Research Progress on Adsorption of Arsenic from Water by Modified Biochar and Its Mechanism: A Review
Abstract
:1. Introduction
2. Biochar Preparation and Modification
2.1. Preparation of Biochar
2.2. Modification of Biochar
3. Characterization of Biochar
3.1. SEM Analysis
3.2. XRD Analysis
3.3. XPS Analysis
3.4. FT-IR Analysis
3.5. TG Analysis
3.6. Other Characterization Methods
4. Influencing Factors of Arsenic Removal by Modified Biochar
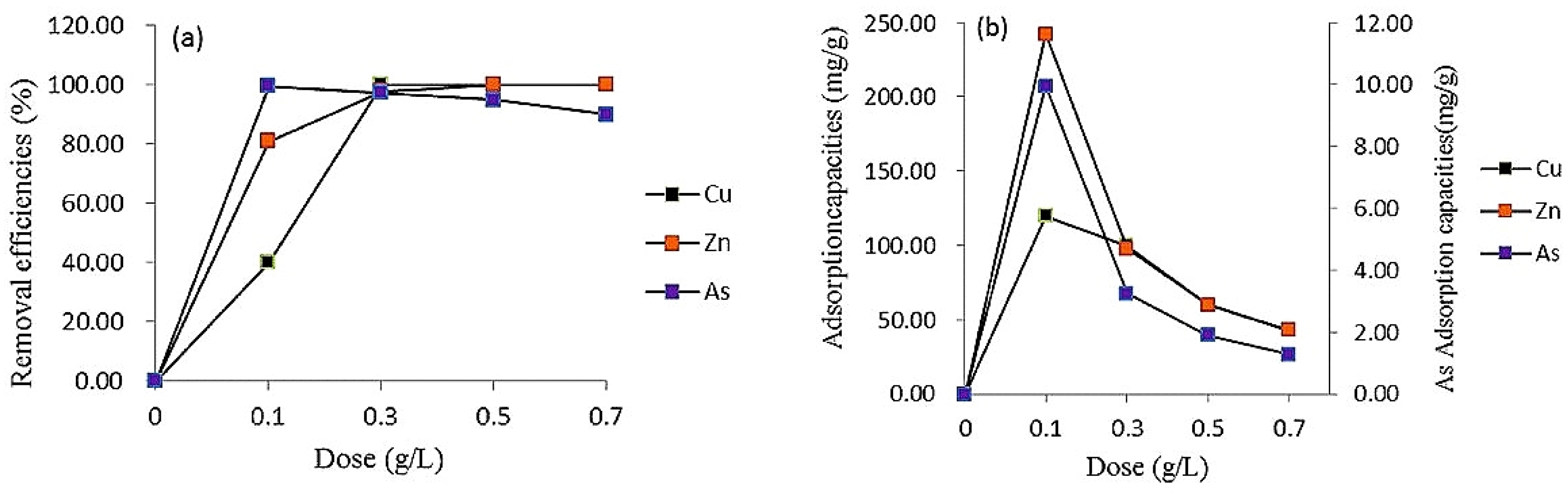
4.1. Effect of Adsorbent Dosage
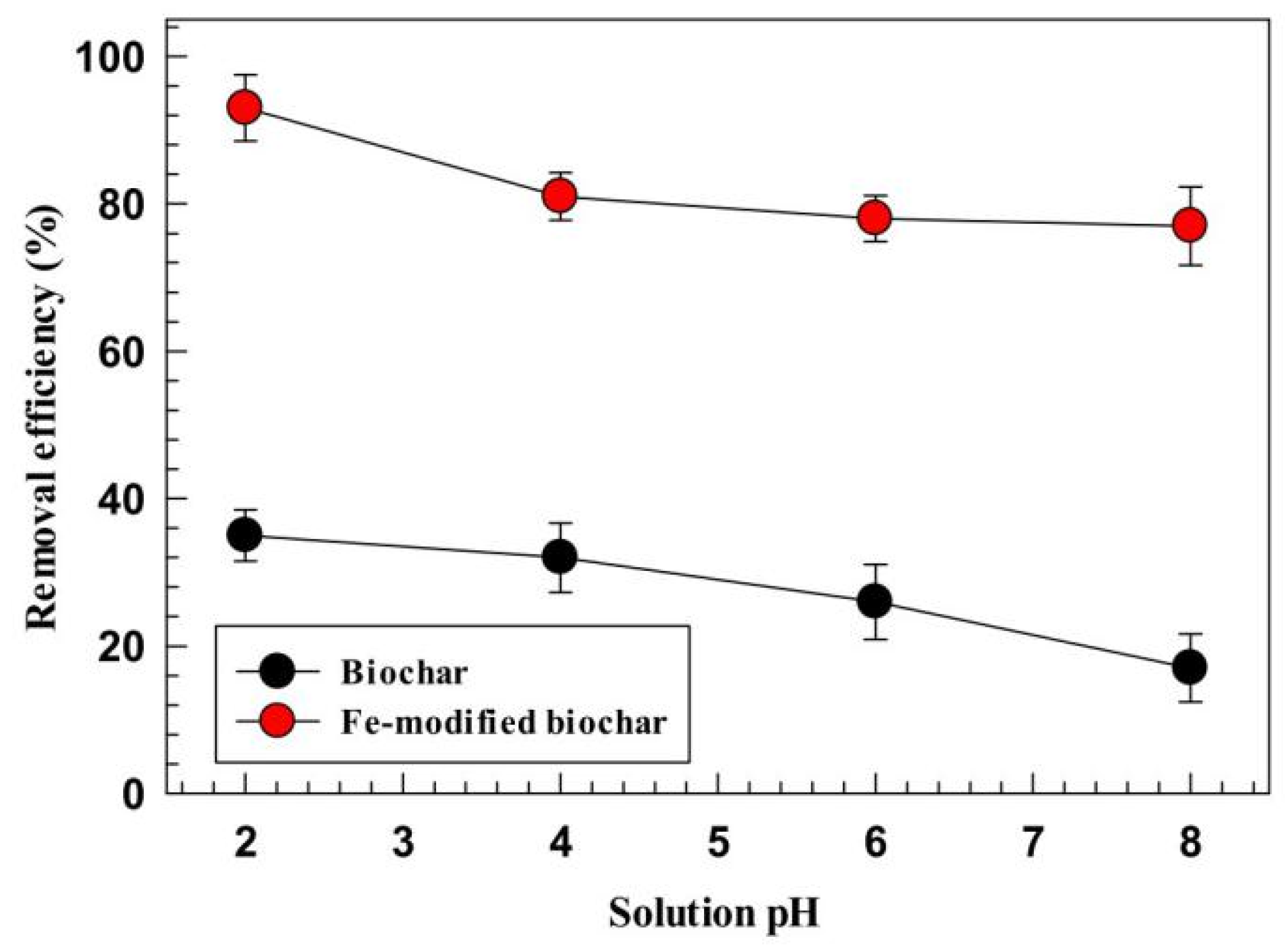
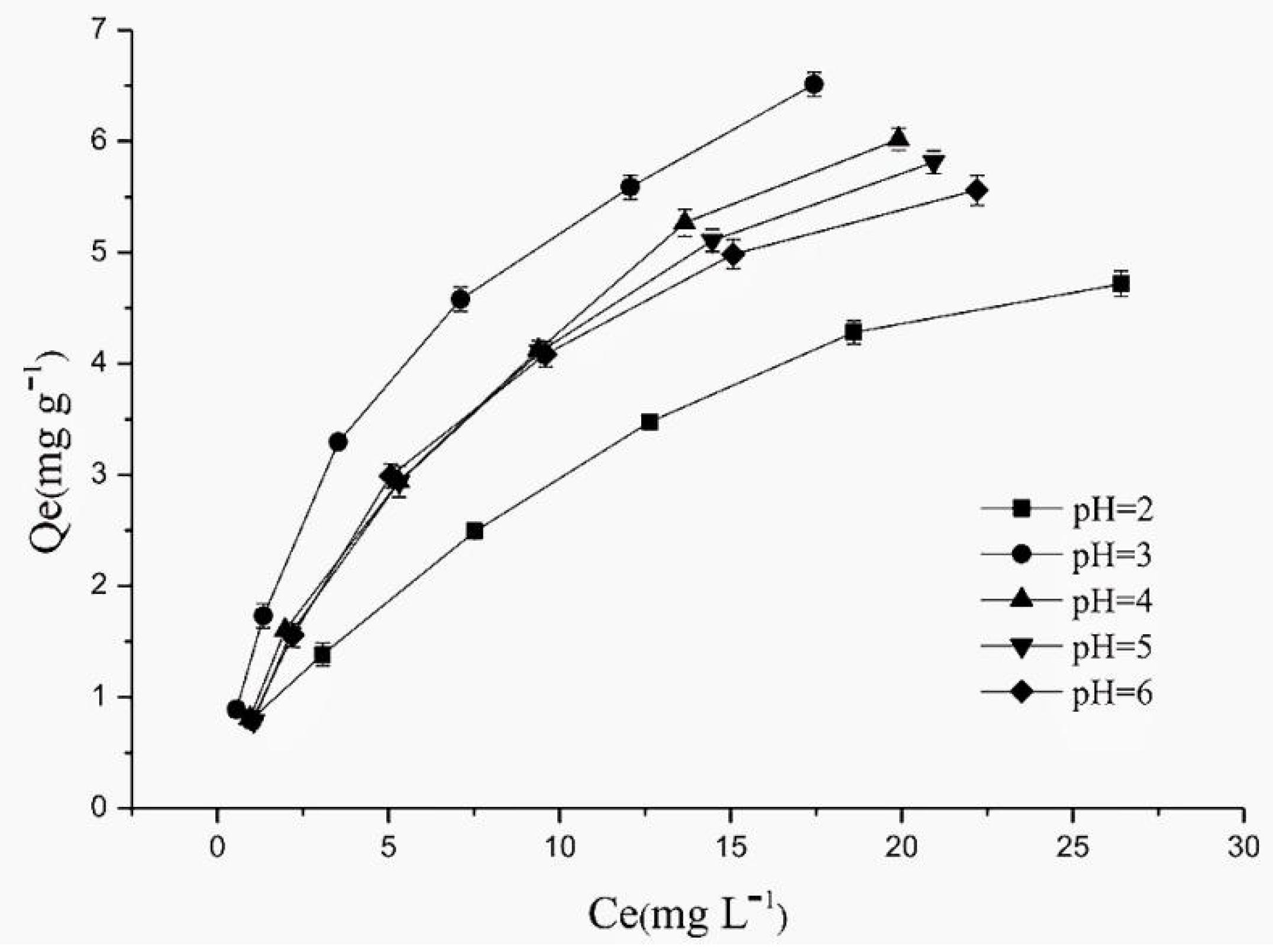
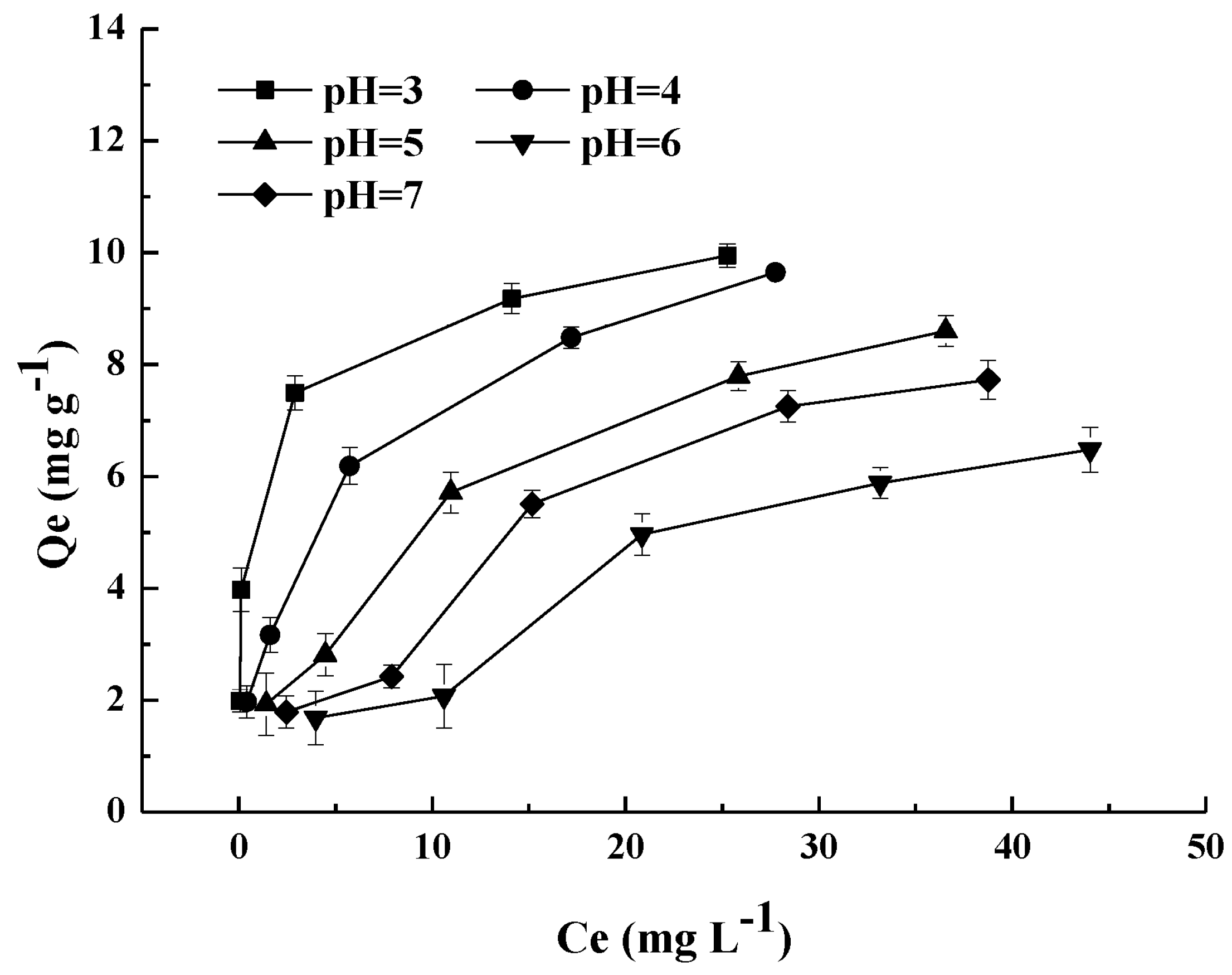
4.2. Effect of Solution pH
4.3. Effect of Reaction Temperature
