Efficient Removal of Organic Matter from Biotreated Coking Wastewater by Coagulation Combined with Sludge-Based Activated Carbon Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. BTCW Characteristics
2.2. Sludge-Based Activated Carbon Preparation
2.3. Adsorbent Characterization
2.4. Coagulation Pretreatment
2.5. Adsorption Treatment
2.6. Dissolved Organic Matter (DOM) Fractionation
2.7. Three-Dimensional Excitation–Emission Matrix (3D-EEM) Analysis
2.8. UV-Vis Spectrum Analysis
2.9. Adsorption–Desorption Performance Analysis
3. Results and Discussion
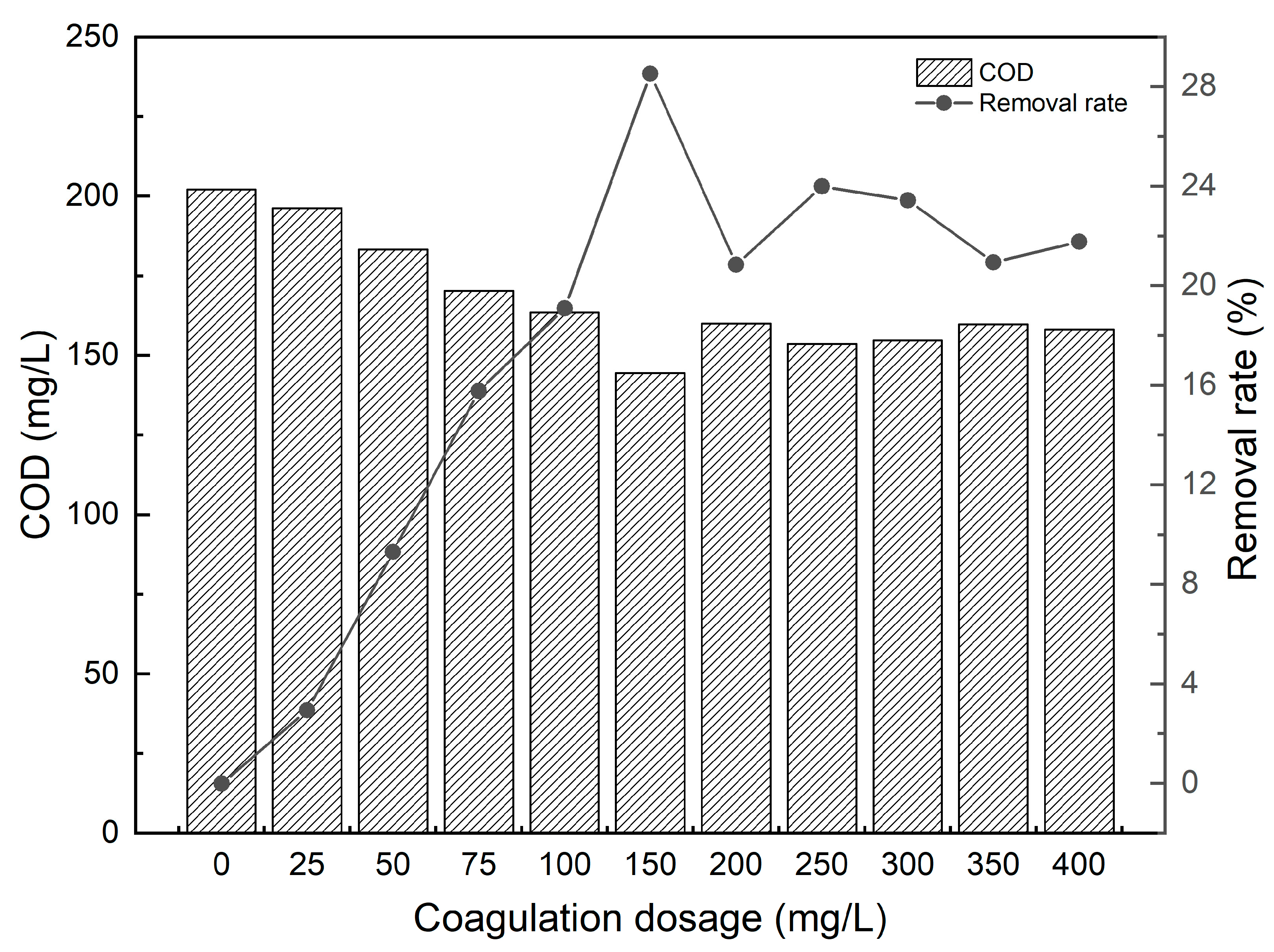
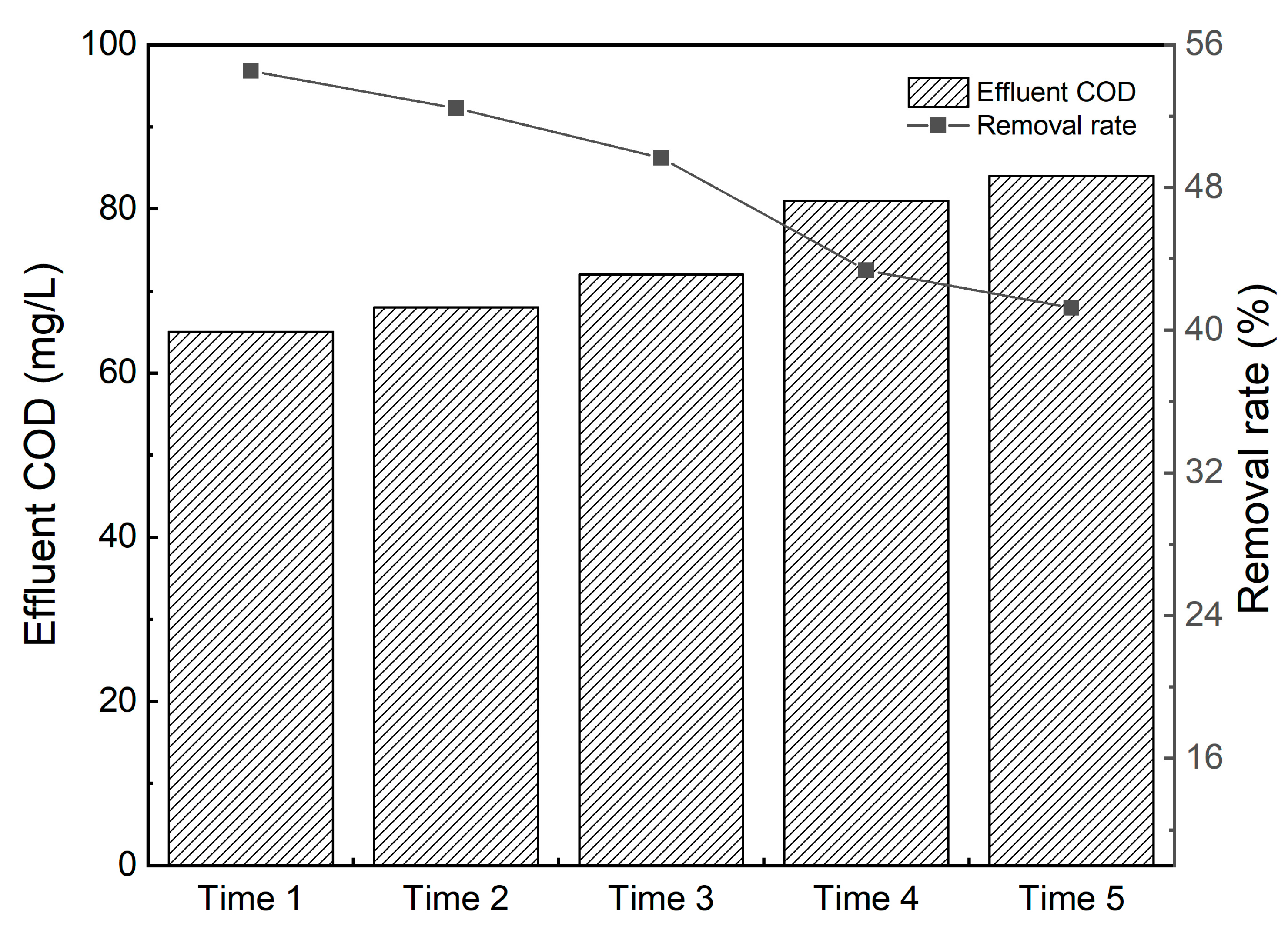
3.1. Optimal Coagulant Dosage and Coagulation Efficiency
3.2. Adsorbent Characterization
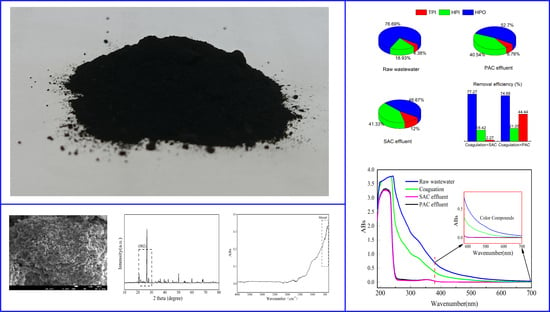
3.2.1. Pore Structure of the Adsorbents
3.2.2. Surface Morphology of the Adsorbents
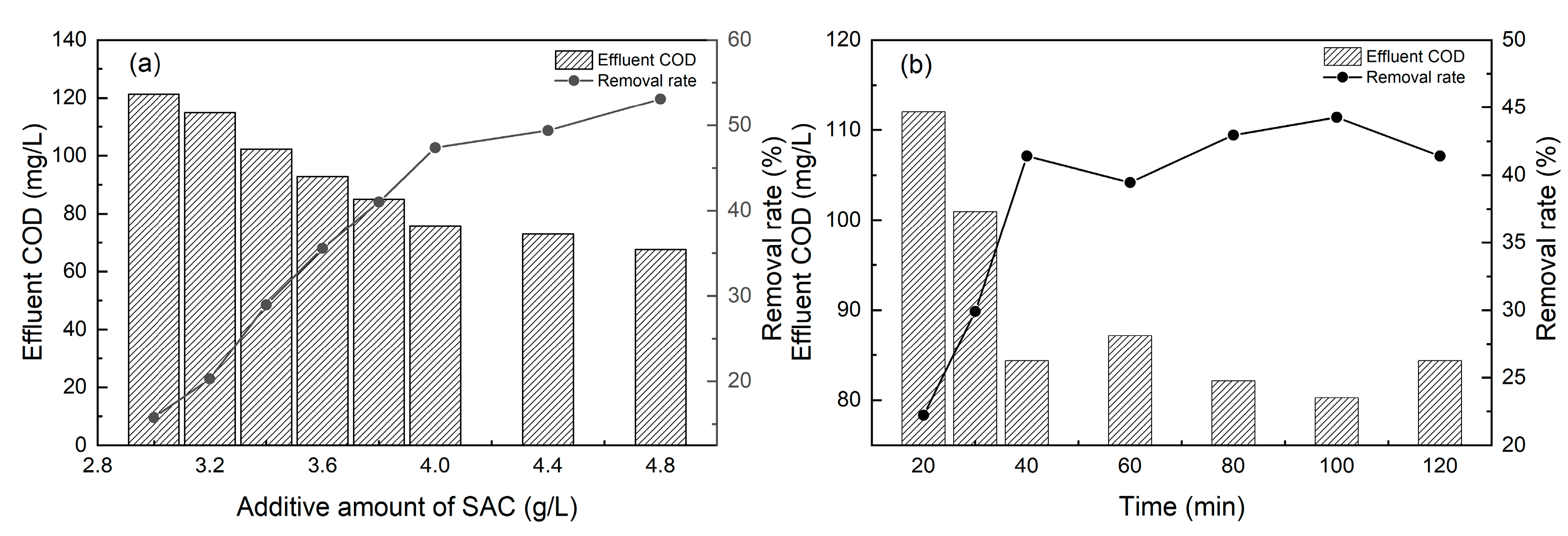
3.3. Optimal Conditions of Adsorption
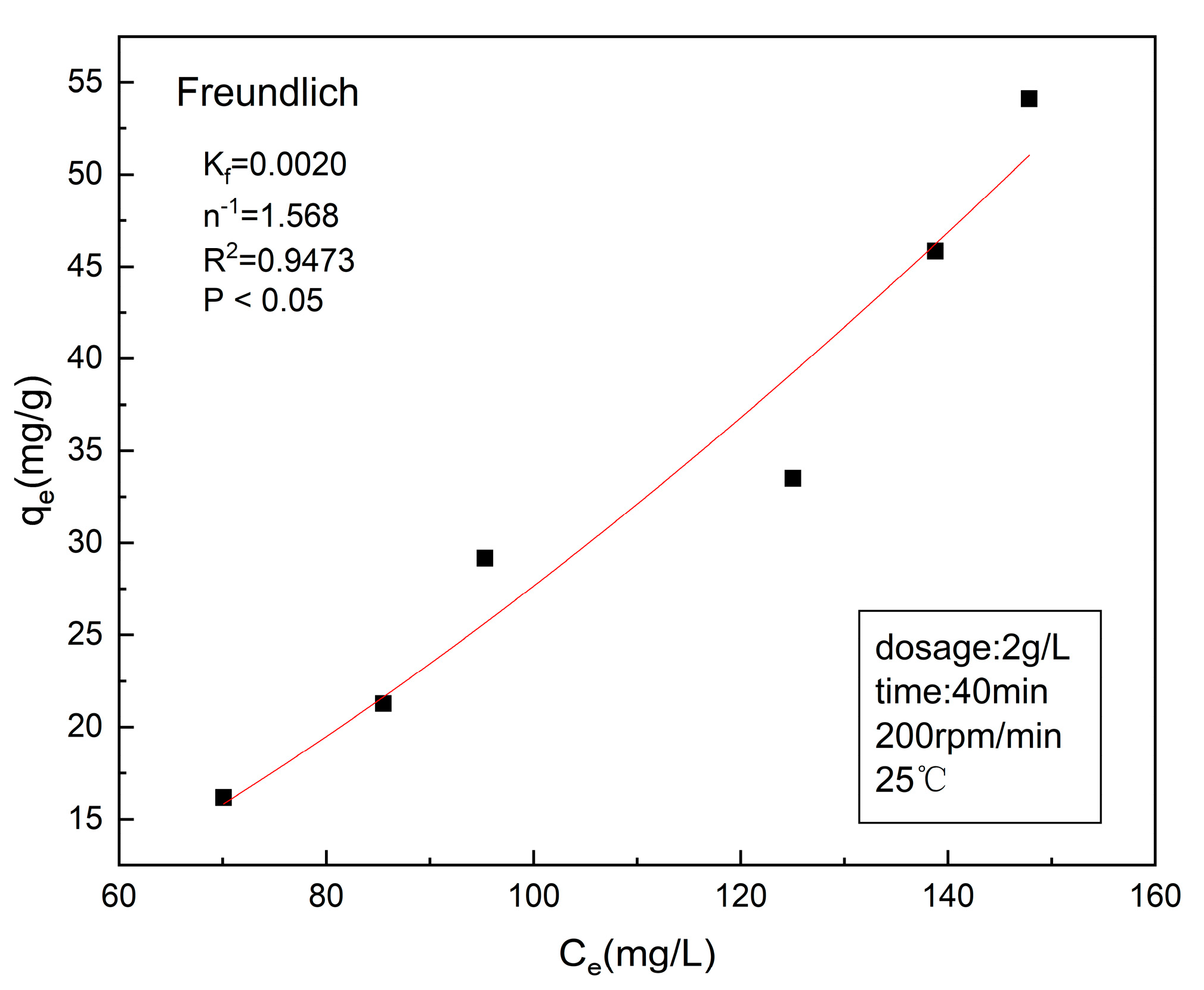
3.4. Adsorption Isotherm
3.5. Removal Characteristics of Organic Matter
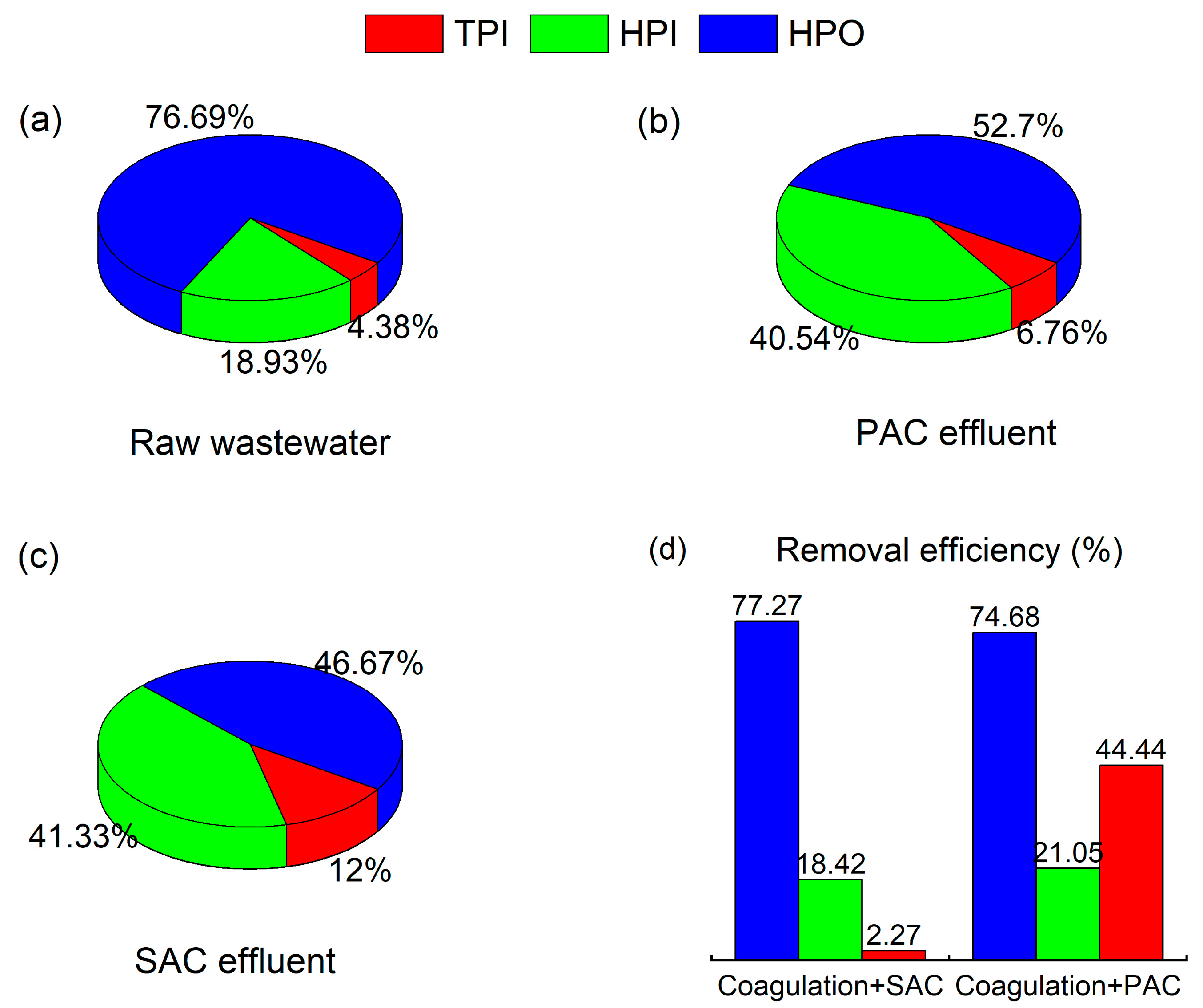
3.5.1. Removal of Hydrophilic and Hydrophobic Components by Coagulation Combined with SAC Adsorption
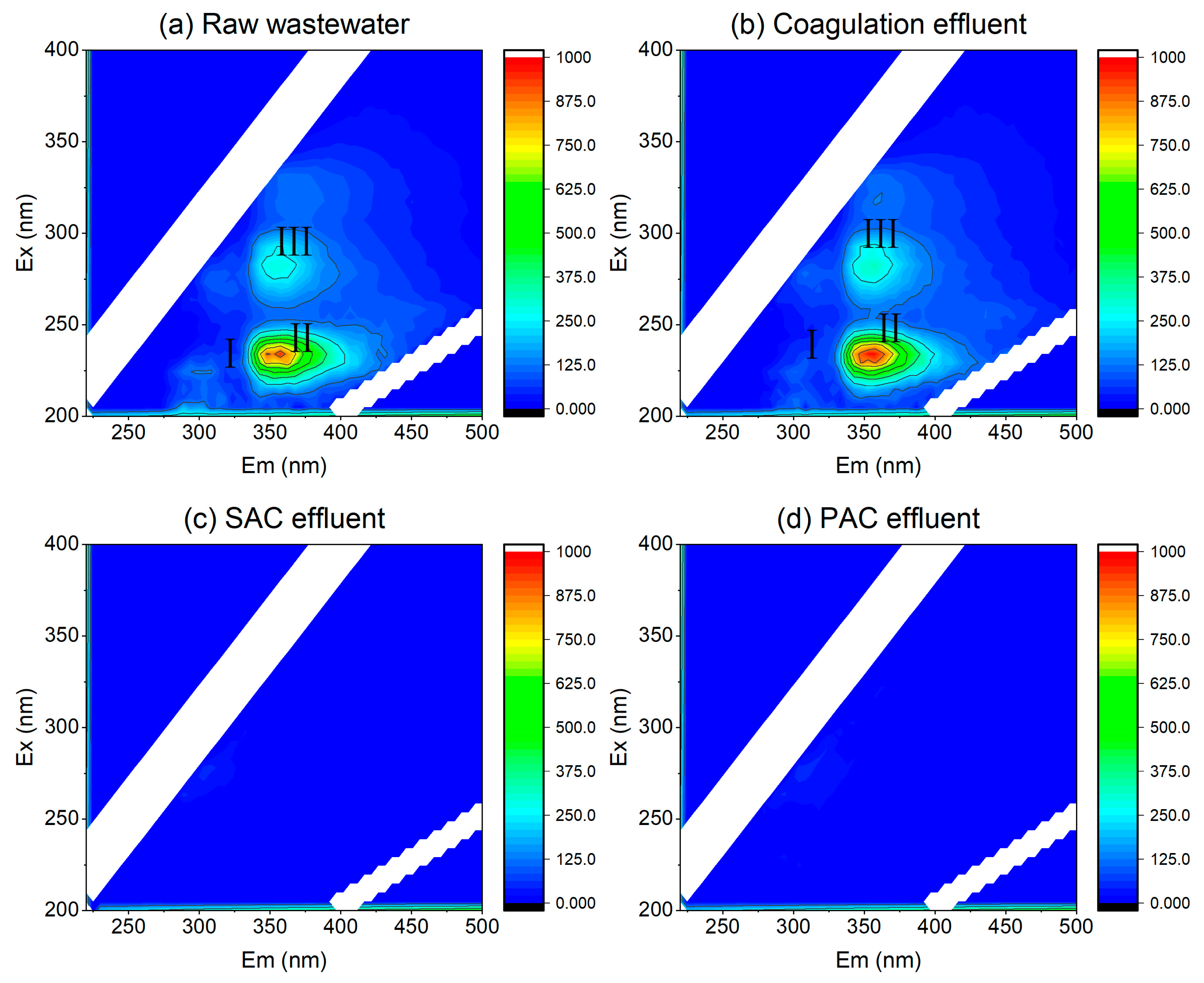
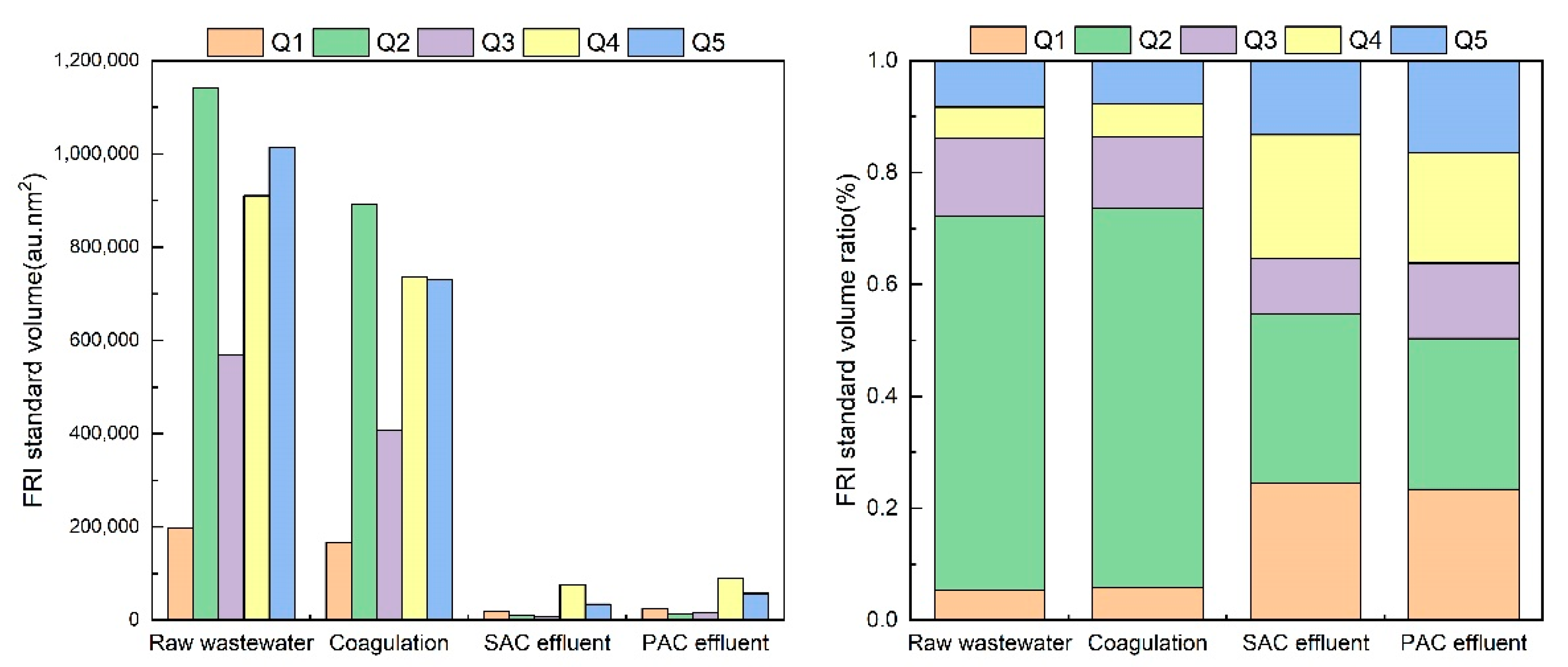
3.5.2. Fluorescence Regional Integration (FRI) Analysis of Organic Matter Removal Characteristics
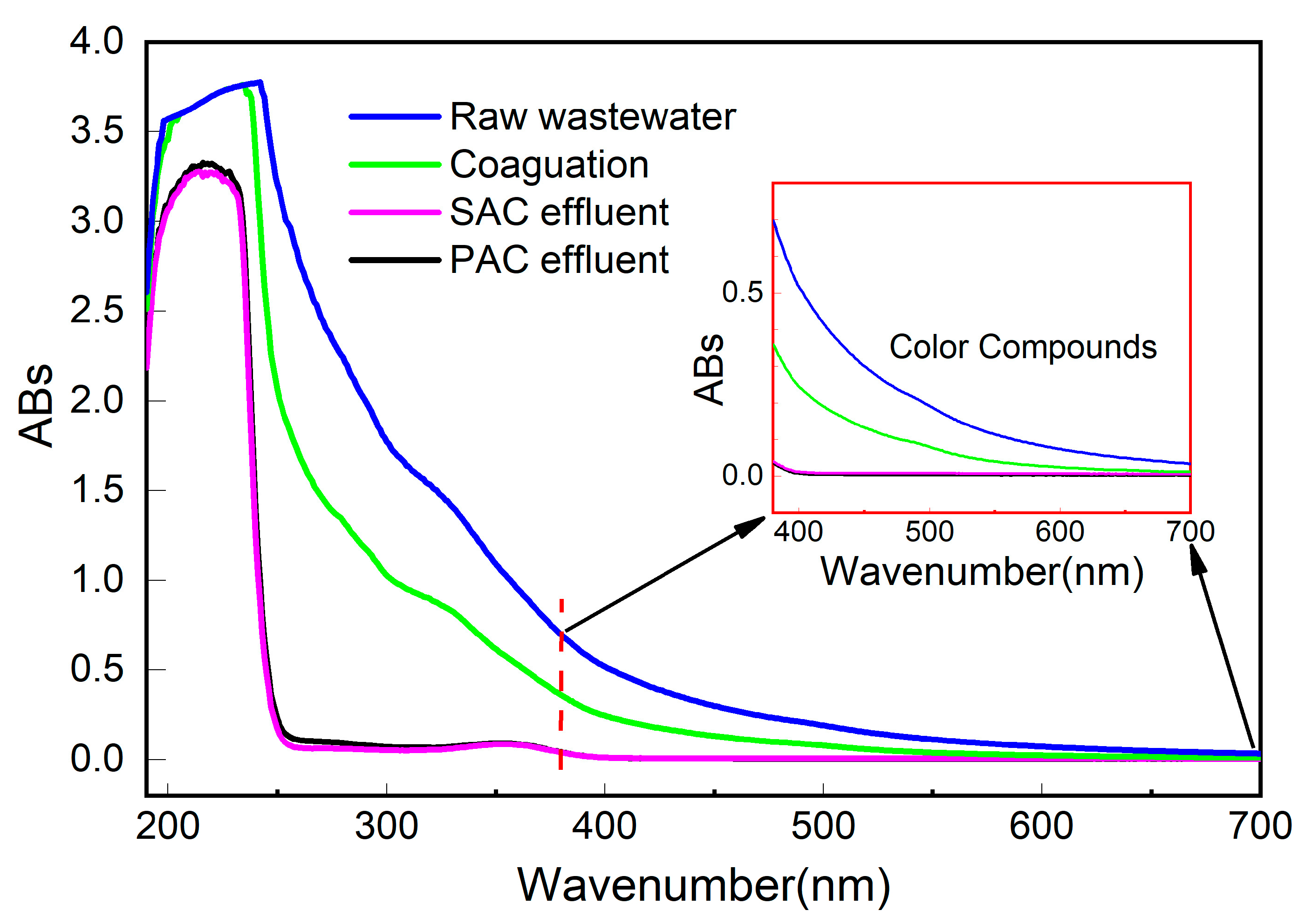
3.5.3. UV-Vis Spectroscopic Analysis of Organic Matter Removal Properties
3.6. SAC Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.; He, C.; Wang, X.; Zhang, Y.; Cheng, Z.; Dai, B.; Zhang, L. Dissolved organic matter (DOM) removal from bio-treated coking wastewater using a new polymeric adsorbent modified with dimethylamino groups. Bioresour. Technol. 2017, 241, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Sun, Y. Combination of Coagulation and Ozone Catalytic Oxidation for Pretreating Coking Wastewater. Int. J. Environ. Res. Public Health 2019, 16, 1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, P.; Zhao, H.; Wang, C.; Ni, J. Advanced treatment of coking wastewater by coagulation and zero-valent iron processes. J Hazard. Mater. 