Effects of Caffeine and COD from Coffee Wastewater on Anaerobic Ammonium Oxidation (Anammox) Activities
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Setup and Preparation of Wastewaters
2.1.1. Preparation of Synthetic Wastewater
2.1.2. Coffee Wastewater
2.1.3. Acute Effects of Caffeine Dosing on Anammox Activity
2.1.4. Long-Term Effect of Caffeine Dosing on Anammox Activity
2.1.5. Effect of Cod from Coffee Wastewater on Anammox Activity. Recovery of Anammox Cultures after Stopping the Addition of Coffee Wastewater
2.2. Experimental Tests
2.2.1. Acute Effects of Caffeine Dosing on Anammox Activity
2.2.2. Long-Term Effect of Caffeine Dosing on Anammox Activity
2.2.3. Effect of Cod from Coffee Wastewater on Anammox Activity and Recovery of Anammox Cultures after Stopping the Addition of Coffee Wastewater
2.3. Specific Anammox Activity (SAA)
2.4. Free Ammonia Calculation
2.5. Analytical Methods
2.5.1. Chemical Analysis
2.5.2. Solid-Phase Extraction Procedure for Caffeine Extraction
2.5.3. Determination of Caffeine Concentration
2.5.4. Statistical Analysis
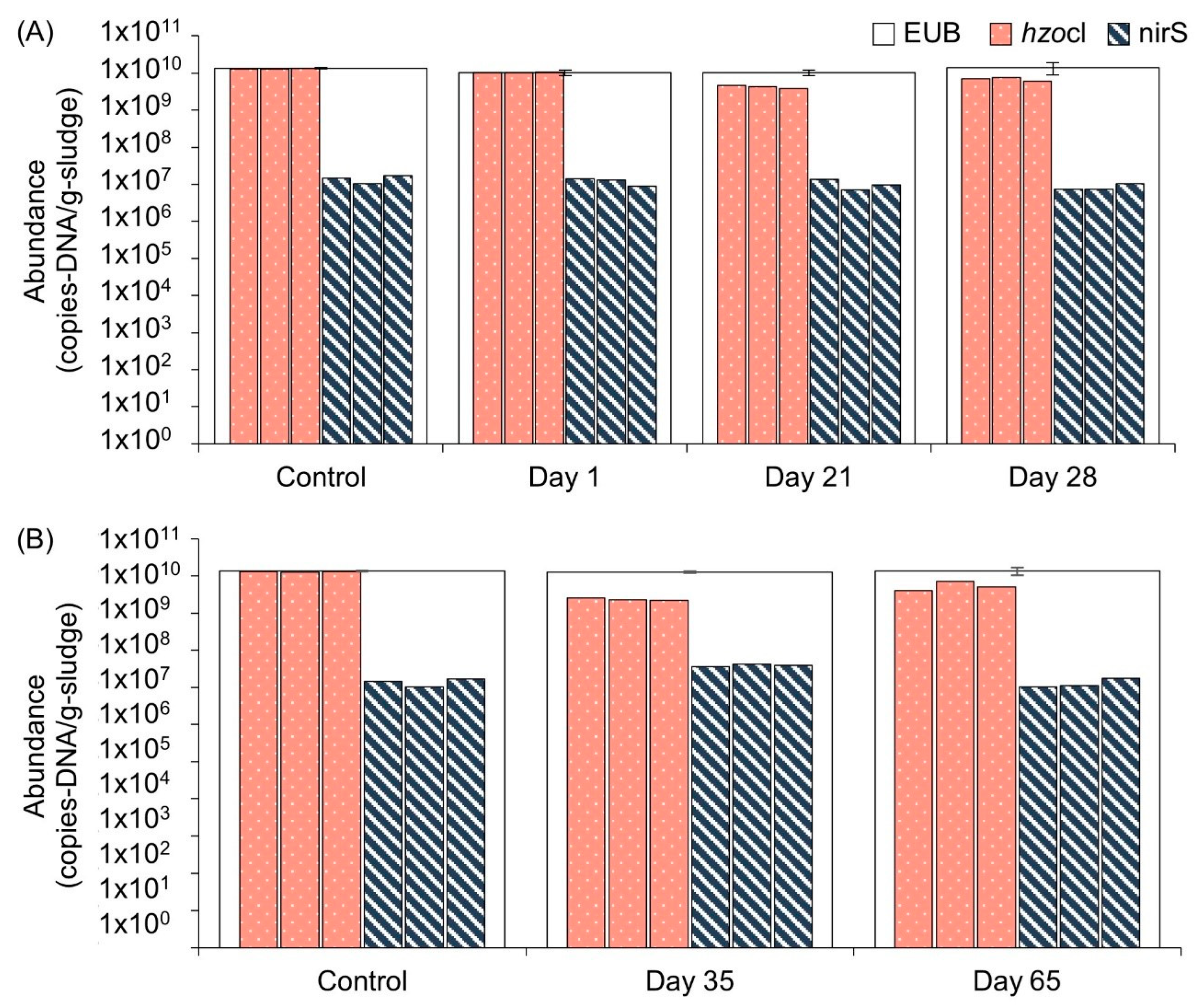
2.5.5. Quantitative Polymerase Chain Reaction (qPCR)
3. Results and Discussion
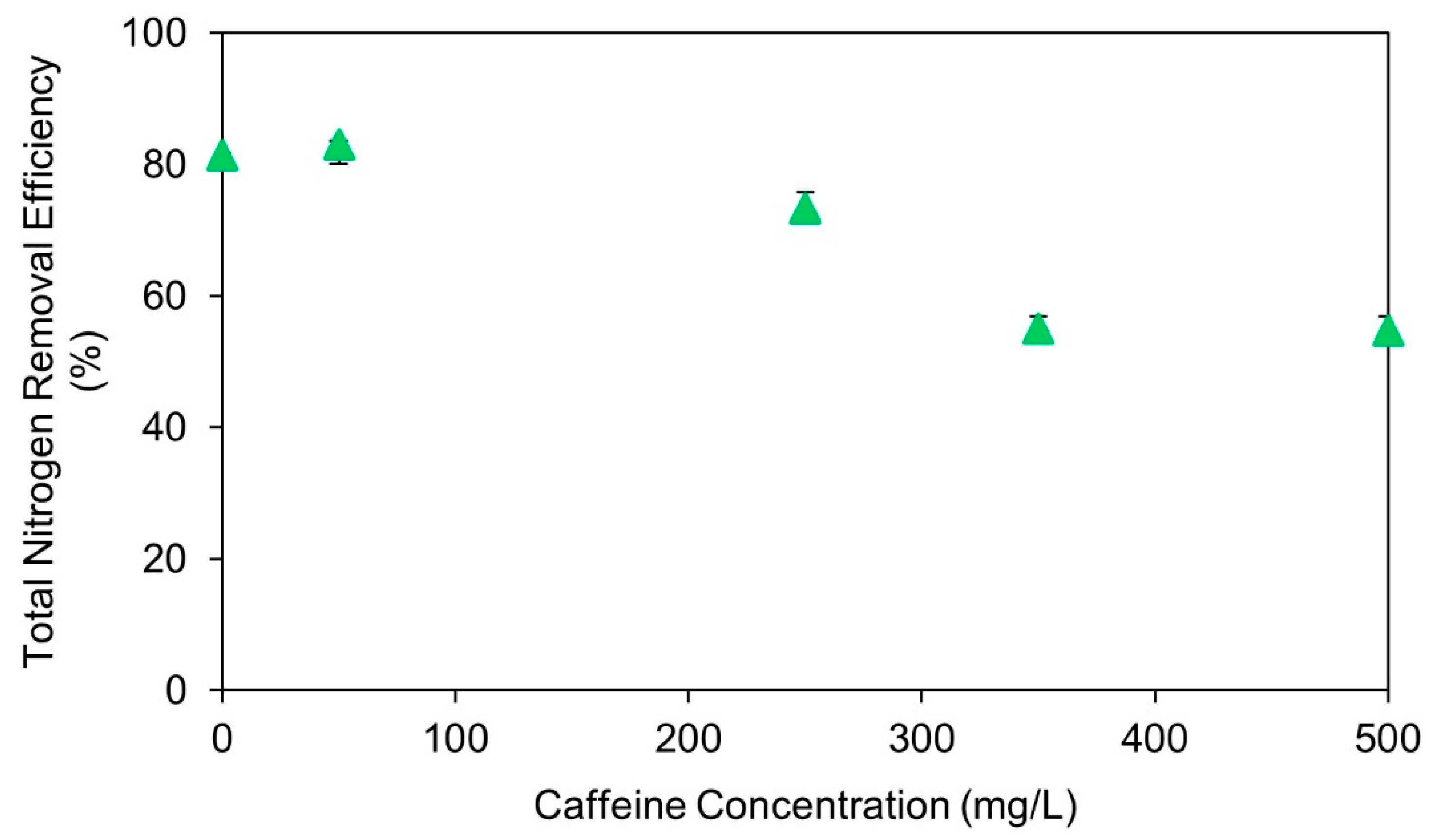
