The Adsorptive Removal of Bengal Rose by Artichoke Leaves: Optimization by Full Factorials Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemical Reagents
2.2. BR Solution
2.3. Bio-Adsorbent
2.4. Methods
2.4.1. Bio-Adsorbent Characterization
pH at the Point of Zero Charge (pHpzc)
Iodine Number
- -
- (VB − VS) is the difference between titration volumes calculated for blank test and test with bio-sorbent. Such difference is measured in ml of sodium thiosulfate (0.1 N) solution;
- -
- N is the normality of the sodium thiosulfate solution (0.1 N);
- -
- 126.9 is the atomic mass of iodine, measured in g/mol;
- -
- m is the mass of the bio-adsorbent, measured in g.
Methylene Blue Index
- -
- Q is the apparent adsorption capacity (mg/g) of activated carbon related to the adsorbate;
- -
- ci is the initial concentration (mg/L) of methylene blue solution (20 mg/L)
- -
- ce is the residual concentration (mg/L) of methylene blue solution (0.855 mg/L)
- -
- V is the volume of methylene blue solution (0.25 L)
- -
- m is the mass (g) of the adsorbent(1 g)
Bio-Adsorbent Surface Analysis
2.5. Experimental Design
2.6. Mathematical Model Design
- -
- Y is the response of the experiment, i.e., removal efficiency (R %);
- -
- X1, X2, X3 are the different factors investigated. (X1 = pH; X2 = bio-sorbent dose; X3 = Temperature);
- -
- a1, …, a23 are the effects of factors (coefficients).
- -
- ε is the error of the model.
2.7. Experimental Protocol of Kinetic and Isotherm Adsorption
- -
- c0 is the initial concentration of the BR dyes (mg/L);
- -
- ce is the residual concentration of BR dye at equilibrium (mg/L);
2.8. Thermodynamic Study
- -
- Kads is theequilibrium constant;
- -
- ΔG° is the free energy variation (kJ/mol);
- -
- R is the perfect gas constant (8.314 J/(mol·K−)
- -
- T is the absolute temperature (K);
- -
- ΔH° and ΔS° are calculated by Van’t Hoff Equation (6) as follows:
3. Results and Discussions
3.1. Bio-Adsorbent Characterization
3.1.1. BET Surface Area Analysis
