Effect of Particle Character and Calcite Dissolution on the Hydraulic Conductivity and Longevity of Biosand Filters Treating Winery and Other Acidic Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Column Experiments: Set-Up and Operation
2.1.1. Calculation of Operational Parameters
Calculation of Hydraulic Conductivity by the Falling Head Method
2.1.2. Hydraulic Loading Rate
2.2. Biosand Filters: Set Up and Operation
2.3. Sampling
2.4. Analytical Procedures
2.4.1. Effluent
2.4.2. Sand
3. Results and Discussion
3.1. Calcite Dissolution in Raw Sand and Fractionated Sand: Column Experiments
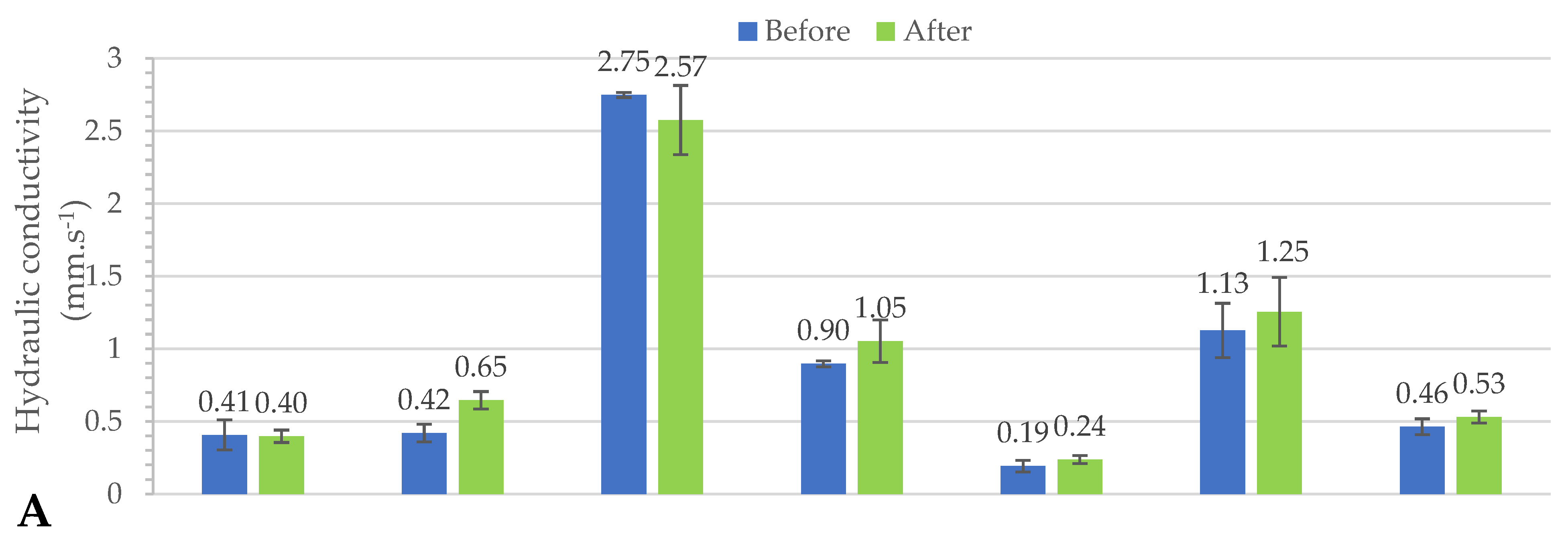
3.2. Hydraulic Conductivities of Raw and Fractionated Sand: Column Experiments
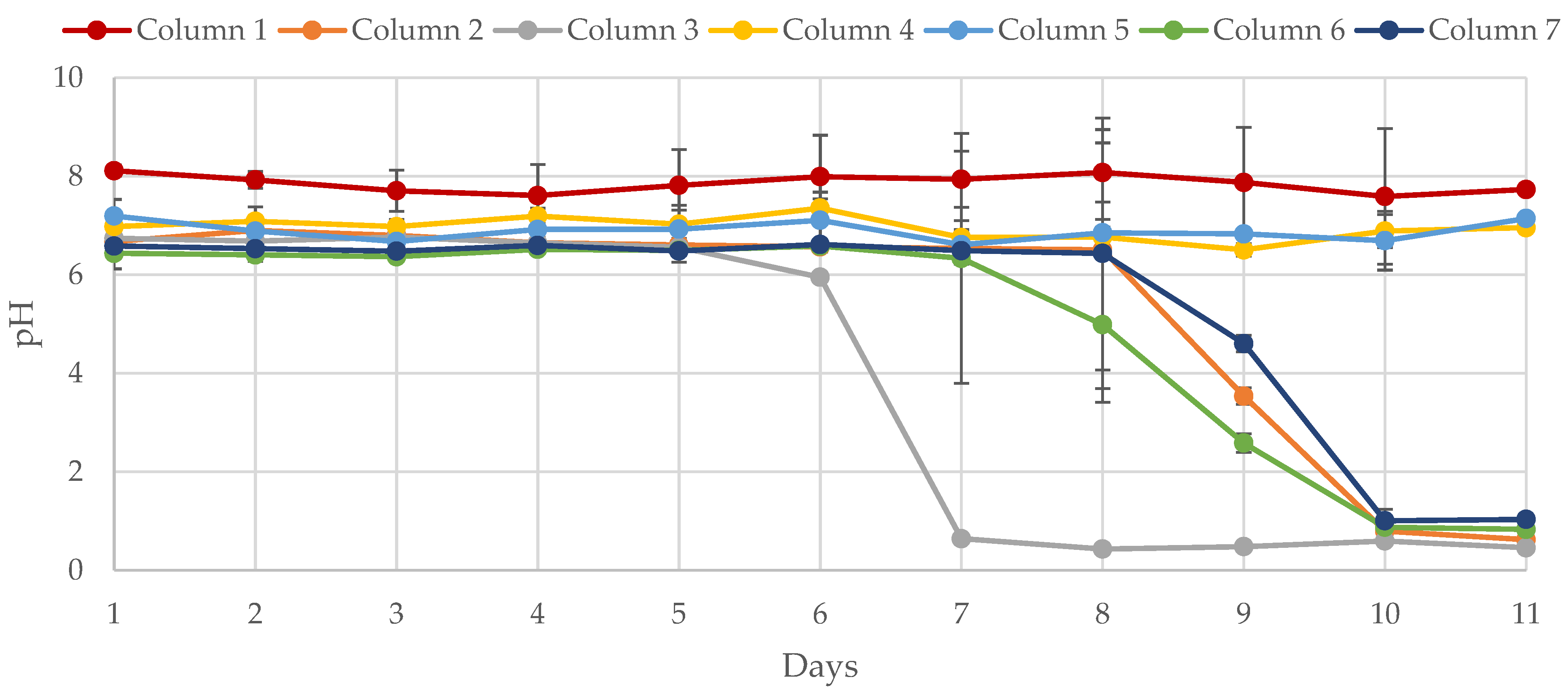
3.3. Temporal Abiotic Neutralization Capacity (TANC) of Biological Sand Reactors
3.3.1. Theoretical Values Based on Data Obtained from Column Experiments
3.3.2. Validation of Theoretical Results with Data Obtained from Operational Biological Sand Reactors
Results Based on the Calcium Concentrations in Core Samples
Results Based on Influent and Effluent Calcium Concentrations
Results Based on Influent pH Values
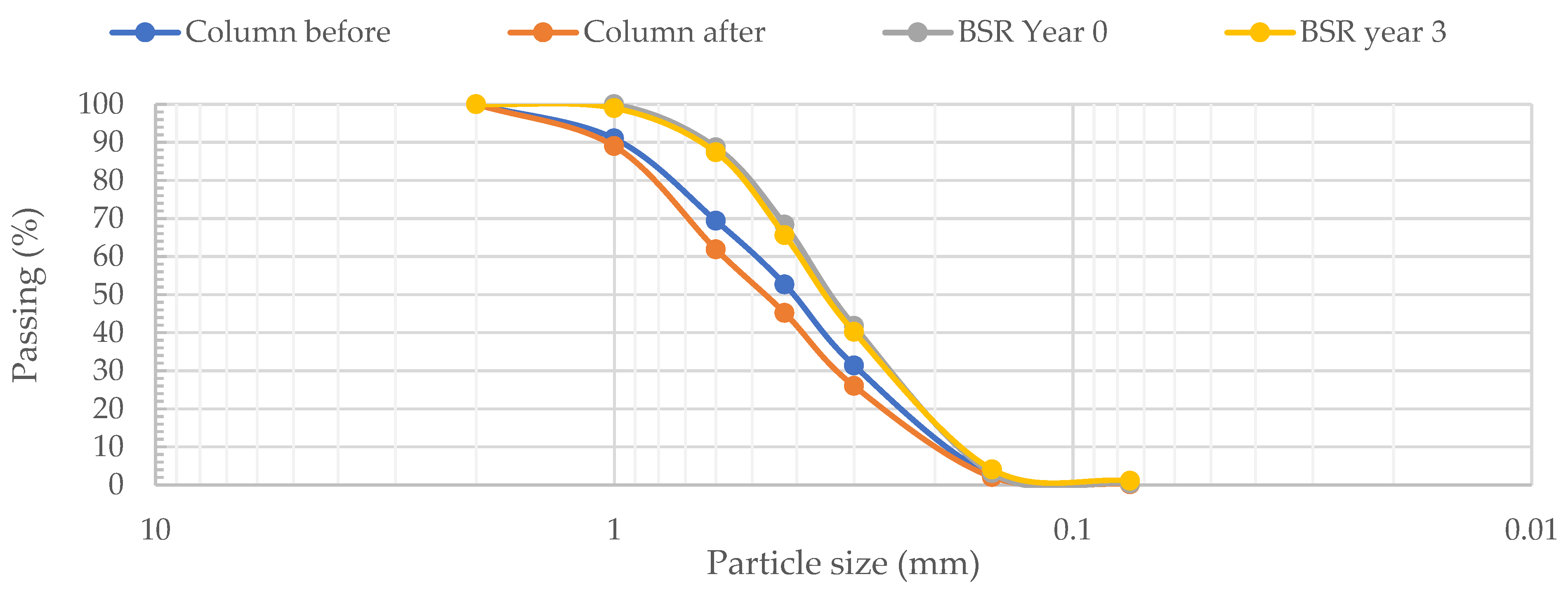
3.4. Changes in the Character of the Sand Particles before and after Calcite Dissolution in Columns and Biological Sand Reactors
3.4.1. Chemical Composition of Sand
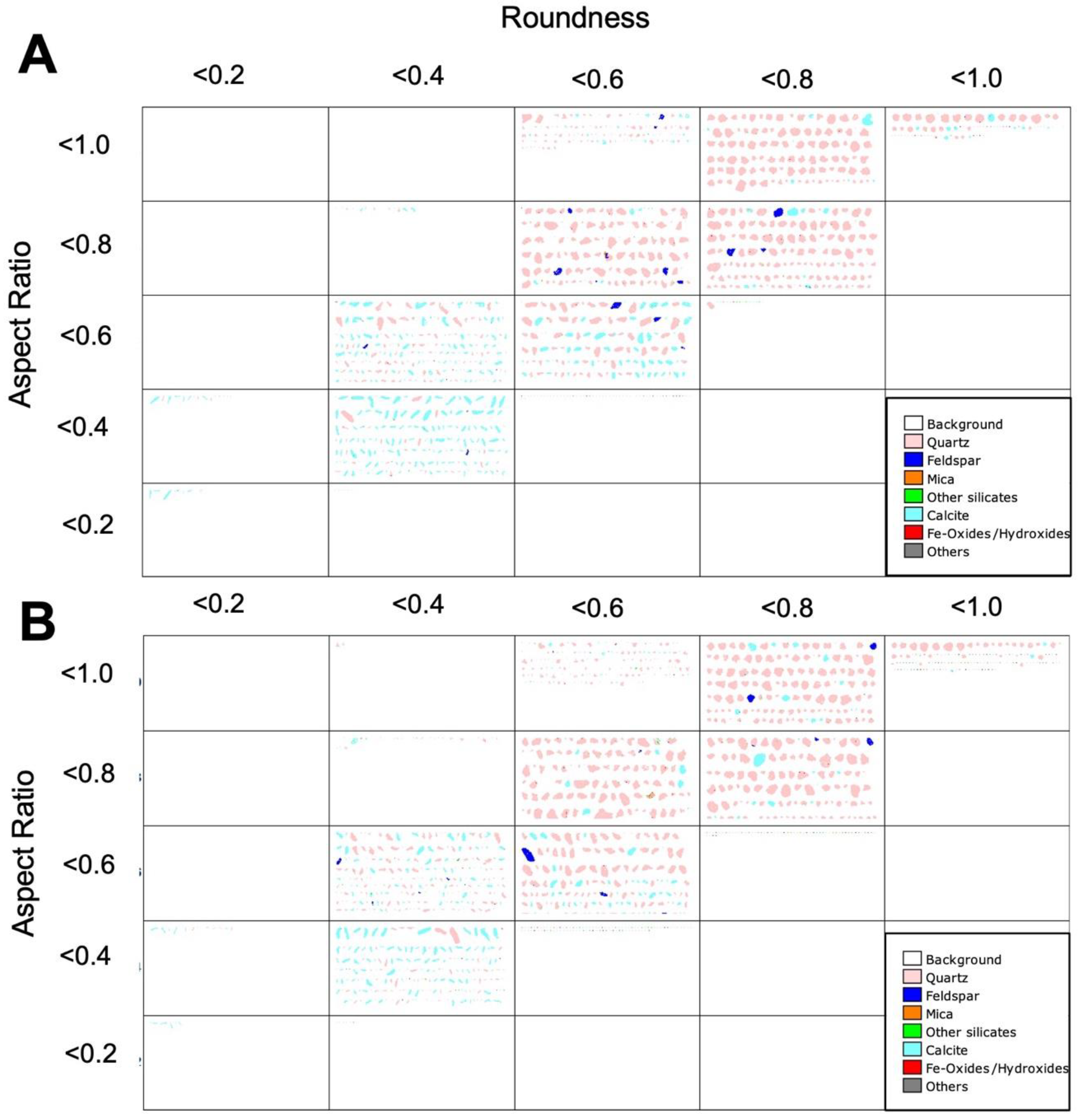
3.4.2. Size and Shape of Sand Particles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, K.; Pang, S.; Yang, B.; Ji, Y.; Zhou, Y.; Gao, C. Polyethyleneimine/4,4′-Bis(chloromethyl)-1,1′-biphenyl nanofiltration membrane for metal ions removal in acid wastewater. J. Membr. Sci. 2020, 614, 118497. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, J.; Chen, C.; Wan, Y. Highly permeable acid-resistant nanofiltration membrane based on a novel sulfonamide aqueous monomer for efficient acidic wastewater treatment. Chem. Eng. J. 2021, 425, 131791. [Google Scholar] [CrossRef]
- Shang, Y.; Hei, P.; Lu, S.; Shang, L.; Li, X.; Wei, Y.; Jia, D.; Jiang, D.; Ye, Y.; Gong, J.; et al. China’s energy-water nexus: Assessing water conservation synergies of the total coal consumption cap strategy until 2050. Appl. Energy 2018, 210, 643–660. [Google Scholar] [CrossRef]
- Alam, P.N.; Yulianis; Pasya, H.L.; Aditya, R.; Aslam, I.N.; Pontas, K. Acid mine wastewater treatment using electrocoagulation method. Mater. Today Proc. 2022, 63, S434–S437. [Google Scholar] [CrossRef]
- Yu, L.; Guo, Q.; Hao, J.; Jiang, W. Recovery of acetic acid from dilute wastewater by means of bipolar membrane electrodialysis. Desalination 2000, 129, 283–288. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, H.; Zhang, P.; Gao, Z.; Zhao, N. Utilizing the supernatant of waste sulfuric acid after dolomite neutralization to recover nutrients from swine wastewater. Chem. Eng. J. 2018, 337, 265–274. [Google Scholar] [CrossRef]
- Aguiar, A.; Andrade, L.; Grossi, L.; Pires, W.; Amaral, M. Acid mine drainage treatment by nanofiltration: A study of membrane fouling, chemical cleaning, and membrane ageing. Sep. Purif. Technol. 2018, 192, 185–195. [Google Scholar] [CrossRef]
- Holtman, G.; Haldenwang, R.; Welz, P. Biological sand filter system treating winery effluent for effective reduction in organic load and pH neutralisation. J. Water Process Eng. 2018, 25, 118–127. [Google Scholar] [CrossRef]
