The Effects of Algaecides and Herbicides on a Nuisance Microcystis wesenbergii-Dominated Bloom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Collection, Water Chemistry, and Maintenance of Bloom
2.2. Cyanobacterial Exposures to Algaecides and Herbicides
2.3. Chemical Concentration Validation
2.4. Statistical Analysis
3. Results
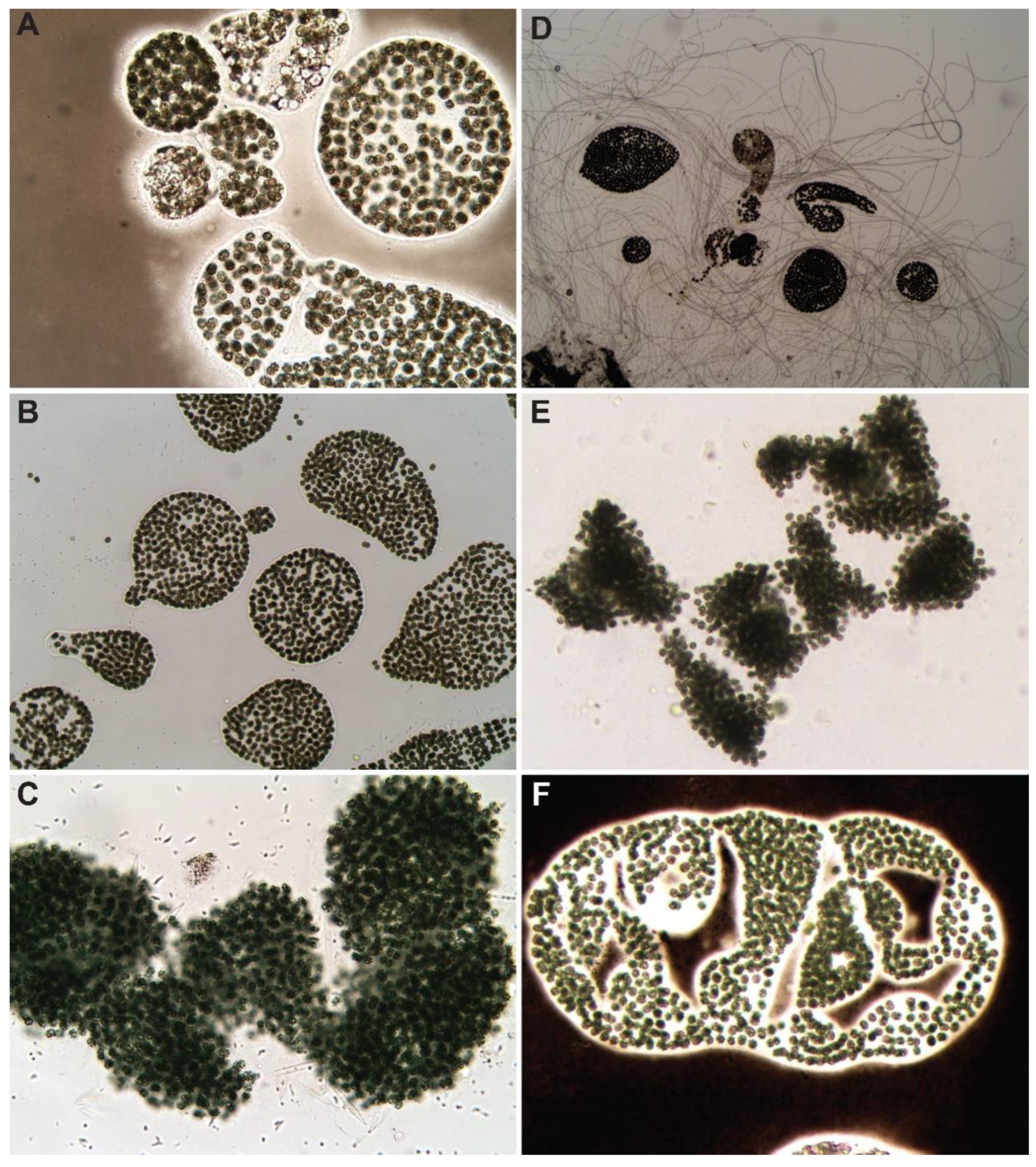
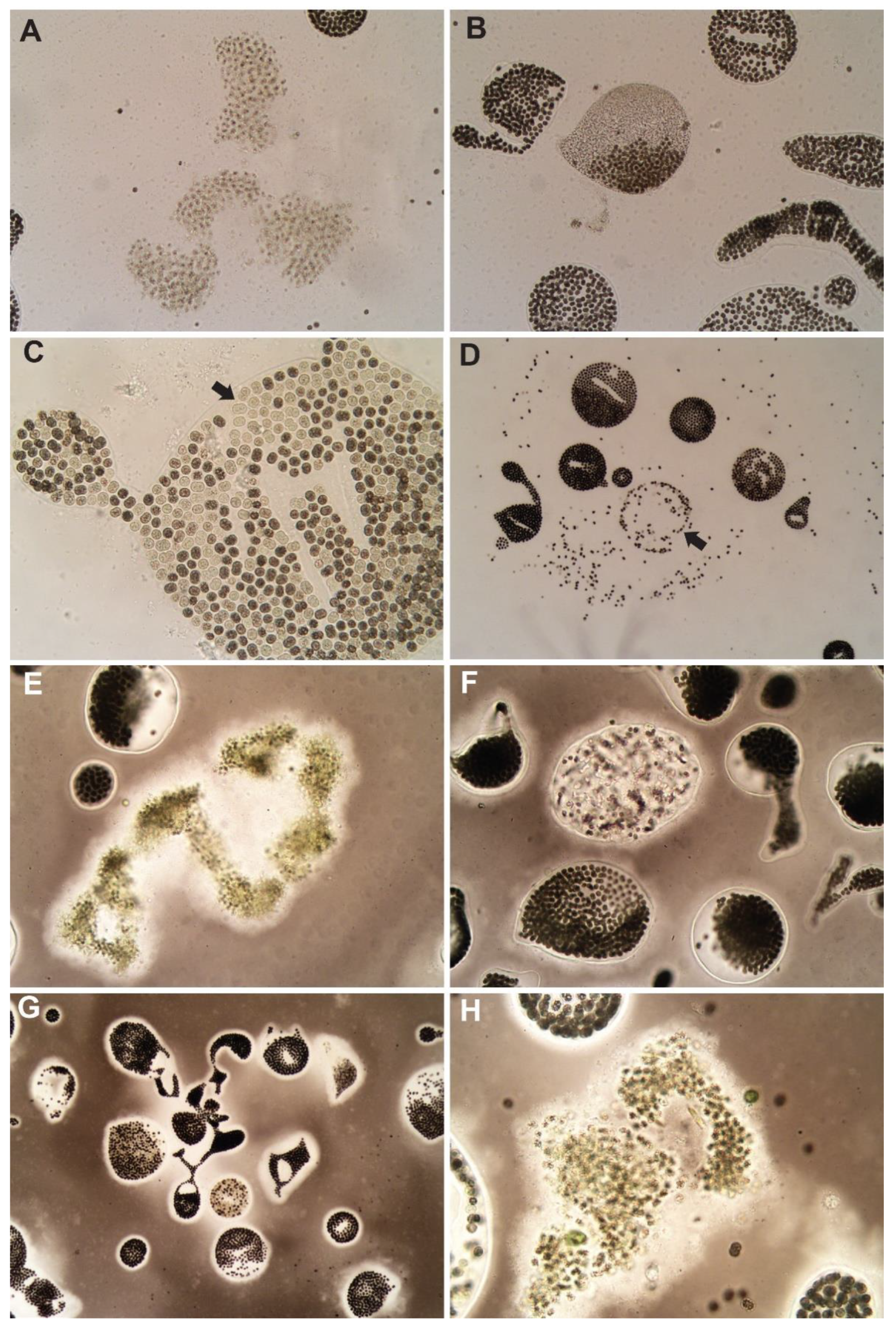
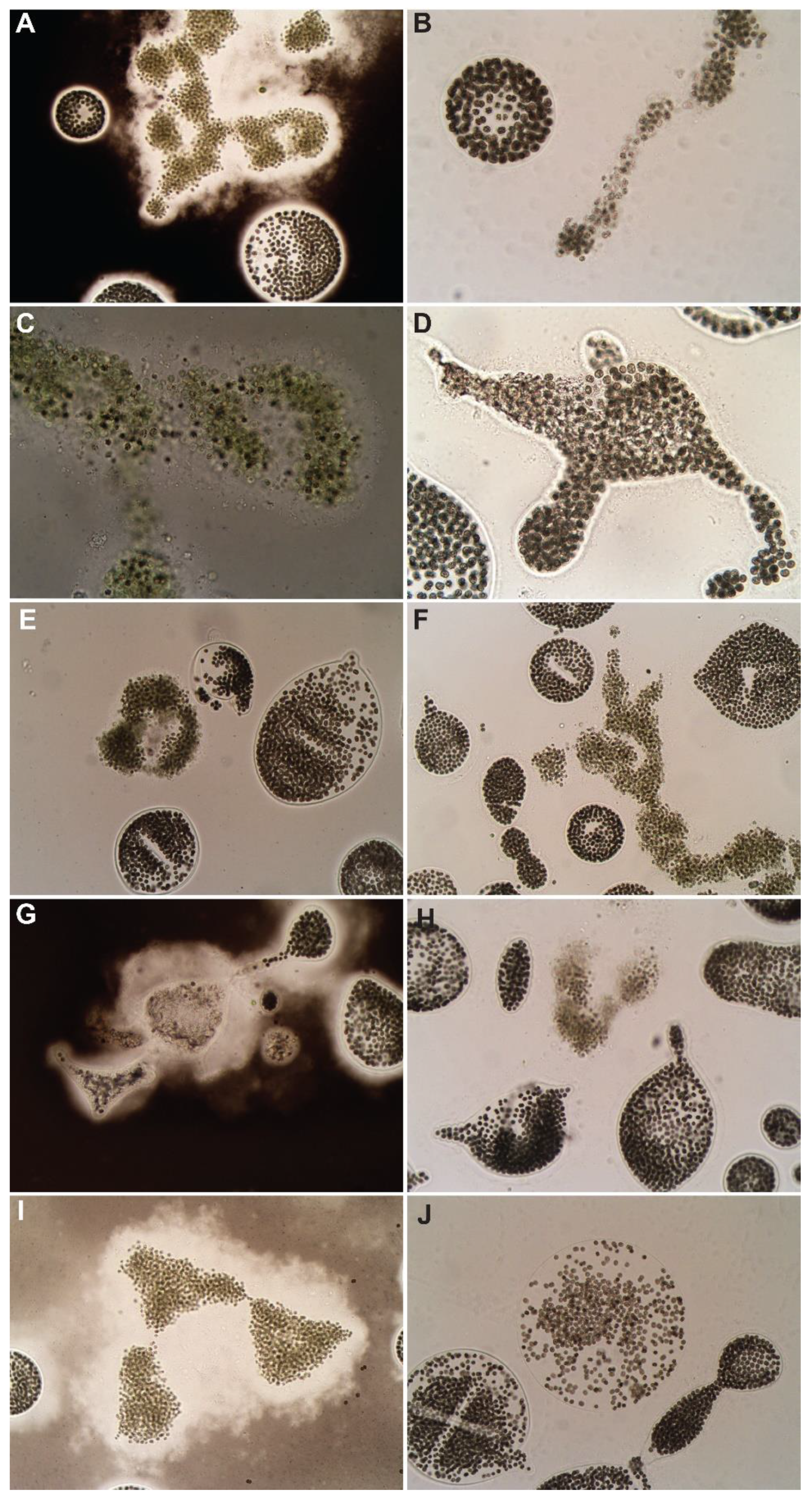
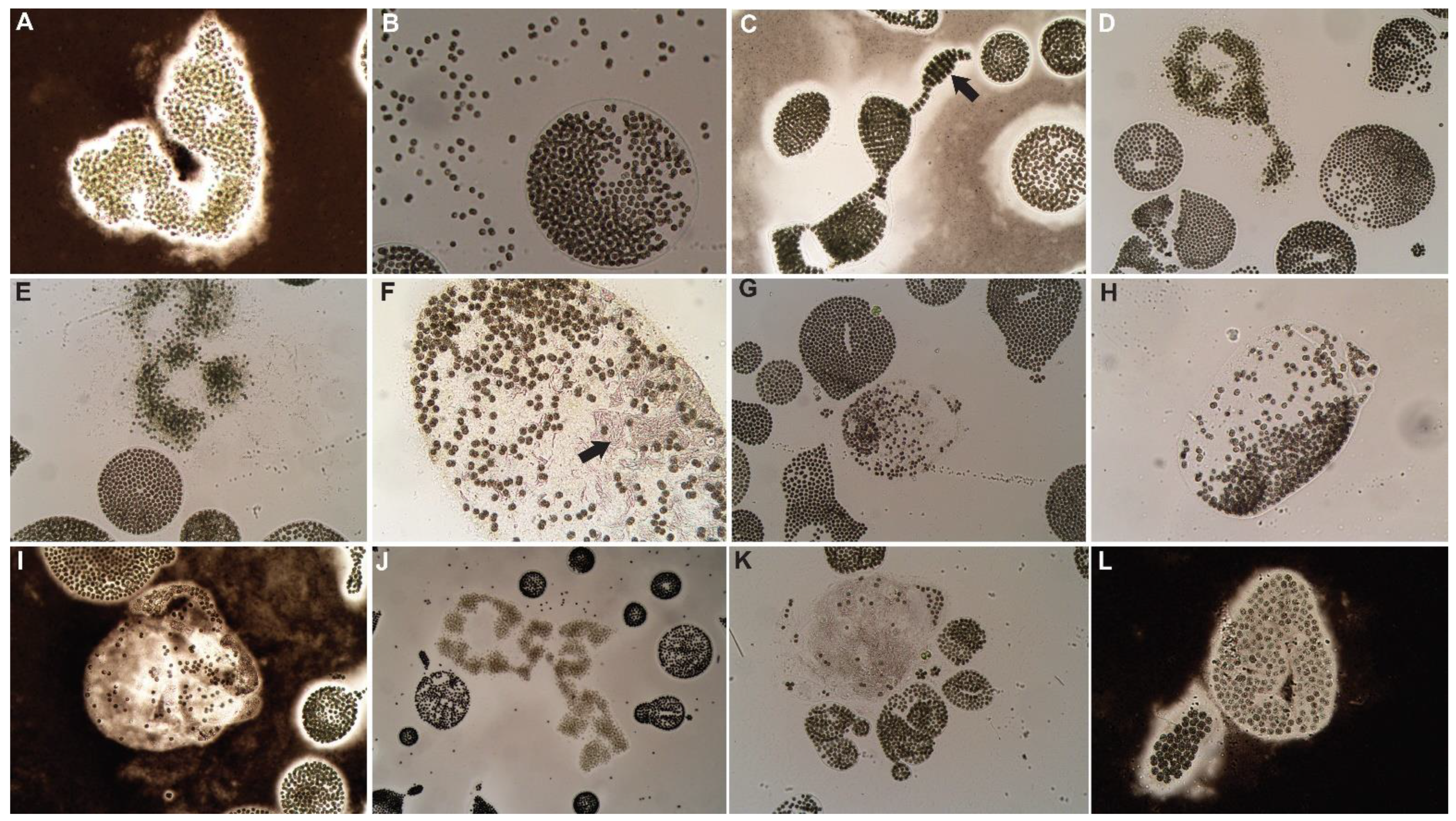
3.1. Control
3.2. Hydrogen Peroxide-Based Algaecides
3.3. Copper-Based Algaecides
3.4. Endothall-Based Algaecide
3.5. Combination Treatments
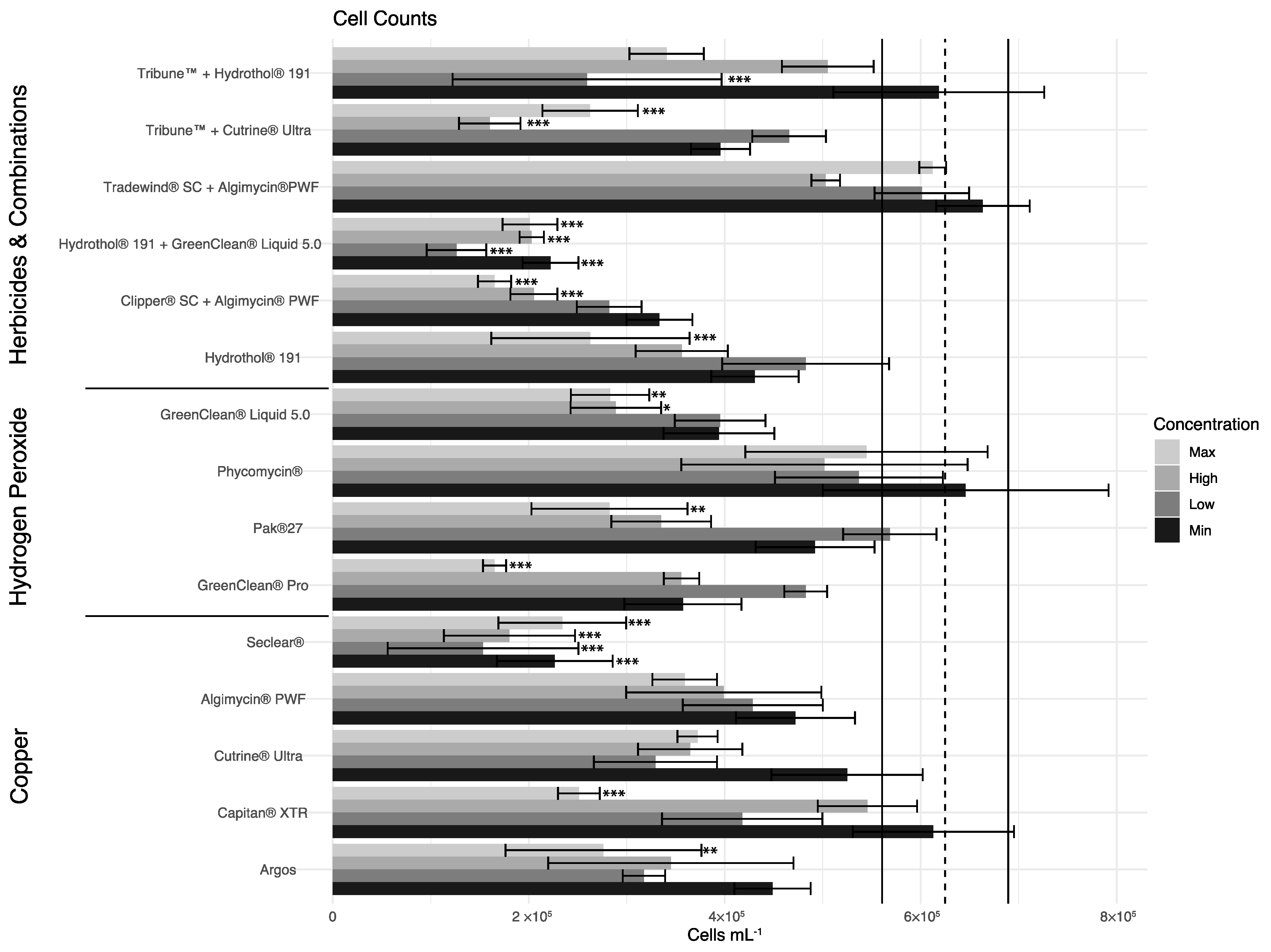
3.6. Statistical Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almanza, V.; Pedreros, P.; Laughinghouse, H.D., IV; Félez, J.; Parra, O.; Azócar, M.; Urrutia, R. Association between trophic state, watershed use, and blooms of cyanobacteria in south-central Chile. Limnologica 2019, 75, 30–41. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.-S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 2019, 86, 139–209. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.F.; de Lima, S.T.; Carmichael, W.W.; McKinnie, S.M.K.; Chekan, J.R.; Moore, B.S. Guanitoxin, re-naming a cyanobacterial organophosphate toxin. Harmful Algae 2020, 92, 101737. [Google Scholar] [CrossRef]
- Jang, M.; Berthold, D.E.; Yu, Z.; Silva-Sanchez, C.; Laughinghouse, H.D., IV; Denslow, N.D.; Han, S. Atmospheric Progression of Microcystin-LR from Cyanobacterial Aerosols. Environ. Sci. Technol. Lett. 2020, 7, 740–745. [Google Scholar] [CrossRef]
- Moretto, J.A.; Freitas, P.N.N.; Souza, J.P.; Oliveira, T.M.; Brites, I.; Pinto, E. Off-flavors in Aquacultured Fish: Origins and Implications for consumers. Fishes 2022, 7, 34. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T.; McCarthy, M.J.; Newell, S.E.; Gardner, W.S.; Havens, K.E.; Hoffman, D.K.; Wilhelm, S.W.; Wurtsbaugh, W.A. It Takes Two to Tango: When and Where Dual Nutrient (N & P) Reductions Are Needed to Protect Lakes and Downstream Ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar]
- Xu, H.; McCarthy, M.J.; Paerl, H.W.; Brookes, J.D.; Zhu, G.; Hall, N.S.; Qin, B.; Zhang, Y.; Zhu, M.; Hampel, J.J.; et al. Contributions of external nutrient loading and internal cycling to cyanobacterial bloom dynamics in Lake Taihu, China: Implications for nutrient management. Limnol. Oceanogr. 2021, 66, 1492–1509. [Google Scholar] [CrossRef]
- Laughinghouse, H.D., IV; Berthold, D.E.; Bishop, W.M. Approaches to managing cyanobacterial blooms and altering water quality. Aquatics 2020, 42, 13–16. [Google Scholar]
- Peterson, H. Toxicity of hexazinone and diquat to green algae, diatoms, cyanobacteria and duckweed. Aquat. Toxicol. 1997, 39, 111–134. [Google Scholar] [CrossRef]
- Ruzycki, E.M.; Axler, R.P.; Owen, C.J.; Martin, T.B. Response of phytoplankton photosynthesis and growth to the aquatic herbicide hydrothol 191. Environ. Toxicol. Chem. 1998, 17, 1530–1537. [Google Scholar] [CrossRef]
- Hadjoudja, S.; Vignoles, C.; Deluchat, V.; Lenain, J.-F.; Le Jeune, A.-H.; Baudu, M. Short term copper toxicity on Microcystis aeruginosa and Chlorella vulgaris using flow cytometry. Aquat. Toxicol. 2009, 94, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, H.C.P.