Adsorptive Removal of Methylene Blue Dye Using Biodegradable Superabsorbent Hydrogel Polymer Composite Incorporated with Activated Charcoal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Acrylic Acid Neutralization
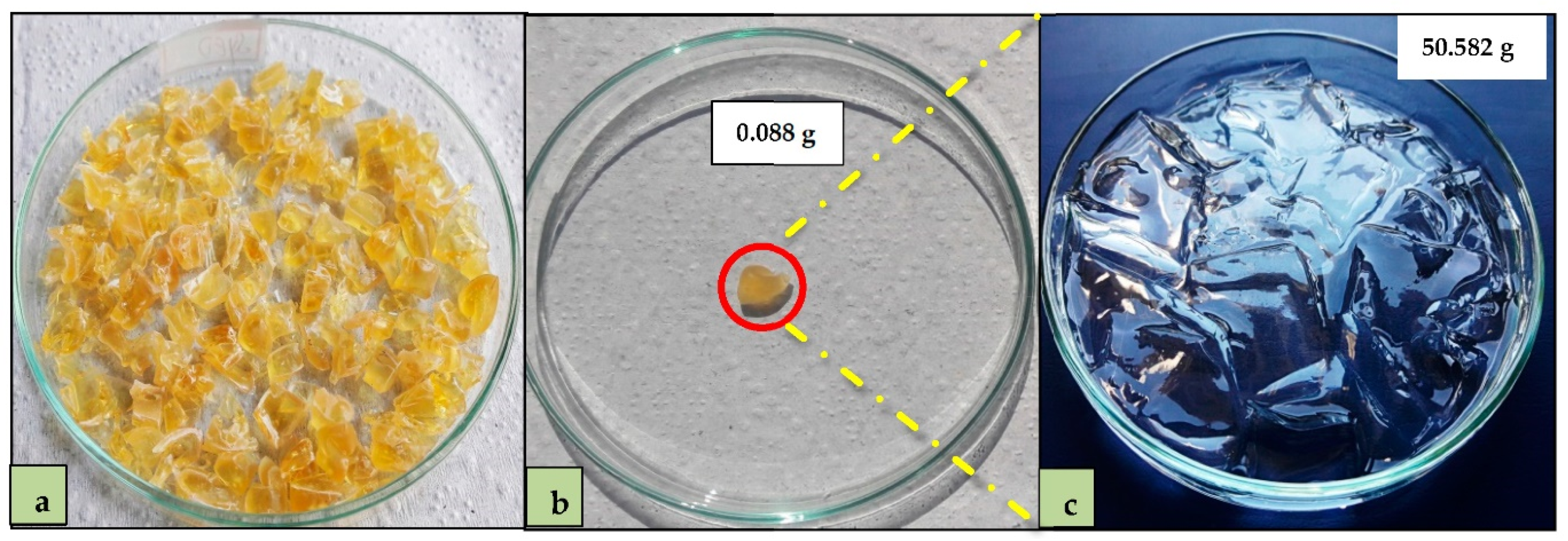
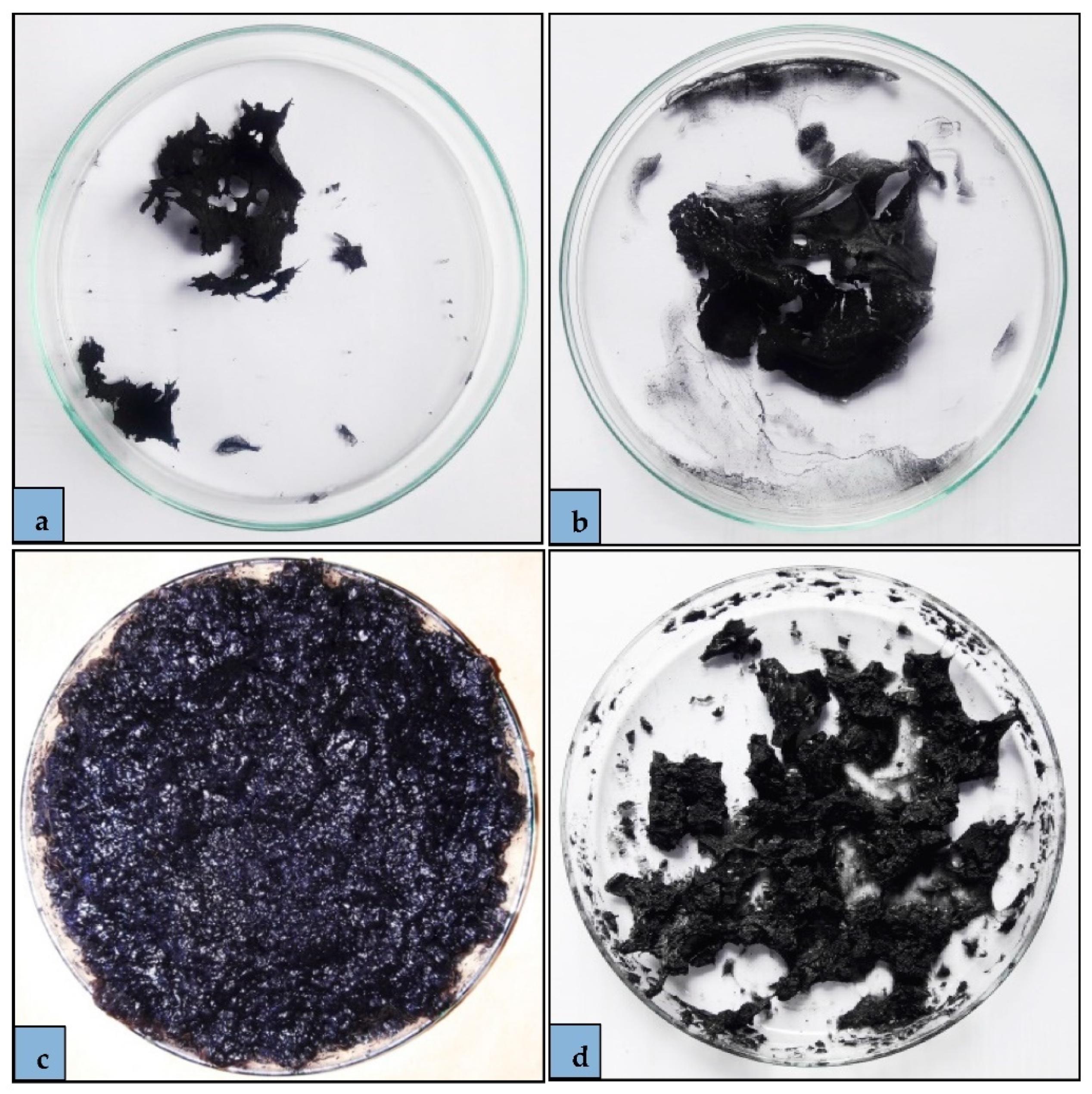
2.3. Adsorbent Preparation
2.4. Water Retention Capacity
2.5. Column Adsorption Studies
3. Results and Discussion
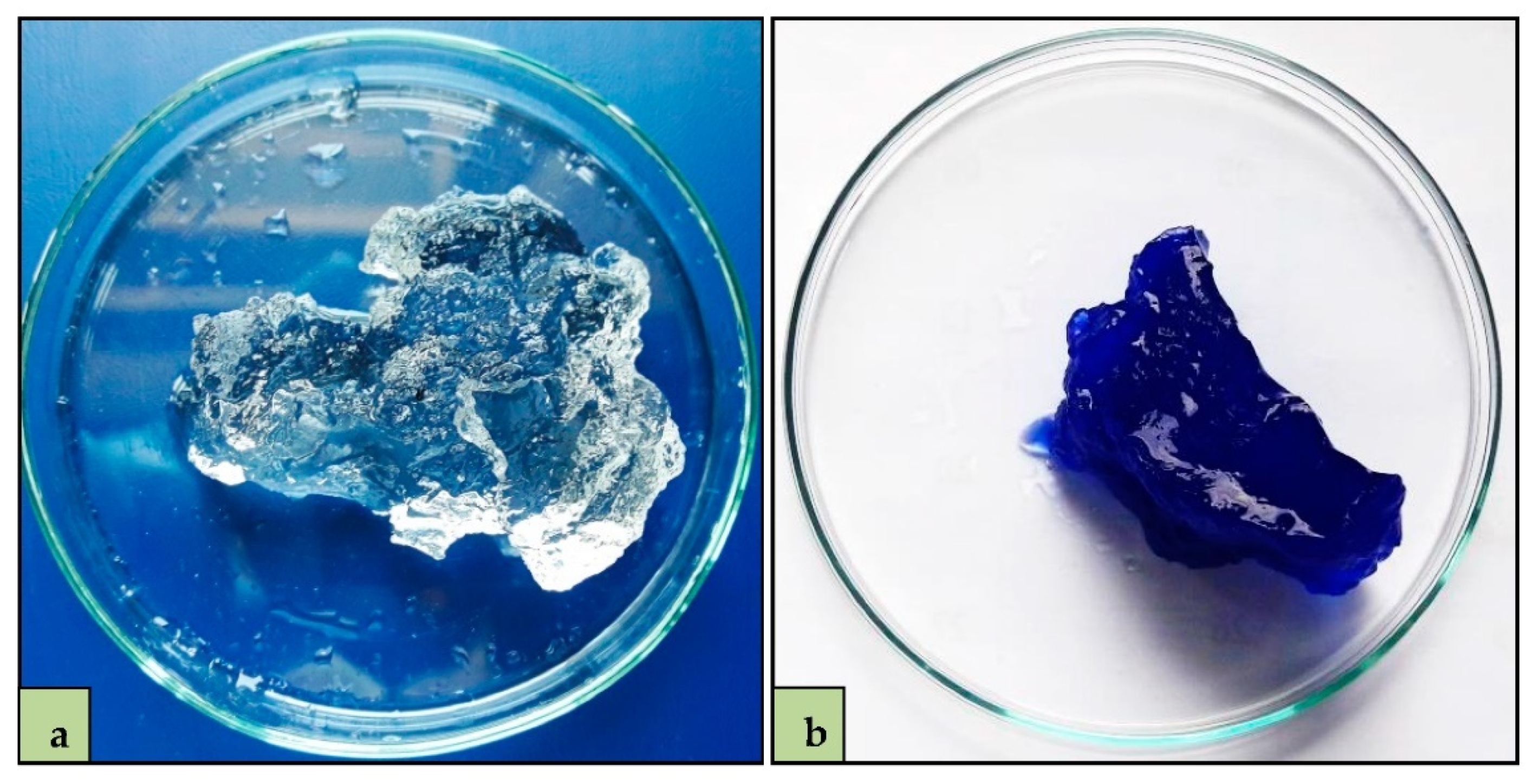
3.1. Swelling Capacity
3.2. MB Adsorption Experiments
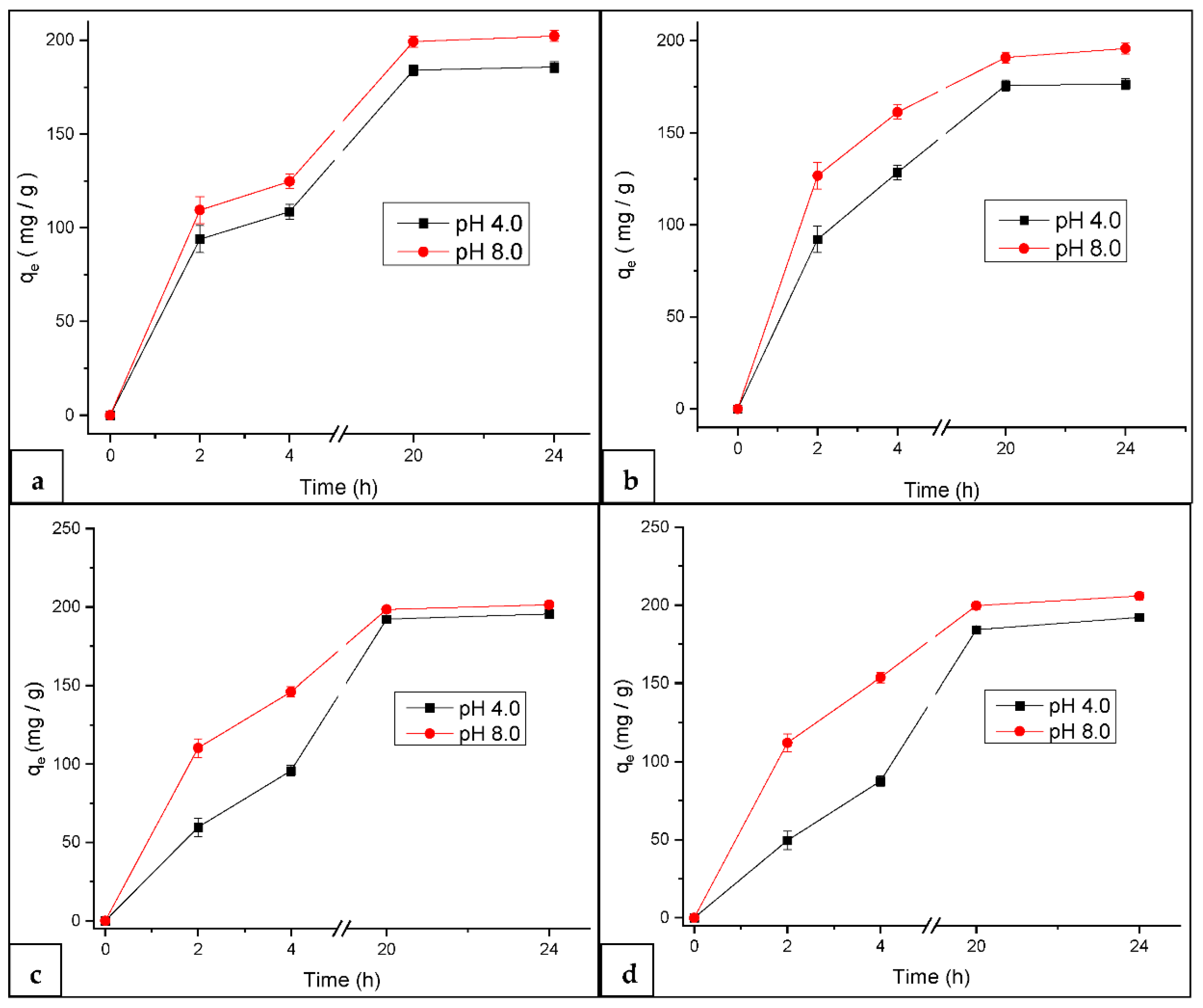
3.2.1. Effect of pH on MB Adsorption at Constant Temperature
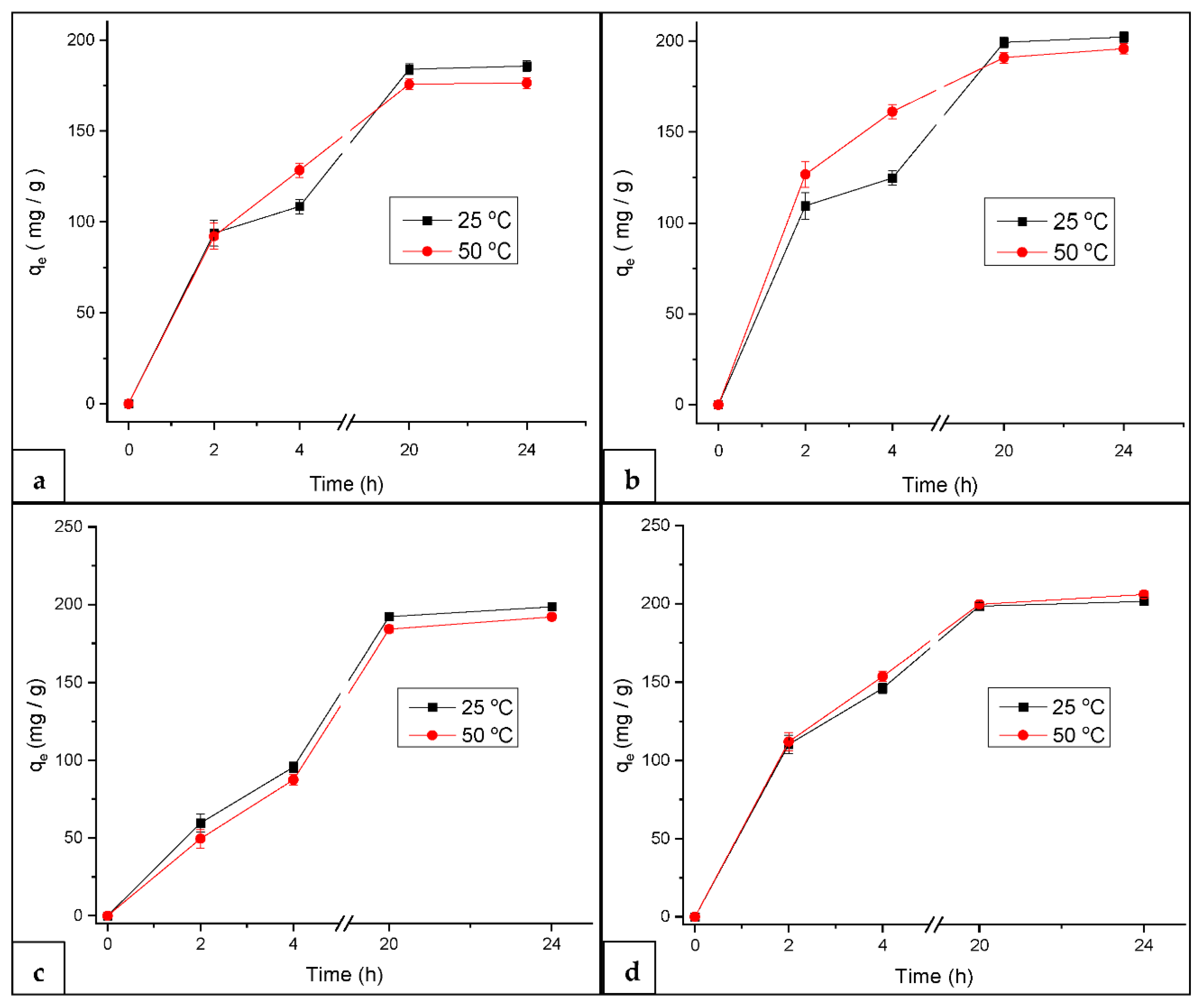
3.2.2. Effect of Temperature on MB Adsorption at Constant pH
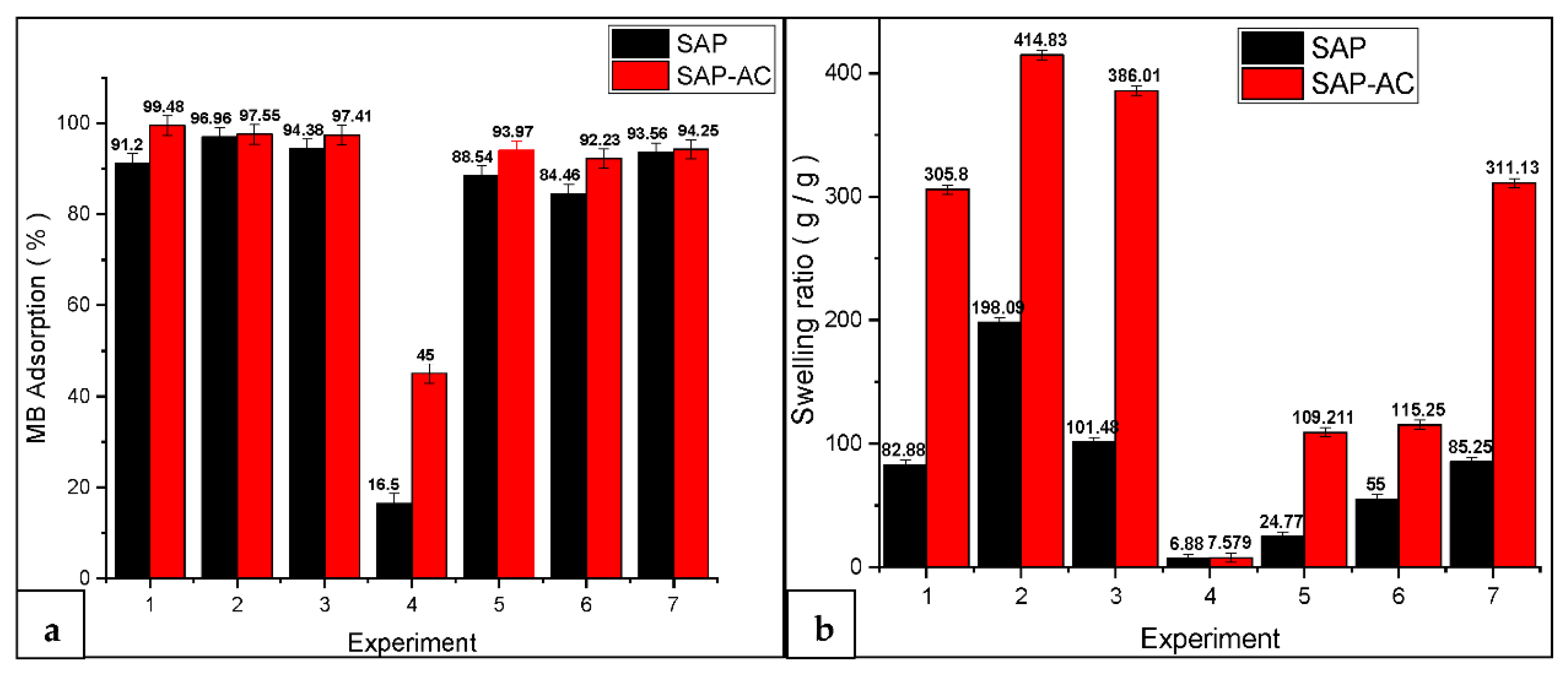
3.3. MB Adsorption Percentage and Swelling Ratio
3.4. Adsorption Capacity
3.5. MB Adsorption at Higher Initial Concentration
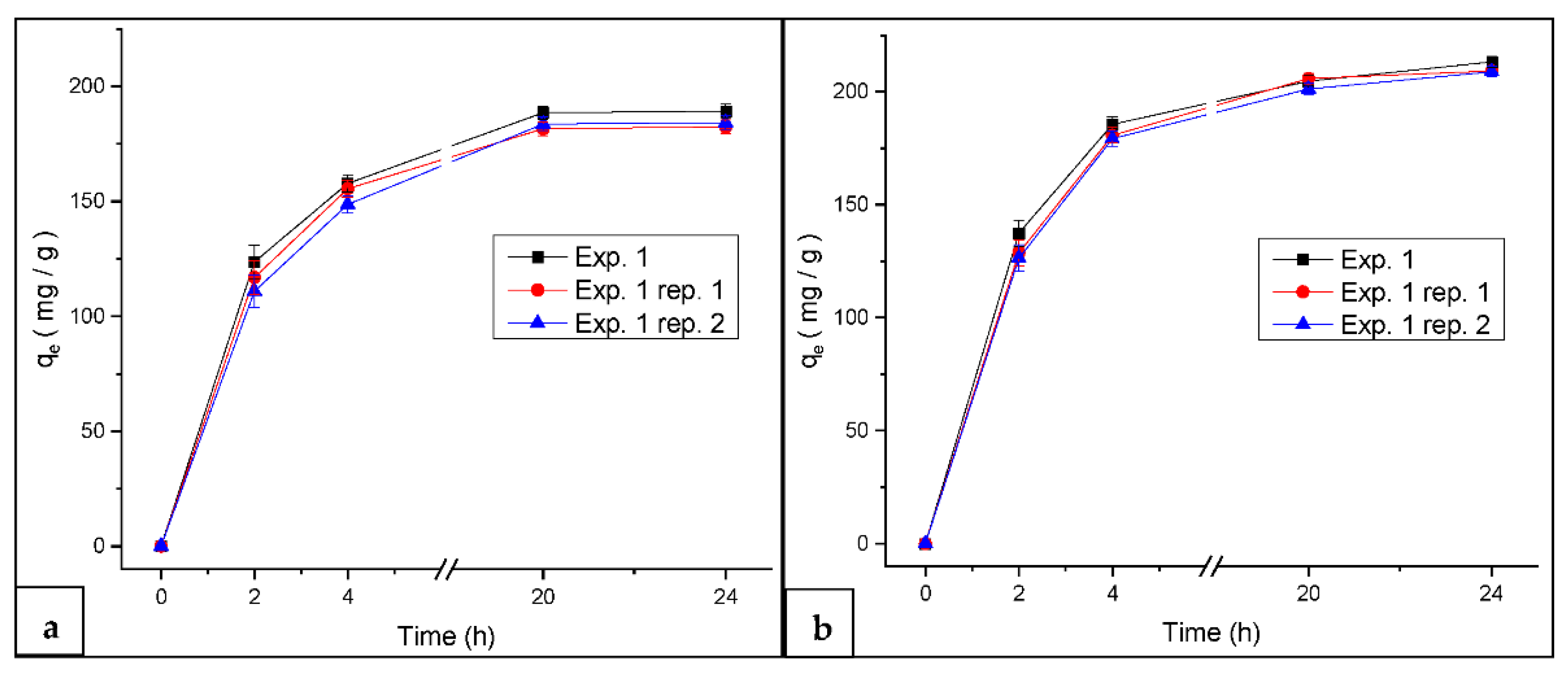
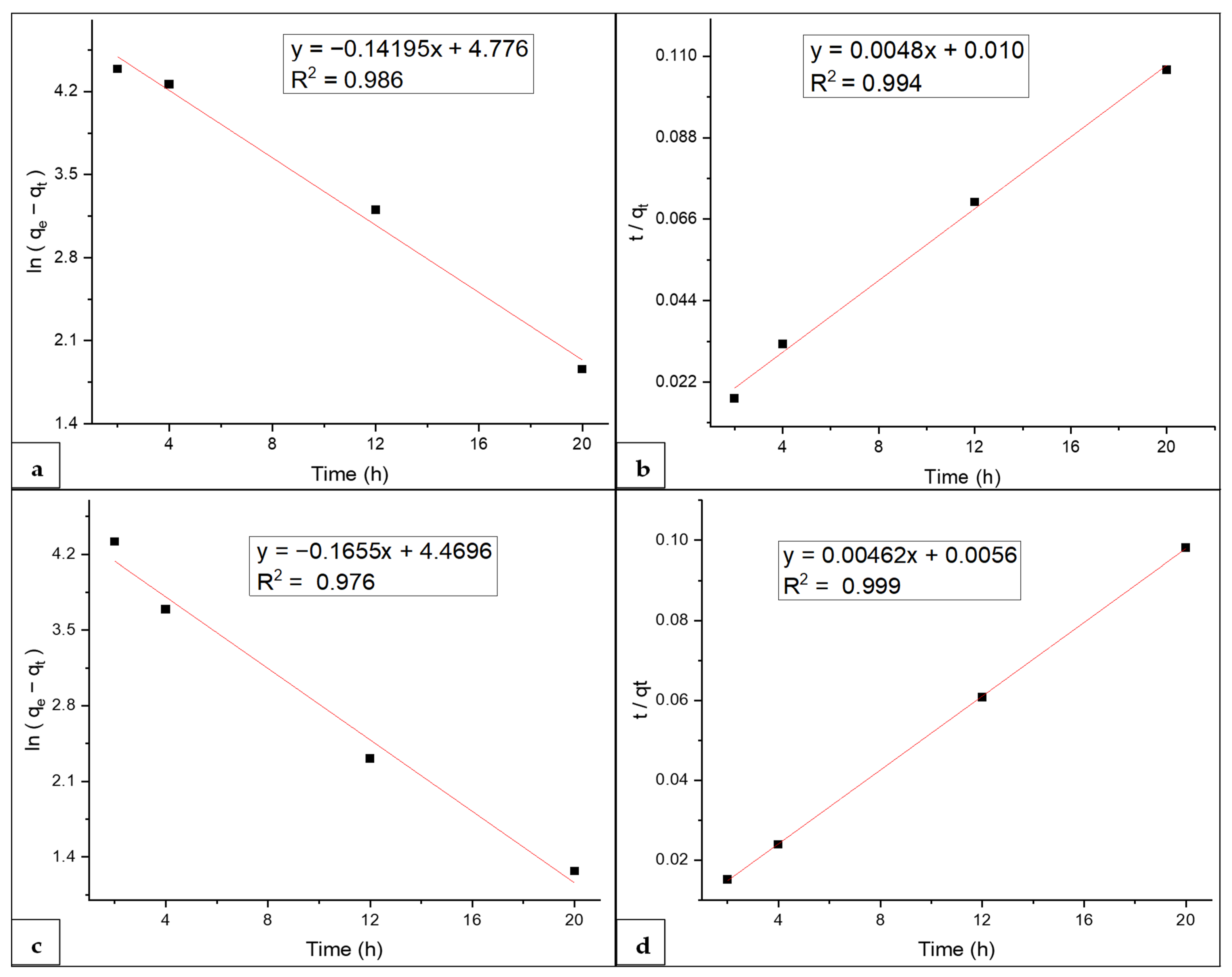
3.6. Kinetics Study
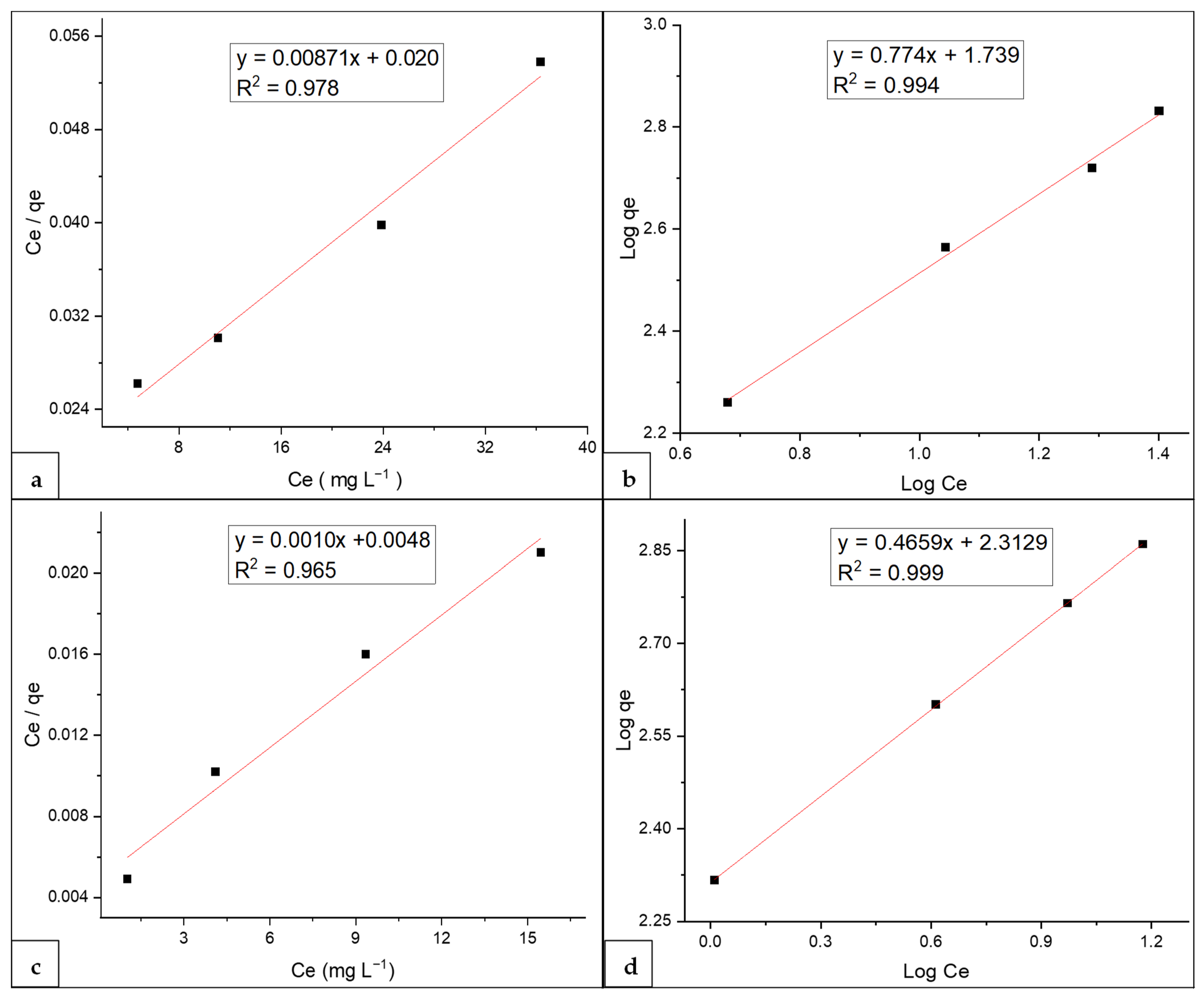
3.7. Adsorption Isotherms
3.8. Physicochemical Characterization of SAP Adsorbents
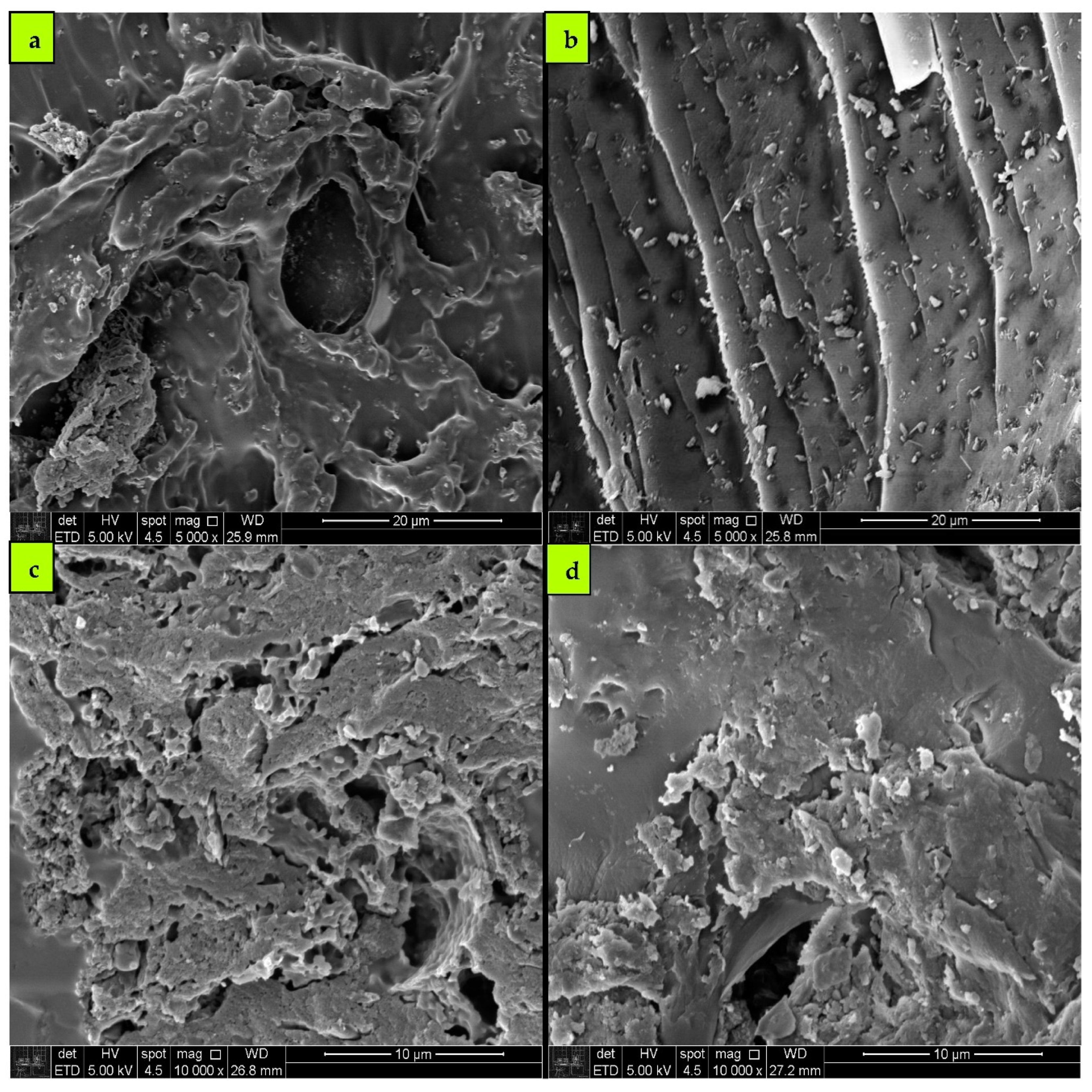
3.8.1. SEM Analysis
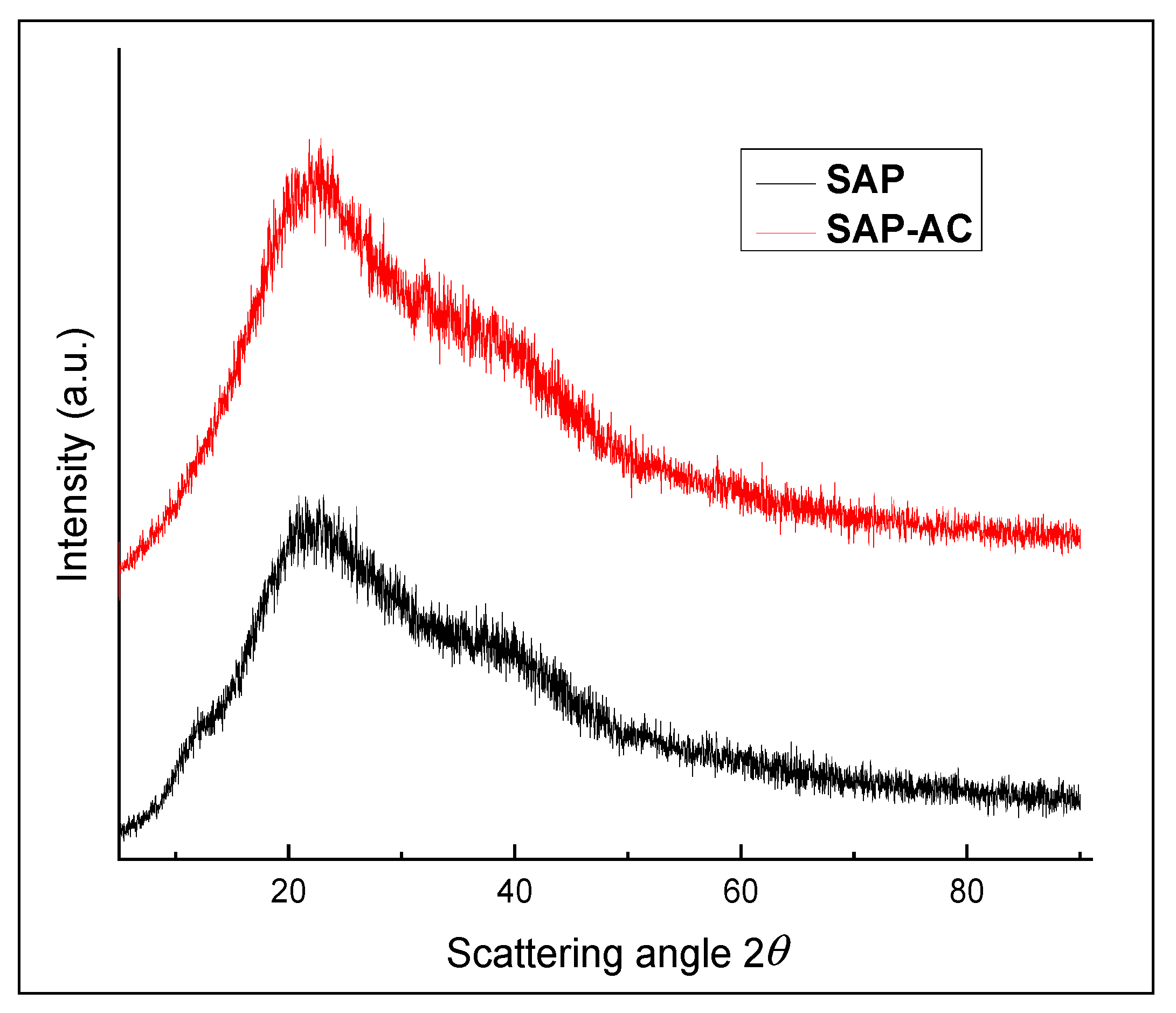
3.8.2. XRD Analysis
3.8.3. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vilela, D.; Parmar, J.; Zeng, Y.; Zhao, Y.; Sánchez, S. Graphene-Based Microbots for Toxic Heavy Metal Removal and Recovery from Water. Nano Lett. 2016, 16, 2860–2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Bhattacharya, A. Drinking Water Contamination and Treatment Techniques. Appl. Water Sci. 2016, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, Fate and Transformation of Emerging Contaminants in Water: An Overarching Review of the Field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Liu, Y.; Yang, Q.; Jiang, L.; Li, G. Occurrence and Distribution of Pharmaceuticals and Personal Care Products (PPCPs) in Wastewater Related Riverbank Groundwater. Sci. Total Environ. 2022, 821, 153372. [Google Scholar] [CrossRef] [PubMed]