- Holtman, G.; Haldenwang, R.; Welz, P. Comparison of continuous and pulse mode of operation of pilot biosand reactors treating winery effluent. Ecol. Eng. 2022, 182, 106706. [Google Scholar] [CrossRef]
- Turingan, C.O.A.; Singson, G.B.; Melchor, B.T.; Alorro, R.D.; Beltran, A.B.; Orbecido, A.H. Evaluation of Efficiencies of Locally Available Neutralizing Agents for Passive Treatment of Acid Mine Drainage. Minerals 2020, 10, 845. [Google Scholar] [CrossRef]
- Larraguibel, A.; Navarrete-Calvo, A.; García, S.; Armijos, V.F.; Caraballo, M.A. Exploring sulfate and metals removal from Andean acid mine drainage using CaCO3-rich residues from agri-food industries and witherite (BaCO3). J. Clean. Prod. 2020, 274, 123450. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J. Constitutive modelling of natural sands using a deep learning approach accounting for particle shape effects. Powder Technol. 2022, 404, 117439. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, B.; Wang, J. Tracking particles in sands based on particle shape parameters. Adv. Powder Technol. 2020, 31, 2005–2019. [Google Scholar] [CrossRef]
- Xie, W.-Q.; Zhang, X.-P.; Yang, X.-M.; Liu, Q.-S.; Tang, S.-H.; Tu, X.-B. 3D size and shape characterization of natural sand particles using 2D image analysis. Eng. Geol. 2020, 279, 105915. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Li, J. Fractal dimension, particle shape, and particle breakage analysis for calcareous sand. Bull. Eng. Geol. Environ. 2022, 81, 1–20. [Google Scholar] [CrossRef]
- Welz, P.; Mbasha, W.; Smith, I.; Holtman, G.; Terblanche, G.; Le Roes-Hill, M.; Haldenwang, R. The influence of grain physicochemistry and biomass on hydraulic conductivity in sand-filled treatment wetlands. Ecol. Eng. 2018, 116, 21–30. [Google Scholar] [CrossRef]
