Occurrence and Reduction of Shiga Toxin-Producing Escherichia coli in Wastewaters in the Kathmandu Valley, Nepal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Wastewater Samples
2.2. Enumeration of E. coli in Wastewater
2.3. Performance of Heat and Kit DNA Extraction
2.4. DNA Extraction and quantitative Polymerase Chain Reaction (qPCR)
2.5. Data Analysis
3. Results
3.1. Performance of Heat and Kit DNA Extraction
3.2. Detection of E. coli Genes in Wastewater Samples
3.3. Concentrations of E. coli and Its Genes in Wastewater Samples
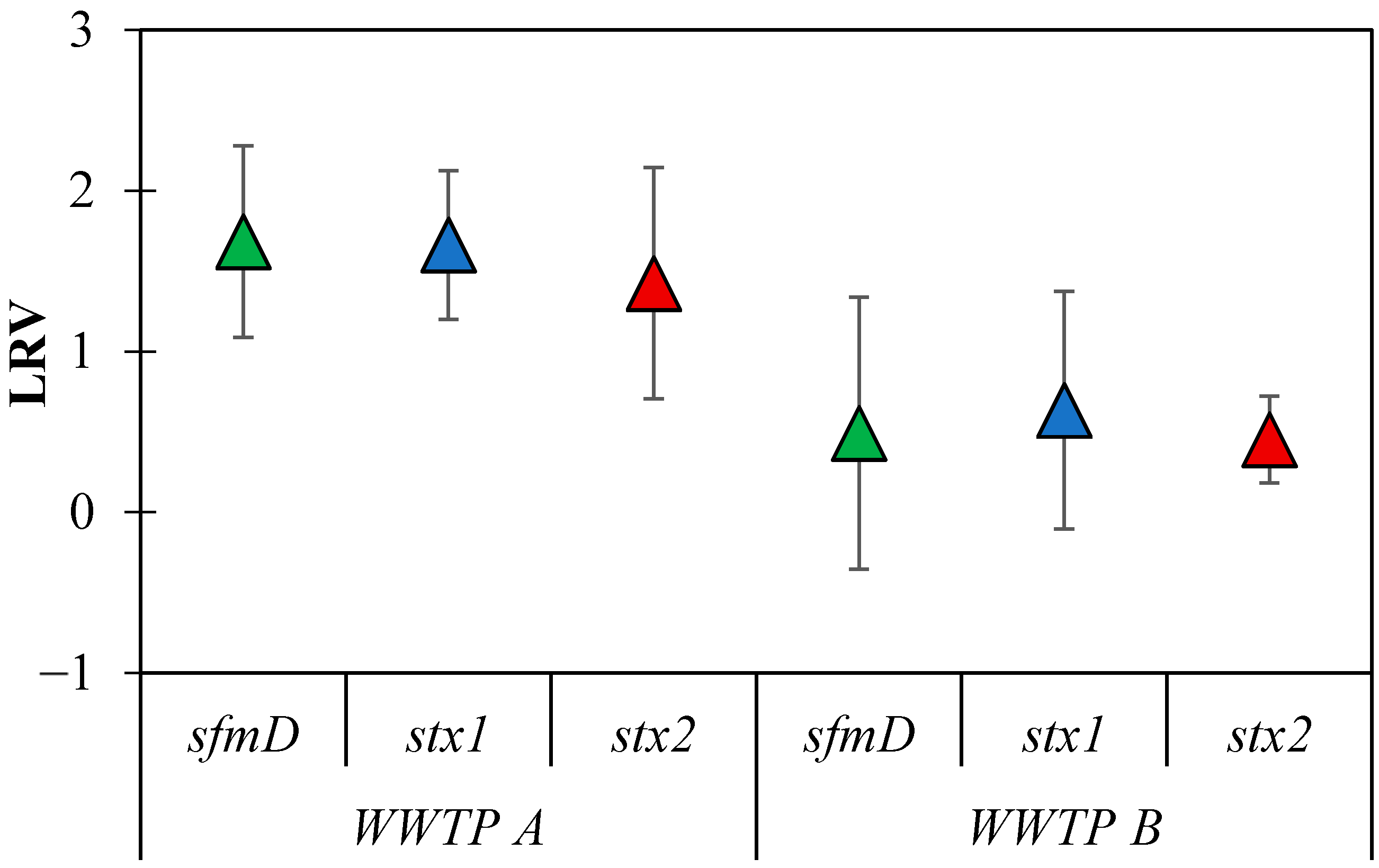
3.4. LRVs of E. coli Genes in Municipal WWTP
3.5. Ratio of Virulent E. coli Genes
4. Discussion
4.1. Performance of DNA Extraction Methods
4.2. Detection of E. coli Genes in Wastewater Samples
4.3. Reduction in E. coli Genes in WWTPs
4.4. Infectivity of Virulent E. coli
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bibbal, D.; Um, M.M.; Diallo, A.A.; Kérourédan, M.; Dupouy, V.; Toutain, P.L.; Bousquet-Mélou, A.; Oswald, E.; Brugère, H. Mixing of Shiga toxin-producing and enteropathogenic Escherichia coli in a wastewater treatment plant receiving city and slaughterhouse wastewater. Int. J. Hyg. Environ. Health 2018, 221, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T. Bacterial pathogen removal in wastewater treatment plants. In Handbook of Water and Wastewater Microbiology; Mara, D., Horan, N., Eds.; Academic Press: London, UK, 2003; pp. 477–490. [Google Scholar]
- Ghernaout, D. Increasing trends towards drinking water reclamation from treated wastewater. World J. Appl. Chem. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Aghalari, Z.; Dahms, H.U.; Sillanpää, M.; Sosa-Hernandez, J.E.; Parra-Saldívar, R. Effectiveness of wastewater treatment systems in removing microbial agents: A systematic review. Global. Health 2020, 16, 1–11. [Google Scholar]
- Thapa, B.; Khatiwada, N.R.; Ghimire, A.; Adhikari, B.; Badaila, R. Study on kinetics of pollutants removal in Guheshwori Wastewater Treatment. J. Water Pollut. Control 2019, 2, 1–7. [Google Scholar]
- Shrestha, S.; Haramoto, E.; Shindo, J. Assessing the infection risk of enteropathogens from consumption of raw vegetables washed with contaminated water in Kathmandu Valley, Nepal. J. Appl. Microbiol. 2017, 123, 1321–1334. [Google Scholar] [CrossRef]
- Naidoo, S.; Olaniran, A.O. Treated wastewater effluent as a source of microbial pollution of surface water resources. Int. J. Environ. Res. Public Health 2013, 11, 249–270. [Google Scholar] [CrossRef] [Green Version]
