Synthesis and Characterization of Ch-PANI-Fe2O3 Nanocomposite and Its Water Remediation Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
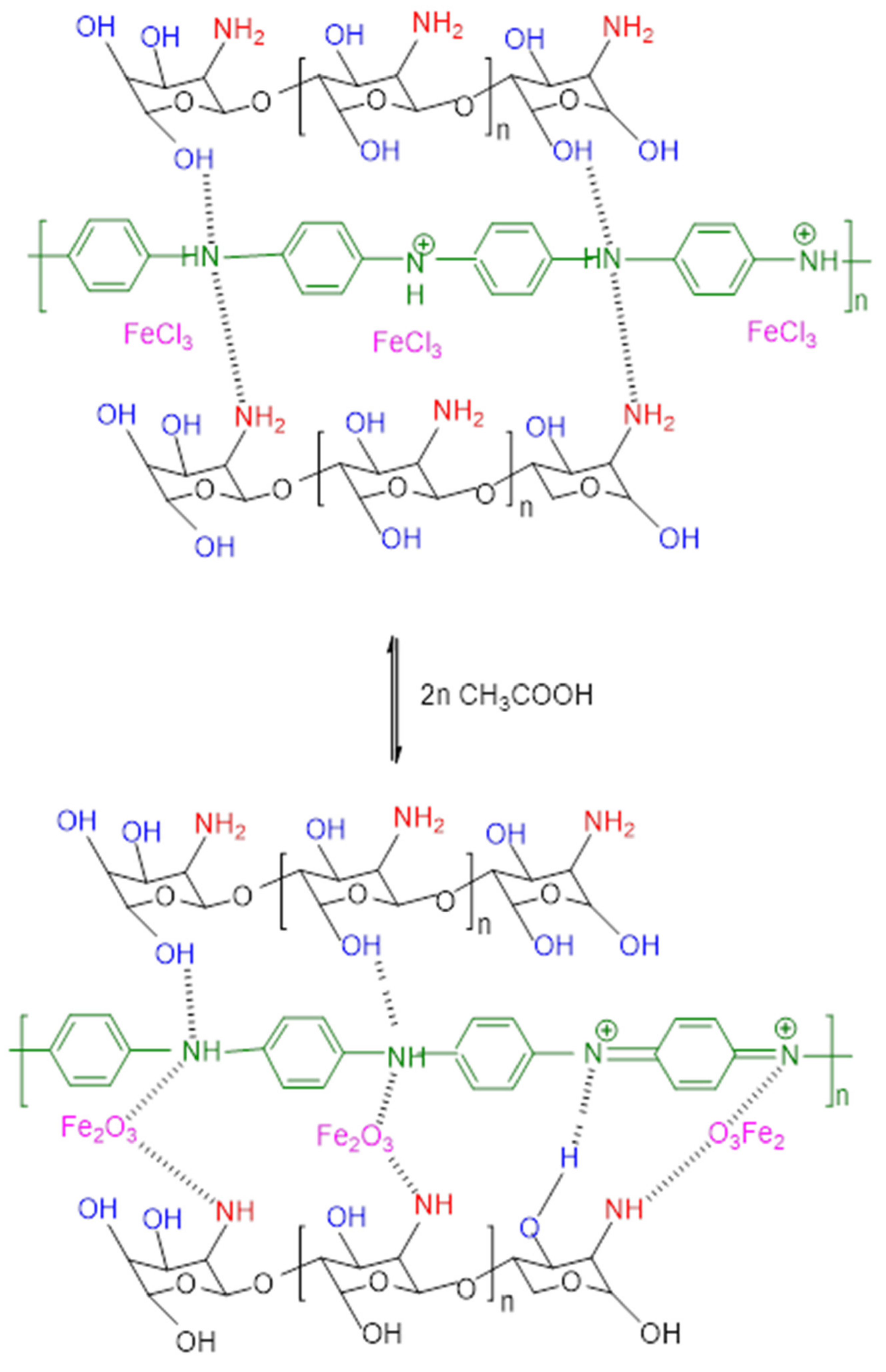
2.2. Method
2.3. Characterization
2.4. Dye Adsorption Study
3. Results and Discussion
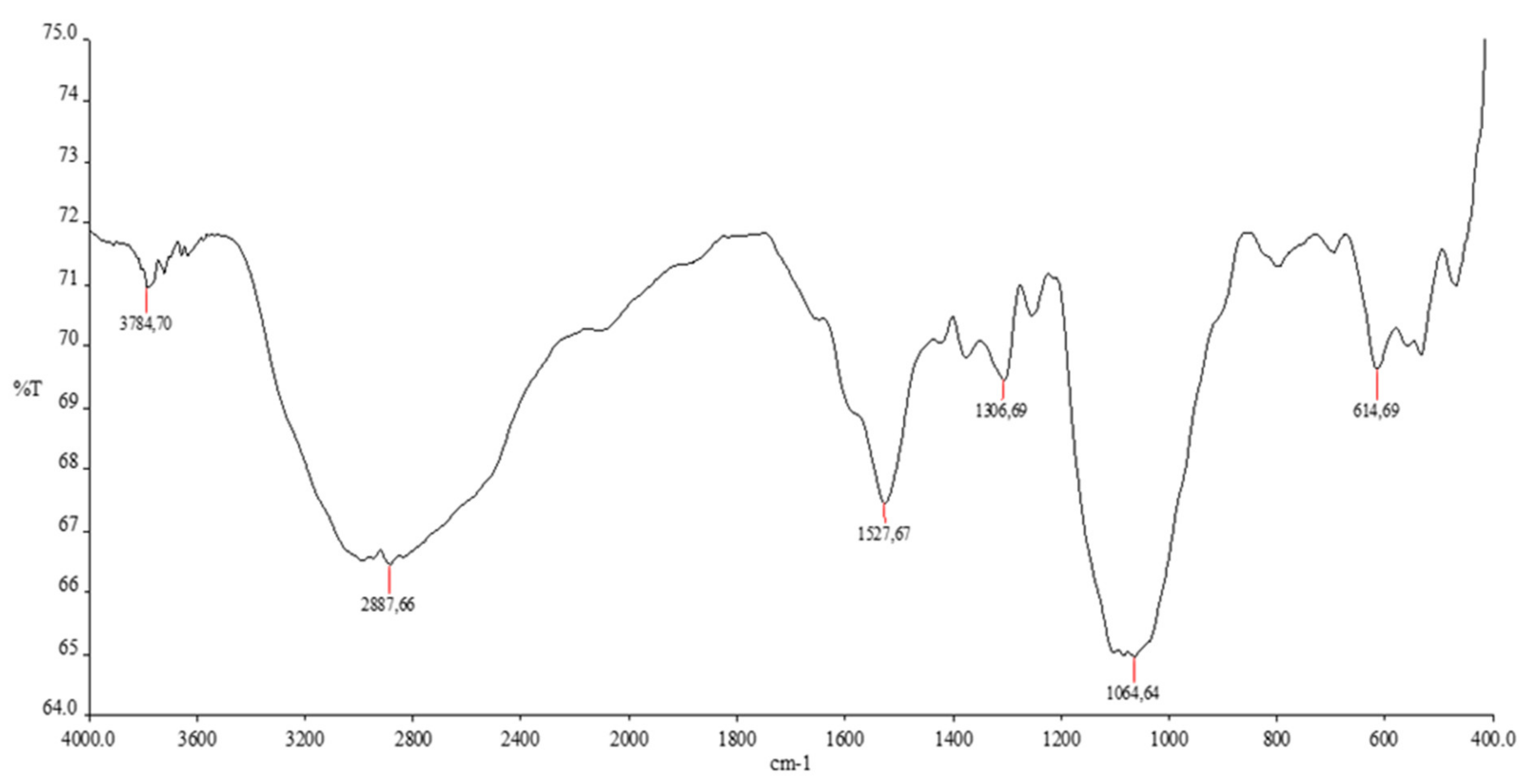
3.1. Spectral Analysis
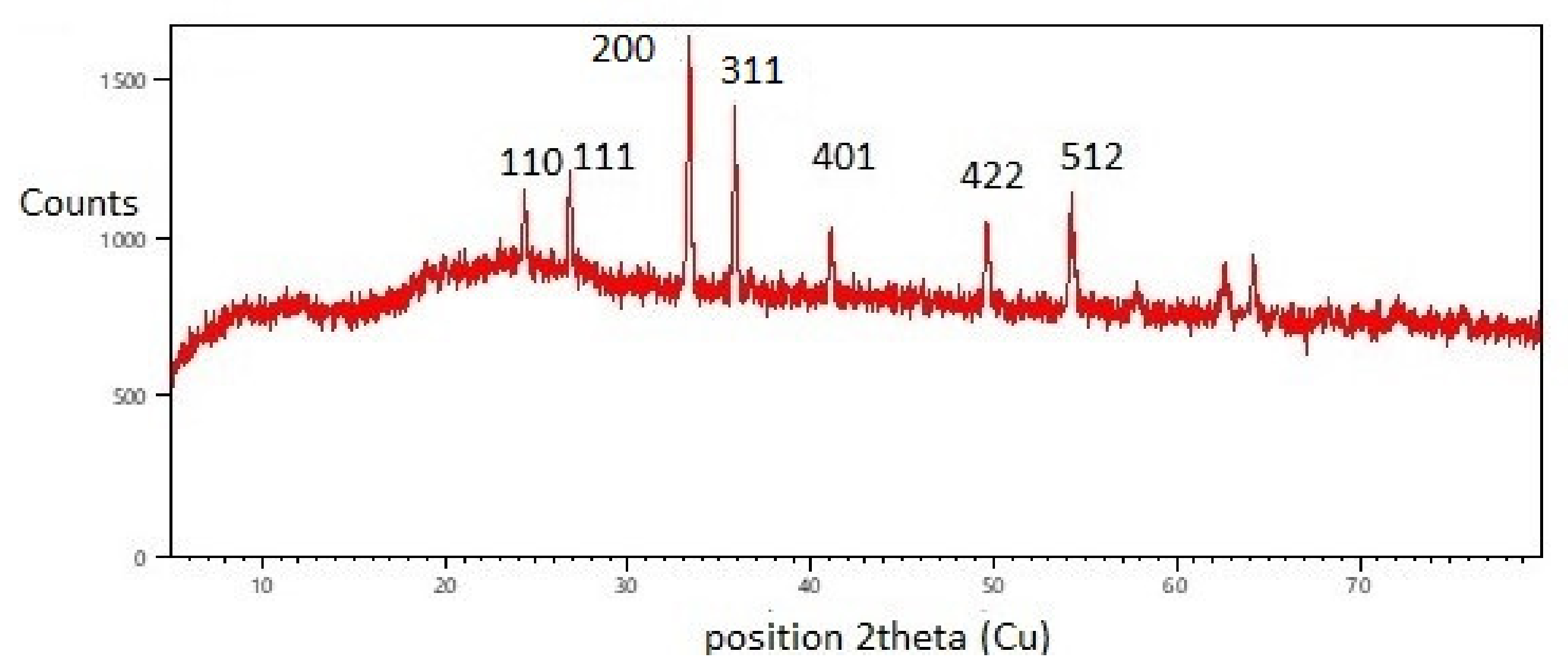
3.2. XRD Analysis
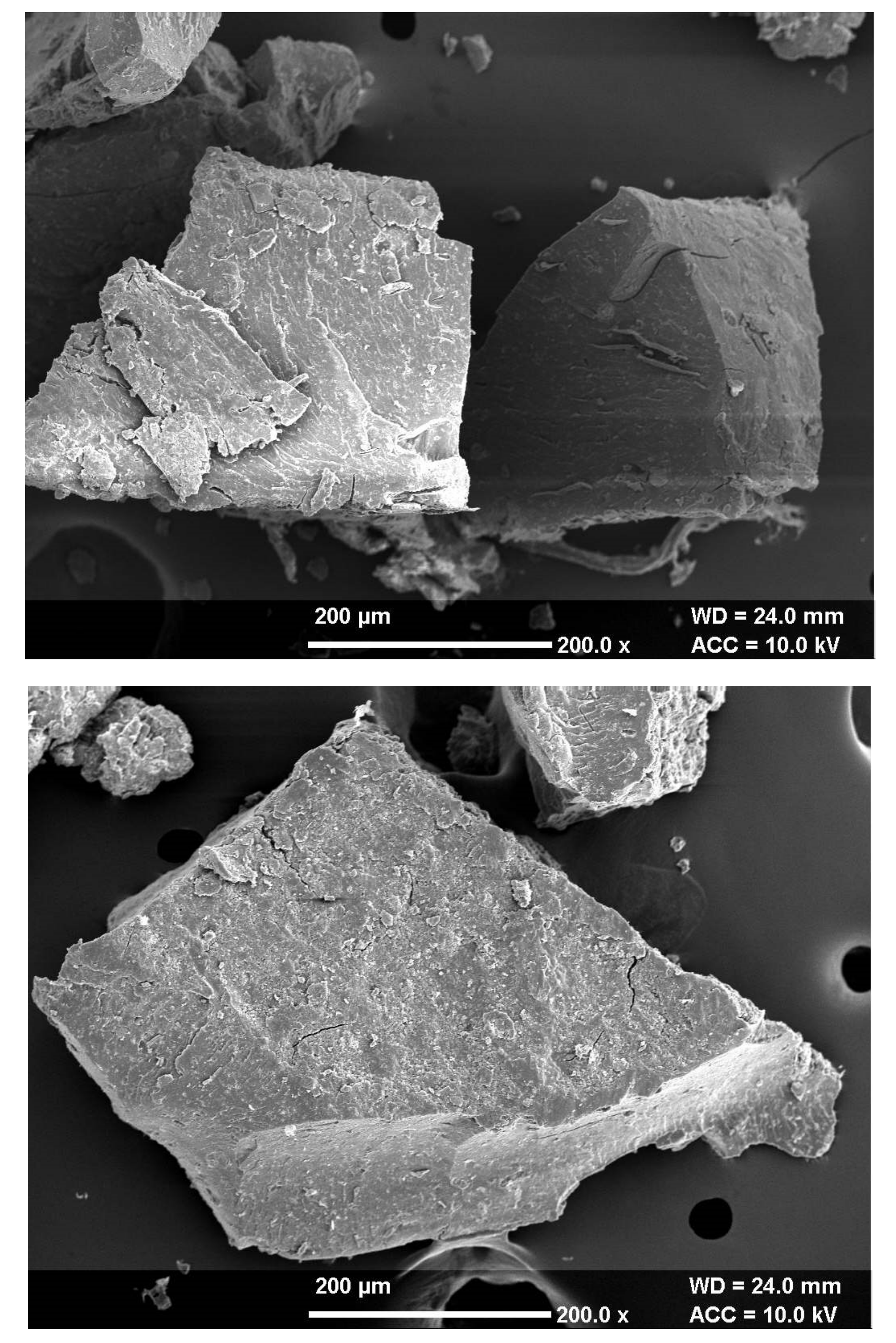
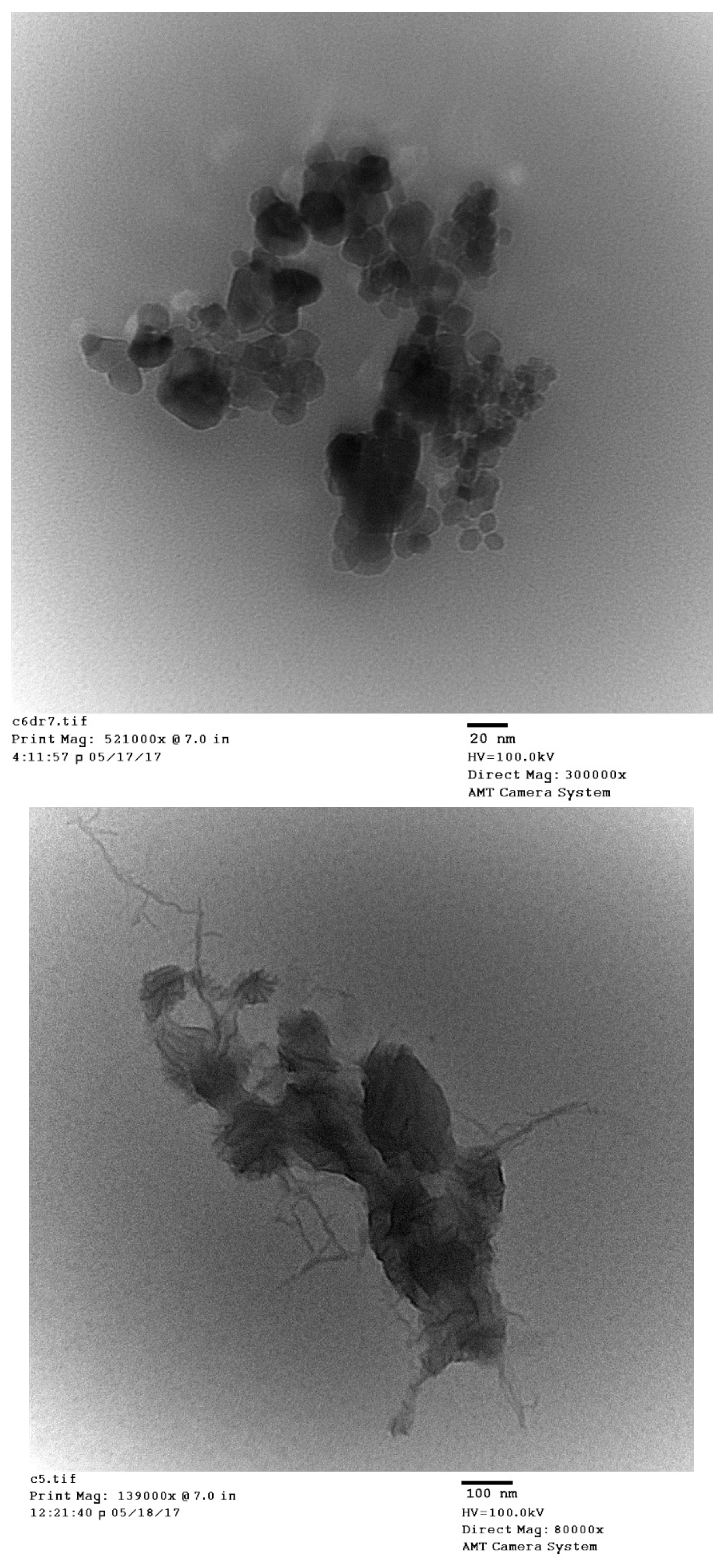
3.3. Topographical Study
3.4. Thermal Stability
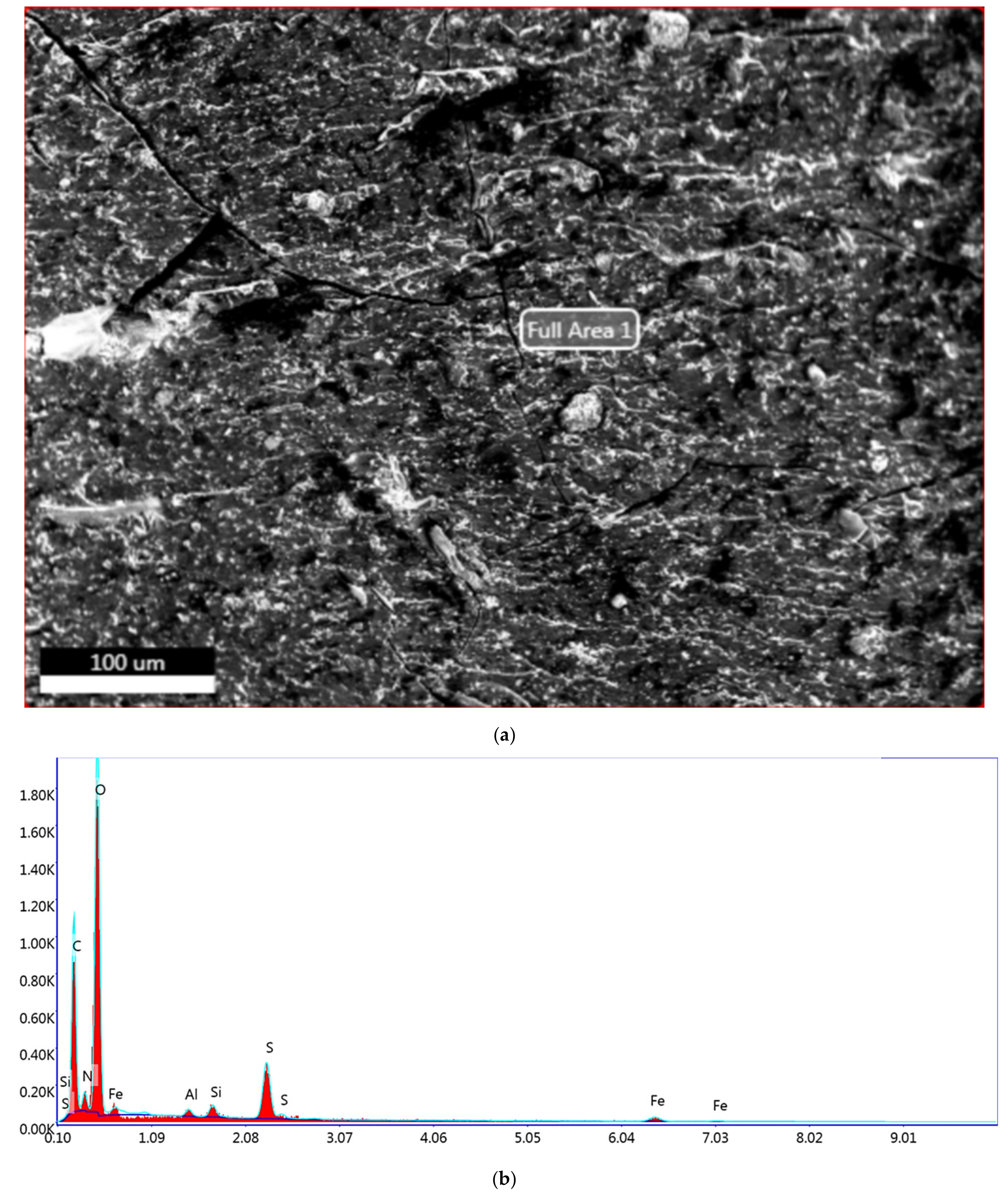
3.5. Elemental Analysis
3.6. Dye Adsorption Study
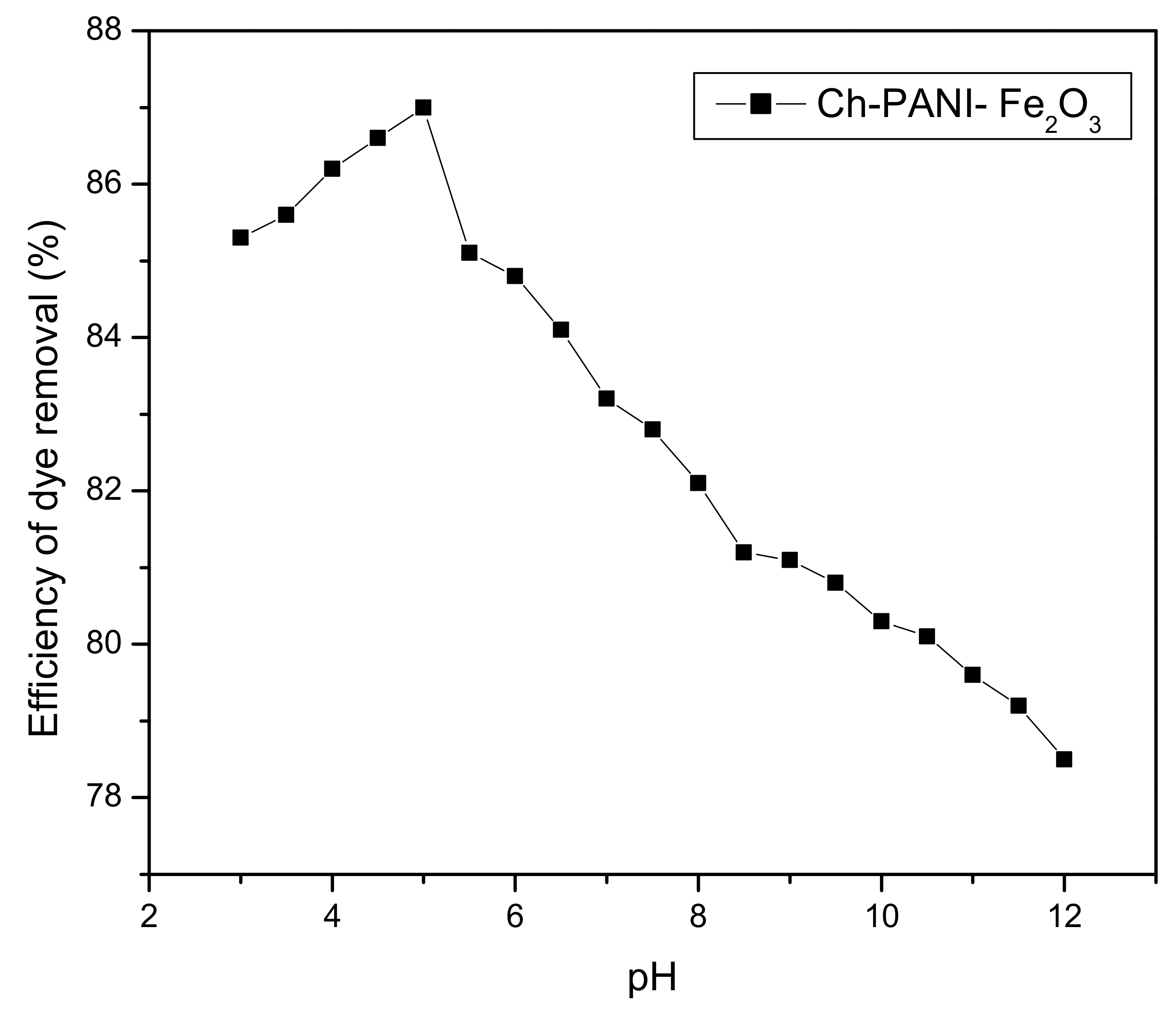
3.6.1. Effect of pH
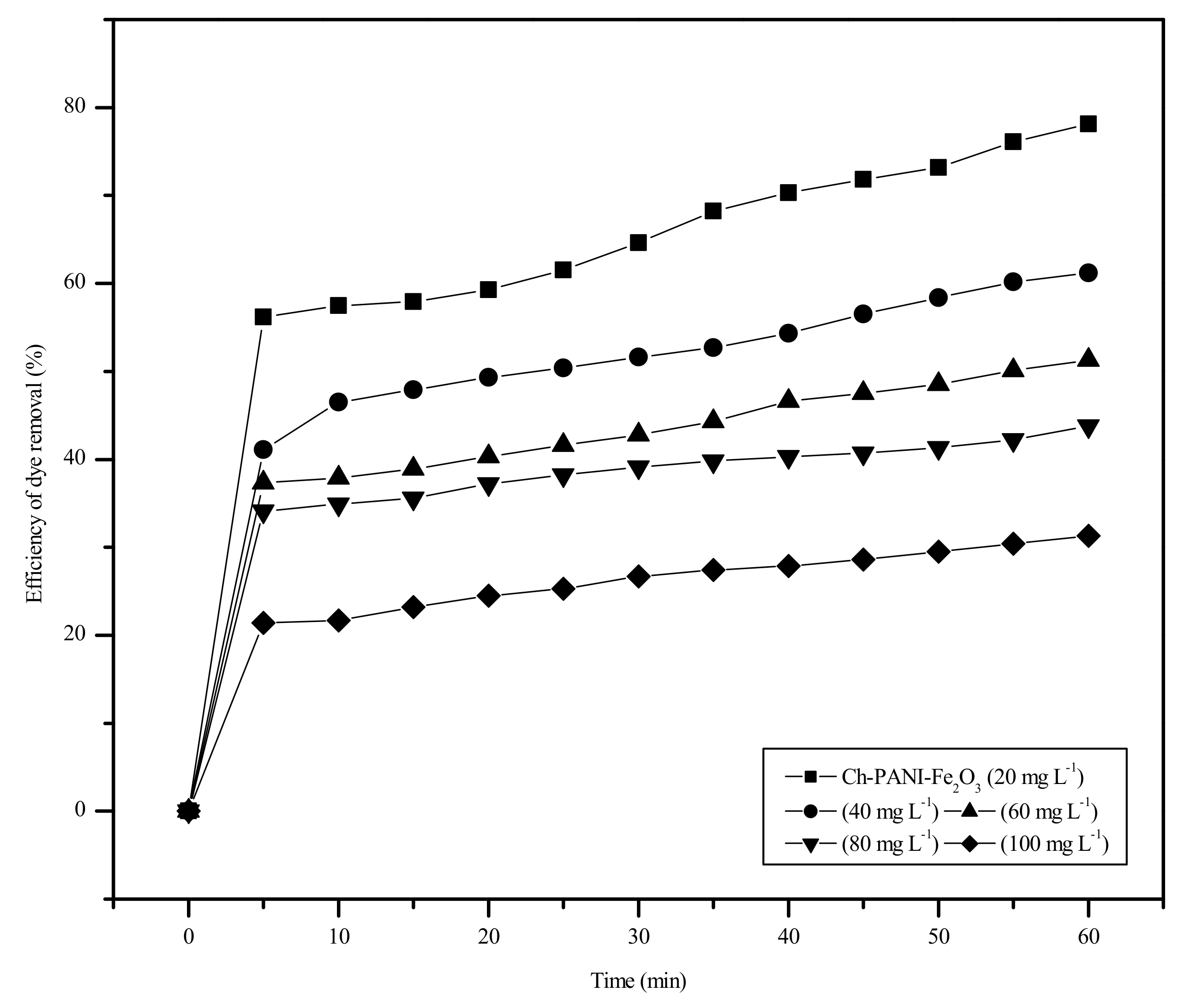
3.6.2. Effect of Dose of Adsorbent and Dye
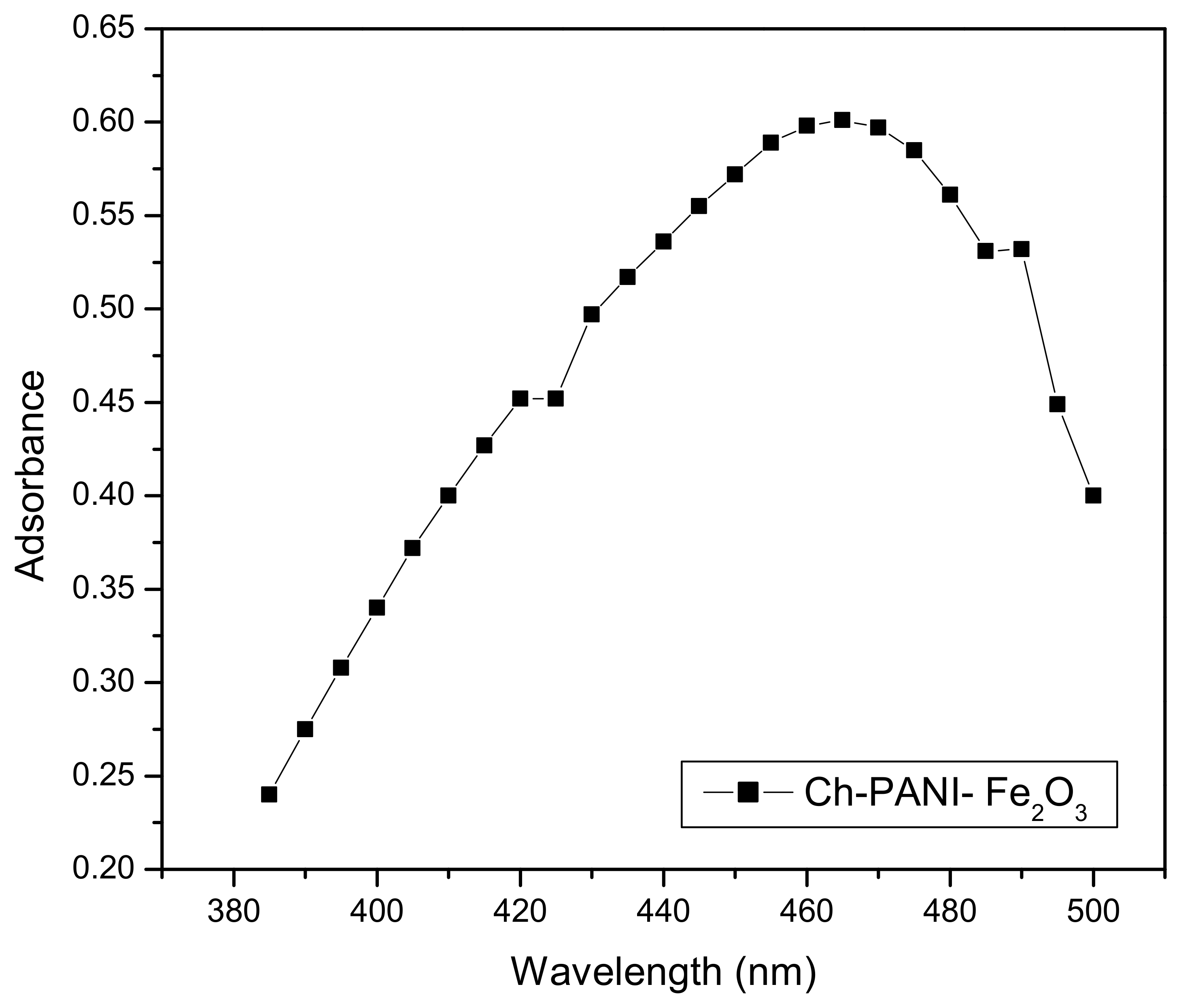
3.6.3. Effect of Wavelength
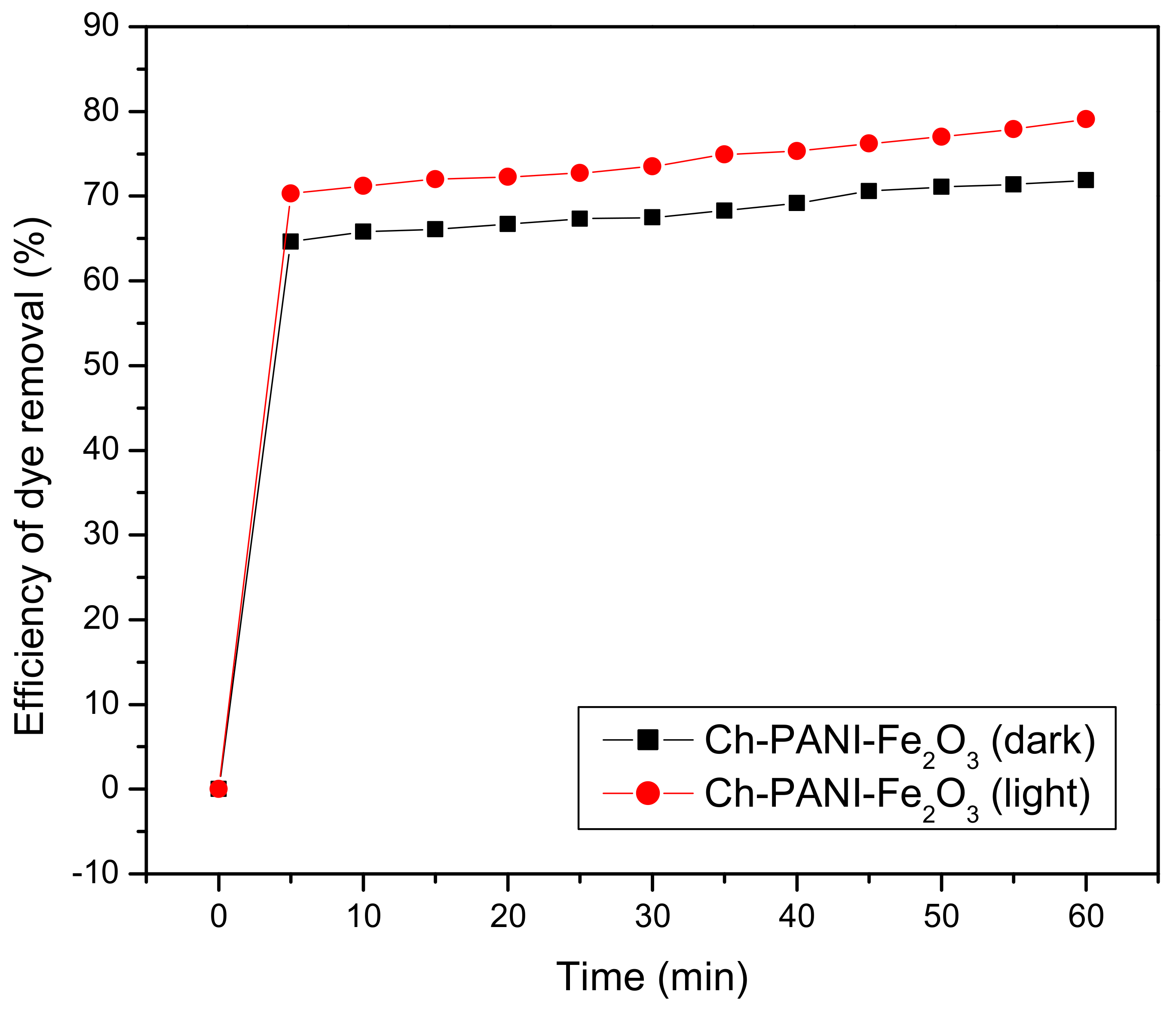
3.6.4. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal properties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]
- Chen, F.; Ma, J.; Zhu, Y.; Li, X.; Yu, H.; Sun, Y. Biodegradation performance and anti-fouling mechanism of an ICME/electro-biocarriers-MBR system in livestock wastewater (antibiotic-containing) treatment. J. Hazard. Mater. 2022, 426, 128064. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, T.; Liu, Y.; Liu, P.; Zhang, J.; Fang, L.; Sun, D. Enhancement of desulfurization by hydroxyl ammonium ionic liquid supported on active carbon. Environ. Res. 2022, 213, 113637. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, K.R.; Viraraghavan, T. Dye removal using low cost adsorbents. Water Sci. Technol. 1997, 36, 189–196. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI-Containing Mg (OH) 2 Nanowaste. Angew. Chem. 2008, 120, 5701–5704. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, Z.; Yu, X.; Jia, Z.; Rosso, M.; Dedman, S.; Zhu, J.; Xia, Y.; Zhang, G.; Yang, J. Can we quantify the aquatic environmental plastic load from aquaculture? Water Res. 2022, 219, 118551. [Google Scholar] [CrossRef]
- Madhu, N.R.; Erfani, H.; Jadoun, S.; Amir, M.; Thiagarajan, Y.; Chauhan, N.P.S. Fused deposition modelling approach using 3D printing and recycled industrial materials for a sustainable environment: A review. Int. J. Adv. Manuf. Technol. 2022, 122, 2125–2138. [Google Scholar] [CrossRef]
- Weisburger, J.H. Comments on the history and importance of aromatic and heterocyclic amines in public health. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2002, 506, 9–20. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Roostaee, M. Coupling of NiO nanoparticles and room temperature ionic liquid for fabrication of highly sensitive voltammetric sensor in tryptophan analysis. Anal. Bioanal. Electrochem. 2016, 8, 578–588. [Google Scholar]
- Mortezagholi, B.; Movahed, E.; Fathi, A.; Soleimani, M.; Forutan Mirhosseini, A.; Zeini, N.; Khatami, M.; Naderifar, M.; Abedi Kiasari, B.; Zareanshahraki, M. Plant-mediated synthesis of silver-doped zinc oxide nanoparticles and evaluation of their antimicrobial activity against bacteria cause tooth decay. Microsc. Res. Tech. 2022, 85, 3553–3564. [Google Scholar] [CrossRef] [PubMed]
- Akbarizadeh, M.R.; Naderifar, M.; Mousazadeh, F.; Zafarnia, N.; Sarani, M. Cytotoxic activity and Magnetic Behavior of green synthesized iron oxide nanoparticles on brain glioblastoma cells. Nanomed. Res. J. 2022, 7, 99–106. [Google Scholar]