- Little, L.; Becker, M.; Wiese, J.; Mainza, A.N. Auto-SEM particle shape characterisation: Investigating fine grinding of UG2 ore. Miner. Eng. 2015, 82, 92–100. [Google Scholar] [CrossRef]
- Kim, Y.C.; Yoon, H. Exploitation of acetic acid for calcite dissolution in small-capacity desalination plants. Desalination 2021, 516, 115227. [Google Scholar] [CrossRef]
- Lehmann, O.; Birnhack, L.; Lahav, O. Design aspects of calcite-dissolution reactors applied for post treatment of desalinated water. Desalination 2013, 314, 1–9. [Google Scholar] [CrossRef]
- Welz, P.J.; Holtman, G.; Haldenwang, R.; Le Roes-Hill, M. Characterisation of winery wastewater from continuous flow settling basins and waste stabilisation ponds over the course of 1 year: Implications for biological wastewater treatment and land application. Water Sci. Technol. 2016, 74, 2036–2050. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Golubev, S.V.; Schott, J. Dissolution kinetics of calcite, dolomite and magnesite at 25 °C and 0 to 50 atm pCO2. Chem. Geol. 2005, 217, 239–255. [Google Scholar] [CrossRef]
- Bories, A.; Sire, Y. Impacts of Winemaking Methods on Wastewaters and their Treatment. S. Afr. J. Enol. Vitic. 2016, 31, 38–44. [Google Scholar] [CrossRef][Green Version]
- Goodall, W.R.; Scales, P.J.; Butcher, A.R. The use of QEMSCAN and diagnostic leaching in the characterisation of visible gold in complex ores. Miner. Eng. 2005, 18, 877–886. [Google Scholar] [CrossRef]
- Mader, A.E.; Holtman, G.A.; Welz, P.J. Treatment wetlands and phyto-technologies for remediation of winery effluent: Challenges and opportunities. Sci. Total Environ. 2021, 807, 150544. [Google Scholar] [CrossRef]
