Unusual Catalytic Effect of Fe3+ on 2,4-dichlorophenoxyacetic Acid Degradation by Radio Frequency Discharge in Aqueous Solution
Abstract
:1. Introduction
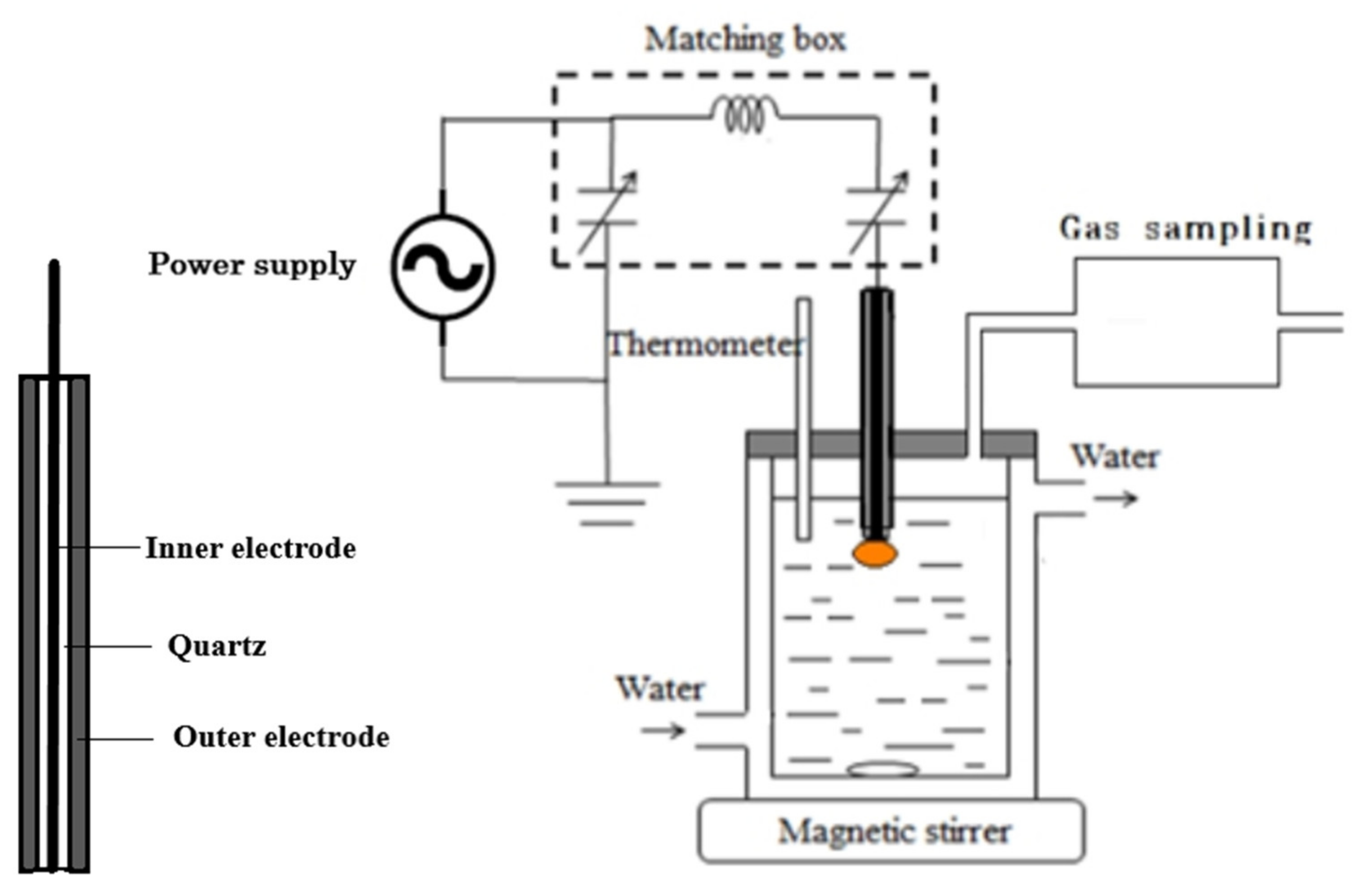
2. Experimental
3. Results and discussion
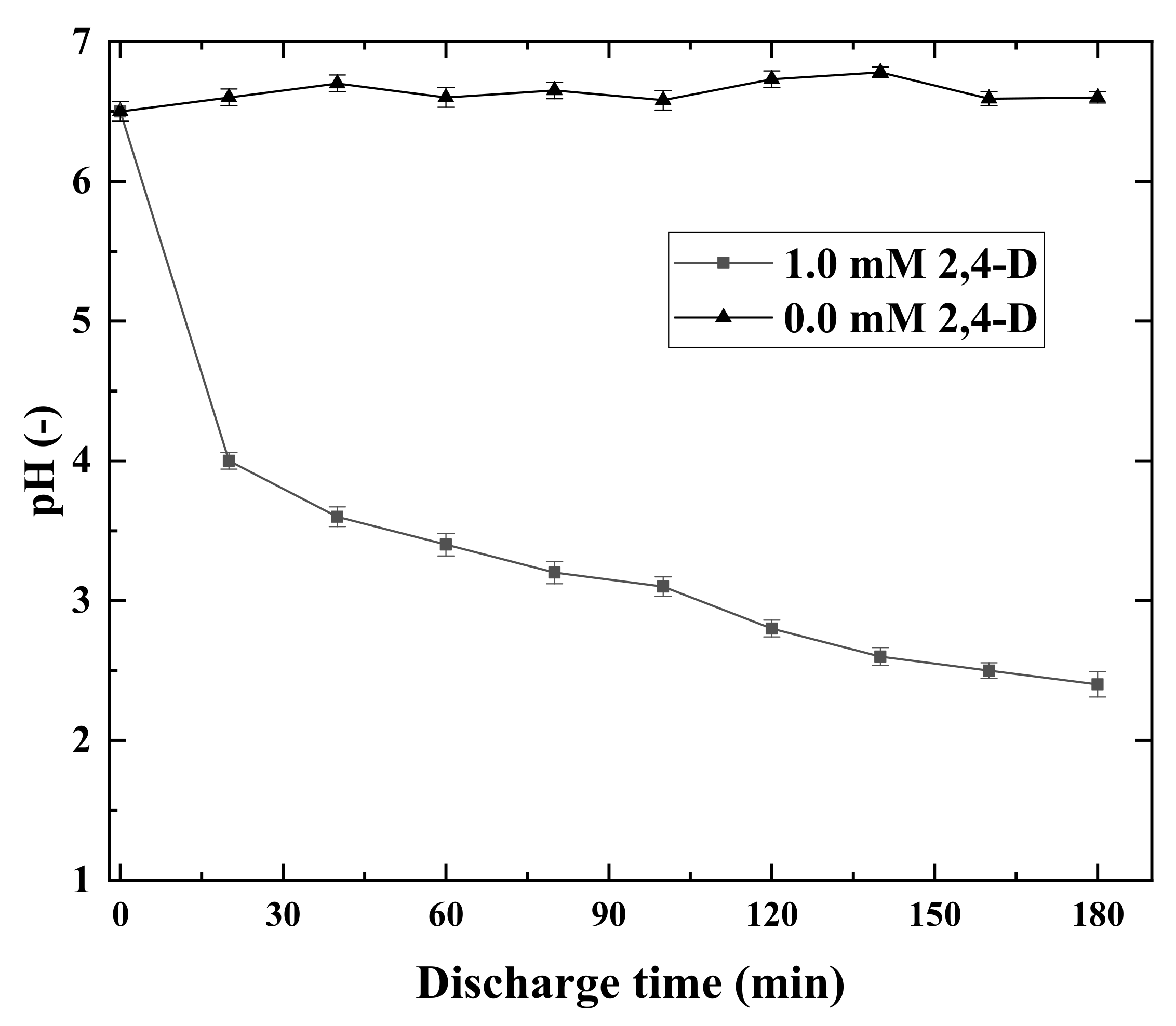
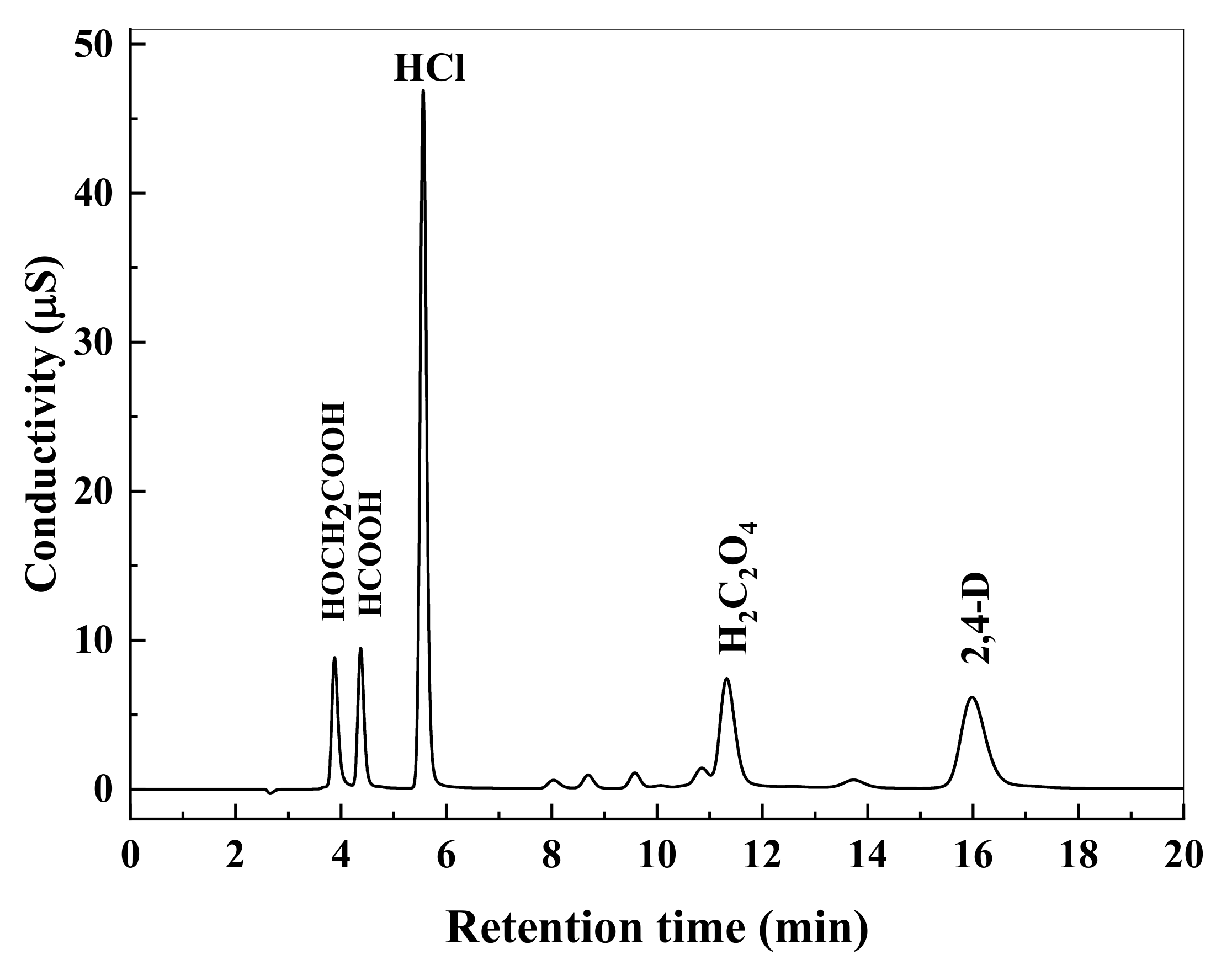
3.1. 2,4-D Degradation and Chloride Formation
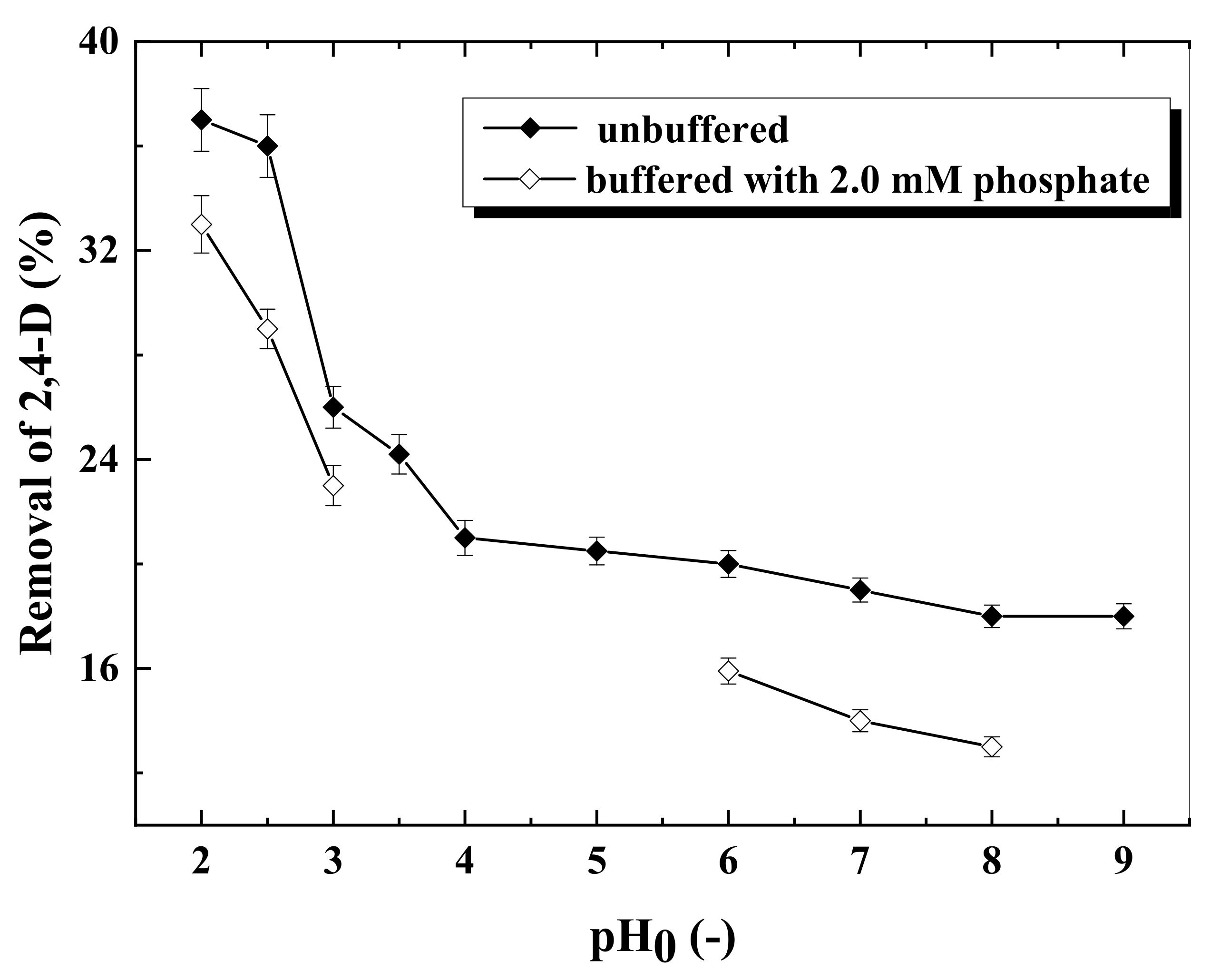
3.2. Effects of Initial pH on 2,4-D Degradation
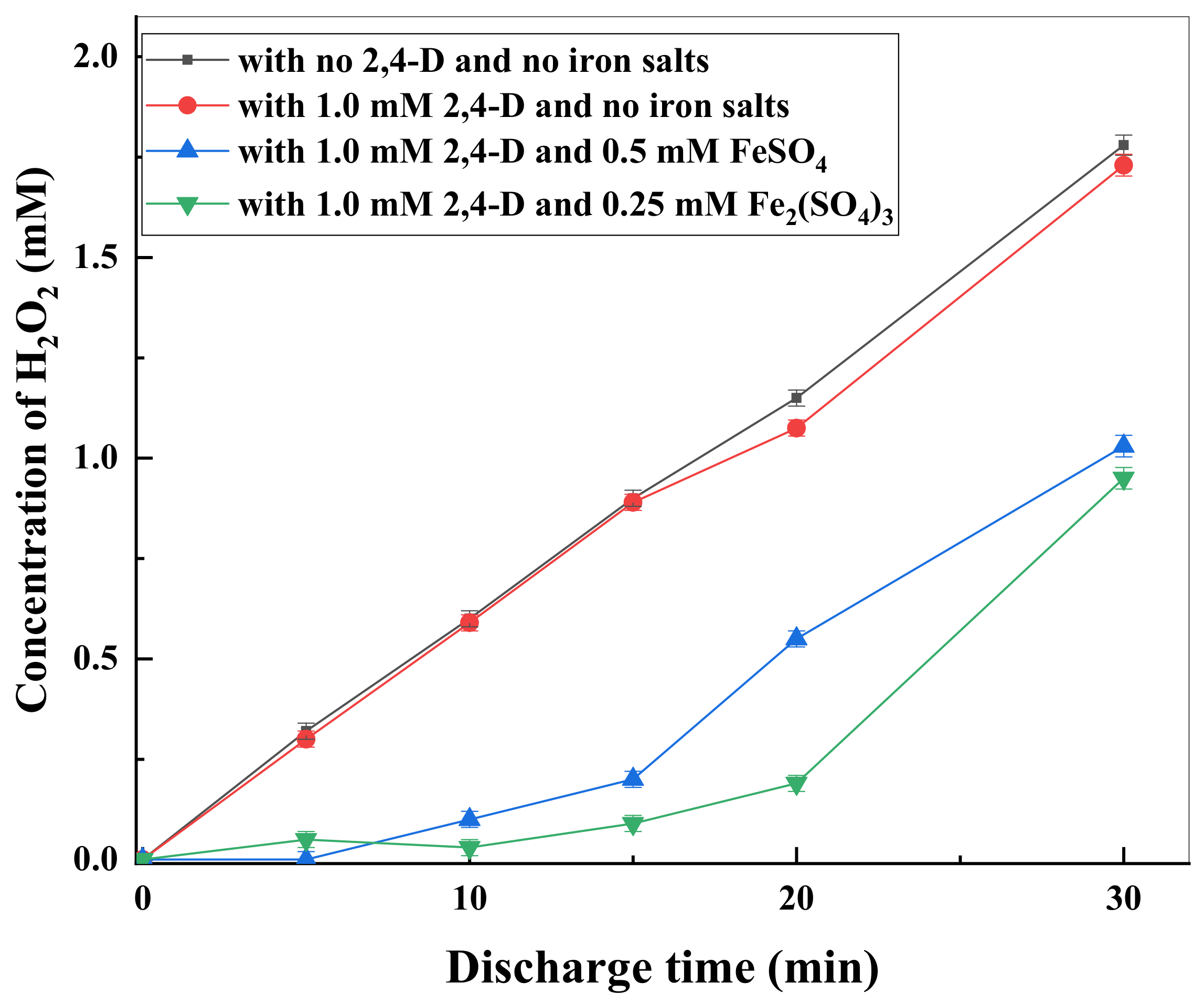
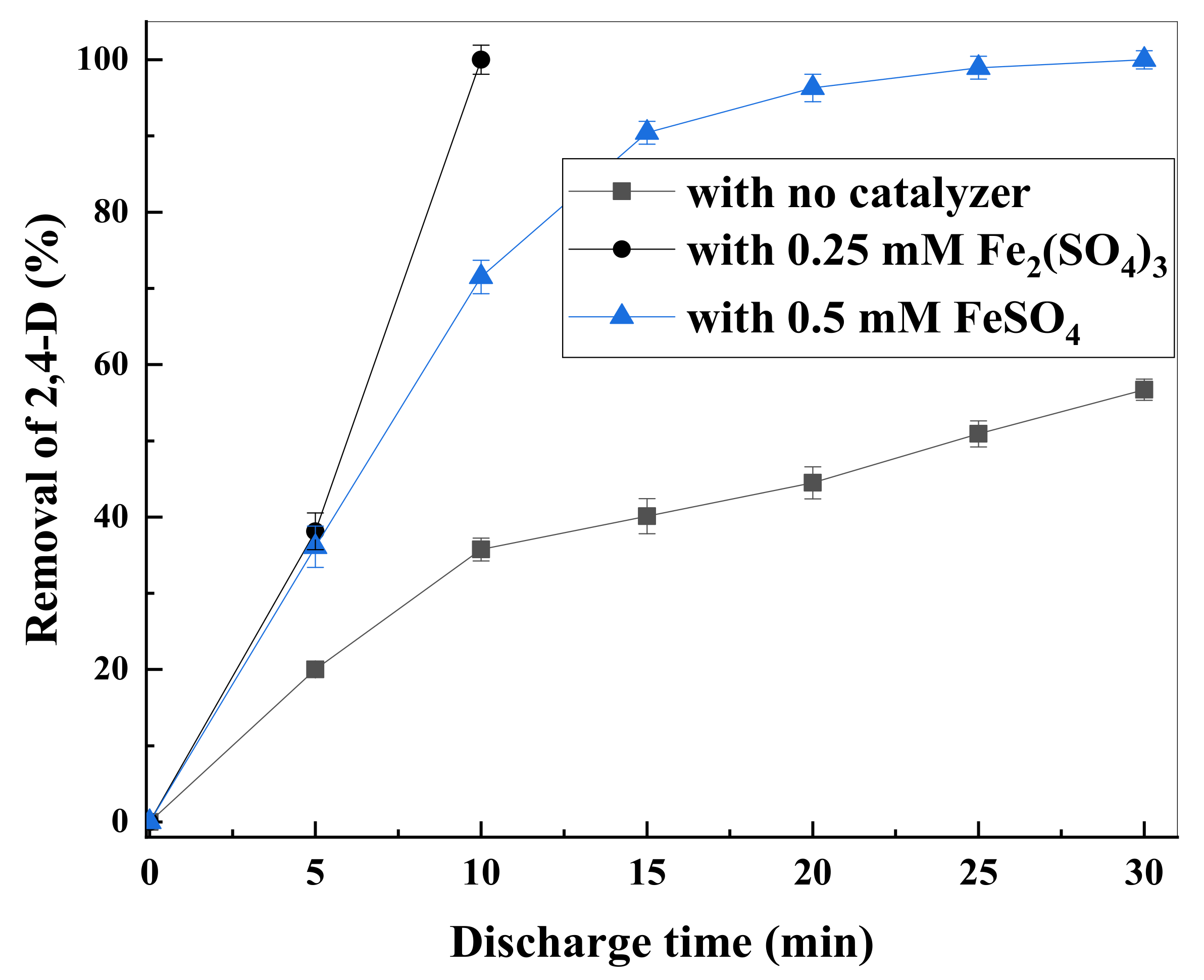
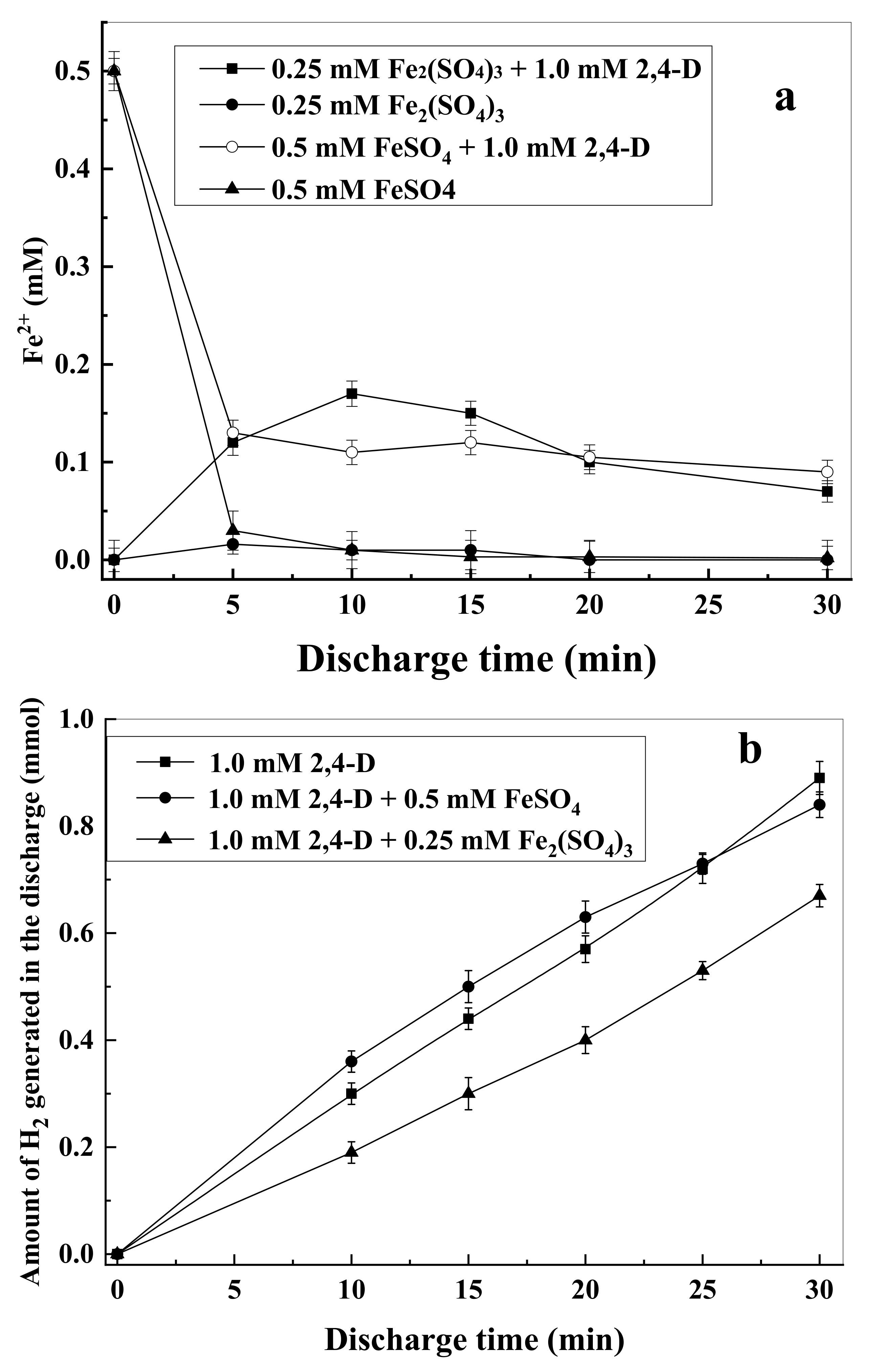
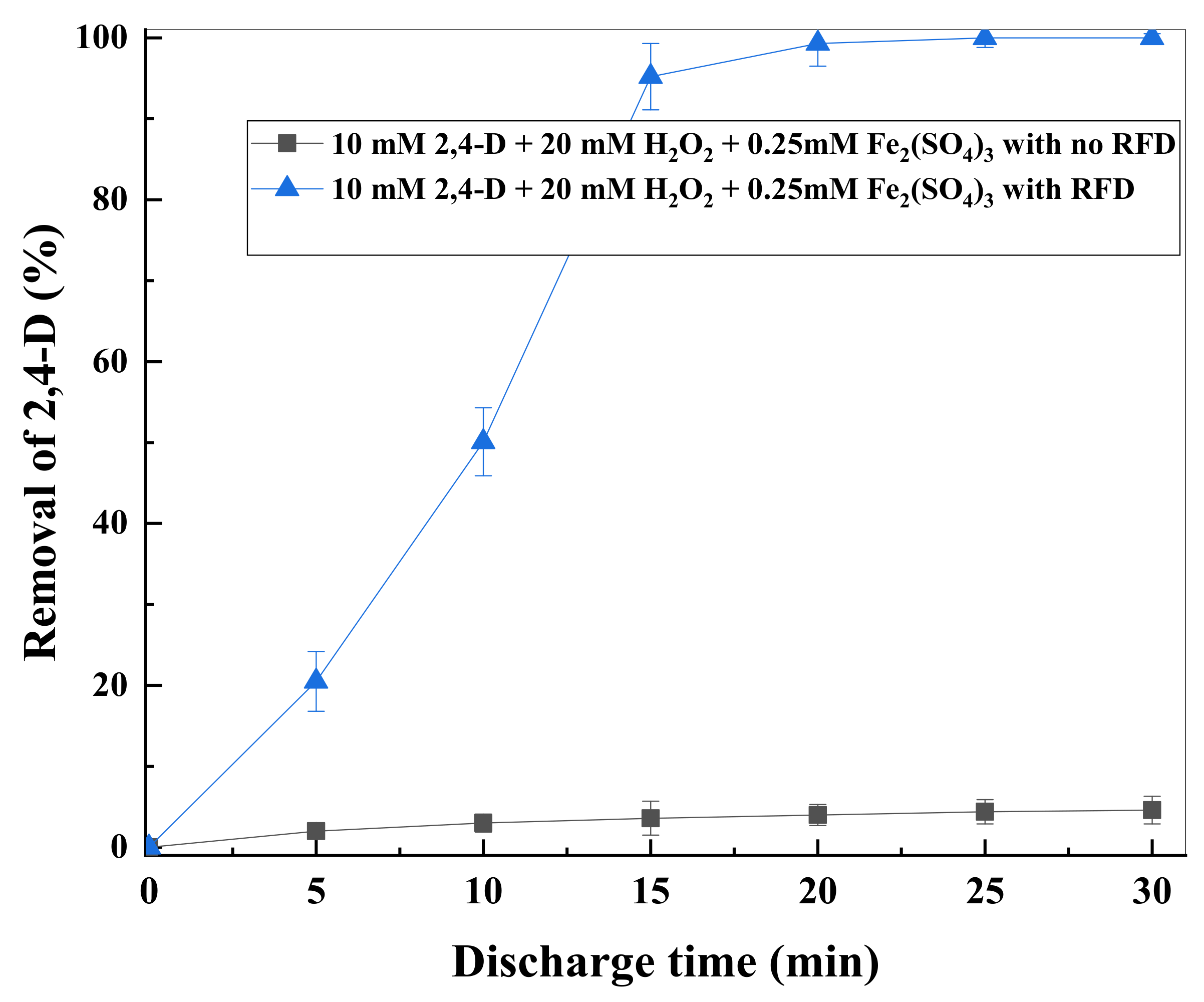
3.3. H2O2 Formation and Effects of Iron Salts on 2,4-D Degradation
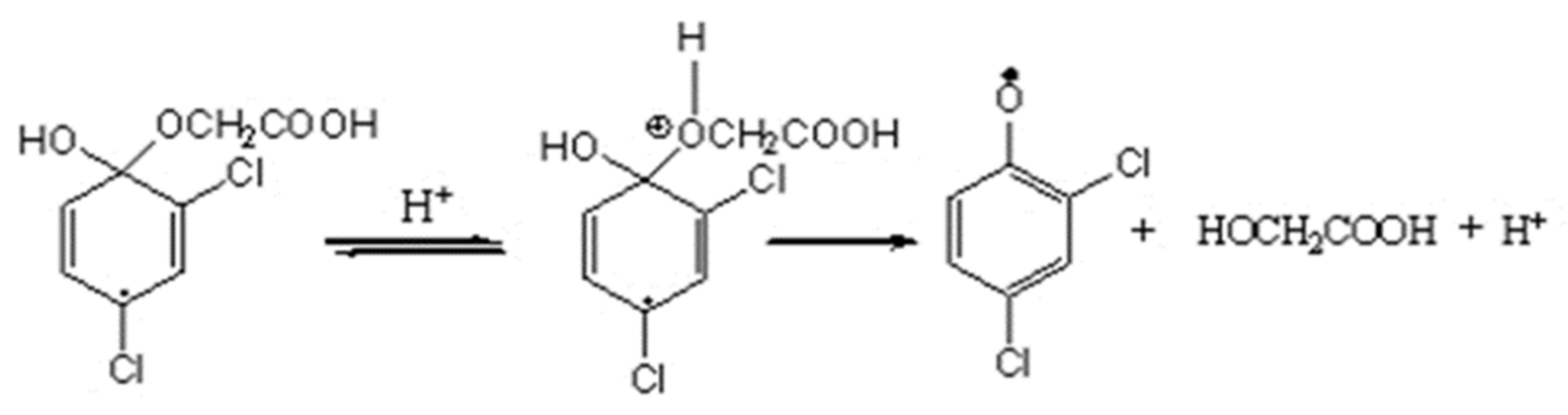
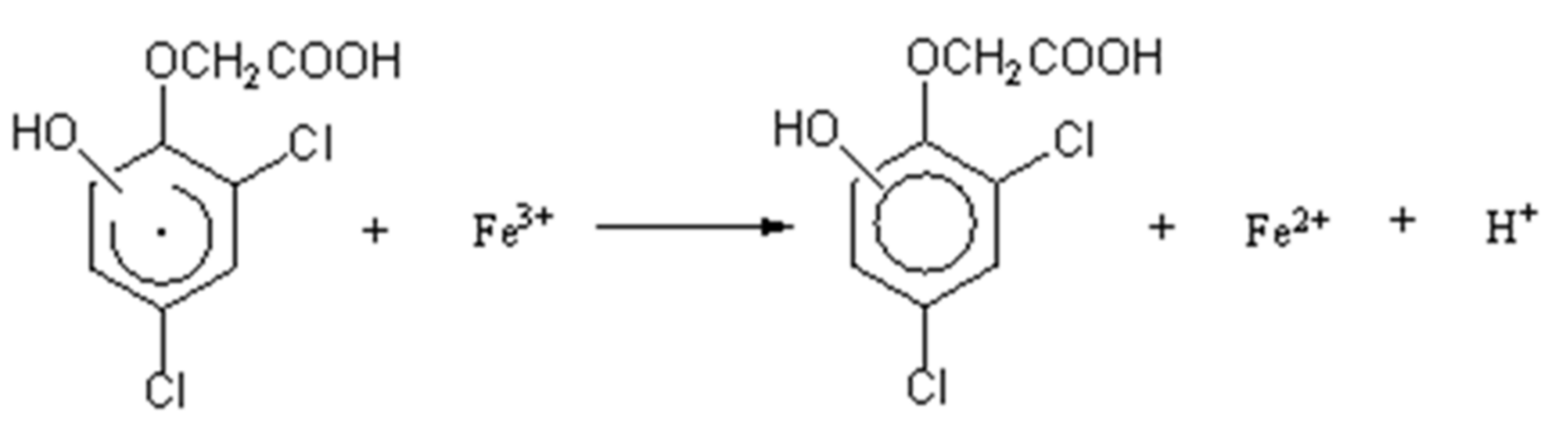
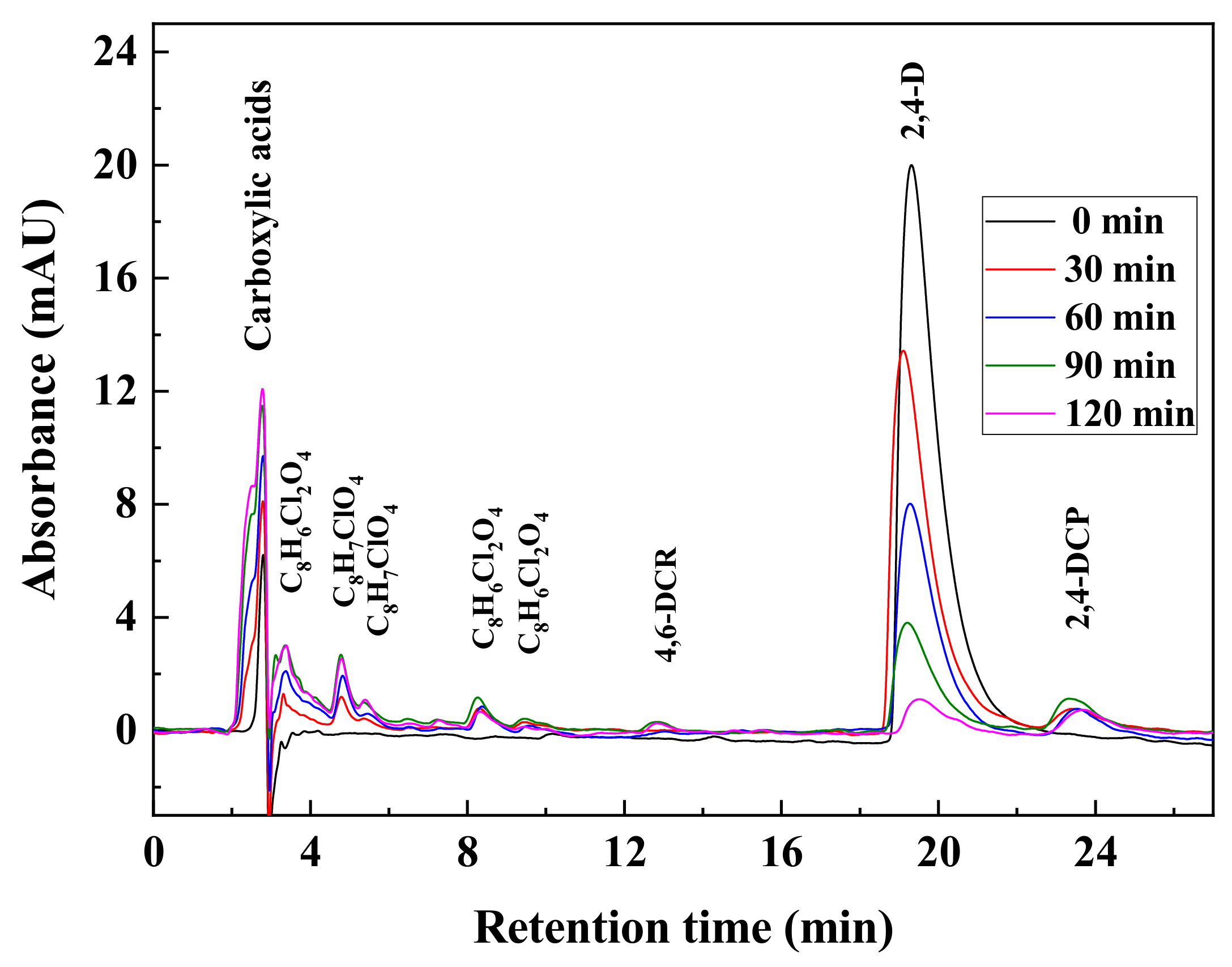
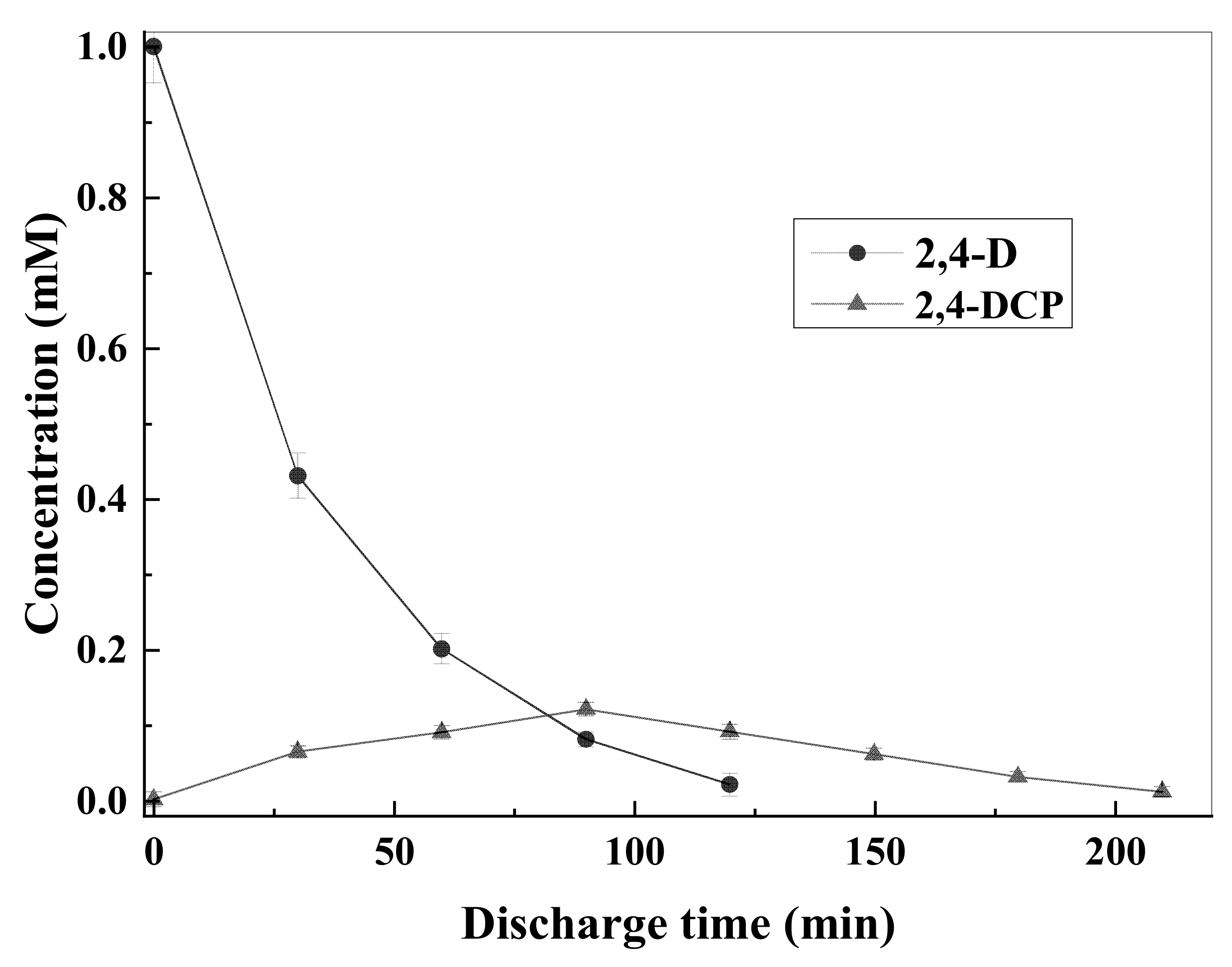
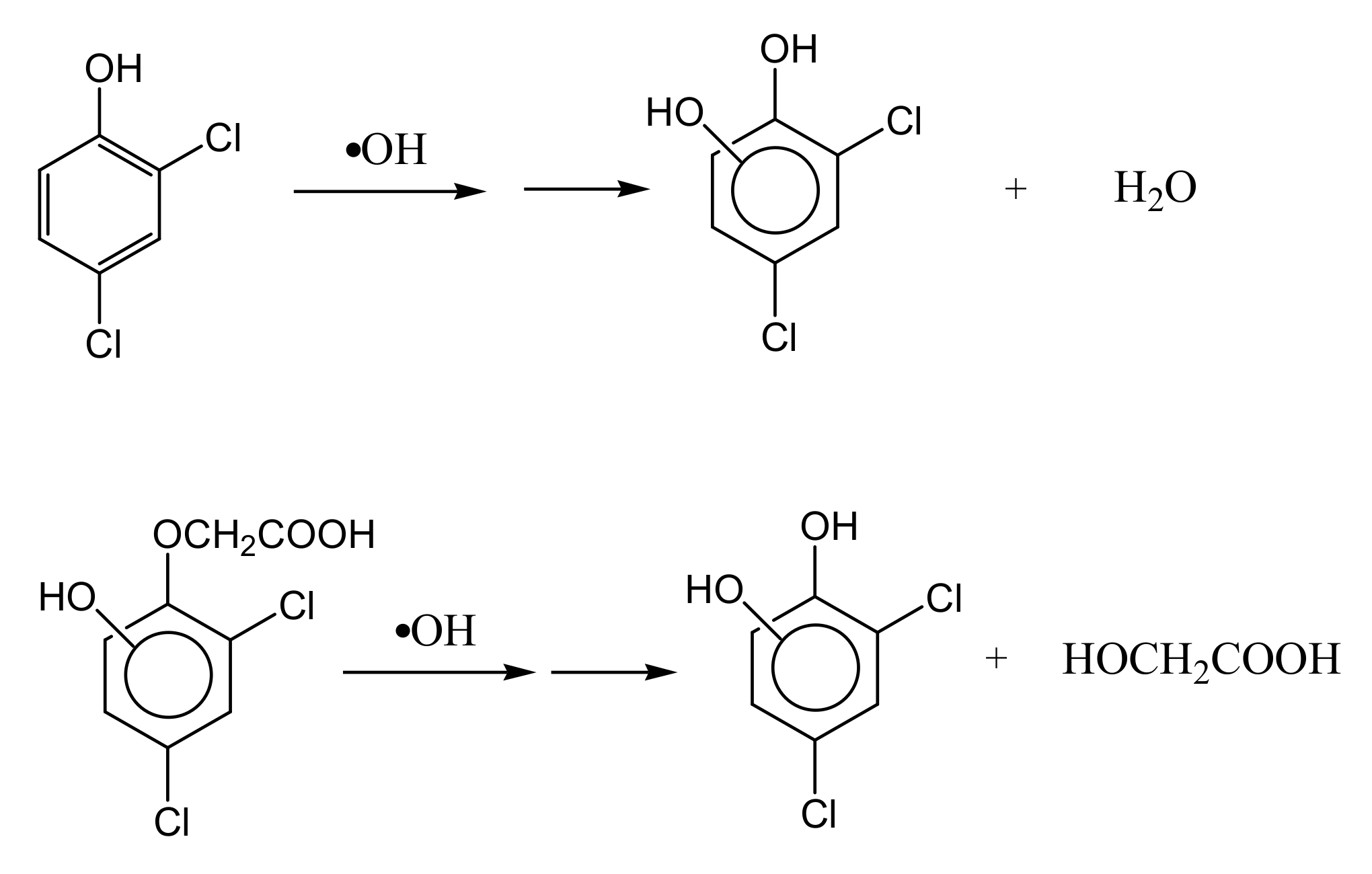
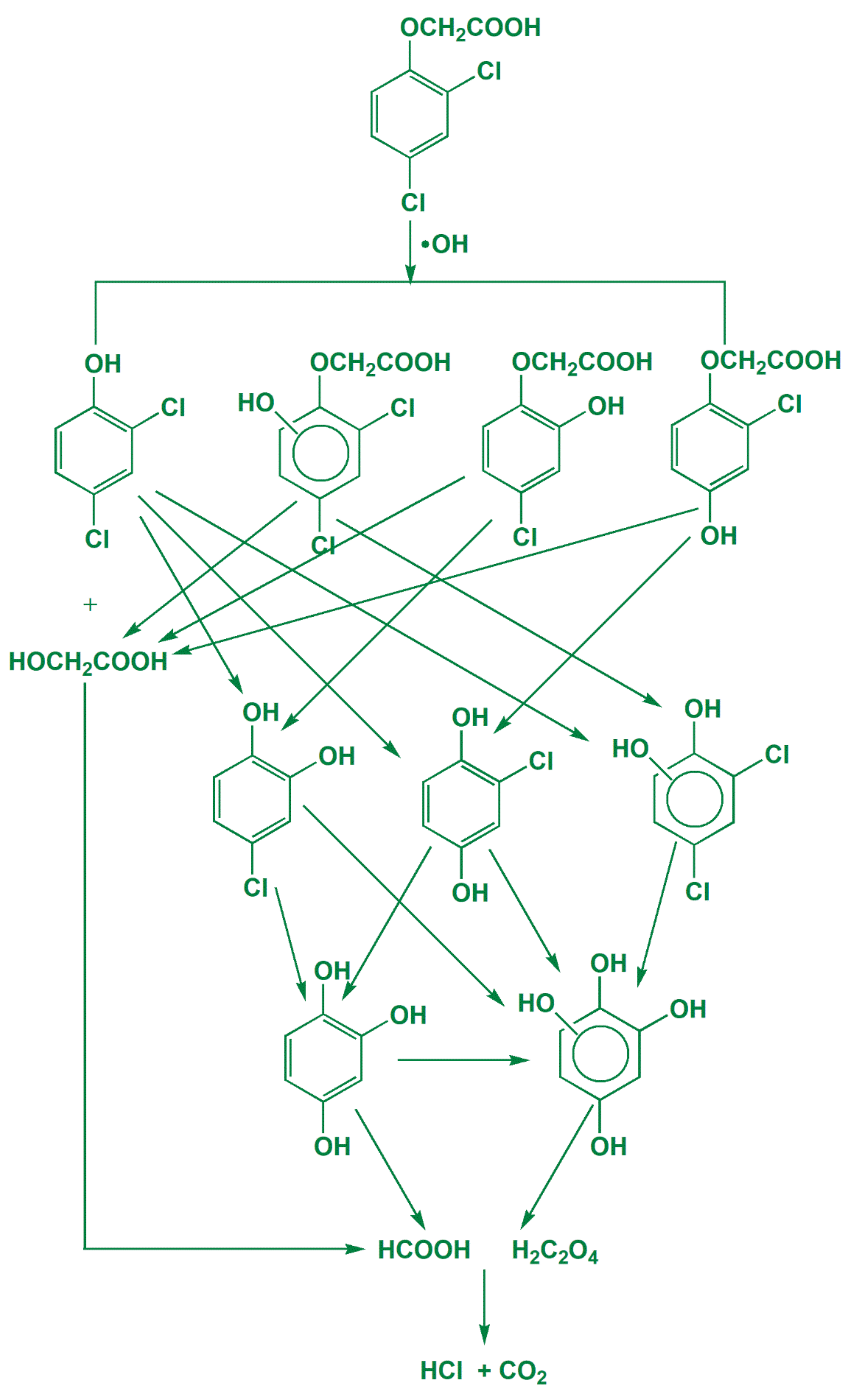
3.4. Intermediate Products Formation and Possible Decomposition Pathway
3.5. Energy Yields Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.invasive.org/gist/products/handbook/10.24-d.pdf (accessed on 24 April 2001).
