1. Introduction
Globally, freshwater use has increased by a factor of 6 in the last 100 years, and it is estimated that the world will face a 40% water deficit by 2030. Although the actual increase in global water use is not known, scientific articles agree that we will face competition for the growing demand of the industrial and energy sectors based on industrial development and the increased coverage of water and sanitation services [
1,
2,
3,
4,
5,
6].
In developing countries, part of the population has suffered from health conditions or illnesses caused by the direct consumption of contaminated water. Drinking water is limited, and its quality is under constant pressure due to the presence of infectious agents from microbial agents such as bacteria and fungus, toxic chemicals, and even radiation, which means it is a global challenge that has increased in both developed and developing countries, weakening the economic growth as well as the socio-environmental sustainability and health of billions of people [
7,
8]. However, the proliferation of heavy metals in water sources is also a growing concern due to limited access to the resources required for implementing effective techniques in pollutant removal, and it is crucial due to their acute toxicity, long-term accumulation, and persistence [
5,
9,
10].
Additionally, the arrangement of large quantities of biomass waste that are continuously produced is one of the primary sources of pollution [
11]. Approximately 100 billion metric tons of biomass waste are generated worldwide [
12]. These include forest and agricultural waste, fruit, and other food processing waste. On top of this agricultural waste, the coffee industry provides 7.4 million tons of spent coffee beans and an additional amount of pulp, shell, and silver skin (husk) [
13,
14]. The outer layer of the coffee bean is called silver skin.
Agricultural waste is commonly burned, emitting pollutants into the atmosphere, such as carbon monoxide, nitrous oxide, nitrogen dioxide, and particulate matter. This is accompanied by the formation of ozone and nitric acid, which contribute to acid deposition, bringing risks to human and environmental health, in addition to methane and CO
2, which are greenhouse gases, all of this resulting in global warming [
13,
15,
16].
In this context, biomass revalorization is considered a technological and sustainable solution. Different applications for biomass waste have been proposed, such as the development of new products for energy generation, sorption, and biosorption of pollutants, among others, replacing or reducing the use of hazardous chemicals, as well as lowering costs in industrial processes [
12]. Likewise, the valorization of various materials has also involved their modification with nanoparticle systems (NPs), connecting interdisciplinary areas such as physics, biology, engineering, and medicine in corresponding applications [
17,
18,
19].
In this study, we present the preparation and characterization of nanocomposite based on revalorized materials: coffee husk, coffee lignin, and coffee husk and lignin modified with silver nanoparticles (AgNPs). In addition, the suitability for the sorption capacity towards different metal ions of Pb(II), Cd(II), Cr(III), and Cu(II) and antifungal activity with Candida fungi species were properly evaluated, with modified materials, kinetic modeling and contact time experiments [
20,
21,
22,
23,
24,
25,
26]. The results obtained contribute with the revalorization of these Colombian agroindustrial wastes due to their modification with silver nanoparticles. The improvement of traditional waste materials in the extraction of heavy metal ions in an aqueous solution with antifungal activity is an alternative to their disposal.
2. Materials and Methods
2.1. Chemical Reagents and Solutions
All the chemicals were of analytic grade. A multiple heavy metals 1000 mg/L stock solution of Pb(II), Cd(II), Cr(III), and Cu(II) is prepared from their nitric salts (all 99% from Panreac, Barcelona, Spain). H2SO4 (>99%), AgNO3 salt (>99%), and NaBH4 salt (>98%) are supplied by Sigma Aldrich (Bucaramanga, Colombia). Standards of heavy metal ions were properly diluted in acidified water with 2% of nitric acid to prepare the calibration set for analysis at a concentration range between 0.01 and 1 µg/L.
2.2. Coffee Husk Preparation and Lignin Obtention
The coffee husk has cellulose, hemicellulose, and lignin contents on a dry basis, as is well known. Lignin provides structural rigidity to the cell walls of many plant species [
27]. Lignin has been used as a potential adsorbent to remove heavy metals due to its unique polyphenolic structure and physicochemical properties [
28].
For that reason, here, lignin is also checked and compared with the original coffee husk biomass.
The coffee husk is obtained from the Café Santander factory in the municipality of Curití, Santander-Colombia. The biomass is obtained in two ways, the first when leaving the coffee roasting production plant, whose shape is an irregular and undefined particle size, and a second that is homogenized in a mill and sieved to a diameter size less than 250 μm. From the sieved coffee husk, acid hydrolysis is performed in H
2SO
4 to 72% (in a ratio of 3 mL of acid per 300 mg of the sample) to extract insoluble lignin. Afterward, it is carried into a thermostatic water bath at 30 °C for one hour, stirring every five minutes. The acidic solution was diluted a concentration of 4%. This solution is autoclaved for two h at 120 °C with a pressure of 103 kPa. Afterward, this mixture is filtered and washed to a neutral pH [
29]. Finally, the solid phase lignin material is obtained [
30].
2.3. Sorbents Modification with AgNPs
The synthesis of AgNPs is carried out using AgNO
3 as a precursor and NaBH
4 as a reducing agent [
31,
32]. For the nano-silver modification of the coffee husk and lignin, this impregnation method is followed in the presence of the biomasses. Thus, the coffee husk and lignin are deposited in falcon tubes, then a 0.1 M silver nitrate solution is added. The biomass and precursor contact time was fixed at 45 min with vigorous agitation. It is then centrifuged at 5000 revolutions per minute (rpm) for 10 min, and then the remaining silver nitrate solution and solid are separated by centrifugation. Once the biomass is impregnated with AgNO
3, 0.3 M is added slowly at room temperature. The volume used for the impregnation with the precursor and the reducing agent was always twice the volume of the biomass used, with a contact time of 45 min. Finally, the liquid and solid phases are separated by centrifugation and filtrated, then the solid phase obtained is washed until a neutral pH is obtained. In this way, both coffee husk and lignin were properly modified with silver nanoparticles (AgNPs). The obtained materials’ morphology is characterized by using Scanning Electron Microscopy (SEM).
2.4. Sorption Experiments
Sorption experiments were carried out at room temperature with aqueous solutions that are prepared at pH 4.0, containing the metals at a concentration of 0.18 mM each, following conditions of previous studies [
25,
26,
33,
34]. The pH is measured with a standardized potentiometer [
35].
