Ecological Quality Assessment of Greek Lowland Rivers with Aquatic Macrophytes in Compliance with the EU Water Framework Directive
Abstract
:1. Introduction
2. Materials and Methods
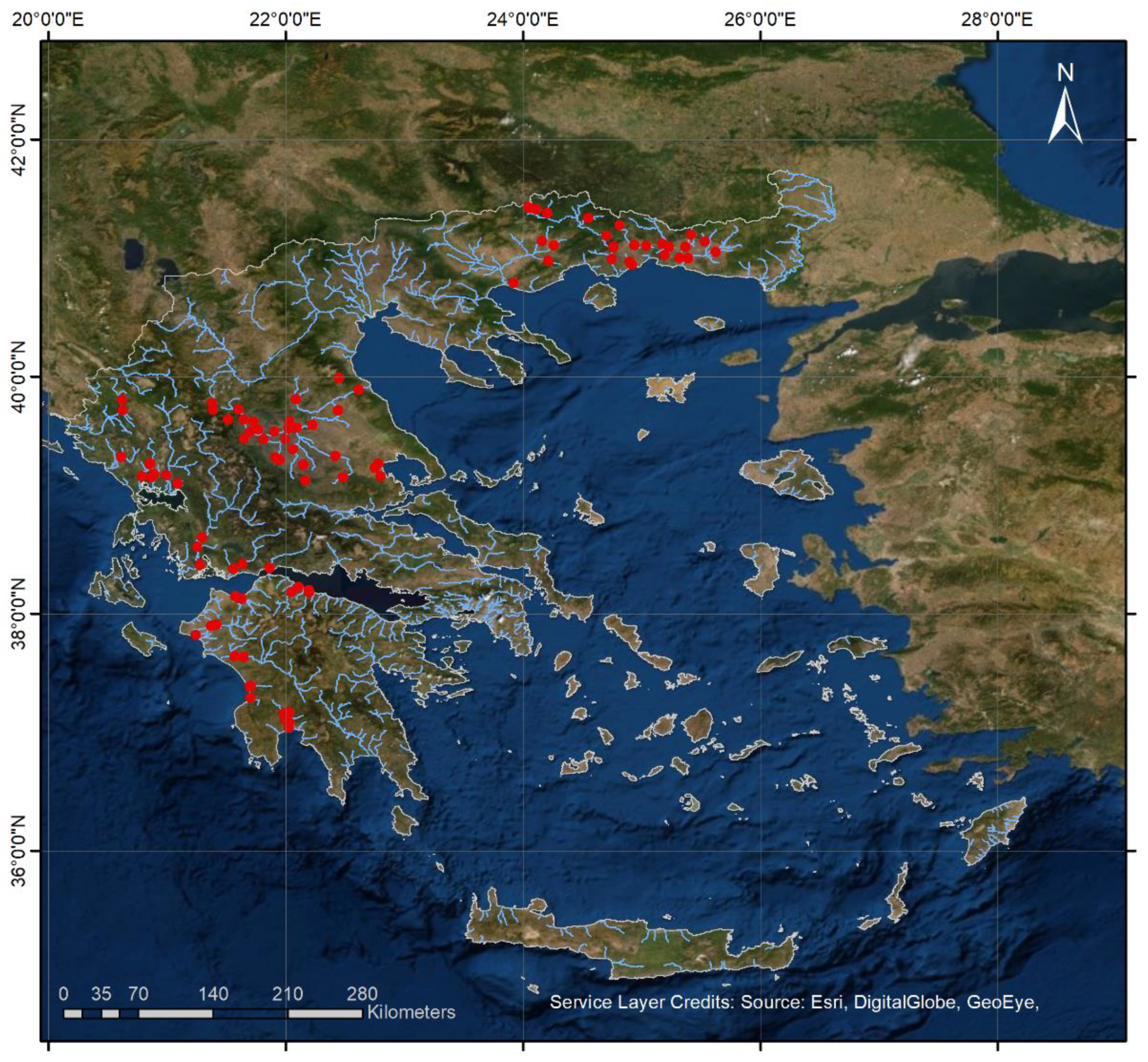
2.1. Field Surveys
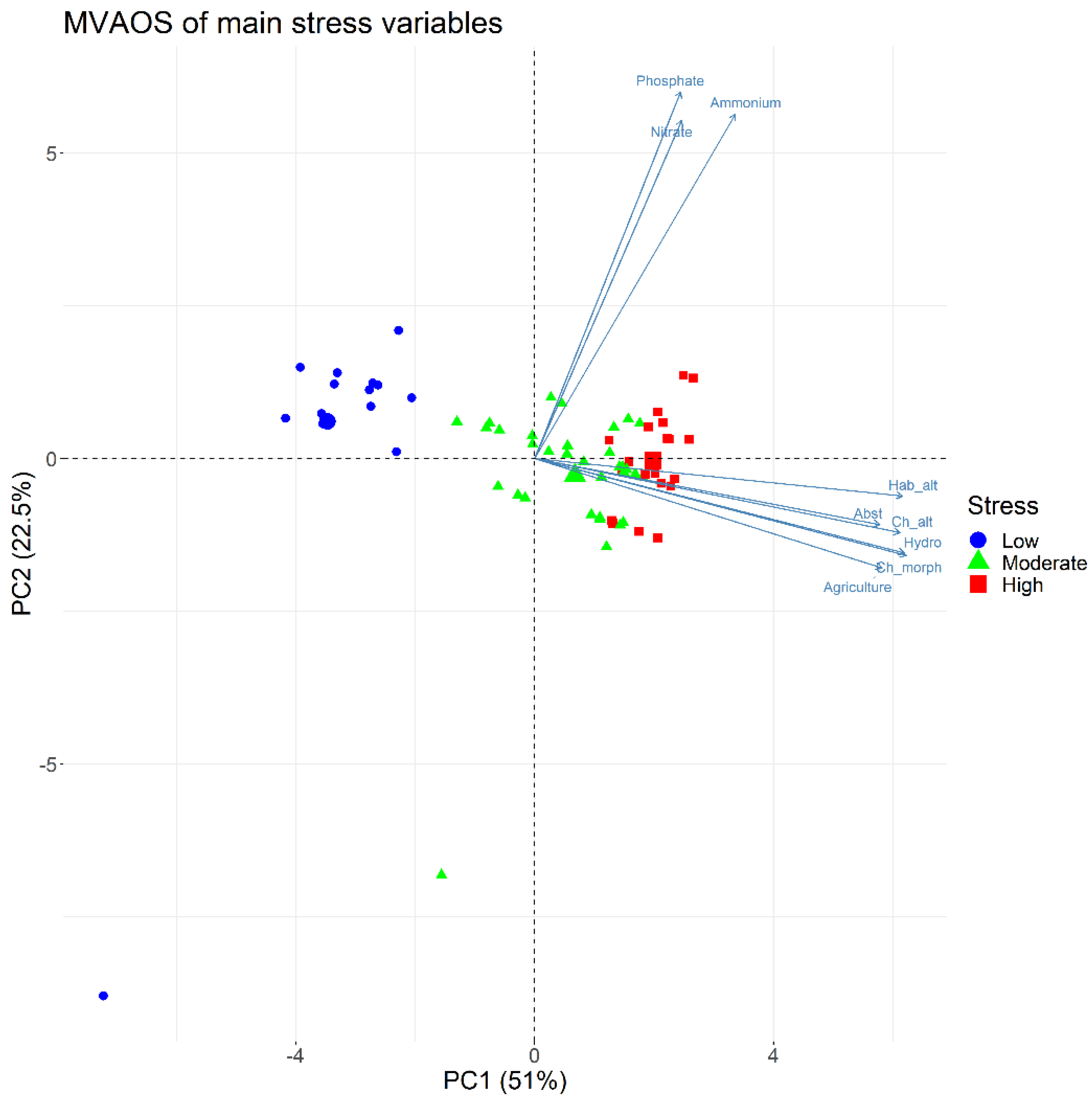
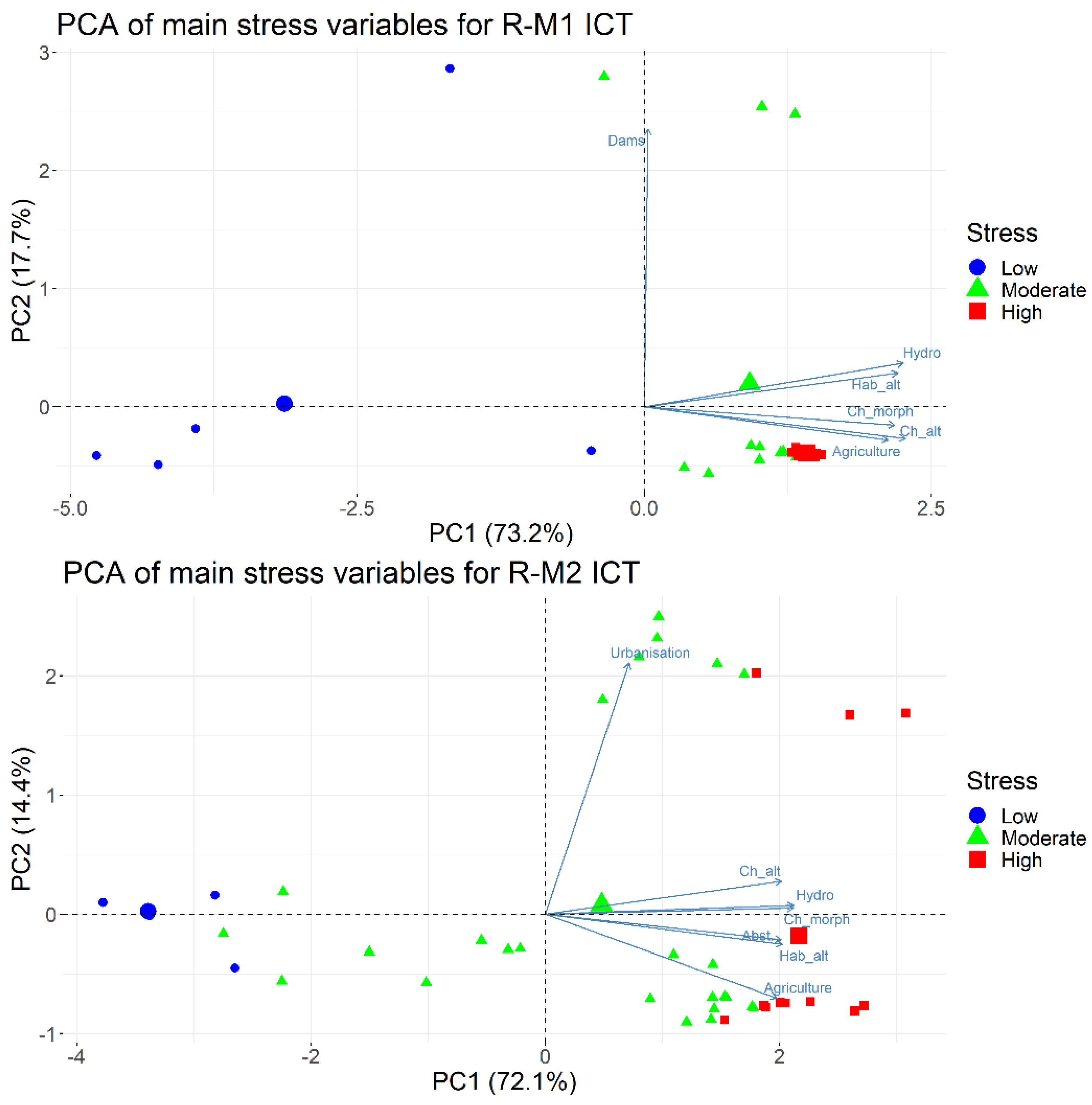
2.2. Statistical Treatment of Environmental Pressure Data—Identification of the Main Stress Gradient
2.3. Development and Implementation of the IBMRGR Index
3. Results and Discussion
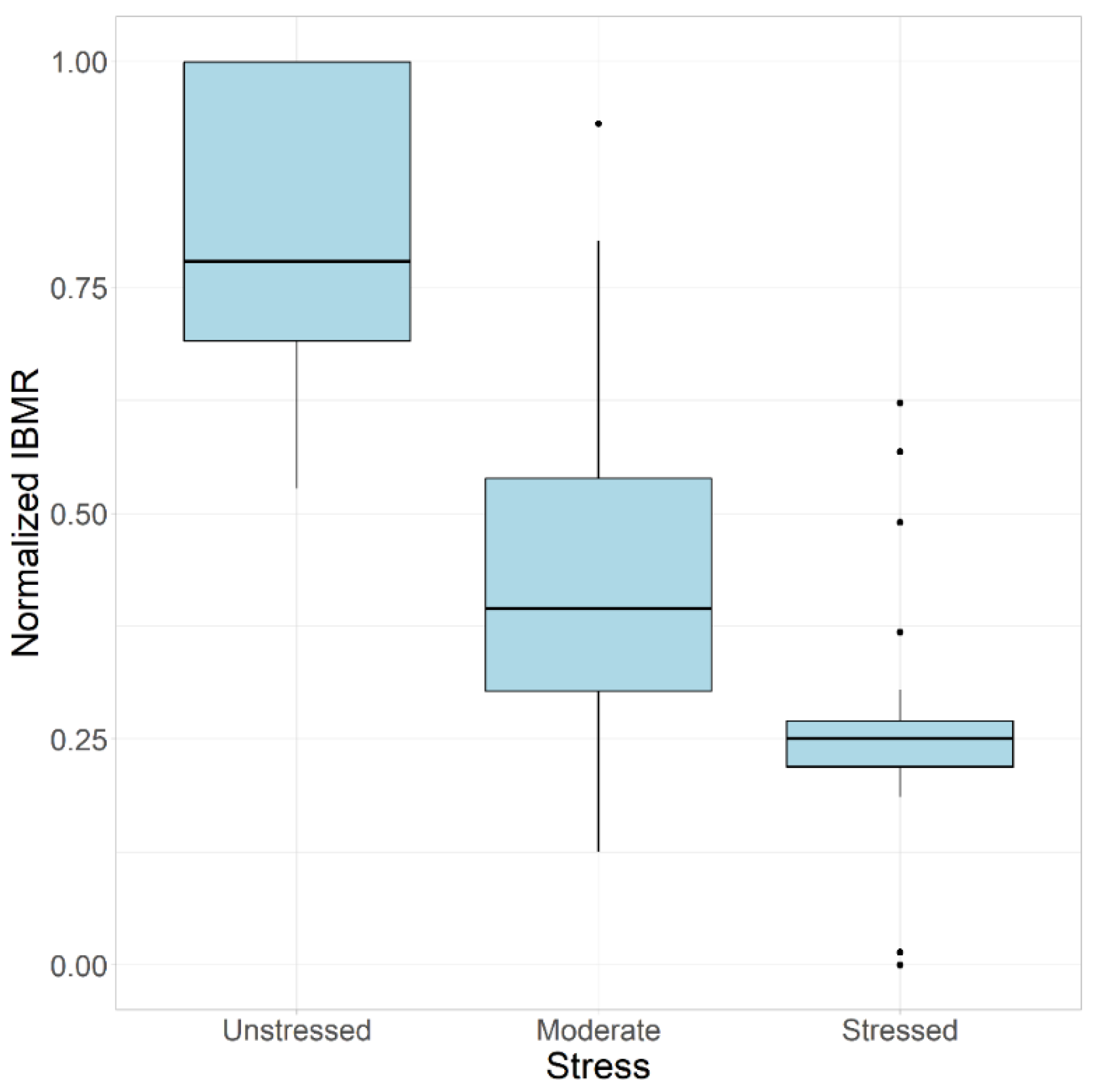
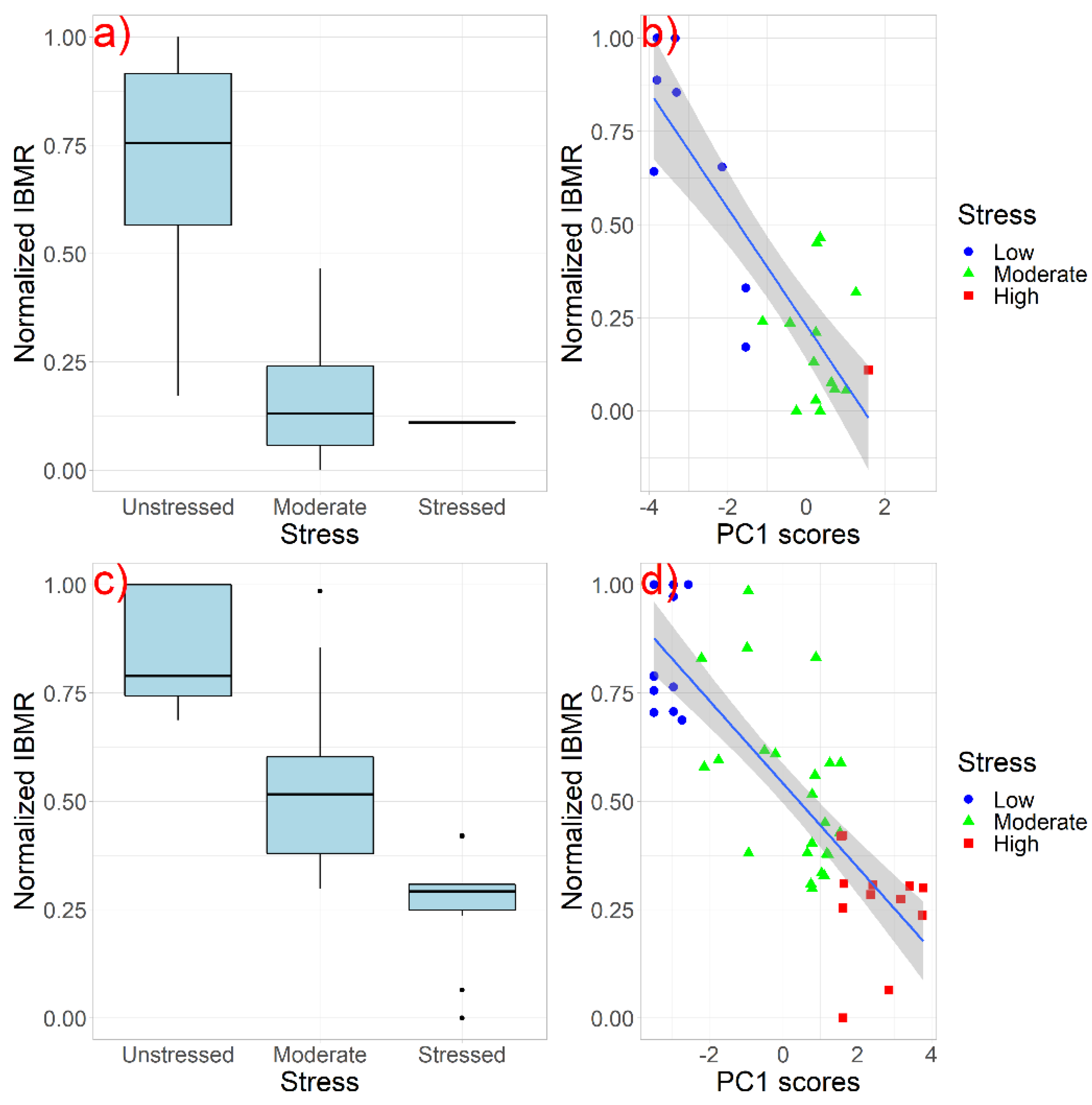
3.1. Determination of the Least Disturbed/Unstressed Sites
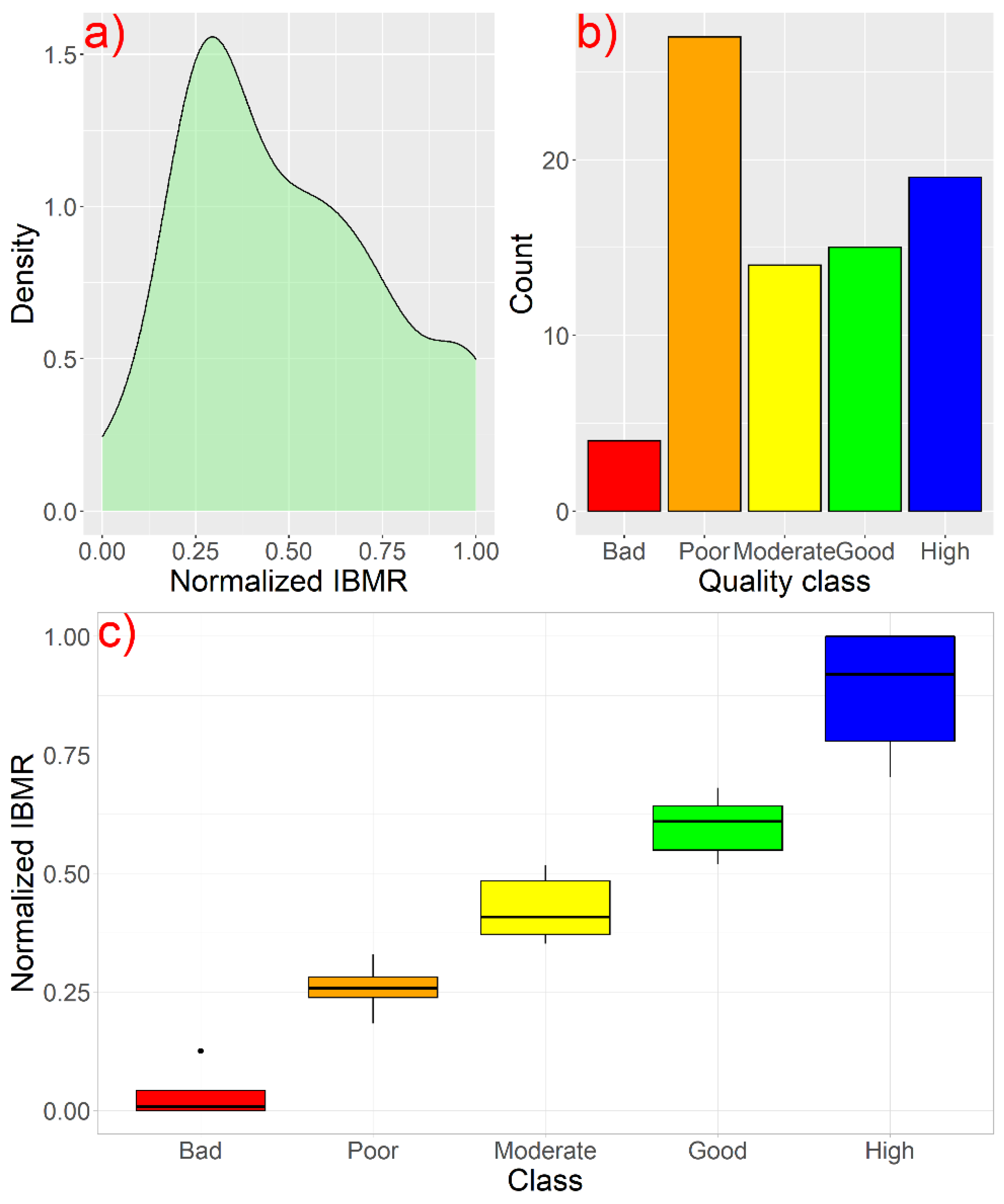
3.2. Definition of the Ecological Class Boundaries of the IBMRGR
3.3. Ecological Classification of the River Sites
3.4. The Use of the IBMRGR Index for Ecological Classification of Greek Running Waters—Strengths and Potential Caveats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguiar, F.C.; Segurado, P.; Urbanič, G.; Cambra, J.; Chauvin, C.; Ciadamidaro, S.; Dörflinger, G.; Ferreira, J.; Germ, M.; Manolaki, P.; et al. Comparability of River Quality Assessment Using Macrophytes: A Multi-Step Procedure to Overcome Biogeographical Differences. Sci. Total Environ. 2014, 476–477, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, K.; Oikonomou, A.; Papastergiadou, E. Responses of Different Facets of Aquatic Plant Diversity along Environmental Gradients in Mediterranean Streams: Results from Rivers of Greece. J. Environ. Manag. 2021, 296, 113307. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, K.; Papastergiadou, E. Linkages between Macrophyte Functional Traits and Water Quality: Insights from a Study in Freshwater Lakes of Greece. Water 2019, 11, 1047. [Google Scholar] [CrossRef]
- Manolaki, P.; Guo, K.; Vieira, C.; Papastergiadou, E.; Riis, T. Hydromorphology as a Controlling Factor of Macrophytes Assemblage Structure and Functional Traits in the Semi-Arid European Mediterranean Streams. Sci. Total Environ. 2020, 703, 134658. [Google Scholar] [CrossRef]
