The Role of the Catchment Area in Shaping Water Quality in the Lowland Springs of the Knyszyn Forest (NE Poland)
Abstract
:1. Introduction
- -
- Drawing attention to the presence and importance of natural groundwater outflows in the lowlands;
- -
- The assessment of the physical and chemical compositions of the waters showing the specificity of the water quality of lowland springs (a significant concentration of biogenic elements that determine their fertility);
- -
- The impact of land use as a factor differentiating the quality of shallow groundwaters flowing onto the surface in the form of springs.
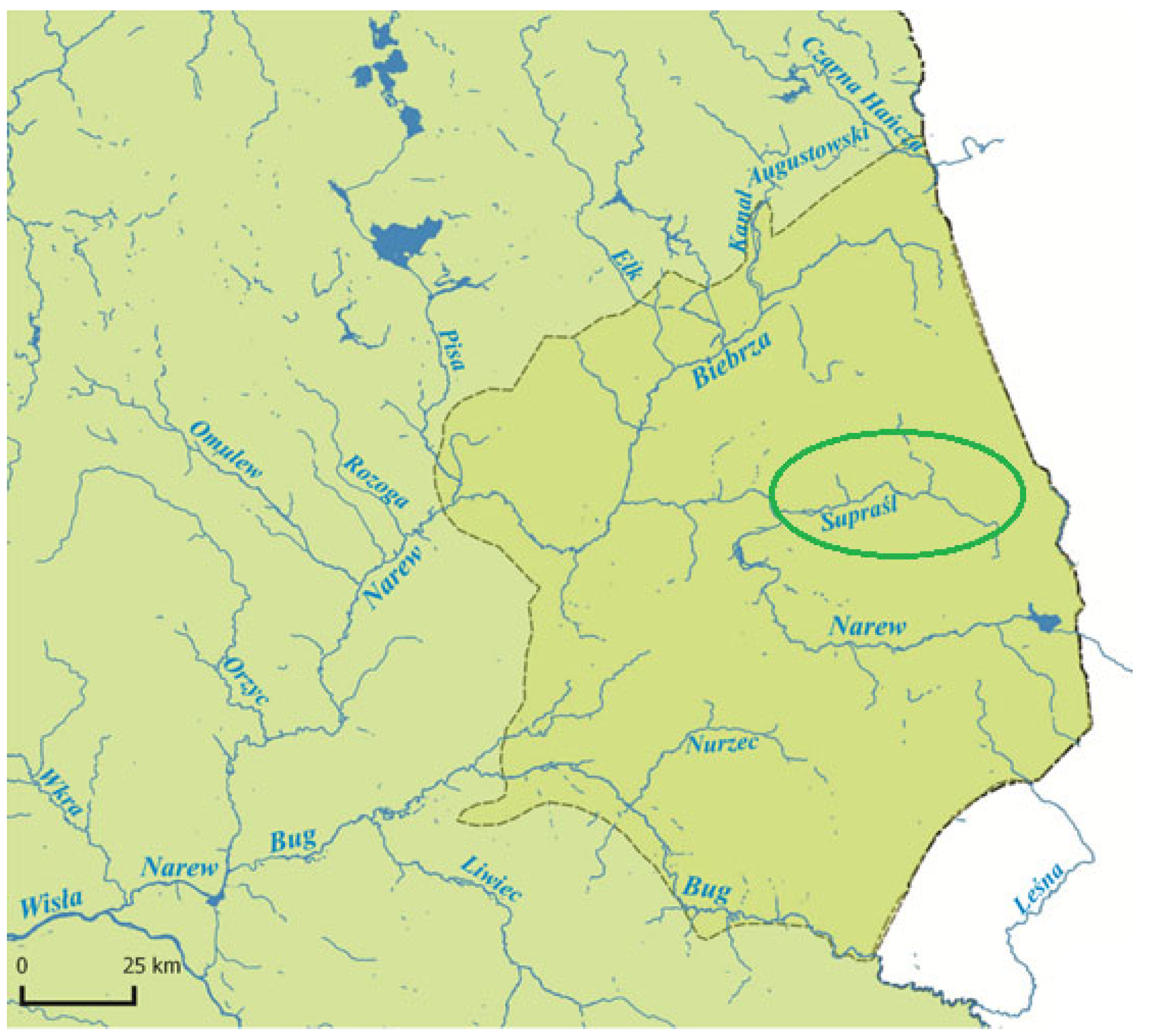
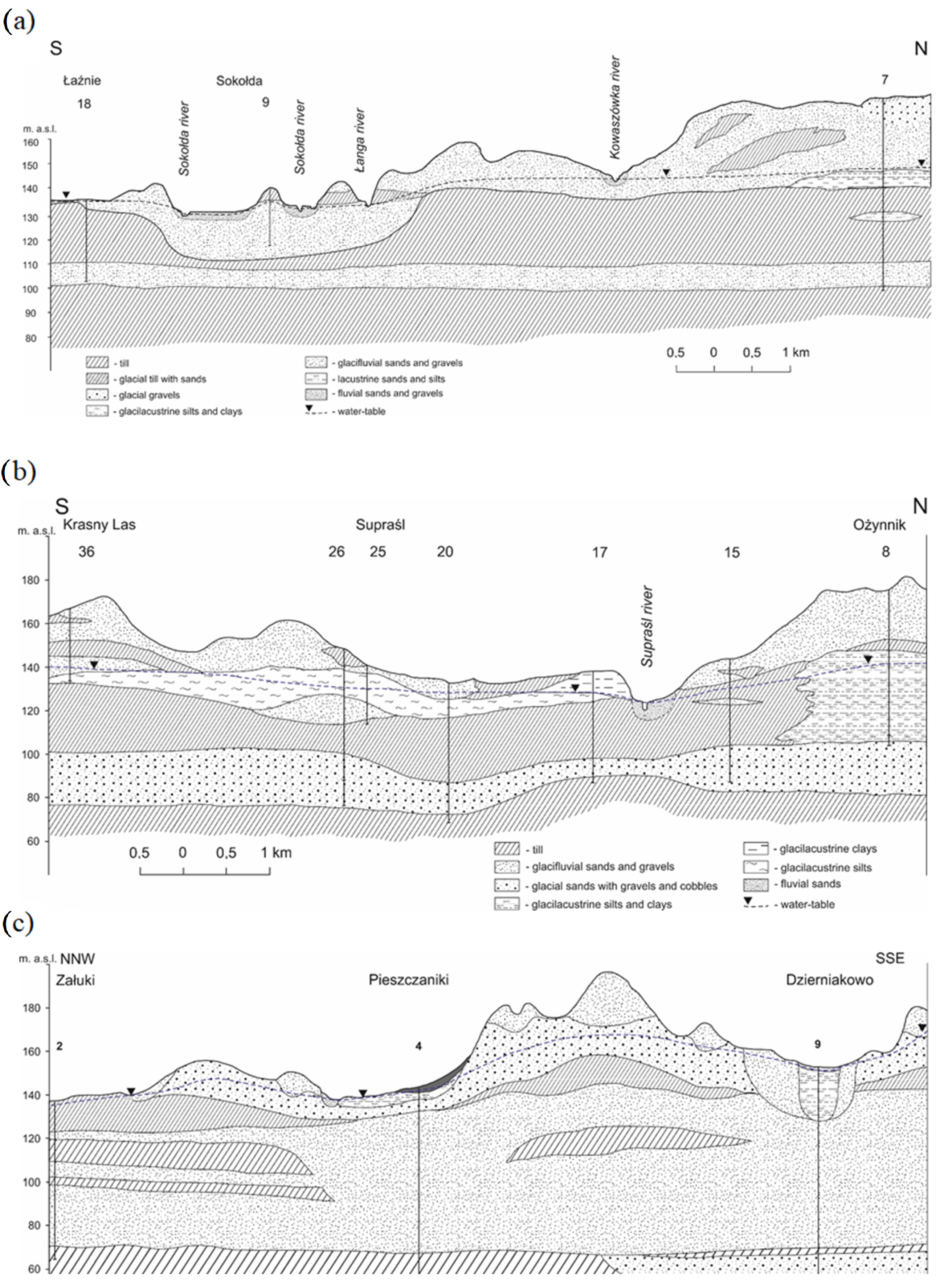
2. Study Area
3. Material and Methods
3.1. Map Analysis and Field Research
3.2. Water Quality Assessment
- Total (TP, TFe) samples of unfiltered and mineralized water;
- Dissolved (DP, DFe) samples of filtered and mineralized water;
- Reactive (SRP, SRFe) samples of unfiltered and non-mineralized water;
- Particle fractions (PP, PFe) were calculated using the following formulas: PP = TP − DP PFe = TFe − Dfe;
- Dissolved organic fractions (ODP, ODFe) were calculated using the following formulas: ODP = DP − SRP ODFe = DFe − SRFe.
