Improving Environmental DNA Sensitivity for Dreissenid Mussels by Targeting Tandem Repeat Regions of the Mitochondrial Genome
Abstract
:1. Introduction
2. Methods
2.1. Estimation of Gene Copy Number per Genome
2.2. Assay Design
2.3. Assay Validation
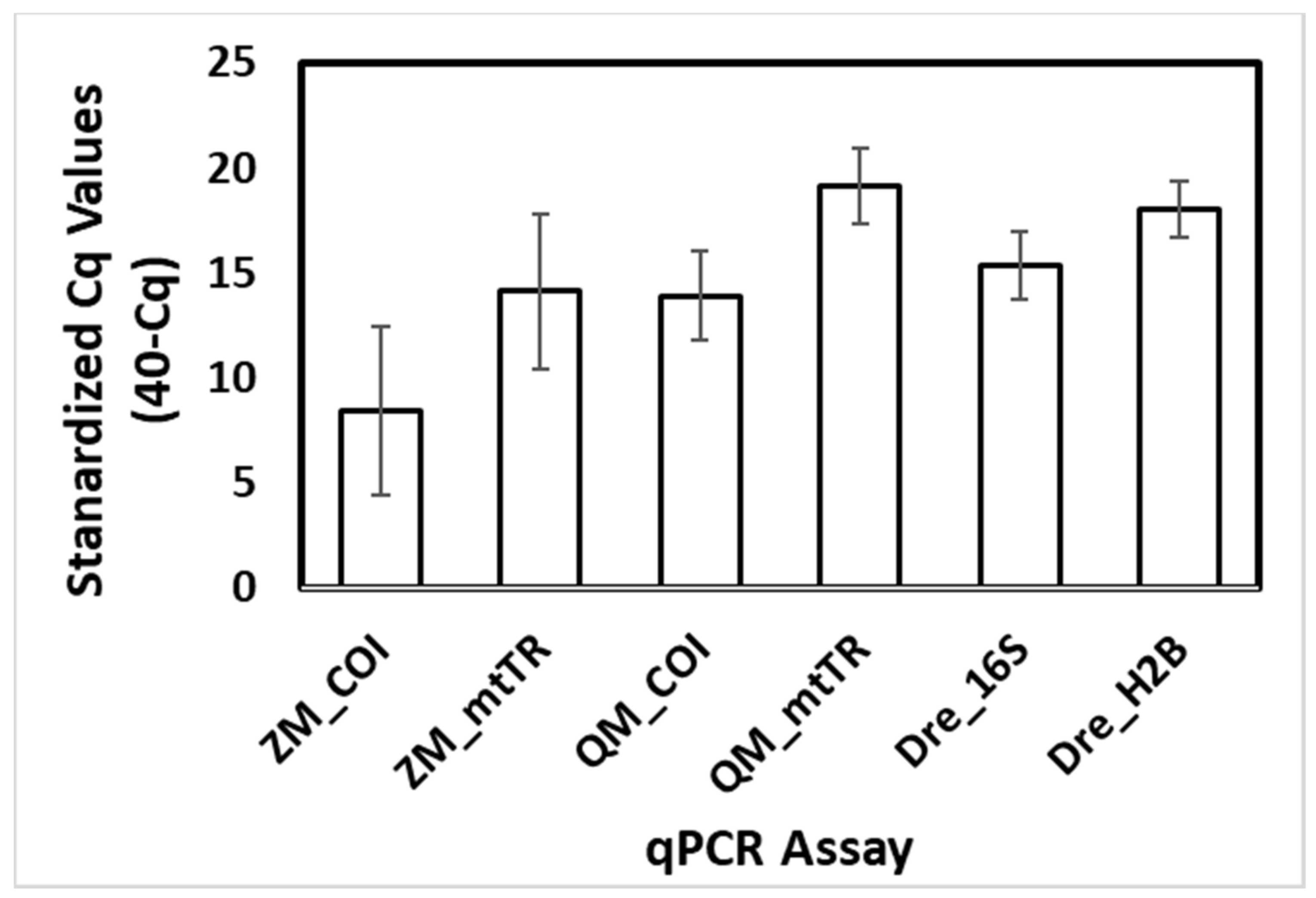
2.4. Comparisons of Assay Sensitivity
3. Results
3.1. Copy Number per Genome
3.2. Assay Specificity
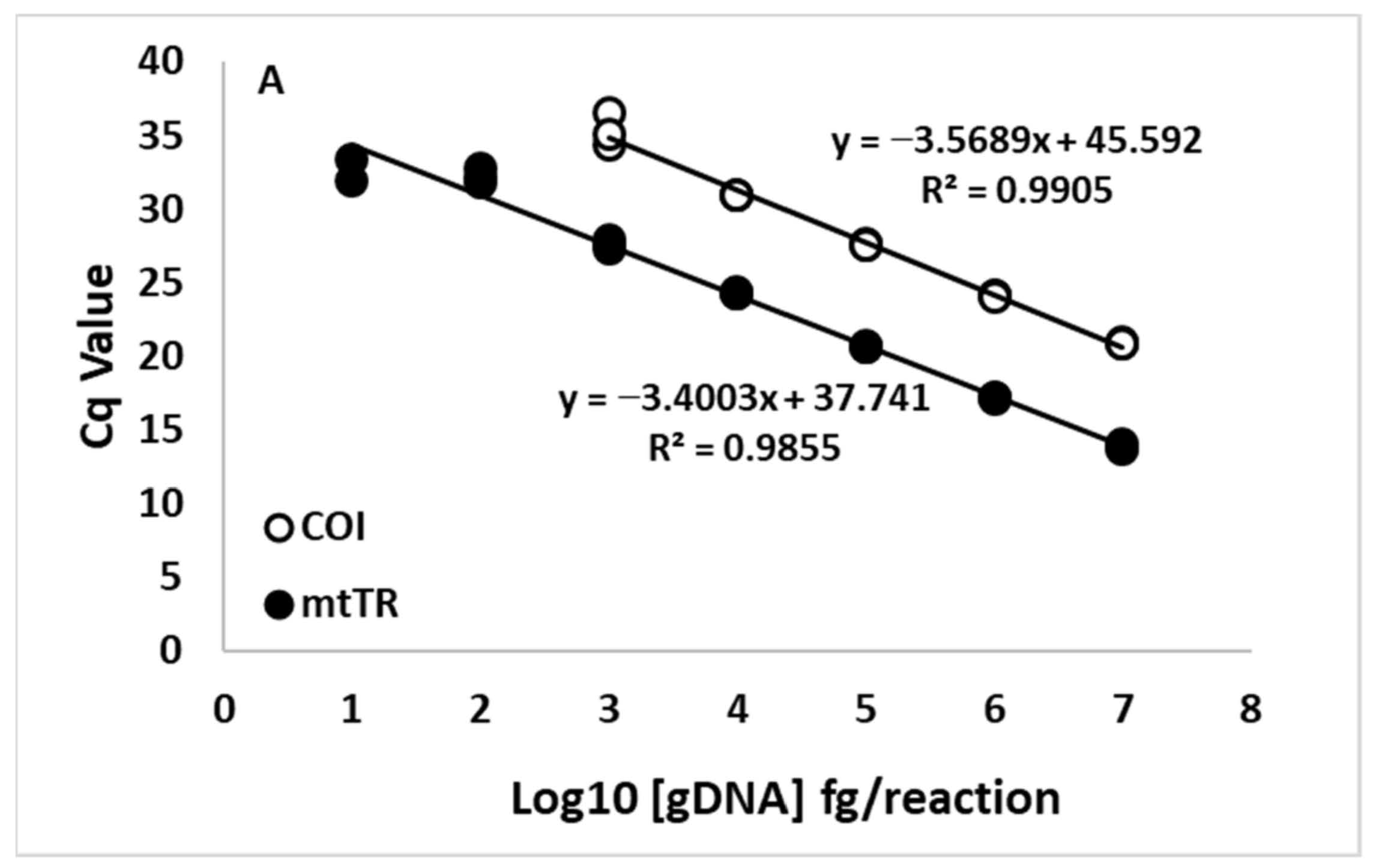
3.3. Assay Sensitivity—Standard Curves
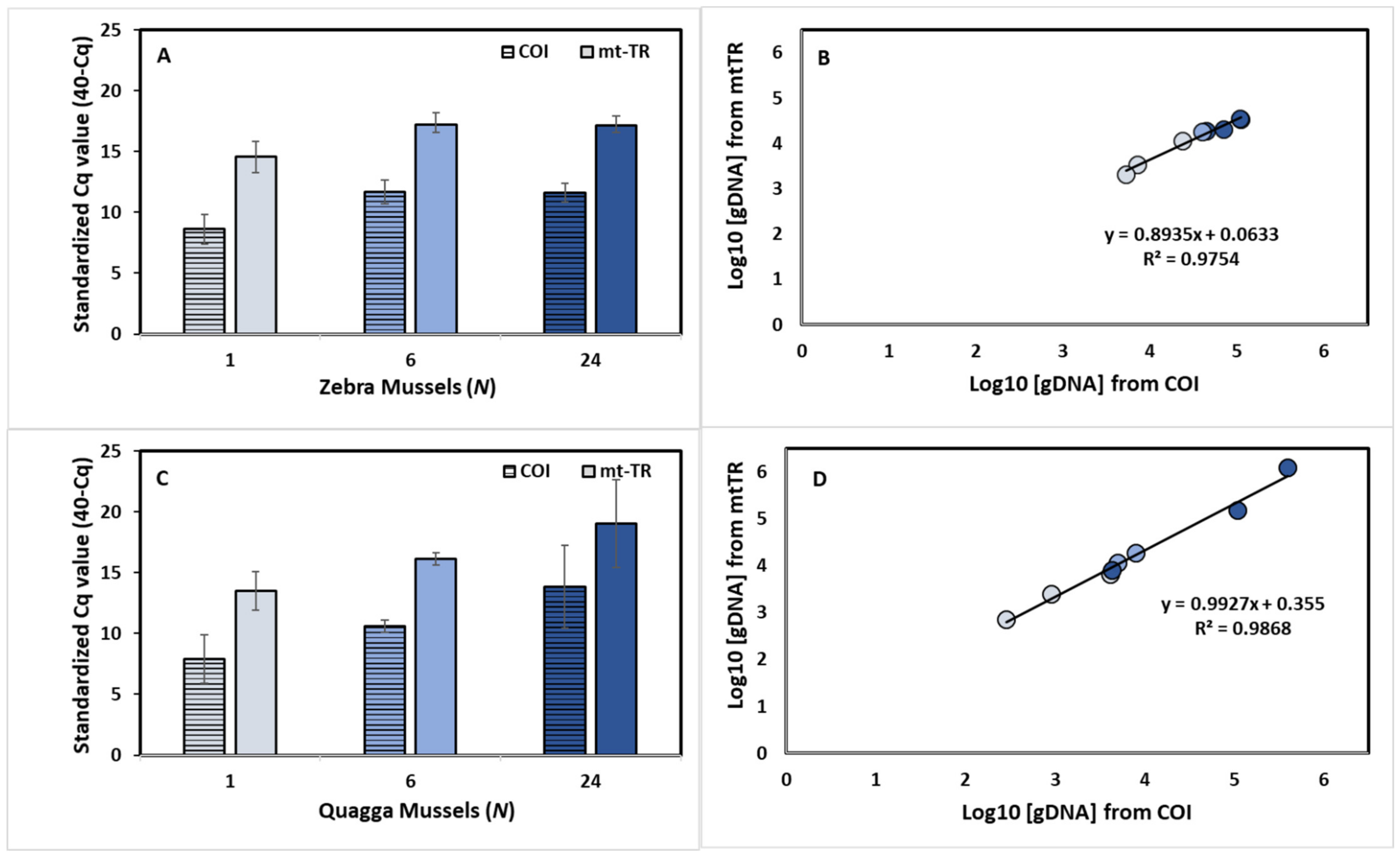
3.4. Assay Sensitivity—Mesocosm Experiments
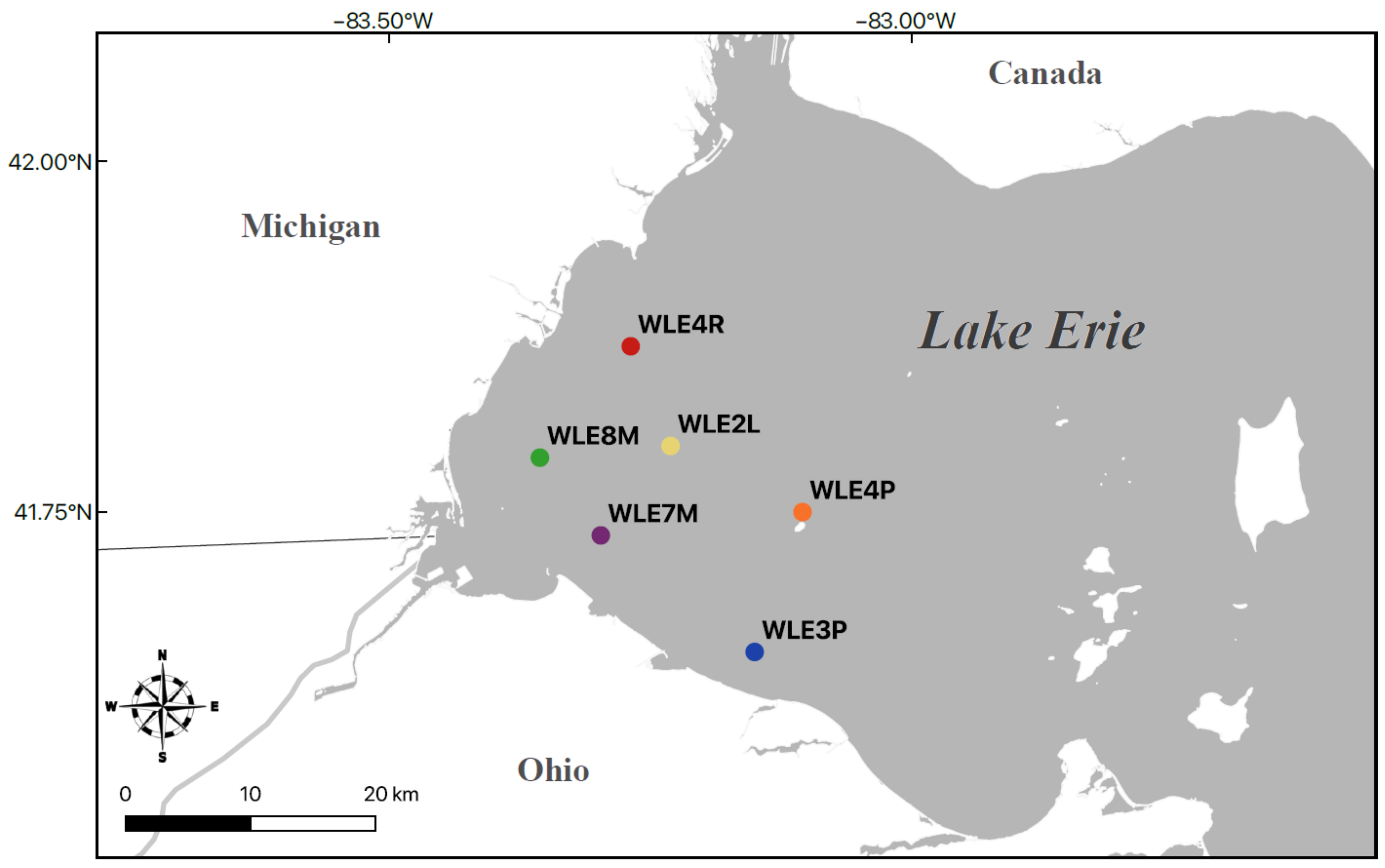
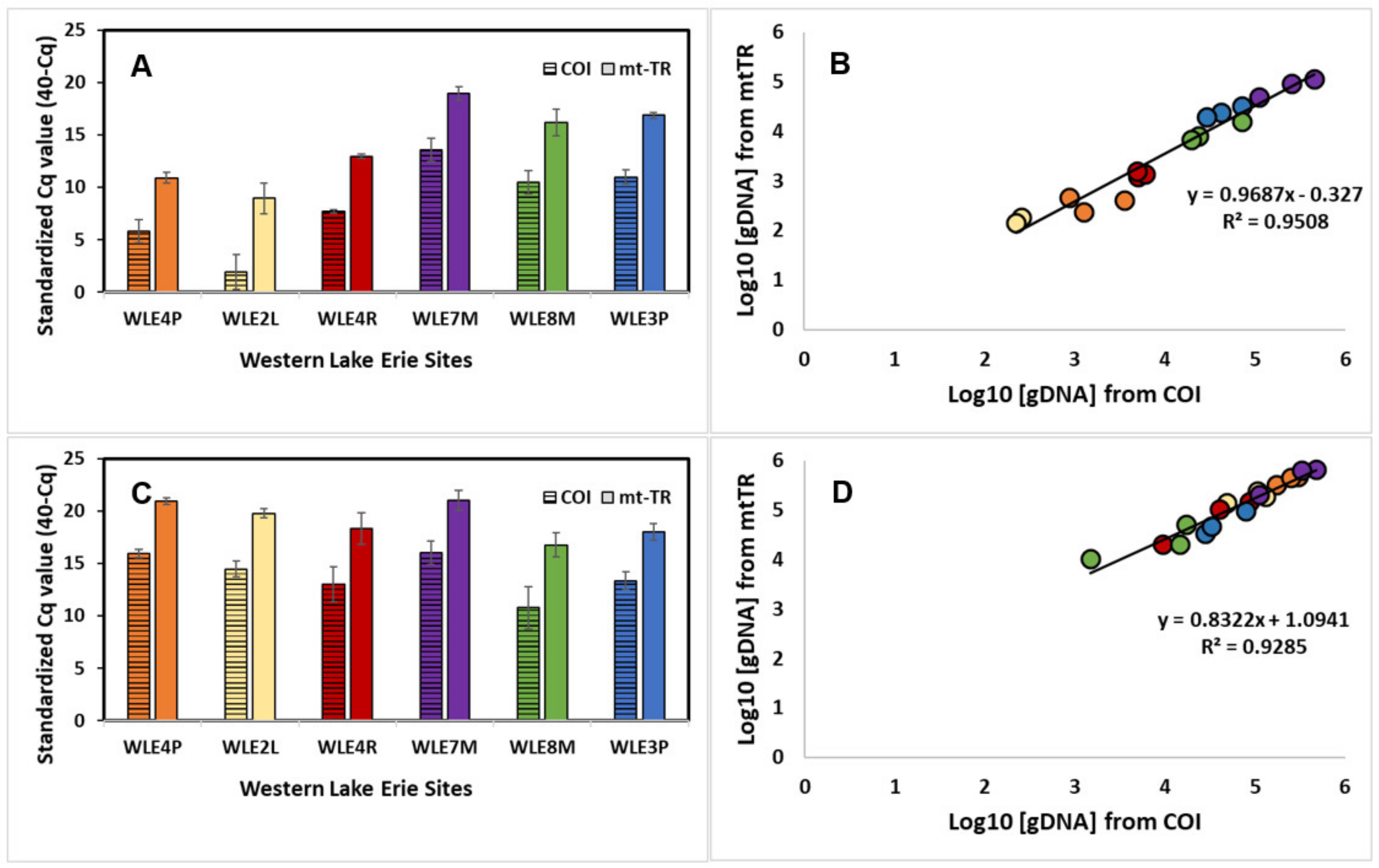
3.5. Assay Sensitivity—Western Lake Erie
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nalepa, T.F.; Schloesser, D.W. (Eds.) Quagga and Zebra Mussels: Biology, Impacts, and Control; CRC Press: Boca Raton, FL, USA, 2013; pp. 9–32. [Google Scholar]
- Marshall, N.T.; Stepien, C.A. The family Dreissenidae. In Freshwater Mollusk Families of the World; Cummings, K., Lydeard, C., Eds.; JHU Press: Baltimore, MD, USA, 2019; pp. 193–196. [Google Scholar]
- Vanderploeg, H.A.; Nalepa, T.F.; Jude, D.J.; Mills, E.L.; Holeck, K.T.; Liebig, J.R.; Grigorovich, I.A.; Ojaveer, H. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Can. J. Fish. Aquat. Sci. 2002, 59, 1209–1228. [Google Scholar] [CrossRef] [Green Version]
- Higgins, S.N.; Zanden, M.J.V. What a difference a species makes: A meta–analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol. Monogr. 2010, 80, 179–196. [Google Scholar] [CrossRef] [Green Version]
- Carlton, J.T. The zebra mussel Dreissena polymorpha found in North America in 1986 and 1987. J. Great Lakes Res. 2008, 34, 770–773. [Google Scholar] [CrossRef]
- May, B.; Marsden, J.E. Genetic identification and implications of another invasive species of dreissenid mussel in the Great Lakes. Can. J. Fish. Aquat. Sci. 1992, 49, 1501–1506. [Google Scholar] [CrossRef]
- De Ventura, L.; Weissert, N.; Tobias, R.; Kopp, K.; Jokela, J. Overland transport of recreational boats as a spreading vector of zebra mussel Dreissena polymorpha. Biol. Invasions 2016, 18, 1451–1466. [Google Scholar] [CrossRef]