- Kalal, S.; Singh Chauhan, N.P.; Ameta, N.; Ameta, R.; Kumar, S.; Punjabi, P.B. Role of copper pyrovanadate as heterogeneous photo-Fenton like catalyst for the degradation of neutral red and azure-B: An eco-friendly approach. Korean J. Chem. Eng. 2014, 31, 2183–2191. [Google Scholar] [CrossRef]
- Anuma, S.; Mishra, P.; Bhat, B.R. Polypyrrole functionalized Cobalt oxide Graphene (COPYGO) nanocomposite for the efficient removal of dyes and heavy metal pollutants from aqueous effluents. J. Hazard. Mater. 2021, 416, 125929. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, S.; Anand, K.; Gengan, R.M.; Nayunigari, M.K.; Maity, A. Sorption isotherms, kinetic and optimization process of amino acid proline based polymer nanocomposite for the removal of selected textile dyes from industrial wastewater. J. Photochem. Photobiol. B Biol. 2016, 165, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Alam, J.; Rahaman, M.; Alrehaili, A.; Alhoshan, M.; Aldalbahi, A. A facile approach for elimination of electroneutral/anionic organic dyes from water using a developed carbon-based polymer nanocomposite membrane. Water Air Soil Pollut. 2020, 231, 104. [Google Scholar] [CrossRef]
- Jagadish, K.; Chandrashekar, B.; Byrappa, K.; Rangappa, K.; Srikantaswamy, S. Simultaneous removal of dye and heavy metals in a single step reaction using PVA/MWCNT composites. Anal. Methods 2016, 8, 2408–2412. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Abd El-Monaem, E.M.; El-Subruiti, G.M.; Abd El-Latif, M.M.; Omer, A.M. Fabrication of UiO-66/MIL-101 (Fe) binary MOF/carboxylated-GO composite for adsorptive removal of methylene blue dye from aqueous solutions. RSC Adv. 2020, 10, 19008–19019. [Google Scholar] [CrossRef]