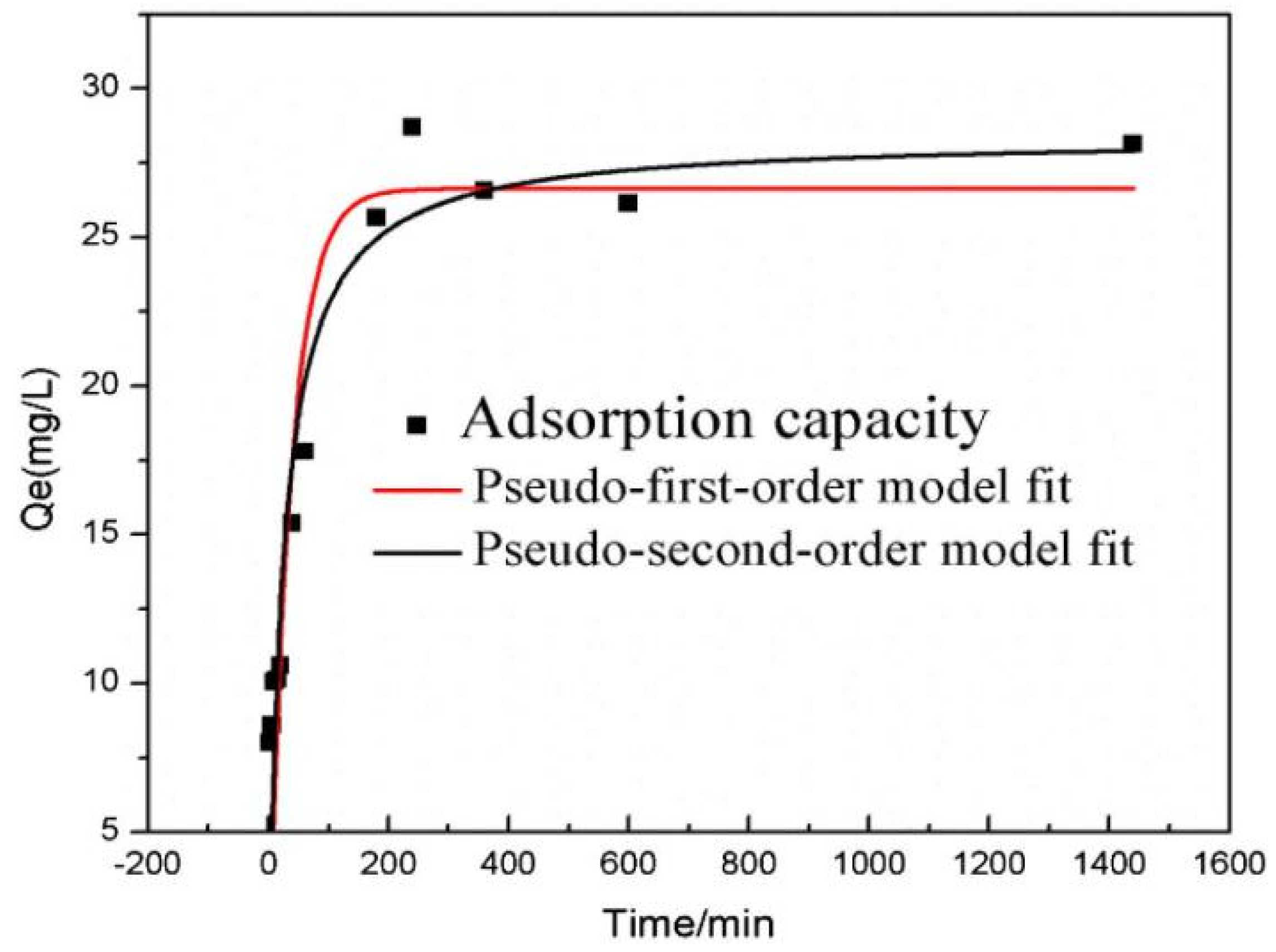
4.4. Effect of Reaction Time
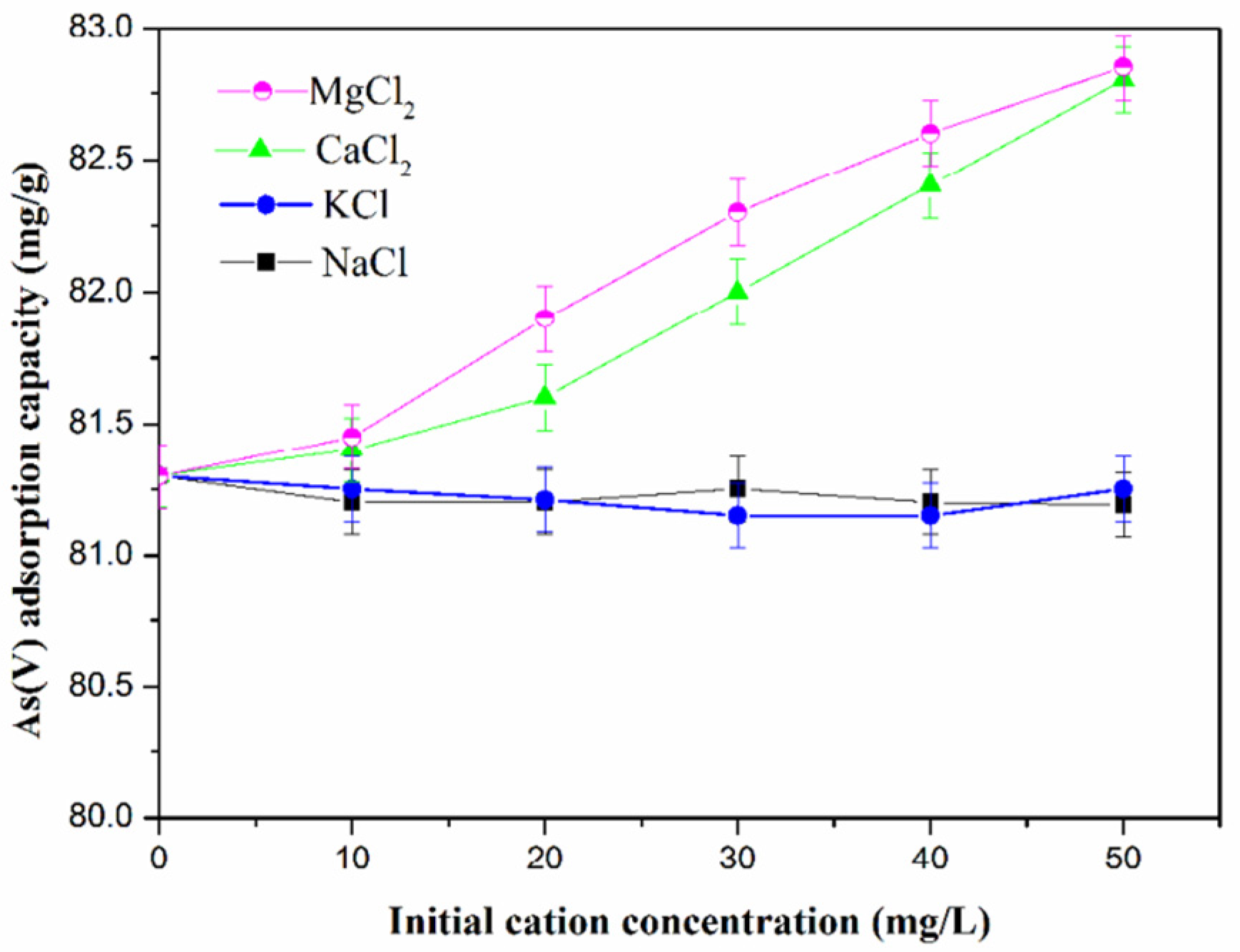
4.5. Influence of Ion Concentration
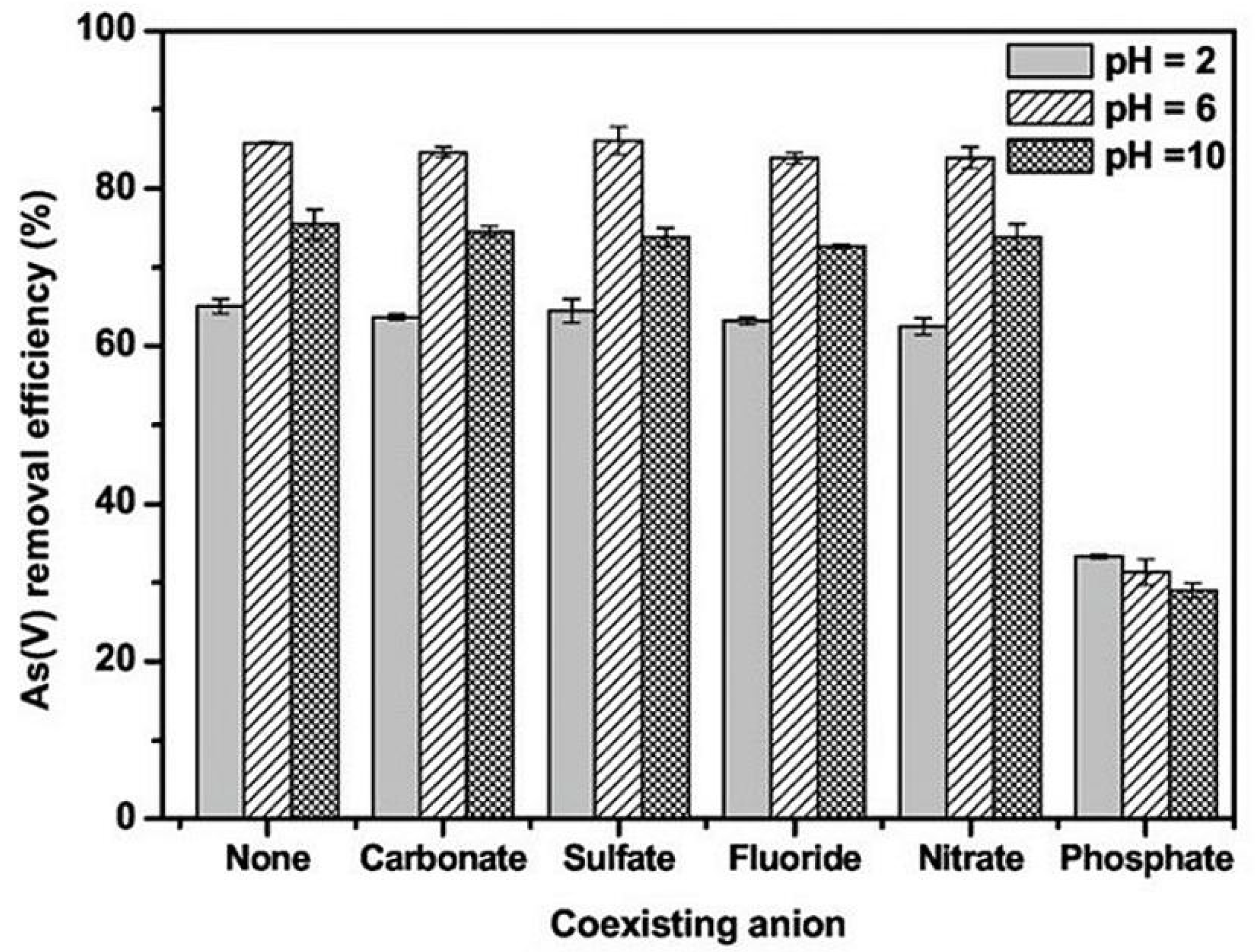
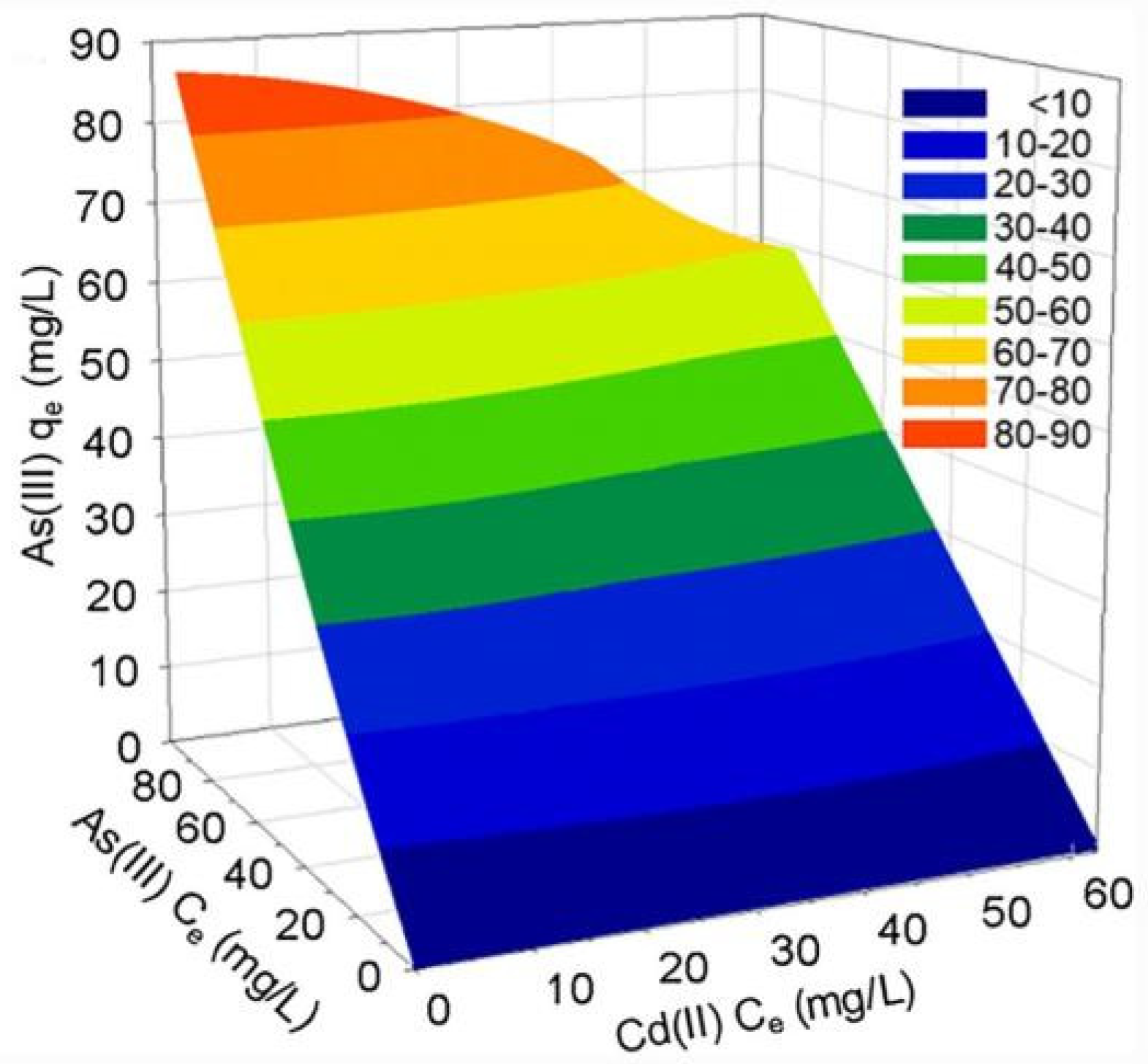
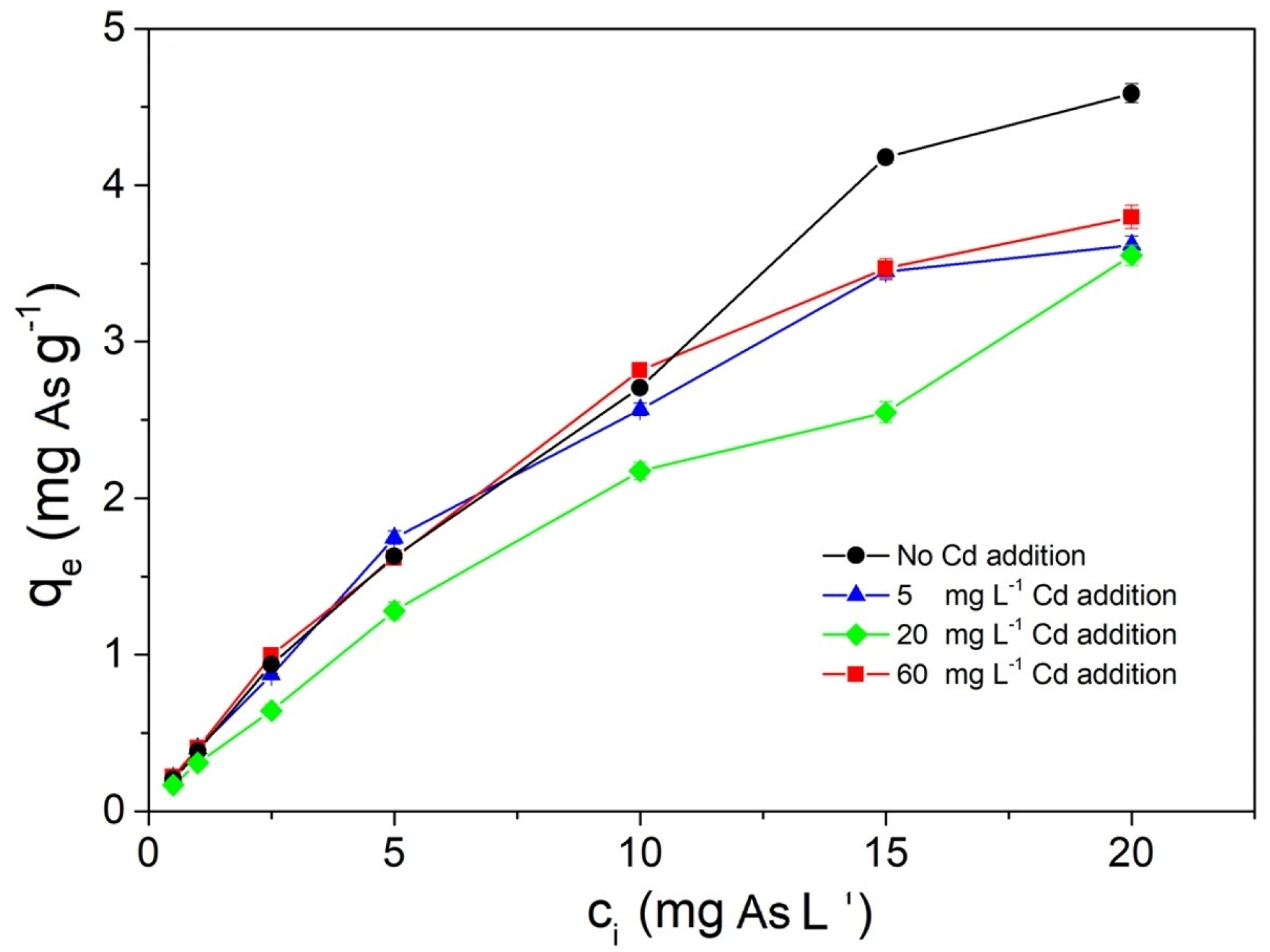
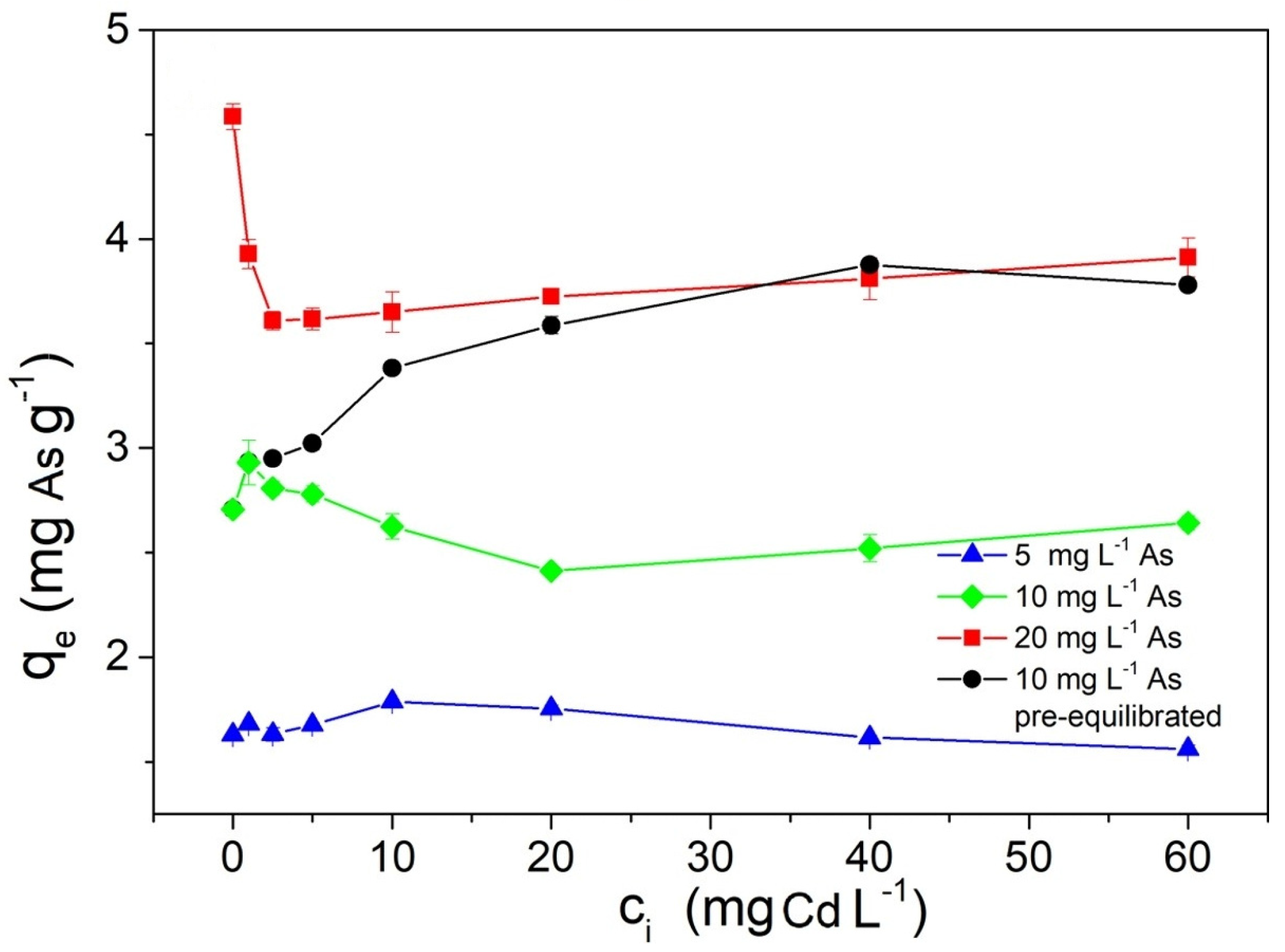
4.6. Influence of Co-Existing Ions
