Fish Species Composition, Distribution and Community Structure in Relation to Environmental Variation in a Semi-Arid Mountainous River Basin, Iran
Abstract
:1. Introduction
2. Material and Methods
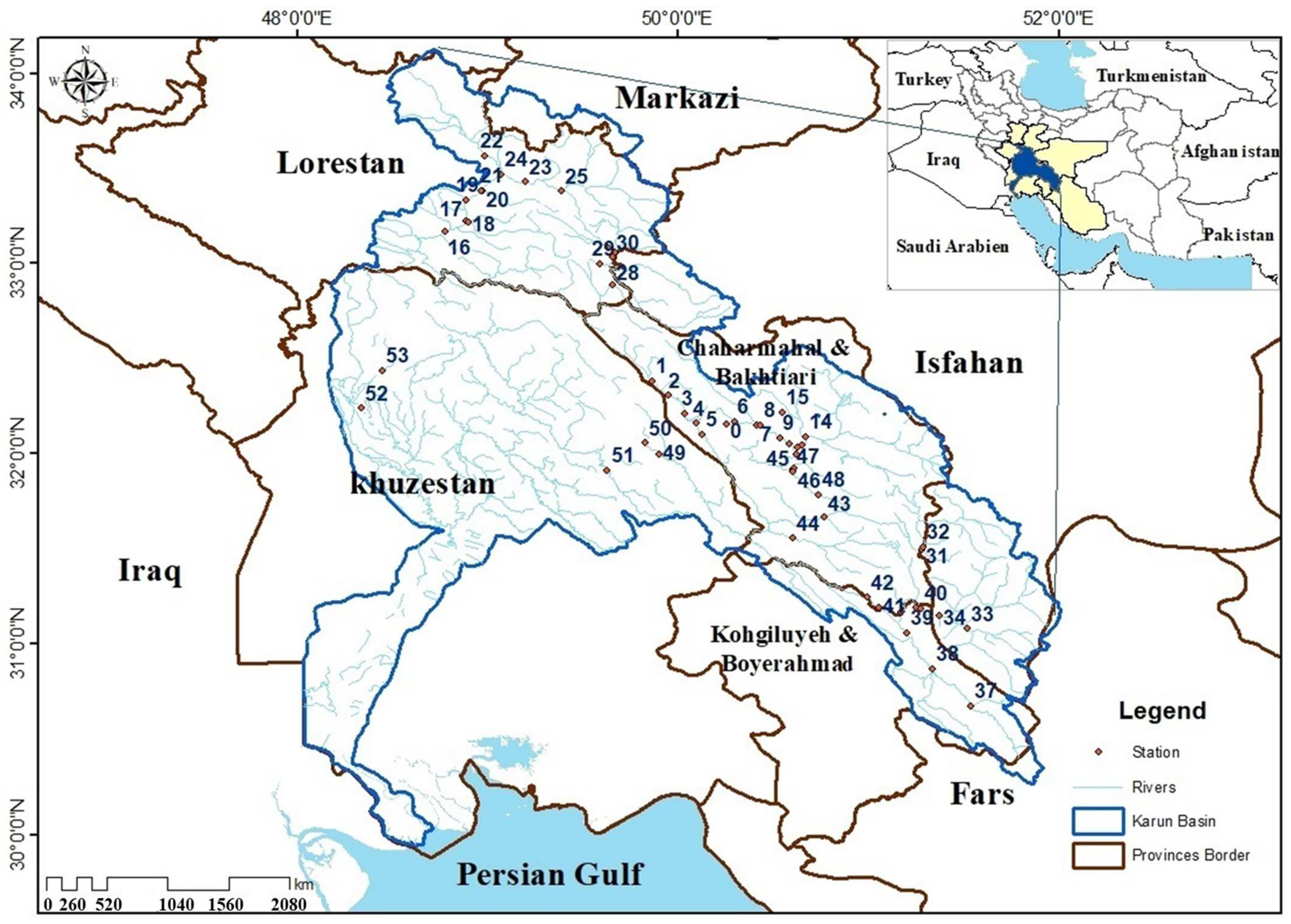
2.1. Study Area
2.2. Field Sampling
2.3. Physico-Chemical and Habitat Parameters
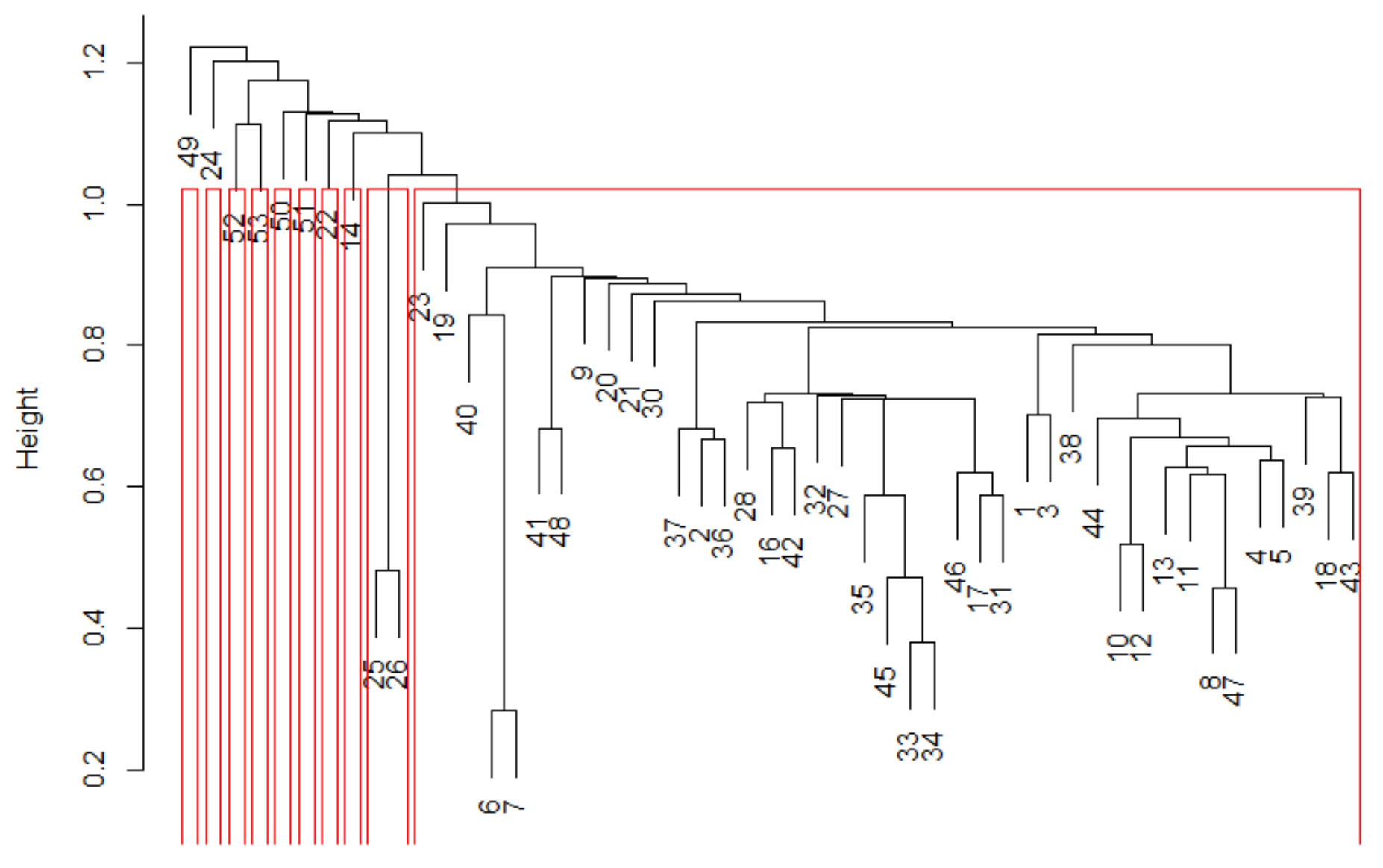
2.4. Data Analysis
3. Results
3.1. Species Composition
3.2. Species Distribution in the Karun River Basin
3.3. Spatial Variation in Fish Composition
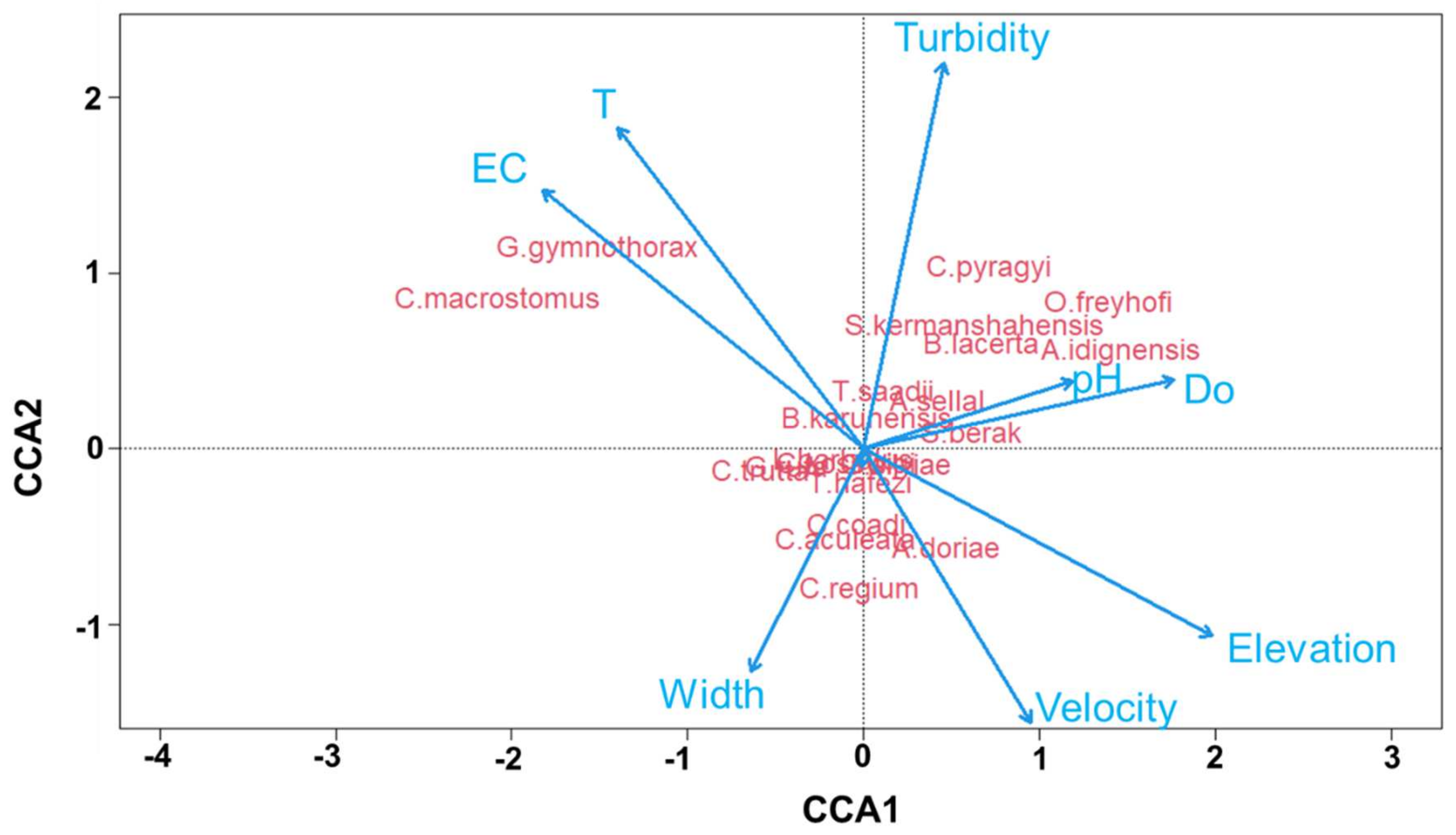
3.4. Environmental Variables
4. Discussion
4.1. Environmental Parameters
4.2. Fish Community Structure and Diversity
4.3. Current Threats to Fish Communities in the Karun River Basin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. The fish species presence/absence status in the Karun River basin
| Family | Cyprinidae | Leuciscidae | Xenocyprididae | Nemacheilidae | Sisoridae | Mugilidae | Cyprinodontidae | Mastacembelidae | Salmonidae | Oxudercidae | Gobionidae | Poeciliidae | |||||||||||||||||||||||||
| Subfamily | Cyprininae | Labeoninae | Barbinae | - | Leuciscinae | Alburninae | Cultrinae | Squaliobarbinae | - | Glyptosterninae | - | Cyprinodontina | - | - | Gobionellinae | Gobioninae | - | ||||||||||||||||||||
| Site | Capoeta coadi | Capoeta aculeata | Capoeta pyragyi | Capoeta trutta | Carassius gibelio | Arabibarbus grypus | Cyprinus carpio | Garra rufa | Garra gymnothorax | Barbus lacerta | Barbus karunensis | Luciobarbus barbulus | Carasobarbus luteus | Carasobarbus kosswigi | Cyprion macrostomus | Chondrostoma regium | Squalius berak | Squalius lepidus | Acanthobrama marmid | Alburnus sellal | Alburnus caeruleus | Alburnus doriae | Alburnoides idignesis | Hemiculter leucisculus | Ctenopharyngodon idella | Sasanidus kermanshahensis | Turcinoemacheilus saadii | Turcinoemacheilus hafezi | Oxynoemacheilus freyhofi | Glyptothorax silviae | PlaniPlaniliza abu | Aphanius vladykovi | Mastacembelus mastacembelus | Oncorhynchus mykiss | Rhinogobius lindbergi | Pseudorasbora parva | Gambusia holbrooki |
| S0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S1 | + | + | - | - | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - |
| S2 | + | + | - | - | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - |
| S3 | + | + | - | + | - | - | - | + | + | - | - | + | - | + | + | + | + | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - |
| S4 | + | + | - | + | - | - | - | + | + | - | - | + | - | - | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S5 | + | + | - | + | - | - | - | + | - | - | - | - | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S6 | + | + | - | - | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - |
| S7 | + | + | - | - | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - |
| S8 | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - |