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Calomeni, A.J.; Rodgers, J.H.; Kinley, C.M. Responses of Planktothrix agardhii and Pseudokirchneriella subcapitata to Copper Sulfate (CuSO4·5H2O) and a Chelated Copper Compound (Cutrine® Ultra). Water Air Soil Pollut. 2014, 225, 2231. [Google Scholar] [CrossRef]
- Calomeni, A.J.; Iwinski, K.J.; Kinley, C.M.; McQueen, A.; Rodgers, J.H. Responses of Lyngbya wollei to algaecide exposures and a risk characterization associated with their use. Ecotoxicol. Environ. Saf. 2015, 116, 90–98. [Google Scholar] [CrossRef]
- Iwinski, K.J.; Calomeni, A.J.; Geer, T.D.; Rodgers, J.H. Cellular and aqueous microcystin-LR following laboratory exposures of Microcystis aeruginosa to copper algaecides. Chemosphere 2016, 147, 74–81. [Google Scholar] [CrossRef]
- Buley, R.P.; Adams, C.; Belfiore, A.P.; Fernandez-Figueroa, A.G.; Gladfelter, M.F.; Garner, B.; Wilson, A.E. Field evaluation of seven products to control cyanobacterial blooms in aquaculture. Environ. Sci. Pollut. Res. 2021, 28, 29971–29983. [Google Scholar] [CrossRef]
- Kinley-Baird, C.M.; Calomeni, A.; Berthold, D.E.; Lefler, F.W.; Barbosa, M.; Rodgers, J.H.; Laughinghouse, H.D., IV. Laboratory-scale evaluation of algaecide effectiveness for control of microcystin-producing cyanobacteria from Lake Okeechobee, Florida (USA). Ecotoxicol. Environ. Saf. 2021, 207, 111233. [Google Scholar]
