Enhanced Methylene Blue Adsorption by Cu-BTC Metal-Organic Frameworks with Engineered Particle Size Using Surfactant Modulators
Abstract
:1. Introduction
2. Experimental and Characterization
2.1. Materials
2.2. Preparation of Surfactant-Modulated Cu-BTC and Pristine Cu-BTC
2.3. Characterization
2.4. Adsorption Experiments
3. Results and Discussion
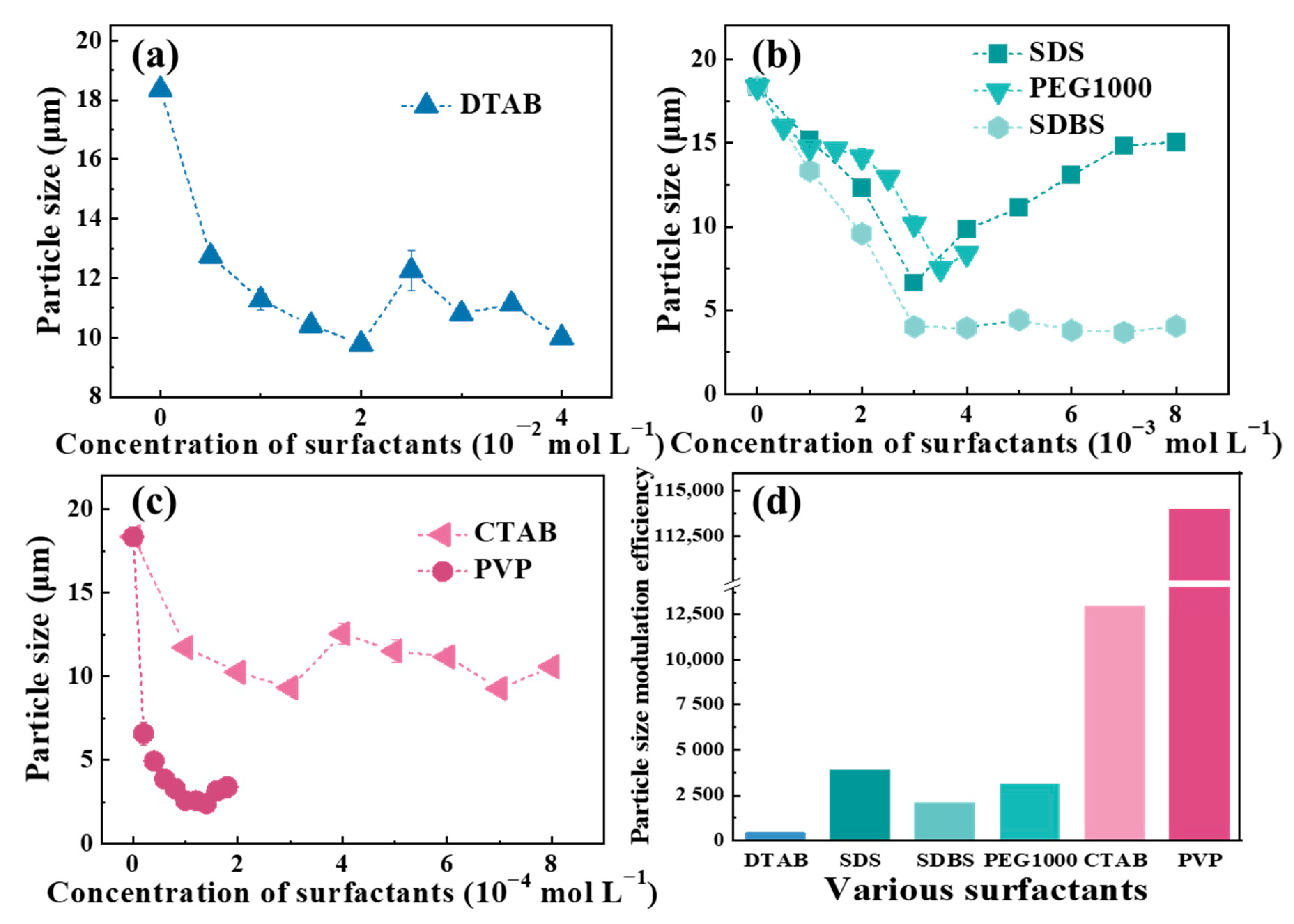
3.1. Modulation Ability of Surfactants on the Particle Size of Cu-BTC
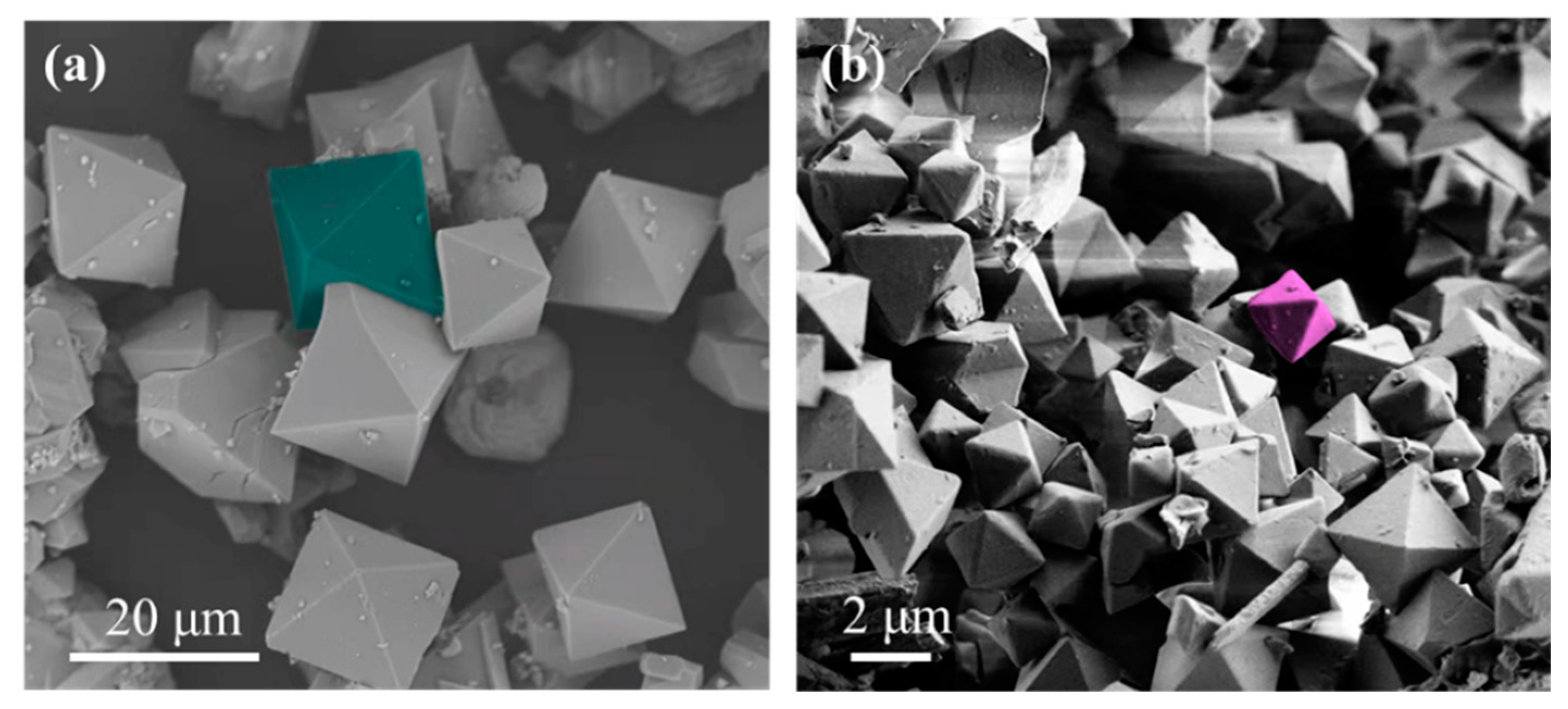
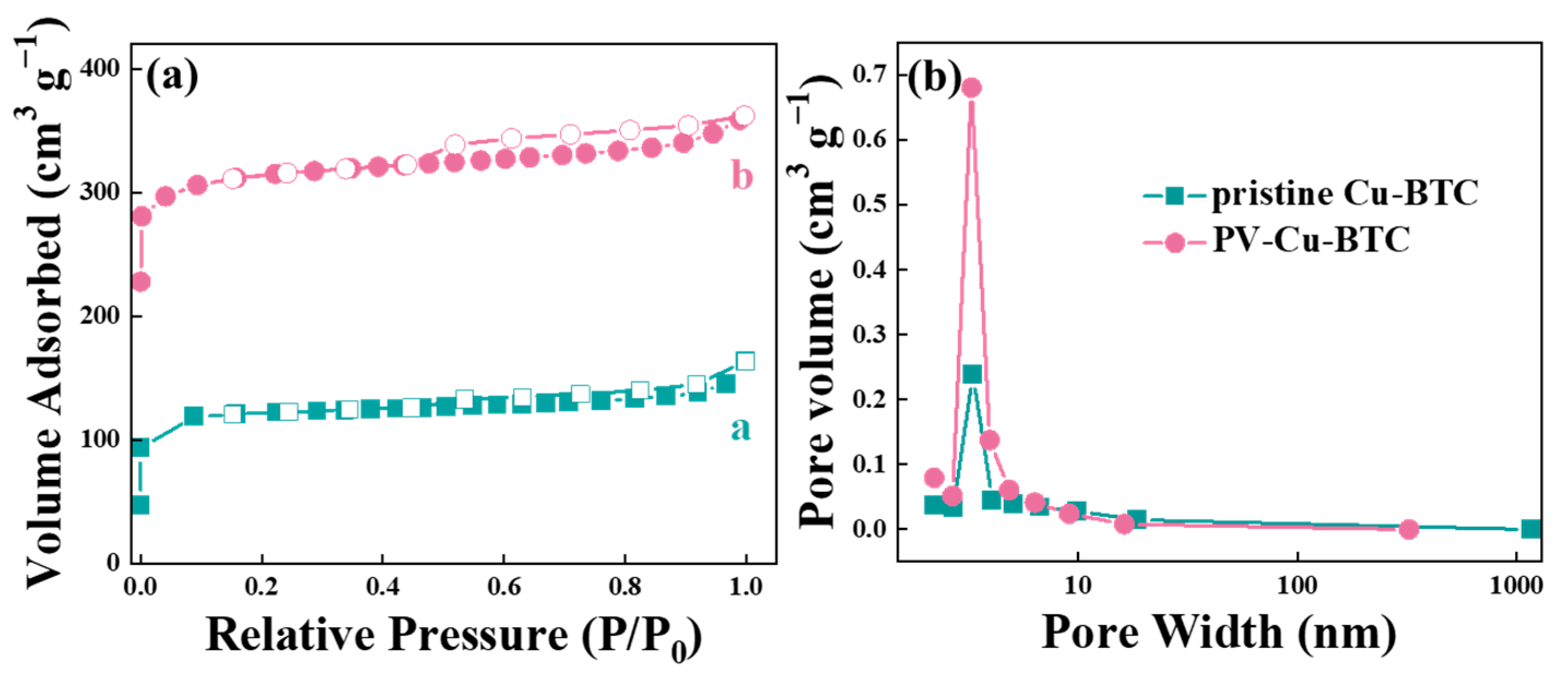
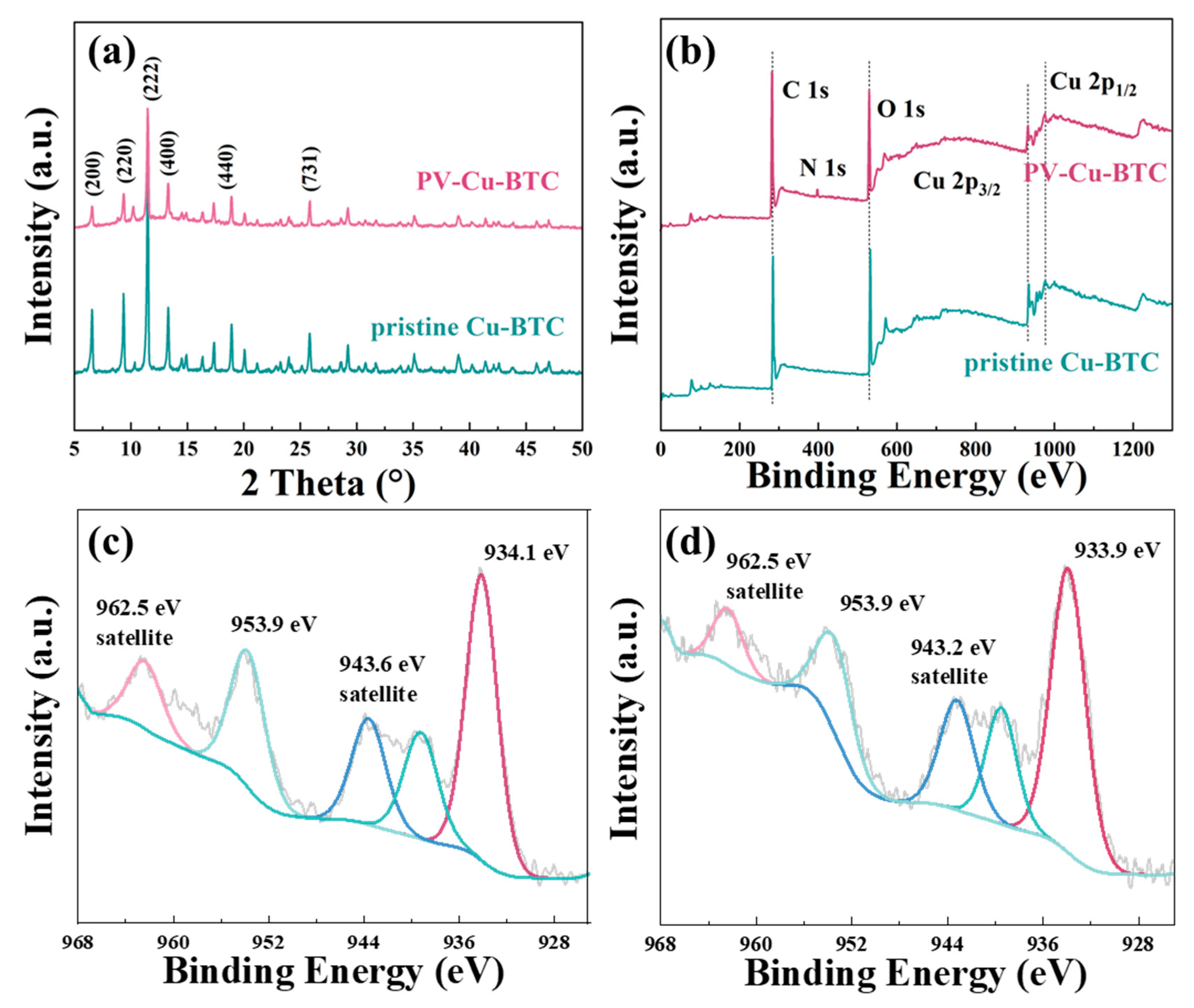
3.2. Structural Analysis of Surfactant Modulated and Pristine Cu-BTC
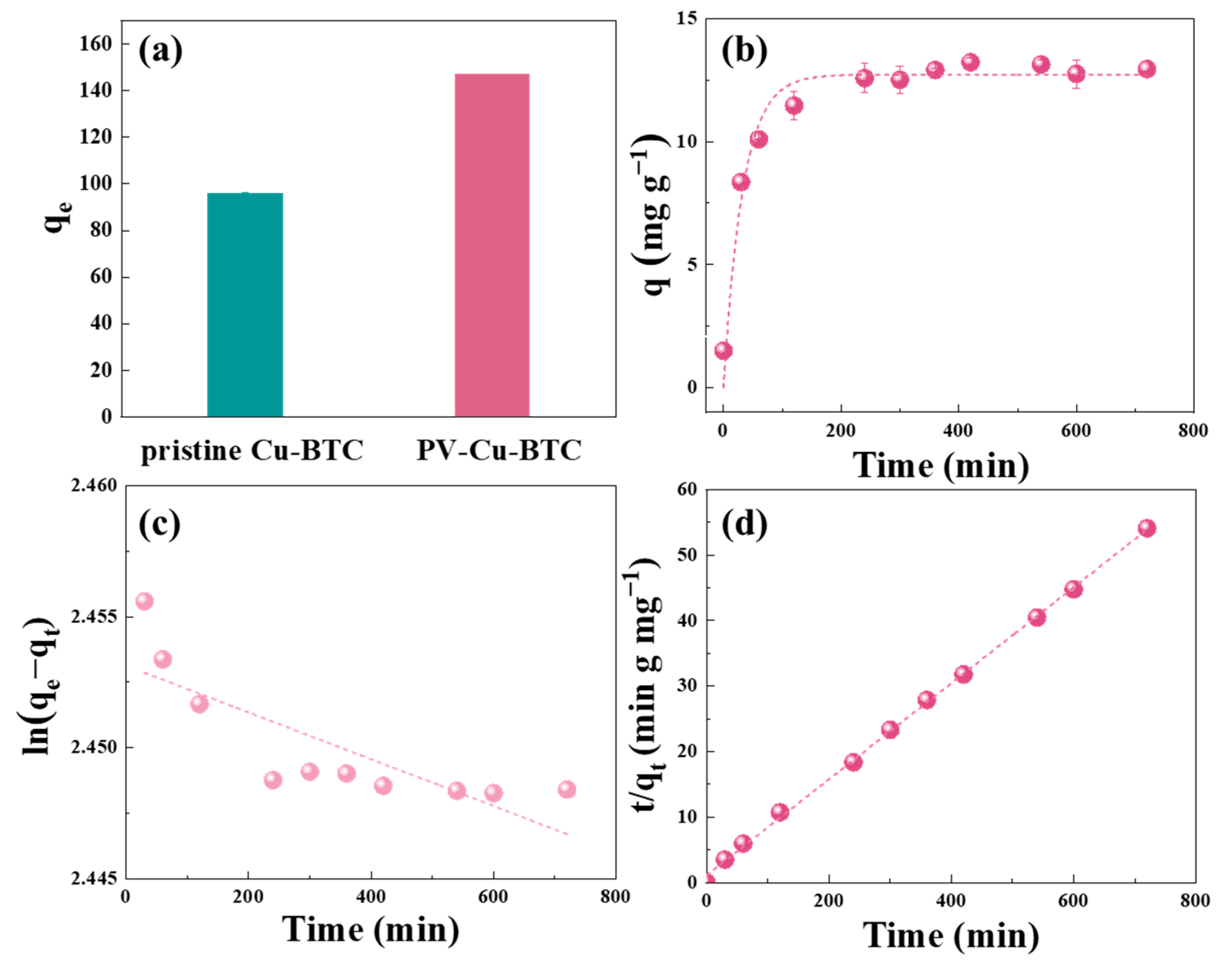
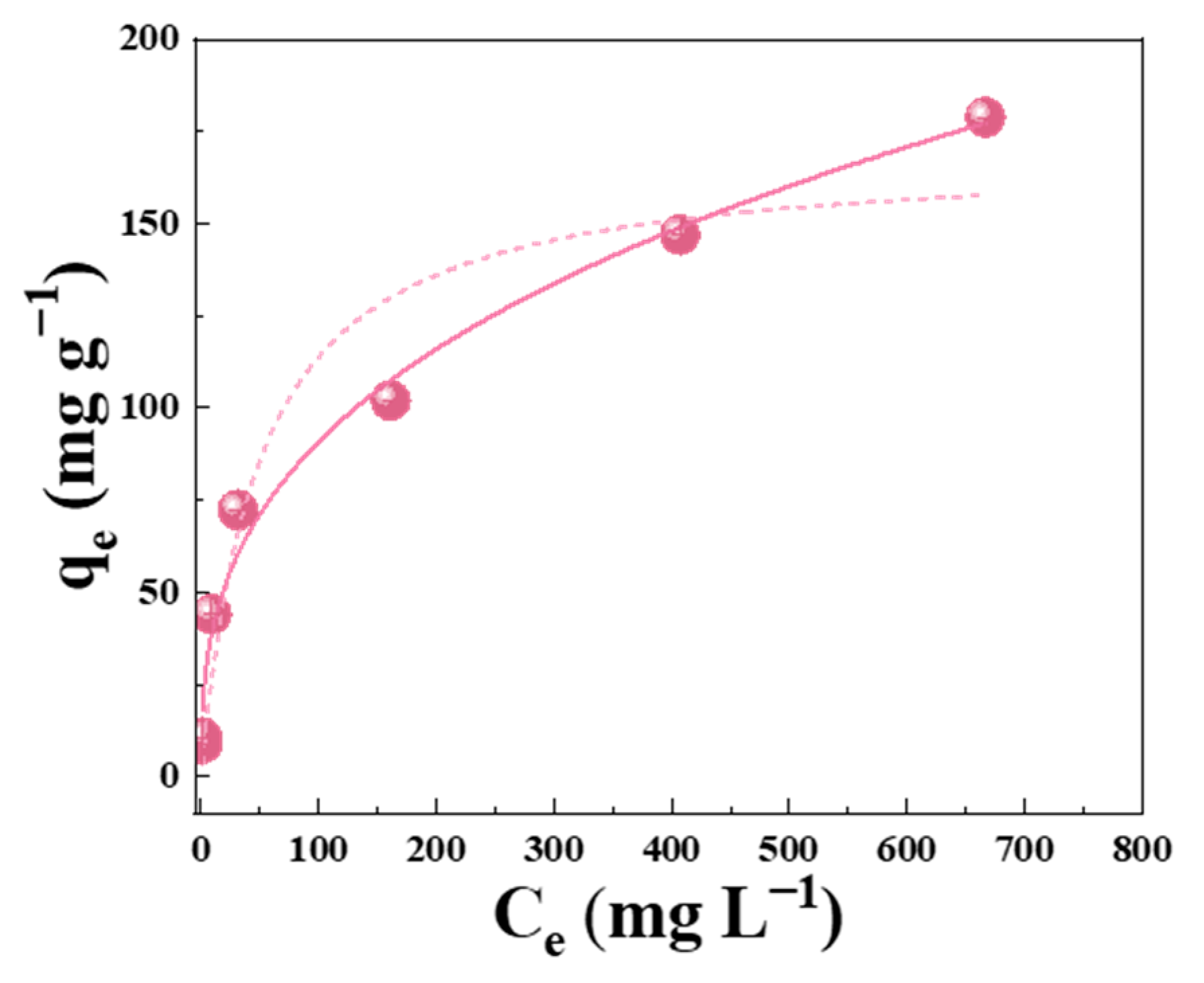
3.3. Enhanced Adsorption Performance of PV-Cu-BTC
3.4. Adsorption Stability of PV-Cu-BTC
3.5. Desorption and Regeneration of PV-Cu-BTC
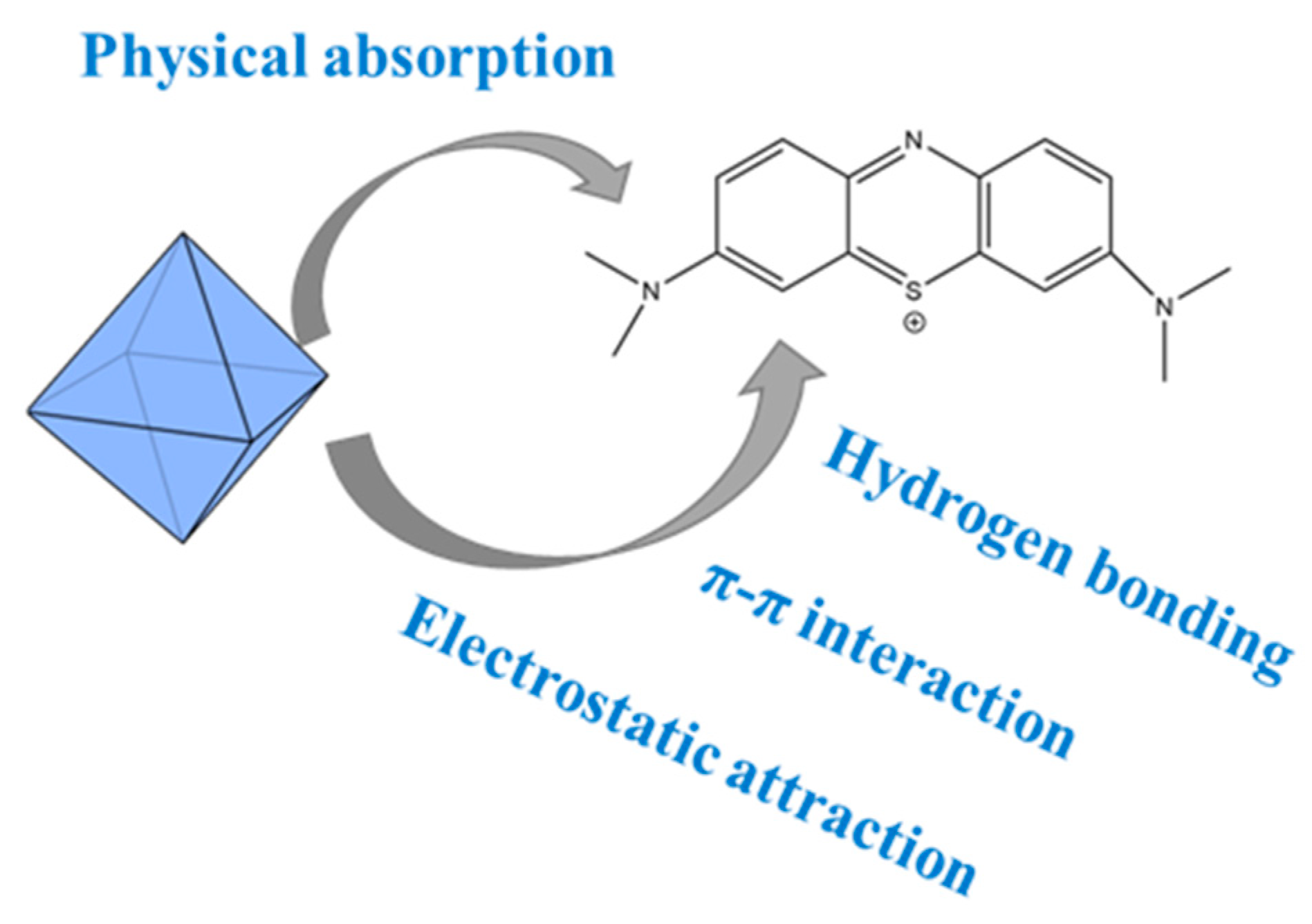
3.6. Possible Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Liu, X.L.; Geng, H.Y.; Hu, B.; Song, G.W.; Xu, Z.S. A MOF/graphite oxide hybrid (MOF: HKUST-1) material for the adsorption of methylene blue from aqueous solution. J. Mater. Chem. A 2013, 1, 10292–10299. [Google Scholar] [CrossRef]
- Luo, J.M.; Fu, K.X.; Yu, D.Y.; Hristovski, K.D.; Westerhoff, P.; Crittenden, J.C. Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Synthesis and Microstructure Impacts. ACS EST Eng. 2021, 1, 623–661. [Google Scholar] [CrossRef]
- Johari, N.A.; Yusof, N.; Lau, W.J.; Abdullah, N.; Salleh, W.N.W.; Jaafar, J.; Aziz, F.; Ismail, A.F. Polyethersulfone ultrafiltration membrane incorporated with ferric-based metal-organic framework for textile wastewater treatment. Sep. Purif. Technol. 2021, 270, 118819. [Google Scholar] [CrossRef]