- Terajima, J.; Izumiya, H.; Hara-Kudo, Y.; Ohnishi, M. Shiga Toxin (Verotoxin)-producing Escherichia coli and Foodborne Disease: A Review. Food Saf. 2017, 5, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Pires, S.M.; Majowicz, S.; Gill, A.; Devleesschauwer, B. Global and regional source attribution of shiga toxin-producing Escherichia coli infections using analysis of outbreak surveillance data. Epidemiol. Infect. 2019, 147, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Melton-celsa, A.R. Shiga Toxin (Stx) classification, structure, and function. Microbiol. Spectr. 2014, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kaclíková, E.; Pangallo, D.; Oravcová, K.; Drahovská, H.; Kuchta, T. Quantification of Escherichia coli by kinetic 5′-nuclease polymerase chain reaction (real-time PCR) oriented to sfmD gene. Lett. Appl. Microbiol. 2005, 41, 132–135. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Gyles, C.L. Pathogenic Shiga toxin-producing Escherichia coli in the intestine of calves. Can. J. Vet. Res. 2002, 66, 65–72. [Google Scholar] [PubMed]
- Gyles, C.L. Shiga toxin-producing Escherichia coli: An overview. J. Anim. Sci. 2007, 85, E45–E62. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.M. Shiga toxin-producing Escherichia coli infection. Clin. Infect. Dis. 2004, 38, 1298–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, K.L.; Wickersham, E.; Strockbine, N.A. Escherichia coli O157:H7 from water. Lancet 1989, 333, 967–968. [Google Scholar] [CrossRef]
- Olsen, S.J.; Miller, G.; Breuer, T.; Kennedy, M.; Higgins, C.; Walford, J.; McKee, G.; Fox, K.; Bibb, W.; Mead, P. A waterborne outbreak of Escherichia coli O157:H7 infections and hemolytic uremic syndrome: Implications for rural water systems. Emerg. Infect. Dis. 2002, 8, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, J.S.; Kim, S.; Shin, E.; Oh, K.H.; Kim, Y.; Kim, C.H.; Hwang, M.A.; Jin, C.M.; Na, K.; et al. A waterborne outbreak of multiple diarrhoeagenic Escherichia coli infections associated with drinking water at a school camp. Int. J. Infect. Dis. 2018, 66, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, D.L.; Woodruff, B.A.; Brady, R.C.; Griffin, P.M.; Tippen, S.; Donnell, H.D.; Geldreich, E.; Payne, B.J.; Meyer, A.; Wells, J.G.; et al. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann. Intern. Med. 1992, 117, 812–819. [Google Scholar] [CrossRef]
- Keene, W.E.; McAnulty, J.M.; Hoesly, F.C.; Williams, P., Jr.; Hedberg, K.; Oxman, G.L.; Barrett, T.J.; Pfaller, M.A.; Fleming, D.W. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli 157:H7 and Shigella sonnei. N. Engl. J. Med. 1994, 331, 579–584. [Google Scholar] [CrossRef]
- Etcheverría, A.I.; Padola, N.L. Shiga toxin-producing Escherichia coli: Factors involved in virulence and cattle colonization. Virulence 2013, 4, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Loukiadis, E.; Kérourédan, M.; Beutin, L.; Oswald, E.; Brugère, H. Characterization of Shiga toxin gene (stx)-positive and intimin gene (eae)-positive Escherichia coli isolates from wastewater of slaughterhouses in France. Appl. Environ. Microbiol. 2006, 72, 3245–3251. [Google Scholar] [CrossRef] [Green Version]