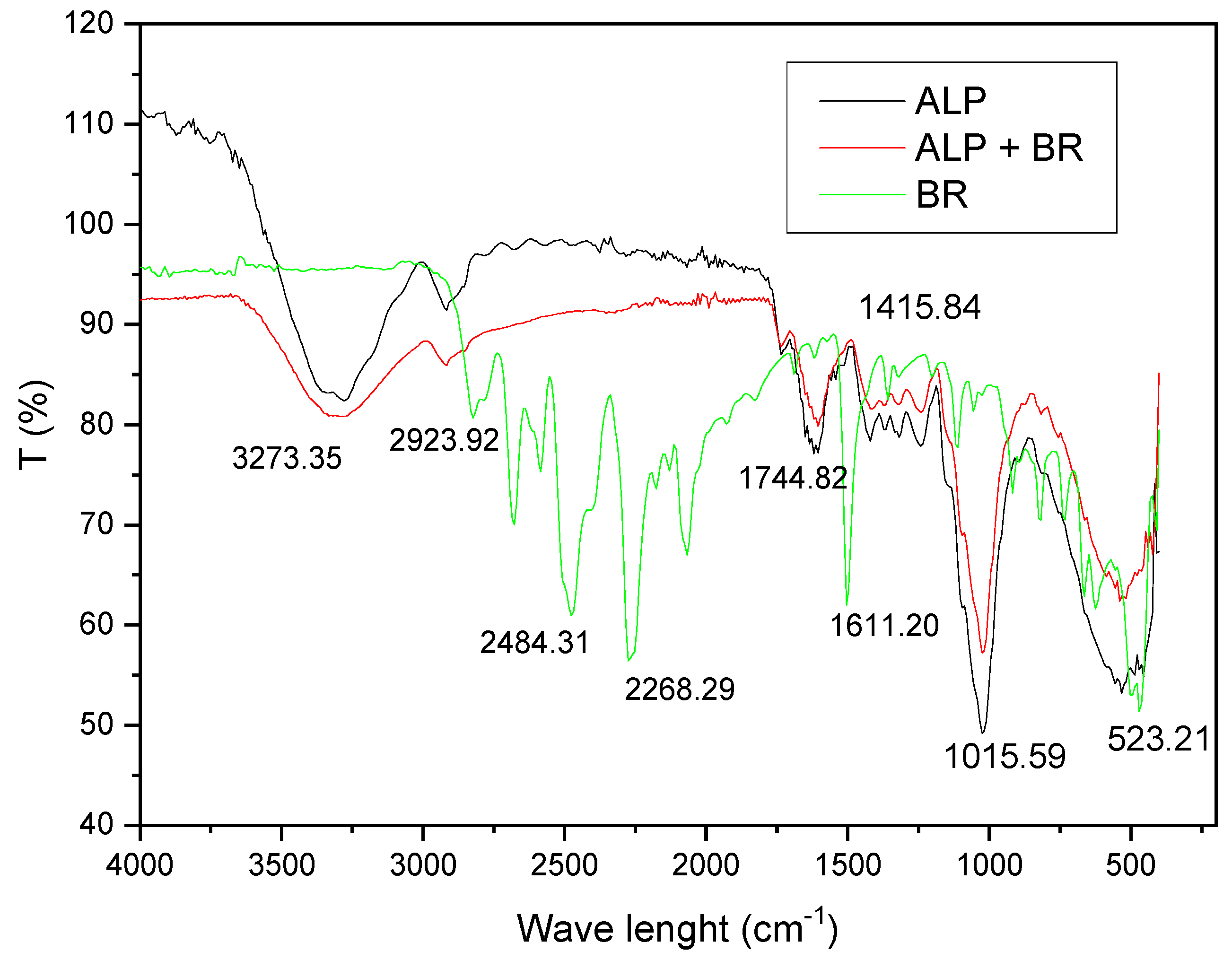
3.1.2. FTIR Analysis
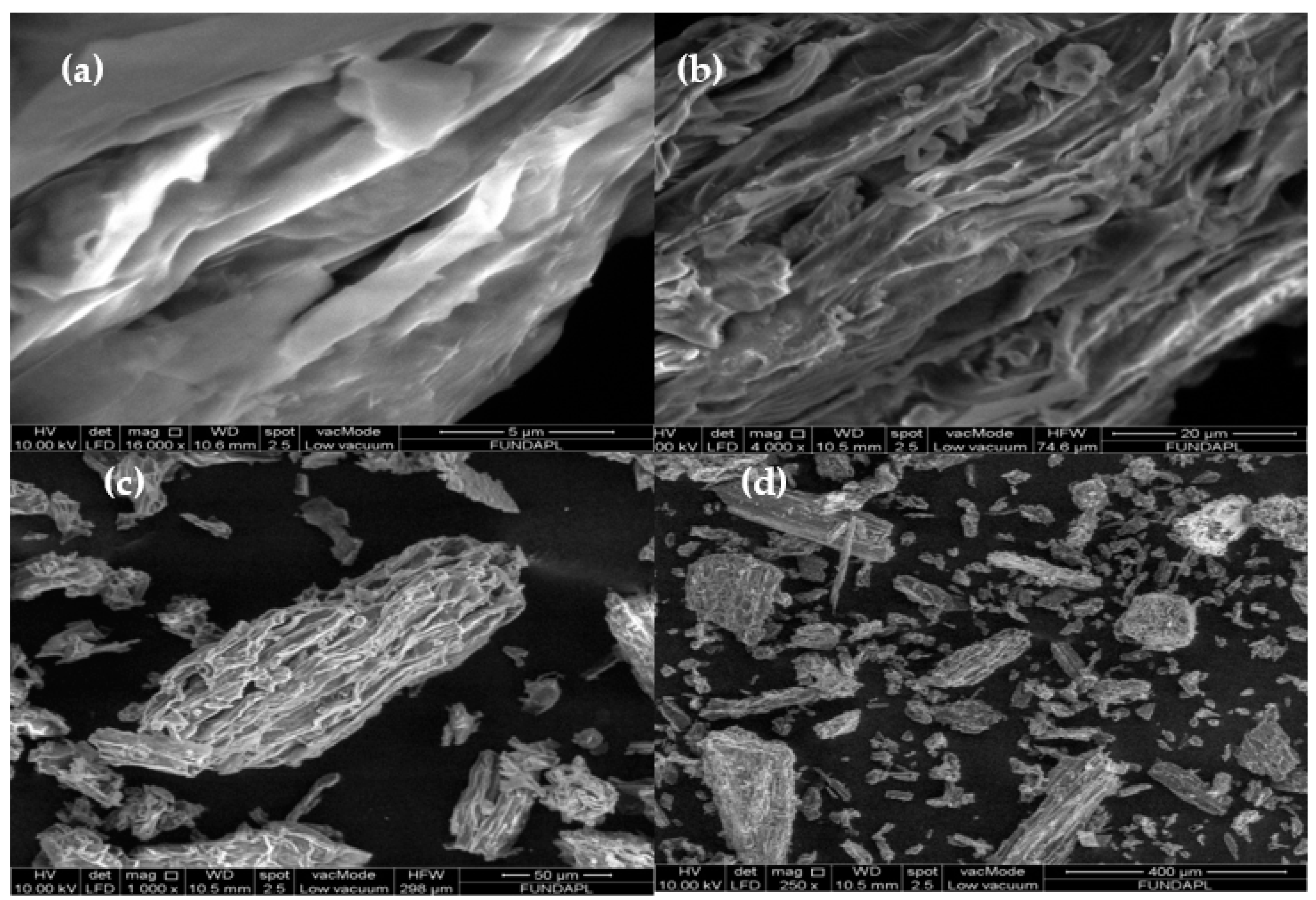
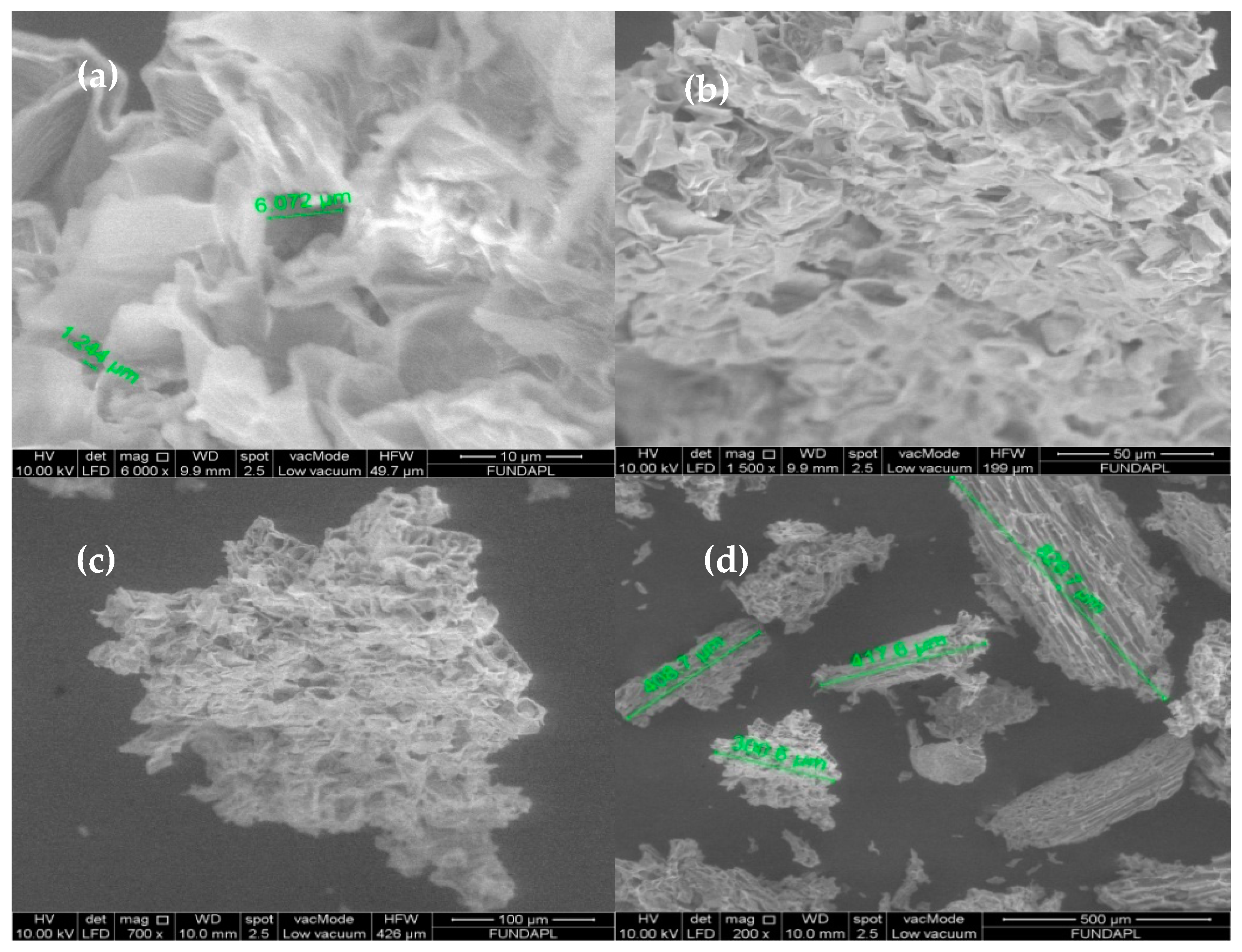
3.1.3. SEM Analysis
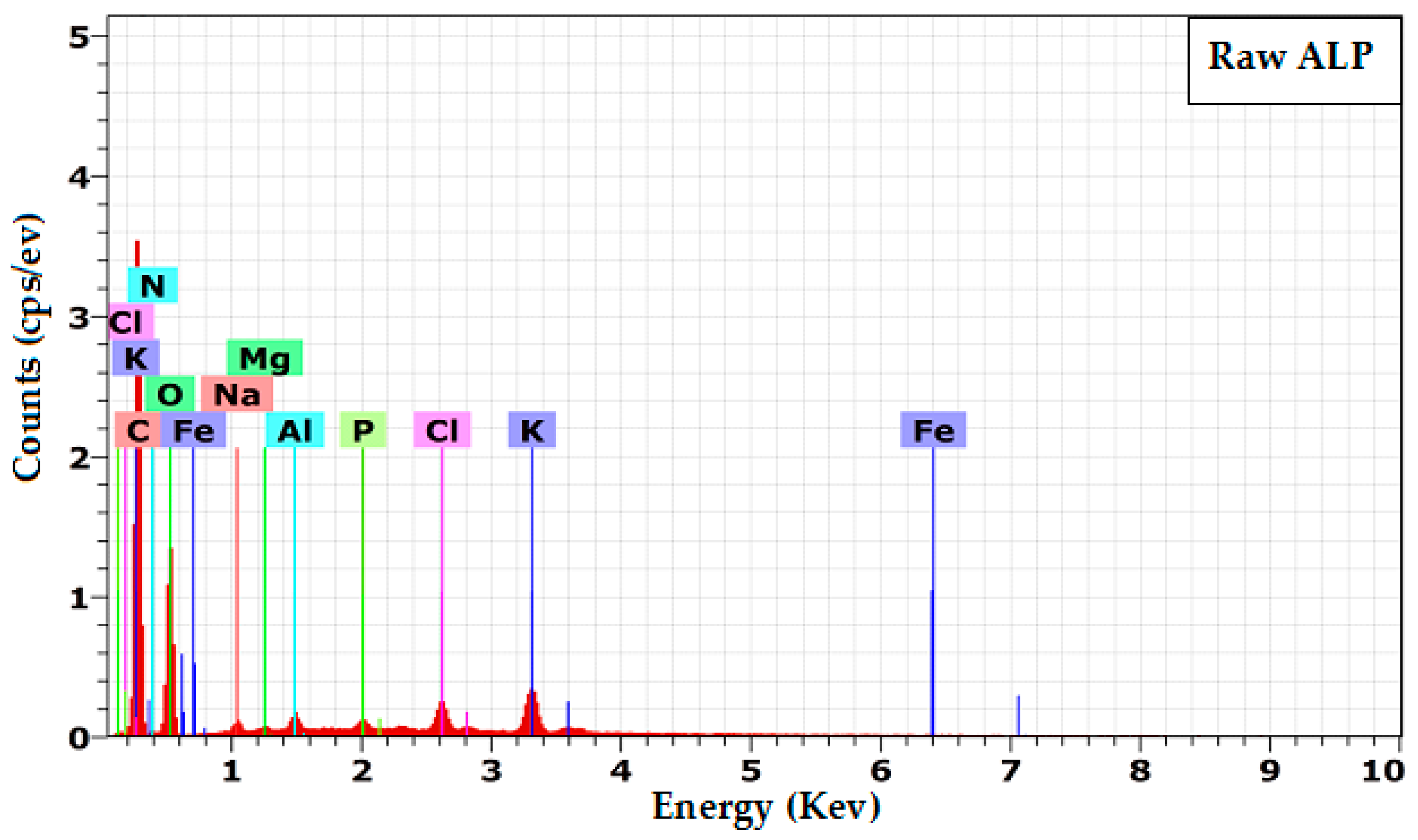
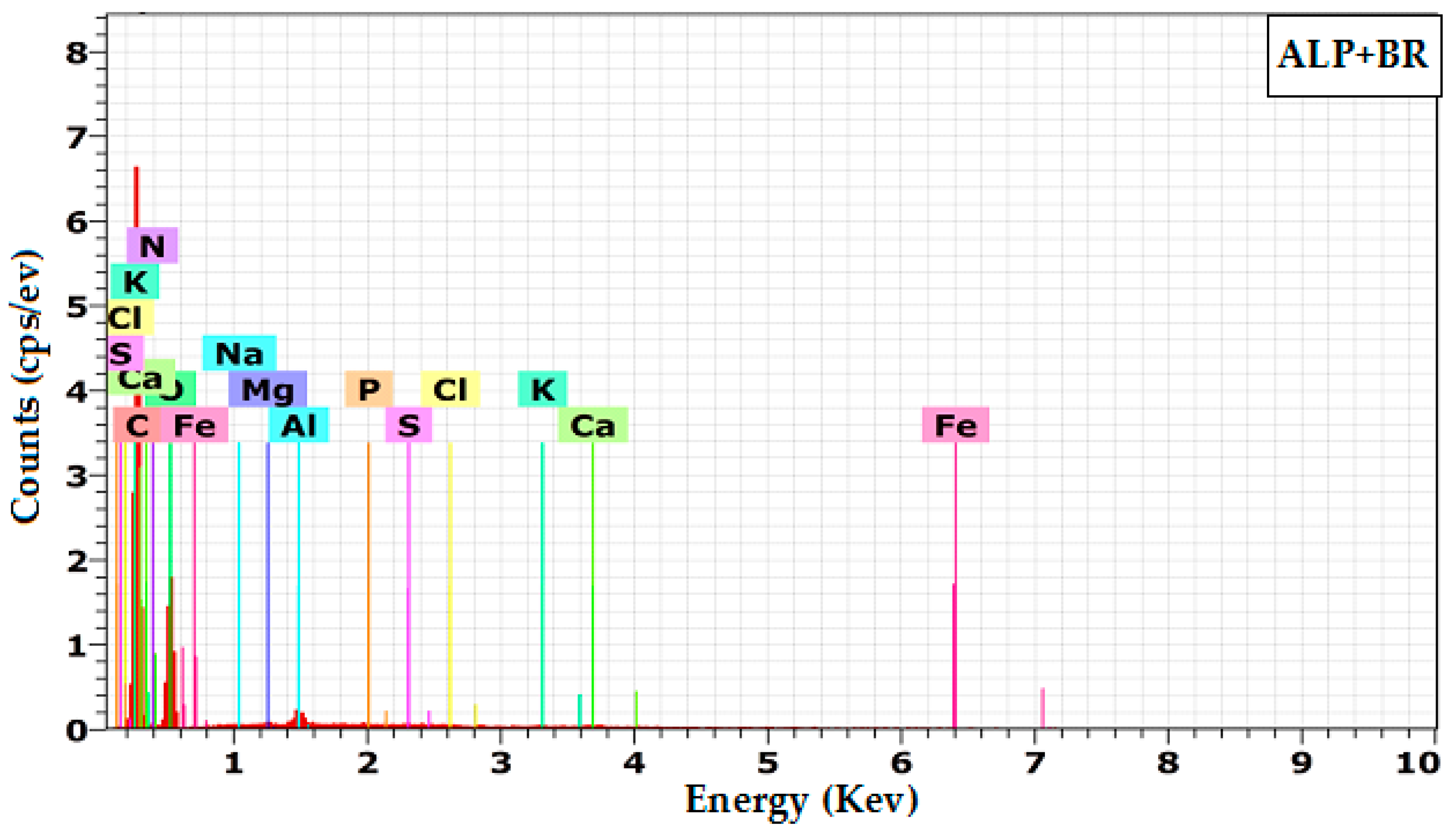
3.1.4. Energy Dispersive Spectroscopy (EDX)
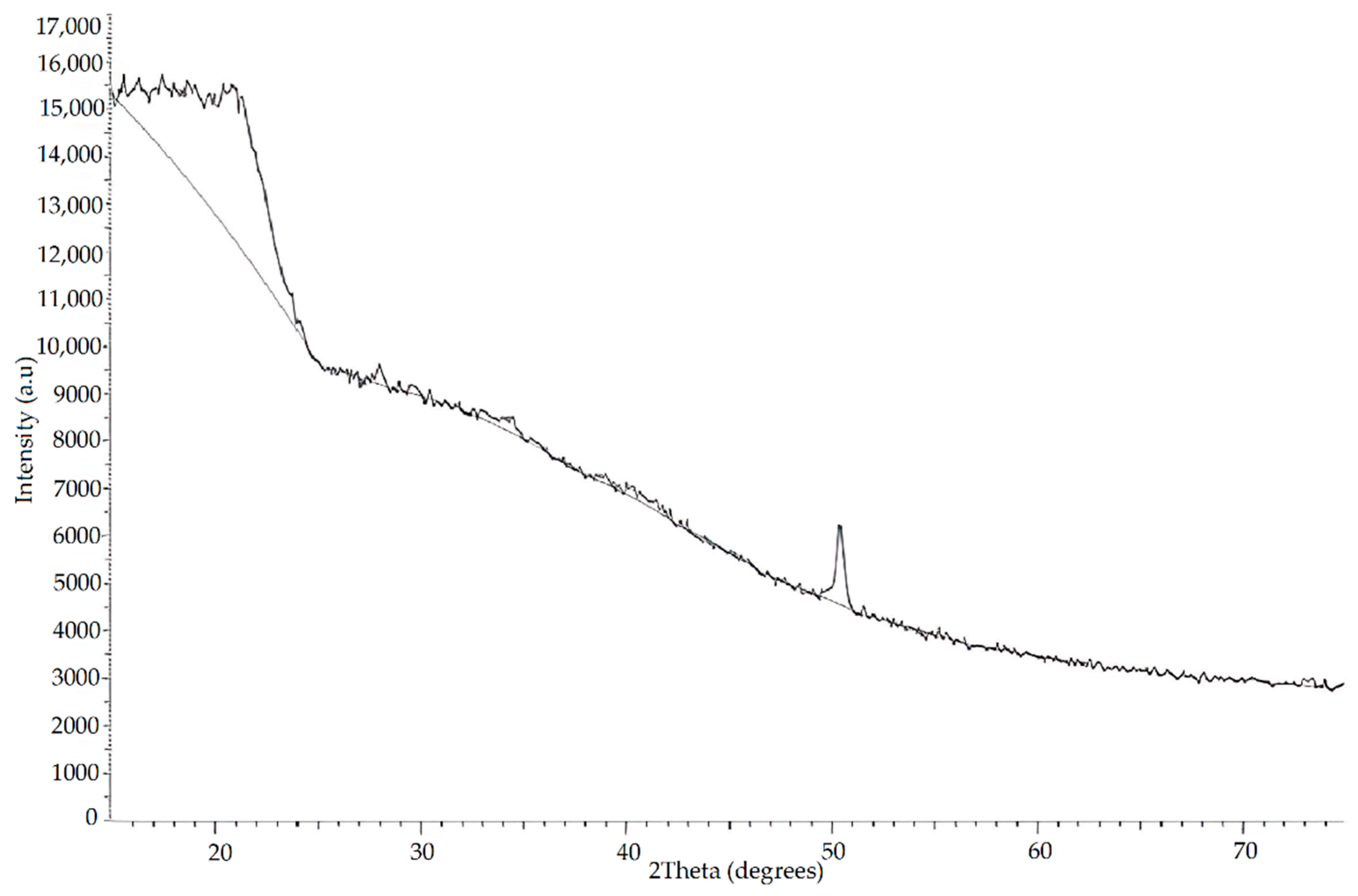
3.1.5. X-ray Analysis
3.1.6. Thermo Gravimetric Analysis
3.1.7. The pH Zero Charge Point (pHpzc)
3.1.8. Iodine and Methylene Blue Index
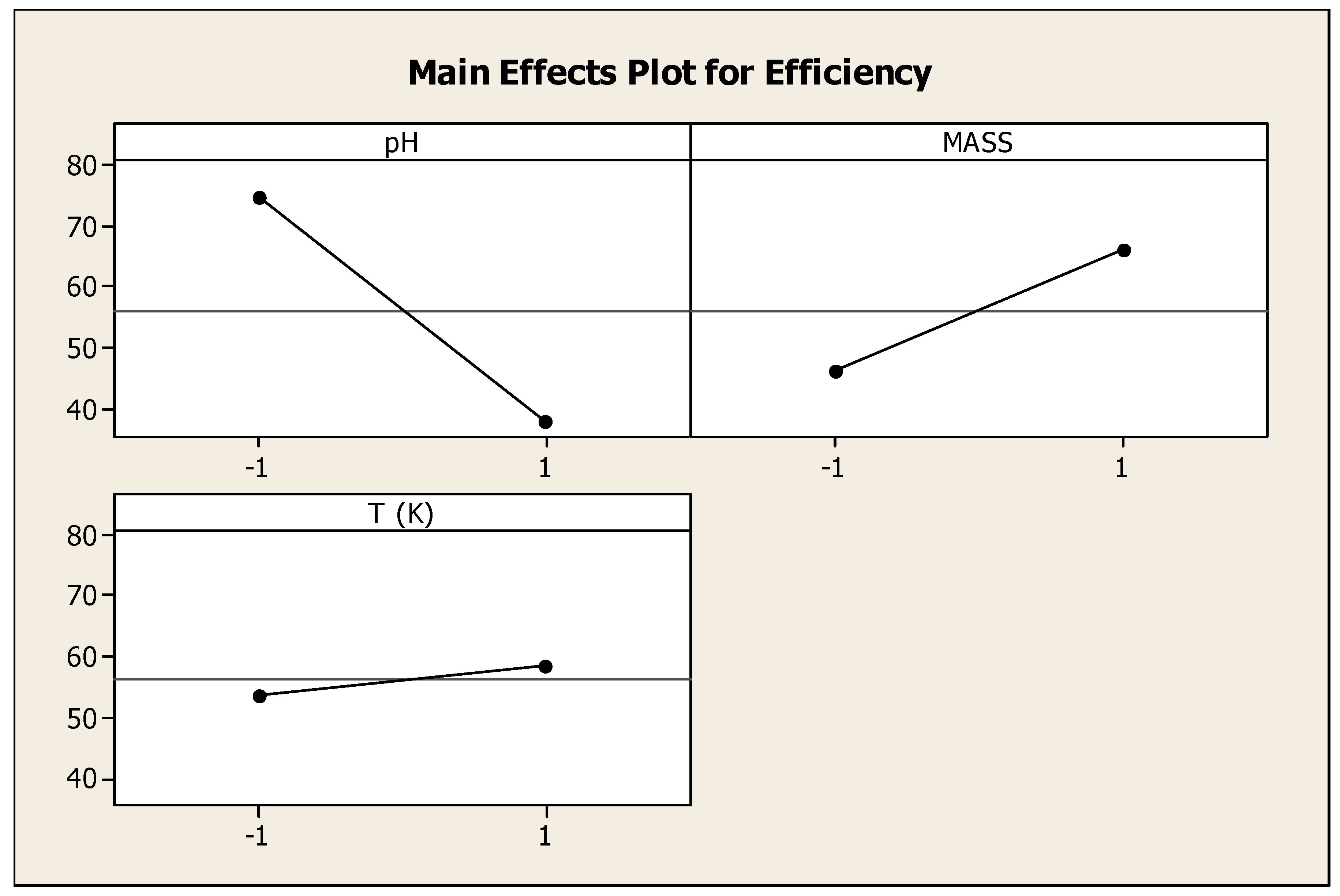
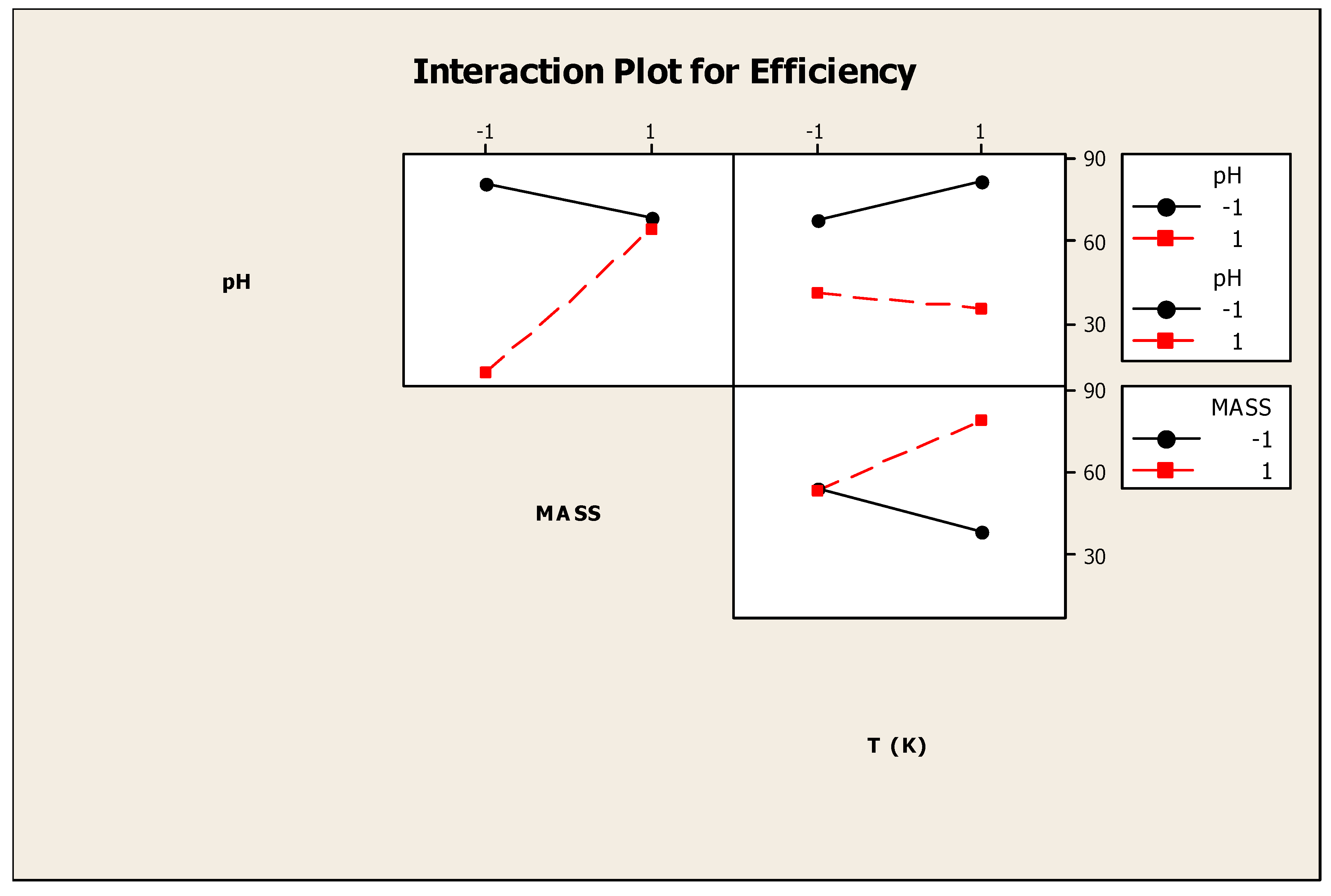
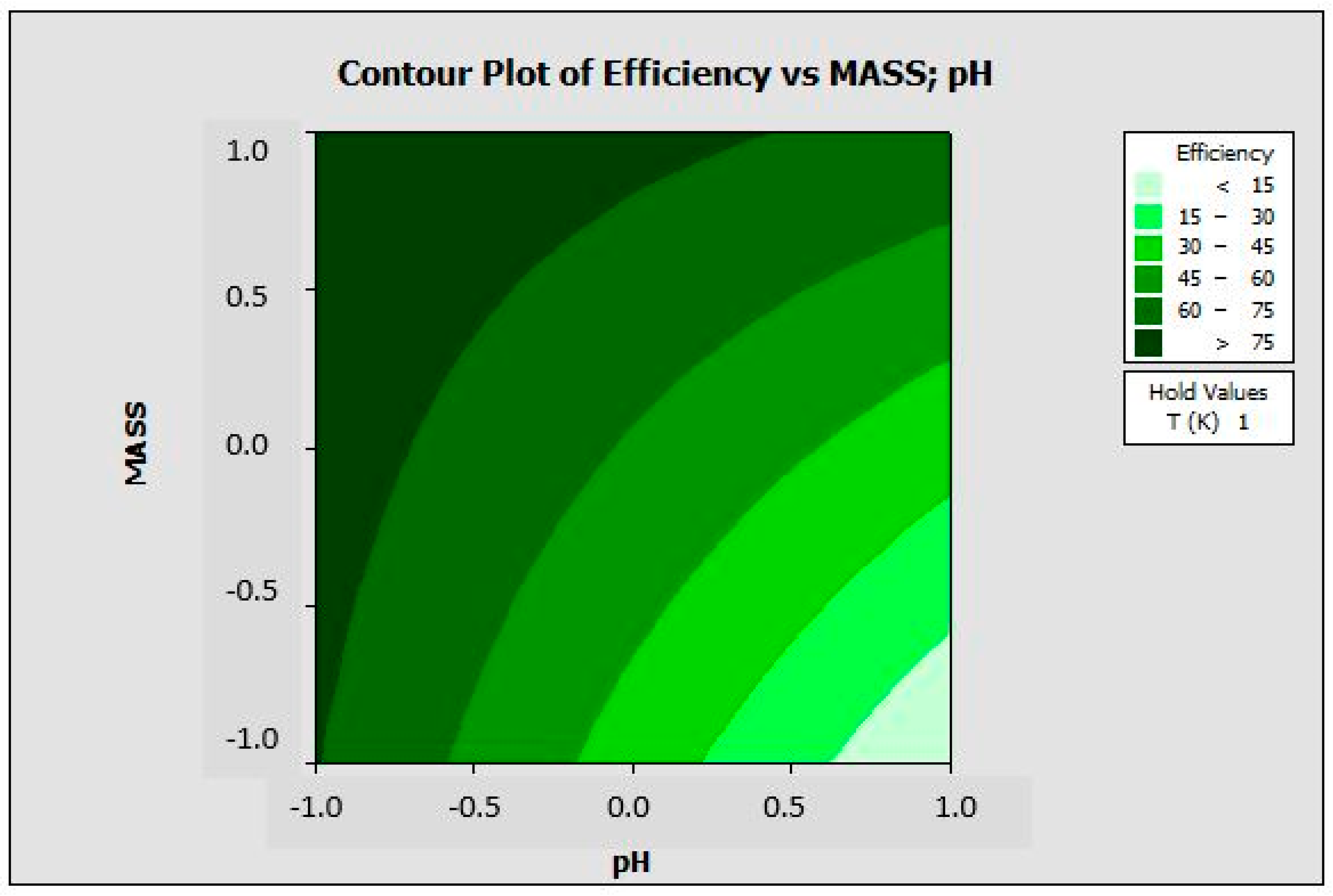
3.2. Factorial Design
3.3. Parametric Study of the Adsorption of BR Dye onto the ALP Surface
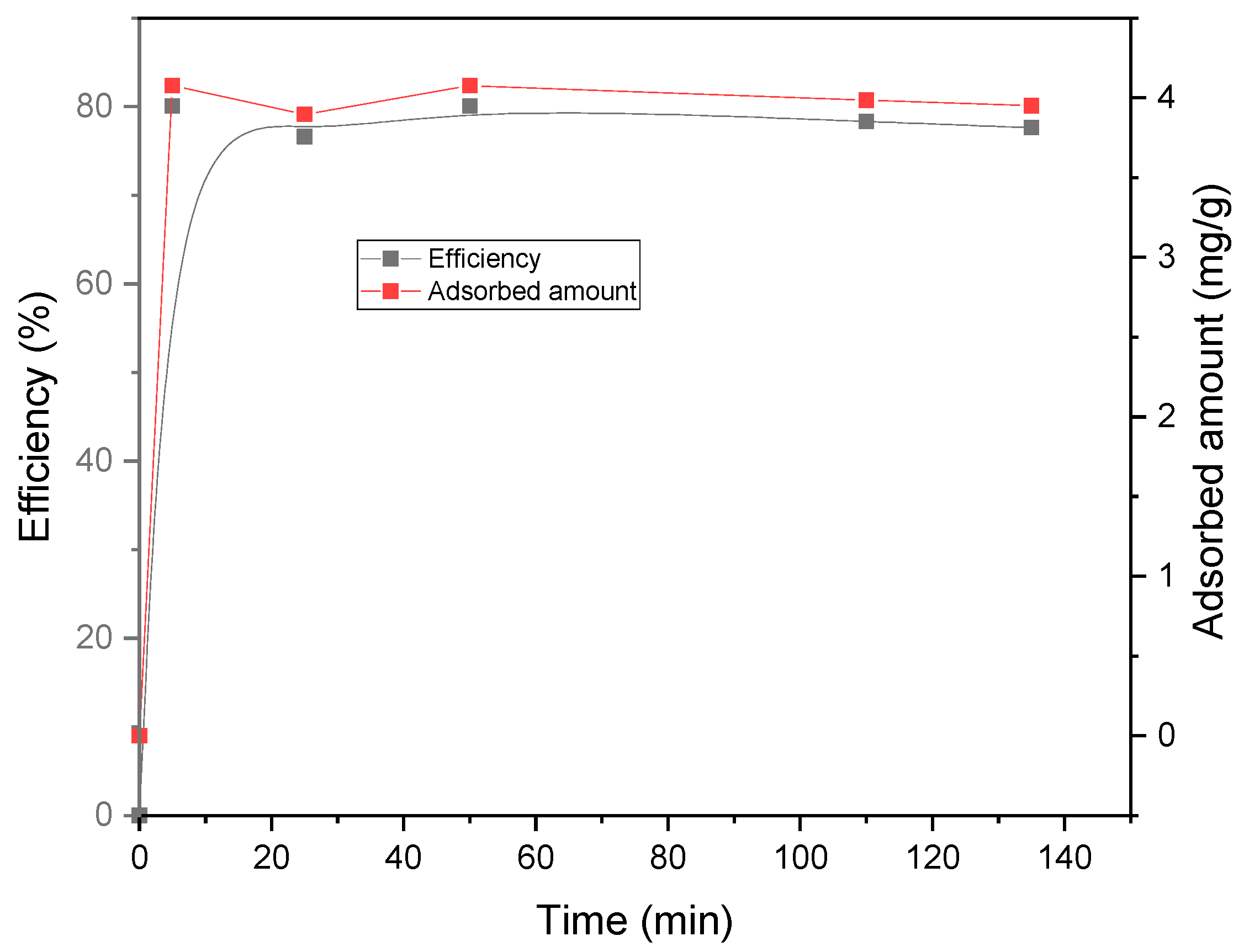
3.3.1. Effect of Contact Time
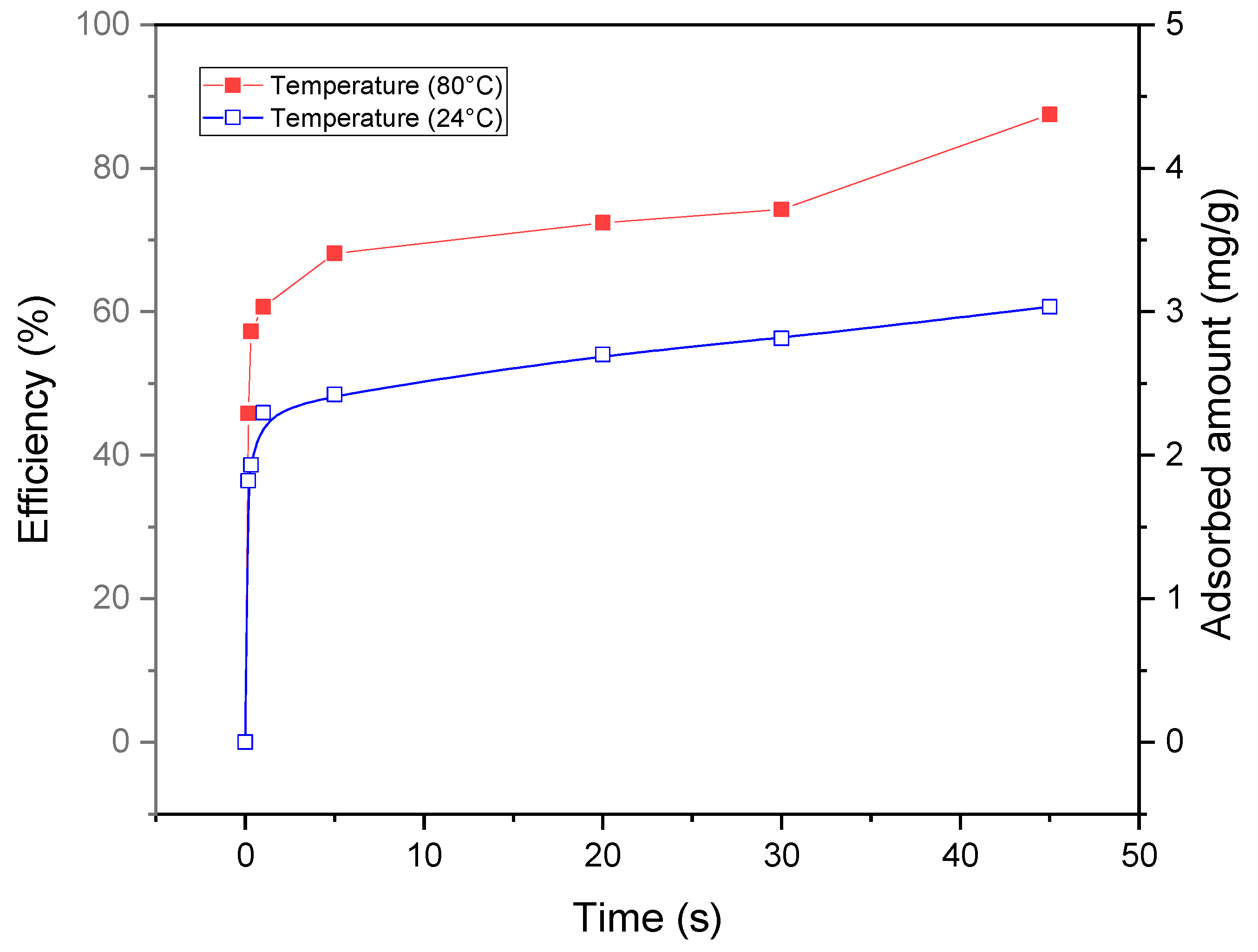
3.3.2. Effect of Temperature
3.3.3. Effect of Initial Concentration of RB Dye
3.4. Kinetics Study of the Adsorption of BR Dye by ALP
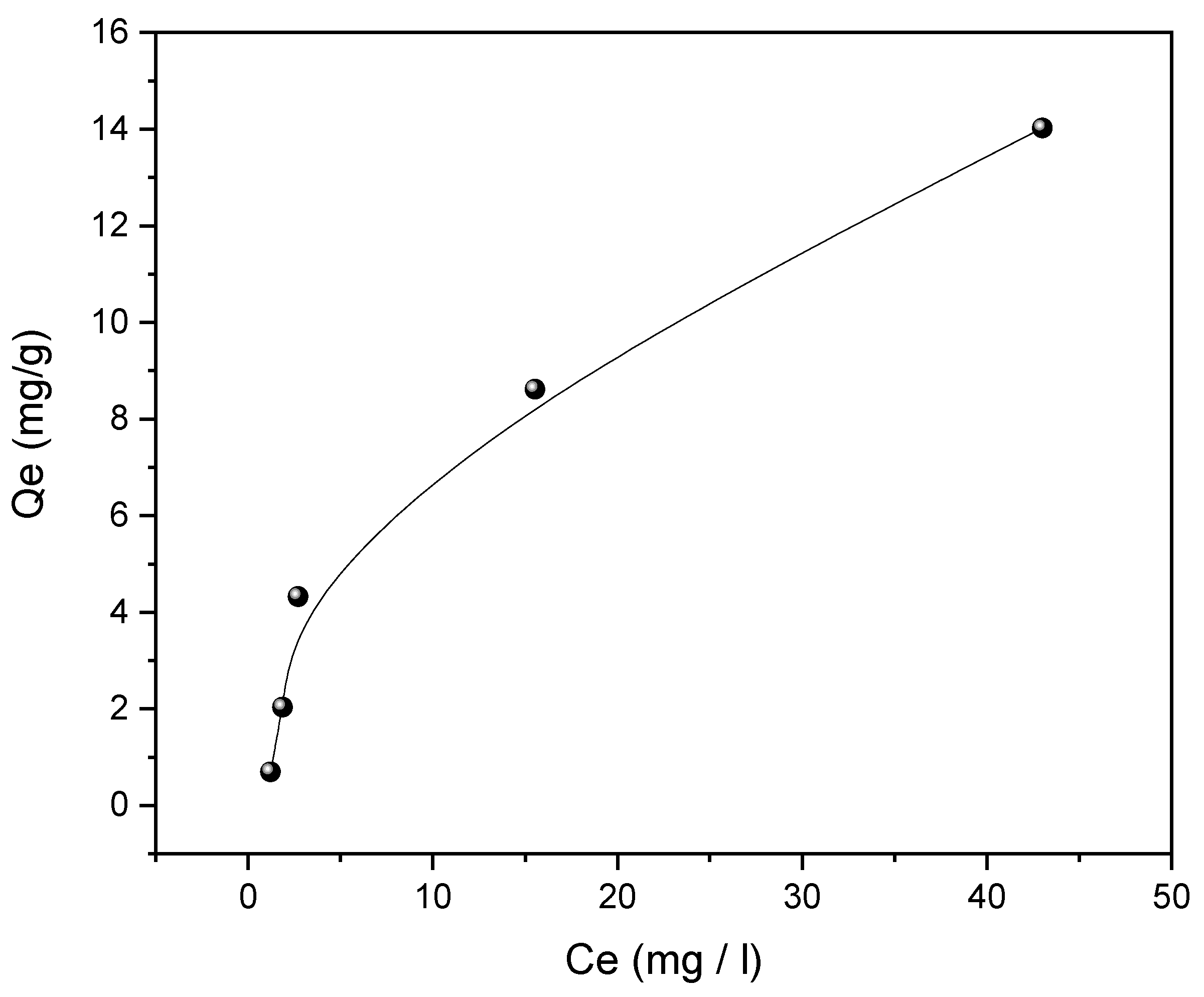
3.5. Adsorption Isotherm
| Type of Isotherm | Linearization of Equations | Constants | R2 |
|---|---|---|---|
| Langmuir | qmax = 11.11 (mg/g) KL = 0.058 (L/mg) | 0.912 | |
| Freundlich | Kf = 1.13 (mg·g−1·(L·mg−1)1/n) 1/n = 0.725 | 0.92 | |
| Temkin | RT/bT = 3.52 ln(AT) = 0.074 | 0.98 | |
| Elovich | qm = 13.3 (mg/g) KE = 0.083 | 0.65 |
- -
- qe and qmax are the equilibrium and maximum capacity of adsorption (mg/g);
- -
- Ce is the equilibrium concentration of BR (mg/L);
- -
- KL, Kf, KE are the Langmuir, Freundlich and Elovich constants, respectively;
- -
- R is the universal gas constant, 8.314 J/(mol·K);
- -
- T is the absolute temperature (K);
- -
- bT is the change in the adsorption energy (J/mole)
- -
- AT is the Temkin constant (L/mg)
3.6. Thermodynamic Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Ben Slama, H.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z. Current status of textile industry wastewater management and research progress in Malaysia: A review. Clean Soil Air Water 2013, 41, 751–764. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Chung, K.