| S9 | + | + | - | - | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S10 | + | - | - | - | - | - | - | + | + | - | - | - | - | - | - | + | - | - | - | + | - | + | - | - | - | - | + | + | - | + | - | - | - | - | - | - | - |
| S11 | + | + | - | - | - | - | - | + | - | + | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - |
| S12 | + | + | - | - | - | - | - | + | - | - | + | - | - | - | - | + | - | - | - | + | - | + | - | - | - | - | + | + | - | + | - | - | - | - | - | - | - |
| S13 | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S14 | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - |
| S15 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S16 | - | - | + | + | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
| S17 | - | - | + | - | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
| S18 | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S19 | + | - | - | + | - | - | + | + | + | - | + | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | + | + | - | - | - | - | - | - | - | - | - |
| S20 | - | - | + | - | - | - | - | + | + | + | + | - | - | - | - | + | - | - | - | + | - | + | + | - | - | + | + | + | - | + | - | - | - | - | - | - | - |
| S21 | - | - | + | - | - | - | - | + | - | + | + | - | - | - | - | + | - | - | - | + | - | - | + | - | - | + | - | - | - | + | - | - | - | - | - | - | - |
| S22 | - | - | + | - | + | - | - | + | + | + | - | - | - | - | + | - | + | - | - | + | - | - | - | - | - | - | - | + | + | + | + | - | - | - | + | + | + |
| S23 | - | - | + | - | - | - | - | + | + | + | - | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | + | - | - | - |
| S24 | - | - | + | - | + | - | - | - | + | + | - | - | - | - | - | - | + | - | - | - | - | - | + | - | + | - | + | + | - | - | - | - | - | - | - | - | - |
| S25 | - | - | + | - | - | - | - | - | - | + | - | - | - | - | - | - | + | - | - | + | - | - | + | - | - | + | - | - | + | + | - | - | - | - | - | - | - |
| S26 | - | - | + | - | - | - | - | - | - | + | - | - | - | - | - | - | + | - | - | + | - | - | + | - | - | + | - | - | + | + | - | - | - | - | - | - | - |
| S27 | - | - | + | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - |
| S28 | - | - | + | - | - | - | - | + | - | + | - | + | - | - | - | - | - | - | - | + | - | - | + | - | - | + | - | - | + | + | - | - | - | - | - | - | - |
| S29 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S30 | - | - | + | - | - | - | - | - | - | + | - | - | - | - | - | - | - | + | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S31 | + | + | + | - | - | - | - | + | + | + | - | + | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
| S32 | + | + | - | - | - | - | - | + | + | - | - | + | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
| S33 | + | + | - | - | - | - | - | + | + | - | + | + | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - | - | - |
| S34 | + | + | - | - | - | - | - | + | - | - | + | + | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - | - | - |
| S35 | + | + | - | - | - | - | - | - | - | - | + | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
| S36 | + | + | - | + | - | - | - | + | + | - | - | - | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - | - | - |
| S37 | + | + | - | + | - | - | - | + | - | - | - | - | - | - | + | + | - | - | - | + | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - |
| S38 | + | + | - | - | - | - | - | + | + | - | + | - | - | - | + | + | + | - | - | + | - | - | - | - | - | - | + | + | - | + | - | - | - | - | - | - | - |
| S39 | + | + | - | + | - | - | - | + | - | - | - | + | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
| S40 | + | + | - | - | - | - | - | + | - | - | + | + | - | + | - | - | + | - | - | + | - | - | - | - | - | - | + | - | - | - | - | - | - | + | - | - | - |
| S41 | + | - | - | + | - | - | - | + | + | - | - | + | - | + | - | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S42 | - | - | - | + | + | - | - | - | + | - | - | + | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S43 | + | + | - | + | - | - | - | + | + | - | - | + | - | + | + | + | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - |
| S44 | + | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - |
| S45 | + | + | - | + | - | - | - | + | - | + | - | - | - | - | - | + | + | - | - | + | - | - | - | - | - | - | + | - | - | + | - | - | - | - | - | - | - |
| S46 | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
| S47 | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S48 | + | + | - | + | - | - | - | + | - | - | - | + | - | + | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S49 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S50 | - | - | - | - | - | - | - | + | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - |
| S51 | - | - | - | + | - | + | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | + | - | - | - | - |
| S52 | - | - | - | + | + | - | - | + | - | - | - | - | + | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - |
| S53 | - | - | - | - | + | - | + | - | - | - | - | - | + | - | + | + | - | - | + | - | + | - | - | + | - | - | - | - | - | - | + | - | - | - | - | - | - |
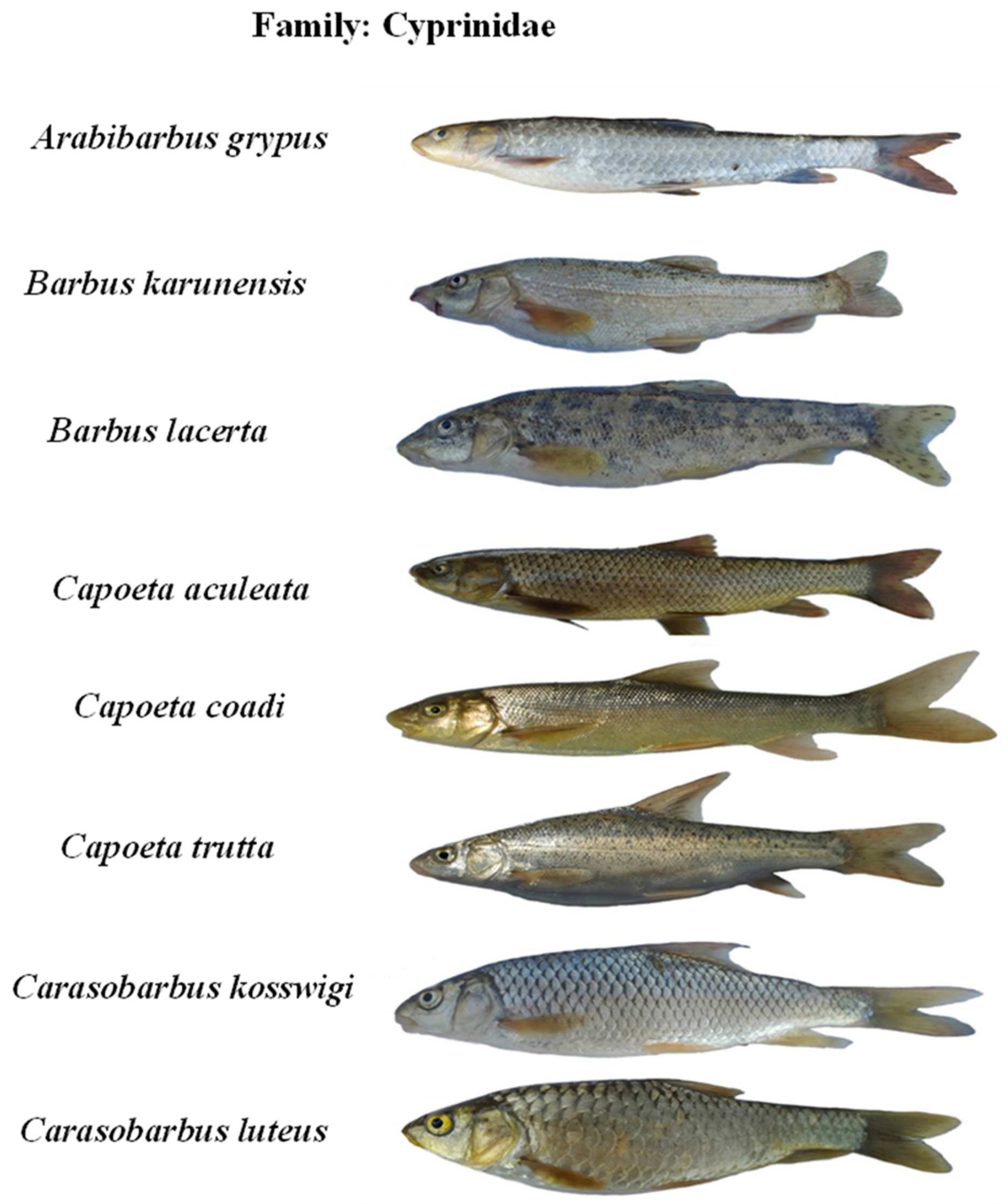
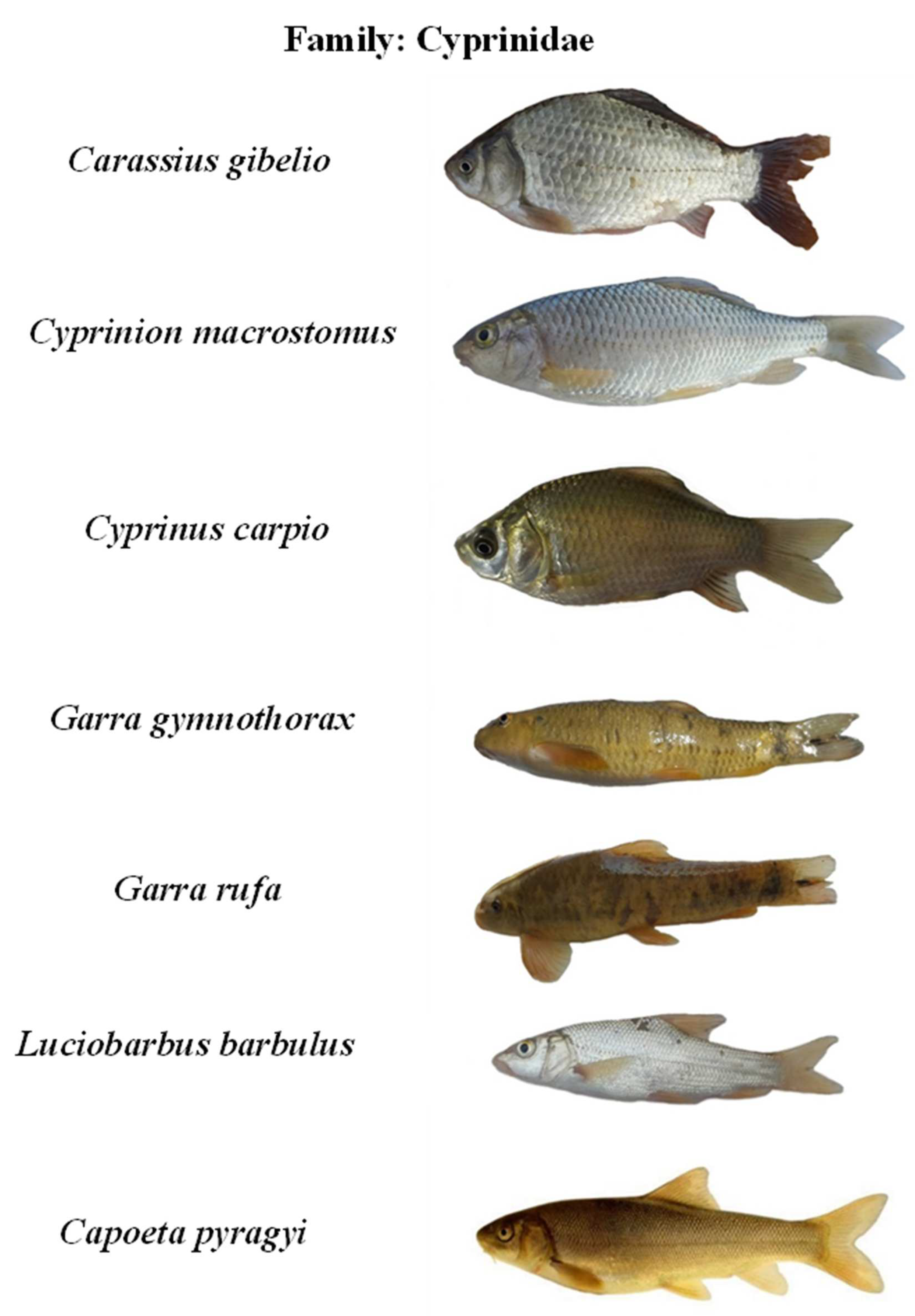
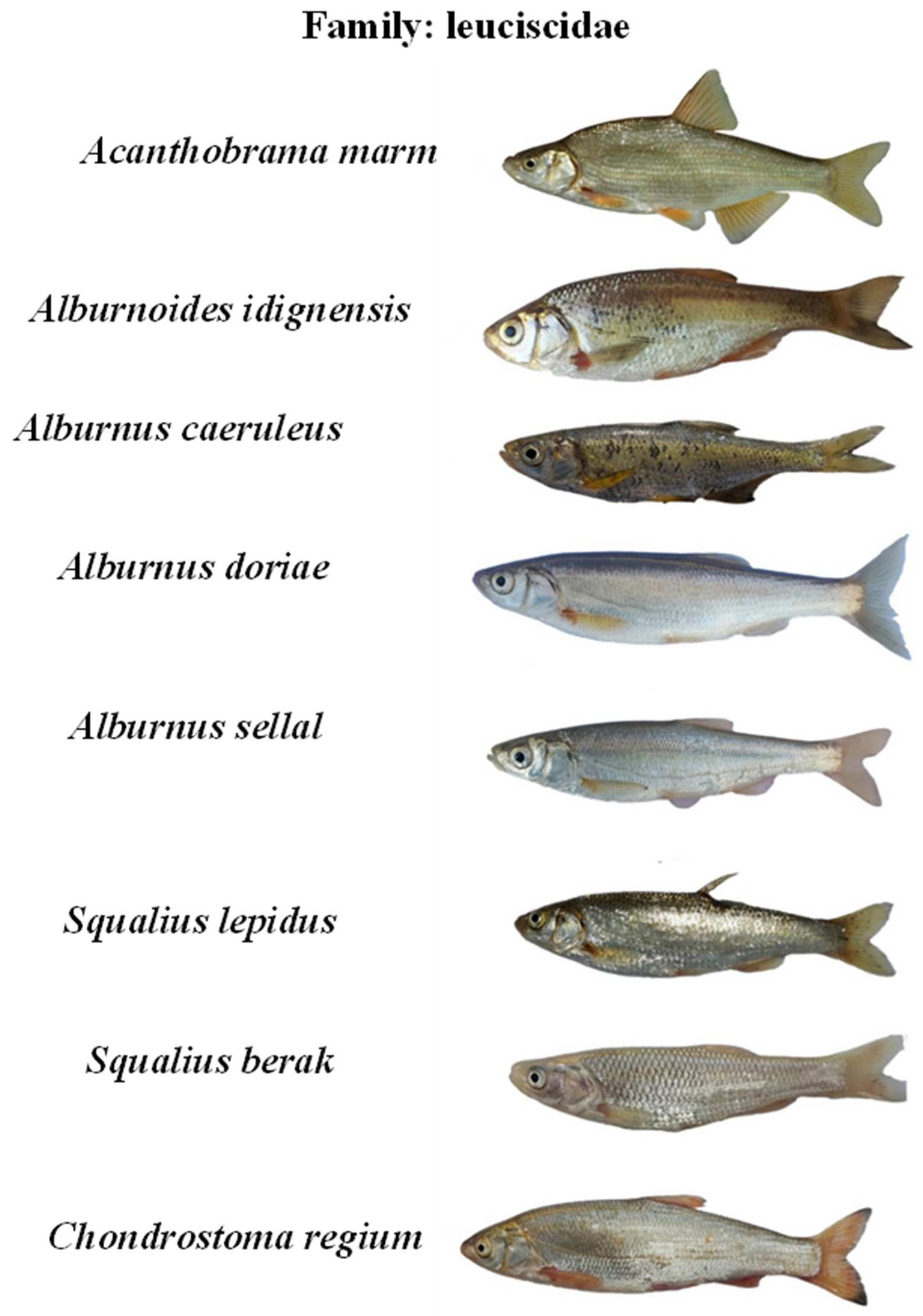
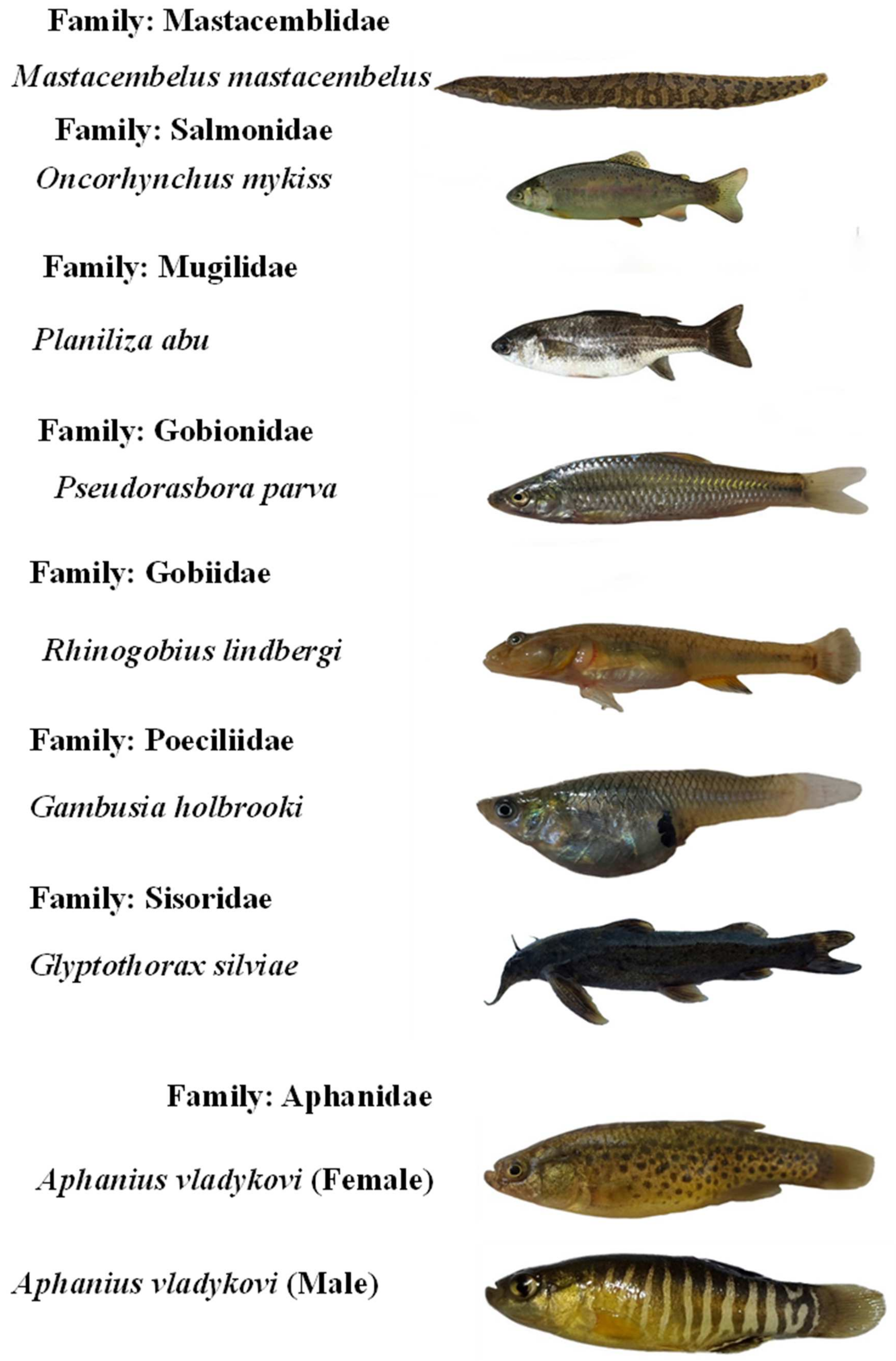
Appendix B. The photos of all recorded fish species in the Karun River basin, Iran

References
- Cianfaglione, K. Plant Landscape and Models of French Atlantic Estuarine Systems, Extended Summary of the Doctoral Thesis. Transylv. Rev. of Syst. and Ecol. Res. 2021, 1, 15–36. [Google Scholar] [CrossRef]
- Fang, Y.; Jawitz, J.W. The Evolution of Human Population Distance to Water in the USA from 1790 to 2010. Nat. Commun. 2019, 10, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marić, S.; Stanković, D.; Wazenbök, J.; Šanda, R.; Erös, T.; Takács, A.; Specziár, A.; Sekulić, A. Phylogeography and population genetics of the European mudminnow (Umbra krameri) with a time-calibrated phylogeny for the family Umbridae. Hidrobiologia 2017, 792, 151–168. [Google Scholar] [CrossRef] [Green Version]