5. Application of Modified Biochar in Arsenic Removal from Water
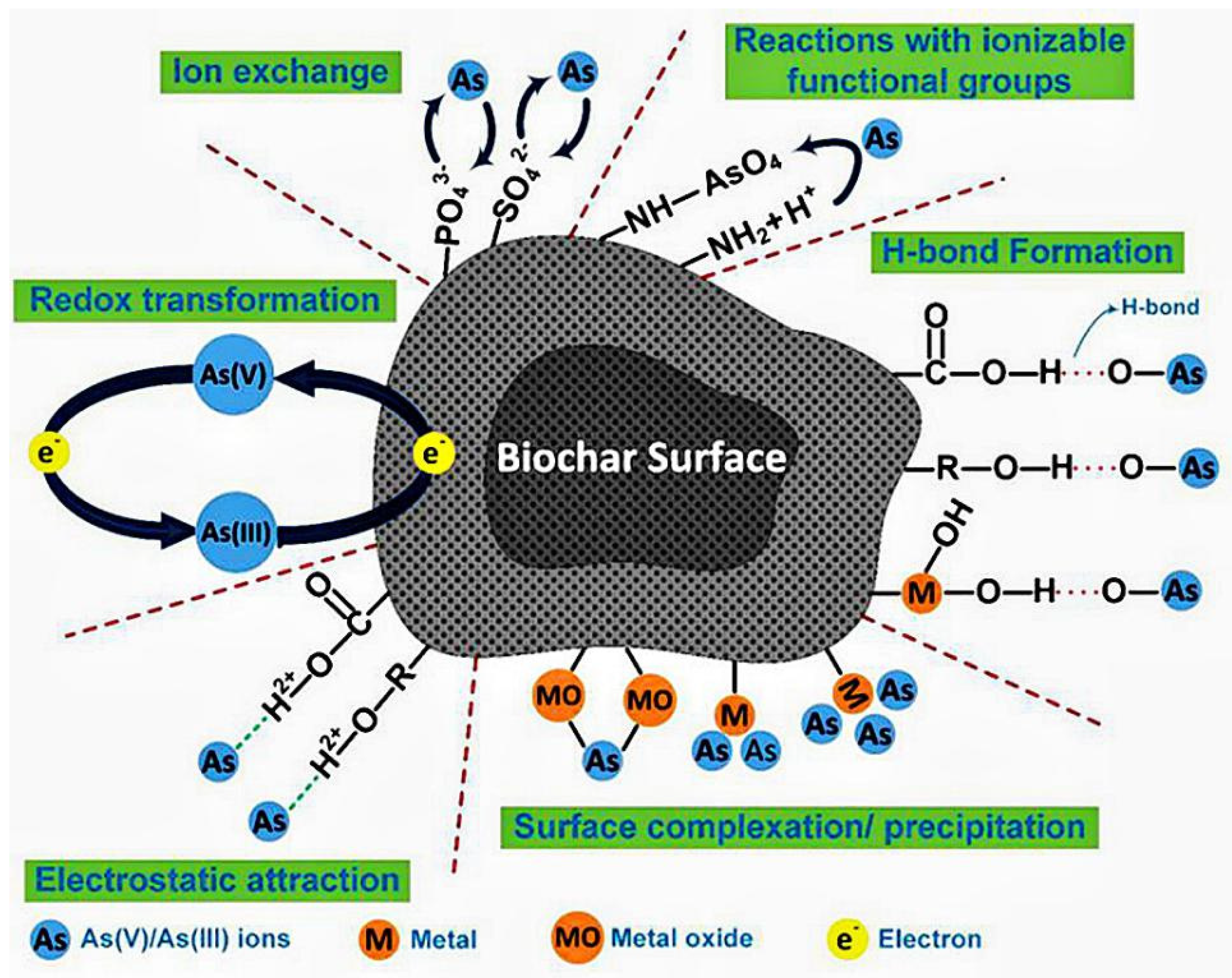
6. Mechanism of Arsenic Removal by Modified Biochar
7. Summary and Prospect
- (1)
- Recently, the most frequently used technique for preparing biochar is pyrolysis technology. However, due to the time-consuming, and low efficiency of pyrolysis technology, and with the deepening of research, the requirements for adsorption materials are increasing. In order to obtain biochar with better performance, the research and use of emerging technologies such as hydrothermal carbonization and microwave can be strengthened.
- (2)
- The toxicity of trivalent methylated arsenic is far greater than that of inorganic arsenic, and there are few reports on this aspect.
- (3)
- Most of the research stays on the laboratory scale, and they are batch processing systems for a short time, while in the actual situation, they are continuous processing systems. Therefore, the practical application research of modified biochar in As removal should be investigated.
- (4)
- For field application, cost-effectiveness is a key constraint, and many commercial adsorbents have been widely used in actual water treatment. In order to increase the competitiveness of biochar, a cost analysis must be carried out. In addition, it is also necessary to increase the comparative study of arsenic removal efficiency with competitive adsorbents (e.g., activated carbon).
