Active Protection of Endangered Species of Peat Bog Flora (Drosera intermedia, D. anglica) in the Łęczna-Włodawa Lake District
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Investigation
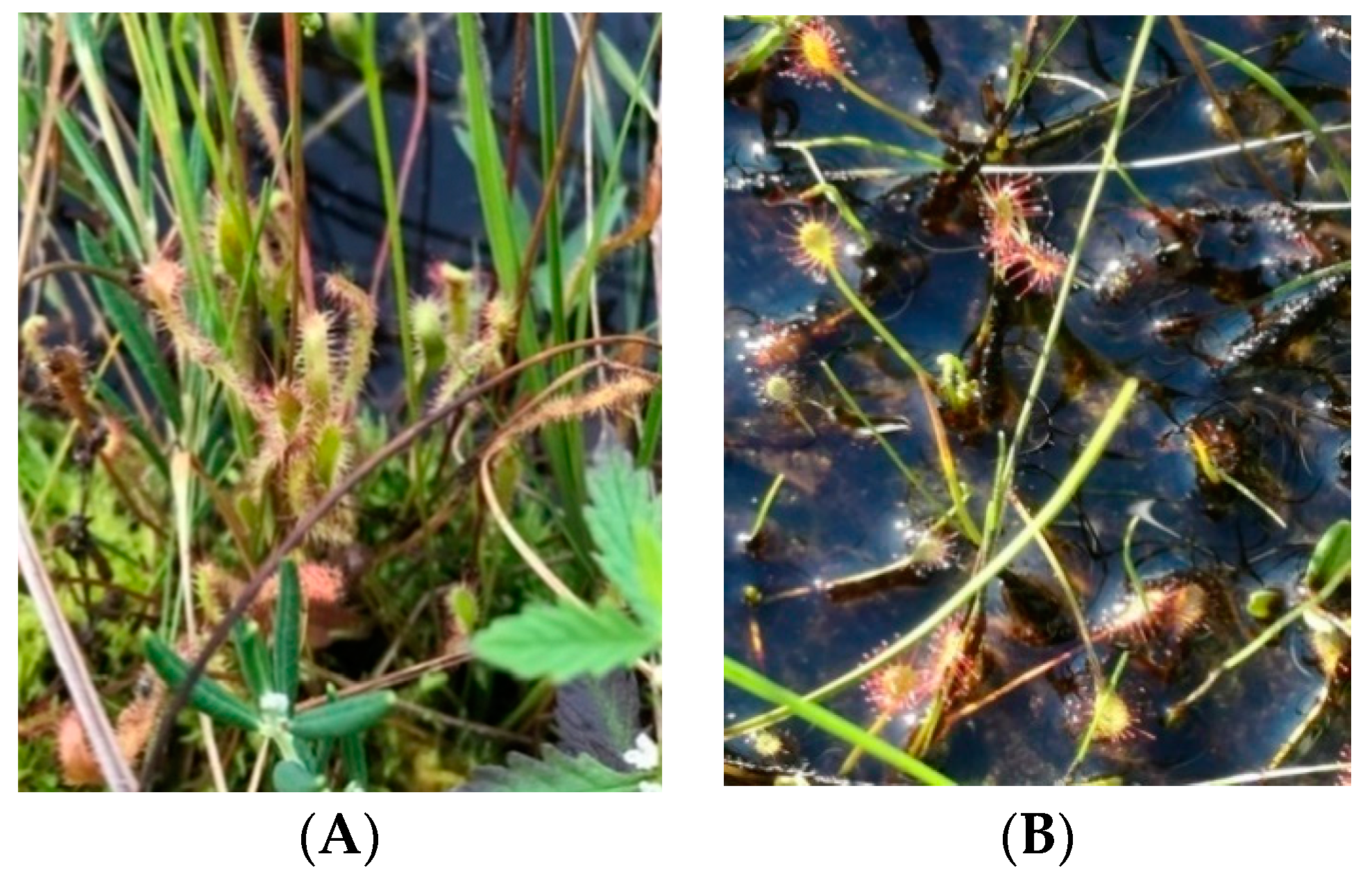
2.2.1. Obtaining Sundew and Identifying Reintroduction Sites
2.2.2. Environment Factors of the Sites
2.2.3. Climatic Factors
2.2.4. Floristic Characteristics
- —numerical fraction of the plants of a given species in relation to all designated species;
- S—number of all species.