- Chen, B.; Liu, Y.; Chen, S.; Zhao, X.; Meng, X.; Pan, X. Magnetically recoverable cross-linked polyethylenimine as a novel adsorbent for removal of anionic dyes with different structures from aqueous solution. J. Taiwan Inst. Chem. Eng. 2016, 67, 191–201. [Google Scholar] [CrossRef]
- Cojocaru, C.; Samoila, P.; Pascariu, P. Chitosan-based magnetic adsorbent for removal of water-soluble anionic dye: Artificial neural network modeling and molecular docking insights. Int. J. Biol. Macromol. 2019, 123, 587–599. [Google Scholar] [CrossRef]
- Jadoun, S.; Rathore, D.S.; Riaz, U.; Chauhan, N.P.S. Tailoring of conducting polymers via copolymerization—A review. Eur. Polym. J. 2021, 155, 110561. [Google Scholar] [CrossRef]
- Chauhan, N.P.; Ameta, R.; Ameta, R.; Ameta, S.C. Thermal and conducting behaviour of emeraldine base (EB) form of polyaniline (PANI). Indian J. Chem. Technol. 2011, 18, 118–122. [Google Scholar]
- Mozafari, M.; Chauhan, N.P.S. Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Supriya, S.; Palanisamy, P. Adsorptive removal of acid orange 7 from industrial effluents using activated carbon and conducting polymer composite—A comparative study. Indian J. Chem. Technol. 2016, 23, 506–512. [Google Scholar]
- Abbasian, M.; Niroomand, P.; Jaymand, M. Cellulose/polyaniline derivatives nanocomposites: Synthesis and their performance in removal of anionic dyes from simulated industrial effluents. J. Appl. Polym. Sci. 2017, 134, 45352. [Google Scholar] [CrossRef]
