Adsorption of Contaminants of Emerging Concern (CECs) with Varying Hydrophobicity on Macro- and Microplastic Polyvinyl Chloride, Polyethylene, and Polystyrene: Kinetics and Potential Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gas Chromatography/Mass Spectrometry (GC/MS)
2.3. Adsorption Experimental Setup
2.4. CECs Concentration Calculation, Kinetic Modeling, and Statistical Analysis
2.5. Material Characterization: Specific Surface Area and Pore Size Distribution
3. Results and Discussion
3.1. Control
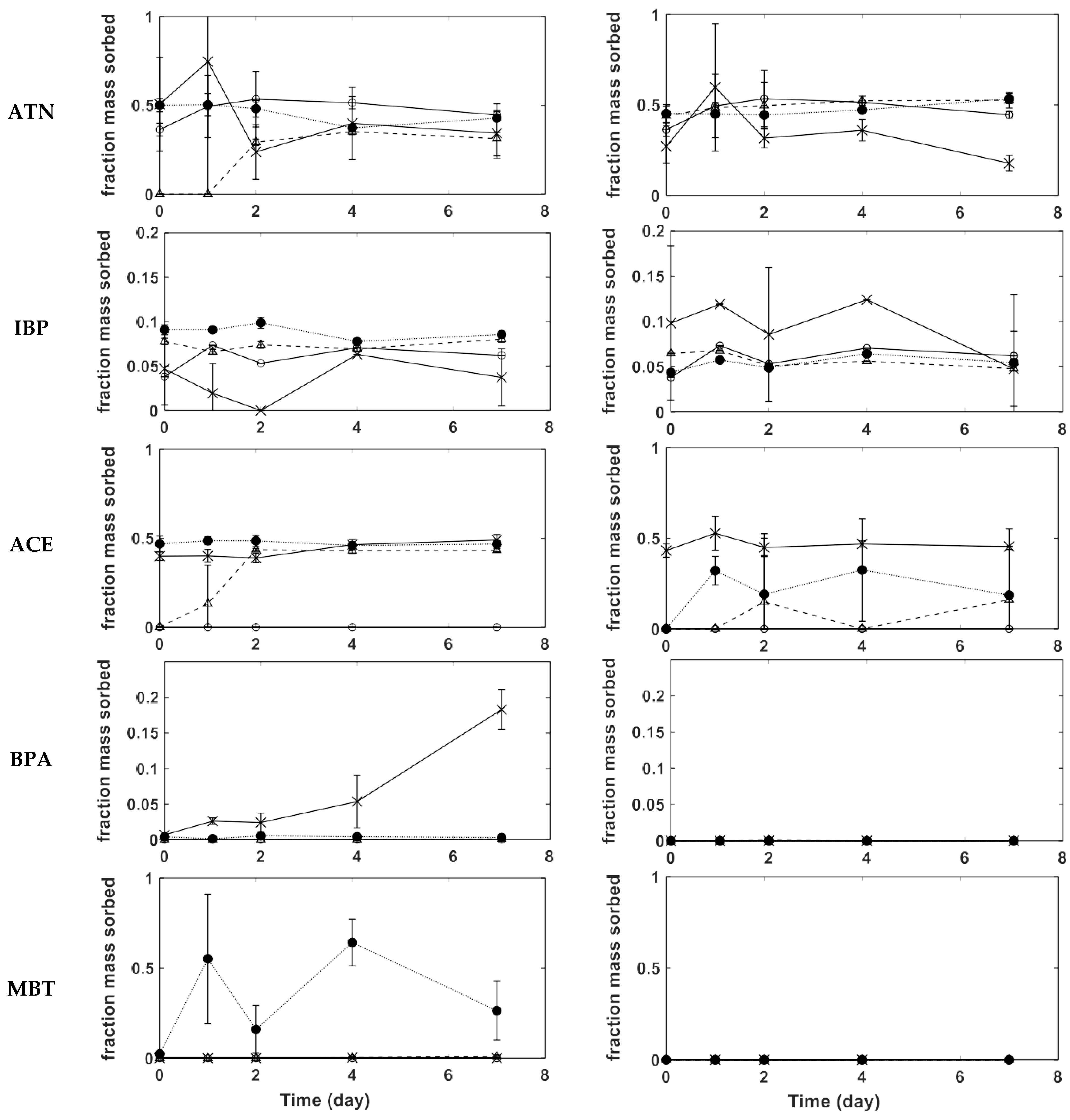
3.2. CECs Adsorption on PVC
3.3. CECs Adsorption on PE
3.4. Adsorption on PS
3.5. Kinetics and Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artham, T.; Doble, M. Biodegradation of aliphatic and aromatic polycarbonates. Macromol. Biosci. 2008, 8, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Discard Studies. Discard Studies Homepage. Available online: discardstudies.com (accessed on 29 September 2021).