- Loomis, D.; Guyton, K.; Grosse, Y.; Ghissasi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of lindane, DDT, and 2,4-Dichlorophenoxyacetic acid. Lancet Oncol. 2015, 16, 891–892. [Google Scholar] [CrossRef]
- Song, Y. Insight into the mode of action of 2,4-Dichlorophenoxyacetic acid (2,4-D) as an herbicide. J. Integr. Plant Biol. 2014, 56, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.G.; Borgonovi, G.E.; Jones, S.H.; Alexander, M. Anomalies in mineralization of low concentrations of organic compounds in lake water and sewage. Appl. Environ. Microbiol. 1986, 51, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksu, Z.; Kabasakal, E. Adsorption characteristics of 2,4-Dichlorophenoxyacetic acid (2,4-D) from aqueous solution on powdered activated carbon. J. Environ. Sci. Health B 2005, 40, 545–570. [Google Scholar] [CrossRef]
- Trillas, M.; Peral, J.; Domènech, X. Redox photodecomposition of 2,4-Dichlorophenoxyacetic acid over TiO2. Appl. Catal. B Environ. 1995, 5, 377–387. [Google Scholar] [CrossRef]
- Brillas, E.; Calpe, J.C.; Casado, J. Mineralization of 2,4-D by advanced electrochemical oxidation processes. Water Res. 2000, 34, 2253–2262. [Google Scholar] [CrossRef]