- Giraldi, D.; Vitturi, M.D.; Zaramella, M.; Marion, A.; Iannelli, R. Hydrodynamics of vertical subsurface flow constructed wetlands: Tracer tests with rhodamine WT and numerical modelling. Ecol. Eng. 2009, 35, 265–273. [Google Scholar] [CrossRef]
- Suliman, F.; Futsaether, C.; Oxaal, U.; Haugen, L.; Jenssen, P. Effect of the inlet–outlet positions on the hydraulic performance of horizontal subsurface-flow wetlands constructed with heterogeneous porous media. J. Contam. Hydrol. 2006, 87, 22–36. [Google Scholar] [CrossRef]
- Narsilio, A.G.; Yun, T.S.; Kress, J.; Evans, T.M. Hydraulic and thermal conduction phenomena in soils at the particle-scale: Towards realistic FEM simulations. IOP Conf. Ser. Mater. Sci. Eng. 2010, 10, 012086. [Google Scholar] [CrossRef]
- Bruch, I.; Alewell, C.; Hahn, A.; Hasselbach, R. Influence of soil physical parameters on removal efficiency and hydraulic conductivity of vertical flow constructed wetlands. Ecol. Eng. 2014, 68, 124–132. [Google Scholar] [CrossRef]
- Urumović, K. The effective porosity and grain size relations in permeability functions. Hydrol Earth Syst. Sci. Discuss 2014, 11, 6675–6714. [Google Scholar]
- Rakotonimaro, T.V.; Neculita, C.M.; Bussière, B.; Zagury, G.J. Comparative column testing of three reactive mixtures for the bio-chemical treatment of iron-rich acid mine drainage. Miner. Eng. 2017, 111, 79–89. [Google Scholar] [CrossRef]
- Ji, X.; Zhao, C.; Lv, Y.; Yang, J.; Li, B. Influence of Particle Size of River Sand on the Decontamination Process in the Slow Sand Filter Treatment of Micro-Polluted Water. Water 2022, 14, 100. [Google Scholar] [CrossRef]


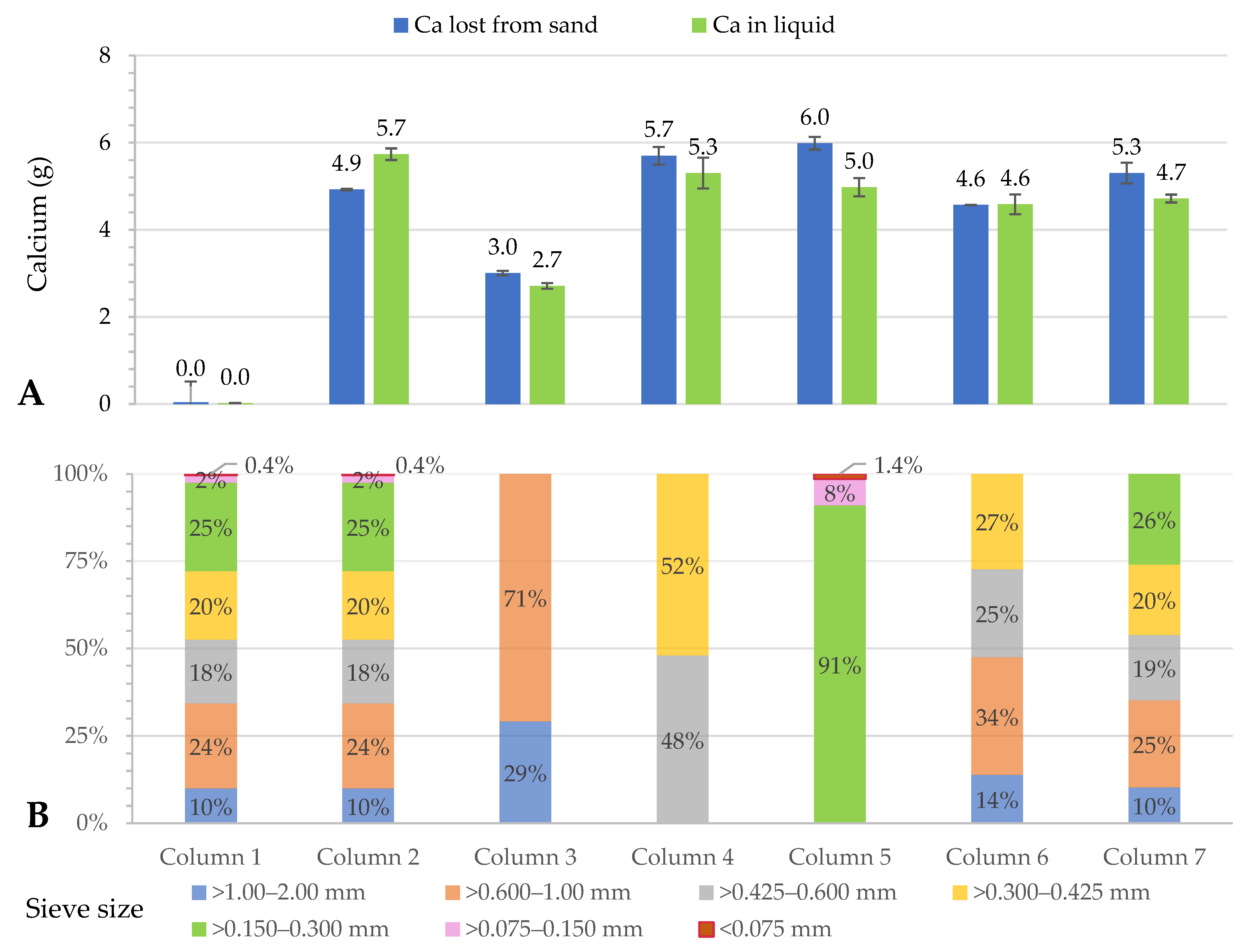
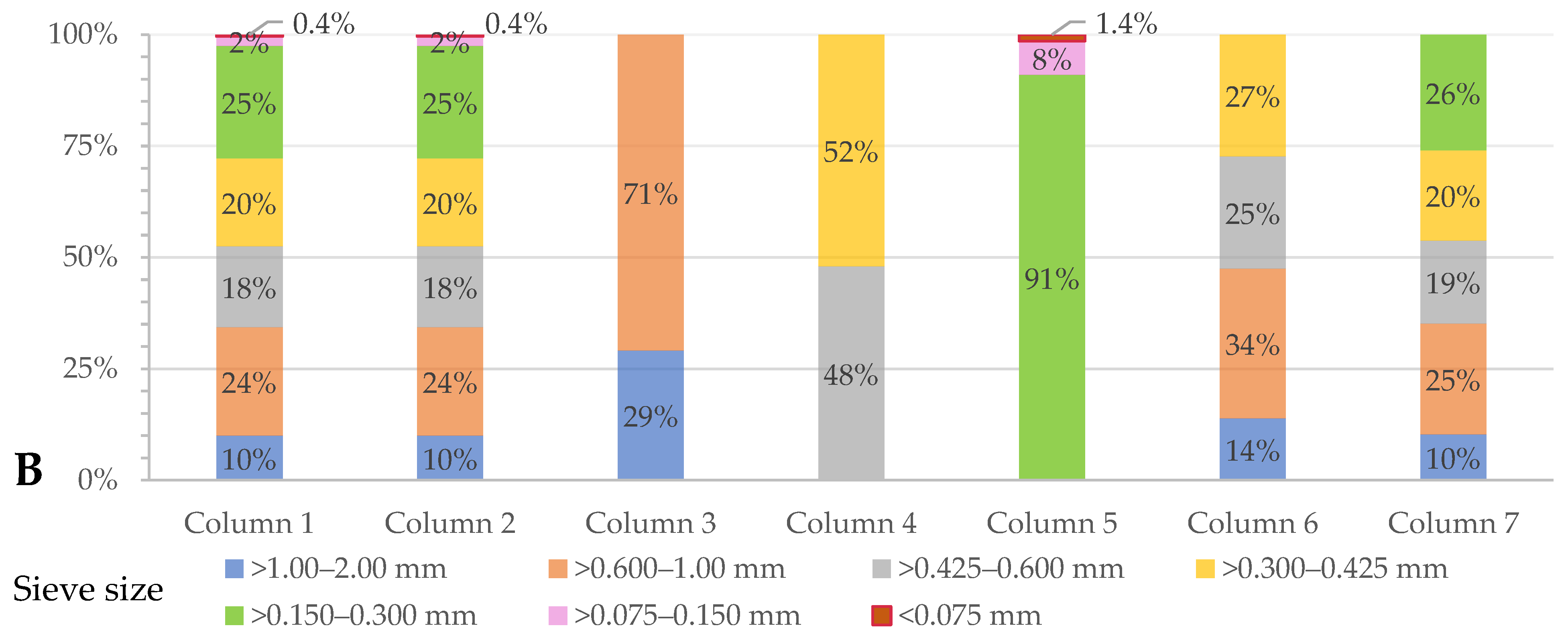
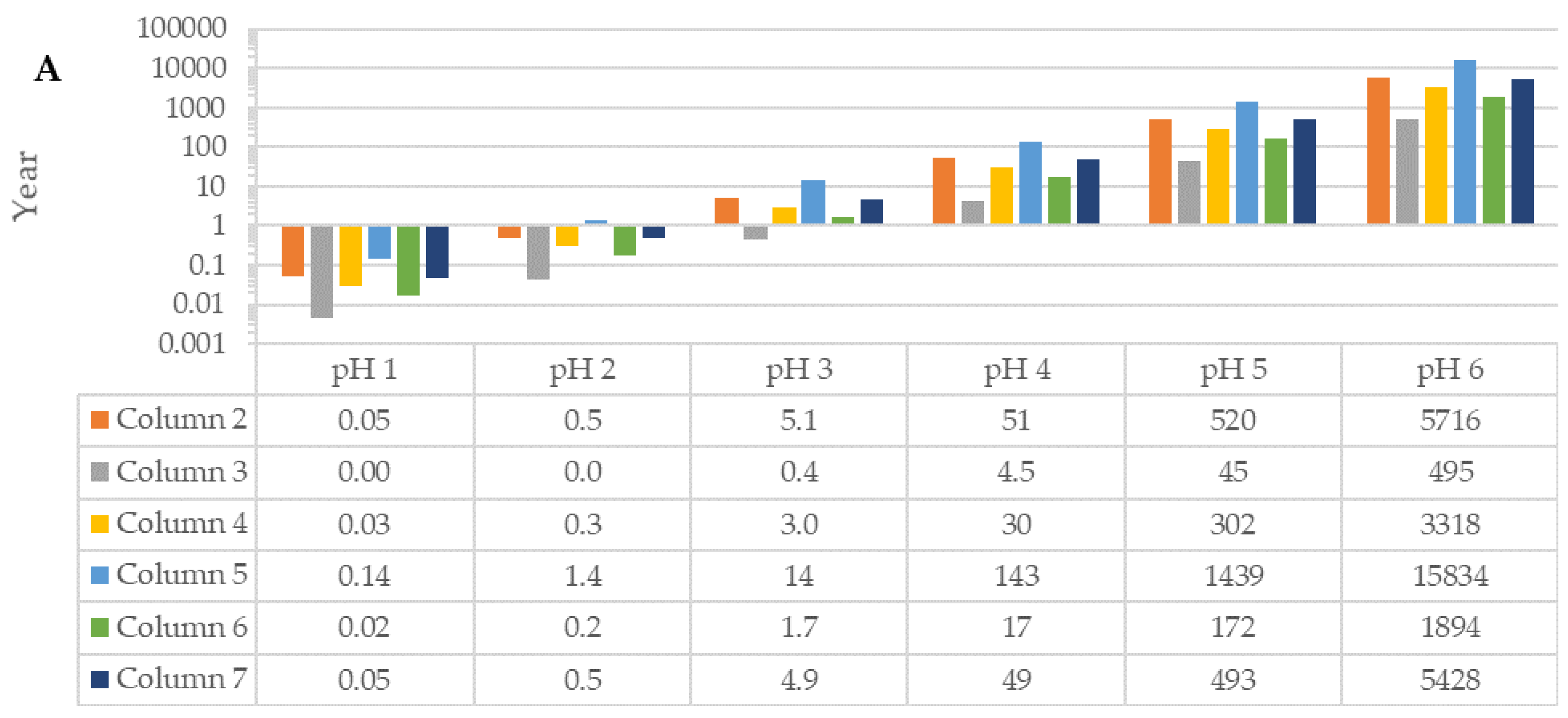
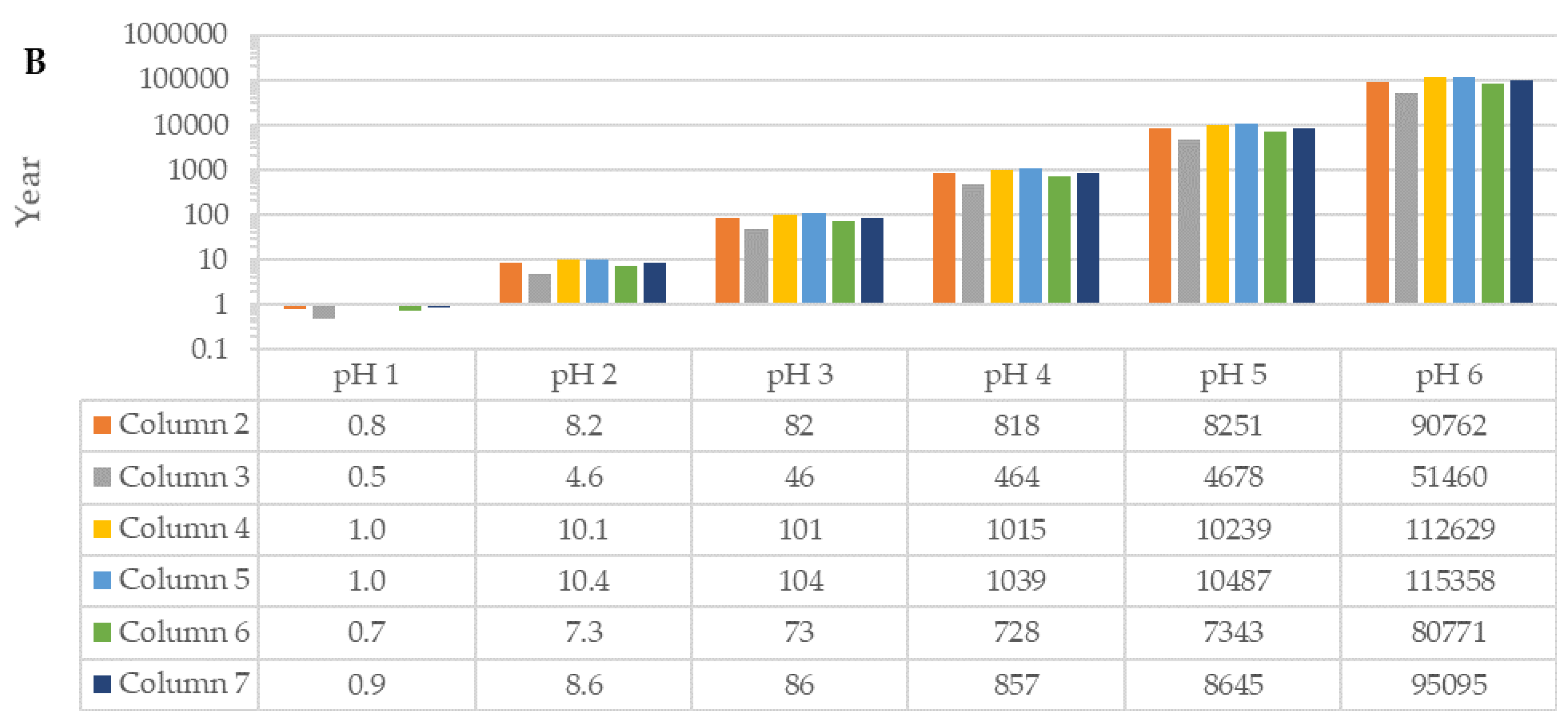