- Ofrydopoulou, A.; Nannou, C.; Evgenidou, E.; Christodoulou, A.; Lambropoulou, D. Assessment of a Wide Array of Organic Micropollutants of Emerging Concern in Wastewater Treatment Plants in Greece: Occurrence, Removals, Mass Loading and Potential Risks. Sci. Total Environ. 2022, 802, 149860. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, Q.; Islam, S.M.; Liu, Y.; Ma, S.; Kanatzidis, M.G. Highly Selective and Efficient Removal of Heavy Metals by Layered Double Hydroxide Intercalated with the MoS42− Ion. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef]
- Ma, L.; Islam, S.M.; Liu, H.; Zhao, J.; Sun, G.; Li, H.; Ma, S.; Kanatzidis, M.G. Selective and Efficient Removal of Toxic Oxoanions of As(III), As(V), and Cr(VI) by Layered Double Hydroxide Intercalated with MoS42−. Chem. Mater. 2017, 29, 3274–3284. [Google Scholar] [CrossRef]
- Dotto, G.L.; Moura, J.M.; Cadaval, T.R.S.; Pinto, L.A.A. Application of Chitosan Films for the Removal of Food Dyes from Aqueous Solutions by Adsorption. Chem. Eng. J. 2013, 214, 8–16. [Google Scholar] [CrossRef]
- Dotto, G.L.; Lima, E.C.; Pinto, L.A.A. Biosorption of Food Dyes onto Spirulina Platensis Nanoparticles: Equilibrium Isotherm and Thermodynamic Analysis. Bioresour. Technol. 2012, 103, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Lin, S.; Yue, T.; Lee, T.-C. Adsorption of Food Dyes from Aqueous Solution by Glutaraldehyde Cross-Linked Magnetic Chitosan Nanoparticles. J. Food Eng. 2014, 126, 133–141. [Google Scholar] [CrossRef]
- Muniyandi, M.; Govindaraj, P.; Bharath Balji, G. Potential Removal of Methylene Blue Dye from Synthetic Textile Effluent Using Activated Carbon Derived from Palmyra (Palm) Shell. Mater. Today Proc. 2021, 47, 299–311. [Google Scholar] [CrossRef]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A. Fabrication of Sphere-like Bi2MoO6/ZnO Composite Catalyst with Strong Photocatalytic Behavior for the Detoxification of Harmful Organic Dyes. Opt. Mater. 2020, 109, 110218. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y.; Tian, F.; Zhang, J.; Yu, J. Structure Tuning of Bi2MoO6 and Their Enhanced Visible Light Photocatalytic Performances. Crit. Rev. Solid State Mater. Sci. 2017, 42, 347–372. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Yuan, X.; Huang, J.; Zhang, J.; Zeng Yu, G.H.; Jiang, L.; Wang, H.; et al. Modulation of Bi2MoO6-Based Materials for Photocatalytic Water Splitting and Environmental Application: A Critical Review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef]
- Krishna Moorthy, A.; Govindarajan Rathi, B.; Shukla, S.P.; Kumar, K.; Shree Bharti, V. Acute Toxicity of Textile Dye Methylene Blue on Growth and Metabolism of Selected Freshwater Microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef]
- Yao, H.; You, X.-M.; Lin, Q.; Li, J.-J.; Guo, Y.; Wei, T.-B.; Zhang, Y.-M. Multi-Stimuli Responsive Metal-Organic Gel of Benzimidazol-Based Ligands with Lead Nitrate and Their Use in Removal of Dyes from Waste-Water. Chin. Chem. Lett. 2013, 24, 703–706. [Google Scholar] [CrossRef]
- Gokturk, S.; Kaluc, S. Removal of Selected Organic Compounds in Aqueous Solutions by Activated Carbon. J. Environ. Sci. Technol. 2008, 1, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Shi, Q.; Zhang, C.; Xu, J.; Zhai, B.; Zhang, B. Adsorption of Neutral Red onto Mn-Impregnated Activated Carbons Prepared from Typha Orientalis. Bioresour. Technol. 2008, 99, 8974–8980. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, L.; Ma, A.; Xia, H.; Peng, J.; Li, C.; Shu, J. Comparison of Activated Carbon and Iron/Cerium Modified Activated Carbon to Remove Methylene Blue from Wastewater. J. Environ. Sci. 2018, 65, 92–102. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M.; Pétrier, C. Influence of Bicarbonate and Carbonate Ions on Sonochemical Degradation of Rhodamine B in Aqueous Phase. J. Hazard. Mater. 2010, 175, 593–599. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Derome, C.; Buleté, A.; Vulliet, E.; Bressy, A.; Varrault, G.; Chebbo, G.; Rocher, V. Removal of Emerging Micropollutants from Wastewater by Activated Carbon Adsorption: Experimental Study of Different Activated Carbons and Factors Influencing the Adsorption of Micropollutants in Wastewater. J. Environ. Chem. Eng. 2016, 4, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Abramian, L.; El-Rassy, H. Adsorption Kinetics and Thermodynamics of Azo-Dye Orange II onto Highly Porous Titania Aerogel. Chem. Eng. J. 2009, 150, 403–410. [Google Scholar] [CrossRef]
- Cechinel, M.A.P.; Ulson De Souza, S.M.A.G.; Ulson De Souza, A.A. Study of Lead (II) Adsorption onto Activated Carbon Originating from Cow Bone. J. Clean. Prod. 2014, 65, 342–349. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J. Enhanced Removal of Nitrate from Water Using Surface Modification of Adsorbents—A Review. J. Environ. Manag. 2013, 131, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Ahmad, I.; Ahmad, W.; Ishaq, M.; Gul, K.; Khan, R.; Khan, H. Study on Adsorptive Capability of Acid Activated Charcoal for Desulphurization of Model and Commercial Fuel Oil Samples. J. Environ. Chem. Eng. 2018, 6, 4037–4043. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.S.; Ahmad, I.; Ahmad, W.; Ishaq, M.; Khan, H. Deep Desulphurization Study of Liquid Fuels Using Acid Treated Activated Charcoal as Adsorbent. Energy Fuels 2017, 31, 7867–7873. [Google Scholar] [CrossRef]
- Shah, S.S.; Ahmad, I.; Ahmad, W. Adsorptive Desulphurization Study of Liquid Fuels Using Tin (Sn) Impregnated Activated Charcoal. J. Hazard. Mater. 2016, 304, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Spagnol, C.; Rodrigues, F.H.A.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogel Nanocomposites Based on Starch-g-Poly(Sodium Acrylate) Matrix Filled with Cellulose Nanowhiskers. Cellulose 2012, 19, 1225–1237. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel Biodegradable Hydrogel Based on Natural Polymers: Synthesis, Characterization, Swelling/Reswelling and Biodegradability. Eur. Polym. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Alam, A.; Zhang, Y.; Kuan, H.C.; Lee, S.H.; Ma, J. Polymer Composite Hydrogels Containing Carbon Nanomaterials—Morphology and Mechanical and Functional Performance. Prog. Polym. Sci. 2018, 77, 1–18. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Dos Santos, W.N.L.; Quintella, C.M.; Neto, B.B.; Bosque-Sendra, J.M. Doehlert Matrix: A Chemometric Tool for Analytical Chemistry—Review. Talanta 2004, 63, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press Inc.: Boca Raton, FL, USA, 2005. [Google Scholar]
- Thakur, S.; Arotiba, O.A. Synthesis, Swelling and Adsorption Studies of a PH-Responsive Sodium Alginate–Poly(Acrylic Acid) Superabsorbent Hydrogel. Polym. Bull. 2018, 75, 4587–4606. [Google Scholar] [CrossRef]
- Kong, Y.; Zhuang, Y.; Han, Z.; Yu, J.; Shi, B.; Han, K.; Hao, H. Dye Removal by Eco-Friendly Physically Cross-Linked Double Network Polymer Hydrogel Beads and Their Functionalized Composites. J. Environ. Sci. 2019, 78, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Momina; Mohammad, S.; Suzylawati, I. Study of the Adsorption/Desorption of MB Dye Solution Using Bentonite Adsorbent Coating. J. Water Process Eng. 2020, 34, 101155. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of Methylene Blue by Adsorption onto Activated Carbon Developed from Ficus Carica Bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef] [Green Version]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene Blue Adsorption on Magnetic Alginate/Rice Husk Bio-Composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Jamroz, N.U.; Sharif, Q.M. Micellization Parameters and Electrostatic Interactions in Micellar Solution of Sodium Dodecyl Sulfate (SDS) at Different Temperatures. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 178, 199–206. [Google Scholar] [CrossRef]
- Ravi; Pandey, L.M. Enhanced Adsorption Capacity of Designed Bentonite and Alginate Beads for the Effective Removal of Methylene Blue. Appl. Clay Sci. 2019, 169, 102–111. [Google Scholar] [CrossRef]