- Pazdzior, K.; Bilinska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.; Feng, Z.; Zhao, H.; Zhao, G. Fast Generation of Hydroxyl Radicals by Rerouting the Electron Transfer Pathway via Constructed Chemical Channels during the Photo-Electro-Reduction of Oxygen. Environ. Sci. Technol. 2022, 56, 1331–1340. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Son, N.; Kang, M. Synergistic sorption performance of karaya gum crosslink poly(acrylamide-co-acrylonitrile) @ metal nanoparticle for organic pollutants. Int. J. Biol. Macromol. 2022, 210, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Khapre, M.; Shekhawat, A.; Saravanan, D.; Pandey, S.; Jugade, R. Mesoporous Fe–Al-doped cellulose for the efficient removal of reactive dyes. Mater. Adv. 2022, 3, 3278–3285. [Google Scholar] [CrossRef]
- Gupta, R.; Pandit, C.; Pandit, S.; Gupta, P.K.; Lahiri, D.; Agarwal, D.; Pandey, S. Potential and future prospects of biochar-based materials and their applications in removal of organic contaminants from industrial wastewater. J. Mater. Cycles Waste Manag. 2022, 24, 852–876. [Google Scholar] [CrossRef]
- Huang, T.; Yan, M.; He, K.; Huang, Z.; Zeng, G.; Chen, A.; Peng, M.; Li, H.; Yuan, L.; Chen, G. Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. J. Colloid Interface Sci. 2019, 543, 43–51. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Wang, B.; Long, Y.; Zhang, M.; Ma, J.; Khan, A.; Chowdhury, S.P.; Zhou, X.; Ni, Y. Cellulose-supported magnetic Fe3O4-MOF composites for enhanced dye removal application. Cellulose 2019, 26, 4909–4920. [Google Scholar] [CrossRef]
- Luo, J.; Maier, R.M.; Yu, D.; Liu, B.; Zhu, N.; Amy, G.L.; Crittenden, J.C. Double-Network Hydrogel: A Potential Practical Adsorbent for Critical Metals Extraction and Recovery from Water. Environ. Sci. Technol. 2022, 56, 4715–4717. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.G.; Ma, J.P.; Li, S.; Guan, J.; Jiang, B.; Wang, L.Y.; Li, J.H.; Wang, X.Y.; Chen, L.X. Magnetic copper-based metal organic framework as an effective and recyclable adsorbent for removal of two fluoroquinolone antibiotics from aqueous solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef]