- Bănăduc, D.; Rey, S.; Trichkova, T.; Lenhardt, M.; Curtean-Bănăduc, A. The Lower Danube River–Danube Delta–North West Black Sea: A Pivotal Area of Major Interest for the Past, Present and Future of Its Fish Fauna—A Short Review. Sci. Total Environ. 2016, 545–546, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Bănăduc, D.; Joy, M.; Olosutean, H.; Afanasyev, S.; Curtean-Bănăduc, A. Natural and Anthropogenic Driving Forces as Key Elements in the Lower Danube Basin–South-Eastern Carpathians–North-Western Black Sea Coast Area Lakes: A Broken Stepping Stones for Fish in a Climatic Change Scenario? Environ. Sci. Eur. 2020, 32, 73. [Google Scholar] [CrossRef]
- Caleta, M.; Marcic, Z.; Buj, I.; Zanella, D.; Mustafic, P.; Duplic, A.; Horvatic, S. A Review of Extant Croatian Freshwater Fish and Lampreys: Annotated List and Distribution. Ribar. Croat. J. Fish. 2019, 77, 137–234. [Google Scholar] [CrossRef] [Green Version]
- Coad, B.W. Zoogeography of the Fishes of the Tigris-Euphrates Basin. Zool. Middle East 1996, 13, 51–70. [Google Scholar] [CrossRef]
- Ghadiri, H.; Afkhami, M. Pollution of Karun-Arvand Rood River System in Iran. In Proceedings of the The First International Conference on Environmental Science and Technology, New Orleans, LA, USA, 23–26 January 2005; American Science Press: New Orleans, LA, USA, 2005. [Google Scholar]
- Khoshnood, Z.; Khoshnood, R. Effect of Industrial Wastewater on Fish in Karoon River. Transylv. Rev. Syst. Ecol. Res. 2016, 17, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Woodbridge, K.P.; Parsons, D.R.; Heyvaert, V.M.; Walstra, J.; Frostick, L.E. Characteristics of Direct Human Impacts on the Rivers Karun and Dez in Lowland South-West Iran and Their Interactions with Earth Surface Movements. Quat. Int. 2016, 392, 315–334. [Google Scholar] [CrossRef]
- Alwan, N.H.; Zareian, H.; Esmaeili, H.R. Capoeta Coadi, a New Species of Cyprinid Fish from the Karun River Drainage, Iran Based on Morphological and Molecular Evidences (Teleostei, Cyprinidae). Zookeys 2016, 572, 155–180. [Google Scholar]
- Coad, B.W. Freshwater Fishes of Iran. Available online: http://www.briancoad.com/species%20accounts/Contents%20new.htm (accessed on 1 October 2020).
- Esmaeili, H.R.; Coad, B.W.; Gholamifard, A.; Nazari, N.; Teimori, A. Annotated Checklist of the Freshwater Fishes of Iran. Zoosystematica Ross. 2010, 19, 361–386. [Google Scholar] [CrossRef]
- Jouladeh-Roudbar, A.; Ghanavi, H.R.; Doadrio, I. Ichthyofauna from Iranian Freshwater: Annotated Checklist, Diagnosis, Taxonomy, Distribution and Conservation Assessment. Zool. Stud. 2020, 59, e21. [Google Scholar] [CrossRef] [PubMed]