2007, 147, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Sun, H.; Cheng, F.; Liu, Y. Advanced treatment of biologically treated coking wastewater by membrane distillation coupled with pre-coagulation. Desalination 2016, 380, 43–51. [Google Scholar] [CrossRef]
- Jiang, B.; Tan, L.; Ning, S.; Shi, S. A novel integration system of magnetically immobilized cells and a pair of graphite plate-stainless iron mesh electrodes for the bioremediation of coking wastewater. Bioresour. Technol. 2016, 216, 684–690. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Pan, B.; Lv, L.; Zhang, W. Effective removal of effluent organic matter (EfOM) from bio-treated coking wastewater by a recyclable aminated hyper-cross-linked polymer. Water Res. 2013, 47, 4730–4738. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Chen, Z.; Cheng, F. Factors cont rolling adsorption of recalcitrant organic contaminant from bio-treated coking wastewater using lignite activated coke and coal tar-derived activated carbon. J. Chem. Technol. Biotechnol. 2018, 93, 112–120. [Google Scholar] [CrossRef]
- Li, N.; Xia, Y.; He, X.; Li, W.; Yuan, L.; Wu, X.; Qin, Y.; Yuan, R.; Gong, X. Glucose Addition Enhanced the Advanced Treatment of Coking Wastewater. Water 2021, 13, 3365. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Zhao, H.; Li, F.; Lei, Z.; Zhang, Z. Highly efficient one-step advanced treatment of biologically pretreated coking wastewater by an integration of coagulation and adsorption process. Bioresour. Technol. 2018, 247, 1206–1209. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, G.; Ying, W. Enhanced Carbon Adsorption Process for Advanced Treatment of Biotreated Coking Plant Wastewater. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 10–12 June 2010; pp. 1–5. [Google Scholar] [CrossRef]
- Haberkamp, J.; Ruhl, A.; Ernst, M.; Jekel, M. Impact of coagulation and adsorption on DOC fractions of secondary effluent and resulting fouling behaviour in ultrafiltration. Water Res. 2007, 41, 3794–3802. [Google Scholar] [CrossRef]