- Han, P.P.; Xia, Y. Thiol-functionalized metal-organic framework for highly efficient removal of bromate from water. J. Environ. Chem. Eng. 2018, 6, 3384–3391. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Schindler, B.J.; Killops, K.L.; Browe, M.A.; Mahle, J.J. The effect of water adsorption on the structure of the carboxylate containing metal-organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66. J. Mater. Chem. A 2013, 1, 11922–11932. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.G.; Yuan, Y.P.; Peng, F.M.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef]
- Yang, Y.; Shukla, P.; Wang, S.; Rudolph, V.; Chen, X.-M.; Zhu, Z. Significant improvement of surface area and CO2 adsorption of Cu-BTC via solvent exchange activation. RSC Adv. 2013, 3, 17065–17072. [Google Scholar] [CrossRef]
- Hamon, L.; Jolimaitre, E.; Pirngruber, G.D. CO2 and CH4 Separation by Adsorption Using Cu-BTC Metal-Organic Framework. Ind. Eng. Chem. Res. 2010, 49, 7497–7503. [Google Scholar] [CrossRef]
- Bordiga, S.; Regli, L.; Bonino, F.; Groppo, E.; Lamberti, C.; Xiao, B.; Wheatley, P.S.; Morris, R.E.; Zecchina, A. Adsorption properties of HKUST-1 toward hydrogen and other small molecules monitored by IR. Phys. Chem. Chem. Phys. 2007, 9, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Chiericatti, C.; Carlos Basilico, J.; Zapata Basilico, M.L.; Manuel Zamaro, J. Novel application of HKUST-1 metal-organic framework as antifungal: Biological tests and physicochemical characterizations. Microporous Mesoporous Mater. 2012, 162, 60–63. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Karimi, M.; Daasbjerg, K. Efficient removal of crystal violet and methylene blue from wastewater by ultrasound nanoparticles Cu-MOF in comparison with mechanosynthesis method. Ultrason. Sonochem. 2017, 37, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Zhao, R.; Qin, J.; Wang, H.; Wang, D. Ultra-efficient removal of NO in a MOFs-NTP synergistic process at ambient temperature. Chem. Eng. J. 2019, 358, 291–298. [Google Scholar] [CrossRef]