- Wang, N.; Chen, J.; Wang, J.; Feng, J.; Yan, W. Removal of methylene blue by Polyaniline/TiO2 hydrate: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2019, 347, 93–102. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.U.H.A.; Bilal, S. Comparative study of the adsorption of Acid Blue 40 on polyaniline, magnetic oxide and their composites: Synthesis, characterization and application. Materials 2019, 12, 2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- AlYahya, S.; Rani, B.J.; Ravi, G.; Yuvakkumar, R.; Arun, A.; Ameen, F.; AlNadhary, S. Size dependent magnetic and antibacterial properties of solvothermally synthesized cuprous oxide (Cu2O) nanocubes. J. Mater. Sci. Mater. Electron. 2018, 29, 17622–17629. [Google Scholar] [CrossRef]
- Swathi, S.; Ameen, F.; Ravi, G.; Yuvakkumar, R.; Hong, S.; Velauthapillai, D.; AlKahtani, M.D.; Thambidurai, M.; Dang, C. Cancer targeting potential of bioinspired chain like magnetite (Fe3O4) nanostructures. Curr. Appl. Phys. 2020, 20, 982–987. [Google Scholar] [CrossRef]
- Jasni, M.; Sathishkumar, P.; Sornambikai, S.; Yusoff, A.R.M.; Ameen, F.; Buang, N.A.; Kadir, M.R.A.; Yusop, Z. Fabrication, characterization and application of laccase–nylon 6, 6/Fe3+ composite nanofibrous membrane for 3, 3′-dimethoxybenzidine detoxification. Bioprocess Biosyst. Eng. 2017, 40, 191–200. [Google Scholar] [CrossRef]
- Hu, X.; Derakhshanfard, A.H.; Khalid, I.; Jalil, A.T.; Opulencia, M.J.C.; Dehkordi, R.B.; Toghraie, D.; Hekmatifar, M.; Sabetvand, R. The microchannel type effects on water-Fe3O4 nanofluid atomic behavior: Molecular dynamics approach. J. Taiwan Inst. Chem. Eng. 2022, 135, 104396. [Google Scholar] [CrossRef]