- Tremp, H.; Kohler, A. The Usefulness of Macrophyte Monitoring-Systems, Exemplified on Eutrophication and Acidification of Running Waters. Acta Bot. Gall. 1995, 142, 541–550. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Ciecierska, H.; Kolada, A.; Schneider, S.C.; Szwabińska, M.; Ruszczyńska, J. Parameters Structuring Macrophyte Communities in Rivers and Lakes—Results from a Case Study in North-Central Poland. Knowl. Manag. Aquat. Ecosyst. 2014, 145, 08. [Google Scholar] [CrossRef]
- O’Hare, M.T.; Baattrup-Pedersen, A.; Baumgarte, I.; Freeman, A.; Gunn, I.D.; Lázár, A.N.; Sinclair, R.; Wade, A.J.; Bowes, M.J. Responses of Aquatic Plants to Eutrophication in Rivers: A Revised Conceptual Model. Front. Plant Sci. 2018, 9, 451. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Göthe, E.; Riis, T.; O’Hare, M.T. Functional Trait Composition of Aquatic Plants Can Serve to Disentangle Multiple Interacting Stressors in Lowland Streams. Sci. Total Environ. 2016, 543, 230–238. [Google Scholar] [CrossRef]
- Stromberg, J.C.; Lite, S.J.; Dixon, M.D. Effects of Stream Flow Patterns on Riparian Vegetation of a Semiarid River: Implications for a Changing Climate. River Res. Appl. 2010, 26, 712–729. [Google Scholar] [CrossRef]
- Son, D.; Cho, H.; Lee, E.J. Determining Factors for the Occurrence and Richness of Submerged Macrophytes in Major Korean Rivers. Aquat. Bot. 2018, 150, 82–88. [Google Scholar] [CrossRef]
- Gecheva, G.; Pall, K.; Todorov, M.; Traykov, I.; Gribacheva, N.; Stankova, S.; Birk, S. Anthropogenic Stressors in Upland Rivers: Aquatic Macrophyte Responses. a Case Study from Bulgaria. Plants 2021, 10, 2708. [Google Scholar] [CrossRef] [PubMed]