-T. Azo dyes and human health: A review. Environ. Sci. Health Care 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Ismail, M.; Gul, S.; Khan, M.I.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange. Green Process. Synth. 2019, 8, 135–143. [Google Scholar] [CrossRef]
- Malik, A.; Akhtar, R.; Grohmann, E. Environmental deterioration and human health: Natural and anthropogenic determinants. In Environmental Deterioration and Human Health: Natural and Anthropogenic Determinants; University Freiburg: Freiburg, Germany, 2014; pp. 1–421. ISBN 9789400778900. [Google Scholar]
- Sadri Moghaddam, S.; Alavi Moghaddam, M.R.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef]
- Liu, M.; Yin, W.; Zhao, T.L.; Yao, Q.Z.; Fu, S.Q.; Zhou, G.T. High-efficient removal of organic dyes from model wastewater using Mg(OH)2-MnO2 nanocomposite: Synergistic effects of adsorption, precipitation, and photodegradation. Sep. Purif. Technol. 2021, 272, 118901. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Paździor, K. Recent achievements in dyes removal focused on advanced. Molecules 2021, 26, 870. [Google Scholar] [CrossRef] [PubMed]
- Malek, N.N.A.; Jawad, A.H.; Ismail, K.; Razuan, R.; ALOthman, Z.A. Fly ash modified magnetic chitosan-polyvinyl alcohol blend for reactive orange 16 dye removal: Adsorption parametric optimization. Int. J. Biol. Macromol. 2021, 189, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Hendaoui, K.; Trabelsi-Ayadi, M.; Ayari, F. Optimization and mechanisms analysis of indigo dye removal using continuous electrocoagulation. Chin. J. Chem. Eng. 2021, 29, 242–252. [Google Scholar] [CrossRef]
- Sultana, S.; Khan, M.Z.; Umar, K.; Ahmed, A.S.; Shahadat, M. SnO2–SrO based nanocomposites and their photocatalytic activity for the treatment of organic pollutants. J. Mol. Struct. 2015, 1098, 393–399. [Google Scholar] [CrossRef]