- Snyder, M.R.; Stepien, C.A.; Marshall, N.; Scheppler, H.B.; Black, C.L.; Czajkowski, K.P. Detecting aquatic invasive species in bait and pond stores with targeted environmental (e)DNA high-throughput sequencing metabarcode assays: Angler, retailer, and manager implications. Biol. Conserv. 2020, 245, 108430. [Google Scholar] [CrossRef]
- Patoka, J.; Patoková, B. Hitchhiking Exotic Clam: Dreissena polymorpha (Pallas, 1771) transported via the ornamental plant trade. Diversity 2021, 13, 410. [Google Scholar] [CrossRef]
- Sepulveda, A.; Smith, D.; O’Donnell, K.; Owens, N.; White, B.; Richter, C.; Merkes, C.; Wolf, S.; Rau, M.; Neilson, M.; et al. Using structured decision making to evaluate potential management responses to detection of dreissenid mussel (Dreissena spp.) environmental DNA. Manag. Biol. Invasions 2022, 13, 344–368. [Google Scholar] [CrossRef]
- Ginn, B.K.; Bolton, R.; Coulombe, D.; Fleischaker, T.; Yerex, G. Quantifying a shift in benthic dominance from zebra (Dreissena polymorpha) to quagga (Dreissena rostriformis bugensis) mussels in a large, inland lake. J. Great Lakes Res. 2018, 44, 271–282. [Google Scholar] [CrossRef]
- Larson, C.E.; Barge, J.T.; Hatzenbuhler, C.L.; Hoffman, J.C.; Peterson, G.S.; Pilgrim, E.M.; Wiechman, B.; Rees, C.B.; Trebitz, A.S. Invasive Dreissena mussel coastal transport from an already invaded estuary to a nearby archipelago detected in DNA and zooplankton surveys. Front. Mar. Sci. 2022, 9, 818738. [Google Scholar] [CrossRef]
- Feist, S.M.; Lance, R.F. Advanced molecular-based surveillance of quagga and zebra mussels: A review of environmental DNA/RNA (eDNA/eRNA) studies and considerations for future directions. NeoBiota 2021, 66, 117–159. [Google Scholar] [CrossRef]
- Frischer, M.E.; Hansen, A.S.; Wyllie, J.A.; Wimbush, J.; Murray, J.; Nierzwicki-Bauer, S.A. Specific amplification of the 18S rRNA gene as a method to detect zebra mussel (Dreissena polymorpha) larvae in plankton samples. Hydrobiologia 2002, 487, 33–44. [Google Scholar] [CrossRef]