Batch sorption experiments were performed with the following steps: 25 mg of each sorbent were placed in falcon tubes, and then 2.5 mL of a heavy metal aqueous solution was added into the tube. Later, samples are placed on a rotary mixer (CE 2000 ABT-4, SBS Instruments SA, Barcelona, Spain) and shaken at 25 rpm. The two phases are separated by decantation and later the aqueous supernatant phase is filtered through 0.22 μm Millipore filters (Millex-GS, Millipore, Burlington, MA, USA), diluted, and analyzed, ensuring a concentration range into the calibration range (as mentioned at 0.01–1 µg/L). Finally, the multielement analysis is carried out by ICP-MS (XSERIES 2 ICP-MS, Thermo Scientific, Waltham, MA, USA) [
35].
All the results are expressed as the mean value of minimum duplicate experiments, and the standard deviation (SD) is used to analyze data errors.
To optimize sorption conditions, and mainly the contact time, the corresponding experiments were carried out by using all biomass materials (the coffee husk and lignin, and modified ones both with AgNPs) [
27,
28,
29]. A properly weighed amount of each biomass system (25 mg) with corresponding metal ions solutions (2.5 mL) was shaking both at different contact times (10, 15, 30, 45, 60 min, and 2, 4, 12, and 24 h) [
25].
2.5. Kinetic Modeling
The sorption mechanism of the metal ions’ sorption process is evaluated using first- and second-order kinetic models with the experimentally obtained data [
36]. The Lagergren pseudo-first-order model is based on the range of change in the solute uptake with time, which is generally applicable over the initial stage of an adsorption process (Equation (1)) [
36,
37,
38,
39].
where
Q1 is the equilibrium adsorption capacity,
qt is the adsorption capacity at the time
t, and
k1 (min
−1) is the adsorption rate of the pseudo-first-order kinetic model.
The pseudo-second-order kinetic model can be represented in the linear form as (Equation (2)) [
40]:
where
k2 (g∙mg
−1∙min
−1) is the rate constant,
qe is the amount of solute adsorbed at equilibrium and
qt is the amount of solute adsorbed (mg∙g
−1) at time
t.
2.6. Antifungal Assays
Antifungal assays of each material are performed with
Candida fungi species such as
Candida albicans,
Candida parapsilosis,
Candida glabrata,
Candida krusei and
Candida guilliermondii. These fungus species here selected are well known as opportunistic pathogens, are frequently found in different anatomical sites, and clinical samples could induce systemic, superficial, and nosocomial infections under optimal environmental conditions by their relative resistance to the external environment [
30,
31,
32,
33].
The antifungal evaluation of the biomass materials is carried out using the Time Kill method. This method is appropriate to evaluate the bactericidal and/or fungicidal activity of certain compounds and allows obtaining information on the dynamic interaction between the antifungal agent and the fungal strain. RPMI 1640 culture medium for different species of
Candida are inoculated and incubated for 24 h in a shaker at 37 ± 1 °C. Then, 1 mL of this inoculum is deposited in a glass container together with the different biomass materials under the study and at different biomass relative concentrations (0.37, 0.75, 1.5 mg/mL). After 24 h of contact, its viability is evaluated by sowing on the agar surface and evaluating the decrease in the logarithm of CFU (Colony Forming Units), as a measure of the antifungal activity of the biomass materials. The CFU/mL was determined following the procedure described in previous work [
41].
2.7. Infrared Spectroscopy
FT-IR analyses were carried out to establish which functional groups are present in the biomass, and which could take part in the modification of coffee and lignin with AgNPs.
2.8. Electron Microscopy Characterization
The morphology of the different nanocomposite materials is analyzed by scanning electron microscopy (SEM) coupled with energy dispersive X-ray (EDX). The samples are coated with gold for the surface study. Images were obtained with the Zeiss EVO® MA10 (Carl Zeiss, Jena, Germany), Zeiss Merlin™ (Carl Zeiss, Germany), and Quanta™ 650 FEG units (FEI Company, Hillsboro, AL, USA).
3. Results and Discussion
3.1. Characterization of Sorbents
Scanning Electron Microscopy (SEM) was performed to analyze the morphological features and evaluate the changes on the material surfaces across the material’s modification and sorption experiments (see
Figure 1).
It is observed that the coffee material changed its morphology through the different treatments received, such as modification with AgNPs and the possible accumulation of metal ions on the surface of the material after the metal sorption experiments.
The coffee husk sample, as observed in
Figure 1a, has a rough and amorphous surface (corrugated). The modification of coffee with AgNPs leads to identifying the presence of scattered particles on the surface, as can be shown in
Figure 1b, and they also do not show any morphological change, except in the deposition of AgNPs. Thus, as expected, nanoparticles of Ag were properly immobilized onto the coffee husk.
The black patches on
Figure 1a,b are probably pore apertures and cavities, which may influence the increase in sorption kinetics [
42,
43,
44]. On the other hand,
Figure 1c shows some changes in the coffee husk morphology. It looks like the roughness and porosity decreased after the heavy metal ions’ adsorption experiment. Notice that there is an evident morphological difference between the surface of the material before (
Figure 1a) and after the adsorption of metals (
Figure 1c). It is observed that the surface of the coffee husk after the sorption of metal ions became smoother than it was before, which is usually related to being in contact with an acidic aqueous solution, as is the case here (pH 4). These results are consistent with those demonstrated previously in the literature [
45], where the same behavior is observed working with copper, zinc, and cadmium metal ion aqueous solutions at pH 4, obtaining different degrees of smoothness of the surface and systems with removal efficacy [
26]. The elemental analysis confirmed the presence of Ag on the surface of
Figure 1b. The presence of synthesized AgNPs was confirmed by the peak at 3.0 keV in EDS [
46,
47,
48].
FT–IR measurements were used to understand the role of the AgNPs’ modification on the biomass materials. The FT–IR spectra is presented in
Figure 2. The peak around 3328 cm
−1 is attributed to the −OH stretching as a result of functional groups of cellulose, hemicellulose, and lignin [
44,
49]. The peak of 2919 cm
−1 corresponds to C–H groups, 1731 cm
−1, 1639 cm
−1 C=O of aldehyde and ketone groups, 1458 cm
−1 from aromatic C=C stretching groups and CH
2 and CH
3 groups, around 1371 cm
−1 from aromatic C–H stretching and carboxyl−carbonate structures, 1238 cm
−1 from CHOH groups, and around 1029 cm
−1 Si–O–Si group [
42,
44,
49,
50].
3.2. Sorption Kinetics of Raw Materials and Nanocomposite Materials
The sorption kinetics experimental data for multi-metal aqueous solutions (Pb, Cd, Cr, and Cu) by using the raw and the nanocomposite biomass materials are collected in
Figure 3.