- Wang, B.; Shui, Y.; Ren, H.; He, M. Research of combined adsorption-coagulation process in treating petroleum refinery effluent. Environ. Technol. 2017, 38, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.K.; Zaher, K. Hybrid treatment system for real textile wastewater remediation based on coagulation/flocculation, adsorption and filtration processes: Performance and economic evaluation. J. Water Process Eng. 2021, 40, 101963. [Google Scholar] [CrossRef]
- El Mrabet, I.; Nawdali, M.; Rafqah, S.; Valdés, H.; Benzina, M.; Zaitan, H. Low-cost biomass for the treatment of landfill leachate from Fez City: Application of a combined coagulation–adsorption process. Euro-Mediterranean J. Environ. Integr. 2020, 5, 63. [Google Scholar] [CrossRef]
- Premarathna, K.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Zhou, H.; Wei, C.; Zhang, F.; Hu, Y.; Wu, H.; Kraslawski, A. A comprehensive evaluation method for sludge pyrolysis and adsorption process in the treatment of coking wastewater. J. Environ. Manag. 2019, 235, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Hu, Y.; Zhang, L.; Xu, L.; Yang, Z. Promoting the sustainable fabrication of bricks from municipal sewage sludge through modifying calcination: Microstructure and performance characterization. Constr. Build. Mater. 2022, 324, 126401. [Google Scholar] [CrossRef]
- Jiang, X.; Xie, Y.; Liu, M.; Bin, S.; Liu, Y.; Huan, C.; Ji, G.; Wang, X.; Yan, Z.; Lyu, Q. Study on anaerobic co-digestion of municipal sewage sludge and fruit and vegetable wastes: Methane production, microbial community and three-dimension fluorescence excitation-emission matrix analysis. Bioresour. Technol. 2022, 347, 126748. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef]