- Wee, L.H.; Lohe, M.R.; Janssens, N.; Kaskel, S.; Martens, J.A. Fine tuning of the metal-organic framework Cu-3(BTC)(2) HKUST-1 crystal size in the 100 nm to 5 micron range. J. Mater. Chem. 2012, 22, 13742–13746. [Google Scholar] [CrossRef]
- Van Assche, T.R.C.; Desmet, G.; Ameloot, R.; De Vos, D.E.; Terryn, H.; Denayer, J.F.M. Electrochemical synthesis of thin HKUST-1 layers on copper mesh. Microporous Mesoporous Mater. 2012, 158, 209–213. [Google Scholar] [CrossRef]
- Zou, F.; Yu, R.; Li, R.; Li, W. Microwave-assisted synthesis of HKUST-1 and functionalized HKUST-1-@H3PW12O40: Selective adsorption of heavy metal ions in water analyzed with synchrotron radiation. Chemphyschem 2013, 14, 2825–2832. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Hill, P.; Torrente-Murciano, L.; Garforth, A.; Gorgojo, P.; Siperstein, F.; Fan, X. Mapping the Cu-BTC metal-organic framework (HKUST-1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases. Chem. Eng. J. 2015, 281, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Osborn Popp, T.M.; Plantz, A.Z.; Yaghi, O.M.; Reimer, J.A. Precise Control of Molecular Self-Diffusion in Isoreticular and Multivariate Metal-Organic Frameworks. Chemphyschem 2020, 21, 32–35. [Google Scholar] [CrossRef]
- Senkovska, I.; Kaskel, S. Ultrahigh porosity in mesoporous MOFs: Promises and limitations. Chem. Commun. 2014, 50, 7089–7098. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-p.; Zou, C.-d.; Zhao, B.-g.; Zhai, Q.-j.; Gao, Y.-l. Size control and its mechanism of SnAg nanoparticles. Trans. Nonferrous Met. Soc. China 2014, 24, 750–757. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Zhang, M.; Wang, X. Molecular and textural engineering of conjugated carbon nitride catalysts for selective oxidation of alcohols with visible light. Chem. Sci. 2013, 4, 3244–3248. [Google Scholar] [CrossRef]