- Malik, A.; Hameed, S.; Siddiqui, M.J.; Haque, M.M.; Umar, K.; Khan, A.; Muneer, M. Electrical and optical properties of nickel- and molybdenum-doped titanium dioxide nanoparticle: Improved performance in dye-sensitized solar cells. J. Mater. Eng. Perform. 2014, 23, 3184–3192. [Google Scholar] [CrossRef]
- Eslek Koyuncu, D.D.; Okur, M. Removal of AV 90 dye using ordered mesoporous carbon materials prepared via nanocasting of KIT-6: Adsorption isotherms, kinetics and thermodynamic analysis. Sep. Purif. Technol. 2021, 257, 117657. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, K.; Dixit, U. Adsorption, kinetics and thermodynamics of phenol removal by ultrasound-assisted sulfuric acid-treated pea (Pisum sativum) shells. Sustain. Chem. Pharm. 2021, 22, 100491. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhao, R.; Li, C.; Li, Y.; Zhang, C. Adsorption of basic dyes on activated carbon prepared from Polygonum orientale Linn: Equilibrium, kinetic and thermodynamic studies. Desalination 2010, 254, 68–74. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef]
- Amel, K.; Hassen, M.A.; Kerroum, D. Isotherm and kinetics study of biosorption of cationic dye onto banana peel. Energy Procedia 2012, 19, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Khalfaoui, A.; Meniai, A.H. Application of chemically modified orange peels for removal of copper(II) from aqueous solutions. Theor. Found. Chem. Eng. 2012, 46, 732–739. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Bendjamaa, I.; Bensid, T.; Meniai, A.H.; Derbal, K. Effect of calcination on orange peels characteristics: Application of an industrial dye adsorption. Chem. Eng. Trans. 2014, 38, 361–366. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Ghanam, A.M.; Saad, S.R.; Mohamed, R.H.A. Promoted removal of metformin hydrochloride anti-diabetic drug from water by fabricated and modified nanobiochar from artichoke leaves. Sustain. Chem. Pharm. 2020, 18, 100336. [Google Scholar] [CrossRef]