- Saito, H.; Taguchi, T.; Aoki, H.; Murabayashi, S.; Mitamura, Y.; Tanaka, J.; Tateishi, T. PH-Responsive Swelling Behavior of Collagen Gels Prepared by Novel Crosslinkers Based on Naturally Derived Di- or Tricarboxylic Acids. Acta Biomater. 2007, 3, 89–94. [Google Scholar] [CrossRef]
- Khare, A.R.; Peppas, N.A. Swelling/Deswelling of Anionic Copolymer Gels. Biomaterials 1995, 16, 559–567. [Google Scholar] [CrossRef]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and Properties of Quaternary Ammonium Chitosan-g-Poly(Acrylic Acid-Co-Acrylamide) Superabsorbent Hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Sanati, A.M.; Kamari, S.; Ghorbani, F. Application of Response Surface Methodology for Optimization of Cadmium Adsorption from Aqueous Solutions by Fe3O4@SiO2@APTMS Core–Shell Magnetic Nanohybrid. Surf. Interfaces 2019, 17, 100374. [Google Scholar] [CrossRef]
- Lei, C.; Bian, Y.; Zhi, F.; Hou, X.; Lv, C.; Hu, Q. Enhanced Adsorption Capacity of Cellulose Hydrogel Based on Corn Stalk for Pollutants Removal and Mechanism Exploration. J. Clean. Prod. 2022, 375, 134130. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; McClements, D.J.; Goodarzi, V.; Chen, W.H. Removal of Methylene Blue from Wastewater Using Ternary Nanocomposite Aerogel Systems: Carboxymethyl Cellulose Grafted by Polyacrylic Acid and Decorated with Graphene Oxide. J. Hazard. Mater. 2022, 421, 126752. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Y.; Ma, L.; Zhang, H.; Huang, H. Synthesis and Response of Pineapple Peel Carboxymethyl Cellulose-g-Poly (Acrylic Acid-Co-Acrylamide)/Graphene Oxide Hydrogels. Carbohydr. Polym. 2019, 215, 366–376. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption Removal of Methylene Blue onto Activated Carbon/Cellulose Biocomposite Films: Equilibrium and Kinetic Studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Hameed, B.H.; Ahmad, A.L. Equilibrium and Kinetic Studies on Basic Dye Adsorption by Oil Palm Fibre Activated Carbon. Chem. Eng. J. 2007, 127, 111–119. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Junlapong, K.; Maijan, P.; Chaibundit, C.; Chantarak, S. Effective Adsorption of Methylene Blue by Biodegradable Superabsorbent Cassava Starch-Based Hydrogel. Int. J. Biol. Macromol. 2020, 158, 258–264. [Google Scholar] [CrossRef]