- Liu, Z.; Singer, S.; Tong, Y.; Kimbell, L.; Anderson, E.; Hughes, M.; Zitomer, D.; McNamara, P. Characteristics and applications of biochars derived from wastewater solids. Renew. Sustain. Energy Rev. 2018, 90, 650–664. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, Y.; Meng, T.; Zhang, R.; Song, M.; Ren, J. Removal of sulfonamide antibiotics and human metabolite by biochar and biochar/H2O2 in synthetic urine. Water Res. 2018, 147, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Igalavithana, A.D.; Oh, S.-E.; Nam, H.; Zhang, M.; Wang, C.-H.; Kwon, E.E.; Tsang, D.C.; Ok, Y.S. Characterization of bioenergy biochar and its utilization for metal/metalloid immobilization in contaminated soil. Sci. Total Environ. 2018, 640, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, H.; Ren, H. Dissolved organic matter (DOM) removal from biotreated coking wastewater by chitosan-modified biochar: Adsorption fractions and mechanisms. Bioresour. Technol. 2020, 297, 122281. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fan, Q. Investigating the effect of landfill leachates on the characteristics of dissolved organic matter in groundwater using excitation–emission matrix fluorescence spectra coupled with fluorescence regional integration and self-organizing map. Environ. Sci. Pollut. Res. 2016, 23, 21229–21237. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef]
- Wang, S.; Li, E.; Li, J.; Du, Z.; Cheng, F. Preparation and coagulation-flocculation performance of covalently bound organic hybrid coagulant with excellent stability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124966. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Zhang, P.; Cui, J.; Chen, P.; Ding, Q.; Cui, X.; Xing, H. Highly Microporous Activated Carbons with Industrial Potential for Selective Adsorption of Ethane over Ethylene. Ind. Eng. Chem. Res. 2021, 60, 13301–13308. [Google Scholar] [CrossRef]
- Liu, S.; Peng, Y.; Chen, J.; Yan, T.; Zhang, Y.; Liu, J.; Li, J. A new insight into adsorption state and mechanism of adsorbates in porous materials. J. Hazard. Mater. 2020, 382, 121103. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Chang, F.-M.; Huang, B.; Song, Y.-P.; Zhao, H.-Y.; Wang, K.-J. Activated carbon preparation from pyrolysis char of sewage sludge and its adsorption performance for organic compounds in sewage. Fuel 2020, 266, 117053. [Google Scholar] [CrossRef]