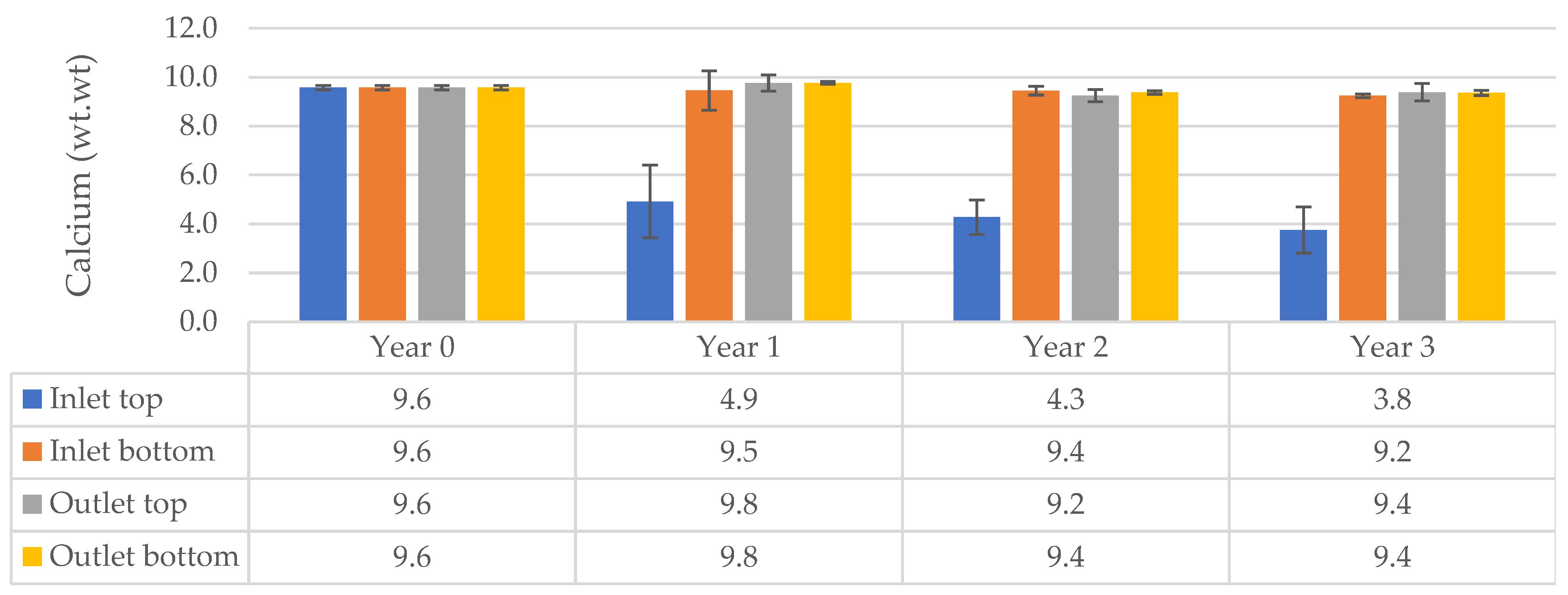
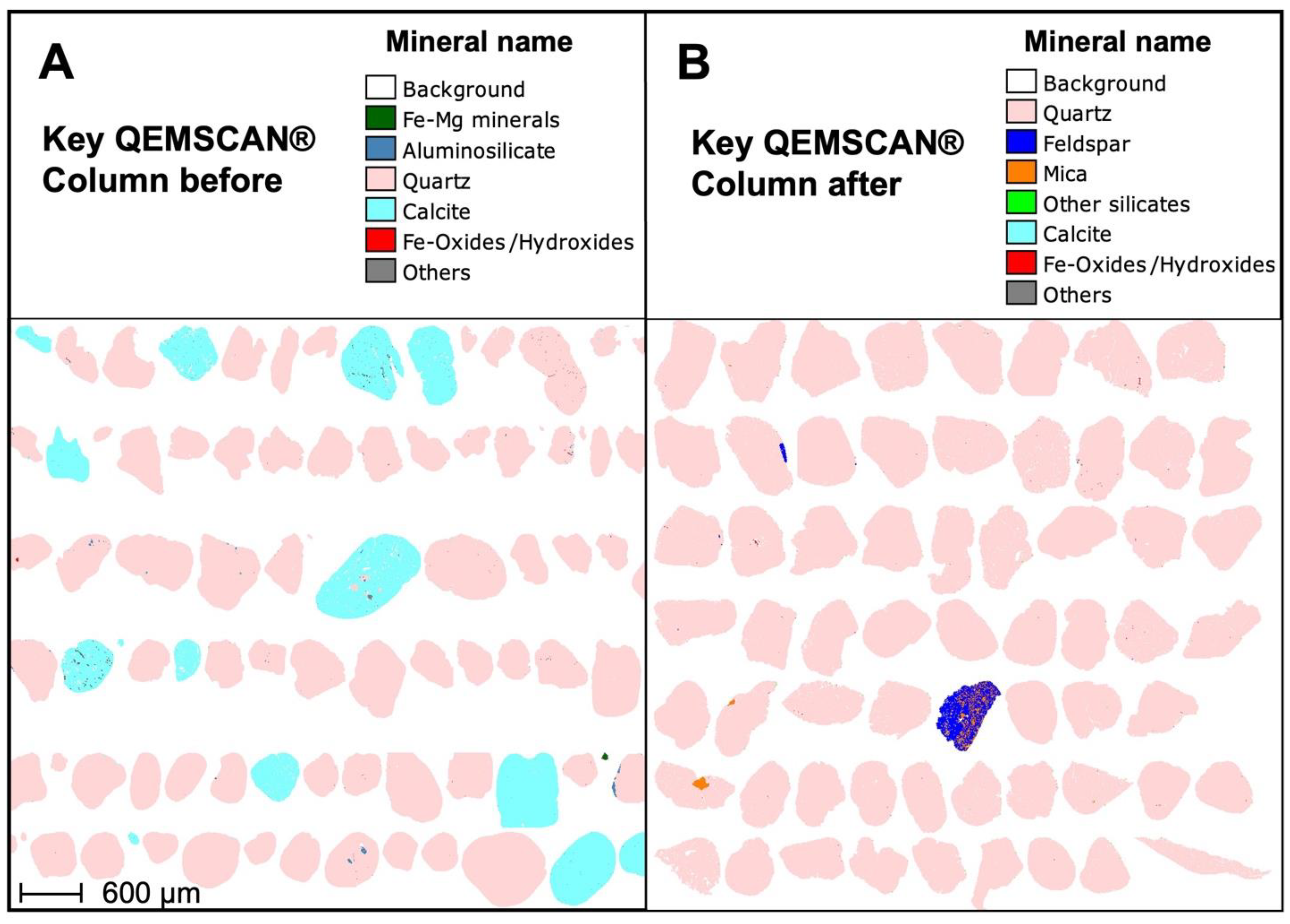
| Column (n = 3) | Particle Size (mm) | Amount (% wt.wt) | Ca in Sand (% wt.wt) | Comments |
|---|---|---|---|---|
| 1 & 2 | >1.00–2.00 >0.600–1.00 >0.425–0.600 >0.300–0.425 >0.150–0.300 >0.075–0.150 <0.075 | 10.0 24.3 18.2 19.7 25.3 2.1 0.4 | 5.4 ± 0.01 | Raw (unfractionated) sand |
| 3 | >1.00–2.00 >0.600–1.00 | 29.2 70.8 | 3.0 ± 0.05 | Homogenized fractions |
| 4 | 0.425–0.600 >0.300–0.425 | 48.0 52.0 | 6.7 ± 0.07 | Homogenized fractions |
| 5 | 0.150–0.300 >0.075–0.150 <0.075 | 91.0 7.6 1.4 | 6.8 ± 0.03 | Homogenized fractions |
| 6 | 1.00–2.00 >0.600–1.00 >0.425–0.600 >0.300–0.425 | 13.9 13.7 25.2 27.3 | 4.8 ± 0.04 | Homogenized fractions |
| 7 | 1.00–2.00 >0.600–1.00 >0.425–0.600 >0.300–0.425 >0.150–0.300 | 10.3 24.9 18.7 20.2 25.9 | 5.6 ± 0.019 | Homogenized fractions |
| Position | Inlet (ca wt.wt) | Middle (ca wt.wt) | Outlet (ca wt.wt) |
| Top (0–5 cm) | 4.5 | 9.0 | 9.2 |
| Middle (25–30 cm) | 9.2 | 9.3 | 9.4 |
| Bottom (45–50 cm) | 9.4 | 9.2 | 9.5 |
| Al | Ca | Cr | Fe | K | Mg | Mn | Na | P | Si | Ti | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Column before | 0.15 | 5.39 | bdl | 0.05 | 0.11 | 0.01 | bdl | 0.06 | 0.01 | 43.0 | 0.03 |
| Column after | 0.16 | 0.54 | bdl | 0.04 | 0.12 | bdl | 0.01 | 0.02 | 0.00 | 49.0 | 0.02 |
| BSR year 0 | 0.18 | 9.57 | bdl | 0.06 | 0.13 | 0.06 | bdl | 0.10 | 0.01 | 37.7 | 0.03 |
| BSR year 3 | 0.24 | 4.26 | bdl | 0.09 | 0.17 | 0.06 | 0.01 | 0.04 | 0.02 | 43.9 | 0.03 |
| Column before | Column after | |||||||
|---|---|---|---|---|---|---|---|---|
| Size fraction (µm) | Combined | 1180> | >425 | <425 | Combined | 600> | >300 | <300 |
| Quartz | 81.3 | 87.8 | 80.0 | 82.5 | 87.49 | 97.30 | 99.36 | 97.91 |