3.3. Statistical Analyses
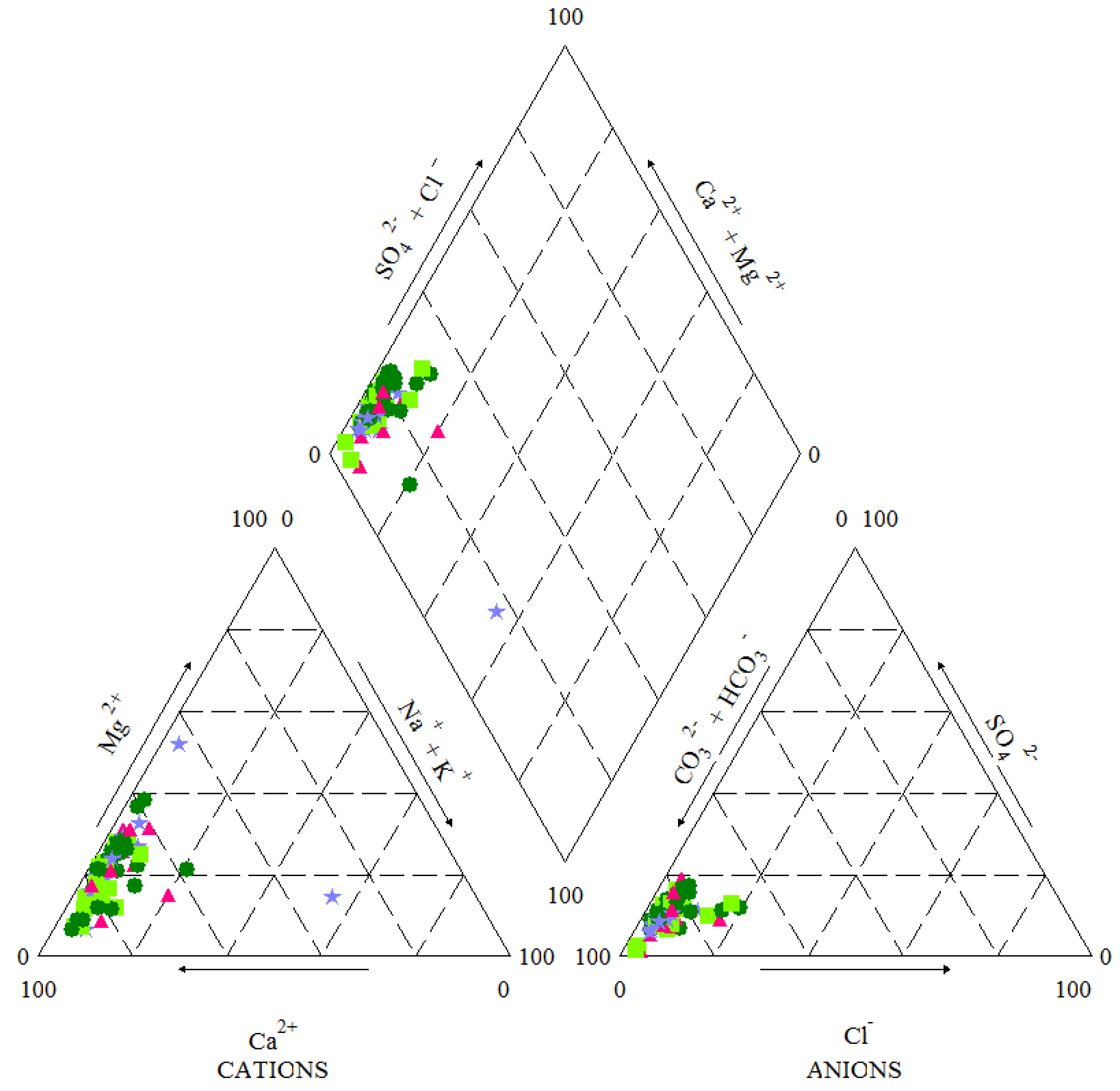
4. Results
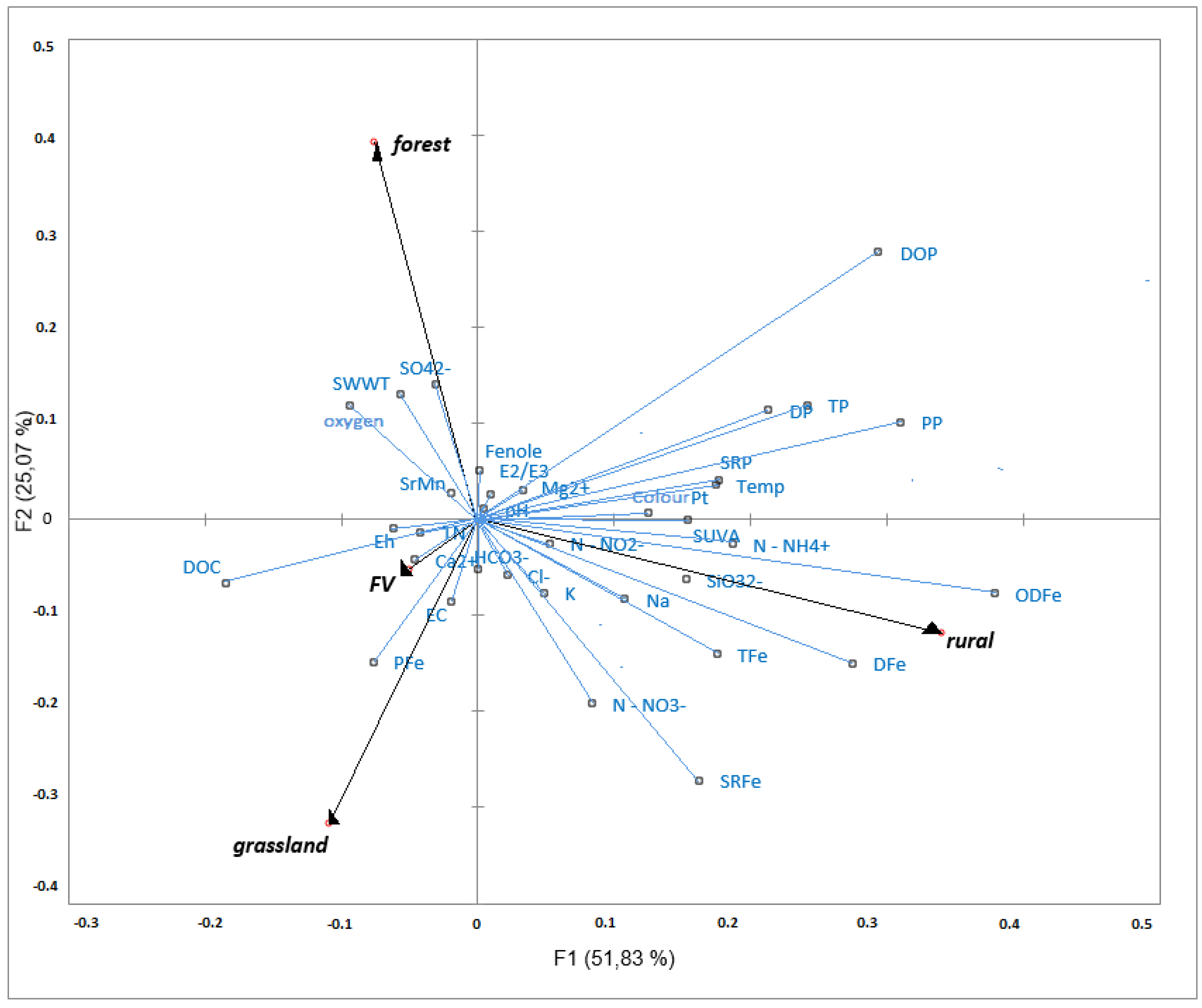
5. Discussion
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, D.D. The spring as an interface between groundwater and lotie faunas and as a tool in assessing groundwater quality. Verh. Internat. Verein. Limnol. 1991, 24, 1621–6124. [Google Scholar]
- Webb, D.W.; Wetzel, M.J.; Reed, P.C.; Philippe, L.R.; Young, T.C. The macroninvertebrate biodiversity, water quality, and hydrogeology of ten karst springs in the Salem Plateau of Illinois. In Studies in Crenobiology; The Biology of Springs and Springbrooks; Botosaneanu, L., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1988; pp. 39–48. [Google Scholar]
- Cantonati, M.; Gerecke, R.; Bertuzzi, E. Springs of the Alps—Sensitive ecosystems to environmental change: From biodiversity assessments to longterm studies. Hydrobiologia 2006, 562, 59–96. [Google Scholar] [CrossRef]
- Zieliński, P.; JekatierynczukRudczyk, E.; Puczko, K. Factors affecting the abundance and activity of the bacterioplankton in lowland forest springs in northeastern Poland. Ecohydrol. Hydrobiol. 2020, 20, 675–686. [Google Scholar] [CrossRef]
- Cantonati, M.; LangeBertalot, H. Diatom Biodiversity of springs in the Berchtesgaden National Park (north—Eastern Alps, Germany), with the ecological and morphological characterization of two species new to science. Diatom Res. 2010, 25, 251–280. [Google Scholar] [CrossRef]
- Scarsbrook, M.; Barquín, J.; Gray, D. Science for Conservation 278, New Zealand Coldwater Springs and Their Biodiversity; Science & Technical Publishing: Wellington, New Zeland, 2007; 71p. [Google Scholar]
- Lai, G.G.; Burato, S.; Padedda, B.M.; Zorza, R.; Pizzul, E.; Delgado, C.; Lugliè, A.; Cantonati, M. Diatom Biodiversity in Karst Springs of Mediterranean Geographic Areas with Contrasting Characteristics: Islands vs Mainland. Water 2019, 11, 2602. [Google Scholar] [CrossRef] [Green Version]
- Chełmicki, W.; Jokiel, P.; Michalczyk, Z.; Moniewski, P. Distribution, discharge and regional characteristics of springs in Poland. Episodes 2011, 34, 244256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokiel, P.; Michalczyk, Z. Springs of Poland—Save for the Future. Pr. Geogr. 2019, 157, 3–7, (In Polish with English summary). [Google Scholar]
- JekatierynczukRudczyk, E. Effects of drainage basin managment on the chemical composition of waters in lowlands springs. Acta Hydrobiol. 1999, 41, 97–105. [Google Scholar]
- JekatierynczukRudczyk, E. Threats to a small river and its urban catchment. Ecohydrol. Hydrobiol. 2008, 8, 77. [Google Scholar] [CrossRef]
- Puczko, K.; Zieliński, P.; Jusik, S.; Kołakowska, A.; JekatierynczukRudczyk, E. Vascular plant and bryophyte species richness in response to water quality in lowland spring niches with different anthropogenic impacts. Environ. Monit. Assess. 2018, 190, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- JekatierynczukRudczyk, E. Holy waters in the Podlasie region, Poland, as a witness of hydrological and hydrochemical changes. Ecohydrol. Hydrobiol. 2020, 20, 610–621. [Google Scholar] [CrossRef]
- Łoszewski, H. Springs in Bialystok and the need to protect them. Białostocczyzna 1995, 4, 140–153. [Google Scholar]
- Lehosmaa, K.; Jyväsjärvi, J.; Virtanen, R.; Rossi, P.M.; Rados, D.; Chuzhekova, T.; Markkola, A.M.; Ilmonen, J.; Muotka, T. Does habitat restoration enhance spring biodiversity and ecosystem functions? Hydrobiologia 2017, 793, 161–173. [Google Scholar] [CrossRef]