- Imane, B.; Karima, A.; Jamal, M.; Souad, E.H.; Ahmed, M.; Najoua, L. The use and the performance of chemically treated artichoke leaves for textile industrial effluents treatment. Value Health 2021, 31, 100597. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, M.I.; Doval Miñarro, M.; Angosto, J.M.; Fernández-López, J.A. Reuse potential of residues of artichoke (Cynara scolymus L.) from industrial canning processing as sorbent of heavy metals in multimetallic effluents. Ind. Crops Prod. 2019, 141, 111751. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef]
- de Oliveira Brito, S.M.; Andrade, H.M.C.; Soares, L.F.; de Azevedo, R.P. Brazil nut shells as a new biosorbent to remove methylene blue and indigo carmine from aqueous solutions. J. Hazard. Mater. 2010, 174, 84–92. [Google Scholar] [CrossRef]
- Wibowo, N.; Setyadhi, L.; Wibowo, D.; Setiawan, J.; Ismadji, S. Adsorption of benzene and toluene from aqueous solutions onto activated carbon and its acid and heat treated forms: Influence of surface chemistry on adsorption. J. Hazard. Mater. 2007, 146, 237–242. [Google Scholar] [CrossRef]
- Loredo-Cancino, M.; Soto-Regalado, E.; Cerino-Córdova, F.J.; García-Reyes, R.B.; García-León, A.M.; Garza-González, M.T. Determining optimal conditions to produce activated carbon from barley husks using single or dual optimization. J. Environ. Manag. 2013, 125, 117–125. [Google Scholar] [CrossRef]
- Saka, C. BET, TG-DTG, FT-IR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2. J. Anal. Appl. Pyrolysis 2012, 95, 21–24. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Utilization of punica granatum peel as an eco-friendly biosorbent for the removal of methylene blue dye from aqueous solution. J. Appl. Biotechnol. Bioeng. 2018, 5, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.E.; Saad, E.A.; El-Khatib, A.M.; Soliman, M.A.; Allam, E.A.; Fekry, N.A. Green solid synthesis of polyaniline-silver oxide nanocomposite for the adsorptive removal of ionic divalent species of Zn/Co and their radioactive isotopes 65Zn/ 60Co. Environ. Sci. Pollut. Res. 2018, 25, 22120–22135. [Google Scholar] [CrossRef] [PubMed]