- Peternel, A.; Gaberščik, A.; Zelnik, I.; Holcar, M.; Germ, M. Long-Term Changes in Macrophyte Distribution and Abundance in a Lowland River. Plants 2022, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.; Willby, N. Towards Harmonization of Ecological Quality Classification: Establishing Common Grounds in European Macrophyte Assessment for Rivers. Hydrobiologia 2010, 652, 149–163. [Google Scholar] [CrossRef]
- European Commission. Directive of the European Parliament and of the Council 2000/60/EC. Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Parliam. 2000, L327, 1–82. [Google Scholar]
- Feio, M.J.; Aguiar, F.C.; Almeida, S.F.P.; Ferreira, J.; Ferreira, M.T.; Elias, C.; Serra, S.R.Q.; Buffagni, A.; Cambra, J.; Chauvin, C.; et al. Least Disturbed Condition for European Mediterranean Rivers. Sci. Total Environ. 2014, 476–477, 745–756. [Google Scholar] [CrossRef]
- Demars, B.O.L.; Potts, J.M.; Trémolières, M.; Thiébaut, G.; Gougelin, N.; Nordmann, V. River Macrophyte Indices: Not the Holy Grail! Freshw. Biol. 2012, 57, 1745–1759. [Google Scholar] [CrossRef]
- Haury, J.; Peltre, M.C.; Trémolières, M.; Barbe, J.; Thiébaut, G.; Bernez, I.; Daniel, H.; Chatenet, P.; Haan-Archipof, G.; Muller, S.; et al. A New Method to Assess Water Trophy and Organic Pollution—The Macrophyte Biological Index for Rivers (IBMR): Its Application to Different Types of River and Pollution. Hydrobiologia 2006, 570, 153–158. [Google Scholar] [CrossRef]
- Skoulikidis, N.T.; Karaouzas, I.; Amaxidis, Y.; Lazaridou, M. Impact of EU Environmental Policy Implementation on the Quality and Status of Greek Rivers. Water 2021, 13, 1858. [Google Scholar] [CrossRef]
- Manolaki, P.; Papastergiadou, E. Environmental Factors Influencing Macrophytes Assemblages in a Middle-Sized Mediterranean Stream. River Res. Appl. 2016, 32, 639–651. [Google Scholar] [CrossRef]
- Manolaki, P.; Papastergiadou, E. The Impact of Environmental Factors on the Distribution Pattern of Aquatic Macrophytes in a Middle-Sized Mediterranean Stream. Aquat. Bot. 2013, 104, 34–46. [Google Scholar] [CrossRef]