- Springer, A.; Stevens, L.E. Spheres of discharge of springs. Hydrogeol. J. 2009, 17, 83–89. [Google Scholar] [CrossRef]
- Kondracki, J. Regional Geography of Poland; Scientific PWN: Warsaw, Poland, 2011; 635p. [Google Scholar]
- Banaszuk, H. Problems of glacial morphogenesis of the North Podlasie Lowland. Work. Geogr. Stud. 2019, 64, 89–112, (In Polish with English Summary). [Google Scholar]
- Czarnecka, H. (Ed.) Atlas of the Hydrographic Division of Poland; Part I: Maps at a scale of 1: 200 000; IMGW: Warsaw, Poland, 2005. [Google Scholar]
- Czarnecka, H. (Ed.) Atlas of the Hydrographic Division of Poland; Part II: Lists of catchments; IMGW: Warsaw, Poland, 2005; 262p. [Google Scholar]
- Ber, A. Plejstocene of NorthEastern Poland and Neighbouring Areas Against Crystallone and Sedimentary Basement; Prace PIG, CLXX: Warszawa, Poland, 2000; 89p, (In Polish with English Summary). [Google Scholar]
- Banaszuk, H. Location of the Knyszyn Forest, outline of the relief and geological structure. In Puszcza Knyszyńska; Czerwiński, A., Ed.; Nature Monograph: Poland, 1995; pp. 11–22. (In Polish) [Google Scholar]
- Polish Geological Institute (PGI). Hydrogeological Map of Poland at the Scale 1:50 000, Sheet Bialystok; PIG: Warszawa, Poland, 2009. (In Polish) [Google Scholar]
- Musiał, A. Study of the Glacial Landscape of the Northern Podlasie; UW 403: Warszawa, Poland, 1992; 203p, (In Polish with English Summary). [Google Scholar]
- Mojski, E.J. Polish Territory During the Quaternary. An Outline of Morphogenesis; PIG: Warszawa, Poland, 2005; 404p, (In Polish with English Summary). [Google Scholar]
- JekatierynczukRudczyk, E. Transformation in Physicochemical Composition of Groundwaters within Lowland Drainage Zones; UwB: Bialystok, Poland, 2010; 224p, (In Polish with English Summary). [Google Scholar]
- Walter, W.G. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Puczko, K.; Zieliński, P.; JekatirynczukRudczyk, E. Dissolved organic matter in freshwaters. Kosmos 2017, 66, 457–464. [Google Scholar]
- Macioszczyk, A.; Dobrzyński, D. Hydrogeochemistry of Active Groundwater Exchange Zones; Scientific PWN: Warszawa, Poland, 2002; 440p. (In Polish) [Google Scholar]
- Łomotowski, J.; Siwoń, Z. Assessing the chemical stability of tap water by means of the software for ionic composition simulation. Ochr. Sr. 2004, 26, 13–16, (In Polish with English Summary). [Google Scholar]
- Małecki, J.J. Role of the Aeration Zone in Forming Chemical Composition of Shallow Ground Waters, Based on Cases of Selected Hydrogeochemical Environments; Biuletyn PIG 381: Warszawa, Poland, 1998; 219p, (In Polish with English Summary). [Google Scholar]
- Wiktorowicz, B. The forms of migration of constituents dissolved in acidulous waters in the Klodzko area. Prace Nauk. In. Gór. Politechniki Wrocławskiej 2007, 33, 121128, (In Polish with English Summary). [Google Scholar]
- Sliva, L.; Williams, D.D. Buffer zone versus whole catchment approaches to studying land use impact on river water quality. Water Res. 2001, 35, 3462–3472. [Google Scholar] [CrossRef]
- Goldstein, M.I.; S DellaSala, D.A. Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, Netherlands, 2020; pp. 131–133. [Google Scholar]
- Barquín, P.; Scarsbrook, M. Management and conservation strategies for coldwater springs. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 580–591. [Google Scholar] [CrossRef]
- Pradhan, N. Conserving Springs and its Significance for Water Security in Himalayas. 2005. Available online: http://thesarcist.org/Opinion/103 (accessed on 26 February 2021).
