Sci. 2020, 246, 107048. [Google Scholar] [CrossRef]
- Breckenridge, J.K.; Bollens, S.M.; Rollwagen-Bollens, G.; Roegner, G.C. Plankton assemblage variability in a river-dominated temperate estuary during late spring (high-flow) and late summer (low-flow) periods. Estuaries Coast. 2015, 38, 93–103. [Google Scholar] [CrossRef]
- Belmonte, G.; Rubino, F. Cysts and resting eggs from marine zooplankton: Dimension of the phenomenon, physiology of rest, and ecological and biogeographic implications. In Dormancy in Aquatic Organisms. Theory, Human Use and Modeling; Springer: Cham, Switzerland, 2019; pp. 71–94. [Google Scholar] [CrossRef]
- Carrasco, N.K.; Perissinotto, R. Development of a halotolerant community in the St. Lucia Estuary (South Africa) during a hypersaline phase. PLoS ONE 2012, 7, e29927. [Google Scholar] [CrossRef]
- Largier, J. Low-inflow estuaries: Hypersaline, inverse and thermal scenarios. In Contemporary Issues in Estuarine Physics; Cambridge University Press: Cambridge, UK, 2010; pp. 247–272. [Google Scholar] [CrossRef]
- Abril, G.; Libardoni, B.G.; Brandini, N.; Cotovicz, L.C., Jr.; Medeiros, P.R.; Cavalcante, G.H.; Knoppers, B.A. Thermodynamic uptake of atmospheric CO2 in the oligotrophic and semiarid São Francisco estuary (NE Brazil). Mar. Chem. 2021, 233, 103983. [Google Scholar] [CrossRef]
- Ezhilarasan, P.; Basuri, C.K.; Gera, A.; Kumaraswami, M.; Rao, V.R.; Murthy, M.R. Mesozooplankton distribution in relation to the salinity gradient in a tropical hypersaline lake. J. Sea Res. 2021, 178, 102138. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Dittmann, S.R.; Whitfield, A.K.; Withers, K.; Hoeksema, S.D.; Potter, I.C. Hypersalinity: Global distribution, causes, and present and future effects on the biota of estuaries and lagoons. In Coasts and Estuaries; Elsevier: Amsterdam, The Netherlands, 2019; pp. 523–546. [Google Scholar] [CrossRef]
- Stocker, T.; Plattner, G.; Dahe, Q. IPCC climate change 2013: The physical science basis-findings and lessons learned. In Proceedings of the EGU General Assembly Conference Abstracts 2014, Vienna, Austria, 27 April–2 May 2014; p. 17003. [Google Scholar]
- Soares, M.D.O.; Campos, C.C.; Carneiro, P.B.M.; Barroso, H.S.; Marins, R.V.; Teixeira, C.E.P.; Menezes, M.O.B.; Pinheiro, L.D.S.; Viana, M.B.; Feitosa, C.V.; et al. Challenges and perspectives for the Brazilian semi-arid coast under global environmental changes. Perspect. Ecol. Conserv. 2021, 19, 267–278. [Google Scholar] [CrossRef]
- Andrade, M.P.; Magalhães, A.; Pereira, L.C.; Flores-Montes, M.J.; Pardal, E.C.; Andrade, T.P.; Costa, R.M. Effects of a La Niña event on hydrological patterns and copepod community structure in a shallow tropical estuary (Taperaçu, Northern Brazil). J. Mar. Syst. 2016, 164, 128–143. [Google Scholar] [CrossRef]
- Atique, P.; da Costa, K.G.; Monteiro, M.C.; Pereira, L.C.; da Costa, R.M. Copepod assemblages in a highly dynamic equatorial estuary on the Brazilian Amazon Coast. Mar. Ecol. 2017, 38, e12385. [Google Scholar] [CrossRef]
- Marengo, J.A.; Torres, R.R.; Alves, L.M. Drought in Northeast Brazil—Past, present, and future. Theor. Appl. Climatol. 2016, 129, 1189–1200. [Google Scholar] [CrossRef]
- de Sousa Barroso, H.; Tavares, T.C.L.; de Oliveira Soares, M.; Garcia, T.M.; Rozendo, B.; Vieira, A.S.C.; Viana, P.B.; Pontes, T.M.; Ferreira, T.J.T.; Pereira Filho, J.; et al. Intra-annual variability of phytoplankton biomass and nutrients in a tropical estuary during a severe drought. Estuarine Coastal Shelf Sci. 2018, 213, 283–293. [Google Scholar] [CrossRef]
- Signorini, S.R.; De Miranda, L.B. Tidal and low-frequency currents near the shelf-break: Northeastern coast of Brazil. J. Phys. Oceanogr. 1983, 13, 2107–2115. [Google Scholar] [CrossRef]
- Dias, C.O.; Bonecker, S.L.C. The copepod assemblage (Copepoda: Crustacea) on the inner continental shelf adjacent to Camamu Bay, northeast Brazil. Zoologia 2009, 26, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.N.B.; Morais, J.O. Avaliação do Uso Potencial de Áreas Estuarinas a Partir da Identificação e Caracterização do Comportamento de Variáveis Hidro-Climáticas, Oceanográficas e Ambientais—Estudo de Caso: Rio Piranji-CE; FUNCEME: Fortaleza, Brazil, 2007; p. 219. [Google Scholar]
- Paula, D.P.; Pinheiro, L.S.; Morais, J.O.; Dias, C.B. A hidrodinâmica do estuário do rio Piranji, Ceará-Brasil: Implicações para o Manejo e Uso Sustentável. In XI Congresso da ABEQUA; Universidade Federal do Pará: Belém, Brazil; Volume 6, pp. 1–3.