- Benalia, A.; Derbal, K.; Panico, A.; Pirozzi, F. Use of acorn leaves as a natural coagulant in a drinking water treatment plant. Water Res. 2019, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- Mashkoor, F.; Nasar, A.; Inamuddin; Asiri, A.M. Exploring the reusability of synthetically contaminated wastewater containing crystal violet dye using tectona grandis sawdust as a very low-cost adsorbent. Sci. Rep. 2018, 8, 8314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Shahid, S.; Amura, I.; Sarihan, A.; Tian, M.; Emanuelsson, E.A. Enhanced adsorption of cationic and anionic dyes from aqueous solutions by polyacid doped polyaniline. Synth. Met. 2018, 245, 151–159. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sarı, A.; Tuzen, M. Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem. Eng. J. 2017, 307, 230–238. [Google Scholar] [CrossRef]
- Nabili, A.; Fattoum, A.; Passas, R.; Elaloui, E.; Nabili, A. Extraction and characterization of cellulose from date palm (Phoenix dactylifera L.). Cellul. Chem. Technol. 2014, 50, 9–10. [Google Scholar]
- Jianxin, H.; Yuyuan, T.; Shan-Yuan, W. Differences in morphological characteristics of bamboo fibres and other natural cellulose fibres: Studies on X-ray diffraction, solid-state 13C-CP/MAS NMR, and second derivative FTIR spectroscopy data. Iran. Polym. J. 2007, 8, 597–613. [Google Scholar]
- Bazan, A.; Nowicki, P.; Półrolniczak, P.; Pietrzak, R. Thermal analysis of activated carbon obtained from residue after supercritical extraction of hops. J. Therm. Anal. Calorim. 2016, 125, 1199–1204. [Google Scholar] [CrossRef] [Green Version]
- Lyu, W.; Yu, M.; Feng, J.; Yan, W. Highly crystalline polyaniline nanofibers coating with low-cost biomass for easy separation and high efficient removal of anionic dye ARG from aqueous solution. Appl. Surf. Sci. 2018, 458, 413–424. [Google Scholar] [CrossRef]
- Roonasi, P.; Holmgren, A. A fourier transform infrared (FTIR) and thermogravimetric analysis (TGA) study of oleate adsorbed on magnetite nano-particle surface. Appl. Surf. Sci. 2009, 255, 5891–5895. [Google Scholar] [CrossRef]