- Czarnecka, B.; Rysiak, A.; Chabudziński, Ł. Spring flora diversity versus ecosystem stressors: Case studies from the Western Roztocze and Lublin Upland border, SouthEast Poland. Ecohydrol. Hydrobiol. 2020, 20, 693–706. [Google Scholar] [CrossRef]
- Baścik, M.; Chełmicki, W.; Urban, J. Geoconservation of Springs in Poland. Episodes 2009, 32, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P. Hydrochemical Assessment of Ground Water of Springs (Bowlis) of Udhampur District, J&K, India. Int. J. Sci. Res. 2014, 3, 1313–1321. [Google Scholar]
- AlKhashman, O.A.; Alnawafleh, H.M.; Jrai, A.M.; AlMuhtaseb, A. Monitoring and Assessing of Spring Water Quality in Southwestern Basin of Jordan. Open J. Mod. Hydrol. 2017, 7, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Machona, L.; Chikodzi, D.; Sithole, N. Water Quality Analysis for Springs in Bvumba Catchment Area, Manicaland Province, Zimbabwe. Journal of Geography. Environ. Earth Sci. Int. 2017, 11, 1–13. [Google Scholar]
- Batool, A.; Samad, N.; Kazmi, S.S.; Ghufran, M.A.; Imad, S.; Shafqat, M.; Mahmood, T. Spring water quality and human health: An assessment of natural springs of margalla hills Islamabad zone III. Int. J. Hydrol. 2018, 2, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Ameen, H.A. Spring water quality assessment using water quality index in villages of Barwari Bala, Duhok, Kurdistan Region, Iraq. Appl. Water Sci. 2019, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Bhat, S.; Hamid, A.; Bhat, A. Quality assessment of springs for drinking water in the Himalaya of South Kashmir, India. Environ. Sci. Pollut. Res. 2020, 28, 2279–2300. [Google Scholar]
- Bhat, S.; Mushtaq, S.; Qayoom, U.; Sabha, I. Water Quality Scenario of Kashmir Himalayan Springs—A Case Study of Baramulla District, Kashmir Valley. Water Air Soil 2020, 231, 1–17. [Google Scholar] [CrossRef]
- Cartwright, J.; Johnson, H.M. Springs as hydrologic refugia in a changing climate? A remote-sensing approach. Ecosphere 2018, 9, e02155. [Google Scholar] [CrossRef]
- Freed, Z.; Aldous, A.; Gannet, M.W. Landscape controls on the distribution and ecohydrology of central Oregon springs. Ecohydrology 2019, 12, e2065. [Google Scholar] [CrossRef]
- Puczko, K.; JekatierynczukRudczyk, E. Analysis of urban land cover influence to organic carbon and nutrients in surface water via impacted groundwater. Environ. Monit. Assess. 2020, 192, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, C.A.; Palmieri, H.E.L.; Menezes, M.Â.; Auler, L.M.L.A. Hydrochemical Assessment of Spring Waters from the Iron Quadrangle Region, Minas Gerais, Brazil. Am. J. Water Resour. 2017, 5, 29–40. [Google Scholar]
- JekatierynczukRudczyk, E.; Zieliński, P.; Puczko, K. Is the protection of springs in Knyszyn Forest effective and satisfactory? Prot. Nativ. Nat. 2017, 73, 135–147, (In Polish with English Summary). [Google Scholar]
- Van der Kamp, R.O. The hydrogeology of springs in relation to the biodiversity of spring fauna: A review. In Ferrington L C Jr (ed.) Biodiversity of Aquatic Insects and Other Invertebrates in Springs. J. Kans. Entomol. Soc. 1995, 68, 4–17. [Google Scholar]
- Pei, Y.S.; Wang, J.; Wang, Z.Y.; Yang, Z.F. Characteristics of ammoniaoxidizing and denitrifying bacteria at the riversediment interface. Procedia Environ. Sci. 2010, 2, 1988–1996. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Qi, Y.; Wang, J.; Pei, Y. Characteristics of aerobic and anaerobic ammoniumoxidizing bacteria in the hyporheic zone of a contaminated river. World J. Microbiol. Biotechnol. 2012, 28, 2801–2811. [Google Scholar] [CrossRef]
- Ali, A. Nitrate assimilation pathway in higher plants: Critical role in nitrogen signalling and utilization. Plant Sci. Today 2020, 7, 182–192. [Google Scholar] [CrossRef]
- McDowell, R.W. Relationship between Sediment Chemistry, Equilibrium Phosphorus Concentrations, and Phosphorus Concentrations at Baseflow in Rivers of the New Zealand National River Water Quality Network. J. Environ. Qual. 2015, 44, 921–929. [Google Scholar] [CrossRef]
- Baltes, B. Einfluss der Gewässerversauerung auf aquatische Insekten. Mitt. Der Dtsch. Ges. Für Allg. Und Angew. Entomol. 2000, 12, 231–236. [Google Scholar]
- Hahn, H.J. Studies on classifying of undisturbed springs in Southwestern Germany by macrobenthic communities. Limnologica 2000, 30, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Jasik, M.; Małek, S. Quality assessment of spring water from the area of the Łysogóry Mts. in Świętokrzyski National Park in 2010. Folia For. Pol. 2013, 55, 27–32. [Google Scholar] [CrossRef]
- Gaglioti, S.; Infusino, E.; Caloiero, T.; Callegari, G. Hydrochemical and qualitative assessment of natural water spring in southern Italy. Eur. Water 2017, 57, 399–405. [Google Scholar]
- Govoni, D.P.; Kristjánsson, B.K.; Olafsson, J.S. Spring type influences invertebrate communities at cold spring sources. Hydrobiologia 2018, 808, 315–332. [Google Scholar] [CrossRef]