- Numberger, D.; Ganzert, L.; Zoccarato, L.; Mühldorfer, K.; Sauer, S.; Grossart, H.P.; Greenwood, A.D. Characterization of bacterial communities in wastewater with enhanced taxonomic resolution by full-length 16S rRNA sequencing. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sthapit, N.; Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Sherchand, J.B.; Haramoto, E.; Kazama, F. Investigation of Shiga toxin-producing Escherichia coli in groundwater, river water, and fecal sources in the Kathmandu Valley, Nepal. Water. Air. Soil Pollut 2020, 231, 557. [Google Scholar] [CrossRef]
- Shrestha, S.; Aihara, Y.; Bhattarai, A.P.; Bista, N.; Rajbhandari, S.; Kondo, N.; Kazama, F.; Nishida, K.; Shindo, J. Dynamics of domestic water consumption in the urban area of the Kathmandu Valley: Situation analysis pre and post 2015 Gorkha Earthquake. Water 2017, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Jha, A.K.; Bajracharya, T.R. Wastewater treatment technologies in Nepal. In Proceedings of the IOE Graduate Conference, Kathmandu, Nepal, 15 July 2014. [Google Scholar]
- van Elsas, J.D.; Bailey, M.J. The ecology of transfer of mobile genetic elements. FEMS Microbiol. Ecol. 2002, 42, 187–197. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Mellmann, A.; Zhang, W.; Köck, R.; Fruth, A.; Bauwens, A.; Peters, G.; Karch, H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. Dis. 2011, 11, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Reid, S.D.; Herbelin, C.J.; Bumbaugh, A.C.; Selander, R.K.; Whittam, T.S. Parallel evolution of virulence in pathogenic Escherichia coli. Nature 2000, 406, 64–67. [Google Scholar] [CrossRef]
- Ayaz, N.D.; Gencay, Y.E.; Erol, I. Prevalence and molecular characterization of sorbitol fermenting and non-fermenting Escherichia coli O157:H7+/H7- isolated from cattle at slaughterhouse and slaughterhouse wastewater. Int. J. Food Microbiol. 2014, 174, 31–38. [Google Scholar] [CrossRef]
- Yang, K.; Pagaling, E.; Yan, T. Estimating the prevalence of potential enteropathogenic Escherichia coli and intimin gene diversity in a human community by monitoring sanitary sewage. Appl. Environ. Microbiol. 2014, 80, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Frigon, D.; Biswal, B.K.; Mazza, A.; Masson, L.; Gehr, R. Biological and physicochemical wastewater treatment processes reduce the prevalence of virulent Escherichia coli. Appl. Environ. Microbiol. 2013, 79, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Tandukar, S.; Ghaju Shrestha, R.; Malla, B.; Sthapit, N.; Sherchand, J.B.; Sherchan, S.P.; Haramoto, E. Virus reduction at wastewater treatment plants in Nepal. Environ. Challenges 2021, 5, 100281. [Google Scholar] [CrossRef]
- Gautam, B.; Rajbhanshi, A.; Adhikari, R. Bacterial load reduction after primary and secondary treatment in Guheswori sewage treatment plant, Kathmandu, Nepal. J. Coll. Med. Sci. 2019, 15, 40–44. [Google Scholar]