- Stefanidis, K.; Papaioannou, G.; Markogianni, V.; Dimitriou, E. Water Quality and Hydromorphological Variability in Greek Rivers: A Nationwide Assessment with Implications for Management. Water 2019, 11, 1680. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, A.; Leprieur, F.; Leonardos, I.D. Biogeography of Freshwater Fishes of the Balkan Peninsula. Hydrobiologia 2014, 738, 205–220. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Baird, R.B., Eaton, A.D., Rice, E.W., Eds.; American Public Health Association, American Water Works Association, Water Environment Federation: Denver, CO, USA, 2017; ISBN 978-0875532875. [Google Scholar]
- Najafzadeh, M.; Niazmardi, S. A Novel Multiple-Kernel Support Vector Regression Algorithm for Estimation of Water Quality Parameters. Nat. Resour. Res. 2021, 30, 3761–3775. [Google Scholar] [CrossRef]
- Najafzadeh, M.; Homaei, F.; Farhadi, H. Reliability Assessment of Water Quality Index Based on Guidelines of National Sanitation Foundation in Natural Streams: Integration of Remote Sensing and Data-Driven Models; Springer: Amsterdam, The Netherlands, 2021; Volume 54, ISBN 0123456789. [Google Scholar]
- Gifi, A. Nonlinear Multivariate Analysis; Wiley: Hoboken, NJ, USA, 1990; ISBN 978-0-471-92620-7. [Google Scholar]
- Mair, P.; Leeuw, J. De Gifi: Multivariate Analysis with Optimal Scaling. R Package Version 0.3-9. 2019. Available online: https://cran.r-project.org/web/packages/Gifi/Gifi.pdf (accessed on 1 July 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 253–258. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Böhmer, J.; Rawer-Jost, C.; Zenker, A. Multimetric Assessment of Data Provided by Water Managers from Germany: Assessment of Several Different Types of Stressors with Macrozoobenthos Communities. Hydrobiologia 2004, 516, 215–228. [Google Scholar] [CrossRef]
- Pollard, P.; van de Bund, W. Template for the Development of a Boundary Setting Protocol for the Purposes of the Intercalibration Exercise; Version 1.2; CIS—Ecostat Working Group: Brussels, Belgium, 2005. [Google Scholar]