- Von Fumetti, S.; Nagel, P.; Scheifhacken, N.; Baltes, B. Factors governing macrozoobenthic assemblages in perennial springs in northwestern Switzerland. Hydrobiologia 2006, 568, 467–475. [Google Scholar] [CrossRef]
- Chełmicki, W.; Siwek, J. Natural and anthropogenic factors controlling spring water quality in the southern part of the Malopolska Upland (Southern Poland). Impact of Human Activity on Groundwater Dynamics (Proceedings of a symposium held during the Sixth IAHS Scientific Assembly at Maastricht, The Netherlands, July 2001). IAHS Publ. 2001, 269, 317–322. [Google Scholar]
- Ågren, A.; Buffam, I.; Berggren, M.; Bishop, K.; Jansson, M.; Laudon, L. Dissolved organic carbon characteristics in boreal streams in a forestwetland gradient during the transition between winter and summer. J. Geophys. Res. 2008, 113, G3. [Google Scholar]
- Laudon, H.; Hedtjärn, J.; Schelker, J.; Bishop, K.; Sørensen, R.; Ågren, A. Response of dissolved organic carbon following forest harvesting in a boreal forest. AMBIO: A J. Hum. Environ. 2009, 38, 381–386. [Google Scholar] [CrossRef]
- Clément, F.; Ruiz, J.; Rodríguez, M.A.; Blais, D.; Campeau, S. Landscape diversity and forest edge density regulate stream water quality in agricultural catchments. Ecol. Indic. 2017, 72, 627–639. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, Y.; Fu, L.; Liu, Q.; Peng, Q.; Kang, M. Impacts of Land Use on Surface Water Quality in a Subtropical River Basin: A Case Study of the Dongjiang River Basin, Southeastern China. Water 2015, 7, 4427–4445. [Google Scholar] [CrossRef] [Green Version]
- Eftimi, R.; Zojer, H. Human impacts on karst aquifers of Albania. Environ. Earth Sci. 2015, 74, 57–70. [Google Scholar] [CrossRef]
- Hossain, M.S. Impact of land use change on stream water quality: A review of modelling approaches. JREAS 2017, 2, 1–6. [Google Scholar] [CrossRef]
- Ramos, S.T.B.; Mafra, M.S.H.; Rech, T.D.; Siegloch, A.E.; Rech, Â.F. Water quality of springs in areas under different land uses in the southern highlands of Santa Catarina. Rev. Ambiente Água 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Rudczyk, E.; Puczko, K.; Żukowska, J.; Sawicka, A. Biota communities influence on nutrients circulation in hyporheic zone—A case study in urban spring niches in Bialystok (NE Poland). Aquat. Sci. 2021, 83, 75. [Google Scholar] [CrossRef]
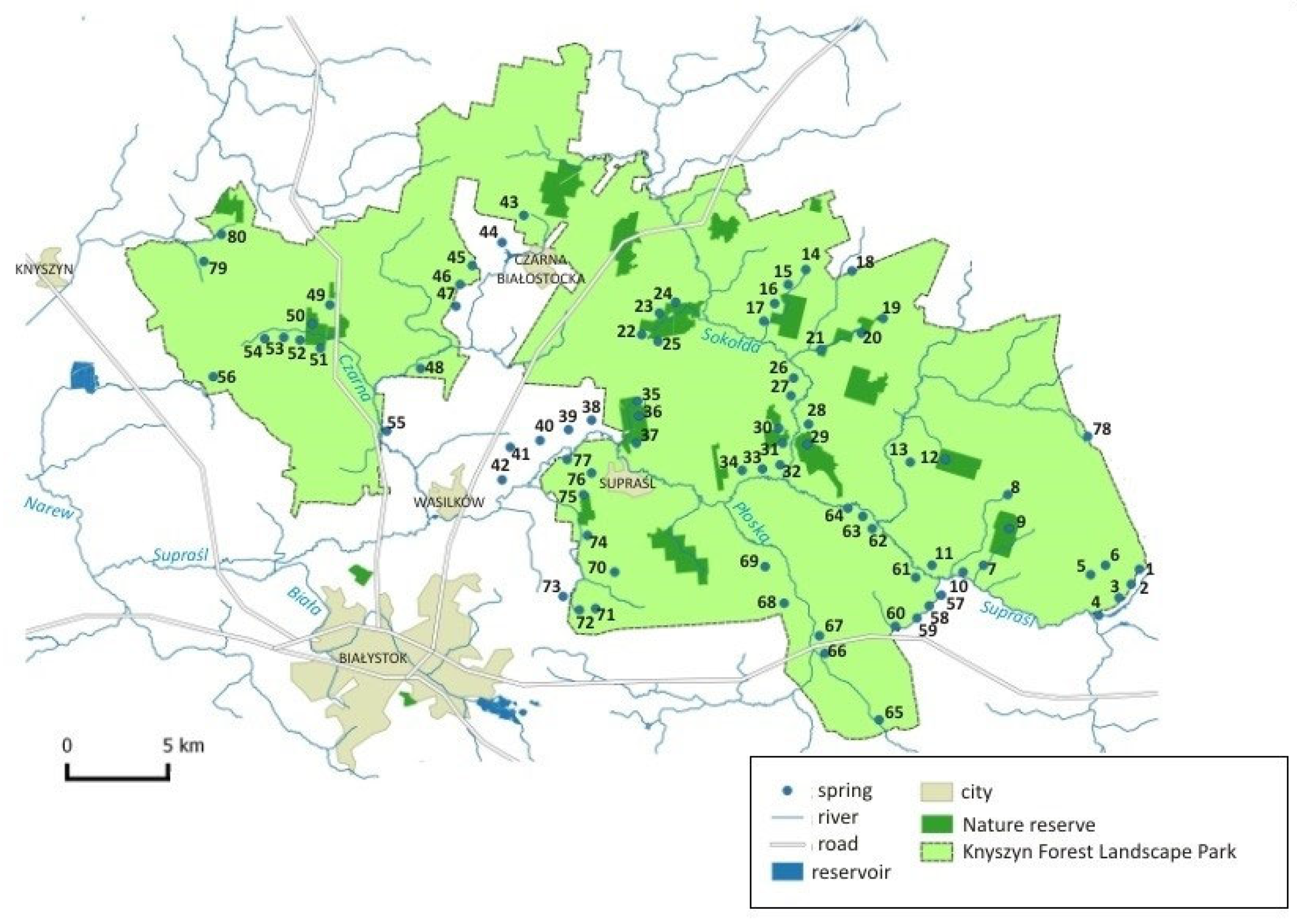



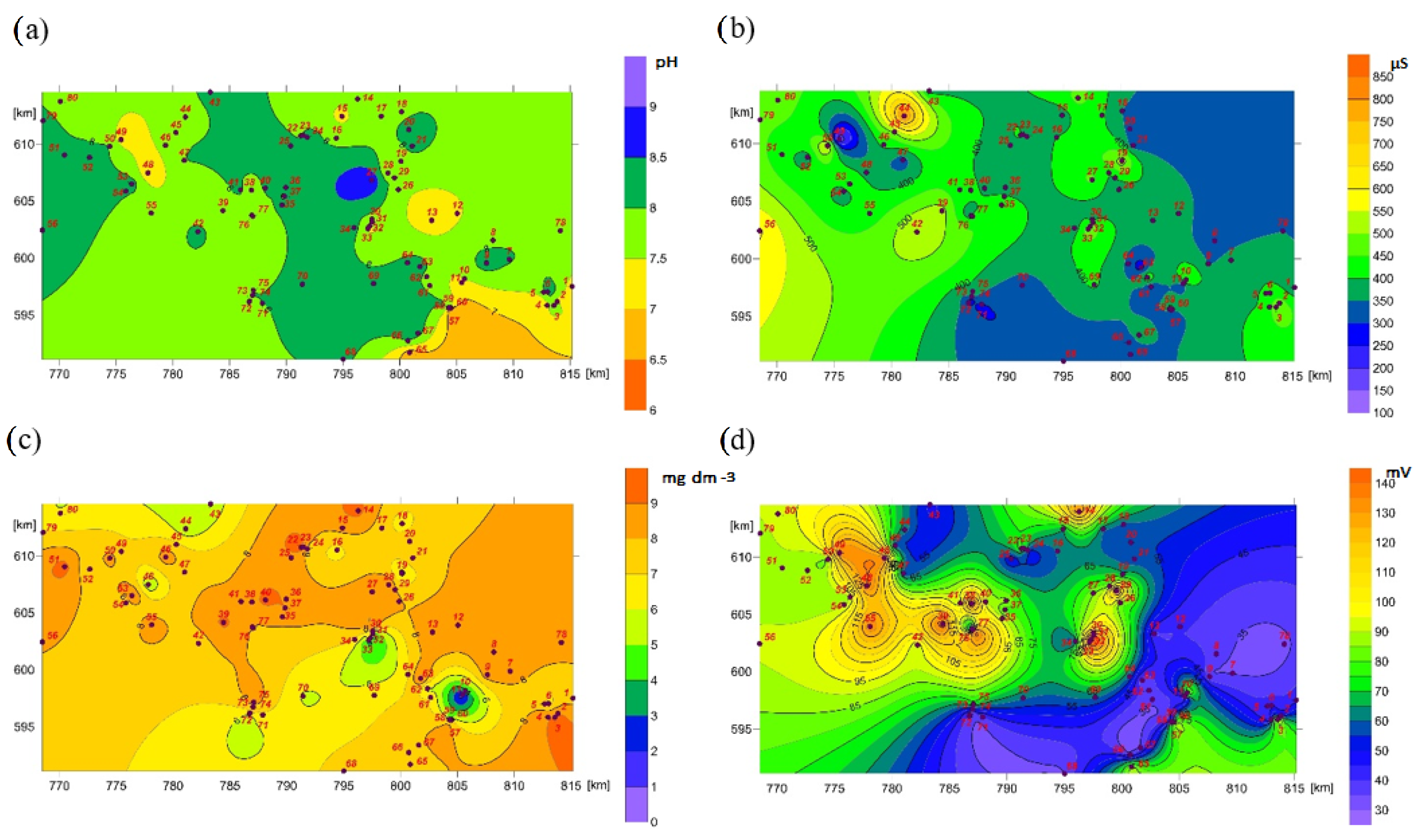
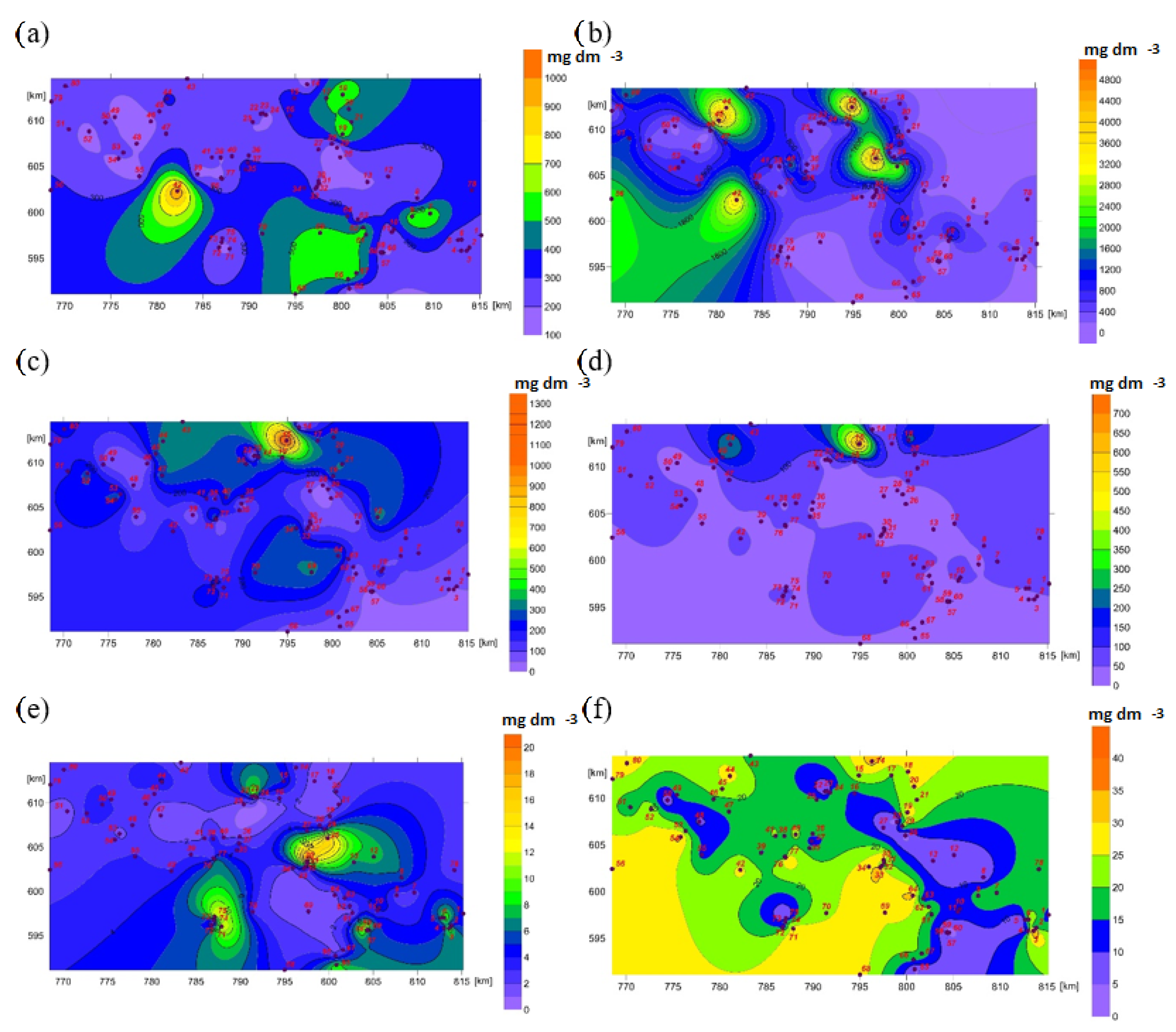

| No | Geographical Coordinates | Hydrological Location | Geomorphological Type of Spring | Hydrobiological Type of Spring | Hydrochemical Type of Water | Land Use |
|---|---|---|---|---|---|---|
| 1 | N: 53°8′58.19″ E: 23°42′56..39″ | right-side direct tributary of the Supraśl river → Breszczeczka catchment | hillslope | rheocrene | HCO3-Ca | forest |
| 2 | N: 53°8′17.31″ E: 23°41′41.48″ | right-side direct tributary of the Supraśl river → Breszczeczka catchment | hillslope | rheocrene | HCO3-Ca-Mg | grassland |
| 3 | N: 53°8′7.23″ E: 23°41′24.34″ | right-side direct tributary of the Supraśl river → Breszczeczka catchment | hillslope | helocrene | HCO3-Ca-Mg | grassland |
| 4 | N: 53°8′7.52″ E: 23°40′52.87″ | right-side direct tributary of the Supraśl river → Breszczeczka catchment | hillslope | rheocrene | HCO3-Ca-Mg | rural area |
| 5 | N: 53°8′47.39″ E: 23°40′39.82″ | right-side direct tributary of the Supraśl river → Breszczeczka catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest |
| 6 | N: 53°8′47.03″ E: 23°40′58.74″ | right-side direct tributary of the Supraśl river → Breszczeczka catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest |
| 7 | N: 53°10′25.56″ E: 23°38′7.25″ | right-side direct tributary of the Supraśl river → Średnica catchment | valley | helocrene | HCO3-Ca | grassland |
| 8 | N: 53°11′22.72″ E: 23°36′56.28″ | right-side direct tributary of the Supraśl river → Radulinka catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 9 | N: 53°10′19.79″ E: 23°36′18.84″ | right-side direct tributary of the Supraśl river → Radulinka catchment | valley | rheocrene | HCO3-Ca | grassland |
| 10 | N: 53°9′38.68″ E: 23°34′29.02″ | right-side direct tributary of the Supraśl river → Radulinka catchment | valley | helocrene | HCO3-Ca-Mg | grassland |
| 11 | N: 53°9′29.32″ E: 23°34′16.04″ | right-side direct tributary of the Supraśl river → Radulinka catchment | valley | artificial limnocrene | HCO3-Ca-Mg | rural area |
| 12 | N: 53°12′46.19″ E: 23°34′14.97″ | Słoja catchment → Skrobacianka catchment | hillslope | helocrene | HCO3-Ca | forest |
| 13 | N: 53°12′30.63″ E: 23°32′11.91″ | direct tributary of the Słoja river → Słoja catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest |
| 14 | N: 53°18′28.05″ E: 23°26′57.28″ | Sokołda catchment → Karnicha catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest/grassland |
| 15 | N: 53°17′42.44″ E: 23°25′37.06″ | Sokołda catchment → Karnicha catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 16 | N: 53°16′41.53″ E: 23°25′4.8″ | Sokołda catchment → Karnicha catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 17 | N: 53°17′35.3″ E: 23°28′44.07″ | Sokołda catchment → Kowszówka catchment | valley | helocrene | HCO3-Ca-Mg | forest |
| 18 | N: 53°17′43.72″ E: 23°30′21.1″ | Sokołda catchment → Łanga catchment | valley | rheocrene | HCO3-Ca-Mg | rural area |
| 19 | N: 53°15′23.85″ E: 23°30′3.88″ | Sokołda catchment → Łanga catchment | valley | rheocrene | HCO3-Ca-Mg | rural area |
| 20 | N: 53°16′52.31″ E: 23°30′51.01″ | Sokołda catchment → Łanga catchment | valley | rheocrene | HCO3-Ca | rural area |
| 21 | N: 53°16′5.05″ E: 23°31′1.89″ | Sokołda catchment → Łanga catchment | hillslope | rheocrene | HCO3-Ca-Mg | rural area |
| 22 | N: 53°16′53.67″ E: 23°22′15.75″ | Sokołda catchment → Migówka catchment | hillslope | rheocrene | HCO3-Ca | forest |
| 23 | N: 53°16′54.62″ E: 23°22′27.36″ | Sokołda catchment → Migówka catchment | hillslope | rheocrene | CO3-Ca-Mg | forest |
| 24 | N: 53°16′49.47″ E: 23°22′44.93″ | Sokołda catchment → Migówka catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 25 | N: 53°16′26.7″ E: 23°21′24.73″ | Sokołda catchment → Migówka catchment | valley | rheocrene | CO3-Ca-Mg | forest |
| 26 | N: 53°14′4.31″ E: 23°29′43.08″ | direct tributary of the Sokołda river → Sokołda catchment | valley | rheocrene | HCO3-Ca | grassland |
| 27 | N: 53°14′35.73″ E: 23°27′38.92″ | direct tributary of the Sokołda river → Sokołda catchment | valley | helocrene | HCO3-Ca | grassland |
| 28 | N: 53°14′53.01″ E: 23°28′59.46″ | direct tributary of the Sokołda river → Sokołda catchment | hillslope | limnocrene | HCO3-Ca-Mg | forest |
| 29 | N: 53°14′38.13″ E: 23°29′28.2″ | direct tributary of the Sokołda river → Sokołda catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest |
| 30 | N: 53°12′44.98″ E: 23°27′28.53″ | direct tributary of the Sokołda river → Sokołda catchment | hillslope | helocrene | HCO3-Ca-Mg | grassland |
| 31 | N: 53°12′37.01″ E: 23°27′29.49″ | direct tributary of the Sokołda river → Sokołda catchment | hillslope | helocrene | HCO3-Ca | forest/grassland |
| 32 | N: 53°12′24.64″ E: 23°27′16.42″ | direct tributary of the Sokołda river → Sokołda catchment | hillslope | helocrene | HCO3-Ca-Mg | forest/grassland |
| 33 | N: 53°12′19.2″ E: 23°27′7.29″ | direct tributary of the Sokołda river → Sokołda catchment | hillslope | helocrene | HCO3-Ca | forest/grassland |
| 34 | N: 53°12′23.77″ E: 23°26′2.44″ | direct tributary of the Sokołda river → Sokołda catchment | hillslope | helocrene | HCO3-Ca-Mg | rural area |
| 35 | N: 53°13′40.52″ E: 23°20′26.84″ | Sokołda catchment → Jałówka catchment | valley | rheocrene | HCO3-Ca-Mg | forest/grassland |
| 36 | N: 53°14′30.49″ E: 23°20′50.34″ | Sokołda catchment → Jałówka catchment | valley | rheocrene | HCO3-Ca | forest/grassland |
| 37 | N: 53°14′4.58″ E: 23°20′41.57″ | Sokołda catchment → Jałówka catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 38 | N: 53°14′28.01″ E: 23°18′7.49″ | right-side direct tributary of the Supraśl river → Supraśl catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest/grassland |
| 39 | N: 53°13′34.82″ E: 23°15′45.74″ | right-side direct tributary of the Supraśl river → Supraśl catchment | hillslope | rheocrene | HCO3-Ca | forest/grassland |
| 40 | N: 53°14′31.25″ E: 23°19’12.46″ | right-side direct tributary of the Supraśl river → Supraśl catchment | hillslope | rheocrene | HCO3-Ca | grassland |
| 41 | N: 53°14′31.38″ E: 23°17′15.09″ | right-side direct tributary of the Supraśl river → Supraśl catchment | hillslope | rheocrene | HCO3-Ca | grassland |
| 42 | N: 53°12′39.65″ E: 23°13′42.02″ | right-side direct tributary of the Supraśl river → Supraśl catchment | hillslope | helocrene | HCO3-Ca | grassland |
| 43 | N: 53°19′13.31″ E: 23°15′18.6″ | Czarna catchment → Czapielówka catchment | valley | rheocrene | HCO3-Ca | rural area |
| 44 | N: 53°18′7.34″ E: 23°13′13.77″ | Czarna catchment → Jurczycha catchment | valley | helocrene | HCO3-Ca | rural area |
| 45 | N: 53°17′24.1″ E: 23°12′25.18″ | Czarna catchment → Czarna Rzeczka catchment | valley | helocrene | HCO3-Ca-Mg | forest |
| 46 | N: 53°16′50.22″ E: 23°11′31.01″ | direct tributary of the Czarna river → Czarna catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 47 | N: 53°16′3.72″ E: 23°12′56.16″ | Czarna catchment → Czarna Rzeczka catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 48 | N: 53°15′35.27″ E: 23°10′0.62″ | direct tributary of the Czarna river → Czarna catchment | valley | helocrene | HCO3-Ca | grassland |
| 49 | N: 53°17′12.6″ E: 23°8′2.38″ | Czarna catchment → Czarna Rzeczka catchment | hillslope | helocrene | HCO3-Ca-Mg | forest |