As can be observed in
Figure 3a, in the case of lead comparing lignin and nanocomposite AgNPs’ modified lignin materials, a potentiating effect in the metal sorption in the presence of AgNPs is observed, reaching around 46% of Pb sorption at 45 min and with a maximum sorption of 56% after 24 h (with raw lignin of just 31% reached). In the case of coffee husk and AgNPs’ modified coffee husk materials, it is observed that the AgNPs’ potentiating effect is even higher than with lignin-based materials. In this case, the Pb sorption percentage increases from around 57% with raw coffee husk up to 99% with the nanocomposite AgNPs’ modified coffee husk. These results demonstrate that the best material for the sorption of Pb is the coffee husk modified with AgNPs, which also highlights that just 4 h of the experiment are needed under the experimental conditions here studied for all cases (2 h for AgNPs–coffee husk).
In the case of the sorption kinetics experiments for cadmium, the results collected in
Figure 3b also show the maximum sorption percentage for the AgNPs’ modified coffee husk, reaching 83% after 24 h. The sorption of Cd with the other biomass materials under study does not give significant results. Both the raw lignin and coffee husk, and the AgNPs’ modified lignin, have Cd extraction percentages not higher than 20%.
Like Pb, the improved sorption results for chromium are observed with the AgNPs modified coffee husk reaching 95% of chromium sorption after 24 h, highlighting that from around 2 h values above 90% are obtained (as can be observed in
Figure 3c). The addition of silver nanoparticles in the coffee husk shows an evident enhancing effect in the extraction of Cr. On the other hand, the modification of the lignin with AgNPs yields a maximum adsorption value of around 50% after 24 h of the sorption experiment, almost double that of raw lignin (around 25%).
Cooper’s sorption behavior (see
Figure 3d) is similar to those of Pb and Cr, even with less efficient sorption when using coffee husk silver nanocomposite (which is AgNPs–coffee husk), just around 80%. It was also observed that the use of nanocomposite lignin with AgNPs reached an extraction percentage after 24 h of around 60%.
Thus, both coffee husk and lignin modified with AgNPs show an enhancement in the extraction of metal ions compared with corresponding raw materials, probably due to the presence of the silver nanoparticles. This can be deduced since the sorption percentages found for both raw coffee husk and lignin are under 30% of metal sorption, except for Pb than raw coffee husk gives up to 50%. Furthermore, the nanocomposite AgNPs–coffee husk reaches quite a high metal sorption just with 2 h of adsorption experiments for Pb, Cd, and Cr ions. The behavior of the sorption capacity of this AgNPs–coffee husk shows the following decreasing order: Pb(II) ≈ Cr(III) > Cu(III) ≈ Cd(II). The AgNPs’ modification enhances the affinity towards the heavy metal ions due to nanocomposite electrostatic charges.
3.3. Evaluation of Sorption Capacity
The results shown in
Table 1 confirm that the modification of the coffee husk and lignin with AgNPs achieves an enhancing effect on the adsorption of metal ions at pH 4 and after 24 h of the sorption experiments. It should be noticed that the coffee husk with AgNPs is the material that achieves the highest values of sorption capacity for each metal ion evaluated, as mentioned previously.
The results evidenced that the modification of the coffee husk with AgNPs has a potentiating effect on the sorption of the studied metal ions. Thus, from the above results, it can be inferred that the increase in the sorption of heavy metals of the AgNPs’ modified materials could be due to two reasons. The first can be related to the use of NaBH
4 as a reducing agent in the synthesis of AgNPs on biomass, inducing a kind of pretreatment on the surface and increasing the availability of active sites; and the second, the possible effect of AgNPs on the biomass surface to increase the sorption. The AgNPs’ modification process could be considered alkaline, modifying the biomasses moieties, and improving the heavy metal ions’ sorption system [
58]. However, this assumption cannot be verified in the morphological characterization carried out [
58,
59]. According to [
60], the nanocomposites have enhanced active sites that demonstrate stronger sorption on Pb ions than Cr, Cd, and Cu ions.
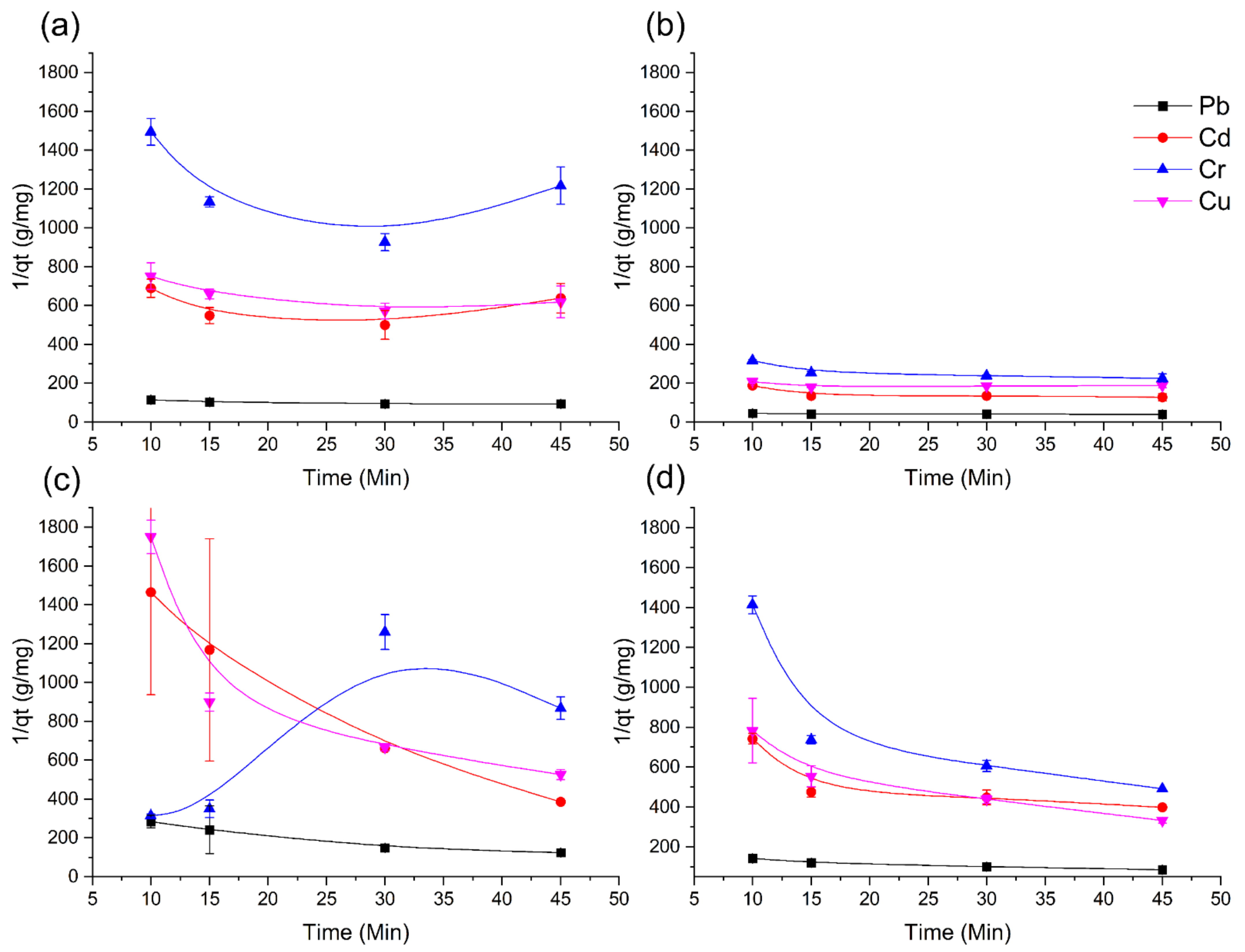
3.4. Kinectic Modeling
Figure 4 shows the pseudo-first-order kinetics modeling with all the four materials here under study for the metal sorption [
28]. It is highlighted that AgNPs–coffee husk achieved better values of 1/
qt (see corresponding equation from the Materials and Methods), where the order of best sorption was Pb > Cd > Cu > Cr, which is like those obtained for the other materials (mainly for AgNPs–coffee husk and AgNPs–lignin).