- Stefanidis, K.; Oikonomou, A.; Stoumboudi, M.; Dimitriou, E.; Skoulikidis, N.T. Do Water Bodies Show Better Ecological Status in Natura 2000 Protected Areas than Non-Protected Ones?—The Case of Greece. Water 2021, 13, 3007. [Google Scholar] [CrossRef]
- Masouras, A.; Karaouzas, I.; Dimitriou, E.; Tsirtsis, G.; Smeti, E. Benthic Diatoms in River Biomonitoring-Present and Future Perspectives within the Water Framework Directive. Water 2021, 13, 478. [Google Scholar] [CrossRef]
- Lemm, J.U.; Venohr, M.; Globevnik, L.; Stefanidis, K.; Panagopoulos, Y.; Gils, J.; Posthuma, L.; Kristensen, P.; Feld, C.K.; Mahnkopf, J.; et al. Multiple Stressors Determine River Ecological Status at the European Scale: Towards an Integrated Understanding of River Status Deterioration. Glob. Chang. Biol. 2021, 27, 1962–1975. [Google Scholar] [CrossRef]
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M.; et al. Impacts of Multiple Stressors on Freshwater Biota across Spatial Scales and Ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068. [Google Scholar] [CrossRef]
- European Commision. Common Implementation Strategy for the Water Framework Directive (2000/60/EC). Guidance Document n. 10. River and Lakes—Typology, Reference Conditions and Classification Systems. 2003. Available online: https://circabc.europa.eu/sd/a/dce34c8d-6e3d-469a-a6f3-b733b829b691/Guidance%20No%2010%20-%20references%20conditions%20inland%20waters%20-%20REFCOND%20(WG%202.3).pdf (accessed on 15 July 2022).
- Wiegleb, G.; Gebler, D.; van de Weyer, K.; Birk, S. Comparative Test of Ecological Assessment Methods of Lowland Streams Based on Long-Term Monitoring Data of Macrophytes. Sci. Total Environ. 2016, 541, 1269–1281. [Google Scholar] [CrossRef]