- Pantha, K.; Acharya, K.; Mohapatra, S.; Khanal, S.; Amatya, N.; Ospina-Betancourth, C.; Butte, G.; Shrestha, S.D.; Rajbhandari, P.; Werner, D. Faecal pollution source tracking in the holy Bagmati River by portable 16S rRNA gene sequencing. NPJ Clean Water 2021, 4, 1–10. [Google Scholar] [CrossRef]
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Junior, J.C.R.; Tamanini, R.; Soares, B.F.; de Oliveira, A.M.; Silva, F.d.G.; da Silva, F.F.; Augusto, N.A.; Beloti, V. Efficiency of boiling and four other methods for genomic DNA extraction of deteriorating spore-forming bacteria from milk. Semin. Agric. Sci. 2016, 37, 3069–3078. [Google Scholar]
- Kathmandu Upatyaka Khanepani Limited NEP: Kathmandu Valley Wastewater Management Project 2013. Available online: https://www.adb.org/projects/documents/kathmandu-valley-wastewater-management-project-iee (accessed on 15 December 2021).
- Ghaju Shrestha, R.; Tanaka, Y.; Malla, B.; Bhandari, D.; Tandukar, S.; Inoue, D.; Sei, K.; Sherchand, J.B.; Haramoto, E. Next-generation sequencing identification of pathogenic bacterial genes and their relationship with fecal indicator bacteria in different water sources in the Kathmandu Valley, Nepal. Sci. Total Environ. 2017, 601, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Sherchand, J.B.; Haramoto, E. Performance Evaluation of Human-Specific Viral Markers and Application of Pepper Mild Mottle Virus and CrAssphage to Environmental Water Samples as Fecal Pollution Markers in the Kathmandu Valley, Nepal. Food Environ. Virol. 2019, 11, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Bhandari, D.; Inoue, D.; Sei, K.; Tanaka, Y.; Sherchand, J.B.; Haramoto, E. Validation of host-specific Bacteroidales quantitative PCR assays and their application to microbial source tracking of drinking water sources in the Kathmandu Valley, Nepal. J. Appl. Microbiol. 2018, 125, 609–619. [Google Scholar] [CrossRef]
- Malla, B.; Shrestha, R.G.; Tandukar, S.; Bhandari, D.; Inoue, D.; Sei, K.; Tanaka, Y.; Sherchand, J.B.; Haramoto, E. Identification of human and animal fecal contamination in drinking water sources in the Kathmandu Valley, Nepal, using host-associated Bacteroidales Quantitative PCR Assays. Water 2018, 10, 1796. [Google Scholar] [CrossRef] [Green Version]
- Perelle, S.; Dilasser, F.; Grout, J.; Fach, P. Detection by 5′-nuclease PCR of Shiga-toxin producing Escherichia coli O26, O55, O91, O103, O111, O113, O145 and O157:H7, associated with the world’s most frequent clinical cases. Mol. Cell. Probes 2004, 18, 185–192. [Google Scholar] [CrossRef]