| Feldspar | 0.8 | 0.1 | 0.9 | 0.8 | 1.25 | 2.46 | 0.50 | 1.56 |
| Mica | ND | ND | ND | ND | 0.07 | 0.14 | 0.02 | 0.09 |
| Other silicates | ND | ND | ND | ND | 0.06 | 0.03 | 0.04 | 0.13 |
| Calcite | 17.6 | 11.9 | 19.0 | 16.2 | 0.02 | 0.01 | 0.02 | 0.06 |
| Fe-oxides/hydroxides | 0.1 | 0.1 | 0.1 | 0.1 | 0.08 | 0.05 | 0.05 | 0.18 |
| Others * | 0.1 | 0.0 | 0.1 | 0.2 | 0.03 | 0.01 | 0.01 | 0.08 |
| Column | |||||||||
| Aspect ratio | <0.2 | <0.3 | <0.4 | <0.5 | <0.6 | <0.7 | <0.8 | <0.9 | <1.0 |
| %Quartz before (n = 2572) | 0.00 | 0.02 | 0.15 | 2.19 | 8.24 | 18.19 | 28.93 | 26.88 | 15.40 |
| %Quartz after (n = 5191) | 0.00 | 0.18 | 0.96 | 4.40 | 12.48 | 23.30 | 27.44 | 23.03 | 8.15 |
| %Calcite before(n = 253) | 0.53 | 1.81 | 4.60 | 15.85 | 13.77 | 21.58 | 18.48 | 15.10 | 8.28 |
| %Calcite after(n = 478) * | 0.00 | 0.00 | 0.79 | 2.33 | 33.39 | 16.38 | 1.76 | 3.28 | 42.07 |
| Roundness | <0.2 | <0.3 | <0.4 | <0.5 | <0.6 | <0.7 | <0.8 | <0.9 | <1.0 |
| %Quartz before (n = 2572) | 0.00 | 0.10 | 1.17 | 8.13 | 18.86 | 33.26 | 31.47 | 7.00 | 0.00 |
| %Quartz after = 5191) | 0.10 | 0.66 | 3.99 | 14.72 | 29.13 | 32.06 | 16.69 | 2.66 | 0.01 |
| %Calcite before (n = 253) | 0.53 | 1.81 | 4.60 | 15.85 | 13.77 | 21.58 | 18.48 | 15.10 | 8.28 |
| %Calcite after(n = 478) * | 0.00 | 4.78 | 6.95 | 9.26 | 32.95 | 5.38 | 0.00 | 1.17 | 39.50 |
| BSR | |||||||||
| Aspect ratio | <0.2 | <0.3 | <0.4 | <0.5 | <0.6 | <0.7 | <0.8 | <0.9 | <1.0 |
| %Quartz year 0 (n = 5267) | 0.00 | 0.08 | 1.17 | 5.35 | 14.22 | 21.47 | 29.24 | 20.33 | 8.13 |
| %Quartz year 3 (n = 5472) | 0.00 | 0.12 | 1.12 | 5.34 | 13.90 | 23.78 | 25.59 | 20.11 | 10.04 |
| %Calcite year 0 (n = 4768) | 0.96 | 4.20 | 16.69 | 18.99 | 22.18 | 14.48 | 11.20 | 7.87 | 3.42 |