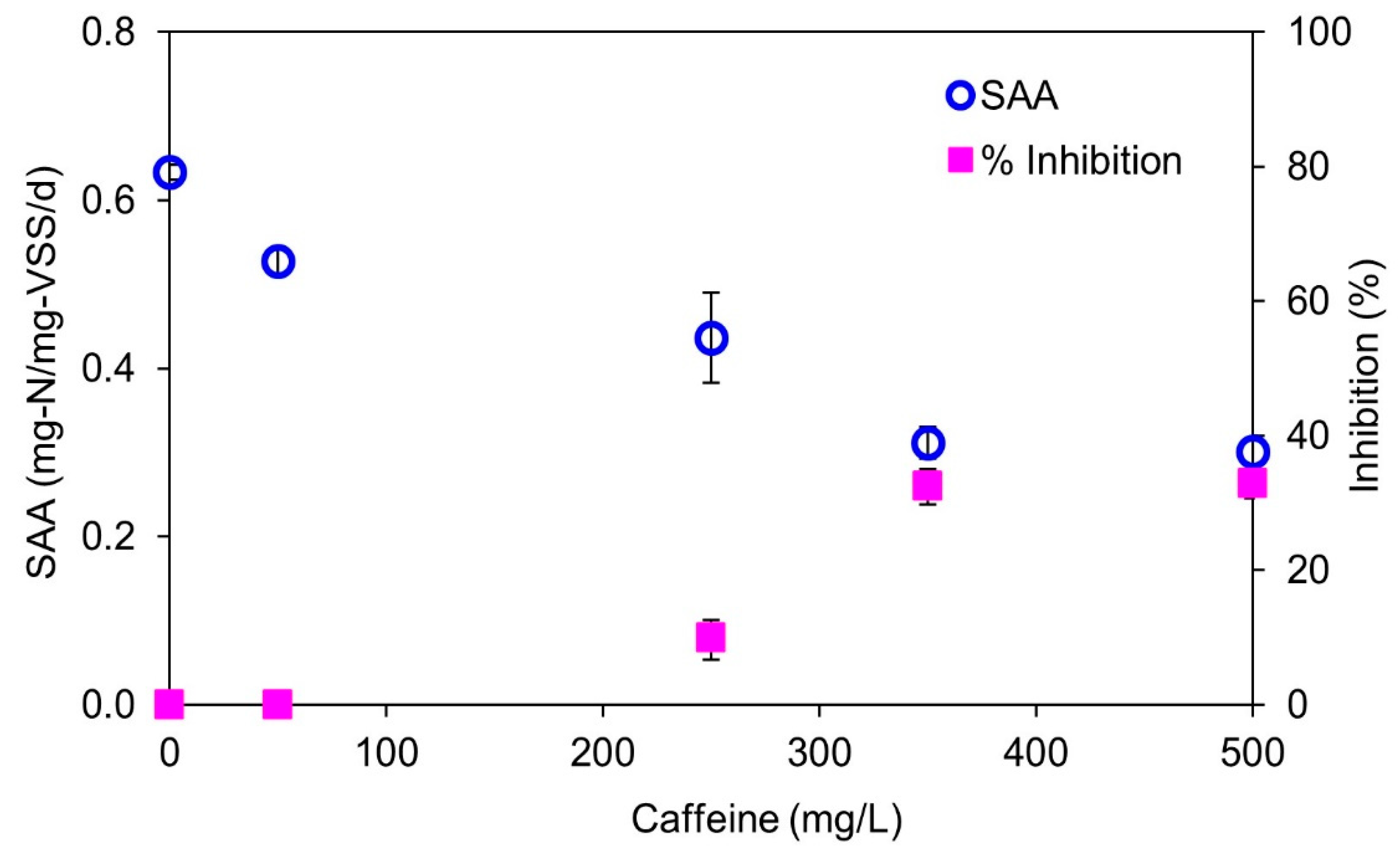
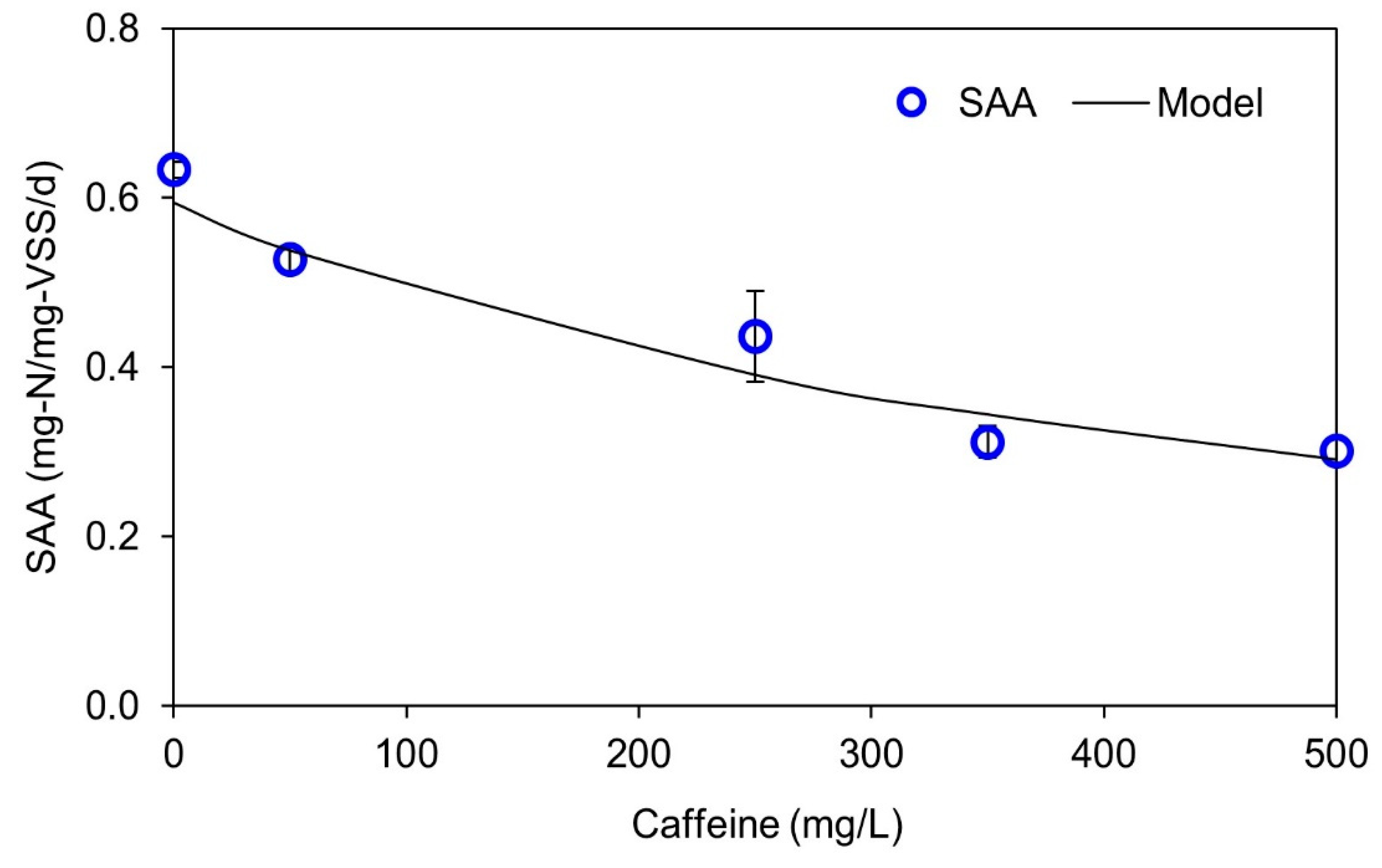
3.1. Acute Effects of Caffeine Dosing on Anammox Activity
3.2. Long-Term Effect of Caffeine Dosing on Anammox Activity
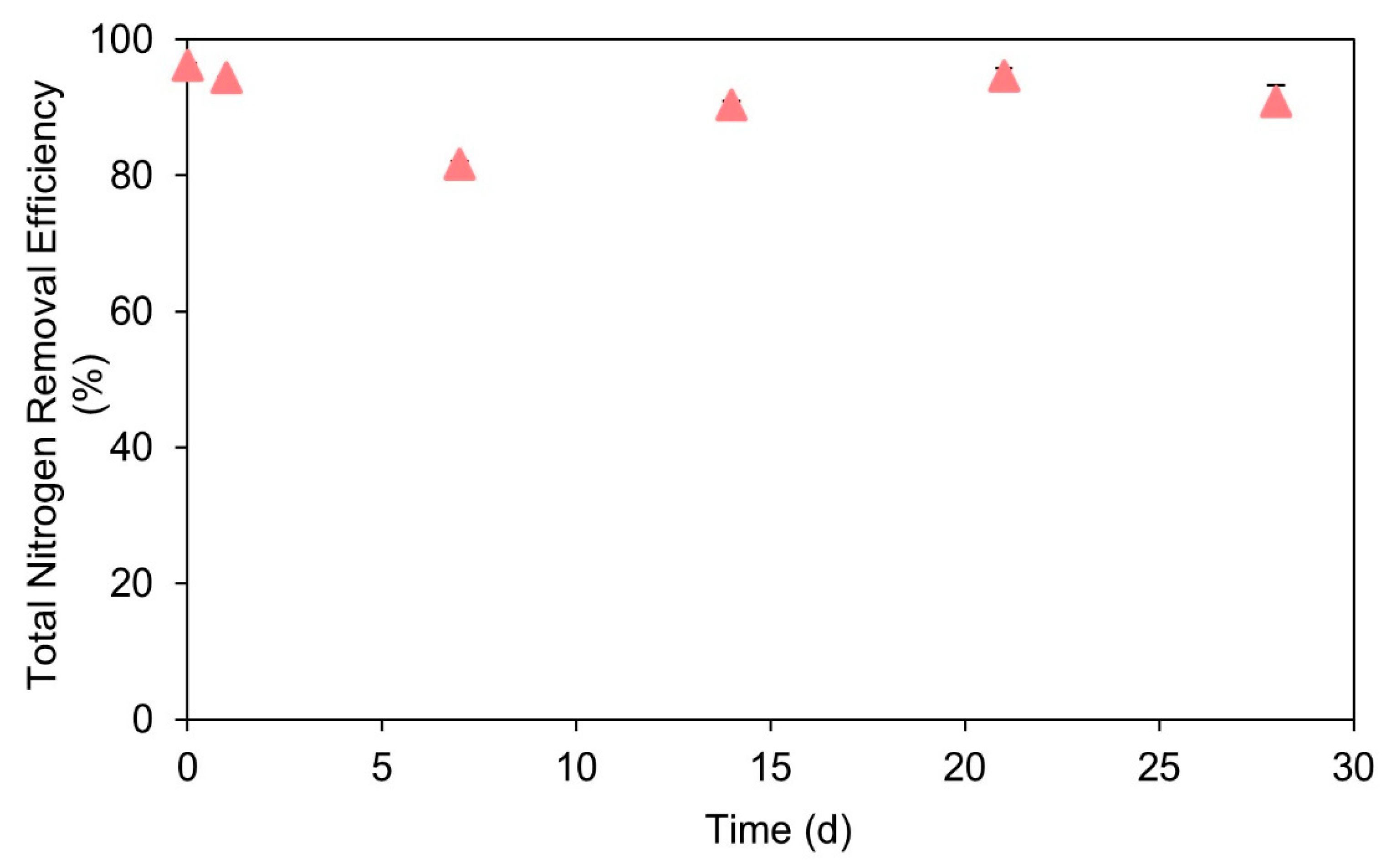
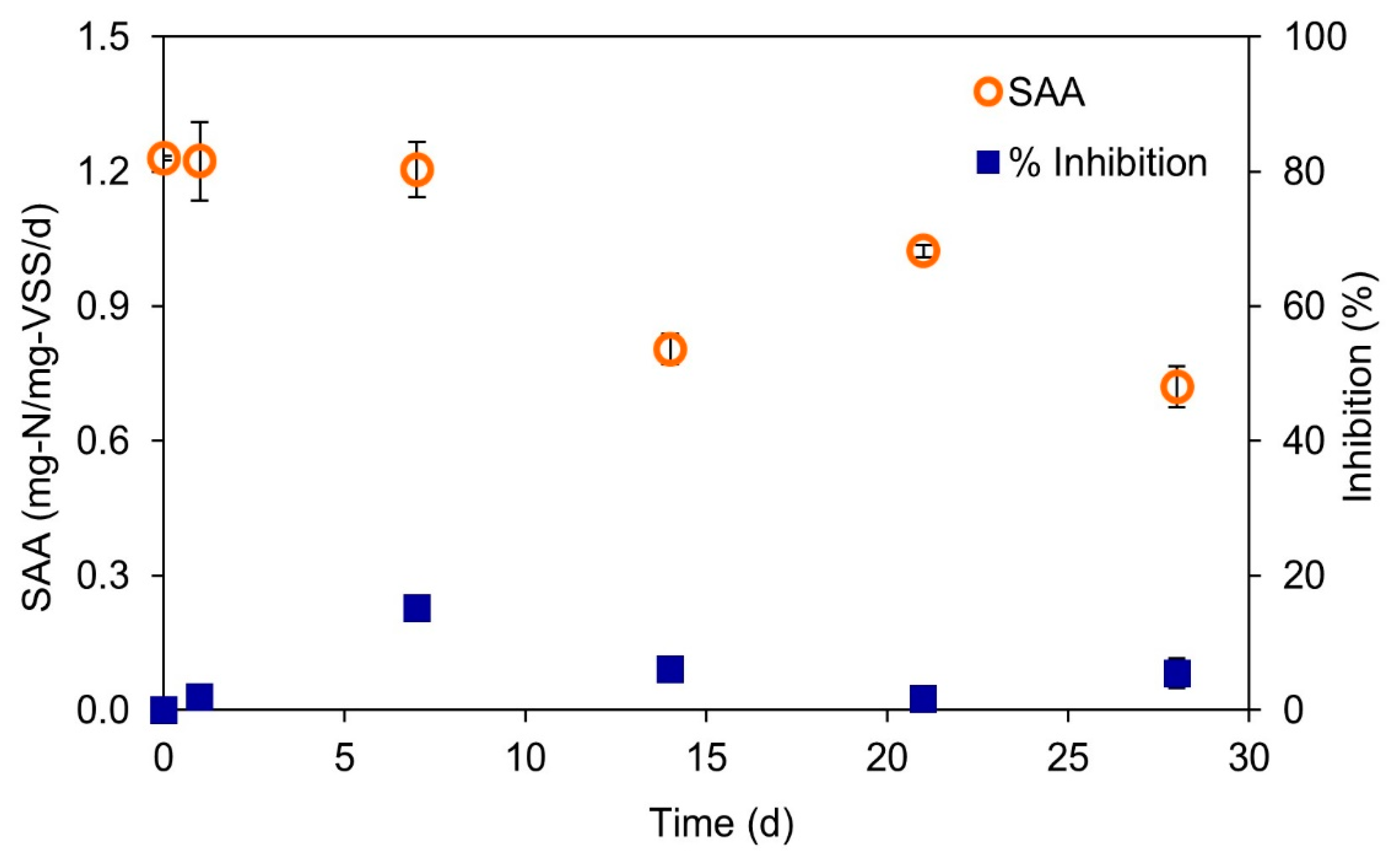
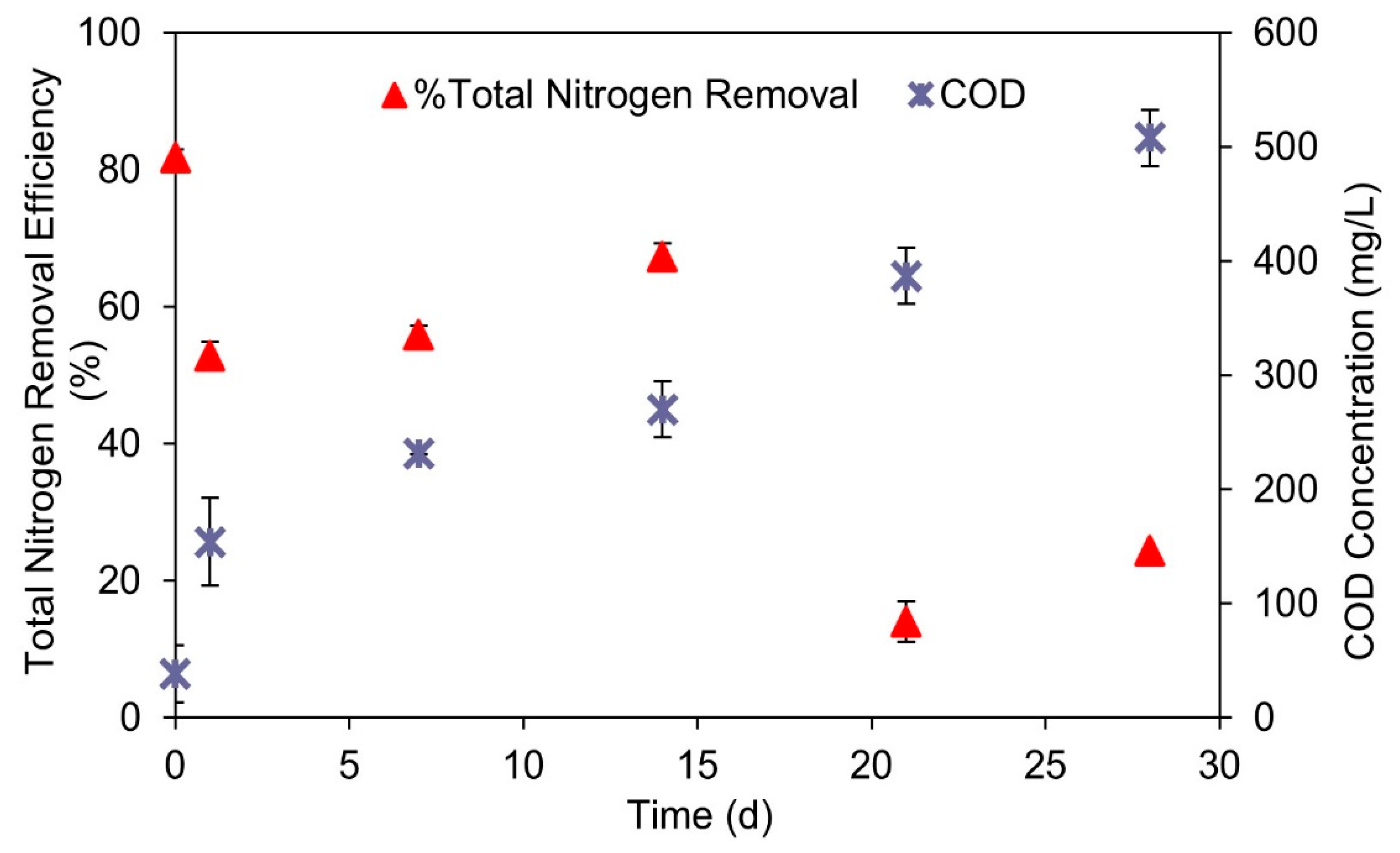
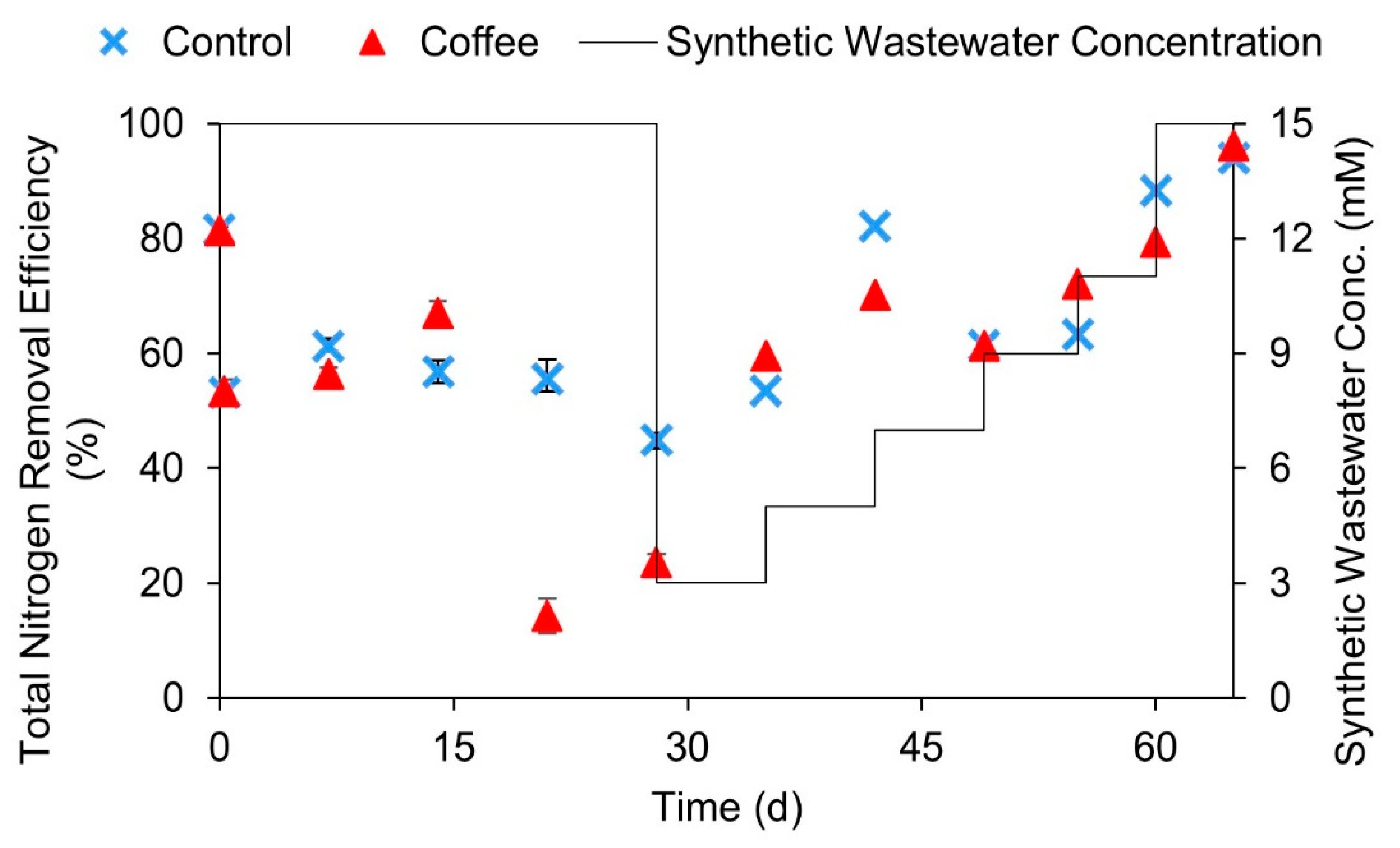
3.3. Treatment of Coffee Wastewater Using Anammox Process and the Recovery of Anammox Cultures after Discontinuance of Coffee Wastewater Additions
4. Conclusions
- The addition of caffeine in concentrations greater than 350 mg/L caffeine significantly inhibits anammox activity.
- There is no long-term effect with low caffeine concentration (2.5 mg/L) on anammox activity.
- The mechanism for caffeine inhibition on anammox bacteria could not be explained clearly because the not-competitive inhibition model could not distinguish between non- and un-competitive inhibition.
- The presence of ≥387 mg/L COD from coffee wastewater could significantly inhibit the anammox activity. However, this inhibition effect is reversible. After the discontinuance of coffee wastewater additions to a batch reactor, the anammox activity could recover in 28 days.