- Zareian, H.; Esmaeili, H.R.; Nejad, R.Z.; Vatandoust, S. Hemiculter Leucisculus (Basilewsky, 1855) and Alburnus Caeruleus Heckel, 1843: New Data on Their Distributions in Iran. Casp. J. Environ. Sci. 2015, 13, 11–20. [Google Scholar]
- Durand, J.D.; Tsigenopoulos, C.S.; Unlu, E.; Berrebi, P. Phylogeny and Biogeography of the Family Cyprinidae in the Middle East Inferred from Cytochrome b DNA—Evolutionary Significance of This Region. Mol. Phylogenet. Evol. 2002, 22, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Krupp, F.; Al-Jumaily, M.; Bariche, M.; Khalaf, M.; Malek, M.; Streit, B. The Middle Eastern Biodiversity Network: Generating and Sharing Knowledge for Ecosystem Management and Conservation. Zookeys 2009, 31, 3–15. [Google Scholar] [CrossRef]
- Kamalifar, R.; Amiri-Moghaddam, J.; Maniei, F. Induction of Spawning in Capoeta Aculeata, (Valenciennes in Cuv. & Val., 1844) (Teleostei, Cyprinidae), Using Carp Pituitary Extract. Int. J. Bioflux Soc. 2009, 1, 6. [Google Scholar]
- Teimori, A.; Esmaeili, H.R.; Gholamhosseini, A. The Ichthyofauna of Kor and Helleh River Basins in Southwest of Iran with Reference to Taxonomic and Zoogeographic Features of Native Fishes. Iran. J. Anim. Biosyst. 2010, 6, 1–8. [Google Scholar]
- Sabbaghi, M.; Masihi, S. Valuation of the Water Pollution in Karun River (Case Study of Ahvaz City). Aust. J. Basic Appl. Sci. 2012, 6, 25–34. [Google Scholar]
- Hosseini-Zare, N.; Gholami, A.; Panahpour, E.; Jafarnejadi, A. Pollution Load Assessment in the Soil and Water Resources: A Case Study in Karun River Drainage Basin, Southwest of Iran. Eur. Online J. Nat. Soc. Sci. 2014, 3, 427–434. [Google Scholar]
- Maktabi, P.; Javaheri Baboli, M.; Jafarnejadi, A.R.; Askary Sary, A. Mercury Concentrations in Common Carp (Cyprinus Carpio) Tissues, Sediment and Water from Fish Farm along the Karoun River in Iran. Vet. Res. Forum 2015, 6, 217–221. [Google Scholar]
- Lawrence, A.J.; Hemingway, K.L. Effects of Pollution on Fish: Molecular Effects and Population Responses; Wiley-Blackwell: Hoboken, NJ, USA, 2003. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols For Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates, and Fish, 2nd ed.; Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999.
- Keivany, Y.; Nasri, M.; Abbasi, K.; Abdoli, A. Atlas of Inland Water Fishes of Iran; Iran Department of Environment: Tehran, Iran, 2016. [Google Scholar]
- Froese, R.; Pauly, D. Fish Base. Available online: https://fishbase.in/search.php (accessed on 1 December 2019).
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L.K. Food Habits of Albacore, Bluefin Tuna, and Bonito in California Waters; Department of Fish and Game: Sacramento, CA, USA, 1971.
- Peet, R.K. The Measurement of Species Diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988. [Google Scholar]
- R Core team. R: A Language and Environment for Statistical Computing 2020. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 1 June 2020).