- Shikata, T.; Okuzono, M. Are All Polar Molecules Hydrophilic? Hydration Numbers of Ketones and Esters in Aqueous Solution. J. Phys. Chem. B 2013, 117, 7718–7723. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.I.; Jiao, S.; Davis, R.J.; Brown, D.R.; Katz, L.E.; Shell, M.S. Affinity of small-molecule solutes to hydrophobic, hydrophilic, and chemically patterned interfaces in aqueous solution. Proc. Natl. Acad. Sci. USA 2021, 118, e2020205118. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Johir, A.H.; Khourshed, C.; Zhou, J.L.; Ngo, H.H.; Nghiem, L.; Moni, M.; Sun, L. Sorptive removal of dissolved organic matter in biologically-treated effluent by functionalized biochar and carbon nanotubes: Importance of sorbent functionality. Bioresour. Technol. 2018, 269, 9–17. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent advances in engineered biochar productions and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef]
- Gao, L.; Wen, H.; Tian, Q.; Wang, Y.; Li, G. Influence of surface modification by sulfuric acid on coking coal′s adsorption of coking wastewater. Water Sci. Technol. 2017, 76, 555–566. [Google Scholar] [CrossRef]
- Wei, C.; Wu, H.; Kong, Q.; Wei, J.; Feng, C.; Qiu, G.; Wei, C.; Li, F. Residual chemical oxygen demand (COD) fractionation in bio-treated coking wastewater integrating solution property characterization. J. Environ. Manag. 2019, 246, 324–333. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wu, C.; Luo, X.; Yu, X.; Chen, M. The inappropriate application of the regression Langmuir Qm for adsorption capacity comparison. Sci. Total Environ. 2020, 699, 134222. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; He, Y. Application and Mechanism of Sludge-Based Activated Carbon for Phenol and Cyanide Removal from Bio-Treated Effluent of Coking Wastewater. Processes 2020, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Nayl, A.E.A.; Elkhashab, R.A.; El Malah, T.; Yakout, S.M.; El-Khateeb, M.A.; Ali, M.M.S.; Ali, H. Adsorption studies on the removal of COD and BOD from treated sewage using activated carbon prepared from date palm waste. Environ. Sci. Pollut. Res. 2017, 24, 22284–22293. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Majumdar, P. Application of response surface methodology for optimization of heavy metal biosorption using surfactant modified chitosan bead. Chem. Eng. J. 2011, 175, 376–387. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Hua, M.; Zhang, Y.; Shi, X. Characterization of effluent organic matter from different coking wastewater treatment plants. Chemosphere 2018, 203, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Wang, K.; Zhao, Q.-L.; Wei, L.-L.; Zhang, J.; Yang, J.-C. Characterization of dissolved organic matter during landfill leachate treatment by sequencing batch reactor, aeration corrosive cell-Fenton, and granular activated carbon in series. J. Hazard. Mater. 2010, 179, 1096–1105. [Google Scholar] [CrossRef]
- Wang, D.; Hu, Q.; Li, M.; Wang, C.; Ji, M. Evaluating the removal of organic fraction of commingled chemical industrial wastewater by activated sludge process augmented with powdered activated carbon. Arab. J. Chem. 2016, 9, 1951–1961. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Wu, C.; Su, Z.; Fu, X.; Xu, Y. Micro-electrolysis biological fluidized bed process for coking wastewater treatment. J. Water Process Eng. 2020, 38, 101624. [Google Scholar] [CrossRef]
- Chen, W.; Habibul, N.; Liu, X.; Sheng, G.; Yu, H. FTIR and Synchronous Fluorescence Heterospectral Two-Dimensional Correlation Analyses on the Binding Characteristics of Copper onto Dissolved Organic Matter. Environ. Sci. Technol. 2015, 49, 2052–2058. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, K.; Han, X.; Du, B.; Wei, Q.; Wei, D. Achievement, performance and characteristics of microbial products in a partial nitrification sequencing batch reactor as a pretreatment for anaerobic ammonium oxidation. Chemosphere 2017, 183, 212–218. [Google Scholar] [CrossRef]