- Schettini, C.A.; Valle-Levinson, A.; Truccolo, E.C. Circulation and transport in short, low-inflow estuaries under anthropogenic stresses. Reg. Stud. Mar. Sci. 2017, 10, 52–64. [Google Scholar] [CrossRef]
- Silva, J.M.O.; da Silva, E.V. Análise Geoambiental do baixo curso da Bacia Hidrográfica do Rio Pirangi–CE. Rev. Geonorte 2012, 3, 593–605. [Google Scholar]
- Savenije, H.H.; Pagès, J. Hypersalinity: A dramatic change in the hydrology of Sahelian estuaries. J. Hydrol. 1992, 135, 157–174. [Google Scholar] [CrossRef]
- Funceme. Available online: https://funceme.br/app/calendario/produto/municipios/maxima/2016 (accessed on 13 February 2016).
- Omori, M.; Ikeda, T. Methods in Marine Zooplankton Ecology; John Wiley & Sons: New York, NY, USA, 1984; Volume 332. [Google Scholar]
- Tregouboff, G.; Rose, M. Manuel de Planctonologia Méditerraneénne; C.N.R.S.: Paris, France, 1957. [Google Scholar]
- Bjönberg, T.K.S. Copepoda. In Atlas del Zooplancton del Atlántico Sudoccidental y Métodos de Trabajos con el Zooplancton Marino; Boltovskoy, D., Ed.; INIDEP: Mar del Plata, Argentina, 1981; pp. 587–679. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Baumgarten, M.G.Z.; De Barros Rocha, J.M.; Niencheski, L.F.H. Manual de Análises em Oceanografia Química; Furg: Rio Grande, Brazil, 1996; p. 132. [Google Scholar]
- UNESCO. Chemical Methods for Use in Marine Environment Monitoring; Intergovernmental Oceanographic Commission: Paris, France, 1983; Volume 56. [Google Scholar]
- Rossi, S.; Soares, M.D.O. Effects of El Niño on the coastal ecosystems and their related services. Mercator 2017, 16, e16030. [Google Scholar] [CrossRef] [Green Version]
- Marengo, J.A.; Alves, L.M.; Alvala, R.; Cunha, A.P.; Brito, S.; Moraes, O.L. Climatic characteristics of the 2010-2016 drought in the semiarid Northeast Brazil region. Anais da Academia Brasileira de Ciências 2017, 90, 1973–1985. [Google Scholar] [CrossRef]
- Campos, J.N.B.; Alves, J.M.B.; Servain, J. Relationship between ocean climatic variability and rain-fed agriculture in northeast Brazil. Clim. Res. 2009, 38, 225–236. [Google Scholar]
- Lawrence, D.; Valiela, I.; Tomasky, G. Estuarine calanoid copepod abundance in relation to season, salinity, and land-derived nitrogen loading, Waquoit Bay, MA. Estuar. Coast. Shelf Sci. 2004, 61, 547–557. [Google Scholar] [CrossRef]
- Matias-Peralta, H.; Yusoff, F.M.; Shariff, M.; Arshad, A. Effects of some environmental parameters on the reproduction and development of a tropical marine harpacticoid copepod Nitocra affinis f. californica Lang. Mar. Pollut. Bull. 2005, 51, 722–728. [Google Scholar] [CrossRef]
- Lance, J. The salinity tolerance of some estuarine planktonic copepods. Limnol. Oceanogr. 1963, 8, 440–449. [Google Scholar] [CrossRef]
- Rippingale, R.J.; Hodgkin, E.P. Food availability and salinity tolerance in a brackish water copepod. Mar. Freshwater Res. 1977, 28, 1–7. [Google Scholar] [CrossRef]
- Froneman, P.W. Seasonal changes in zooplankton biomass and grazing in a temperate estuary, South Africa. Estuar. Coast. Shelf Sci. 2001, 52, 543–553. [Google Scholar] [CrossRef]
- Costa, K.G.; Pereira, L.C.C.; Costa, R.M. Short and long-term temporal variation of the zooplankton in a tropical estuary (Amazon region, Brazil). Bol. Mus. Emílio Goeldi Ciências Nat. 2008, 3, 127–141. [Google Scholar] [CrossRef]
- Champalbert, G.; Pagano, M.; Arfi, R.; Chevalier, C. Effects of the sandbar breaching on hydrobiological parameters and zooplankton communities in the Senegal River Estuary (West Africa). Mar. Pollut. Bull. 2014, 82, 86–100. [Google Scholar] [CrossRef]
- Blanda, E.; Drillet, G.; Huang, C.C.; Hwang, J.S.; Højgaard, J.K.; Jakobsen, H.H.; Rayner, T.A.; Su, H.M.; Hansen, B.W. An analysis of how to improve production of copepods as live feed from tropical Taiwanese outdoor aquaculture ponds. Aquaculture 2017, 479, 432–441. [Google Scholar] [CrossRef]