- In using an anammox treatment with real wastewater (coffee wastewater) as substrate, bCOD:TN should be used rather than TCOD:TN as the control parameter because bCOD and TCOD are not equal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, R.C.; Pinto, V.R.A.; Melo, L.F.; da Rocha, S.J.S.S.; Coimbra, J.S. New sustainable perspectives for “Coffee Wastewater” and other by-products: A critical review. Future Foods 2021, 4, 100058. [Google Scholar] [CrossRef]
- Von Enden, J.C.; Calvert, K.C.; Sanh, K.; Hoa, H.; Tri, Q.; Vietnam, S.; Consulting, C. Review of coffee waste water characteristics and approaches to treatment. In PPP Project, Improvement of Coffee Quality and Sustainability of Coffee Production in Vietnam; German Technical Cooperation Agency (GTZ): Bonn, Germany, 2002; pp. 1–10. [Google Scholar]
- Rattan, S.; Parande, A.; Nagaraju, V.; Ghiwari, G.K. A comprehensive review on utilization of wastewater from coffee processing. Environ. Sci. Pollut. Res. 2015, 22, 6461–6472. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Stadler, L.B.; Love, N.G.; Skerlos, S.J.; Raskin, L. Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: A critical review. Bioresour. Technol. 2012, 122, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Delgado Vela, J.; Stadler, L.B.; Martin, K.J.; Raskin, L.; Bott, C.B.; Love, N.G. Prospects for biological nitrogen removal from anaerobic effluents during mainstream wastewater treatment. Environ. Sci. Technol. Lett. 2015, 2, 234–244. [Google Scholar] [CrossRef]
- Ijanu, E.; Kamaruddin, M.; Norashiddin, F. Coffee processing wastewater treatment: A critical review on current treatment technologies with a proposed alternative. Appl. Water Sci. 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Peng, Y.; Liu, X.; Zeng, W.; Mino, T.; Satoh, H. Nitrogen removal via nitrite from municipal wastewater at low temperatures using real-time control to optimize nitrifying communities. Environ. Sci. Technol. 2007, 41, 8159–8164. [Google Scholar] [CrossRef]
- Law, Y.; Ye, L.; Pan, Y.; Yuan, Z. Nitrous oxide emissions from wastewater treatment processes. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1265–1277. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Hu, S.; Guo, J. Enhancing mainstream nitrogen removal by employing nitrate/nitrite-dependent anaerobic methane oxidation processes. Crit. Rev. Biotechnol. 2019, 39, 732–745. [Google Scholar] [CrossRef]
- Jetten, M.S.; Wagner, M.; Fuerst, J.; van Loosdrecht, M.; Kuenen, G.; Strous, M. Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Curr. Opin. Biotechnol. 2001, 12, 283–288. [Google Scholar] [CrossRef]
- Joss, A.; Salzgeber, D.; Eugster, J.; König, R.; Rottermann, K.; Burger, S.; Fabijan, P.; Leumann, S.; Mohn, J.; Siegrist, H. Full-scale nitrogen removal from digester liquid with partial nitritation and anammox in one SBR. Environ. Sci. Technol. 2009, 43, 5301–5306. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Peng, Y.; Li, B.; Wang, S. Novel two stage partial denitrification (PD)-Anammox process for tertiary nitrogen removal from low carbon/nitrogen (C/N) municipal sewage. Chem. Eng. J. 2019, 362, 107–115. [Google Scholar] [CrossRef]
- Kuenen, J.G. Anammox bacteria: From discovery to application. Nat. Rev. Microbiol. 2008, 6, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Lee, P.-H.; Kumar, M.; Huang, Y.-T.; Sung, S.; Lin, J.-G. Simultaneous partial nitrification, anaerobic ammonium oxidation and denitrification (SNAD) in a full-scale landfill-leachate treatment plant. J. Hazard. Mater. 2010, 175, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yang, Y.-C.; Lin, J.-G.; Denecke, M.; Gu, J.-D. Occurrence of anammox bacteria in a traditional full-scale wastewater treatment plant and successful inoculation for new establishment. Int. Biodeterior. Biodegrad. 2017, 120, 224–231. [Google Scholar] [CrossRef]
- Ramanavičienė, A.; Mostovojus, V.; Bachmatova, I.; Ramanavičius, A. Antibacterial effect of caffeine on Escherichia coli and Pseudomonas fluorescens. Acta Med. Litu. 2003, 10, 185–188. [Google Scholar]
- Dash, S.S.; Gummadi, S.N. Inhibitory effect of caffeine on growth of various bacterial strains. Res. J. Microbiol. 2008, 3, 457–465. [Google Scholar]
- Norizan, S.N.M.; Yin, W.-F.; Chan, K.-G. Caffeine as a potential quorum sensing inhibitor. Sensors 2013, 13, 5117–5129. [Google Scholar] [CrossRef]
- Esimone, C.; Okoye, F.; Nworu, C.; Agubata, C. In vitro interaction between caffeine and some penicillin antibiotics against Staphylococcus aureus. Trop. J. Pharm. Res. 2008, 7, 969–974. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Chatterjee, S. Radiomimetic property of furazolidone and the caffeine enhancement of its lethal action on the vibrios. Chem.-Biol. Interact. 1981, 37, 321–335. [Google Scholar] [CrossRef]
- Egli, K.; Fanger, U.; Alvarez, P.J.; Siegrist, H.; van der Meer, J.R.; Zehnder, A.J. Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch. Microbiol. 2001, 175, 198–207. [Google Scholar] [CrossRef]