- Santos, A.; Guedes, D.O.; Barros, M.U.G.; Oliveira, S.; Pacheco, A.B.F.; Azevedo, S.M.F.O.; Magalhães, V.F.; Pestana, C.J.; Edwards, C.; Lawton, L.A.; et al. Effect of hydrogen peroxide on natural phytoplankton and bacterioplankton in a drinking water reservoir: Mesocosm-scale study. Water Res. 2021, 197, 117096. [Google Scholar] [CrossRef]
- Papadimitriou, T.; Katsiapi, M.; Stefanidou, N.; Paxinou, A.; Poulimenakou, V.; Laspidou, C.S.; Moustaka-Gouni, M.; Kormas, K.A. Differential Effect of Hydroxen Peroxide on Toxic Cyanobacteria of Hypertrophic Mediterranean Waterbodies. Sustainability 2022, 14, 123. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Bishop, W.M.; Grasso, C.R.; Fernando, B.M.; Sperry, B.P.; Berthold, D.E.; Laughinghouse, H.D., IV; VanGoethem, E.M.; Volk, K.; Heilman, M.; et al. Evaluation of a Peroxide-Based Algaecide for Cyanobacteria Control: A Mesocosm Trial in Lake Okeechobee, FL, USA. Water 2022, 14, 169. [Google Scholar] [CrossRef]