- Sepulveda, A.J.; Nelson, N.M.; Jerde, C.L.; Luikart, G. Are environmental DNA methods ready for aquatic invasive species management? Trends Ecol. Evol. 2020, 35, 668–678. [Google Scholar] [CrossRef]
- Gingera, T.; Bajno, R.; Docker, M.; Reist, J. Environmental DNA as a detection tool for zebra mussels Dreissena polymorpha (Pallas, 1771) at the forefront of an invasion event in Lake Winnipeg, Manitoba, Canada. Manag. Biol. Invasions 2017, 8, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Amberg, J.J.; Merkes, C.M.; Stott, W.; Rees, C.B.; Erickson, R.A. Environmental DNA as a tool to help inform zebra mussel, Dreissena polymorpha, management in inland lakes. Manag. Biol. Invasions 2019, 10, 96–110. [Google Scholar] [CrossRef] [Green Version]
- Marshall, N.; Stepien, C.A. Invasion genetics from eDNA and thousands of larvae: A targeted metabarcoding assay that distinguishes species and population variation of zebra and quagga mussels. Ecol. Evol. 2019, 9, 3515–3538. [Google Scholar] [CrossRef]
- Peñarrubia, L.; Alcaraz, C.; De Vaate, A.B.; Sanz, N.; Pla, C.; Vidal, O.; Viñas, J. Validated methodology for quantifying infestation levels of dreissenid mussels in environmental DNA (eDNA) samples. Sci. Rep. 2016, 6, 39067. [Google Scholar] [CrossRef]
- De Ventura, L.; Kopp, K.; Seppälä, K.; Jokela, J. Tracing the quagga mussel invasion along the Rhine river system using eDNA markers: Early detection and surveillance of invasive zebra and quagga mussels. Manag. Biol. Invasions 2017, 8, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Shogren, A.J.; Tank, J.L.; Egan, S.P.; Bolster, D.; Riis, T. Riverine distribution of mussel environmental DNA reflects a balance among density, transport, and removal processes. Freshw. Biol. 2019, 64, 1467–1479. [Google Scholar] [CrossRef]
- Blackman, R.C.; Ling, K.K.S.; Harper, L.R.; Shum, P.; Hänfling, B.; Lawson-Handley, L. Targeted and passive environmental DNA approaches outperform established methods for detection of quagga mussels, Dreissena rostriformis bugensis in flowing water. Ecol. Evol. 2020, 10, 13248–13259. [Google Scholar] [CrossRef]