2.3. Statistical Analysis
3. Results
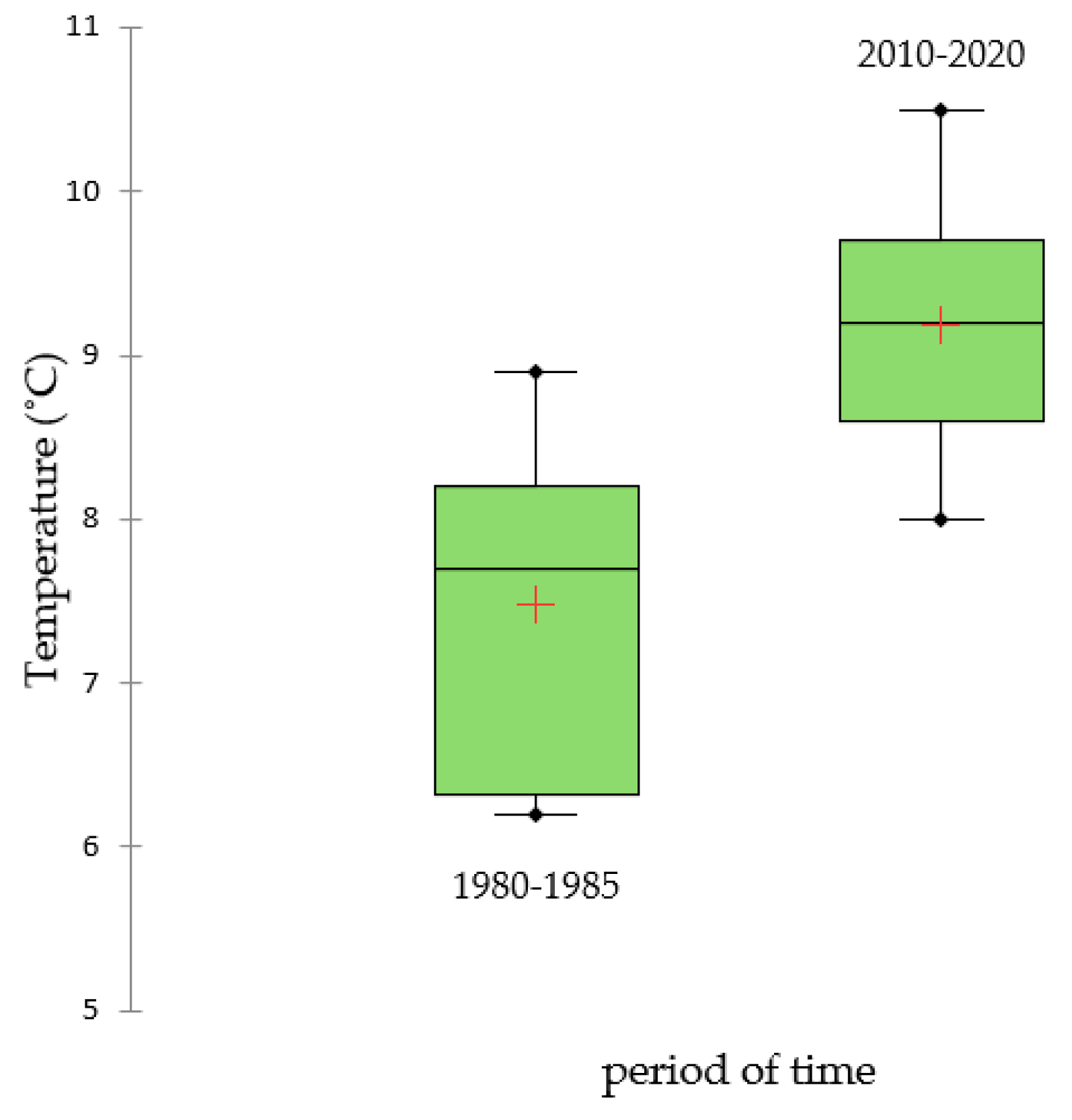
3.1. Long-Term Changes in Temperature and Precipitation
3.2. State of Current and Potential Habitats
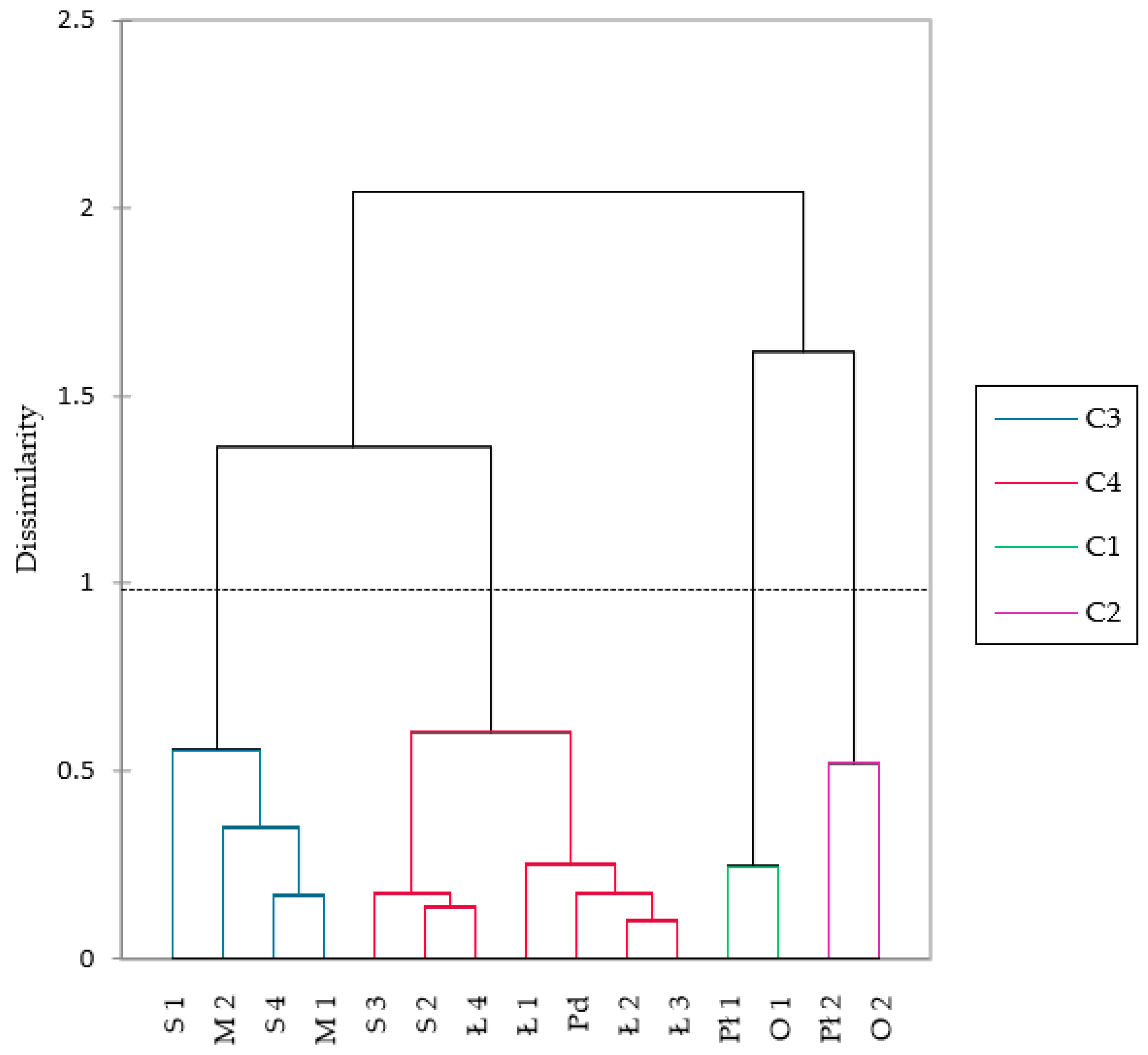
3.3. Phytosociological Characteristics of Sundew Sites