- Seoane, B.; Castellanos, S.; Dikhtiarenko, A.; Kapteijn, F.; Gascon, J. Multi-scale crystal engineering of metal organic frameworks. Coord. Chem. Rev. 2016, 307, 147–187. [Google Scholar] [CrossRef]
- Pan, Y.; Heryadi, D.; Zhou, F.; Zhao, L.; Lestari, G.; Su, H.; Lai, Z. Tuning the crystal morphology and size of zeolitic imidazolate framework-8 in aqueous solution by surfactants. CrystEngComm 2011, 13, 6937–6940. [Google Scholar] [CrossRef]
- Loera-Serna, S.; Oliver-Tolentino, M.A.; de Lourdes López-Núñez, M.; Santana-Cruz, A.; Guzmán-Vargas, A.; Cabrera-Sierra, R.; Beltrán, H.I.; Flores, J. Electrochemical behavior of [Cu3(BTC)2] metal–organic framework: The effect of the method of synthesis. J. Alloys Compd. 2012, 540, 113–120. [Google Scholar] [CrossRef]
- Hartmann, M.; Kunz, S.; Himsl, D.; Tangermann, O.; Ernst, S.; Wagener, A. Adsorptive separation of isobutene and isobutane on Cu3(BTC)2. Langmuir 2008, 24, 8634–8642. [Google Scholar] [CrossRef]
- Wi-Afedzi, T.; Kwon, E.; Tuan, D.D.; Lin, K.-Y.A.; Ghanbari, F. Copper hexacyanoferrate nanocrystal as a highly efficient non-noble metal catalyst for reduction of 4-nitrophenol in water. Sci. Total Environ. 2020, 703, 134781. [Google Scholar] [CrossRef]
- Luo, Q.-x.; An, B.-w.; Ji, M.; Park, S.-E.; Hao, C.; Li, Y.-q. Metal–organic frameworks HKUST-1 as porous matrix for encapsulation of basic ionic liquid catalyst: Effect of chemical behaviour of ionic liquid in solvent. J. Porous Mater. 2014, 22, 247–259. [Google Scholar] [CrossRef]
- Eren, M.Ş.A.; Arslanoğlu, H.; Çiftçi, H. Production of microporous Cu-doped BTC (Cu-BTC) metal-organic framework composite materials, superior adsorbents for the removal of methylene blue (Basic Blue 9). J. Environ. Chem. Eng. 2020, 8, 104247. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Wang, Y.; Wu, M.; Zhang, L.; Wang, L.; Ni, H. Surface functionalization of cellulose with hyperbranched polyamide for efficient adsorption of organic dyes and heavy metals. J. Clean. Prod. 2019, 232, 774–783. [Google Scholar] [CrossRef]