- Marshall, N.T.; Stepien, C.A. Macroinvertebrate community diversity and habitat quality relationships along a large river from targeted eDNA metabarcode assays. Environ. DNA 2020, 2, 572–586. [Google Scholar] [CrossRef]
- Johansson, M.L.; Lavigne, S.Y.; Ramcharan, C.W.; Heath, D.D.; MacIsaac, H.J. Detecting a spreading non-indigenous species using multiple methodologies. Lake Reserv. Manag. 2020, 36, 432–443. [Google Scholar] [CrossRef]
- Xia, Z.; Johansson, M.L.; Gao, Y.; Zhang, L.; Haffner, G.D.; MacIsaac, H.J.; Zhan, A. Conventional versus real-time quantitative PCR for rare species detection. Ecol. Evol. 2018, 8, 11799–11807. [Google Scholar] [CrossRef] [PubMed]
- Trebitz, A.S.; Hatzenbuhler, C.L.; Hoffman, J.C.; Meredith, C.S.; Peterson, G.S.; Pilgrim, E.M.; Barge, J.T.; Cotter, A.M.; Wick, M. Dreissena veligers in western Lake Superior—Inference from new low-density detection. J. Great Lakes Res. 2019, 45, 691–699. [Google Scholar] [CrossRef]
- Robin, E.D.; Wong, R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J. Cell. Physiol. 1988, 136, 507–513. [Google Scholar] [CrossRef]
- Thalinger, B.; Deiner, K.; Harper, L.R.; Rees, H.C.; Blackman, R.C.; Sint, D.; Traugott, M.; Goldberg, C.S.; Bruce, K. A validation scale to determine the readiness of environmental DNA assays for routine species monitoring. Environ. DNA 2021, 3, 823–836. [Google Scholar] [CrossRef]
- Braun, P.; Nguyen, M.D.-T.; Walter, M.C.; Grass, G. Ultrasensitive Detection of Bacillus anthracis by Real-Time PCR Targeting a Polymorphism in Multi-Copy 16S rRNA Genes and Their Transcripts. Int. J. Mol. Sci. 2021, 22, 12224. [Google Scholar] [CrossRef]
- Shan, J.; Jia, Y.; Teulières, L.; Patel, F.; Clokie, M.R.J. Targeting multicopy prophage genes for the increased detection of Borrelia burgdorferi sensu lato (s.l.), the causative agents of Lyme disease, in blood. Front. Microbiol. 2021, 12, 464. [Google Scholar] [CrossRef]