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of Size and Temperature on Metabolic Rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IUCN. Red Book of Threatened Fishes of Bangladesh; IUCN: Dhaka, Bangladesh, 2013. [Google Scholar]
- Yağcı, A.; Yağcı, M.A.; Bilgin, F.; Erbatur, İ. The Effects of Physicochemical Parameters on Fish Distribution in Egirdir Lake, Turkey. Iran. J. Fish. Sci. 2016, 15, 846–857. [Google Scholar]
- Fialho, A.P.; Oliveira, L.G.; Tejerina-Garro, F.L.; De Mérona, B. Fish-Habitat Relationship in a Tropical River under Anthropogenic Influences. Hydrobiologia 2008, 598, 315–324. [Google Scholar] [CrossRef]
- Islam, M.A.; Siddik, M.A.B.; Hanif, M.A.; Chaklader, M.R.; Nahar, A.; Ilham, I. Length-Weight Relationships of Four Small Indigenous Fish Species from an Inland Artisanal Fishery, Bangladesh. J. Appl. Ichthyol. 2017, 33, 851–852. [Google Scholar] [CrossRef]
- Paujiah, E.; Solihin, D.D.; Affandi, R. Community Structure of Fish and Environmental Characteristics in Cisadea River, West Java, Indonesia. J. Biodjati 2019, 4, 278–290. [Google Scholar] [CrossRef]
- Magnuson, J.J.; Crowder, L.B.; Medvick, P.A. Temperature as an Ecological Resource. Integr. Comp. Biol. 1979, 19, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Scrimgeour, G.; Prowse, T.; Culp, J.; Chambers, P. Ecological Effects of River Ice Break-up: A Review and Perspective. Freshw. Biol. 1994, 32, 261–275. [Google Scholar] [CrossRef]
- Masese, F.; Muchiri, M.; Raburu, P. Macroinvertebrate Assemblages as Biological Indicators of Water Quality in the Moiben River, Kenya. Afr. J. Aquat. Sci. 2009, 34, 15–26. [Google Scholar] [CrossRef]
- Siddik, M.; Chaklader, M.; Hanif, M.; Islam, M.; Sharker, M.; Rahman, M. Stock Identification of Critically Endangered Olive Barb, Puntius Sarana (Hamilton, 1822) with Emphasis on Management Implications. J. Aquac. Res. Dev. 2016, 7, 1000411. [Google Scholar] [CrossRef] [Green Version]
- Kramer, D.L. Dissolved Oxygen and Fish Behavior. Environ. Biol. Fishes 1987, 18, 81–92. [Google Scholar] [CrossRef]
- Ruma, M.; Hossain, M.M.; Rahman, M.B.; Nahar, A.; Siddik, M.A.B. Fish Community Structure of Sandha River: A Link Analysis towards Fisheries Management and Conservation. J. Biodivers. Endanger. Species 2017, 5, 92. [Google Scholar] [CrossRef] [Green Version]
- Dettinger, M.D.; Diaz, H.F. Global Characteristics of Stream Flow Seasonality and Variability. J. Hydrometeorol. 2000, 1, 289–310. [Google Scholar] [CrossRef] [Green Version]
- Negi, R.; Mamgain, S. Species Diversity, Abundance and Distribution of Fish Community and Conservation Status of Tons River of Uttarakhand State, India. Fish. Aquat. Sci. 2013, 8, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Rachmatika, I.; Sjafei, D.S.; Nurcahyadi, W. Fish Fauna Of Gunung Halimun National Park Region: Additional Information On The Utilization. Ber. Biol. 2002, 6, 13–24. [Google Scholar]
- Shahnawaz, A.; Venkateshwarlu, M.; Somashekar, D.S.; Santosh, K. Fish Diversity with Relation to Water Quality of Bhadra River of Western Ghats (INDIA). Environ. Monit. Assess. 2010, 161, 83–91. [Google Scholar] [CrossRef]
- Rowe, D.C.; Pierce, C.L.; Wilton, T.F. Fish Assemblage Relationships with Physical Habitat in Wadeable Iowa Streams. N. Am. J. Fish. Manag. 2009, 29, 1314–1332. [Google Scholar] [CrossRef] [Green Version]
- Cunico, A.M.; Ferreira, E.A.; Agostinho, A.A.; Beaumord, A.C.; Fernandes, R. The Effects of Local and Regional Environmental Factors on the Structure of Fish Assemblages in the Pirapó Basin, Southern Brazil. Landsc. Urban Plan. 2012, 105, 336–344. [Google Scholar] [CrossRef]
- Bănăduc, A.; Bănăduc, D.; Bucşa, C. Watersheds Management (Transylvania/Romania): Implications, Risks, Solutions. In Strategies to Enhance Environmental Security in Transition Countries; Springer: Dordrecht, The Netherlands, 2007; pp. 225–238. ISBN 978-1-4020-5994-0. [Google Scholar]
- Fricke, R.; Bilecenoglu, M.; Sari, H.M. Annotated Checklist of Fish and Lamprey Species (Gnathostomata and Petromyzontomorphi) of Turkey, Including a Red List of Threatened and Declining Species; Stuttgarter Beiträge zur NaturkundeSerie A, Staatliches Museum für Naturkunde: Stuttgarter, Germany, 2007. [Google Scholar]
- Leprieur, F.; Beauchard, O.; Blanchet, S.; Oberdorff, T.; Brosse, S. Fish Invasions in the World’s River Systems: When Natural Processes Are Blurred by Human Activities. PLoS Biol. 2008, 6, e28. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, C.; Fan, E.; Zhao, Y. Freshwater Fishes of China: Species Richness, Endemism, Threatened Species and Conservation. Divers. Distrib. 2016, 22, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Nyanti, L.; Noor-Azhar, N.I.; Soo, C.L.; Ling, T.Y.; Sim, S.F.; Grinang, J.; Ganyai, T.; Lee, K.S.P. Physicochemical Parameters and Fish Assemblages in the Downstream River of a Tropical Hydroelectric Dam Subjected to Diurnal Changes in Flow. Int. J. Ecol. 2018, 2018, 8690948. [Google Scholar] [CrossRef] [Green Version]
- Angel, A.; Ojeda, F.P. Structure and Trophic Organization of Subtidal Fish Assemblages on the Northern Chilean Coast: The Effect of Habitat Complexity. Mar. Ecol. Prog. Ser. 2001, 217, 81–91. [Google Scholar] [CrossRef]
- Yeager, L.A.; Layman, C.A.; Allgeier, J.E. Effects of Habitat Heterogeneity at Multiple Spatial Scales on Fish Community Assembly. Oecologia 2011, 167, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, N.; Rostami, S.; Sepehrfar, K.; Lahijanzadeh, A. Identification of the Water Pollutant Industries in Khuzestan Province. Iran. J. Environ. Health Sci. Eng. 2004, 1, 36–42. [Google Scholar]
- Angermeier, P.L.; Karr, J.R. Biological Integrity Versus Biological Diversity as Policy Directives: Protecting Biotic Resources. In Ecosystem Management; Springer: New York, NY, USA, 1994; pp. 264–275. [Google Scholar]
- Daufresne, M.; Boët, P. Climate Change Impacts on Structure and Diversity of Fish Communities in Rivers. Glob. Chang. Biol. 2007, 13, 2467–2478. [Google Scholar] [CrossRef]
- Jaramillo-Villa, U.; Maldonado-Ocampo, J.A.; Escobar, F. Altitudinal Variation in Fish Assemblage Diversity in Streams of the Central Andes of Colombia. J. Fish Biol. 2010, 76, 2401–2417. [Google Scholar] [CrossRef]
- Suarez, Y.R.; de Souza, M.M.; Ferreira, F.S.; Pereira, M.J.; da Silva, E.A.; Ximenes, L.Q.L.; de Azevedo, L.G.; Martins, O.C.; Junior, S.E.L. Patterns of Species Richness and Composition of Fish Assemblages in Streams of the Ivinhema River Basin, Upper Paraná River. Acta Limnol. Bras. 2011, 23, 177–188. [Google Scholar] [CrossRef]