- Van Dongen, U.; Jetten, M.S.; Van Loosdrecht, M. The SHARON®-Anammox® process for treatment of ammonium rich wastewater. Water Sci. Technol. 2001, 44, 153–160. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Motora, K.G.; Beyene, T.T. Determination of caffeine in raw and roasted coffee beans of ilu abba bora zone, South West Ethiopia. Indo Am. J. Pharm. Res. 2017, 7, 463–470. [Google Scholar]
- Zhou, J.; Bruns, M.A.; Tiedje, J.M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 1996, 62, 316–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, M.C.; Hooper, A.B.; Klotz, M.G.; Woebken, D.; Lam, P.; Kuypers, M.M.; Pommerening-Roeser, A.; Op Den Camp, H.J.; Jetten, M.S. Environmental detection of octahaem cytochrome c hydroxylamine/hydrazine oxidoreductase genes of aerobic and anaerobic ammonium-oxidizing bacteria. Environ. Microbiol. 2008, 10, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Zhang, Z.Z.; Sun, F.Q.; Shi, Z.J.; Huang, B.C.; Fan, N.S.; Jin, R.C. Insight into the short-and long-term effects of quinoline on anammox granules: Inhibition and acclimatization. Sci. Total Environ. 2019, 651, 1294–1301. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Cao, X.; Chen, J. Insights into simultaneous anammox and denitrification system with short-term pyridine exposure: Process capability, inhibition kinetics and metabolic pathways. Front. Environ. Sci. Eng. 2021, 15, 1–14. [Google Scholar] [CrossRef]
- Waki, M.; Tokutomi, T.; Yokoyama, H.; Tanaka, Y. Nitrogen removal from animal waste treatment water by anammox enrichment. Bioresour. Technol. 2007, 98, 2775–2780. [Google Scholar] [CrossRef]
- Fernández, I.; Dosta, J.; Fajardo, C.; Campos, J.; Mosquera-Corral, A.; Méndez, R. Short-and long-term effects of ammonium and nitrite on the Anammox process. J. Environ. Manag. 2012, 95, S170–S174. [Google Scholar] [CrossRef]
- Bettazzi, E.; Caffaz, S.; Vannini, C.; Lubello, C. Nitrite inhibition and intermediates effects on Anammox bacteria: A batch-scale experimental study. Process Biochem. 2010, 45, 573–580. [Google Scholar] [CrossRef]
- Kimura, Y.; Isaka, K.; Kazama, F.; Sumino, T. Effects of nitrite inhibition on anaerobic ammonium oxidation. Appl. Microbiol. Biotechnol. 2010, 86, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Dapena-Mora, A.; Campos, J.; Mosquera-Corral, A.; Jetten, M.; Méndez, R. Stability of the ANAMMOX process in a gas-lift reactor and a SBR. J. Biotechnol. 2004, 110, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Chamchoi, N.; Nitisoravut, S.; Schmidt, J.E. Inactivation of ANAMMOX communities under concurrent operation of anaerobic ammonium oxidation (ANAMMOX) and denitrification. Bioresour. Technol. 2008, 99, 3331–3336. [Google Scholar] [CrossRef]
- Leal, C.D.; Pereira, A.D.; Nunes, F.T.; Ferreira, L.O.; Coelho, A.C.C.; Bicalho, S.K.; Mac Conell, E.F.A.; Ribeiro, T.B.; de Lemos Chernicharo, C.A.; de Araújo, J.C. Anammox for nitrogen removal from anaerobically pre-treated municipal wastewater: Effect of COD/N ratios on process performance and bacterial community structure. Bioresour. Technol. 2016, 211, 257–266. [Google Scholar] [CrossRef]
- He, S.; Yang, W.; Qin, M.; Mao, Z.; Niu, Q.; Han, M. Performance and microbial community of anammox in presence of micro-molecule carbon source. Chemosphere 2018, 205, 545–552. [Google Scholar] [CrossRef]
- Wang, X.; Yang, R.; Guo, Y.; Zhang, Z.; Kao, C.M.; Chen, S. Investigation of COD and COD/N ratio for the dominance of anammox pathway for nitrogen removal via isotope labelling technique and the relevant bacteria. J. Hazard. Mater. 2019, 366, 606–614. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| pH | 7.75 ± 0.11 |
| Total Nitrogen (mg N/L) | 471.67 ± 3.94 |
| Ammonium (mg N/L) | 209.44 ± 6.16 |
| Nitrite (mg N/L) | 262.23 ± 5.06 |
| Parameter | Value |
|---|---|
| pH | 4.21 |
| COD (mg/L) | 387.05 |
| bCOD (mg/L) | 120.23 |
| Caffeine (mg/L) | 3.12 |
| Target Gene | Primer | Sequence (5′-3′) | References |
|---|---|---|---|
| EUB (Total bacteria) | 338F 518R | ACTCCTACGGGAGGCAGCAG ATTACCGCGGCTGCTGG | Muyzer et al. [26] |
| hzo (Anammox bacteria) | hzocl1F1 hzocl1R2 | TGYAAGACYTGYCAYTGG ACTCCAGATRTGCTGACC | Schmid et al. [27] |
| nirS (Denitrifying bacteria, DNB) | Cd3aF R3cd | GTSAACGTSAAGGARACSGG GASTTCGGRTGSGTCTTGA | Throback et al. [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongphoom, T.; Saleepochn, T.; Noophan, P.L.; Li, C.-W. Effects of Caffeine and COD from Coffee Wastewater on Anaerobic Ammonium Oxidation (Anammox) Activities. Water 2022, 14, 2238. https://doi.org/10.3390/w14142238
Wongphoom T, Saleepochn T, Noophan PL, Li C-W. Effects of Caffeine and COD from Coffee Wastewater on Anaerobic Ammonium Oxidation (Anammox) Activities. Water. 2022; 14(14):2238. https://doi.org/10.3390/w14142238
Chicago/Turabian StyleWongphoom, Titima, Tharinee Saleepochn, Pongsak Lek Noophan, and Chi-Wang Li. 2022. "Effects of Caffeine and COD from Coffee Wastewater on Anaerobic Ammonium Oxidation (Anammox) Activities" Water 14, no. 14: 2238. https://doi.org/10.3390/w14142238