- Minamoto, T.; Uchii, K.; Takahara, T.; Kitayoshi, T.; Tsuji, S.; Yamanaka, H.; Doi, H. Nuclear internal transcribed spacer-1 as a sensitive genetic marker for environmental DNA studies in common carp Cyprinus carpio. Mol. Ecol. Resour. 2017, 17, 324–333. [Google Scholar] [CrossRef]
- Dysthe, J.C.; Franklin, T.W.; McKelvey, K.S.; Young, M.K.; Schwartz, M.K. An improved environmental DNA assay for bull trout (Salvelinus confluentus) based on the ribosomal internal transcribed spacer I. PLoS ONE 2018, 13, e0206851. [Google Scholar] [CrossRef]
- Jo, T.; Arimoto, M.; Murakami, H.; Masuda, R.; Minamoto, T. Estimating shedding and decay rates of environmental nuclear DNA with relation to water temperature and biomass. Environ. DNA 2020, 2, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Marshall, N.T.; Vanderploeg, H.A.; Chaganti, S.R. Environmental (e)RNA advances the reliability of eDNA by predicting its age. Sci. Rep. 2021, 11, 2769. [Google Scholar] [CrossRef] [PubMed]
- McCartney, M.A.; Auch, B.; Kono, T.; Mallez, S.; Zhang, Y.; Obille, A.; Becker, A.; Abrahante, J.E.; Garbe, J.; Badalamenti, J.P.; et al. The genome of the zebra mussel, Dreissena polymorpha: A resource for comparative genomics, invasion genetics, and biocontrol. G3 Genes Genom. Genet. 2022, 12, jkab423. [Google Scholar] [CrossRef] [PubMed]
- Calcino, A.; Baranyi, C.; Wanninger, A. Heteroplasmy and repeat expansion in the plant-like mitochondrial genome of a bivalve mollusc. bioRxiv 2020, 1–32. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Klymus, K.E.; Merkes, C.M.; Allison, M.J.; Goldberg, C.S.; Helbing, C.C.; Hunter, M.E.; Jackson, C.A.; Lance, R.F.; Mangan, A.M.; Monroe, E.M.; et al. Reporting the limits of detection and quantification for environmental DNA assays. Environ. DNA 2019, 2, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Blackman, R.; Benucci, M.; Donnelly, R.; Hänfling, B.; Harper, L.; Sellers, G.; Lawson-Handley, L. Simple, sensitive and species-specific assays for detecting quagga and zebra mussels (Dreissena rostriformis bugensis and D. polymorpha) using environmental DNA. Manag. Biol. Invasions 2020, 11, 218–236. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, R.-H.; Jiang, L.; Hu, X.-D.; Wu, Z.-J.; Yao, Y.-J. rRNA pseudogenes in filamentous ascomycetes as revealed by genome data. G3 Genes Genom. Genet. 2017, 7, 2695–2703. [Google Scholar] [CrossRef] [Green Version]