| 50 | N: 53°16′55.79″ E: 23°7′5.98″ | Czarna catchment → Krzemianka catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 51 | N: 53°16′38.56″ E: 23°3′30.18″ | Czarna catchment → Krzemianka catchment | valley | rheocrene | HCO3-Ca-Mg | forest/FV |
| 52 | N: 53°16′27.53″ E: 23°5′28.25″ | Czarna catchment → Krzemianka catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 53 | N: 53°15′5.65″ E: 23°8′40.17″ | Czarna catchment → Krzemianka catchment | valley | rheocrene | HCO3-Ca | forest |
| 54 | N: 53°14′46.25″ E: 23°8′10.17″ | Czarna catchment → Krzemianka catchment | valley | helocrene | HCO3-Ca-Mg | forest |
| 55 | N: 53°13′39.73″ E: 23°10′4.77″ | direct tributary of the Czarna river → Czarna catchment | hillslope | rheocrene | HCO3-Ca-Mg | grassland |
| 56 | N: 53°13′9.44″ E: 23°1′24.03″ | right-side direct tributary of the Supraśl river → Supraśl catchment | hillslope | helocrene | HCO3-Ca | rural area |
| 57 | N: 53°8′19.35″ E: 23°33′17.86″ | left-side direct tributary of the Supraśl river → stream from Sofipol catchment | hillslope | artificial limnocrene | HCO3-Ca | grassland |
| 58 | N: 53°8′17.93″ E: 23°33′12.44″ | left-side direct tributary of the Supraśl river → stream from Sofipol catchment | hillslope | rheocrene | HCO3-Ca | grassland |
| 59 | N: 53°8′20.26″ E: 23°33′5.42″ | left-side direct tributary of the Supraśl river → stream from Sofipol catchment | hillslope | rheocrene | HCO3-Ca-Mg | grassland |
| 60 | N: 53°8′21.2″ E: 23°33′7.51″ | left-side direct tributary of the Supraśl river → stream from Sofipol catchment | hillslope | rheocrene | HCO3-Ca-Mg | grassland/FV |
| 61 | N: 53°9′26.11″ E: 23°31′44.04″ | left-side direct tributary of the Supraśl river → stream from Sofipol catchment | valley | rheocrene | HCO3-Ca | grassland |
| 62 | N: 53°9′52.42″ E: 23°31′30.91″ | left-side direct tributary of the Supraśl river → Supraśl catchment | valley | rheocrene | HCO3-Ca | rural area |
| 63 | N: 53°10′21.9″ E: 23°31′2.85″ | left-side direct tributary of the Supraśl river → Supraśl catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest |
| 64 | N: 53°10′35.29″ E: 23°30′3.76″ | left-side direct tributary of the Supraśl river → Supraśl catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 65 | N: 53°6′20.46″ E: 23°29′48.26″ | Płoska catchment → Świniobródka catchment | valley | rheocrene | HCO3-Ca | forest |
| 66 | N: 53°6′53.57″ E: 23°29′43.86″ | Płoska catchment → Świniobródka catchment | valley | rheocrene | HCO3-Ca-Mg | forest/grassland |
| 67 | N: 53°7′13.04″ E: 23°30′34.1″ | Płoska catchment → Świniobródka catchment | valley | rheocrene | HCO3-Mg-Ca | forest/grassland |
| 68 | N: 53°6′12.95″ E: 23°24′31.8″ | direct tributary of the Płoska river → Płoska catchment | hillslope | helocrene | HCO3-Ca-Mg | forest/grassland |
| 69 | N: 53°9′42.33″ E: 23°27′18.34″ | direct tributary of the Płoska river → Płoska catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest |
| 70 | N: 53°9′52.69″ E: 23°21′41.36″ | left-side direct tributary of the Supraśl river → Starzynka catchment | valley | rheocrene | HCO3-Ca-Mg | forest |
| 71 | N: 53°9′5.69″ E: 23°18′25.66″ | Supraśl catchment → Krasna Rzeczka catchment | hillslope | helocrene | HCO3-Ca-Mg | forest |
| 72 | N: 53°9′13.86″ E: 23°17′26.2″ | Supraśl catchment → Krasna Rzeczka catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest |
| 73 | N: 53°9′12.1″ E: 23°17′24.11″ | Supraśl catchment → Krasna Rzeczka catchment | hillslope | limnocrene | HCO3-Ca | forest/FV |
| 74 | N: 53°9′29.54″ E: 23°17′42.11″ | left-side direct tributary of the Supraśl river → Supraśl catchment | valley | rheocrene | HCO3-Ca | forest |
| 75 | N: 53°9′43.62″ E: 23°17′47.22″ | left-side direct tributary of the Supraśl river → Supraśl catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest |
| 76 | N: 53°13′17.37″ E: 23°18′1.96″ | left-side direct tributary of the Supraśl river → Supraśl catchment | valley | rheocrene | HCO3-Ca | forest |
| 77 | N: 53°13′13.9″ E: 23°18′5.54″ | left-side direct tributary of the Supraśl river → Supraśl catchment | valley | rheocrene | HCO3-Ca-Mg | forest/grassland |
| 78 | N: 53°11′37.9″ E: 23°42′17.62″ | Niemen catchment → Nietupa catchment | hillslope | rheocrene | HCO3-Ca-Mg | forest/grassland/FV |
| 79 | N: 53°18′19.94″ E: 23°1′55.69″ | Narew catchment → Jaskranka catchment | hillslope | helocrene | HCO3-Ca-Mg | rural area |
| 80 | N: 53°19′12.49″ E: 23°3′23.81″ | Narew catchment → Jaskranka catchment | hillslope | rheocrene | HCO3-Ca | rural area |
| Parameter | Mean | Min. | Max. | SD | CV | |
|---|---|---|---|---|---|---|
| Water temperature | °C | 14.2 | 9.4 | 21.2 | 3.13 | 22 |
| Reaction | pH | 7.81 | 6.22 | 9.02 | 0.44 | 6 |
| Electrolytic conductivity | µS cm−1 | 399 | 153 | 846 | 90 | 23 |
| ORP | mV | 73.0 | 28.3 | 145.0 | 34.1 | 47 |