- Zona, R.; Solar, S. Oxidation of 2,4-Dichlorophenoxyacetic acid by ionizing radiation: Decomposition, detoxification and mineralization. Radiat. Phys. Chem. 2003, 66, 137–143. [Google Scholar] [CrossRef]
- Peller, J.; Wiest, O.; Kamat, P.V. Synergy of combining sonolysis and photocatalysis in the decomposition and mineralization of chlorinated aromatic compounds. Environ. Sci. Technol. 2003, 37, 1926–1932. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Miessner, H.; Mahyar, A.; Mueller, S.; Moeller, D.; Mustafa, F.; Omer, K.M. Degradation of perfluorosurfactant in aqueous solution using non-thermal plasma generated by nano-second pulse corona discharge reactor. Arab. J. Chem. 2021, 14, 103366. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Miessner, H.; Mahyar, A.; Mueller, S.; Kalass, D.; Moeller, D.; Omer, K.M. Removal of dichloroacetic acid from aqueous solution using non-thermal plasma generated by dielectric barrier discharge and nano-pulse corona discharge. Sep. Purif. Technol. 2019, 216, 51–57. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A. Recent advances of cold plasma technology for water and soil remediation: A critical review. Chem. Eng. J. 2022, 428, 131657. [Google Scholar] [CrossRef]