- Morisette, J.; Burgiel, S.; Brantley, K.; Daniel, W.; Darling, J.; Davis, J.; Franklin, T.; Gaddis, K.; Hunter, M.; Lance, R.; et al. Strategic considerations for invasive species managers in the utilization of environmental DNA (eDNA): Steps for incorporating this powerful surveillance tool. Manag. Biol. Invasions 2021, 12, 747–775. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.; Takao, K.; Minamoto, T. Linking the state of environmental DNA to its application for biomonitoring and stock assessment: Targeting mitochondrial/nuclear genes, and different DNA fragment lengths and particle sizes. Environ. DNA 2022, 4, 271–283. [Google Scholar] [CrossRef]
- Langlois, V.S.; Allison, M.J.; Bergman, L.C.; To, T.A.; Helbing, C.C. The need for robust qPCR-based eDNA detection assays in environmental monitoring and species inventories. Environ. DNA 2021, 3, 519–527. [Google Scholar] [CrossRef]
- Kinkar, L.; Gasser, R.; Webster, B.; Rollinson, D.; Littlewood, D.; Chang, B.; Stroehlein, A.; Korhonen, P.; Young, N. Nanopore sequencing resolves elusive long tandem-repeat regions in mitochondrial genomes. Int. J. Mol. Sci. 2021, 22, 1811. [Google Scholar] [CrossRef] [PubMed]
- Wynn, E.L.; Christensen, A.C. Repeats of unusual size in plant mitochondrial genomes: Identification, incidence and evolution. G3 Genes Genom. Genet. 2019, 9, 549–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.R.; Stedtfeld, R.D.; Engle, C.; Salach, P.; Fakher, U.; Stedtfeld, T.; Dreelin, E.; Stevenson, R.J.; Latimore, J.; Hashsham, S.A. Isothermal amplification of environmental DNA (eDNA) for direct field-based monitoring and laboratory confirmation of Dreissena sp. PLoS ONE 2017, 12, e0186462. [Google Scholar] [CrossRef]
- Hoy, M.S.; Kelly, K.; Rodriguez, R.J. Development of a molecular diagnostic system to discriminate Dreissena polymorpha (zebra mussel) and Dreissena bugensis (quagga mussel). Mol. Ecol. Resour. 2010, 10, 190–192. [Google Scholar] [CrossRef]