- Wang, W.; Ni, J.; Chen, L.; Ai, Z.; Zhao, Y.; Song, S. Synthesis of Carboxymethyl Cellulose-Chitosan-Montmorillonite Nanosheets Composite Hydrogel for Dye Effluent Remediation. Int. J. Biol. Macromol. 2020, 165, 1–10. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Modibane, K.D.; Kang, M.; Hato, M.J. Sequestration of Methylene Blue Dye Using Sodium Alginate Poly(Acrylic Acid)@ZnO Hydrogel Nanocomposite: Kinetic, Isotherm, and Thermodynamic Investigations. Int. J. Biol. Macromol. 2020, 162, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Binma-ae, H.; Prasertsan, P.; Choorit, W. Preparation and Characterization of Biopolymers Recovered from Palm Oil Mill Effluent and Their Complex Hydrogels Compared to Commercial Xylan. Waste Biomass Valoriz. 2020, 11, 5109–5121. [Google Scholar] [CrossRef]





| Coded Values | Real Values | ||||
|---|---|---|---|---|---|
| Exp. no. | pH | Temperature (°C) | Exp. no. | pH | Temperature (°C) |
| 1 | 0 | 0 | 1 | 6.00 | 37.5 |
| 1 rep. 1 | 0 | 0 | 1 rep. 1 | 6.00 | 37.5 |
| 1 rep. 2 | 0 | 0 | 1 rep. 2 | 6.00 | 37.5 |
| 2 | 1 | 0 | 2 | 10.00 | 37.5 |
| 3 | 0.5 | 0.866 | 3 | 8.00 | 50.0 |
| 4 | −1 | 0 | 4 | 2.00 | 37.5 |
| 5 | −0.5 | −0.866 | 5 | 4.00 | 25.0 |
| 6 | −0.5 | 0.866 | 6 | 4.00 | 50.0 |
| 7 | 0.5 | −0.866 | 7 | 8.00 | 25.0 |
| S. no. | Adsorbent | Initial MB conc. (mg L−1) | MB Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|
| 1. | Sodium alginate/cellulose | 100 | 328.36 | [44] |
| 2. | Carboxymethylcellulose/PAA */GO * | 100 | 138.4 | [45] |
| 3. | * PCMC/AA/acrylamide/GO | 200 | 133.32 | [46] |
| 4. | Cellulose/activated carbon | 100 | 103.66 | [47] |
| 5. | SAP-AC | 50 | 213.2 | Current study |
| 6. | SAP-AC | 100 | 399.03 | Current study |
| 7. | SAP-AC | 200 | 736.75 | Current study |