The adjustment of the pseudo-second-order model demonstrates behavior that tends towards linearity, regardless of the material used, except for Cr with both raw biomass systems (as can be seen from
Figure 5), where the AgNPs–coffee husk shows the best correlation coefficient (values collected in
Table 2. The pseudo-second-order kinetic model assumes that the rate-limiting step is chemical sorption or chemisorption [
36].
However, the correlation coefficient values show that the sorption mechanisms of the metals on all the sorption materials used do not follow the pseudo-first-order kinetic model. The results of the correlation coefficients of both the pseudo-first-order model and the pseudo-second-order model are presented in
Table 2.
The experimental data exhibits good fitting with the pseudo-second-order model with R
2 values ranging from 0.9579 to 1.0000, better than those of the pseudo-first-order model (from 0.0007 to 0.9884) (
Table 2). Although such negative values (
Table 2) are not usually observed, as in another study reported previously, this may be associated with the electrostatic nature of the adsorption process [
61] In the case of the pseudo-first-order model, the correlation coefficients are quite low, which can probably be related to the adsorption of the metals not occurring exclusively by the ion exchange mechanism, which is mostly explained when experimental data can be adjusted by this model. The pseudo-second-order model assumes that the adsorption is controlled by the chemical adsorption or chemisorption process based on valence forces by either sharing or exchanging electrons between the adsorbent and the metal ions of Pb, Cd, Cr, and Cu [
62] The pseudo-first-order and the pseudo-second-order kinetic models both assume that the metal ions’ adsorption may be [
63].
The materials used as adsorbents have a variety of functional groups that allow inducing chemical adsorption together with physical adsorption on the adsorbent surface. Taking that into account, in the case of the use of modified materials, such as AgNPs–coffee husk or AgNPs–lignin, the obtained improvement in the adsorption rate can be related to the modification itself. The modification of the surface can increment the electrostatic interactions between the adsorbent surface and the metal ions [
62]. In addition, the kinetics is here checked with a mixed solution of metals that may imply a competition between them. The adsorption of metals could be related to the ionic radius and the electronegativity of each metal ion [
34]. Thus, Pb ions with higher ionic radius and electronegativity show the best R
2 and the highest rate constant
k2 of the pseudo-second-order kinetic model with AgNPs–coffee husk as sorbent, and this correlates to being the one with the highest adsorption rate.
3.5. Antifungal Assays
As in all Candida species checked (C. albicans, C. parapsilosis, C. glabrata, C. krusei and C. guilliermondii) lignin and its modification give less antifungal activity; thus, just coffee husk and its modifications will be presented here.
Thus, the antifungal behavior of coffee husk and its modification with silver nanoparticles against the species of
Candida albicans and
Candida parapsilosis can be observed in
Figure 6, respectively. As can be observed, the modified and unmodified coffee husk’s best performance is observed at a biomass relative concentration of 1.5 mg/mL, when a fungistatic effect is achieved. In turn, it can be evidenced that the AgNPs’ modified coffee husk presents a potentiating effect on the antifungal activity of both
Candida species, especially when comparing results of experiments with biomass relative concentration of 0.75 mg/mL, in terms of a material to be considered bacteriostatic.
Figure 7 presents the results of
Candida glabrata that evidence a higher antimicrobial activity in the case of the modified coffee husk AgNPs–coffee husk, in comparison with the previous mentioned species. In this case, the bacteriostatic effect of the modified coffee husk is also present at low relative biomass concentrations.