- Ram, J.L.; Karim, A.S.; Acharya, P.; Jagtap, P.; Purohit, S.; Kashian, D.R. Reproduction and genetic detection of veligers in changing Dreissena populations in the Great Lakes. Ecosphere 2011, 2, 1–16. [Google Scholar] [CrossRef]
- Ardura, A.; Zaiko, A.; Borrell, Y.J.; Samuiloviene, A.; Garcia-Vazquez, E. Novel tools for early detection of a global aquatic invasive, the zebra mussel Dreissena polymorpha. Aquatic. Conserv. 2017, 27, 165–176. [Google Scholar] [CrossRef]
- Mahon, A.R.; Barnes, M.A.; Senapati, S.; Feder, J.L.; Darling, J.A.; Chang, H.C.; Lodge, D.M. Molecular detection of invasive species in heterogeneous mixtures using a microfluidic carbon nanotube platform. PLoS ONE 2011, 6, e17280. [Google Scholar] [CrossRef] [Green Version]
- Bronnenhuber, J.E.; Wilson, C.C. Combining species-specific COI primers with environmental DNA analysis for targeted detection of rare freshwater species. Conserv. Genet. Resour. 2013, 5, 971–975. [Google Scholar] [CrossRef]
- Egan, S.P.; Barnes, M.A.; Hwang, C.T.; Mahon, A.R.; Feder, J.L.; Ruggiero, S.T.; Tanner, C.E.; Lodge, D.M. Rapid invasive species detection by combining environmental DNA with light transmission spectroscopy. Conserv. Lett. 2013, 6, 402–409. [Google Scholar] [CrossRef]
- Egan, S.P.; Grey, E.; Olds, B.; Feder, J.L.; Ruggiero, S.T.; Tanner, C.E.; Lodge, D.M. Rapid molecular detection of invasive species in ballast and harbor water by integrating environmental DNA and light transmission spectroscopy. Environ. Sci. Technol. 2015, 49, 4113–4121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepulveda, A.J.; Amberg, J.J.; Hanson, E. Using environmental DNA to extend the window of early detection for dreissenid mussels. Manag. Biol. Invasion. 2019, 10, 342. [Google Scholar] [CrossRef] [Green Version]

| Species | Assay | Zebra Mussel Primer | Length (bps) | Efficiency | R2 | LOD | LOQ | Source |
|---|---|---|---|---|---|---|---|---|