- Sant’Anna, C.L.; Azevedo, M.T.P. Contribution to the knowledge of potentially toxic Cyanobacteria from Brazil. Nova Hedwig. 2000, 71, 359–385. [Google Scholar] [CrossRef]
- Komárek, O.; Keršner, V. On the dominance of the planktic cyanobacterium Microcystis wesenbergii—Ecological and statistical analysis of the population. Algol. Stud. 2000, 97, 29–42. [Google Scholar]
- Komárek, J.; Komárková-Legnerová, J.; Snat’Anna, C.L.; Azevedo, M.T.P.; Senna, P.A. Two common Microcystis species from tropical America. Criptogam. Algol. 2002, 23, 159–177. [Google Scholar]
- Carvalho, L.R.; Pipole, F.; Werner, V.R.; Laughinghouse, H.D., IV; Camargo, A.C.; Rangel, M.; Konno, K.; Sant’Anna, C.L. A toxic cyanobacterial bloom in an urban coastal lake, Rio Grande do Sul state, Southern Brazil. Braz. J. Microbiol. 2008, 39, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H. Mitigating Harmful Cyanobacterial Blooms in a Human- and Climatically-Impacted World. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 22 May 2022).
- U.S. EPA. Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry; Revision 4.4; U.S. EPA: Cincinnati, OH, USA, 1994.