- Campos, C.C.; Garcia, T.M.; Neumann-Leitão, S.; Soares, M.O. Ecological indicators and functional groups of copepod assemblages. Ecol. Indic. 2017, 83, 416–426. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Halsey, S.H. An Introduction to Copepod Diversity; Ray Society: London, UK, 2004. [Google Scholar]
- Sterza, J.M.; Fernandes, L.L. Zooplankton community of the Vitória Bay estuarine system (Southeastern Brazil): Characterization during a three-year study. Braz. J. Oceanogr. 2006, 54, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, A.; Leite, N.D.R.; Silva, J.G.; Pereira, L.C.; Costa, R.M.D. Seasonal variation in the copepod community structure from a tropical Amazon estuary, Northern Brazil. An. Acad. Bras.Ciências 2009, 81, 187–197. [Google Scholar] [CrossRef] [Green Version]
- da Conceicao, L.R.; Demoner, L.E.; Pereira, J.B.; Perassoli, F.; Ghisolfi, R.D.; Bastos, A.C.; Junior, C.D.; Lázaro, G.C.S.; do Nascimento Lemos, K.; Fernandes, L.F.L. Copepod community structure after a mining dam disaster in the Southwestern Atlantic Ocean. Estuar. Coast. Shelf Sci. 2021, 254, 107325. [Google Scholar] [CrossRef]
- Truong, T.; Nguyen, C.; Lam, N.N.; Jensen, K. Seasonal and spatial distribution of mesozooplankton in a tropical estuary, Nha Phu, South Central Viet Nam. Biologia 2014, 69, 80–91. [Google Scholar] [CrossRef]
- Tseng, L.C.; Hsiao, S.H.; Sarkar, S.K.; Bhattacharya, B.D.; Chen, Q.C.; Hwang, J.S. Influence of Kuroshio water on the annual copepod community structure in an estuary in the northwest Pacific Ocean. Cont. Shelf Res. 2016, 118, 165–176. [Google Scholar] [CrossRef]
- Rahman, M.M. Factors influencing the vertical distribution of copepods in a tropical oligotrophic estuary, South China sea. Estuar. Coast. Shelf Sci. 2021, 250, 107165. [Google Scholar] [CrossRef]
- Sloterdijk, H.; Brehmer, P.; Sadio, O.; Müller, H.; Döring, J.; Ekau, W. Composition and structure of the larval fish community related to environmental parameters in a tropical estuary impacted by climate change. Estuar. Coast. Shelf Sci. 2017, 197, 10–26. [Google Scholar] [CrossRef]
- Rosa, J.D.C.L.D.; Batista, L.L.; Monteiro-Ribas, W.M. Tracking of spatial changes in the structure of the zooplankton community according to multiple abiotic factors along a hypersaline lagoon. Nauplius 2020, 28. [Google Scholar] [CrossRef]
- Toumi, N.; Ayadi, H.; Abid, O.; Carrias, J.F.; Sime-Ngando, T.; Boukhris, M.; Bouain, A. Zooplankton distribution in four ponds of different salinity: A seasonal study in the solar salterns of Sfax (Tunisia). Hydrobiologia 2005, 534, 1–9. [Google Scholar] [CrossRef]
- Cyrus, D.; Jerling, H.; MacKay, F.; Vivier, L. Lake St Lucia, Africa’s largest estuarine lake in crisis: Combined effects of mouth closure, low levels and hypersalinity. S. Afr. J. Sci. 2011, 107, 1–13. [Google Scholar] [CrossRef]
- Moscatello, S.; Belmonte, G. Egg banks in hypersaline lakes of the South-East Europe. Saline Syst. 2009, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Moscatello, S.; Rubino, F.; Saracino, O.D.; Fanelli, G.; Belmonte, G.; Boero, F. Plankton biodiversity around the Salento Peninsula (South East Italy): An integrated water/sediment approach. Sci. Mar. 2004, 68, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Turner, J.T. Predation and respiration by the small cyclopoid copepod Oithona similisr: How important is feeding on ciliates and heterotrophic flagellates? J. Plankton Res. 1997, 74, 1275–1288. [Google Scholar] [CrossRef] [Green Version]
- Calbet, A.; Landry, M.R.; Scheinberg, R.D. Copepod grazing in a subtropical bay: Species-specific responses to a midsummer increase in nanoplankton standing stock. Mar. Ecol. Prog. Ser. 2000, 193, 75–84. [Google Scholar] [CrossRef]