- Khok, Y.T.; Ooi, C.H.; Matsumoto, A.; Yeoh, F.Y. Reactivation of spent activated carbon for glycerine purification. Adsorption 2020, 26, 1015–1025. [Google Scholar] [CrossRef]
- Bencheikh, I.; Azoulay, K.; Mabrouki, J.; El Hajjaji, S.; Dahchour, A.; Moufti, A.; Dhiba, D. The adsorptive removal of MB using chemically treated artichoke leaves: Parametric, kinetic, isotherm and thermodynamic study. Sci. Afr. 2020, 9, e00509. [Google Scholar] [CrossRef]
- Amel, K. Etude Expérimentale de L’élimination de Polluants Organiques et Inorganiques Par Adsorption Sur Des Matériaux Naturels: Application Aux Peaux d’Orange et de Banane. Ph.D. Thesis, University of Constantine, Constantine, Algeria, 2012. [Google Scholar]
- Xiao, J.; Lv, W.; Xie, Z.; Tan, Y.; Song, Y.; Zheng, Q. Environmentally friendly reduced graphene oxide as a broad-spectrum adsorbent for anionic and cationic dyes: Via π-π interactions. J. Mater. Chem. A 2016, 4, 12126–12135. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, M.; Nishioka, H. Adsorption of methylene blue dye from aqueous solution by novel biomass eucalyptus sheathiana bark: Equilibrium, kinetics, thermodynamics and mechanism. Desalin. Water Treat. 2016, 57, 5858–5878. [Google Scholar] [CrossRef]




| Parameters | Minimum Value | Maximum Value |
|---|---|---|
| pH | 4 | 10 |
| Bio-adsorbent dosage (g) | 0.5 | 2 |
| Temperature (°C) | 24 | 80 |
| Tests | pH (X1) | Bio-Adsorbent Dosage (g) (X2) | Temperature (°C) (X3) |
|---|---|---|---|
| 1 | 4 | 0.5 | 24 |
| 2 | 10 | 0.5 | 24 |
| 3 | 4 | 2 | 24 |
| 4 | 10 | 2 | 24 |
| 5 | 4 | 0.5 | 80 |
| 6 | 10 | 0.5 | 80 |
| 7 | 4 | 2 | 80 |
| 8 | 10 | 2 | 80 |
| Time (min) | 5 | 10 | 30 | 60 | 90 | 120 |
|---|---|---|---|---|---|---|
| absorbance (664 nm) | 0.3279 | 0.2975 | 0.2961 | 0.1758 | 0.1782 | 0.201 |
| Q (mg/g) (v = 250 mL) | 4.60 | 4.64 | 4.64 | 4.79 | 4.78 | 4.75 |
| R (%) | 92.02 | 92.76 | 92.79 | 95.72 | 95.66 | 95.11 |
| pH | Mass (g) | Temperature (°C) | Q (mg/g) | Efficiency (%) |