- PlasticsEurope. Plastics—The Facts 2013: An Analysis of European Lastest Plastics Production, Demand and Waste Data; PlasticsEurope Association of Plastics Manufactures, Ed.; PlasticsEurope: Brussels, Belgium, 2013. [Google Scholar]
- PlasticsEurope. Plastics—The Facts 2020: An Analysis of European Lastest Plastics Production, Demand and Waste Data; PlasticsEurope Association of Plastics Manufactures, Ed.; PlasticsEurope: Brussels, Belgium, 2020. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- NOAA. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastics Marine Debris, Tacoma, WA, USA, 9–11 September 2008; National Oceanic and Atmospheric Administration: Washington, DC, USA; University of Washington Tacoma: Tacoma, WA, USA, 2009; 49p.
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A global Assessment; 1020-4873; International Maritime Organization (IMO): London, UK, 2015; 96p. [Google Scholar]
- WHO. Microplastics in Drinking-Water; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Al-Jaibachi, R.; Cuthbert, R.N.; Callaghan, A. Up and away: Ontogenic transference as a pathway for aerial dispersal of microplastics. Biol. Lett. 2018, 14, 20180479. [Google Scholar] [CrossRef] [Green Version]
- Fowler, C.W. Marine debris and northern fur seals: A case study. Mar. Pollut. Bull. 1987, 18, 326–335. [Google Scholar] [CrossRef]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.S.; Turra, A. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Windsor, F.M.; Tilley, R.M.; Tyler, C.R.; Ormerod, S.J. Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environ. 2019, 646, 68–74. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef]
- Rubin, A.E.; Zucker, I. Interactions of microplastics and organic compounds in aquatic environments: A case study of augmented joint toxicity. Chemosphere 2022, 289, 133212. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thelakkat, M.; Greiner, A.; Schmalz, H.; Kress, H.; Laforsch, C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef]
- Wu, J.; Xu, P.; Chen, Q.; Ma, D.; Ge, W.; Jiang, T.; Chai, C. Effects of polymer aging on sorption of 2,2′,4,4′-tetrabromodiphenyl ether by polystyrene microplastics. Chemosphere 2020, 253, 126706. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Tian, L.; Liu, X.; Qi, Z.; Ma, Y.; Ji, R.; Chen, W. Aging Significantly Affects Mobility and Contaminant-Mobilizing Ability of Nanoplastics in Saturated Loamy Sand. Environ. Sci. Technol. 2019, 53, 5805–5815. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical Pollutants Sorbed to Ingested Microbeads from Personal Care Products Accumulate in Fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M. Microplastics research-from sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Chua, E.M.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Clarke, B.O. Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environ. Sci. Technol. 2014, 48, 8127–8134. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.-A.; Watanuki, Y. Facilitated leaching of additive-derived PBDEs from plastic by seabirds’ stomach oil and accumulation in Tissues. Environ. Sci. Technol. 2015, 49, 11799–11807. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J. M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and wildlife. Philos. Trans. R. Soc. B 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [Green Version]
- Bakir, A.; O’Connor, I.A.; Rowland, S.J.; Hendriks, A.J.; Thompson, R.C. Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environ. Pollut. 2016, 219, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Ziccardi, L.M.; Edgington, A.; Hentz, K.; Kulacki, K.J.; Kane Driscoll, S. Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: A state-of-the-science review. Environ. Toxicol. Chem. 2016, 35, 1667–1676. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics—A review. Sci. Total. Environ. 2021, 797, 149140. [Google Scholar] [CrossRef]