- Yamagishi, J.; Sato, Y.; Shinozaki, N.; Ye, B.; Tsuboi, A.; Nagasaki, M.; Yamashita, R. Comparison of boiling and robotics automation method in DNA extraction for metagenomic sequencing of human oral microbes. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Dimitrakopoulou, M.-E.; Stavrou, V.; Kotsalou, C.; Vantarakis, A. Boiling extraction method vs commercial kits for bacterial DNA isolation from food samples. J. Food Sci. Nutr. Res. 2020, 03, 311–319. [Google Scholar] [CrossRef]
- Peng, X.; Yu, K.Q.; Deng, G.H.; Jiang, Y.X.; Wang, Y.; Zhang, G.X.; Zhou, H.W. Comparison of direct boiling method with commercial kits for extracting fecal microbiome DNA by Illumina sequencing of 16S rRNA tags. J. Microbiol. Methods 2013, 95, 455–462. [Google Scholar] [CrossRef] [PubMed]
- García-Aljaro, C.; Muniesa, M.; Blanco, J.E.; Blanco, M.; Blanco, J.; Jofre, J.; Blanch, A.R. Characterization of Shiga toxin-producing Escherichia coli isolated from aquatic environments. FEMS Microbiol. Lett. 2005, 246, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Niestępski, S. The prevalence of virulence genes specific for Escherichia coli in wastewater samples from wastewater treatment plants with the activated sludge process. In Proceedings of the 10th Conference on Interdisciplinary Problems in Environmental Protection and Engineering EKO-DOK 2018, Online, 16–18 April 2018. [Google Scholar]
- Razzolini, M.T.P.; dos Santos, F.A.; Matte, M.H.; Matte, G.R.; Gunther, W.M.R. Occurrence of Escherichia coli O157 in raw and treated domestic sewage stabilization ponds with potential reuse in agricultural irrigation.; XXVIII Inter-American Congress of Sanitary and Environmental Engineering: Cancun, Mexico, 2002; pp. 1–3. [Google Scholar]
- Ahmed, W.; Gyawali, P.; Toze, S. Quantitative PCR measurements of Escherichia coli including Shiga toxin-producing E. coli (STEC) in animal feces and environmental waters. Environ. Sci. Technol. 2015, 49, 3084–3090. [Google Scholar] [CrossRef] [PubMed]
- Dumke, R.; Schröter-Bobsin, U.; Jacobs, E.; Röske, I. Detection of phages carrying the Shiga toxin 1 and 2 genes in waste water and river water samples. Lett. Appl. Microbiol. 2006, 42, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Grau-Leal, F.; Quirós, P.; Martínez-Castillo, A.; Muniesa, M. Free Shiga toxin 1-encoding bacteriophages are less prevalent than Shiga toxin 2 phages in extraintestinal environments. Environ. Microbiol. 2015, 17, 4790–4801. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ohta, Y.; Noda, M. Shiga toxin 2 is specifically released from bacterial cells by two different mechanisms. Infect. Immun. 2009, 77, 2813–2823. [Google Scholar] [CrossRef] [Green Version]
- Karmali, M.A. Emerging public health challenges of Shiga toxin-producing Escherichia coli related to changes in the pathogen, the population, and the environment. Clin. Infect. Dis. 2017, 64, 371–376. [Google Scholar]
- Allison, H.E. Stx-phages: Drivers and mediators of the evolution of STEC and STEC-like pathogens. Future Microbiol. 2007, 2, 165–174. [Google Scholar] [CrossRef]