- Homaeigohar, S. The nanosized dye adsorbents for water treatment. Nanomaterials 2020, 10, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roostaee, M.; Sheikhshoaie, I. Fabrication of a sensitive sensor for determination of xanthine in the presence of uric acid and ascorbic acid by modifying a carbon paste sensor with Fe3O4@Au core–shell and an ionic liquid. J. Food Meas. Charact. 2022, 16, 731–739. [Google Scholar] [CrossRef]
- Pandiselvi, K.; Thambidurai, S. Synthesis of adsorption cum photocatalytic nature of polyaniline-ZnO/chitosan composite for removal of textile dyes. Desalination Water Treat. 2016, 57, 8343–8357. [Google Scholar] [CrossRef]
- Kannusamy, P.; Sivalingam, T. Chitosan–ZnO/polyaniline hybrid composites: Polymerization of aniline with chitosan–ZnO for better thermal and electrical property. Polym. Degrad. Stab. 2013, 98, 988–996. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Ismail, F.H.; Shahid, M.M.; Huang, N.M. Synthesis of chitosan grafted-polyaniline/Co3O4 nanocube nanocomposites and their photocatalytic activity toward methylene blue dye degradation. RSC Adv. 2015, 5, 83857–83867. [Google Scholar] [CrossRef]
- Kannusamy, P.; Sivalingam, T. Synthesis of porous chitosan–polyaniline/ZnO hybrid composite and application for removal of reactive orange 16 dye. Colloids Surf. B: Biointerfaces 2013, 108, 229–238. [Google Scholar] [CrossRef]
- Vellakkat, M.; Hundekal, D. Electrical conductivity and supercapacitor properties of polyaniline/chitosan/nickel oxide honeycomb nanocomposite. J. Appl. Polym. Sci. 2017, 134, 44536. [Google Scholar] [CrossRef]
- Rathore, B.S.; Chauhan, N.P.S.; Jadoun, S.; Ameta, S.C.; Ameta, R. Synthesis and characterization of chitosan-polyaniline-nickel (II) oxide nanocomposite. J. Mol. Struct. 2021, 1242, 130750. [Google Scholar] [CrossRef]