| * PAA = Polyacrylic acid, GO = Graphene oxide, PCMC = Pineapple peel carboxymethyl cellulose. | ||||
| SAP | SAP-AC | ||||||
|---|---|---|---|---|---|---|---|
| Pseudo first-order parameters | Pseudo second-order parameters | Pseudo first-order parameters | Pseudo second-order parameters | ||||
| qe (mg g−1) | 118.62 | qe (mg g−1) | 208.33 | qe (mg g−1) | 87.32 | qe (mg g−1) | 216.45 |
| K1 (min−1) | 0.007 | K2 (mg g−1 min−1) | 0.0023 | K1 (min−1) | 0.0082 | K2 (mg g−1 min−1) | 0.0038 |
| R2 | 0.986 | R2 | 0.994 | R2 | 0.976 | R2 | 0.999 |
| SAP | SAP-AC | ||||||
|---|---|---|---|---|---|---|---|
| Langmuir isotherm parameters | Freundlich isotherm parameters | Langmuir isotherm parameters | Freundlich isotherm parameters | ||||
| qm (mg g−1) | 114.94 | n | 1.291 | qm (mg g−1) | 917.43 | n | 2.146 |
| KL | 0.435 | KF | 54.82 | KL | 0.227 | KF | 205.54 |
| R2 | 0.978 | R2 | 0.994 | R2 | 0.965 | R2 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.S.; Ramos, B.; Teixeira, A.C.S.C. Adsorptive Removal of Methylene Blue Dye Using Biodegradable Superabsorbent Hydrogel Polymer Composite Incorporated with Activated Charcoal. Water 2022, 14, 3313. https://doi.org/10.3390/w14203313
Shah SS, Ramos B, Teixeira ACSC. Adsorptive Removal of Methylene Blue Dye Using Biodegradable Superabsorbent Hydrogel Polymer Composite Incorporated with Activated Charcoal. Water. 2022; 14(20):3313. https://doi.org/10.3390/w14203313
Chicago/Turabian StyleShah, Syed Sikandar, Bruno Ramos, and Antonio Carlos Silva Costa Teixeira. 2022. "Adsorptive Removal of Methylene Blue Dye Using Biodegradable Superabsorbent Hydrogel Polymer Composite Incorporated with Activated Charcoal" Water 14, no. 20: 3313. https://doi.org/10.3390/w14203313
APA StyleShah, S. S., Ramos, B., & Teixeira, A. C. S. C. (2022). Adsorptive Removal of Methylene Blue Dye Using Biodegradable Superabsorbent Hydrogel Polymer Composite Incorporated with Activated Charcoal. Water, 14(20), 3313. https://doi.org/10.3390/w14203313