- Oakley, S.; Mihelcic, J. Pathogen reduction and survival in complete treatment works. In Global Water Pathogen Project; Part 4 Management of Risk from Excreta and Wastewater; Rose, J.B., Jiménez-Cisneros, B., Mihelcic, J.R., Verbyla, M.E., Eds.; Michigan State University: East Lansing, MI, USA; UNESCO: Paris, France, 2019; Available online: http://www.waterpathogens.org/book/pathogen-reduction-and-survival-in-complete-treatment-works (accessed on 15 December 2021). [CrossRef]
- García-Aljaro, C.; Ballesté, E.; Muniesa, M.; Jofre, J. Determination of crAssphage in water samples and applicability for tracking human faecal pollution. Microb. Biotechnol. 2017, 10, 1775–1780. [Google Scholar] [CrossRef]
- Worley-Morse, T.; Mann, M.; Khunjar, W.; Olabode, L.; Gonzalez, R. Evaluating the fate of bacterial indicators, viral indicators, and viruses in water resource recovery facilities. Water Environ. Res. 2019, 91, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Foddai, A.C.G.; Grant, I.R. Methods for detection of viable foodborne pathogens: Current state-of-art and future prospects. Appl. Microbiol. Biotechnol. 2020, 104, 4281–4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Assay | Function | Sequence (5′–3′) a | Reference |
|---|---|---|---|
| sfmD | Forward primer | ACTGGAATACTTCGGATTCAGATACGT | [11] |
| Reverse primer | ATCCCTACAGATTCATTCCACGAAA | ||
| TaqMan probe | FAM-CAGCAGCTGGGTTGGCATCAGTTATTCG-TAMRA | ||
| stx1 | Forward primer | TTTGTYACTGTSACAGCWGAAGCYTTACG | [42] |
| Reverse primer | CCCCAGTTCARWGTRAGRTCMACRTC | ||
| TaqMan robe | FAM-CTGGATGATCTCAGTGGGCGTTCTTATGTAA-TAMRA | ||
| stx2 | Forward primer | TTTGTYACTGTSACAGCWGAAGCYTTACG | [42] |
| Reverse primer | CCCCAGTTCARWGTRAGRTCMACRTC | ||
| TaqMan probe | FAM-TCGTCAGGCACTGTCTGAAACTGCTCC-TAMRA |
| Wastewater Type | No. of Tested Samples | sfmD | stx1 | stx2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct qPCR | Cultured qPCR | Direct qPCR | Cultured qPCR | Direct qPCR | Cultured qPCR | ||||||||
| No. of Positive Samples (%) | Ct (Mean ± SD) | No. of Positive Samples (%) | Ct (Mean ± SD) | No. of Positive Samples (%) | Ct (Mean ± SD) | No. of Positive Samples (%) | Ct (Mean ± SD) | No. of Positive Samples (%) | Ct (Mean ± SD) | No. of Positive Samples (%) | Ct (Mean ± SD) | ||
| HWW | 17 | 17 (100) | 28.3 ± 3.9 | 17 (100) | 22.2 ± 2.1 | 6 (35) | 37.7 ± 0.9 | 8 (47) | 34.9 ± 2.1 | 6 (35) | 37.0 ± 0.9 | 9 (53) | 32.4 ± 3.0 |
| STP | 4 | 2 (50) | 31.3 ± 0.4 | 4 (100) | 22.1 ± 0.8 | 0 (0) | NA | 0 (0) | NA | 0 (0) | NA | 1 (25) | 36.3 |
| Not-working WWTP | 5 | 5 (100) | 27.3 ± 2.6 | 5 (100) | 22.7 ± 1.2 | 3 (60) | 38.2 ± 1.2 | 5 (100) | 36.5 ± 2.7 | 3 (60) | 37.5 ± 1.5 | 5 (100) | 35.5 ± 3.1 |
| WWTP A influent | 6 | 6 (100) | 25.3 ± 0.4 | 6 (100) | 21.7 ± 1.0 | 6 (100) | 36.5 ± 0.9 | 6 (100) | 33.9 ± 1.8 | 6 (100) | 35.5 ± 1.2 | 6 (100) | 31.8 ± 2.2 |