- Mauchline, J. The Biology of Calanoid Copepods. Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 33. [Google Scholar]
- Satapoomin, S.; Nielsen, T.G.; Hansen, P.J. Andaman Sea copepods: Spatio-temporal variations in biomass and production, and role in the pelagic food web. Mar. Ecol. Prog. Ser. 2004, 274, 99–122. [Google Scholar] [CrossRef]
- Belmonte, G. The Suspected Contradictory Role of Parental Care in the Adaption of Planktonic Calanoida to Temporary Freshwater. Water 2021, 13, 100. [Google Scholar] [CrossRef]
- Hirst, A.G.; Kiørboe, T. Mortality of marine planktonic copepods: Global rates and patterns. Mar. Ecol. Prog. Ser. 2002, 230, 195–209. [Google Scholar] [CrossRef] [Green Version]
- Webb, D.G.; Weaver, A.J. Predation and the evolution of free spawning in marine calanoid copepods. Oikos 1988, 51, 189–192. [Google Scholar] [CrossRef]
- Castellani, C.; Irigoien, X.; Harris, R.P.; Lampitt, R.S. Feeding and egg production of Oithona similis in the North Atlantic. Mar. Ecol. Prog. Ser. 2005, 288, 173–182. [Google Scholar] [CrossRef]
- Almeda, R.; Augustin, C.B.; Alcaraz, M.; Calbet, A.; Saiz, E. Feeding rates and gross growth efficiencies of larval developmental stages of Oithona davisae (Copepoda, Cyclopoida). J. Exp. Mar. Biol. Ecol. 2010, 387, 24–35. [Google Scholar] [CrossRef]
- Nielsen, T.G.; Sabatini, M. Role of cyclopoid copepods Oithona spp. in North Sea plankton communities. Mar. Ecol. Prog. Ser. 1996, 139, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Terol, S.; Mckinnon, A.D.; Saiz, E. Feeding and egg production of Oithona spp. in tropical waters of North Queensland, Australia. J. Plankton Res. 2014, 36, 1047–1059. [Google Scholar] [CrossRef] [Green Version]
- Statham, P.J. Nutrients in estuaries—An overview and the potential impacts of climate change. Sci. Total Environ. 2012, 434, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Robins, P.E.; Skov, M.W.; Lewis, M.J.; Giménez, L.; Davies, A.G.; Malham, S.K.; Neill, S.P.; McDonald, J.E.; Whitton, T.A.; Jackson, S.E.; et al. Impact of climate change on UK estuaries: A review of past trends and potential projections. Estuar. Coast. Shelf Sci. 2016, 169, 119–135. [Google Scholar] [CrossRef] [Green Version]
- Andersen, V.; Gubanova, A.; Nival, P.; Ruellet, T. Zooplankton community during the transition from spring bloom to oligotrophy in the open NW Mediterranean and effects of wind events. 2. Vertical distributions and migrations. J. Plankton Res. 2001, 23, 243–261. [Google Scholar] [CrossRef]
- Barton, A.D.; Pershing, A.J.; Litchman, E.; Record, N.R.; Edwards, K.F.; Finkel, Z.V.; Ward, B.A. The biogeography of marine plankton traits. Ecol. Lett. 2013, 16, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Björnberg, T.K. Developmental Stages of Some Tropical and Subtropical Planktonic Marine Copepods. Studies on the Fauna of Curacao and Other Caribbean Islands. Available online: https://repository.naturalis.nl/pub/506033 (accessed on 1 October 2022).
- Björnberg, T.K.S.; Wilbur, K.M. Copepod phototaxis and vertical migration influenced by xanthene dyes. Biol. Bull. 1968, 134, 398–410. [Google Scholar] [CrossRef]
- Böttger-Schnack, R. Community structure and vertical distribution of cyclopoid copepods in the Red Sea. Mar. Biol. 1990, 106, 473–485. [Google Scholar] [CrossRef]
- Böttger-Schnack, R. Vertical structure of small metazoan plankton, especially noncalanoid copepods. I. Deep Arabian Sea. J. Plankton Res. 1996, 18, 1073–1101. [Google Scholar] [CrossRef]
- Bottger-Schnack, R. Vertical structure of small metazoan plankton, especially non-calanoid copepods. 2. Deep Eastern Mediterranean (Levantine sea). Oceanol. Acta. 1997, 20, 399–419. Available online: https://archimer.ifremer.fr/doc/00093/20405/ (accessed on 1 October 2022).