- Stefanidis, K.; Latsiou, A.; Kouvarda, T.; Lampou, A.; Kalaitzakis, N.; Gritzalis, K.; Dimitriou, E. Disentangling the Main Components of Hydromorphological Modifications at Reach Scale in Rivers of Greece. Hydrology 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Vukov, D.; Ilić, M.; Ćuk, M.; Igić, R. The Effect of Hydro-Morphology and Habitat Alterations on the Functional Diversity and Composition of Macrophyte Communities in the Large River. Front. Environ. Sci. 2022, 10, 863508. [Google Scholar] [CrossRef]
- Lozanovska, I.; Rivaes, R.; Vieira, C.; Ferreira, M.T.; Aguiar, F.C. Streamflow Regulation Effects in the Mediterranean Rivers: How Far and to What Extent Are Aquatic and Riparian Communities Affected? Sci. Total Environ. 2020, 749, 141616. [Google Scholar] [CrossRef]
- Lozanovska, I.; Bejarano, M.D.; Martins, M.J.; Nilsson, C.; Ferreira, M.T.; Aguiar, F.C. Functional Diversity of Riparian Woody Vegetation Is Less Affected by River Regulation in the Mediterranean Than Boreal Region. Front. Plant Sci. 2020, 11, 857. [Google Scholar] [CrossRef]
- Bejarano, M.D.; Jansson, R.; Nilsson, C. The Effects of Hydropeaking on Riverine Plants: A Review. Biol. Rev. 2018, 93, 658–673. [Google Scholar] [CrossRef]
- Lazaridou, M.; Ntislidou, C.; Karaouzas, I.; Skoulikidis, N.; Birk, S. Harmonization of the Assessment Method for Classifying the Ecological Quality Status of Very Large Greek Rivers. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 50. [Google Scholar] [CrossRef]
- Kelly, M.G.; Chiriac, G.; Soare-Minea, A.; Hamchevici, C.; Birk, S. Defining Ecological Status of Phytobenthos in Very Large Rivers: A Case Study in Practical Implementation of the Water Framework Directive in Romania. Hydrobiologia 2019, 828, 353–367. [Google Scholar] [CrossRef]
- Rodrigues, C.; Alves, P.; Bio, A.; Vieira, C.; Guimarães, L.; Pinheiro, C.; Vieira, N. Assessing the Ecological Status of Small Mediterranean Rivers Using Benthic Macroinvertebrates and Macrophytes as Indicators. Environ. Monit. Assess. 2019, 191, 596. [Google Scholar] [CrossRef]
- Schneider, S. Macrophyte Trophic Indicator Values from a European Perspective. Limnologica 2007, 37, 281–289. [Google Scholar] [CrossRef]