3.4. Rare Species
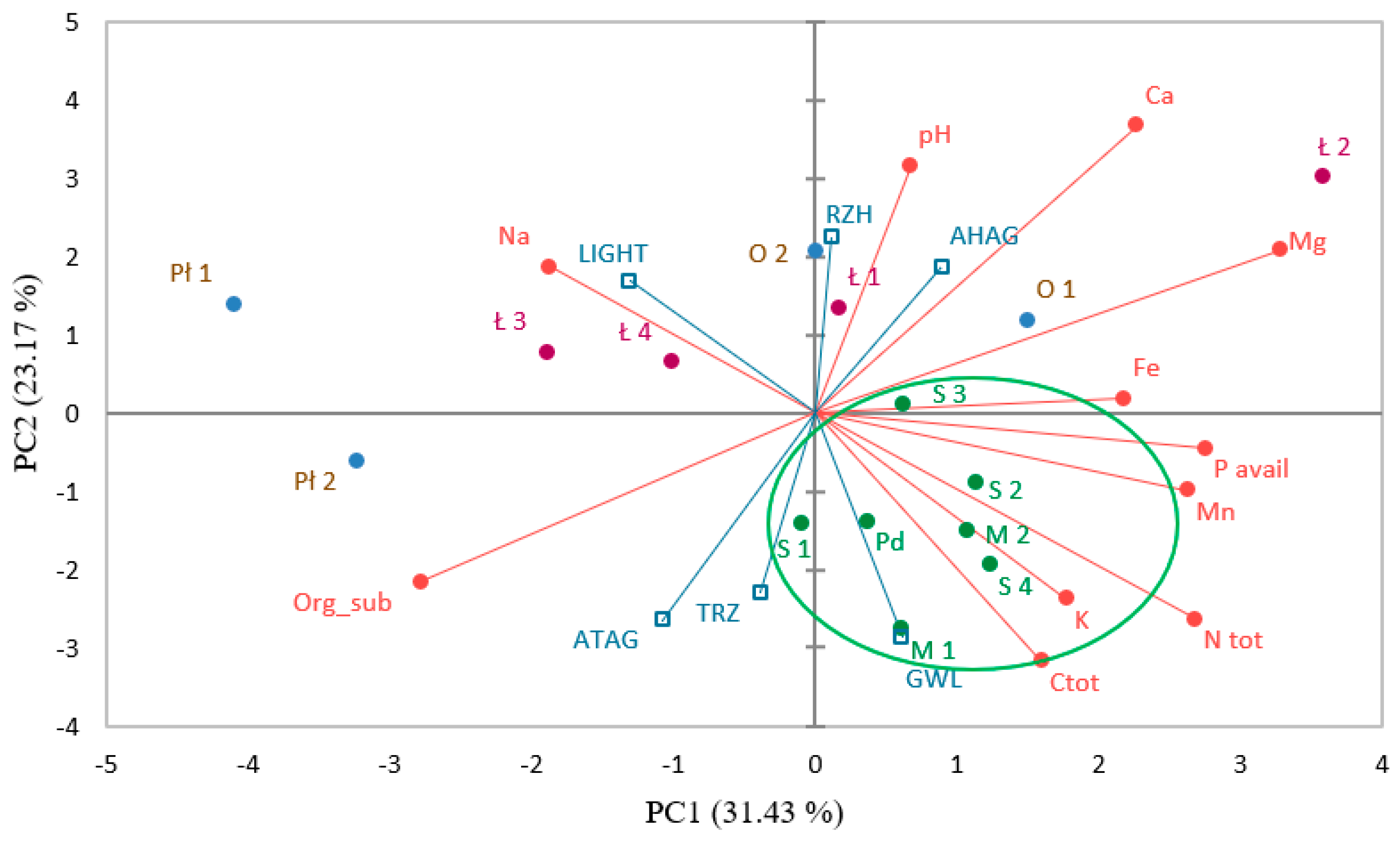
3.5. Physico-Chemical Characteristics of Sundew Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Union for Conservation of Nature Rules of Procedure for IUCN Red List Assessments 2017–2020 (Version 3.0). 2016. Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/Rules_of_Procedure_for_IUCN_Red_List_Assessments_2017–2020.pdf (accessed on 10 April 2022).
- Murray, M.G. Current Issues in Biodiversity Conservation; FAO: Rome, Italy, 2002.