- Broglio, E.; Saiz, E.; Calbet, A.; Trepat, I.; Alcaraz, M. Trophic impact and prey selection by crustacean zooplankton on the microbial communities of an oligotrophic coastal area (NW Mediterranean Sea). Aquat. Microb. Ecol. 2004, 35, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Bunker, A.J.; Hirst, A.G. Fecundity of marine planktonic copepods: Global rates and patterns in relation to chlorophyll a, temperature and body weight. Mar. Ecol. Prog. Ser. 2004, 279, 161–181. [Google Scholar] [CrossRef]
- Calbet, A.; Carlotti, F.; Gaudy, R. The feeding ecology of the copepod Centropages typicus (Kröyer). Prog. Oceanogr. 2007, 72, 137–150. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mukherjee, A.; Mondal, S.; Das, S.; De, M.; De, T.K. A Case Study to Understand the Feeding Strategy of Some Selected Estuarine Copepods in Response to Mixed Phytoplankton Diet. IJRASET 2016, 4, 43–54. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Li, H. Effect of mesozooplankton feeding selectivity on the dynamics of algae in the presence of intermediate grazers a laboratory simulation. Mar. Ecol. Prog. Ser. 2013, 486, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, B.S.; Ianora, A.; Fresi, E.; Hure, J. Vertical zonation patterns for Mediterranean copepods from the surface to 3000 m at a fixed station in the Tyrrhenian Sea. J. Plankton Res. 1984, 6, 1031–1056. [Google Scholar] [CrossRef]
- Gonzalez, H.E.; Smetacek, V. The possible role of the cyclopoid copepod Oithona in retarding vertical flux of zooplankton faecal material. Mar. Ecol. Prog. Ser. Oldendorf 1994, 113, 233–246. [Google Scholar] [CrossRef]
- Kiørboe, T. How zooplankton feed: Mechanisms, traits and tradeoffs. Biol. Rev. 2011, 86, 311–339. [Google Scholar] [CrossRef]
- Kiørboe, T.; Sabatini, M. Reproductive and life cycle strategies in egg-carrying cyclopoid and free-spawning calanoid copepods. J. Plankton Res. 1994, 16, 1353–1366. [Google Scholar] [CrossRef]
- Kiørboe, T.; Andersen, A.; Langlois, V.J.; Jakobsen, H.H.; Bohr, T. Mechanisms and feasibility of prey capture in ambush-feeding zooplankton. Proc. Natl. Acad. Sci. USA 2009, 106, 12394–12399. [Google Scholar] [CrossRef] [Green Version]
- Maar, M.; Visser, A.W.; Nielsen, T.G.; Stips, A.; Saito, H. Turbulence and feeding behaviour affect the vertical distributions of Oithona similis and Microsetella norwegica. Mar. Ecol. Prog. Ser. 2006, 313, 157–172. [Google Scholar] [CrossRef] [Green Version]
- Melo-Júnior, M. Produção Secundária e Aspectos Reprodutivos de Copépodes Pelágicos ao largo de Ubatuba (SP, Brasil). Doctoral Thesis, Instituto Oceanográfico, Universidade de São Paulo, São Paulo, Brazil, 2009. [Google Scholar]
- Ohtsuka, S.; Onbé, T. Relationship between mouthpart structures and in situ feeding habits of species of the family Pontellidae (Copepoda: Calanoida). Mar. Biol. 1991, 111, 213–225. [Google Scholar] [CrossRef]
- Paffenhöfer, G.A. On the ecology of marine cyclopoid copepods (Crustacea, Copepoda). J. Plankton Res. 1993, 15, 37–55. [Google Scholar] [CrossRef]
- Paffenhöfer, G.A. On the relation of structure, perception and activity in marine planktonic copepods. J. Mar. Syst. 1998, 15, 457–473. [Google Scholar] [CrossRef]
- Paffenhöfer, G.A.; Mazzocchi, M.G. On some aspects of the behaviour of Oithona plumifera (Copepoda: Cyclopoida). J. Plankton Res. 2002, 24, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Razouls, C.; Desreumaux, N.; Kouwenberg, J.; de Bovée, F. Biodiversity of Marine Planktonic Copepods (Morphology, Geographical Distribution and Biological Data). Available online: http://copepodes.obs-banyuls.fr/en (accessed on 15 April 2022).