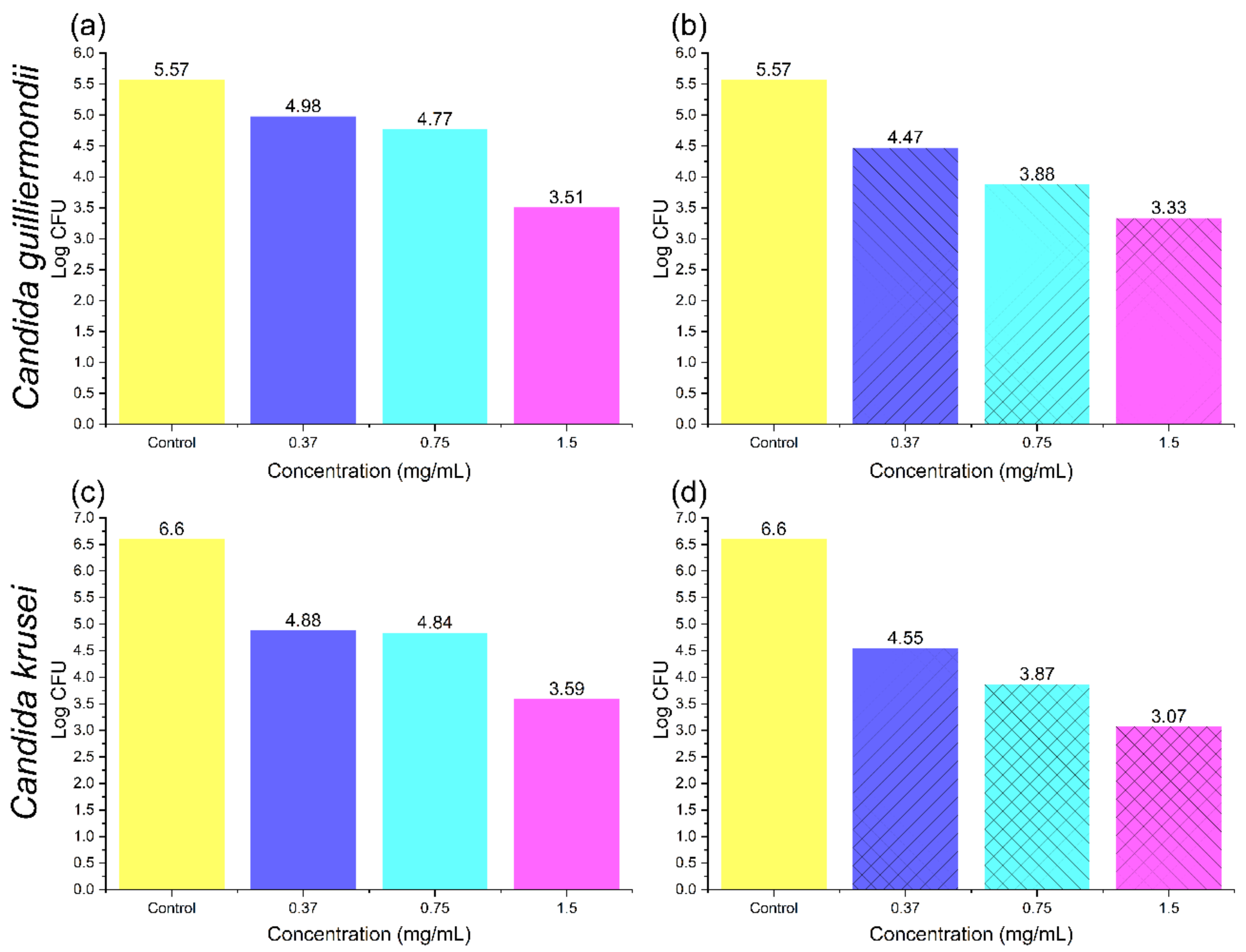
In
Figure 8, greater sensitivity of the
Candida krusei and
Candida guilliermondii species to both materials are evidenced, respectively. Especially at a biomass relative concentration of 1.5 mg/mL, where a bacteriostatic effect is obtained. In these cases, as for previous ones, the use of AgNPs–coffee husk shows higher values of logarithmic reduction, reflected in a higher bacteriostatic effect, with higher effectivity at 1.5 mg/mL.
Candida krusei was more affected than
Candida guilliermondii, as can be observed.
In summary, the modification of the coffee husk with AgNPs demonstrates an improving effect on the antifungal activity; however, using a 1.5 mg/mL concentration that exceeds two and sometimes reaches three logarithmic reductions could be considered fungistatic and antifungal activity, respectively. It should be noted that the use of AgNPs nowadays is increasing, and should also be applied for the reduction of
Candida classification species since they have been demonstrated to improve their microbial reduction and achieve biocompatibility in some cases [
64].
The materials’ concentration at which the highest fungistatic effect is achieved is 1.5 mg/mL. The material with the highest logarithmic reduction values is the modified coffee husk, which demonstrates a higher effect on the reduction of the microbial activity of the evaluated species. C. krusei is the species more sensitive to the general presence of both materials under study (coffee husk and its modifications with AgNPs), with the corresponding higher fungicidal effects, followed by C. glabrata.
4. Conclusions
The use of four different biomass materials as sorbents in this study demonstrates the viability of coffee husk and lignin to be able to remove heavy metals such as Pb, Cd, Cr, and Cu from aqueous solutions. Their modification with silver nanoparticles is here presented as a way of biomass nanocomposites’ preparation and as a comparison of the corresponding raw materials. It is demonstrated that the nanocomposites with silver are an option for the removal of the metals under study, under the conditions here checked (such as pH 4.0 and an initial concentration of 0.18 mmol/L), giving sorption percentages above 90% for Pb and Cr, and around 80% for Cd and Cu. When trying to model the sorption process, the pseudo-second model is the one that best described the heavy metal sorption under the working experimental conditions (24 h of sorption experiments), thus assuming that the adsorption is controlled by the chemisorption process.
When comparing all the sorbents here checked (coffee husk, lignin, and their modifications with AgNPs), the heavy metal sorption efficiency follows the order of AgNPs–coffee husk > coffee husk > AgNPs–lignin > lignin, with sorption capacities of 2.56 mg/g for Pb, 1.02 mg/g for Cd, 0.644 mg/g for Cu, and 0.575 mg/g for Cr with AgNPs–coffee husk.
Finally, the antifungal evaluation demonstrates that the modified and unmodified coffee husk have fungistatic activity on C. albicans, C. glabrata, C. krusei, and C. guilliermondii mainly with a concentration of 1.5 mg/mL, as they do not exceed the two logarithms of reduction, therefore demonstrating some fungicidal effect.
In this research, the implementation of the coffee husk modified with AgNPs was developed, and constitutes a promising sorbent in the sorption of heavy metal ions such as lead, cadmium, copper, and chromium from aqueous solutions, and can also inhibit the growth of fungus pathogens at the same time. To achieve a realistic application with wastewater using these materials, it is necessary to further study the reuse of the material and the cost of the modified materials to produce nanoparticle-modified biomass systems.
Author Contributions
Conceptualization, A.M.C., J.A.G.C., J.B.-A. and C.P.; data curation: M.Y.C.O. and D.F.G.-B.; methodology, D.F.G.-B., A.M.C., J.A.G.C. and M.Y.C.O.; formal analysis, D.F.G.-B. and M.Y.C.O.; investigation, D.F.G.-B., A.M.C., J.A.G.C., J.B.-A. and C.P.; writing—original draft preparation, D.F.G.-B.; writing—review and editing, A.M.C., J.B.-A. and C.P.; visualization, J.B.-A. and C.P.; supervision, A.M.C., J.B.-A. and C.P.; project administration, A.M.C.; Project administration: A.M.C.; funding acquisition: A.M.C., J.A.G.C., C.P. and M.Y.C.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by institutional funds for the development of the research, within the framework of project No. 01946233 Act No. 16-VII-2019 of Universidad Santo Tomás–Bucaramanga, and by one Spanish research project No. CTM2015-65414-C2-1-R.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Acknowledgment to the Grup de Tècniques de Separació en Química (GTS) for allowing and financing the sample analysis, as employees in the study of kinetics modeling. Thanks to the Servei de Microscòpia from Universitat Autónoma de Barcelona for allowing morphological studies of the samples. Moreover, thanks to the Grupo de Investigación en Bioquímica y Microbiología for allowing the microbiology analysis of samples; and thanks to the Grupo en Nuevos Materiales y Energías Alternativas (GINMEA) of the Universidad Santo Tomás for directing the project (GIFQCAAMBUISUPCP62017).