- Mahanta, D.; Madras, G.; Radhakrishnan, S.; Patil, S. Adsorption and desorption kinetics of anionic dyes on doped polyaniline. J. Phys. Chem. B 2009, 113, 2293–2299. [Google Scholar] [CrossRef]
- Rathore, B.S.; Chauhan, N.P.S.; Rawal, M.K.; Ameta, S.C.; Ameta, R. Chitosan–polyaniline–copper (II) oxide hybrid composite for the removal of methyl orange dye. Polym. Bull. 2020, 77, 4833–4850. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Soflaee, F. Synthesis and characterization of α-Fe2O3 nanoparticles by simple co-precipitation method. Phys. Chem. Res. 2015, 3, 191–196. [Google Scholar]
- Hameed, B. Equilibrium and kinetic studies of methyl violet sorption by agricultural waste. J. Hazard. Mater. 2008, 154, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]




| S. No. | Polymer Composite | Method | Properties and Applications | References |
|---|---|---|---|---|
| 1 | PANI/Ch/ZnO | Single-step in situ oxidation polymerization | Better thermal and electrical property | [35] |
| 2 | PANI/Ch/ZnO | Precipitation–oxidation method | Wastewater pollutants | [36] |
| 3 | PANI-g-Ch/Co3O4 | Oxidative radical copolymerization | Photocatalytic activity | [37] |
| 4 | PANI/Ch/ZnO | Chemical polymerization | Removal of dye | [38] |
| 5 | PANI/Ch/NiO | Chemical route and Breath Figure technique | Electrical conductivity and supercapacitor properties | [39,40] |
| Bands (cm−1) | Assignments | |
|---|---|---|
| FTIR | 3784.70 | O-H Stretching |
| 2887.66 | symmetric -CH2- stretching vibration credited to pyranose ring | |
| 1527.67 | -C = O and N-H stretching | |
| 1306.69 | -CH3 bending in alkyl-substituted amide | |
| 1064.64 | C-O stretching vibration in chitosan | |
| 614.69 | Fe-O Stretching |