- Sabouni, R.; Aidan, A.; AlObeidli, A.; Lahib, F.; Bacha, H.H.; Kassermally, R.; Jarmakani, S. Adsorption kinetics and thermodynamics of Methylene Blue by HKUST-1. Desalination Water Treat. 2019, 138, 301–312. [Google Scholar] [CrossRef]
- Luo, J.M.; Yu, D.Y.; Hristovski, K.D.; Fu, K.X.; Shen, Y.W.; Westerhoff, P.; Crittenden, J.C. Critical Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Mechanism Identification and Engineering Design. Environ. Sci. Technol. 2021, 55, 4287–4304. [Google Scholar] [CrossRef]
- Kavak, S.; Durak, O.; Kulak, H.; Polat, H.M.; Keskin, S.; Uzun, A. Enhanced Water Purification Performance of Ionic Liquid Impregnated Metal-Organic Framework: Dye Removal by BMIM PF6 /MIL-53(Al) Composite. Front. Chem. 2021, 8, 1277. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef]
- Huang, X.-X.; Qiu, L.-G.; Zhang, W.; Yuan, Y.-P.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Hierarchically mesostructured MIL-101 metal–organic frameworks: Supramolecular template-directed synthesis and accelerated adsorption kinetics for dye removal. CrystEngComm 2012, 14, 1613–1617. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Chen, D.; Wei, F.H.; Chen, N.N.; Liang, Z.; Luo, Y. Synthesis of graphene oxide/metal-organic frameworks hybrid materials for enhanced removal of Methylene blue in acidic and alkaline solutions. J. Chem. Technol. Biotechnol. 2018, 93, 698–709. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.L.; Gao, M.; Hong, W.; Liu, G.Z.; Fan, L.; Hu, B.; Xia, Q.H.; Liu, L.; Song, G.W.; et al. The adsorption on magnetic hybrid Fe3O4/HKUST-1/GO of methylene blue from water solution. J. Mater. Chem. A 2014, 2, 1795–1801. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Metal organic framework (MOF) porous octahedral nanocrystals of Cu-BTC: Synthesis, properties and enhanced adsorption properties. Mater. Res. Bull. 2019, 109, 124–133. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, S.; Zhao, Q.; Lu, R.; Hang, C.; Chen, Z.; Zheng, H. Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem. Eng. J. 2018, 333, 49–57. [Google Scholar] [CrossRef]
- Wang, R.; Ge, C.; Tianzhu, X.; Yuanyuan, Z.; Zhang, Y.; Zhang, X. Facile synthesis of magnetic hybrid metal organic frameworks with high adsorption capacity for methylene blue. Appl. Organomet. Chem. 2017, 31, e3798. [Google Scholar] [CrossRef]
- Abd El Salam, H.M.; Zaki, T. Removal of hazardous cationic organic dyes from water using nickel-based metal-organic frameworks. Inorg. Chim. Acta 2018, 471, 203–210. [Google Scholar] [CrossRef]
- Bui, T.T.M.; Nguyen, L.T.; Pham, N.P.H.; Tran, C.C.; Nguyen, L.T.; Nguyen, T.A.; Nguyen, H.N.; Nguyen, M.V. A new approach for ultra-high adsorption of cationic methylene blue in a Zr-sulfonic-based metal-organic framework. RSC Adv. 2021, 11, 36626–36635. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Sun, Q. Rapid and selective adsorption of cationic dyes by a unique metal-organic framework with decorated pore surface. Appl. Surf. Sci. 2018, 440, 1219–1226. [Google Scholar] [CrossRef]
- Zhuang, J.-L.; Ceglarek, D.; Pethuraj, S.; Terfort, A. Rapid Room-Temperature Synthesis of Metal Organic Framework HKUST 1 Crystals in Bulk and as Oriented and Patterned Thin Films. Adv. Funct. Mater. 2011, 21, 1442–1447. [Google Scholar] [CrossRef]


| Surfactant | Formula | Molecular Weight | Structural Diagram | * Cost (RMB ton−1 ΔParticle Size−1) |
|---|---|---|---|---|
| SDS | C12H25SO4Na | 288.38 | 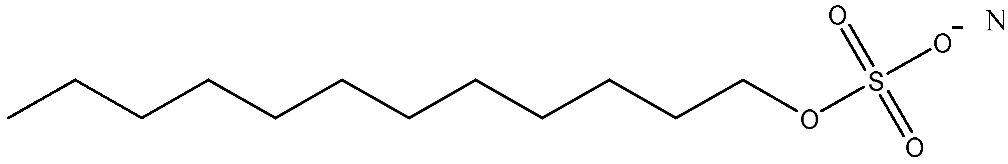 | 4.4 |
| SDBS | C18H29NaO3S | 348.476 | 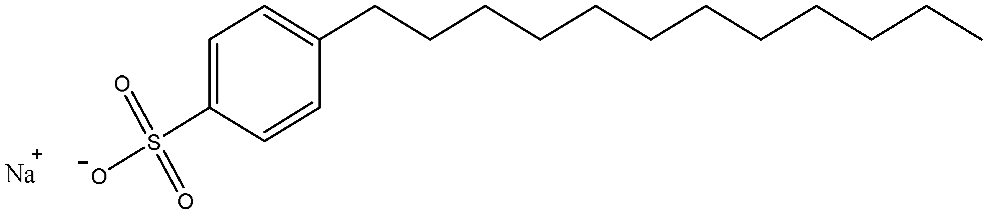 | 0.9 |
| DTAB | C15H34NBr | 308.34 | 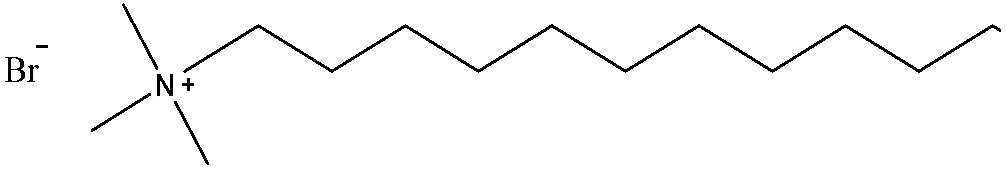 | 7 |
| CTAB | C19H42BrN | 364.46 | 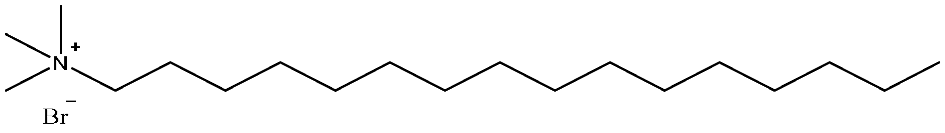 | 2.8 |