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 27 January 2021).
- OECD. OECD Environmental Outlook to 2050: The Consequences of Inaction; OECD Publishing: Paris, France, 2012; ISBN 978-92-64-122161. [Google Scholar]
- Burek, P.; Satoh, Y.; Fischer, G.; Kahil, M.T.; Scherzer, A.; Tramberend, S.; Nava, L.F.; Wada, Y.; Eisner, S.; Hanasaki, N.; et al. Water Futures and Solution—Fast Track Initiative; IIASA: Laxenburg, Austria, 2016. [Google Scholar]
- IEA Water Energy Nexus, Excerpt from the World Energy Outlook 2016; IEA: Paris, France, 2016.
- Genç-Fuhrman, H.; Mikkelsen, P.S.; Ledin, A. Simultaneous Removal of As, Cd, Cr, Cu, Ni and Zn from Stormwater Using High-Efficiency Industrial Sorbents: Effect of PH, Contact Time and Humic Acid. Sci. Total Environ. 2016, 566–567, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations. The United Nations World Water Development Report 2021: Valuing Water; UNESCO: Paris, France, 2021. [Google Scholar]
- Picetti, R.; Deeney, M.; Pastorino, S.; Miller, M.R.; Shah, A.; Leon, D.A.; Dangour, A.D.; Green, R. Nitrate and Nitrite Contamination in Drinking Water and Cancer Risk: A Systematic Review with Meta-Analysis. Environ. Res. 2022, 210, 112988. [Google Scholar] [CrossRef]
- Sagasta, M.; Zadeh, S.M.; Turral, H. More People, More Food, Worse Water?: A Global Review of Water Pollution from Agriculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Joseph, L.; Jun, B.-M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of Heavy Metals from Water Sources in the Developing World Using Low-Cost Materials: A Review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Egodawatta, P.; McGree, J.; Liu, A.; Goonetilleke, A. Human Health Risk Assessment of Heavy Metals in Urban Stormwater. Sci. Total Environ. 2016, 557–558, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishna, I.v.; Manickam, V. Introduction. In Environmental Management; Elsevier, 2017; pp. 1–4. [Google Scholar]
- TerraGreen Global Waste—Solvable Problem as a Renewable Energy Resource. Available online: https://medium.com/@support_61820/global-waste-solvable-problem-as-a-renewable-energy-resource-5d8f05cc1a7d (accessed on 27 January 2021).
- Alatzas, S.; Moustakas, K.; Malamis, D.; Vakalis, S. Biomass Potential from Agricultural Waste for Energetic Utilization in Greece. Energies 2019, 12, 1095. [Google Scholar] [CrossRef] [Green Version]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Spent Coffee Grounds as a Versatile Source of Green Energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Kumar, A.; Roy, S.S. Quantitative Analysis of the Methane Gas Emissions from Municipal Solid Waste in India. Sci. Rep. 2018, 8, 2913. [Google Scholar] [CrossRef]
- Sabiiti, E. Utilising Agricultural Waste to Enhance Food Security and Conserve the Environment. Afr. J. Food Agric. Nutr. Dev. 2011, 11, 3–5. [Google Scholar]
- Radhakrishnan, R.; Patra, P.; Das, M.; Ghosh, A. Recent Advancements in the Ionic Liquid Mediated Lignin Valorization for the Production of Renewable Materials and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2021, 149, 111368. [Google Scholar] [CrossRef]
- Becker, J.; Wittmann, C. A Field of Dreams: Lignin Valorization into Chemicals, Materials, Fuels, and Health-Care Products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Liu, Y.; Ruan, X.; Tsang, D.C.W.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin Valorization for the Production of Renewable Chemicals: State-of-the-Art Review and Future Prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of Nanoparticles: Technological Concepts and Future Applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Królikowska, A.; Kudelski, A.; Michota, A.; Bukowska, J. SERS Studies on the Structure of Thioglycolic Acid Monolayers on Silver and Gold. Surf. Sci. 2003, 532–535, 227–232. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, S.; Selvakannan, P.R.; Pasricha, R.; Mandale, A.B.; Sastry, M. Investigation into the Interaction between Surface-Bound Alkylamines and Gold Nanoparticles. Langmuir 2003, 19, 6277–6282. [Google Scholar] [CrossRef]
- Chandrasekharan, N.; Kamat, P.v. Improving the Photoelectrochemical Performance of Nanostructured TiO 2 Films by Adsorption of Gold Nanoparticles. J. Phys. Chem. B 2000, 104, 10851–10857. [Google Scholar] [CrossRef]
- Pető, G.; Molnár, G.L.; Pászti, Z.; Geszti, O.; Beck, A.; Guczi, L. Electronic Structure of Gold Nanoparticles Deposited on SiOx/Si(100). Mater. Sci. Eng. C 2002, 19, 95–99. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and Nanomaterials: Promises for Improved Tissue Regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Zhang, H.; Carrillo-Navarrete, F.; Palet-Ballús, C. Human Hair Biogenic Fiber as a Biosorbent of Multiple Heavy Metals from Aqueous Solutions. J. Nat. Fibers 2022, 19, 2018–2033. [Google Scholar] [CrossRef]
- Suárez-Forero, S.J.; Candela-Soto, A.M.; Henao-Martínez, J.A.; Bayona-Ayala, O.L. Evaluation of the Performance of the Preteretment with the Hydrogen Peroxide on Sugar Cane Bagasse for Removing Lignin. ITECKNE 2019, 16, 21–28. [Google Scholar] [CrossRef]
- Tokay, B.; Akpınar, I. A Comparative Study of Heavy Metals Removal Using Agricultural Waste Biosorbents. Bioresour. Technol. Rep. 2021, 15, 100719. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Burbano Patiño, A.A. Síntesis y Caracterización de Nanopartículas Magnéticas Del Tipo Core-Shell Fe3O4@Ag Soportadas Sobre Lignina y Cascarilla de Café; Universidad Santo Tomás: Bucaramanga, Colombia, 2018. [Google Scholar]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Silver. Chem.—A Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shen, X.-J.; Domene, X.; Alcañiz, J.-M.; Liao, X.; Palet, C. Comparison of Biochars Derived from Different Types of Feedstock and Their Potential for Heavy Metal Removal in Multiple-Metal Solutions. Sci. Rep. 2019, 9, 9869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Boada, R.; Cibin, G.; Palet, C. Enhancement of Selective Adsorption of Cr Species via Modification of Pine Biomass. Sci. Total Environ. 2021, 756, 143816. [Google Scholar] [CrossRef]
- Zhang, H. Biosorption of Heavy Metals from Aqueous Solutions Using Keratin Biomaterials. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2014. [Google Scholar]