|---|---|---|---|---|
| 4 | 0.5 | 24 | 7.42 | 68.46 |
| 10 | 0.5 | 24 | 2.84 | 26.24 |
| 4 | 2 | 24 | 1.35 | 50.11 |
| 10 | 2 | 24 | 1.59 | 58.6 |
| 4 | 0.5 | 80 | 8.11 | 74.8 |
| 10 | 0.5 | 80 | 0.64 | 5.95 |
| 4 | 2 | 80 | 2.34 | 86.5 |
| 10 | 2 | 80 | 1.88 | 69.66 |
| Pseudo First-Order | Pseudo Second-Order | Intra Particle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|
| Kinetic | Kinetic | Model | ||||||
| C0 (mg·L−1) | R2 | K1 (min−1) | qe (mg/g) | R2 | K2 (g·mg−1·min−1) | qe (mg/g) | R2 | Kint (mg·g−1·min−1/2) |
| 4 | 0.8132 | 0.103 | 4.17 | 0.9994 | 3.4 | 0.96 | 0.801 | 0.0344 |
| 10 | 0.5743 | 0.1038 | 2.32 | 0.9999 | 2.9897 | 2.03 | 0.7039 | 0.0778 |
| 20 | 0.761 | 0.1756 | 1.15 | 0.9999 | 0.5377 | 4.29 | 0.8922 | 0.0601 |
| 50 | 0.8289 | 0.1194 | 2.85 | 1 | 0.4513 | 8.63 | 0.8444 | 0.4482 |
| 100 | 0.8018 | 0.155 | 3.39 | 0.9998 | 0.3575 | 14.06 | 0.7527 | 0.7527 |
| Parameters | Temperature (K) | ||
|---|---|---|---|
| 297 | 323 | 353 | |
| ΔG° (kJ/mol) | −2.411 | −2.314 | −1.948 |
| ΔS° (J/(mol·K) | −8.129 | ||
| ∆H° (kJ/mol) | −4.86 | ||
| R2 | 0.9745 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalfaoui, A.; Khelifi, M.N.; Khelfaoui, A.; Benalia, A.; Derbal, K.; Gisonni, C.; Crispino, G.; Panico, A. The Adsorptive Removal of Bengal Rose by Artichoke Leaves: Optimization by Full Factorials Design. Water 2022, 14, 2251. https://doi.org/10.3390/w14142251
Khalfaoui A, Khelifi MN, Khelfaoui A, Benalia A, Derbal K, Gisonni C, Crispino G, Panico A. The Adsorptive Removal of Bengal Rose by Artichoke Leaves: Optimization by Full Factorials Design. Water. 2022; 14(14):2251. https://doi.org/10.3390/w14142251
Chicago/Turabian StyleKhalfaoui, Amel, Mohamed Nadir Khelifi, Anouar Khelfaoui, Abderrezzaq Benalia, Kerroum Derbal, Corrado Gisonni, Gaetano Crispino, and Antonio Panico. 2022. "The Adsorptive Removal of Bengal Rose by Artichoke Leaves: Optimization by Full Factorials Design" Water 14, no. 14: 2251. https://doi.org/10.3390/w14142251