| WWTP A effluent | 6 | 6 (100) | 30.5 ± 1.6 | 6 (100) | 21.9 ± 0.6 | 1 (17) | 38.8 | 6 (100) | 33.5 ± 1.1 | 4 (67) | 38.9 ± 0.3 | 6 (100) | 31.8 ± 1.7 |
| WWTP B influent | 5 | 5 (100) | 24.9 ± 0.5 | 5 (100) | 21.6 ± 0.8 | 5 (100) | 35.7 ± 0.9 | 5 (100) | 32.9 ± 1.3 | 5 (100) | 34.7 ± 0.9 | 5 (100) | 31.3 ± 2.0 |
| WWTP B effluent | 5 | 5 (100) | 26.4 ± 2.2 | 5 (100) | 21.5 ± 0.9 | 4 (80) | 36.6 ± 1.3 | 5 (100) | 32.6 ± 1.0 | 5 (100) | 36.0 ± 1.3 | 5 (100) | 31.1 ± 1.6 |
| Total | 48 | 46 (96) | 27.6 ± 3.2 | 48 (100) | 22.0 ± 1.5 | 25 (52) | 36.8 ± 1.3 | 35 (73) | 34.1 ± 2.1 | 29 (60) | 36.3 ± 1.7 | 37 (77) | 32.3 ± 2.6 |
| Wastewater Type | No. of Tested Samples | Colilert | sfmD | stx1 | stx2 |
|---|---|---|---|---|---|
| Conc. (log10 MPN/L) (Mean ± SD) | Conc. (log10 Copies/L) (Mean ± SD) | Conc. (log10 Copies/L) (Mean ± SD) | Conc. (log10 Copies/L) (Mean ± SD) | ||
| HWW | 17 | 7.5 ± 1.4 | 10.0 ± 1.2 | 6.5 ± 0.3 | 6.9 ± 0.3 |
| STP | 4 | 6.3 ± 0.8 | 9.1 ± 0.1 | <LOD a | <LOD b |
| Not-working WWTP | 5 | 8.2 ± 0.8 | 10.3 ± 0.8 | 6.3 ± 0.3 | 6.7 ± 0.6 |
| WWTP A Influent | 6 | 8.7 ± 0.2 | 10.9 ± 0.1 | 6.8 ± 0.2 | 7.5 ± 0.3 |
| WWTP A Effluent | 6 | 7.3 ± 0.3 | 9.2 ± 0.6 | 5.2 ± 0.4 | 6.1 ± 0.6 |
| WWTP B Influent | 5 | 8.9 ± 0.3 | 11.0 ± 0.1 | 7.1 ± 0.3 | 7.7 ± 0.3 |
| WWTP B Effluent | 5 | 8.6 ± 0.7 | 10.5 ± 0.8 | 6.5 ± 0.9 | 7.3 ± 0.4 |
| Wastewater Type | No. of Tested Samples | Colilert/sfmD Ratio (%) | stx1/sfmD Ratio (%) | stx2/sfmD Ratio (%) |
|---|---|---|---|---|
| HWW | 17 | 1.43 ± 2.14 | 0.01 ± 0.01 | 0.03 ± 0.02 |
| STP | 4 | 0.60 ± 0.78 | NA | NA |
| Not-working WWTP | 5 | 0.90 ± 0.36 | 0.004 ± 0.002 | 0.01 ± 0.01 |
| WWTP A Influent | 6 | 0.73 ± 0.36 | 0.01 ± 0.01 | 0.05 ± 0.03 |
| WWTP A Effluent | 6 | 1.77 ± 1.39 | 0.02 | 0.15 ± 0.13 |
| WWTP B Influent | 5 | 0.87 ± 0.51 | 0.01 ± 0.01 | 0.06 ± 0.04 |
| WWTP B Effluent | 5 | 1.17 ± 0.51 | 0.01 ± 0.01 | 0.09 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sthapit, N.; Malla, B.; Tandukar, S.; Ghaju Shrestha, R.; Thakali, O.; Sherchand, J.B.; Haramoto, E.; Kazama, F. Occurrence and Reduction of Shiga Toxin-Producing Escherichia coli in Wastewaters in the Kathmandu Valley, Nepal. Water 2022, 14, 2224. https://doi.org/10.3390/w14142224
Sthapit N, Malla B, Tandukar S, Ghaju Shrestha R, Thakali O, Sherchand JB, Haramoto E, Kazama F. Occurrence and Reduction of Shiga Toxin-Producing Escherichia coli in Wastewaters in the Kathmandu Valley, Nepal. Water. 2022; 14(14):2224. https://doi.org/10.3390/w14142224
Chicago/Turabian StyleSthapit, Niva, Bikash Malla, Sarmila Tandukar, Rajani Ghaju Shrestha, Ocean Thakali, Jeevan B. Sherchand, Eiji Haramoto, and Futaba Kazama. 2022. "Occurrence and Reduction of Shiga Toxin-Producing Escherichia coli in Wastewaters in the Kathmandu Valley, Nepal" Water 14, no. 14: 2224. https://doi.org/10.3390/w14142224