- Bradu, C.; Magureanu, M.; Parvulescu, V.I. Degradation of the chlorophenoxyacetic herbicide 2,4-D by plasma-ozonation system. J. Hazard. Mater. 2017, 336, 52–56. [Google Scholar] [CrossRef]
- Singh, R.K.; Philip, L.; Ramanujam, S. Removal of 2,4-dichlorophenoxyacetic acid in aqueous solution by pulsed corona discharge treatment: Effect of different water constituents, decomposition pathway and toxicity assay. Chemosphere 2017, 184, 207–214. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Miessner, H.; Mueller, S.; Mahyar, A.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Comparative study on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J. Hazard. Mater. 2018, 343, 107–115. [Google Scholar] [CrossRef]
- Maehara, T.; Miyamot, I.; Kurokawa, K.; Hashimoto, Y.; Iwamae, A.; Kuramoto, M.; Yamashita, H.; Mukasa, S.; Toyota, H.; Nomura, S.; et al. Decomposition of methylene blue by RF plasma in water. Plasma Chem. Plasma Process. 2008, 28, 467–482. [Google Scholar] [CrossRef]
- Amano, T.; Mukasa, S.; Honjoya, N.; Okumura, H.; Maehara, T. Generation of radio frequency plasma in high-conductivity NaCl solution. Jpn. J. Appl. Phys. 2012, 51, 978–982. [Google Scholar] [CrossRef]
- Ji, L. Research on Characteristics of Radio Frequency Plasma Discharge Underwater and Its Application of Azo Dyes Decomposition; Soochow University: Suzhou, China, 2012. (In Chinese) [Google Scholar]
- Wang, L.; Wang, J.; Lin, J.; Zhang, S.; Liu, Y. Decomposition of metronidazole by radio frequency discharge in an aqueous solution. Plasma Proces. Polym. 2018, 15, 1700176. [Google Scholar] [CrossRef]
- Zona, R.; Solar, S.; Gehringer, P. Decomposition of 2,4-dichlorophenoxyacetic acid by ionizing radiation: Influence of oxygen concentration. Water Res. 2002, 36, 1369–1374. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Y.; Sun, B. Characteristics of gas-liquid diaphragm discharge and its application on decolorization of brilliant red B in aqueous solution. Plasma Sci. Technol. 2017, 19, 115404. [Google Scholar] [CrossRef]
- Harvey, A.E.; Smart, J.A.; Amis, E.S. Simultaneous spectrophotometric determination of iron (II) and total iron with 1,10-phenanthroline. Anal. Chem. 1953, 26, 1854–1856. [Google Scholar] [CrossRef]