- Lee, H.; Shim, W.J.; Kwon, J.H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total. Environ. 2014, 470–471, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, K.; Huang, X.; Liu, J. Sorption of pharmaceuticals and personal care products to polyethylene debris. Environ. Sci. Pollut. Res. Int. 2016, 23, 8819–8826. [Google Scholar] [CrossRef]
- Zrimec, J.; Kokina, M.; Jonasson, S.; Zorrilla, F.; Zelezniak, A. Plastic-Degrading Potential across the Global Microbiome Correlates with Recent Pollution Trends. mBio 2021, 12, e0215521. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wegner, A.; Foekema, E.M.; van den Heuvel-Greve, M.J.; Koelmans, A.A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.H.; Kirkham, M.B. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019, 131, 104937. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Pham, D.N.; Clark, L.; Li, M. Microplastics as hubs enriching antibiotic-resistant bacteria and pathogens in municipal activated sludge. J. Hazard. Mater. Lett. 2021, 2, 100014. [Google Scholar] [CrossRef]
- Castañeda, R.A.; Avlijas, S.; Simard, M.A.; Ricciardi, A.; Smith, R. Microplastic pollution in St. Lawrence River sediments. Can. J. Fish. Aquat. Sci. 2014, 71, 1767–1771. [Google Scholar] [CrossRef]
- Corcoran, P.L.; Norris, T.; Ceccanese, T.; Walzak, M.J.; Helm, P.A.; Marvin, C.H. Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record. Environ. Pollut. 2015, 204, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cozar, A.; Echevarria, F.; Gonzalez-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernandez-Leon, S.; Palma, A.T.; Navarro, S.; Garcia-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.; Zhang, H.; Zhou, Q.; Tian, Y.; Chen, T.; Tu, C.; Fu, C.; Luo, Y. Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environ. Pollut. 2018, 242 Pt B, 1557–1565. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef]
- Frias, J.P.; Sobral, P.; Ferreira, A.M. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J.; Alcolea, A. Microplastics and microfibers in the sludge of a municipal wastewater treatment plant. Int. J. Sustain. Dev. Plan. 2016, 11, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total. Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, S.A.; Garneu, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Loder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Ashton, K.; Holmes, L.; Turner, A. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef]
- Batel, A.; Borchert, F.; Reinwald, H.; Erdinger, L.; Braunbeck, T. Microplastic accumulation patterns and transfer of benzo[a]pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 2018, 235, 918–930. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.A.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; d’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as Vehicles of Environmental PAHs to Marine Organisms: Combined Chemical and Physical Hazards to the Mediterranean Mussels, Mytilus galloprovincialis. Front. Mar. Sci. 2018, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Kleinteich, J.; Seidensticker, S.; Marggrander, N.; Zarfl, C. Microplastics Reduce Short-Term Effects of Environmental Contaminants. Part II: Polyethylene Particles Decrease the Effect of Polycyclic Aromatic Hydrocarbons on Microorganisms. Int. J. Environ. Res. Public Health 2018, 15, 103. [Google Scholar] [CrossRef] [Green Version]
- Endo, S.; Yuyama, M.; Takada, H. Desorption kinetics of hydrophobic organic contaminants from marine plastic pellets. Mar. Pollut. Bull. 2013, 74, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Razanajatovo, R.M.; Ding, J.; Zhang, S.; Jiang, H.; Zou, H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar] [CrossRef]