- Klassen, N.V.; Marchington, D.; McGowan, H.C.E. H2O2 determination by the I3- method and by KMnO4 titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Kinley, C.M.; Rodgers, J.H.; Iwinski, K.J.; McQueen, A.; Calomeni, A.J. Analysis of Algaecide Exposures: An Evaluation of the I3- Method to Measure Sodium Carbonate Peroxyhydrate Algaecides. Water Air Soil Pollut. 2015, 226, 170. [Google Scholar] [CrossRef]
- Ferrell, J.A.; Vencill, W.K. Gas Chromatographic/Mass Spectrometric Determination of Flumioxazin Extracted from Soil and Water. J. AOAC Int. 2004, 87, 56–59. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, G.P. Factors in the testing and application of algicides. Appl. Microbiol. 1964, 12, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kinley, C.M.; Iwinski, K.J.; Hendrikse, M.; Geer, T.D.; Rodgers, J.H., Jr. Cell density dependence of Microcystis aeruginosa responses to copper algaecide concentrations: Implications for microcystin-LR release. Ecotoxicol. Environ. Saf. 2017, 145, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Gaikowski, M.P.; Rach, J.J.; Ramsay, R.T. Acute toxicity of hydrogen peroxide treatments to selected life stages of cold-, cool-, and warmwater fish. Aquaculture 1999, 178, 191–207. [Google Scholar] [CrossRef]
- Bauzá, L.; Aguilera, A.; Echenique, R.; Andrinolo, D.; Giannuzzi, L. Application of Hydrogen Peroxide to the Control of Eutrophic Lake Systems in Laboratory Assays. Toxins 2014, 6, 2657–2675. [Google Scholar] [CrossRef] [PubMed]
- Geer, T.D.; Kinley, C.M.; Iwinski, K.J.; Calomeni, A.J.; Rodgers, J.H., Jr. Comparative toxicity of sodium carbonate peroxyhydrate to freshwater organisms. Ecotoxicol. Environ. Saf. 2016, 132, 202–211. [Google Scholar] [CrossRef]
- Calomeni, A.J.; Kinley, C.M.; Geer, T.D.; Hendrikse, M.; Rodgers, J.H., Jr. Lyngbya wollei responses to copper algaecide exposures predicted using a concentration–exposure time (CET) model: Influence of initial biomass. J. Aquat. Plant Manag. 2018, 56, 73–83. [Google Scholar]
- Bishop, W.M.; Rodgers, J.H. Responses of Lyngbya wollei to Exposures of Copper-Based Algaecides: The Critical Burden Concept. Arch. Environ. Contam. Toxicol. 2012, 62, 403–410. [Google Scholar] [CrossRef]
- Ozturk, S.; Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. Res. Int. 2010, 17, 595–602. [Google Scholar] [CrossRef]
- Yang, Z.; Linlin, G.; Wang, W.; Zhang, J. Combined effects of temperature, light intensity, and nitrogen concentration on the growth and polysaccharide content of Microcystis aeruginosa in batch culture Biochem. Syst. Ecol. 2012, 41, 130–135. [Google Scholar] [CrossRef]
- Pennington, T.G.; Skogerboe, J.G.; Getsinger, K.D. Herbicide/Copper combinations for improved control of Hydrilla verticillata. J. Aquat. Plant Manag. 2001, 39, 56–58. [Google Scholar]
| Parameter | |
|---|---|
| Temperature (°C) | 19.8 |
| Dissolved Oxygen (%) | 83.3 |
| Conductivity (µS/cm) | 321.3 |
| pH (S.U.) | 7.94 |
| Product Name | Active Ingredient (s) | Range of Concentrations Evaluated as Active Ingredient | Concentrations in Terms of Application Concentration of Product |
|---|---|---|---|
| Cutrine® Ultra | Copper ethanolamine Complex | 0.3, 0.5, 0.7, 1.0 mg Cu·L−1 | 0.9, 1.5, 2.1, 3.0 gallons·acre-ft−1 as Cutrine® Ultra |
| Algimycin® PWF | Copper citrate and copper gluconate | 0.3, 0.5, 0.7, 1.0 mg Cu·L−1 | 1.59, 2.66, 3.72, 5.31 gallons·acre-ft−1 as Algimycin® PWF |
| Phycomycin® SCP | Sodium carbonate peroxyhydrate | 2, 5, 7, 10 mg H2O2·L−1 | 20, 49, 69, 100 lbs·acre-ft−1 as Phycomycin® SCP |
| GreenClean® Pro | Sodium carbonate peroxyhydrate | 2, 5, 7, 10 mg H2O2·L−1 | 20, 49, 69, 100 lbs·acre-ft−1 as GreenClean® Pro |
| GreenClean® Liquid 5.0 | Hydrogen peroxide and peroxyacetic acid | 4, 10, 15, 22 mg H2O2·L−1 | 5, 13, 20, 28.5 gallons·acre-ft−1 as GreenClean® Liquid 5.0 |