- Allpike, B.P.; Heitz, A.; Joll, C.A.; Kagi, R.I.; Abbt-Braun, G.; Frimmel, F.H.; Brinkmann, T.; Her, N.; Amy, G. Size Exclusion Chromatography To Characterize DOC Removal in Drinking Water Treatment. Environ. Sci. Technol. 2005, 39, 2334–2342. [Google Scholar] [CrossRef]
- Kamei, N.; Tamiwa, H.; Miyata, M.; Haruna, Y.; Matsumura, K.; Ogino, H.; Hirano, S.; Higashiyama, K.; Takeda-Morishita, M. Hydrophobic Amino Acid Tryptophan Shows Promise as a Potential Absorption Enhancer for Oral Delivery of Biopharmaceuticals. Pharmaceutics 2018, 10, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podorieszach, A.P.; Huttunen-Hennelly, H.E.K. The effects of tryptophan and hydrophobicity on the structure and bioactivity of novel indolicidin derivatives with promising pharmaceutical potential. Org. Biomol. Chem. 2010, 8, 1679. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hur, J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1030. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, Z.; Song, J.; Lv, L. Spectrum evolution of dissolved aromatic organic matters (DAOMs) during electro-peroxi-coagulation pretreatment of coking wastewater. Sep. Purif. Technol. 2020, 235, 116125. [Google Scholar] [CrossRef]
- Xu, R.; Ou, H.; Yu, X.; He, R.; Lin, C.; Wei, C. Spectroscopic characterization of dissolved organic matter in coking wastewater during bio-treatment: Full-scale plant study. Water Sci. Technol. 2015, 72, 1411–1420. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Biswas, P.; Bhunia, P.; Kadukar, S.; Banerjee, S.K.; Ghosh, R.; Sarkar, S. Application of coke breeze for removal of colour from coke plant wastewater. J. Environ. Manag. 2022, 302, 113800. [Google Scholar] [CrossRef]
- Xin, C.; Wang, J.; Zhang, Y.; Liu, Y.; Jia, H.; Li, L. Application of UV-vis absorption spectrum to test the membrane integrity of Membrane bioreactor (MBR). Water Res. 2021, 198, 117153. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Yusaf, T.; Pittaway, P.A. Exploring the correlations between common UV measurements and chemical fractionation for natural waters. Desalination Water Treat. 2016, 57, 16324–16335. [Google Scholar] [CrossRef]