- Magadini, D.L.; Goes, J.I.; Ortiz, S.; Lipscomb, J.; Pitiranggon, M.; Yan, B. Assessing the sorption of pharmaceuticals to microplastics through in-situ experiments in New York City waterways. Sci. Total. Environ. 2020, 729, 138766. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Hou, Z.; Ding, T. Sorption of diclofenac by polystyrene microplastics: Kinetics, isotherms and particle size effects. Chemosphere 2022, 290, 133311. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243 Pt B, 15501557. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption properties of tylosin on four different microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.G.; Lohmann, R.; Fernandez, L.A.; MacFarlane, J.K.; Gschwend, P.M. Polyethylene devices: Passive samplers for measuring dissolved hydrophobic organic compounds in aquatic environments. Environ. Sci. Technol. 2007, 41, 1317–1323. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liu, G.; Zhang, Z.; Wu, H.; Cui, B.; Bai, J.; Zhang, W. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicol. Environ. Saf. 2019, 173, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Seidensticker, S.; Zarfl, C.; Cirpka, O.A.; Grathwohl, P. Microplastic-Contaminant Interactions: Influence of Nonlinearity and Coupled Mass Transfer. Environ. Toxicol. Chem. 2019, 38, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Oulton, R.L.; Kohn, T.; Cwiertny, D.M. Pharmaceuticals and personal care products in effluent matrices: A survey of transformation and removal during wastewater treatment and implications for wastewater management. J. Environ. Monit. 2010, 12, 1956–1978. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Xue, J.; Zhao, Y.; Taylor, A.A.; Zenobio, J.E.; Sun, Y.; Han, Z.; Salawu, O.A.; Zhu, Y. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard. Mater. 2021, 424, 127284. [Google Scholar] [CrossRef]
- Palmgrén, J.J.; Mönkkönen, J.; Korjamo, T.; Hassinen, A.; Auriola, S. Drug adsorption to plastic containers and retention of drugs in cultured cells under in vitro conditions. Eur. J. Pharm. Biopharm. 2006, 64, 369–378. [Google Scholar] [CrossRef]
- Guart, A.; Bono-Blay, F.; Borrell, A.; Lacorte, S. Migration of plasticizers phthalates, bisphenol A and alkylphenols from plastic containers and evaluation of risk. Food Addit. Contam. 2011, 28, 676–685. [Google Scholar] [CrossRef]
- Garcia, A.E.; Wang, C.S.; Sanderson, R.N.; McDevitt, K.M.; Zhang, Y.; Valdevit, L.; Mumm, D.R.; Mohraz, A.; Ragan, R. Scalable synthesis of gyroid-inspired freestanding three-dimensional graphene architectures. Nanoscale Adv. 2019, 1, 3870–3882. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.K.; Anan, A.; Buckley, R.P.; Ouellette, W.; Asefa, T. Toward efficient nanoporous catalysts: Controlling site-isolation and concentration of grafted catalytic sites on nanoporous materials with solvents and colorimetric elucidation of their site-isolation. J. Am. Chem. Soc. 2008, 130, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, K.N.; Karapanagioti, H.K. Surface properties of beached plastics. Environ. Sci. Pollut. Res. Int. 2015, 22, 11022–11032. [Google Scholar] [CrossRef] [PubMed]
- Chinaglia, S.; Tosin, M.; Degli-Innocenti, F. Biodegradation rate of biodegradable plastics at molecular level. Polym. Degrad. Stab. 2018, 147, 237–244. [Google Scholar] [CrossRef]
- Zhan, Z.; Wang, J.; Peng, J.; Xie, Q.; Huang, Y.; Gao, Y. Sorption of 3,3′,4,4′-tetrachlorobiphenyl by microplastics: A case study of polypropylene. Mar. Pollut. Bull. 2016, 110, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Seidensticker, S.; Grathwohl, P.; Lamprecht, J.; Zarfl, C. A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ. Sci. Eur. 2018, 30, 30. [Google Scholar] [CrossRef]
- Seidensticker, S.; Zarfl, C.; Cirpka, O.A.; Fellenberg, G.; Grathwohl, P. Shift in Mass Transfer of Wastewater Contaminants from Microplastics in the Presence of Dissolved Substances. Environ. Sci. Technol. 2017, 51, 12254–12263. [Google Scholar] [CrossRef]
- PubChem. PubChem Search. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 12 April 2022).