| Time (min) | Ch-PANI-Fe2O3 (0.1 mg L−1) | Ch-PANI-Fe2O3 (0.25 mg L−1) | Ch-PANI-Fe2O3 (0.5 mg L−1) |
|---|---|---|---|
| 0.0 | 0.0 | 0.0 | 0.0 |
| 5.0 | 81.2 | 84.3 | 88.2 |
| 10.0 | 81.6 | 84.4 | 88.7 |
| 15.0 | 81.9 | 85.1 | 89.2 |
| 20.0 | 82.5 | 85.3 | 89.9 |
| 25.0 | 83.0 | 85.9 | 90.3 |
| 30.0 | 83.7 | 86.7 | 90.5 |
| 35.0 | 83.9 | 87.0 | 90.8 |
| 40.0 | 84.1 | 87.3 | 91.5 |
| 45.0 | 84.5 | 88.2 | 92.4 |
| 50.0 | 84.9 | 88.6 | 93.5 |
| 55.0 | 85.3 | 89.2 | 93.8 |
| 60.0 | 85.6 | 89.5 | 94.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh Rathore, B.; Pal Singh Chauhan, N.; Panneerselvam, P.; Jadoun, S.; Barani, M.; Ameta, S.C.; Ameta, R. Synthesis and Characterization of Ch-PANI-Fe2O3 Nanocomposite and Its Water Remediation Applications. Water 2022, 14, 3615. https://doi.org/10.3390/w14223615
Singh Rathore B, Pal Singh Chauhan N, Panneerselvam P, Jadoun S, Barani M, Ameta SC, Ameta R. Synthesis and Characterization of Ch-PANI-Fe2O3 Nanocomposite and Its Water Remediation Applications. Water. 2022; 14(22):3615. https://doi.org/10.3390/w14223615
Chicago/Turabian StyleSingh Rathore, Bharatraj, Narendra Pal Singh Chauhan, Perumal Panneerselvam, Sapana Jadoun, Mahmood Barani, Suresh C. Ameta, and Rakshit Ameta. 2022. "Synthesis and Characterization of Ch-PANI-Fe2O3 Nanocomposite and Its Water Remediation Applications" Water 14, no. 22: 3615. https://doi.org/10.3390/w14223615
APA StyleSingh Rathore, B., Pal Singh Chauhan, N., Panneerselvam, P., Jadoun, S., Barani, M., Ameta, S. C., & Ameta, R. (2022). Synthesis and Characterization of Ch-PANI-Fe2O3 Nanocomposite and Its Water Remediation Applications. Water, 14(22), 3615. https://doi.org/10.3390/w14223615