- Sahoo, T.R.; Prelot, B. Adsorption Processes for the Removal of Contaminants from Wastewater. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. Z. Für Chem. Und Ind. Der Kolloide 1907, 2, 15. [CrossRef]
- Zheng, Y.-M.; Li, N.; Zhang, W.-D. Preparation of Nanostructured Microspheres of Zn–Mg–Al Layered Double Hydroxides with High Adsorption Property. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 195–201. [Google Scholar] [CrossRef]
- Rout, S.; Kumar, A.; Mana Ravi, P.; Mangal Tripathi, R. Pseudo Second Order Kinetic Model for the Sorption of U (VI) onto Soil: A Comparison of Linear and Non-Linear Methods. Int. J. Environ. Sci. 2015, 6, 145–154. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-Negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Daffalla, S.B.; Mukhtar, H.; Shaharun, M.S. Preparation and Characterization of Rice Husk Adsorbents for Phenol Removal from Aqueous Systems. PLoS ONE 2020, 15, e0243540. [Google Scholar] [CrossRef]
- Jahirul, M.; Rasul, M.; Chowdhury, A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energy 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Konneh, M.; Wandera, S.M.; Murunga, S.I.; Raude, J.M. Adsorption and Desorption of Nutrients from Abattoir Wastewater: Modelling and Comparison of Rice, Coconut and Coffee Husk Biochar. Heliyon 2021, 7, e08458. [Google Scholar] [CrossRef]
- Oliveira, W.E.; Franca, A.S.; Oliveira, L.S.; Rocha, S.D. Untreated Coffee Husks as Biosorbents for the Removal of Heavy Metals from Aqueous Solutions. J. Hazard. Mater. 2008, 152, 1073–1081. [Google Scholar] [CrossRef]
- Sharma, A.; Sagar, A.; Rana, J.; Rani, R. Green Synthesis of Silver Nanoparticles and Its Antibacterial Activity Using Fungus Talaromyces Purpureogenus Isolated from Taxus Baccata Linn. Micro Nano Syst. Lett. 2022, 10, 2. [Google Scholar] [CrossRef]
- Chandrasekharan, S.; Chinnasamy, G.; Bhatnagar, S. Sustainable Phyto-Fabrication of Silver Nanoparticles Using Gmelina Arborea Exhibit Antimicrobial and Biofilm Inhibition Activity. Sci. Rep. 2022, 12, 156. [Google Scholar] [CrossRef]
- Kaidi, S.; Belattmania, Z.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B. Synthesis and Characterization of Silver Nanoparticles Using Alginate from the Brown Seaweed Laminaria Ochroleuca: Structural Features and Antibacterial Activity. Biointerface Res. Appl. Chem. 2021, 12, 6046–6057. [Google Scholar] [CrossRef]
- Fu, B.; Ge, C.; Yue, L.; Luo, J.; Feng, D.; Deng, H.; Yu, H. Characterization of Biochar Derived from Pineapple Peel Waste and Its Application for Sorption of Oxytetracycline from Aqueous Solution. BioResources 2016, 11, 9017–9035. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Kaur, P.; Kaur, K. Adsorptive Removal of Imazethapyr and Imazamox from Aqueous Solution Using Modified Rice Husk. J. Clean. Prod. 2020, 244, 118699. [Google Scholar] [CrossRef]
- Huang, M.-L.; Yen, P.-L.; Liao, V.H.-C. A Combined Approach to Remediate Cadmium Contaminated Sediment Using the Acidophilic Sulfur-Oxidizing Bacterial SV5 and Untreated Coffee Ground. Chemosphere 2021, 273, 129662. [Google Scholar] [CrossRef]
- Seniūnaitė, J.; Vaiškūnaitė, R.; Bazienė, K. Mathematical Modelling for Copper and Lead Adsorption on Coffee Grounds. In Proceedings of the Proccedings of 10th International Conference “Environmental Engineering”, VGTU Technika, Vilnius, Lithuania, 27 April 2017. [Google Scholar]
- Ben Amar, M.; Walha, K.; Salvadó, V. Evaluation of Olive Stones for Cd(II), Cu(II), Pb(II) and Cr(VI) Biosorption from Aqueous Solution: Equilibrium and Kinetics. Int. J. Environ. Res. 2020, 14, 193–204. [Google Scholar] [CrossRef]
- Mahmood-ul-Hassan, M.; Suthor, V.; Rafique, E.; Yasin, M. Removal of Cd, Cr, and Pb from Aqueous Solution by Unmodified and Modified Agricultural Wastes. Environ. Monit. Assess. 2015, 187, 19. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy Metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) Removal from Water in Malaysia: Post Treatment by High Quality Limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, S.N.M.; Kamari, A.; Putra, W.P.; Ishak, C.F.; Mohamed, A.; Hashim, N.; Isa, I.M. Removal of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Agricultural Wastes: Adsorption and Characterisation Studies. J. Environ. Prot. 2014, 05, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Abdić, Š.; Memić, M.; Šabanović, E.; Sulejmanović, J.; Begić, S. Adsorptive Removal of Eight Heavy Metals from Aqueous Solution by Unmodified and Modified Agricultural Waste: Tangerine Peel. Int. J. Environ. Sci. Technol. 2018, 15, 2511–2518. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Morales De La Rosa, S. Hidrólisis Ácida de Celulosa y Biomasa Lignocelulósica Asistida Con Líquidos Iónicos. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2015. [Google Scholar]
- Ituen, E.; Yuanhua, L.; Verma, C.; Alfantazi, A.; Akaranta, O.; Ebenso, E.E. Synthesis and Characterization of Walnut Husk Extract-Silver Nanocomposites for Removal of Heavy Metals from Petroleum Wastewater and Its Consequences on Pipework Steel Corrosion. J. Mol. Liq. 2021, 335, 116132. [Google Scholar] [CrossRef]
- Kul, A.R.; Caliskan, N. Equilibrium and Kinetic Studies of the Adsorption of Zn(II) Ions onto Natural and Activated Kaolinites. Adsorpt. Sci. Technol. 2009, 27, 85–105. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Wang, Y.; Xu, S.; Li, P.; Yang, C.; Jin, Y.; Sun, Q.; Su, H. Superior Adsorption Performance of Graphitic Carbon Nitride Nanosheets for Both Cationic and Anionic Heavy Metals from Wastewater. Chin. J. Chem. Eng. 2019, 27, 305–313. [Google Scholar] [CrossRef]
- Cuong Nguyen, X.; Thanh Huyen Nguyen, T.; Hong Chuong Nguyen, T.; Van Le, Q.; Yen Binh Vo, T.; Cuc Phuong Tran, T.; Duong La, D.; Kumar, G.; Khanh Nguyen, V.; Chang, S.W.; et al. Sustainable Carbonaceous Biochar Adsorbents Derived from Agro-Wastes and Invasive Plants for Cation Dye Adsorption from Water. Chemosphere 2021, 282, 131009. [Google Scholar] [CrossRef]
- Torres Acosta, L.; Mendieta, I.; Hernández, G.; Núñez, R.; Castaño, V. Citotoxicidad y Genotoxicidad de AgNPs Para Disminuir La Adherencia de Candida Albicans En Prótesis Dentales. Mundo Nano. Rev. Interdiscip. Nanocienc. Nanotecnol. 2011, 5. [Google Scholar]
Figure 1.