- Römermann, C.; Tackenberg, O.; Jackel, A.K.; Poschlod, P. Eutrophication and fragmentation are related to species’ rate of decline but not to species rarity: Results from a functional approach. Biodivers. Conserv. 2008, 17, 591–604. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Guide to the Identification of Plant Communities in Poland; PWN: Warszawa, Poland, 2008. [Google Scholar]
- Sychowa, M.; Zarzycki, K. Our sundews. Chrońmy Przyr. Ojcz. 1968, 24, 16–26. [Google Scholar]
- Piękoś-Mirkowa, H.; Mirek, Z. Protected Plants; Multico Publication: Warszawa, Poland, 2006. [Google Scholar]
- Zarzycki, K.; Trzcińska-Tacik, H.; Różański, W.; Szeląg, Z.; Wołek, J.; Korzeniak, U. Ecological Indicator Numbers of Polish Vascular Plants; Biodiversity of Poland; IB PAN: Kraków, Poland, 2002. [Google Scholar]
- Zając, A.; Zając, M. Atlas of the Distribution of Vascular Plants in Poland; Laboratory of Computer Chorology, Institute of Botany, Jagiellonian University: Kraków, Poland, 2001. [Google Scholar]
- Ciosek, M.T.; Krechowski, J.; Piórek, K. The position of the intermediate sundew Drosera intermedia in the Południowopodlaska Lowland. Chrońmy Przyr. Ojczystą 2012, 68, 139–142. [Google Scholar]
- Zarzycki, K.; Szeląg, Z. Red List of the Vascular Plants in Poland. In Red List of Plants and Fungi in Poland; W. Szafer Institute of Botany Polish Academy of Sciences: Kraków, Poland, 2006; pp. 9–20. [Google Scholar]
- Rozporządzenie Ministra Środowiska z dnia 9 lipca 2004 r. w sprawie gatunków dziko występujących roślin objętych ochroną [Minister of Environment Regulation of 9 July 2004 on naturally occurring protected plants]. Dz. U. 2004, 168, 1764. (In Polish)
- Piękoś-Mirkowa, H.; Mirek, Z. Flora of Poland. In Atlas of Protected Plants; Multico Publication: Warszawa, Poland, 2003. [Google Scholar]
- Kłosowski, S.; Kłosowski, G. Flora of Poland. In Water and Marsh Plants; Multico Publication: Warszawa, Poland, 2015. [Google Scholar]
- Rutkowski, L. The Key to Marking the Vascular Plants of the Polish Lowland; PWN Publication: Warszawa, Poland, 2006. [Google Scholar]
- Fijałkowski, D. Sundews (Drosera L.) in the Lublin province. Folia Scietatis Sci. Lub. 1975, 17, 53–59. [Google Scholar]
- Council Directive 92/43/EEC of 21 May 1992 on the Protection of Natural Habitats and Wild Fauna and Flora. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1992L0043:20070101:en:PDF (accessed on 8 April 2022).
- Plackowski, R. Ecological and biological aspekt new locality of Drosera anglica Huds. near Końskich. Annales Universitatis Mariae Curie-Sklodowska. Sec. C–Biol. 2019, 73, 41–46. [Google Scholar]
- Borowiec, J. Peat bogs of the Lublin Region. In Prace Wydz. Nauk o Ziemi i Nauk Górniczych; PWN Publication: Warszawa, Poland, 1990. [Google Scholar]
- Chmielewski, T.J.; Harasimiuk, M.; Radwan, S. Renaturalization of Water and Peatland Ecosystems in the Łęczna-Włodawa Lake District; Lubelska Fundacja Ochrony Środowiska Naturalnego; UMCS: Lublin, Poland, 1996. [Google Scholar]
- Kaszewski, B.M.; Siwek, K. Climate and its changes. In Polesie Environment Melioration International; Urban, D., Dobrowolski, R., Jeznach, J., Eds.; Scientific Monograph: Baech, Switzerland, 2020; pp. 135–161. [Google Scholar]
- Fijałkowski, D. The vegetation cover of lakes in the Łęczna and Włodawa area and of peat bogs adjacent to these lakes. Ann. UMCS Sect. B 1959, 14, 131–206. [Google Scholar]