| %Calcite year 3 (n = 2999) | 0.52 | 5.88 | 17.11 | 16.90 | 17.31 | 19.77 | 11.89 | 7.53 | 3.07 |
| Roundness | <0.2 | <0.3 | <0.4 | <0.5 | <0.6 | <0.7 | <0.8 | <0.9 | <1.0 |
| %Quartz year 0 (n = 5267) | 0.06 | 0.89 | 5.12 | 17.78 | 30.59 | 31.48 | 12.00 | 2.03 | 0.06 |
| %Quartz year 3(n = 5472) | 0.02 | 0.92 | 5.86 | 16.82 | 32.08 | 26.09 | 15.51 | 2.66 | 0.05 |
| %Calcite year 0 (n = 4768) | 1.67 | 11.11 | 24.11 | 27.00 | 18.75 | 11.48 | 4.50 | 1.16 | 0.22 |
| %Calcite year 3 (n = 2999) | 1.50 | 11.01 | 25.34 | 25.14 | 19.25 | 13.40 | 3.46 | 0.70 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtman, G.A.; Haldenwang, R.; Welz, P.J. Effect of Particle Character and Calcite Dissolution on the Hydraulic Conductivity and Longevity of Biosand Filters Treating Winery and Other Acidic Effluents. Water 2022, 14, 2603. https://doi.org/10.3390/w14172603
Holtman GA, Haldenwang R, Welz PJ. Effect of Particle Character and Calcite Dissolution on the Hydraulic Conductivity and Longevity of Biosand Filters Treating Winery and Other Acidic Effluents. Water. 2022; 14(17):2603. https://doi.org/10.3390/w14172603
Chicago/Turabian StyleHoltman, Gareth Alistair, Rainer Haldenwang, and Pamela Jean Welz. 2022. "Effect of Particle Character and Calcite Dissolution on the Hydraulic Conductivity and Longevity of Biosand Filters Treating Winery and Other Acidic Effluents" Water 14, no. 17: 2603. https://doi.org/10.3390/w14172603
APA StyleHoltman, G. A., Haldenwang, R., & Welz, P. J. (2022). Effect of Particle Character and Calcite Dissolution on the Hydraulic Conductivity and Longevity of Biosand Filters Treating Winery and Other Acidic Effluents. Water, 14(17), 2603. https://doi.org/10.3390/w14172603