- Sautour, B.; Castel, J. Feeding behaviour of the coastal copepod Euterpina acutifrons on small particles. Cah. Biol. Mar. 1993, 34, 239–251. [Google Scholar]
- Steinberg, D.K.; Silver, M.W.; Pilskaln, C.H.; Coale, S.L.; Paduan, J.B. Midwater zooplankton communities on pelagic detritus (giant larvacean houses) in Monterey Bay, California. Limnol. Oceanogr. 1994, 39, 1606–1620. [Google Scholar] [CrossRef]
- Turner, J.T. Zooplankton feeding ecology: Contents of fecal pellets of the cyclopoid copepods Oncaea venusta, Corycaeus amazonicus, Oithona plumifera, and O. simplex from the northern Gulf of Mexico. Mar. Ecol. 1986, 7, 289–302. [Google Scholar] [CrossRef]
- Turner, J.T. The Feeding Ecology of Some Zooplankters that Are Important Prey Items of Larval Fish. Available online: https://aquadocs.org/bitstream/handle/1834/20603/tr7opt.pdf?sequence=1&isAllowed=y (accessed on 15 April 2022).
- VanderLugt, K.; Lenz, P.H. Handbook for the Cultivation of Two Hawaiian Paracalanid Copepods. Available online: https://www.researchgate.net/publication/267237260_Handbook_for_the_Cultivation_of_two_Hawaiian_Paracalanid_Copepods (accessed on 15 April 2022).
- Visser, A.W.; Stips, A. Turbulence and zooplankton production: Insights from PROVESS. J. Sea Res. 2002, 47, 317–329. [Google Scholar] [CrossRef]
- Vives, F. Distribución de la población de copépodos en el Mediterráneo Occidental. Available online: https://digital.csic.es/handle/10261/154639 (accessed on 15 April 2022).