- Karaouzas, I.; Smeti, E.; Kalogianni, E.; Skoulikidis, N.T. Ecological Status Monitoring and Assessment in Greek Rivers: Do Macroinvertebrate and Diatom Indices Indicate Same Responses to Anthropogenic Pressures? Ecol. Indic. 2019, 101, 126–132. [Google Scholar] [CrossRef]
- Urbanič, G.; Politti, E.; Rodríguez-González, P.M.; Payne, R.; Schook, D.; Alves, M.H.; Anđelković, A.; Bruno, D.; Chilikova-Lubomirova, M.; Di Lonardo, S.; et al. Riparian Zones—From Policy Neglected to Policy Integrated. Front. Environ. Sci. 2022, 10, 868527. [Google Scholar] [CrossRef]


| Stressor | Description | |
|---|---|---|
| Hydromorphological | Channel profile/cross section alteration | Degree of channel profile modification present at the site/cross section alteration |
| Channel morphology | Degree of the morphological modification of the channel present at the site | |
| Local habitat alteration | Alteration of instream habitats | |
| Stream hydrology | Degree of the hydrological alteration present at the site | |
| Upstream dams influence | Effect of upstream dams | |
| Water abstraction | Effect of water abstraction at the site | |
| Dykes (flood protection) | Effect of dykes for flood protection | |
| Physicochemical | pH | Sorensen scale |
| Conductivity | Conductivity [mS/cm] | |
| Ammonium | Ammonia concentration in the water [mg/L NH4+] | |
| Nitrate | Nitrate concentration in the water [mg/L NO3−] | |
| Total nitrogen | Total Nitrogen [mg/L TN] | |
| Total phosphorus | Concentration of total phosphorus in the water [mg/L TP] | |
| Orthophosphates | Concentration of Orthophosphates in the water [mg/L PO4 3−] | |
| DO | Concentration of dissolved oxygen [mg/L] | |
| Land use | Urbanization | Urban and industrial areas in immediate vicinity of site |
| Agriculture | Agriculture at the immediate vicinity of site |
| Stressor | PC1 | PC2 |
|---|---|---|
| Channel profile/cross section alteration | 0.882 | −0.082 |
| Channel morphology | 0.872 | −0.099 |
| Habitat alteration | 0.848 | 0.012 |