| COD (mg/L) | BOD5 (mg/L) | pH | NO3−-N (mg/L) | NH4+-N (mg/L) |
|---|---|---|---|---|
| 200~250 | 20~25 | 7.0~8.0 | 11.5~14.0 | 4.0~4.5 |
| Samples | SBET (m2/g) | Smicro (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Vtotal (cm3/g) | Dp (nm) | |
|---|---|---|---|---|---|---|---|
| SAC | B 1 | 710.175 | 414.142 | 0.187 | 0.246 | 0.433 | 2.436 |
| A 2 | 689.674 | 375.874 | 0.163 | 0.259 | 0.422 | 2.447 | |
| PAC | B 1 | 789.189 | 350.021 | 0.147 | 0.687 | 0.834 | 2.113 |
| A 2 | 632.617 | 295.574 | 0.125 | 0.558 | 0.683 | 4.319 | |
| Samples | a254 | a300 | A254/A204 | E2/E3 | E3/E4 |
|---|---|---|---|---|---|
| Raw wastewater | 683.66 | 399.26 | 0.8365 | 3.61 | 40.20 |
| Coagulation effluent | 433.16 | 233.97 | 0.5311 | 4.26 | 73.43 |
| SAC adsorption effluent | 20.49 | 11.28 | 0.0298 | 2.25 | 10.80 |
| PAC adsorption effluent | 32.15 | 15.80 | 0.0449 | 2.90 | 18.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Li, W.; He, X.; Liu, D.; Sun, Y.; Chang, J.; Liu, J. Efficient Removal of Organic Matter from Biotreated Coking Wastewater by Coagulation Combined with Sludge-Based Activated Carbon Adsorption. Water 2022, 14, 2446. https://doi.org/10.3390/w14152446
Xia Y, Li W, He X, Liu D, Sun Y, Chang J, Liu J. Efficient Removal of Organic Matter from Biotreated Coking Wastewater by Coagulation Combined with Sludge-Based Activated Carbon Adsorption. Water. 2022; 14(15):2446. https://doi.org/10.3390/w14152446
Chicago/Turabian StyleXia, Yu, Weijia Li, Xuwen He, Dannuo Liu, Yichen Sun, Jie Chang, and Jing Liu. 2022. "Efficient Removal of Organic Matter from Biotreated Coking Wastewater by Coagulation Combined with Sludge-Based Activated Carbon Adsorption" Water 14, no. 15: 2446. https://doi.org/10.3390/w14152446
APA StyleXia, Y., Li, W., He, X., Liu, D., Sun, Y., Chang, J., & Liu, J. (2022). Efficient Removal of Organic Matter from Biotreated Coking Wastewater by Coagulation Combined with Sludge-Based Activated Carbon Adsorption. Water, 14(15), 2446. https://doi.org/10.3390/w14152446