- Pischedda, A.; Tosin, M.; Degli-Innocenti, F. Biodegradation of plastics in soil: The effect of temperature. Polym. Degrad. Stab. 2019, 170, 109017. [Google Scholar] [CrossRef]
- Aruniit, A.; Kers, J.; Goljandin, D.; Saarna, M.; Tall, K.; Majak, J.; Herranen, H. Particulate Filled Composite Plastic Materials from Recycled Glass Fibre Reinforced Plastics. Mater. Sci. 2011, 17, 276–281. [Google Scholar] [CrossRef]
- Tamada, J.A.; Langer, R. Erosion kinetics of hydrolytically degradable polymers. Proc. Natl. Acad. Sci. USA 1993, 90, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Pignatello, J.J. Soil organic matter as a nanoporous sorbent of organic pollutants. Adv. Colloid Interface Sci. 1998, 76–77, 445–467. [Google Scholar] [CrossRef]
- Tseng, L.Y.; Gori, R.; Rosso, D. Effects of Activated Sludge Process Conditions on the Production of Extracellular Polymeric Substances: Results of Yearlong Monitoring in a Warm Climate. Environ. Eng. Sci. 2015, 32, 582–592. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Cho, H.-H.; Poster, D.L.; Ball, W.P. Evidence for a pore-filling mechanism in the adsorption of aromatic hydrocarbons to a natural wood char. Environ. Sci. Technol. 2007, 41, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Manzano, C.; Hentschel, B.T.; Simonich, S.L.; Hoh, E. Polystyrene plastic: A source and sink for polycyclic aromatic hydrocarbons in the marine environment. Environ. Sci. Technol. 2013, 47, 13976–13984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Fokkink, R.; Koelmans, A.A. Sorption of polycyclic aromatic hydrocarbons to polystyrene nanoplastic. Environ. Toxicol. Chem. 2016, 35, 1650–1655. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar. Coast. Shelf Sci. 2014, 140, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Huang, A.; Cao, S.; Sun, F.; Wang, L.; Guo, H.; Ji, R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016, 219, 166–173. [Google Scholar] [CrossRef]
- Velzeboer, I.; Kwadijk, C.J.; Koelmans, A.A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]


| CEC | Log Kow | Control | Macro- PVC | Micro- PVC | Macro- PE | Micro- PE | Macro- PS | Micro- PS |
|---|---|---|---|---|---|---|---|---|
| DCF | 4.51 | NA | 0.0022 | 0.0080 | 0.0123 | 0.0071 | 0.0047 | 0.0077 |
| IBP | 3.97 | NA | NA | NA | 0.0017 p < 0.001 | NA | 0.0004 | NA |
| BPA | 3.32 | NA | NA | NA | 0.0270 | 0.0000 | 0.0001 p < 0.001 | NA |
| MBT | 2.41 | NA | NA | NA | NA | NA | 0.0047 | NA |
| ACE | 0.46 | NA | NA | NA | NA | NA | 0.0775 p < 0.001 | NA |
| ATN | 0.16 | NA | NA | 0.0239 | NA | NA | 0.0545 p < 0.001 | 0.0233 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, L.Y.; You, C.; Vu, C.; Chistolini, M.K.; Wang, C.Y.; Mast, K.; Luo, F.; Asvapathanagul, P.; Gedalanga, P.B.; Eusebi, A.L.; et al. Adsorption of Contaminants of Emerging Concern (CECs) with Varying Hydrophobicity on Macro- and Microplastic Polyvinyl Chloride, Polyethylene, and Polystyrene: Kinetics and Potential Mechanisms. Water 2022, 14, 2581. https://doi.org/10.3390/w14162581
Tseng LY, You C, Vu C, Chistolini MK, Wang CY, Mast K, Luo F, Asvapathanagul P, Gedalanga PB, Eusebi AL, et al. Adsorption of Contaminants of Emerging Concern (CECs) with Varying Hydrophobicity on Macro- and Microplastic Polyvinyl Chloride, Polyethylene, and Polystyrene: Kinetics and Potential Mechanisms. Water. 2022; 14(16):2581. https://doi.org/10.3390/w14162581
Chicago/Turabian StyleTseng, Linda Y., ChanJu You, Cecilia Vu, Matthew K. Chistolini, Catherine Y. Wang, Kristen Mast, Florence Luo, Pitiporn Asvapathanagul, Phillip B. Gedalanga, Anna Laura Eusebi, and et al. 2022. "Adsorption of Contaminants of Emerging Concern (CECs) with Varying Hydrophobicity on Macro- and Microplastic Polyvinyl Chloride, Polyethylene, and Polystyrene: Kinetics and Potential Mechanisms" Water 14, no. 16: 2581. https://doi.org/10.3390/w14162581
APA StyleTseng, L. Y., You, C., Vu, C., Chistolini, M. K., Wang, C. Y., Mast, K., Luo, F., Asvapathanagul, P., Gedalanga, P. B., Eusebi, A. L., Gorbi, S., Pittura, L., & Fatone, F. (2022). Adsorption of Contaminants of Emerging Concern (CECs) with Varying Hydrophobicity on Macro- and Microplastic Polyvinyl Chloride, Polyethylene, and Polystyrene: Kinetics and Potential Mechanisms. Water, 14(16), 2581. https://doi.org/10.3390/w14162581