| Stream hydrology | 0.877 | −0.093 |
| Dams influence | −0.137 | 0.35 |
| Water abstraction | 0.822 | −0.048 |
| Dykes | 0.619 | −0.282 |
| DO | −0.436 | −0.225 |
| pH | −0.168 | −0.21 |
| Electrical conductivity | 0.596 | −0.109 |
| Ammonium | 0.407 | 0.799 |
| Nitrate | 0.283 | 0.834 |
| Phosphate | 0.268 | 0.82 |
| Urbanization | 0.325 | 0.167 |
| Agriculture | 0.868 | −0.189 |
| Type | Ecological Quality Class Boundaries | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High | Good | Moderate | Poor | Bad | ||||||
| MIN | MAX | MIN | MAX | MIN | MAX | MIN | MAX | MIN | MAX | |
| R-M1 | >0.705 | 1 | >0.529 | ≤0.705 | >0.352 | ≤0.529 | >0.176 | ≤0.352 | 0 | ≤0.176 |
| R-M2 | >0.754 | 1 | >0.567 | ≤0.754 | >0.378 | ≤0.567 | >0.189 | ≤0.378 | 0 | ≤0.189 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanidis, K.; Dimitrellos, G.; Sarika, M.; Tsoukalas, D.; Papastergiadou, E. Ecological Quality Assessment of Greek Lowland Rivers with Aquatic Macrophytes in Compliance with the EU Water Framework Directive. Water 2022, 14, 2771. https://doi.org/10.3390/w14182771
Stefanidis K, Dimitrellos G, Sarika M, Tsoukalas D, Papastergiadou E. Ecological Quality Assessment of Greek Lowland Rivers with Aquatic Macrophytes in Compliance with the EU Water Framework Directive. Water. 2022; 14(18):2771. https://doi.org/10.3390/w14182771
Chicago/Turabian StyleStefanidis, Konstantinos, Georgios Dimitrellos, Maria Sarika, Dionysios Tsoukalas, and Eva Papastergiadou. 2022. "Ecological Quality Assessment of Greek Lowland Rivers with Aquatic Macrophytes in Compliance with the EU Water Framework Directive" Water 14, no. 18: 2771. https://doi.org/10.3390/w14182771
APA StyleStefanidis, K., Dimitrellos, G., Sarika, M., Tsoukalas, D., & Papastergiadou, E. (2022). Ecological Quality Assessment of Greek Lowland Rivers with Aquatic Macrophytes in Compliance with the EU Water Framework Directive. Water, 14(18), 2771. https://doi.org/10.3390/w14182771