| A. ZM | mt-tr285 | F: GTTTTCCAGTTCTTCTGTCG | 97 | 96.83 | 0.990 | 2.2 × 10−6 | 1.2 × 10−4 | Present Study |
| R: CTCTCACTTTTTTCCCCTATCCCTC | ||||||||
| mt-COI | F: TAGAGCTAAGGGCACCTGGAA | 73 | 90.63 | 0.990 | 2.5 × 10−4 | 3.3 × 10−3 | [40] | |
| R: AGCCCATGAGTGGTGACAAT | ||||||||
| mt-16S | F: TGGGGCAGTAAGAAGAAAAAAATAA | 141 | 91.00 | 0.995 | 2.5 × 10−4 | 2.4 × 10−3 | [16] | |
| R: CATCGAGGTCGCAAACCG | ||||||||
| nu-H2B | F: CGCGCGCTCCACTGACAAGA | 251 | 88.36 | 0.999 | 2.2 × 10−6 | 4.1 × 10−5 | [19] | |
| R: CACCAGGCAGCAGGAGACGC | ||||||||
| B. QM | mt-tr258 | F: TCGGTTCAACGGGATTCCC | 232 | 102.32 | 0.995 | 9.3 × 10−6 | 2.8 × 10−4 | Present Study |
| R: CCCCCTTACAAGATTTTCGATTT | ||||||||
| mt-COI | F: GGAAACTGGTTGGTCCCGAT | 188 | 97.16 | 0.998 | 9.8 × 10−5 | 3.5 × 10−4 | [40] | |
| R: GGCCCTGAATGCCCCATAAT | ||||||||
| mt-16S | F: TGGGGCAGTAAGAAGAAAAAAATAA | 141 | 101.15 | 0.996 | 5.7 × 10−5 | 1.1 × 10−3 | [16] | |
| R: CATCGAGGTCGCAAACCG | ||||||||
| nu-H2B | F: CGCGCGCTCCACTGACAAGA | 251 | 95.89 | 0.994 | 5.7 × 10−5 | 1.8 × 10−3 | [19] | |
| R: CACCAGGCAGCAGGAGACGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshall, N.T.; Vanderploeg, H.A.; Chaganti, S.R. Improving Environmental DNA Sensitivity for Dreissenid Mussels by Targeting Tandem Repeat Regions of the Mitochondrial Genome. Water 2022, 14, 2069. https://doi.org/10.3390/w14132069
Marshall NT, Vanderploeg HA, Chaganti SR. Improving Environmental DNA Sensitivity for Dreissenid Mussels by Targeting Tandem Repeat Regions of the Mitochondrial Genome. Water. 2022; 14(13):2069. https://doi.org/10.3390/w14132069
Chicago/Turabian StyleMarshall, Nathaniel T., Henry A. Vanderploeg, and Subba Rao Chaganti. 2022. "Improving Environmental DNA Sensitivity for Dreissenid Mussels by Targeting Tandem Repeat Regions of the Mitochondrial Genome" Water 14, no. 13: 2069. https://doi.org/10.3390/w14132069
APA StyleMarshall, N. T., Vanderploeg, H. A., & Chaganti, S. R. (2022). Improving Environmental DNA Sensitivity for Dreissenid Mussels by Targeting Tandem Repeat Regions of the Mitochondrial Genome. Water, 14(13), 2069. https://doi.org/10.3390/w14132069