- Dubey, V.K.; Sarkar, U.K.; Pandey, A.; Sani, R.; Lakra, W.S. The Influence of Habitat on the Spatial Variation in Fish Assemblage Composition in an Unimpacted Tropical River of Ganga Basin, India. Aquat. Ecol. 2012, 46, 165–174. [Google Scholar] [CrossRef]
- Mondal, R.; Bhat, A. Temporal and Environmental Drivers of Fishcommunity Structure in Tropical Streams from Two Contrasting Regions in India. PLoS ONE 2020, 15, e0227354. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, H.; Teimori, A.; Schinegger, R.; Schmutz, S. A New Fish Based Multi-Metric Assessment Index for Cold-Water Streams of the Southern Caspian Sea Basin in Iran. Environ. Biol. Fishes 2019, 102, 645–662. [Google Scholar] [CrossRef]
- Mostafavi, H.; Schinegger, R.; Melcher, A.; Moder, K.; Mielach, C.; Schmutz, S. A New Fish-Based Multi-Metric Assessment Index for Cyprinid Streams in the Iranian Caspian Sea Basin. Limnologica 2015, 51, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Patil, T.S.; Bhosale, A.R.; Yadav, R.B.; Khandekar, R.S.; Muley, D.V. Study of Endemic and Threatened Fish Species Diversity and Its Assemblage Structure from Northern Western Ghats, Maharashtra, India. Int. J. Zool. Res. 2015, 11, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Pokharel, K.K.; Basnet, K.B.; Majupuria, T.C.; Baniya, C.B. Correlations between Fish Assemblage Structure and Environmental Variables of the Seti Gandaki River Basin, Nepal. J. Freshw. Ecol. 2018, 33, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yan, D.; Yang, Q.; Gong, S.; Shi, Z.; Qiu, Q.; Huang, S.; Zhou, S.; Hu, M. Fish Species Composition, Distribution and Community Structure in the Fuhe River Basin, Jiangxi Province, China. Glob. Ecol. Conserv. 2021, 27, e01559. [Google Scholar] [CrossRef]

| Species | Distribution | Presence Status in Karun Basin | Feeding Behaviour | Substrate Preference | IUCN Status |
|---|---|---|---|---|---|
| Acanthobrama marmid | Tigris basin | Native | Omnivore | Vegetative | Least Concern |
| Alburnoides idignensis | Tigris basin | Endemic | Omnivore | Vegetative | Not Evaluated |
| Alburnus caeruleus | Tigris basin | Native | Omnivore | Vegetative | Least Concern |
| Alburnus doriae | Namak, Esfahan and Tigris basins | Endemic | Benthivore | Rocky | Not Evaluated |
| Alburnus sellal | Tigris, Kor, Maharlu Lake, Persis and Hormuz basins | Native | Omnivore | Rocky | Least Concern |
| Aphanius vladykovi | Tigris and Esfahan basins | Endemic | Omnivore | Vegetative | Not Evaluated |
| Arabibarbus grypus | Tigris, Persis and Hormuz basins | Native | Omnivore | Vegetative | Vulnerable/Decreasing |
| Barbus karunensis | Tigris basin | Endemic | Omnivore | Rocky | Not Evaluated |
| Barbus lacerta | Tigris basin | Native | Omnivore | Rocky | Least Concern |
| Capoeta aculeata | Tigris and Kor basins | Endemic | Herbivore | Rocky | Not Evaluated |
| Capoeta coadi | Tigris and Esfahan Basins | Endemic | Herbivore | Rocky | Not Evaluated |
| Capoeta trutta | Tigris basin | Native | Herbivore | Rocky | Least Concern |
| Carasobarbus kosswigi | Tigris basin | Native | Omnivore | Rocky | Vulnerable/Decreasing |
| Carasobarbus luteus | Tigris, Persis, Hormuz, Maharlu Lake basins | Native | Herbivore | Rocky | Least Concern |
| Carassius gibelio | Introduced widely; found in all basins of Iran. | Non-native | Omnivore | Vegetative | Not Evaluated |
| Chondrostoma regium | Tigris and Esfahan basin. | Native | Omnivore | Rocky | Least Concern |
| Ctenopharyngodon idella | Introduced widely elsewhere, found in all basins of Iran. | Non-native | Herbivore | Vegetative | Least Concern |
| Cyprinion macrostomus | Tigris basin | Native | Omnivore | Rocky | Least Concern |
| Cyprinus carpio | Native to the Caspian Sea basin. Introduced widely to all basins in Iran. | Non-native | Omnivore | Vegetative | Vulnerable |
| Gambusia holbrooki | Introduced widely elsewhere, found in all basins of Iran. | Non-native | Omnivore | Vegetative | Least Concern |
| Garra gymnothorax | Tigris basin | Endemic | Omnivore | Rocky | Not Evaluated |
| Garra rufa | Tigris, Kor, Maharlu Lake and Persis | Native | Omnivore | Rocky | Least Concern |
| Glyptothorax silviae | Tigris and Persis basins | Endemic | Benthivore | Rocky | Not Evaluated |
| Hemiculter leucisculus | Introduced widely everywhere, found in all Iranian basins. | Non-native | Omnivore | Rocky | Least Concern |
| Luciobarbus barbulus | Tigris and Persis basins | Native | Carnivore | Rocky | Not Evaluated |
| Mastacembelus mastacembelus | Tigris and Persis | Native | Carnivore | Rocky | Least Concern |
| Oncorhynchus mykiss | Introduced widely elsewhere, found in all basins of Iran. | Non-native | Carnivore | Rocky | Not Evaluated |
| Oxynoemacheilus freyhofi | Tigris basin | Endemic | Benthivore | Rocky | Not Evaluated |
| Planiliza abu | Tigris River, Persis, Hormuz and Maharlu Lake basins | Native | Benthivore | Rocky | Least Concern |
| Pseudorasbora parva | Introduced widely everywhere, found in all Iranian basins. | Non-native | Omnivore | Vegetative | Least Concern |
| Rhinogobius lindbergi | Caspian, Namak, Hari and Tigris basins | Non-native | Benthivore | Rocky | Not Evaluated |
| Sasanidus kermanshahensis | Tigris basin | Endemic | Benthivore | Rocky | Endangered |
| Squalius berak | Tigris basin | Native | Omnivore | Rocky | Least Concern |
| Squalius lepidus | Tigris basin | Native | Omnivore | Rocky | Least Concern |
| Turcinoemacheilus hafezi | Tigris basin | Endemic | Benthivore | Rocky | Least Concern |
| Turcinoemacheilus saadii | Tigris basin | Endemic | Benthivore | Rocky | Least Concern |
| Capoeta pyragyi | Tigris basin | Endemic | Herbivore | Rocky | Least Concern |
| Family/Species | Total Number of Individuals (N) | Total Biomass W(g) | Index of Relative Importance (IRI) (%) | Frequency of Occurrence (%) |
|---|---|---|---|---|
| Leuciscidae | ||||
| Acanthobrama marmid | 4 | 4.78 | 0.002 | 1.96 |
| Chondrostoma regium | 504 | 10,062 | 10.271 | 54.9 |
| Alburnoides idignensis | 256 | 2299.47 | 1.229 | 17.64 |
| Alburnus caeruleus | 30 | 56.93 | 0.024 | 3.92 |
| Squalius berak | 104 | 5623.12 | 2.771 | 39.21 |
| Squalius lepidus | 76 | 3630.66 | 0.371 | 7.84 |
| Alburnus doriae | 145 | 2051.49 | 0.815 | 17.64 |
| Alburnus sellal | 390 | 4121.19 | 6.786 | 60.78 |
| Cyprinidae | ||||
| Capoeta aculeate | 485 | 18,959.85 | 12.416 | 47.05 |