| PEG-1000 | H(OCH2CH2)nOH | 1000 | 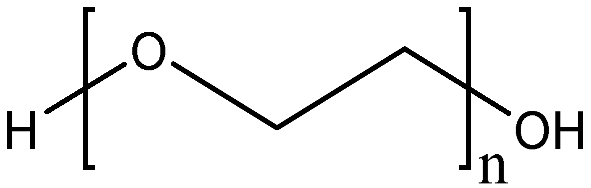 | 3.9 |
| PVP | (C6H9NO)n | 10,000 | 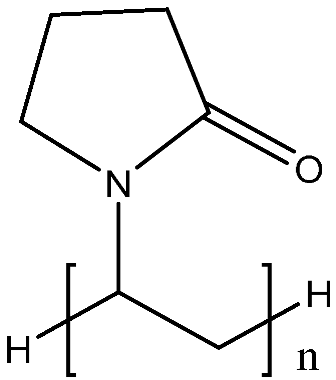 | 0.2 |
| Sample | SBET (m2 g−1) | Average Pore Size (nm) |
|---|---|---|
| pristine Cu-BTC | 424.597 | 2.713 |
| PV-Cu-BTC | 1093.764 | 2.381 |
| Adsorbent | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1 ) | qe (mg g−1) | R2 | |
| PV-Cu-BTC | 8.9209 | 12.7 | 0.6247 | 0.0733 | 13.1 | 0.9991 |
| Temperature (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Kl (L mg−1) | qm (mg g−1) | R2 | f | Kf (g mg−1 min−1) | R2 | |
| 298 | 0.0206 | 169.2 | 0.9228 | 0.3522 | 17.9535 | 0.9832 |
| Adsorbents | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|
| [BMIM][PF6]/MIL-53 | 204.9 | [42] |
| MOF-235 | 187 | [43] |
| MIL-101 MOFs | 21 | [44] |
| Ni-MOFs | 156 | [45] |
| HKUST-1/GO/Fe3O4 | 105.9 | [46] |
| Cu-BTC MOF | 101.2 | [47] |
| Fe3O4@ZIF-67 | 20 | [48] |
| Fe3O4/HKUST-1 | 104 | [49] |
| NiCu-BTC | 798 | [50] |
| VNU-23 | 1992 | [51] |
| Zn-MOF | 326 | [52] |
| PV-Cu-BTC | 169.2 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, L.; Zhang, M.; Xu, L.; Hu, Q.; Yang, T.; Tu, K.; Wu, M.; Yu, D. Enhanced Methylene Blue Adsorption by Cu-BTC Metal-Organic Frameworks with Engineered Particle Size Using Surfactant Modulators. Water 2022, 14, 1864. https://doi.org/10.3390/w14121864
Wang S, Zhang L, Zhang M, Xu L, Hu Q, Yang T, Tu K, Wu M, Yu D. Enhanced Methylene Blue Adsorption by Cu-BTC Metal-Organic Frameworks with Engineered Particle Size Using Surfactant Modulators. Water. 2022; 14(12):1864. https://doi.org/10.3390/w14121864
Chicago/Turabian StyleWang, Shanli, Lu Zhang, Mingyan Zhang, Licong Xu, Qian Hu, Tao Yang, Kaili Tu, Minghua Wu, and Deyou Yu. 2022. "Enhanced Methylene Blue Adsorption by Cu-BTC Metal-Organic Frameworks with Engineered Particle Size Using Surfactant Modulators" Water 14, no. 12: 1864. https://doi.org/10.3390/w14121864