SEM micrographs showing the morphology transformation: (a) Coffee husk, (b) AgNPs–coffee husk and, (c) AgNPs–coffee husk after heavy metal ions’ sorption experiment. EDX analysis of AgNPs-coffee husk (at red + sign) is presented in figure (b), which includes Ag peak.
Figure 1.
SEM micrographs showing the morphology transformation: (a) Coffee husk, (b) AgNPs–coffee husk and, (c) AgNPs–coffee husk after heavy metal ions’ sorption experiment. EDX analysis of AgNPs-coffee husk (at red + sign) is presented in figure (b), which includes Ag peak.
Figure 2.
FT–IR spectra of (a) coffee husk and (b) AgNPs+coffee husk.
Figure 2.
FT–IR spectra of (a) coffee husk and (b) AgNPs+coffee husk.
Figure 3.
Sorption kinetics for (a) lead, (b) cadmium, (c) chromium, and (d) copper.
Figure 3.
Sorption kinetics for (a) lead, (b) cadmium, (c) chromium, and (d) copper.
Figure 4.
Pseudo-first-order model: (a) Coffee husk, (b) AgNPs–coffee husk, (c) lignin, and (d) AgNPs–lignin.
Figure 4.
Pseudo-first-order model: (a) Coffee husk, (b) AgNPs–coffee husk, (c) lignin, and (d) AgNPs–lignin.
Figure 5.
Pseudo-second-order model: (a) Coffee husk, (b) AgNPs–coffee husk, (c) lignin, and (d) AgNPs–lignin.
Figure 5.
Pseudo-second-order model: (a) Coffee husk, (b) AgNPs–coffee husk, (c) lignin, and (d) AgNPs–lignin.
Figure 6.
Antifungal activity on Candida albicans and Candida parapsilosis of (a,c) coffee husk, (b,d) AgNPs–coffee husk.
Figure 6.
Antifungal activity on Candida albicans and Candida parapsilosis of (a,c) coffee husk, (b,d) AgNPs–coffee husk.
Figure 7.
Antifungal activity on Candida glabrata of (a,c) coffee husk, and (b,d) AgNPs–coffee husk.
Figure 7.
Antifungal activity on Candida glabrata of (a,c) coffee husk, and (b,d) AgNPs–coffee husk.
Figure 8.
Antifungal activity on Candida guilliermondii and Candida krusei of (a,c) coffee husk, and (b,d) AgNPs–coffee husk.
Figure 8.
Antifungal activity on Candida guilliermondii and Candida krusei of (a,c) coffee husk, and (b,d) AgNPs–coffee husk.
Table 1.
Sorption capacity in mg/g.
Table 1.
Sorption capacity in mg/g.
| Sorbent | Heavy Metal Ion Sorption Capacity (mg/g) | Reference |
|---|
| Pb(II) | Cd(II) | Cr(III) | Cu(II) |
|---|
| Coffee husk | 1.47 | 0.174 | 0.188 | 0.259 | This study |
| Coffee–AgNPs | 2.560 | 1.02 | 0.575 | 0.644 |
| Lignin | 0.807 | 0.103 | 0.168 | 0.204 |
| Lignin–AgNPs | 1.440 | 0.176 | 0.312 | 0.451 |
| Coffee ground | 0.628 | 3.450 | - | 0.616 | [51,52] |
| Milled olive stones | 0.581 | 0.300 | 2.340 | 0.557 | [53] |
| Banana | 20.898 | 3.658 | 6.855 | - | [54] |
| Corn cob | 29.168 | 13.577 | 18.782 | - | [54] |
| Sunflower | 22.644 | 11.404 | 12.206 | - | [54] |
| Limestone | 0.0128 | 0.016 | 0.016 | 0.013 | [55] |
| Coconut coir | 2.760 | - | - | 2.25 | [56] |
| Tangerine peel | 1.556 | 0.659 | 1.480 | 1.616 | [57] |
Table 2.
Pseudo-first- and pseudo-second-order kinetic model values for sorption experiments of Pb(II), Cd(II), Cr(III), and Cu(II), all with an initial concentration of 0.18 mmol/L. The value k2 is a velocity constant from the pseudo-second-order kinetic model.
Table 2.
Pseudo-first- and pseudo-second-order kinetic model values for sorption experiments of Pb(II), Cd(II), Cr(III), and Cu(II), all with an initial concentration of 0.18 mmol/L. The value k2 is a velocity constant from the pseudo-second-order kinetic model.
| Material | Ion | Pseudo-First-Order | Pseudo-Second-Order |
|---|
| R2 | k2 (g/mg·min) | R2 |
|---|
| Coffee husk | Pb(II) | 0.8525 | 3.767 | 0.9996 |
| Cd(II) | 0.0172 | −198.2 * | 0.9998 |
| Cr(III) | 0.4268 | 7.296 | 0.9841 |
| Cu(II) | 0.8121 | 15.90 | 0.9994 |
| AgNPs–coffee husk | Pb(II) | 0.8247 | 22.90 | 1.000 |
| Cd(II) | 0.9623 | 7.308 | 0.9994 |
| Cr(III) | 0.9490 | 18.79 | 0.9999 |
| Cu(II) | 0.6747 | 9.290 | 0.9976 |
| Lignin | Pb(II) | 0.9103 | 10.05 | 0.9982 |
| Cd(II) | 0.2986 | −77.61 * | 0.9973 |
| Cr(III) | 0.4431 | 10.44 | 0.9579 |
| Cu(II) | 0.9573 | 28.06 | 0.9987 |
| AgNPs–lignin | Pb(II) | 0.9884 | 5.102 | 0.9999 |
| Cd(II) | 0.0007 | −110.4 * | 0.9978 |
| Cr(III) | 0.8474 | 6.089 | 0.9922 |
| Cu(II) | 0.9657 | 6.263 | 0.9988 |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).