| SeClear® | Copper sulfate pentahydrate | 0.3, 0.5, 0.7, 1.0 mg Cu·L−1 | 1.95, 3.25, 4.55, 6.5 gallons·acre-ft−1 as SeClear® |
| PAK® 27 | Sodium carbonate peroxyhydrate | 2, 5, 7, 10 mg H2O2·L−1 | 20, 49, 69, 100 lbs·acre-ft−1 as PAK® 27 |
| Captain® XTR | Copper ethanolamine complex | 0.3, 0.5, 0.7, 1.0 mg Cu·L−1 | 0.9, 1.5, 2.1, 3 gallons·acre-ft−1 as Captain® XTR |
| Hydrothol® 191 | Amine salt of endothall | 0.15, 0.3, 0.5, 1.0 mg acid equivalents endothall·L−1 | 2.25, 4.5, 7.5, 15 gallons·acre-ft−1 as Hydrothol® 191 |
| GreenClean® Liquid 5.0 + Hydrothol® 191 | Hydrogen peroxide with peroxyacetic acid + Amine salt of endothall | 4, 10, 15, 22 mg H2O2·L−1 each with 0.3 mg acid equivalents endothall·L−1 mixed at the same time | 5, 13, 20, 28.5 gallons·acre-ft−1 of GreenClean Liquid 5.0 and 4.5 gallons·acre-ft−1 as Hydrothol® 191 |
| Argos | Copper ethanolamine complex | 0.3, 0.5, 0.7, 1.0 mg Cu·L−1 | 0.9, 1.5, 2.1, 3 gallons·acre-ft−1 as Argos |
| Algimycin® PWF + Clipper® SC | Copper citrate and copper gluconate + Flumioxazin | 0.1, 0.3, 0.5, 0.7 mg Cu·L−1 each mixed with 200 µg·L−1 flumioxazin | 0.53, 1.59, 2.66, 3.72 gallons·acre-ft−1 as Algimycin® PWF with 1.1 pounds·surface acre−1 of Clipper® SC |
| Algimycin® PWF + Tradewind® | Copper citrate and copper gluconate + bispyribac sodium | 0.1, 0.3, 0.5, 0.7 mg Cu·L−1 each mixed with 45 µg·L−1 bispyribac sodium | 0.53, 1.59, 2.66, 3.72 gallons·acre-ft−1 as Algimycin® PWF with 0.15 pounds·acre-ft−1 of Tradewind® |
| Cutrine® Ultra + Tribune™ | Copper ethanolamine complex + Diquat dibromide | 0.3, 0.5, 0.7, 1.0 mg Cu·L−1 each mixed with 0.37 mg diquat cation·L−1 (equivalent to 0.69 mg diquat dibromide·L−1) | 0.9, 1.5, 2.1, 3 gallons·acre-ft−1 of Cutrine® Ultra mixed with 0.5 gallons·acre-ft−1 of Tribune™ |
| Hydrothol® 191 + Tribune™ | Amine salt of endothall + Diquat dibromide | 0.15, 0.3, 0.5, 1.0 mg acid equivalents·L−1 as Hydrothol® 191 and 0.37 mg diquat cation·L−1 (equivalent to 0.69 mg diquat dibromide·L−1) | 2.25, 4.5, 7.5, 15 gallons·acre-ft−1 as Hydrothol® 191 and 0.5 gallons·acre-ft−1 of Tribune™ |
| Active Ingredient | Algaecide/Herbicide | Targeted Stock Concentration (mg·L−1) | Measured Stock Concentration (mg Active Ingredient·L−1 ± Standard Deviation) |
|---|---|---|---|
| Flumioxazin | Clipper® SC | 100 | 103.10 ± 6.05 |
| Endothall | Hydrothol® 191 | 100 | nd |
| Copper (Cu) | SeClear® | 100 | 96.81 ± 0.80 |
| Algimycin® PWF | 100 | 148.8 ± 0.59 | |
| Cutrine® Ultra | 100 | 100.23 ± 1.4 | |
| Argos | 100 | 96.45 ± 0.59 | |
| Captain® XTR | 100 | 99.94 ± 1.46 | |
| Peroxide (H2O2) | GreenClean® Liquid 5.0 | 2200 | 2154.83 ± 20.75 |
| GreenClean® Pro | 1000 | 822.42 ± 28.81 | |
| Phycomycin® SCP | 1000 | 803.98 ± 1.25 | |
| PAK® 27 | 1000 | 793.98 ± 12.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefler, F.W.; Berthold, D.E.; Barbosa, M.; Laughinghouse, H.D., IV. The Effects of Algaecides and Herbicides on a Nuisance Microcystis wesenbergii-Dominated Bloom. Water 2022, 14, 1739. https://doi.org/10.3390/w14111739
Lefler FW, Berthold DE, Barbosa M, Laughinghouse HD IV. The Effects of Algaecides and Herbicides on a Nuisance Microcystis wesenbergii-Dominated Bloom. Water. 2022; 14(11):1739. https://doi.org/10.3390/w14111739
Chicago/Turabian StyleLefler, Forrest W., David E. Berthold, Maximiliano Barbosa, and H. Dail Laughinghouse, IV. 2022. "The Effects of Algaecides and Herbicides on a Nuisance Microcystis wesenbergii-Dominated Bloom" Water 14, no. 11: 1739. https://doi.org/10.3390/w14111739
APA StyleLefler, F. W., Berthold, D. E., Barbosa, M., & Laughinghouse, H. D., IV. (2022). The Effects of Algaecides and Herbicides on a Nuisance Microcystis wesenbergii-Dominated Bloom. Water, 14(11), 1739. https://doi.org/10.3390/w14111739