- Marotta, E.; Ceriani, E.; Schiorlin, M.; Ceretta, C.; Paradisi, C. Comparison of the rates of phenol advanced oxidation in deionized and tap water within a dielectric barrier discharge reactor. Water Res. 2012, 46, 6239–6246. [Google Scholar] [CrossRef]
- Li, X.; Jenks, W.S. Isotope studies of photocatalysis: Dual mechanisms in the conversion of anisole to phenol. J. Am. Chem. Soc. 2000, 122, 11864–11870. [Google Scholar] [CrossRef]
- Zona, R.; Solar, S.; Sehested, K.; Holcman, J.; Mezyk, S.P. OH-radical induced oxidation of phenoxyacetic acid and 2,4-Dichlorophenoxyacetic acid. Primary radical steps and products. J. Phys. Chem. A 2002, 106, 6743–6749. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, X.; Liu, Y. Degradation of bisphenol A and formation of hydrogen peroxide induced by glow discharge plasma in aqueous solutions. J. Hazard. Mater. 2008, 154, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, J.H.; Dixon, R.S.; Stott, D.A. Reactivity of hydrogen atoms with Fe3+, FeOH2+ and Cu2+ in aqueous solutions. Trans. Faraday Soc. 1968, 64, 2398–2401. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Soutrel, I.; Petit, P.; Medimagh, K.; Wolbert, D. A study of pollution removal in exhaust gases from animal quartering centers by combining photocatalysis with surface discharge plasma: From pilot to industrial scale. Chem. Eng. Processing Process Intensif. 2017, 111, 1–6. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (•OH/•O−) in aqueous-solution. J. Phys. Chem. Refer. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Quint, R.M.; Park, H.R.; Krajnik, P.; Solar, S.; Getoff, N.; Sehested, K. γ-radiolysis and pulse radiolysis of aqueous 4-chloroanisole. Radiat. Phys. Chem. 1996, 47, 835–845. [Google Scholar] [CrossRef]
- Albarrán, G.; Mendoza, E. Radiolytic oxidation and degradation of 2,4-dichlorophenol in aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 17055–17065. [Google Scholar] [CrossRef]

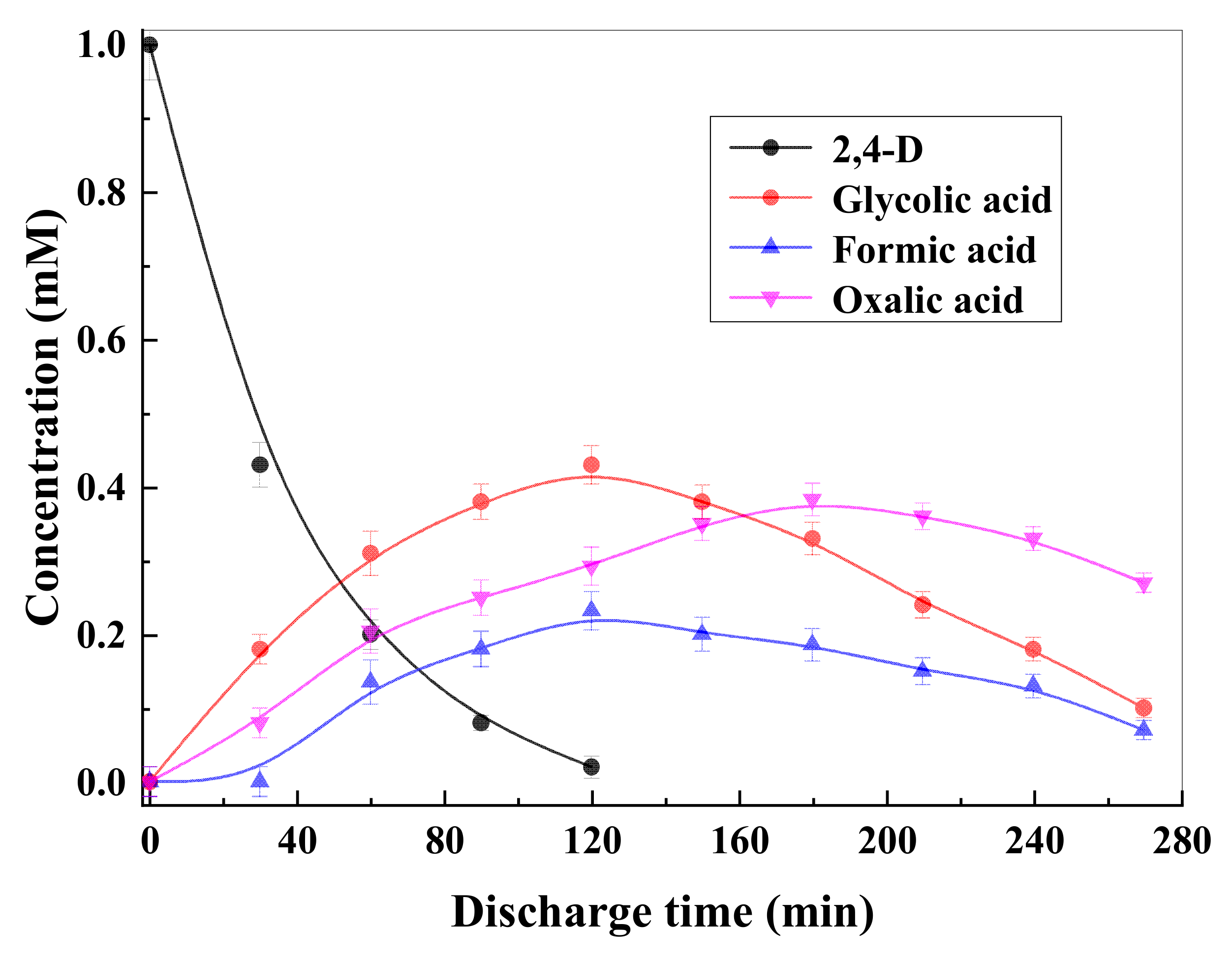
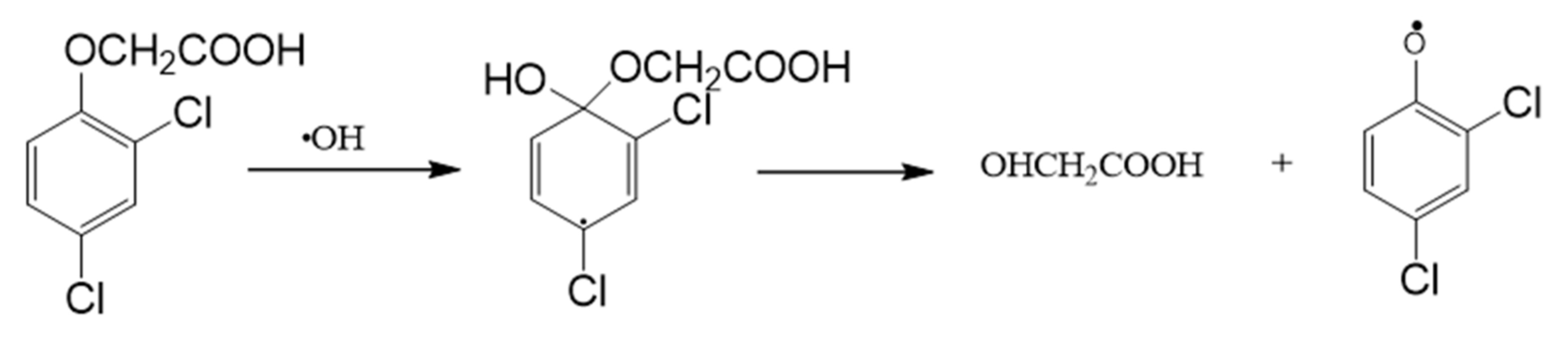
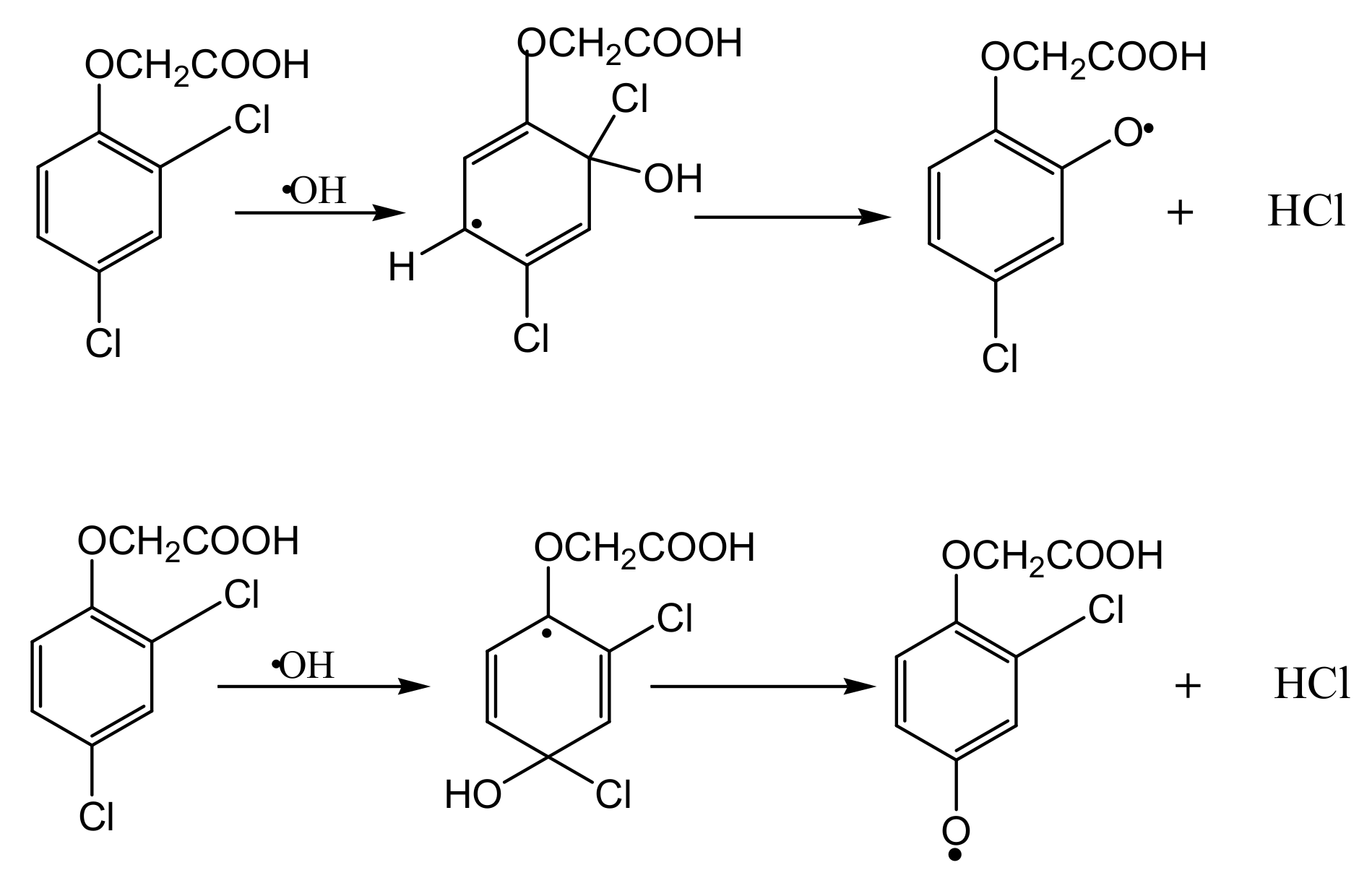
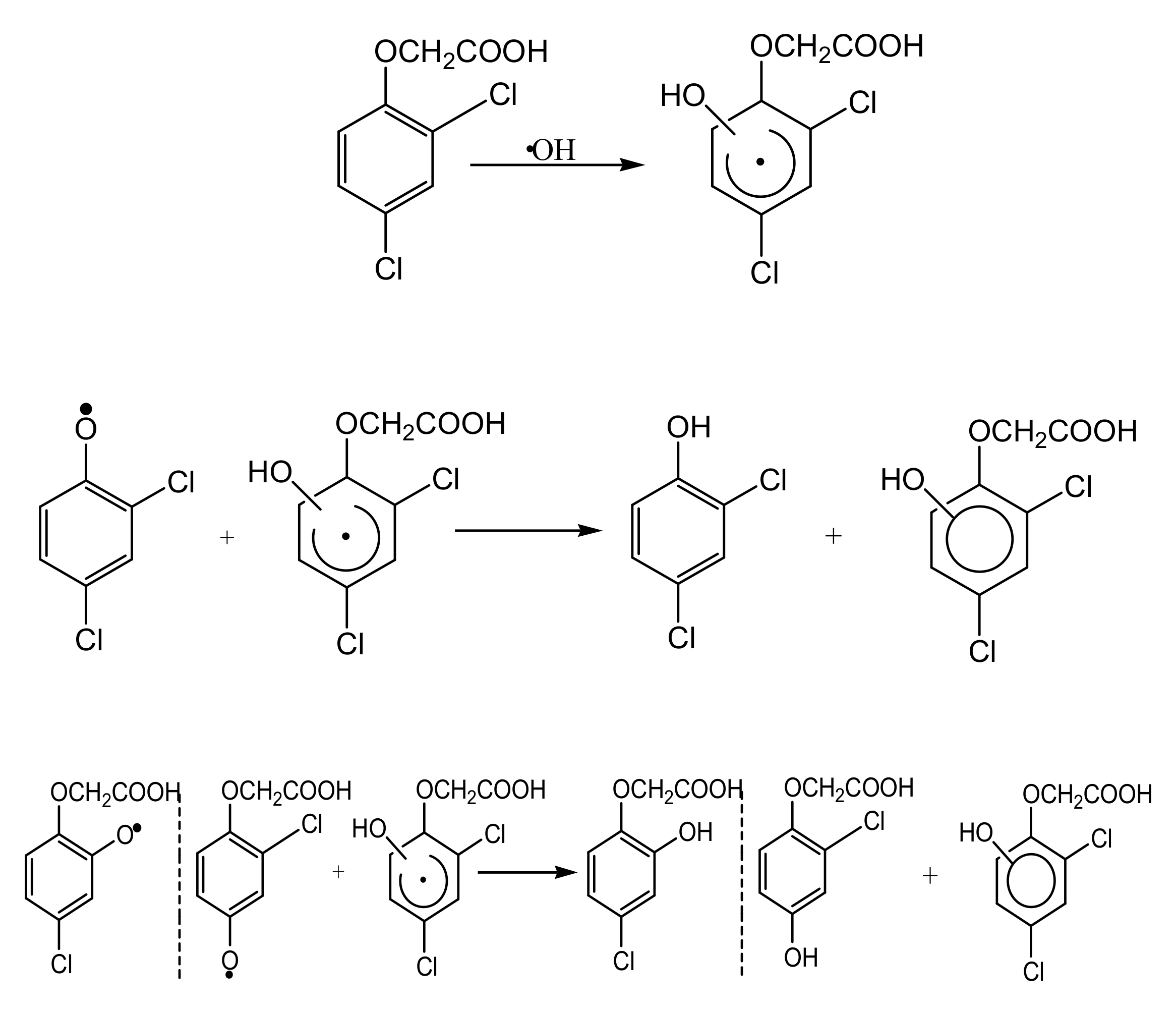
| C0 (mM) | Method | k | J2,4-D | References |
|---|---|---|---|---|
| min−1 | g/kWh | |||
| 1.0 | RFD 200 W, pH0 2.0 | 0.018 | 0.35 | This work |
| 1.0 | RFD 200 W, pH0 2.0, 0.5 mM Fe3+ | 0.078 | 1.51 | This work |