- Sapek, B.; Sapek, A. Methods of Chemical Analysis of Organic Soils; IMUZ Publication: Falenty, Poland, 1997. [Google Scholar]
- Rowell, D.L. Soil Science: Methods and Applications; Longman Group Ltd.: London, UK, 1994. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der vegetationskunde; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- den Hartog, C.; Segal, S. A new classification of the water-plant communities. Acta Bot. Neerl. 1964, 13, 367–393. [Google Scholar] [CrossRef]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridiophytes of Poland. A Check-List; Szafer Institute of Botany; Polish Academy of Sciences: Kraków, Poland, 2002. [Google Scholar]
- Ochyra, R.; Żarnowiec, J.; Bednarek-Ochyra, H. Census Catalogue of Polish Mosses: Biodiversity of Poland; Polish Academy of Sciences: Kraków, Poland, 2003; Volume 3. [Google Scholar]
- Caliński, T.; Harabasz, J. A dendrite method for cluster analysis. Commun. Stat. Theory Methods 1974, 3, 1–27. [Google Scholar] [CrossRef]
- Ricotta, C.; Podani, J. On some properties of the Bray-Curtis dissimilarity and their ecological meaning. Ecol. Complex. 2017, 31, 201–205. [Google Scholar] [CrossRef]
- Crowder, A.A.; Pearson, M.C.; Grubb, P.J.; Langlois, P.H. Biological flora of the British Isles. Drosera L. J. Ecol. 1990, 78, 233–267. [Google Scholar] [CrossRef]
- Keddy, P.A. Wetland ecology: Principles and Conservation; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Dembek, W. Problems of protection and restitution of wetlands in Poland. Inżynieria Ekol. 2002, 6, 68–85. [Google Scholar]
- Huntke, T. The distribution of Drosera anglica Huds. Lower Saxony past and present–the extent of the decline of a raised bog specialist and its causes. Tuexenia 2007, 27, 241–253. [Google Scholar]
- Jennings, D.E.; Rohr, J.R. A review of the conservation threats to carnivorous plants. Biol. Conserv. 2011, 144, 1356–1363. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- McPartland, M.Y.; Montgomery, R.A.; Hanson, P.J.; Phillips, J.R.; Kolka, R.; Palik, B. Vascular plant species response to warming and elevated carbon dioxide in a boreal peatland. Environ. Res. Lett. 2020, 15, 124066. [Google Scholar] [CrossRef]
- Sender, J. Impact of the drainage system on water vegetation of the lowland lakes (Eastern Poland). Turk. J. Fish. Aquat. Sci. 2018, 18, 611–622. [Google Scholar] [CrossRef]
- Urban, D. Soils and vegetation of small interforest bogs of Sobibór forest inspectorate (Wołczyny Forest District). Acta Agrophys. 2002, 68, 235–244. [Google Scholar]
- Pawlaczyk, P. A Swamp Protection Guide; Lubuski Klub Przyrodników Publication: Świebodzin, Poland, 2001. [Google Scholar]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Kadlec, R.H.; Reddy, K.R. Temperature effects in treatment wetlands. Water Environ. Res. 2001, 735, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Hoyo, Y.; Tsuyuzaki, S. Habitat differentiation between Drosera anglica and D. rotundifolia in a post-mined peatland, Northern Japan. Wetlands 2014, 34, 943–953. [Google Scholar] [CrossRef]
- Casper, S.J.; Krausch, H.D. Süßwasserflora von Mitteleuropa. In Pteridophyta und Anthophyta; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1981. [Google Scholar]
- Nordbakken, J.F. Plant niches along the water-table gradient on an ombrotrophic mire expanse. Ecography 1996, 19, 114–121. [Google Scholar] [CrossRef]