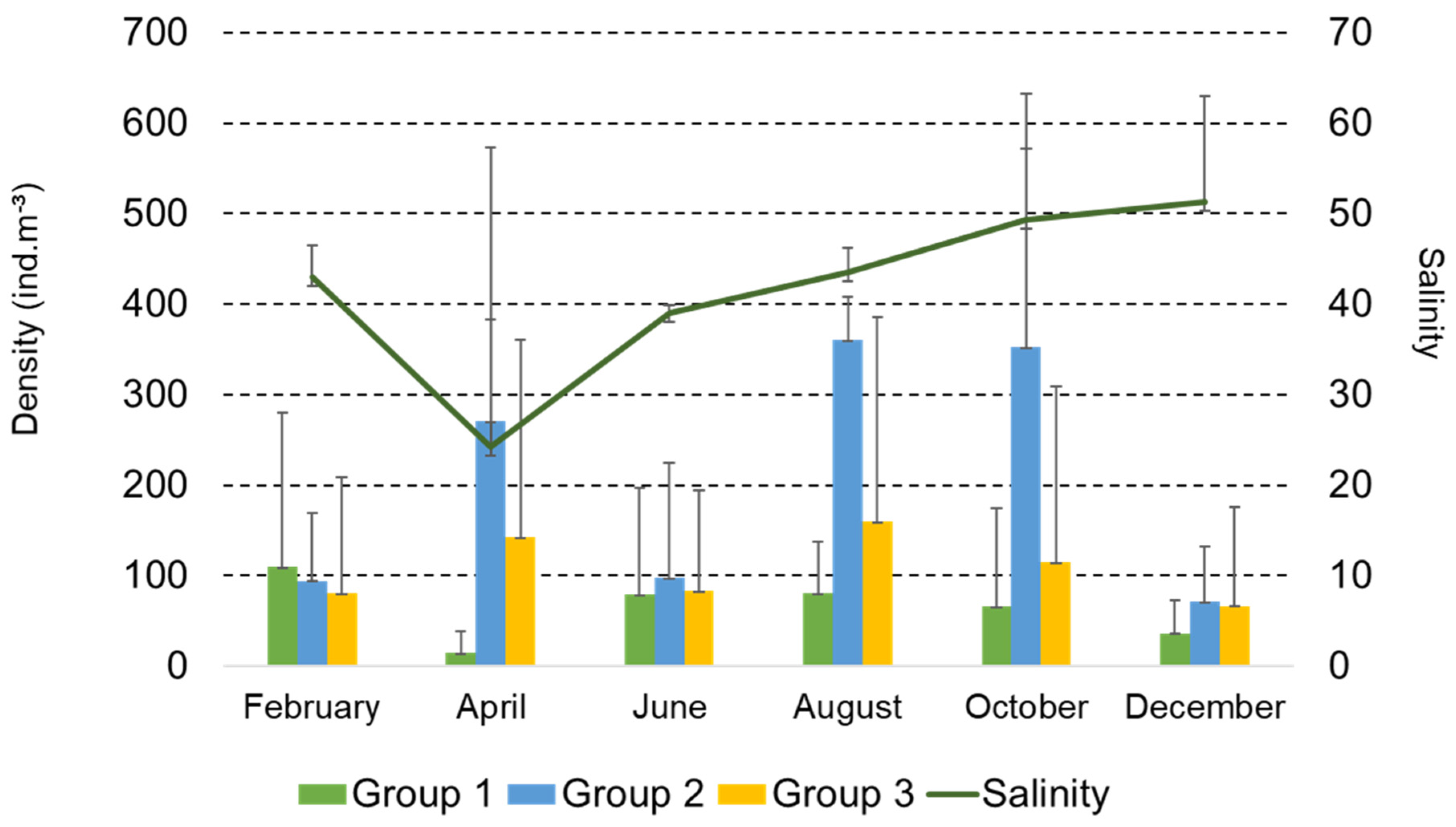
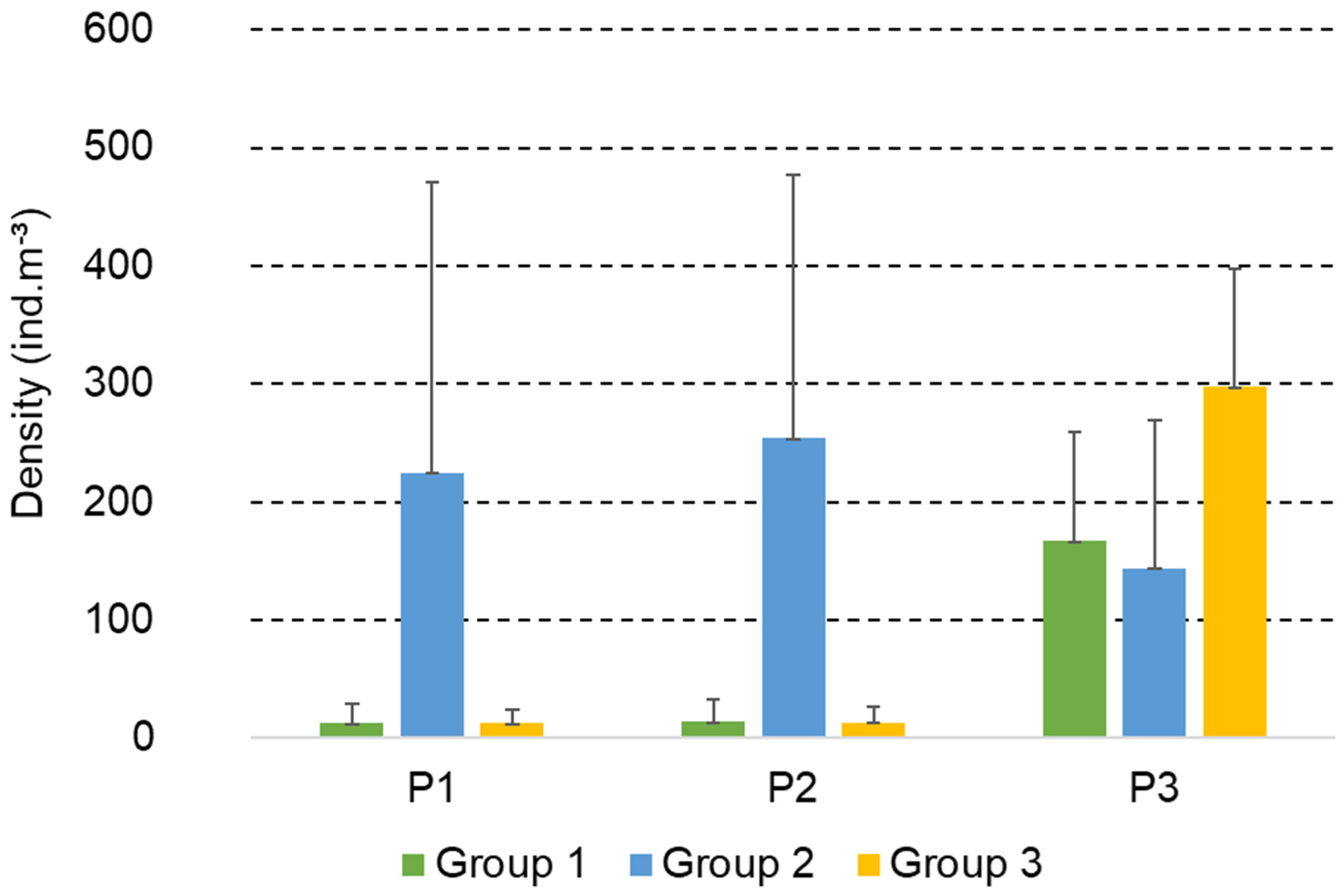
| Month | Station | T (°C) | pH | DO (mg/L) | D (m) |
|---|---|---|---|---|---|
| February | P1 | 29.15 ± 0.09 | 8.83 ± 0.02 | 3.79 ± 0.51 | 1.95 ± 0.63 |
| P2 | 29.06 ± 0.42 | 8.76 ± 0.49 | 3.84 ± 0.73 | 1.47 ± 0.47 | |
| P3 | 28.07 ± 0.25 | 8.75 ± 0.09 | 5.55 ± 0.33 | 2.79 ± 1.40 | |
| April | P1 | 28.19 ± 0.06 | 7.30 ± 0.01 | 4.18 ± 0.59 | 2.87 ± 1.12 |
| P2 | 28.12 ± 0.03 | 7.62 ± 0.01 | 4.71 ± 0.11 | 2.28 ± 0.38 | |
| P3 | 27.94 ± 0.01 | 7.81 ± 0.04 | 5.68 ± 0.06 | 3.08 ± 1.72 | |
| June | P1 | 28.42 ± 0.01 | 8.05 ± 0.01 | 4.77 ± 0.02 | 2.02 ± 0.44 |
| P2 | 28.07 ± 0.03 | 8.07 ± 0.01 | 4.58 ± 0.81 | 2.56 ± 0.73 | |
| P3 | 27.15 ± 0.01 | 8.03 ± 0.07 | 5.48 ± 0.04 | 3.35 ± 1.06 | |
| August | P1 | 27.02 ± 0.01 | 7.92 ± 0.01 | 4.64 ± 0.04 | 1.59 ± 0.27 |
| P2 | 25.89 ± 0.01 | 8.18 ± 0.01 | 5.13 ± 0.10 | 1.65 ± 0.65 | |
| P3 | 25.60 ± 0.01 | 8.13 ± 0.01 | 5.94 ± 0.03 | 3.30 ± 0.90 | |
| October | P1 | 27.58 ± 0.01 | 7.89 ± 0.01 | 6.01 ± 0.05 | 1.78 ± 0.45 |
| P2 | 27.02 ± 0.02 | 7.73 ± 0.01 | 4,65 ± 0.65 | 2.82 ± 1.07 | |
| P3 | 26.15 ± 0.01 | 7.66 ± 0.01 | 5,73 ± 0.10 | 2.86 ± 0.93 | |
| December | P1 | 28.28 ± 0.02 | 8.60 ± 0.05 | 5.77 ± 0.80 | 1.82 ± 0.82 |
| P2 | 27.64 ± 0.20 | 8.48 ± 0.01 | 5.00 ± 0.94 | 1.59 ± 0.41 | |
| P3 | 26.83 ± 0.05 | 8.47 ± 0.03 | 6.04 ± 0.01 | 2.81 ± 0.78 |
| FEB | APR | JUN | AUG | OCT | DEC | |
|---|---|---|---|---|---|---|