| Oxygen | mg dm−3 | 7.42 | 1.12 | 9.74 | 1.66 | 22 |
| Oxygen saturation | % | 74 | 10 | 96 | 74 | 23 |
| Water color (Pt) | mg Pt dm−3 | 14.7 | 0.1 | 64.2 | 14.7 | 82 |
| Ca2+ | mg dm−3 | 78.4 | 43.1 | 122.6 | 13.9 | 18 |
| Mg2+ | mg dm−3 | 14.4 | 3.6 | 35.1 | 6.2 | 43 |
| Na+ | mg dm−3 | 5.2 | 0.01 | 31.62 | 4.36 | 84 |
| K+ | mg dm−3 | 1.6 | 0.01 | 37.14 | 4.58 | 289 |
| HCO3− (C) | mg dm−3 | 53.8 | 28.7 | 82.3 | 9.0 | 17 |
| SO42− | mg dm−3 | 27.7 | 1.6 | 50.0 | 8 | 28 |
| Cl− | mg dm−3 | 11.5 | 5.3 | 52.6 | 8.7 | 76 |
| SiO32− | mg dm−3 | 2.24 | 0.5 | 5.3 | 1.05 | 47 |
| TFe | mg dm−3 | 1.24 | 0.09 | 5.20 | 1.24 | 54 |
| DFe | mg dm−3 | 0.82 | 0.04 | 1.59 | 0.82 | 61 |
| PFe | mg dm−3 | 0.42 | 0.002 | 4.13 | 0.42 | 124 |
| SRFe | mg dm−3 | 0.15 | 0.009 | 0.85 | 0.16 | 98 |
| ODFe | mg dm−3 | 0.67 | 0.012 | 1.53 | 0.67 | 74 |
| TN | mg dm−3 | 1.86 | 0.40 | 18.16 | 21.6 | 116 |
| N-NH4+ | mg dm−3 | 0.24 | 0.049 | 0.939 | 0.244 | 55 |
| N-NO3− | mg dm−3 | 0.66 | 0.052 | 4.576 | 0.916 | 139 |
| N-NO2− | mg dm−3 | 0.002 | 0.0001 | 0.009 | 0.001 | 71 |
| TP | mg dm−3 | 0.17 | 0.028 | 1.311 | 0.16 | 96 |
| DP | mg dm−3 | 0.121 | 0.017 | 1.174 | 0.144 | 119 |
| PP | mg dm−3 | 0.049 | 0 | 0.253 | 0.050 | 103 |
| SRP | mg dm−3 | 0.066 | 0.013 | 0.717 | 0.086 | 130 |
| DOP | mg dm−3 | 0.056 | 0 | 0.457 | 0.056 | 134 |
| DOC | mg dm−3 | 4.16 | 1.09 | 16.27 | 3.32 | 80 |
| SUVA | mg dm−3 | 17.03 | 1.6 | 32.8 | 9.0 | 53 |
| Phenols | mg dm−3 | 0.46 | 0.13 | 1.19 | 0.22 | 48 |
| Minerals | Saturation Index | |
|---|---|---|
| Halite | NaCl | −8.81 |
| Iron (III) hydroxide, hydrated | Fe(OH)3 | 1.97 |
| Goethite | FeOOH | 7.25 |
| Hematite | Fe2O3 | 16.43 |
| Calcite | CaCO3 | 0.28 |
| Aragonite | CaCO3 | 0.12 |
| Dolomite | CaMg(CO3)2 | −0.29 |
| Siderite | Fe CO3 | −0.47 |
| Gypsum | CaSO4 2H2O | −2.01 |
| Anhydrite | CaSO4 | −2.27 |
| Jarosite K | KFe3(SO4)2(OH)6 | −6.65 |
| Melanterite | Fe SO4 7 H2O | −7.31 |
| Hydroxyapatite | Ca5(PO4) 3OH | −0.07 |
| Vivianite | Fe3(PO4)2 8 H2O | −3.84 |
| Quartz | SiO2 | 0.07 |
| Chalcedony | SiO2 | −0.41 |
| Chrysolite | Mg3Si2O5(OH)4 | −7.61 |
| Sepiolite | Mg2Si3O7 5OH 3 H2O | −5.34 |
| Talc | Mg3Si4O10(OH)2 | −4.96 |
| Niche Land Use Type | ||||
|---|---|---|---|---|
| Parameter | Forest | Grassland | Rural Area | FV |
| Water temperature | 0.003 | −0.135 | 0.255 | −0.009 |
| Reaction | 0.125 | −0.145 | 0.042 | −0.010 |
| Electrolytic conductivity | −0.301 | 0.081 | 0.040 | 0.276 |
| Eh | −0.079 | 0.030 | −0.143 | 0.161 |
| Oxygen | 0.272 | −0.194 | −0.266 | 0.070 |
| Oxygen saturation | 0.212 | −0.220 | −0.161 | 0.120 |
| Water color | 0.030 | −0.013 | 0.070 | −0.088 |
| Ca2+ | −0.090 | −0.019 | −0.034 | 0.164 |
| Mg2+ | 0.003 | −0.086 | −0.046 | 0.124 |
| Na+ | −0.306 | 0.082 | 0.185 | 0.182 |
| K+ | −0.115 | 0.145 | 0.082 | −0.071 |
| HCO3− | −0.188 | −0.101 | 0.149 | 0.255 |
| SO42− | 0.215 | −0.091 | −0.068 | −0.142 |
| Cl− | −0.187 | 0.176 | −0.046 | 0.091 |
| SiO32− | −0.090 | −0.073 | 0.267 | 0.015 |
| TFe | −0.145 | −0.029 | 0.212 | 0.053 |
| DFe | −0.133 | 0.009 | 0.227 | 0.024 |
| PFe | −0.126 | −0.013 | 0.067 | 0.065 |
| SRFe | −0.256 | 0.146 | 0.216 | 0.011 |
| ODFe | −0.068 | −0.026 | 0.205 | 0.005 |
| SRMn | −0.046 | −0.125 | −0.021 | 0.211 |
| TN | 0.016 | 0.126 | −0.051 | −0.136 |
| NNH4+ | −0.037 | −0.060 | 0.286 | −0.070 |
| NNO3− | −0.095 | 0.081 | 0.116 | −0.011 |
| NNO2− | −0.033 | 0.056 | 0.060 | −0.049 |
| TP | 0.120 | −0.222 | 0.255 | −0.115 |
| DP | 0.128 | −0.190 | 0.238 | −0.153 |
| PP | 0.039 | −0.096 | 0.130 | −0.054 |
| SRP | 0.099 | −0.046 | 0.217 | −0.264 |
| DOP | 0.086 | −0.279 | 0.157 | 0.065 |
| DOC | 0.028 | 0.184 | −0.227 | −0.085 |
| SUVA | −0.044 | −0.177 | 0.200 | 0.120 |
| Fenols | 0.006 | 0.022 | 0.013 | −0.037 |
| Variance Component | Explanation [%] | |
|---|---|---|
| Land Use | Forest | 46.5 |
| Finetextured vegetation (FV) | 16.9 | |
| Grassland | 25.7 | |
| Rural | 10.7 | |
| Total explained | 68.9 | |
| Unexplained | 31.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jekatierynczuk-Rudczyk, E.; Zieliński, P.; Puczko, K.; Micun, K.; Puczyłowska, E. The Role of the Catchment Area in Shaping Water Quality in the Lowland Springs of the Knyszyn Forest (NE Poland). Water 2022, 14, 3202. https://doi.org/10.3390/w14203202
Jekatierynczuk-Rudczyk E, Zieliński P, Puczko K, Micun K, Puczyłowska E. The Role of the Catchment Area in Shaping Water Quality in the Lowland Springs of the Knyszyn Forest (NE Poland). Water. 2022; 14(20):3202. https://doi.org/10.3390/w14203202
Chicago/Turabian StyleJekatierynczuk-Rudczyk, Elżbieta, Piotr Zieliński, Katarzyna Puczko, Krzysztof Micun, and Emilia Puczyłowska. 2022. "The Role of the Catchment Area in Shaping Water Quality in the Lowland Springs of the Knyszyn Forest (NE Poland)" Water 14, no. 20: 3202. https://doi.org/10.3390/w14203202