- Nordbakken, J.F.; Rydgren, K.; Økland, R.H. Demography and population dynamics of Drosera anglica and D. rotundifolia. J. Ecol. 2004, 92, 110–121. [Google Scholar] [CrossRef]
- Wozniak, L. Chemical degradation of irrationally drained peat soils of the San Valley. Zesz. Probl. Postępów Nauk. Rol. 1999, 467, 371–377. [Google Scholar]

| Feature | Precipitation (mm) | Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| Station | W | Ch | Ł | W | Ch | Ł |
| 1980–1990 | 751.27 | 766.00 | 761.95 | 7.45 | 7.47 | 7.53 |
| 2010–2020 | 710.09 | 712.57 | 710.65 | 9.16 | 9.15 | 9.25 |
| Source of Variability | Precipitation | Temperature |
|---|---|---|
| Period | 0.1170 | 0.0001 * |
| Station | 0.9729 | 0.9454 |
| Period * station | 0.9847 | 0.9972 |
| Site | Stand Area (m2) | Degree of Overgrowth (%) | Expansive and Competitive Species |
|---|---|---|---|
| Potential lake sites (LP) | |||
| Pł1 | 0.09 | 25 | Sphagnum, Vaccinium oxycoccos |
| Pł2 | 0.15 | 80 | Sphagnum, Vaccinium oxycocco, pine seedlings, Andromeda polifonia |
| O1 | 0.06 | 60 | Carex limosa, Vaccinium oxycocco |
| O2 | 0.09 | 80 | Carex limosa, birch seedlings, Molinia caerulea, Vaccinium oxycocco |
| Potential wetland sites (WP) | |||
| S1 | 0.25 | 30 | Sphagnum, Rhynchospora alba |
| S2 | 0.12 | 50 | birch seedlings and pine seedlings, Carex |
| S3 | 0.4 | 30 | Sphagnum, birch seedlings, Rhynchospora alba |
| S4 | 0.09 | 35 | Sphagnum, Vaccinium oxycoccos, pine seedlings |
| M1 | 0.15 | 35 | Sphagnum, Vaccinium oxycoccos, Eriophorum vaginatum |
| M2 | 0.12 | 50 | Sphagnum, Eriophorum vaginatum, Carex lmosa, pine seedlings |
| Pd | 0.16 | 80 | Sphagnum, Eriophorum vaginatum, pine seedlings |
| Current lake sites (LC) | |||
| Ł1 | 0.25 | 20 | Comarum palustre, Vaccinium oxycoccos, Sphagnum, Salix aurita, Vaccinium oxycocco |
| Ł2 | 0.3 | 20 | Comarum palustre, Andromeda polifonia, pine seedlings |
| Ł3 | 3.75 | 5 | Phragmites australis, Nymphea alba, Rhynchospora alba, Sphagnum |
| Ł4 | 0.25 | 20 | Sphagnum, Rhynchospora alba, Rhododendron tomentosum |
| Ł5 | 2 | 5 | Phragmites australis, Nymphea alba |
| Layer Cover | A | B | C | D |
|---|---|---|---|---|
| p-value | 0.5647 | 0.6334 | 0.7043 | 0.0670 |
| Site | Shannon–Wiener Index |
|---|---|
| Pł1 | 1.74 |
| Pł2 | 1.73 |
| O1 | 1.92 |
| O2 | 2.26 |
| S1 | 2.01 |
| S2 | 1.43 |
| S3 | 2.26 |
| S4 | 1.45 |
| M1 | 1.90 |
| M2 | 1.38 |
| Pd | 1.85 |
| Ł1 | 1.45 |
| Ł2 | 1.88 |
| Ł3 | 2.04 |
| Ł4 | 1.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sender, J.; Różańska-Boczula, M.; Urban, D. Active Protection of Endangered Species of Peat Bog Flora (Drosera intermedia, D. anglica) in the Łęczna-Włodawa Lake District. Water 2022, 14, 2775. https://doi.org/10.3390/w14182775
Sender J, Różańska-Boczula M, Urban D. Active Protection of Endangered Species of Peat Bog Flora (Drosera intermedia, D. anglica) in the Łęczna-Włodawa Lake District. Water. 2022; 14(18):2775. https://doi.org/10.3390/w14182775
Chicago/Turabian StyleSender, Joanna, Monika Różańska-Boczula, and Danuta Urban. 2022. "Active Protection of Endangered Species of Peat Bog Flora (Drosera intermedia, D. anglica) in the Łęczna-Włodawa Lake District" Water 14, no. 18: 2775. https://doi.org/10.3390/w14182775
APA StyleSender, J., Różańska-Boczula, M., & Urban, D. (2022). Active Protection of Endangered Species of Peat Bog Flora (Drosera intermedia, D. anglica) in the Łęczna-Włodawa Lake District. Water, 14(18), 2775. https://doi.org/10.3390/w14182775