| P1 | 45 | 9 | 36 | 42 | 55 | 63 |
| P2 | 45 | 26 | 37 | 39 | 46 | 52 |
| P3 | 39 | 37 | 38 | 37 | 39 | 39 |
| Correlation | Number of Factors | Factors |
|---|---|---|
| 0.576 | 4 | Sal, NT, silica e Chl-a |
| 0.564 | 5 | Sal, Ammoniacal N, NT, silica and Chl-a |
| 0.563 | 5 | P, Sal, NT, silica and Chl-a |
| 0.561 | 3 | Sal, silica and Chl-a |
| 0.559 | 7 | P, Sal, Ammoniacal N, NT, PT, silica and Chl-a |
| 0.559 | 6 | P, Sal, Ammoniacal N, NT, silica and Chl-a |
| 0.558 | 7 | P, Sal, Ammoniacal N, NT, silica, SST and Chl-a |
| 0.558 | 5 | Sal, NT, silica, Chl-a and Feofitina-a |
| 0.557 | 5 | Sal, NT, PT, silica and Chl-a |
| 0.555 | 6 | Sal, Ammoniacal N, NT, silica, SST and Chl-a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, C.C.; de Sousa Barroso, H.; Belmonte, G.; Rossi, S.; Soares, M.O.; Garcia, T.M. Copepod Assemblages at the Base of Mangrove Food Webs during a Severe Drought. Water 2022, 14, 3648. https://doi.org/10.3390/w14223648
Campos CC, de Sousa Barroso H, Belmonte G, Rossi S, Soares MO, Garcia TM. Copepod Assemblages at the Base of Mangrove Food Webs during a Severe Drought. Water. 2022; 14(22):3648. https://doi.org/10.3390/w14223648
Chicago/Turabian StyleCampos, Carolina Coelho, Hortência de Sousa Barroso, Genuario Belmonte, Sergio Rossi, Marcelo Oliveira Soares, and Tatiane Martins Garcia. 2022. "Copepod Assemblages at the Base of Mangrove Food Webs during a Severe Drought" Water 14, no. 22: 3648. https://doi.org/10.3390/w14223648