| Capoeta coadi | 857 | 22,480.05 | 23.004 | 62.74 |
| Capoeta trutta | 184 | 5104.41 | 2.548 | 31.37 |
| Carasobarbus kosswigi | 15 | 266.66 | 0.052 | 9.80 |
| Carasobarbus luteus | 42 | 1280.93 | 0.077 | 3.92 |
| Carassius gibelio | 44 | 688.52 | 0.143 | |
| Cyprinion macrostomus | 148 | 1612.6 | 0.839 | 19.60 |
| Cyprinus carpio | 9 | 1348.49 | 0.055 | 3.92 |
| Garra rufa | 619 | 4532.74 | 10.292 | 64.70 |
| Garra gymnothorax | 254 | 990.97 | 2.252 | 39.21 |
| Luciobarbus barbulus | 44 | 825.25 | 0.528 | 33.33 |
| Capoeta pyragyi | 386 | 16,709.63 | 5.726 | 25.49 |
| Arabibarbus grypus | 2 | 17.23 | 0.001 | 1.96 |
| Barbus karunensis | 26 | 786.98 | 0.213 | 17.64 |
| Barbus lacerta | 79 | 1938.33 | 1.022 | 31.37 |
| Xenocyprinidae | ||||
| Ctenopharyngodon idella | 1 | 326.9 | 0.006 | 1.96 |
| Hemiculter leucisculus | 4 | 21.3 | 0.004 | 3.92 |
| Poeciliidae | ||||
| Gambusia holbrooki | 9 | 6.86 | 0.003 | 1.96 |
| Sisoridae | ||||
| Glyptothorax silviae | 64 | 296.78 | 0.613 | 41.17 |
| Mastacembelidae | ||||
| Mastacembelus mastacembelus | 22 | 1034.19 | 0.080 | 5.88 |
| Salmonidae | ||||
| Oncorhynchus mykiss | 7 | 1245.85 | 0.123 | 9.88 |
| Nemacheilidae | ||||
| Oxynoemacheilus freyhofi | 66 | 97.85 | 0.159 | 11.76 |
| Sasanidus kermanshahensis | 36 | 27.81 | 0.112 | 15.68 |
| Turcinoemacheilus hafezi | 173 | 48.88 | 0.853 | 25.49 |
| Turcinoemacheilus saadii | 36 | 17.36 | 0.124 | 17.64 |
| Mugilidae | ||||
| Planiliza abu | 66 | 1513.9 | 0.103 | 3.92 |
| Gobionidae | ||||
| Pseudorasbora parva | 3 | 6.2 | 0.001 | 1.96 |
| Gobiidae | ||||
| Rhinogobius lindbergi | 5 | 2 | 0.002 | 1.96 |
| Aphanidae | ||||
| Aphanius vladykovi | 10 | 11.72 | 0.007 | 3.92 |
| Site_Code | Shannon−Wiener Diversity Index | Simpson’s Index of Diversity | Margalef Species Richness Index | Pielou Evenness Index | Total Number of Species | Total Abundance |
|---|---|---|---|---|---|---|
| 1 | 1.09 | 0.58 | 1.20 | 0.56 | 7 | 151 |
| 2 | 1.58 | 0.75 | 1.63 | 0.81 | 7 | 40 |
| 3 | 1.57 | 0.67 | 2.28 | 0.63 | 12 | 124 |
| 4 | 1.87 | 0.82 | 1.68 | 0.85 | 9 | 118 |
| 5 | 1.72 | 0.78 | 1.57 | 0.88 | 7 | 46 |
| 6 | 1.59 | 0.77 | 1.27 | 0.81 | 7 | 111 |
| 7 | 1.63 | 0.79 | 1.24 | 0.84 | 7 | 125 |
| 8 | 0.76 | 0.51 | 0.50 | 0.69 | 3 | 55 |
| 9 | 1.50 | 0.76 | 0.92 | 0.93 | 6 | 76 |
| 10 | 1.30 | 0.59 | 1.50 | 0.59 | 9 | 205 |
| 11 | 1.32 | 0.66 | 1.39 | 0.68 | 7 | 75 |
| 12 | 1.26 | 0.62 | 1.77 | 0.55 | 10 | 162 |
| 13 | 0.69 | 0.61 | 0.87 | 0.63 | 3 | 10 |
| 14 | 1.29 | 0.7 | 0.84 | 0.93 | 5 | 50 |
| 16 | 1.89 | 0.83 | 2.15 | 0.91 | 8 | 26 |
| 17 | 0.97 | 0.51 | 1.04 | 0.70 | 4 | 18 |
| 18 | 0.69 | 0.5 | 1.44 | 1.00 | 2 | 2 |
| 19 | 1.92 | 0.83 | 2.08 | 0.83 | 10 | 76 |
| 20 | 2.08 | 0.83 | 2.49 | 0.81 | 13 | 123 |
| 21 | 1.73 | 0.74 | 1.89 | 0.83 | 9 | 69 |
| 22 | 2.14 | 0.85 | 2.38 | 0.79 | 15 | 359 |
| 23 | 1.47 | 0.74 | 1.35 | 0.75 | 7 | 85 |
| 24 | 1.94 | 0.83 | 2.08 | 0.84 | 10 | 75 |
| 25 | 1.54 | 0.71 | 1.29 | 0.74 | 8 | 229 |
| 26 | 1.55 | 0.7 | 1.35 | 0.75 | 8 | 177 |
| 27 | 1.01 | 0.53 | 1.00 | 0.63 | 5 | 55 |
| 28 | 1.39 | 0.6 | 1.90 | 0.61 | 10 | 66 |
| 30 | 1.44 | 0.71 | 1.06 | 0.80 | 6 | 110 |
| 31 | 1.84 | 0.72 | 2.96 | 0.70 | 11 | 66 |
| 32 | 1.69 | 0.78 | 2.04 | 0.87 | 7 | 19 |
| 33 | 2.01 | 0.83 | 2.17 | 0.84 | 10 | 95 |
| 34 | 1.88 | 0.81 | 2.05 | 0.78 | 10 | 127 |
| 35 | 1.37 | 0.7 | 1.38 | 0.85 | 5 | 18 |
| 36 | 1.88 | 0.79 | 2.32 | 0.78 | 10 | 123 |
| 37 | 1.30 | 0.67 | 1.50 | 0.59 | 9 | 207 |
| 38 | 1.75 | 0.78 | 1.85 | 0.70 | 12 | 388 |
| 39 | 1.79 | 0.78 | 1.94 | 0.78 | 10 | 103 |
| 40 | 1.87 | 0.79 | 2.36 | 0.78 | 11 | 79 |
| 41 | 2.13 | 0.86 | 2.91 | 0.93 | 8 | 20 |
| 42 | 1.75 | 0.82 | 2.57 | 0.98 | 6 | 7 |
| 43 | 1.46 | 0.4 | 2.02 | 0.63 | 11 | 141 |
| 44 | 0.51 | 0.25 | 0.63 | 0.37 | 4 | 116 |
| 45 | 1.74 | 0.75 | 1.88 | 0.73 | 11 | 202 |
| 46 | 1.10 | 0.67 | 1.82 | 1.00 | 3 | 3 |
| 47 | 1.04 | 0.43 | 0.65 | 0.75 | 4 | 101 |
| 48 | 1.77 | 0.8 | 1.34 | 0.85 | 8 | 184 |
| 49 | 0.00 | 0.00 | 0.00 | 0.00 | 1 | 3 |
| 50 | 0.98 | 0.58 | 0.62 | 0.71 | 4 | 123 |
| 51 | 0.85 | 0.49 | 1.00 | 0.53 | 6 | 152 |
| 52 | 1.55 | 0.72 | 1.56 | 0.75 | 8 | 89 |
| 53 | 1.74 | 0.78 | 1.98 | 0.79 | 9 | 57 |
| Mean | 1.47 | 0.68 | 1.60 | 0.75 | 7.69 | 102.76 |
| Range | 0–2.14 | 0–0.86 | 0–2.96 | 0–1 | 0–15 | 0–388 |
| Factor | Mean | Min. | Max. | S.D. |
|---|---|---|---|---|
| Physico-chemical parameters | ||||
| pH | 7.87 | 7.03 | 8.31 | 0.32 |
| Electrical Conductivity (μS/cm) | 475.75 | 235 | 2250 | 281.98 |
| Dissolved Oxygen (mg/L) | 8.46 | 6.05 | 10.51 | 0.89 |
| Water Temperature (°C) | 18.89 | 10.7 | 28.43 | 3.95 |
| Turbidity (NTU) | 43.69 | 15.93 | 148.84 | 26.46 |
| Habitat parameters | ||||
| Stream Width (m) | 46.49 | 5 | 110 | 24.67 |
| Stream Depth (m) | 48.63 | 27.4 | 91.9 | 10.62 |
| Water Velocity (m/s) | 3.5 | 1.5 | 5.01 | 0.78 |
| Altitude (m) | 1424.94 | 67 | 2012 | 445.05 |
| Environmental Descriptors | Axis 1 | Axis 2 | F-Ratio | p-Value |
|---|---|---|---|---|
| Electrical Conductivity | −0.6787 | 0.54754 | 5.4521 | 0.005 ** |
| Elevation | 0.736671 | −0.39558 | 5.205 | 0.005 ** |
| Water Temperature | −0.51904 | 0.67974 | 4.7298 | 0.005 ** |
| Turbidity | 0.169677 | 0.81554 | 3.6076 | 0.005 ** |
| Dissolved Oxygen | 0.65749 | 0.147 | 3.5576 | 0.005 ** |
| Water Velocity | 0.353703 | −0.57765 | 2.6851 | 0.005 ** |
| pH | 0.441877 | 0.14455 | 2.8554 | 0.01 ** |
| Width | −0.23955 | −0.46967 | 2.1536 | 0.01 ** |
| Depth | −0.00233 | −0.03782 | 1.4982 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zare-Shahraki, M.; Ebrahimi-Dorche, E.; Bruder, A.; Flotemersch, J.; Blocksom, K.; Bănăduc, D. Fish Species Composition, Distribution and Community Structure in Relation to Environmental Variation in a Semi-Arid Mountainous River Basin, Iran. Water 2022, 14, 2226. https://doi.org/10.3390/w14142226
Zare-Shahraki M, Ebrahimi-Dorche E, Bruder A, Flotemersch J, Blocksom K, Bănăduc D. Fish Species Composition, Distribution and Community Structure in Relation to Environmental Variation in a Semi-Arid Mountainous River Basin, Iran. Water. 2022; 14(14):2226. https://doi.org/10.3390/w14142226
Chicago/Turabian StyleZare-Shahraki, Mojgan, Eisa Ebrahimi-Dorche, Andreas Bruder, Joseph Flotemersch, Karen Blocksom, and Doru Bănăduc. 2022. "Fish Species Composition, Distribution and Community Structure in Relation to Environmental Variation in a Semi-Arid Mountainous River Basin, Iran" Water 14, no. 14: 2226. https://doi.org/10.3390/w14142226