| 1.0 | RFD 200 W, pH0 2.0, 0.5 mM Fe2+ | 0.063 | 1.22 | This work |
| 10.0 | RFD 200 W, pH0 2.0, 20 mM H2O2 2,4-D, 0.5 mM Fe3+ | 0.047 | 9.06 | This work |
| 0.0045 | PCD over water surface, air as discharge gas (PCD/Air) | 1.50 | [19] | |
| 0.25 | TiO2 photocatalysis, 125 W, 2.0g/L TiO2, pH0 3.3 | 0.76 | [6] | |
| 0.22 | Sonolysis, 50 W, O2 sparging | 1.75 | [9] | |
| 0.45 | DBD/Ar-Fenton | 8.83 | [15] | |
| 0.45 | DBD/Ar | 3.85 | [15] | |
| 0.45 | DBD/Air, 200 W | 0.25 | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Sun, B. Unusual Catalytic Effect of Fe3+ on 2,4-dichlorophenoxyacetic Acid Degradation by Radio Frequency Discharge in Aqueous Solution. Water 2022, 14, 1719. https://doi.org/10.3390/w14111719
Liu Y, Sun B. Unusual Catalytic Effect of Fe3+ on 2,4-dichlorophenoxyacetic Acid Degradation by Radio Frequency Discharge in Aqueous Solution. Water. 2022; 14(11):1719. https://doi.org/10.3390/w14111719
Chicago/Turabian StyleLiu, Yongjun, and Bing Sun. 2022. "Unusual Catalytic Effect of Fe3+ on 2,4-dichlorophenoxyacetic Acid Degradation by Radio Frequency Discharge in Aqueous Solution" Water 14, no. 11: 1719. https://doi.org/10.3390/w14111719