- (5)
- How to recycle or dispose of the used biochar to prevent secondary pollution of water and soil is also a question worthy of consideration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y. Global solutions to a silent poison. Science 2020, 368, 818–819. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Ghosh, P.K. Simultaneous removal of arsenic and nitrate in absence of iron in an attached growth bioreactor to meet drinking water standards: Importance of sulphate and empty bedcontact time. J. Clean. Prod. 2018, 186, 304–312. [Google Scholar] [CrossRef]
- Manning, B.A.; Fendorf, S.E.; Bostick, B.; Suarez, D.L. Arsenic (III) oxidation and arsenic (V) adsorption reactions on syntheticbirnessite. Environ. Sci. Technol. 2002, 36, 976–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Khan, M.H.; Haque, M. Arsenic contamination in groundwater in bangladesh: Implications and challenges for healthcare policy. Risk Manag. Healthc. Policy 2018, 11, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Arsenite removal from water by electro-coagulation on zinc–zinc and copper–copper electrodes. Int. J. Environ. Sci. Technol. 2013, 10, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Senn, A.; Hug, S.J.; Kaegi, R.; Hering, J.G.; Voegelin, A. Arsenate co-precipitation with Fe (II) oxidation products and retention or release during precipitate aging. Water Res. 2018, 131, 334–345. [Google Scholar] [CrossRef] [Green Version]
- Nobaharan, K.; Bagheri Novair, S.; Asgari Lajayer, B.; van Hullebusch, E. Phosphorus removal from wastewater: The potential use of biochar and the key controlling factors. Water 2021, 13, 517. [Google Scholar] [CrossRef]
- Hu, Y.; Boyer, T.H. Removal of multiple drinking water contaminants by combined ion exchange resin in a completely mixed flow reactor. J. Water Supply Res. Technol. 2018, 67, 659–672. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, X.; Li, J.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Zhao, S. Developing new adsorptive membrane by modification of support layer with iron oxide microspheres for arsenic removal. J. Colloid Interf. Sci. 2018, 514, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, L.; Wang, C.; Chen, J.P. An innovative yttrium nanoparticles/PVA modified PSF membrane aiming at decontamination of arsenate. J. Colloid Interface Sci. 2018, 530, 658–666. [Google Scholar] [CrossRef]
- You, H.J.; Han, I.S. Effects of dissolved ions and natural organic matter on electrocoagulation of As (III) in groundwater. J. Environ. Chem. Eng. 2016, 4, 1008–1016. [Google Scholar] [CrossRef]
- Khan, S.U.; Farooqi, I.H.; Usman, M.; Basheer, F. Energy efficient rapid removal of arsenic in an electrocoagulation reactor with hybrid Fe/Al electrodes: Process optimization using CCD and kinetic modeling. Water 2020, 12, 2876. [Google Scholar] [CrossRef]
- Nyangi, M.J. Remediation of arsenic from water using iron and aluminum electrodes in electrocoagulation technology: Adsorption isotherm and kinetic studies. Chem. Afr. 2021, 4, 943–954. [Google Scholar] [CrossRef]
- Paul, S.; Bhardwaj, S.K.; Kaur, R.; Bhaumik, A.J. Lignin-derived hybrid materials as promising adsorbents for the separation of pollutants. In Multidisciplinary Advances in Efficient Separation Processes; Chernyshova, I., Ponnurangam, S., Liu, Q.X., Eds.; American Chemical Society: Washington, DC, USA, 2020; Volume 1348, pp. 225–261. [Google Scholar]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2011, 185, 1614–1617. [Google Scholar] [CrossRef]
- Merodio-Morales, E.E.; Reynel-ávila, H.E.; Mendoza-Castillo, D.I.; Duran-Valle, C.J.; Bonilla-Petriciolet, A. Lanthanum- and cerium-based functionalization of chars and activated carbons for the adsorption of fluoride and arsenic ions. Int. J. Environ. Sci. Technol. 2020, 17, 115–128. [Google Scholar] [CrossRef]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic removal from water by adsorption onto iron oxide/nano-porous carbon magnetic composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef] [Green Version]
- Walsh, K.; Mayer, S.; Rehmann, D.; Hofmann, T.; Glas, K. Equilibrium data and its analysis with the freundlich model in the adsorption of arsenic (V) on granular ferric hydroxide. Sep. Purif. Technol. 2020, 243, 116704. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.; Bello, M.M. Adsorption of arsenic from aqueous solution using magnetic graphene oxide. IOP Conf. Ser. Mater. Sci. Eng. 2017, 210, 12007. [Google Scholar] [CrossRef] [Green Version]
- Pillai, P.; Kakadiya, N.; Timaniya, Z.; Dharaskar, S.; Sillanpaa, M. Removal of arsenic using iron oxide amended with rice husk nanoparticles from aqueous solution. Mater. Today Proc. 2020, 28, 830–835. [Google Scholar] [CrossRef]
- Gupta, N.K.; Bae, J.; Kim, K.S. Iron-organic frameworks-derived iron oxide adsorbents for hydrogen sulfide removal at room temperature. J. Environ. Chem. Eng. 2021, 9, 106195. [Google Scholar]
- Usman, M.; Katsoyiannis, I.; Rodrigues, J.H.; Ernst, M. Arsenate removal from drinking water using by-products from conventional iron oxyhydroxides production as adsorbents coupled with submerged microfiltration unit. Environ. Sci. Pollut. Res. 2021, 28, 59063–59075. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Kushwaha, K.; MC Chattopadhyaya, A. Adsorption of cobalt (II) from aqueous solution onto hydroxyapatite/zeolite composite. Adv. Mater. Lett. 2011, 2, 309–312. [Google Scholar] [CrossRef]
- Pervez, M.N.; Fu, D.; Wang, X.; Bao, Q.; Yu, T.; Naddeo, V.; Tian, H.; Cao, C.; Zhao, Y. A bifunctional α-FeOOH@GCA nanocomposite for enhanced adsorption of arsenic and photo fenton-like catalytic conversion of As (III). Environ. Technol. Innov. 2021, 22, 101437. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, W.; Zhang, Z.; Yang, Z.; Wang, Y. Adsorption of three pharmaceuticals on two magnetic ion-exchange resins. J. Environ. Sci. 2015, 31, 226–234. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Gao, Q.; Gao, Y.; Liu, S. Adsorption of phosphorus by different biochars. Spectrosc. Lett. 2017, 50, 73–80. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; Da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar]
- Yin, Q.; Liu, M.; Li, Y.; Li, H.; Wen, Z. Computational study of phosphate adsorption on Mg/Ca modified biochar structure in aqueous solution. Chemosphere 2021, 269, 129374. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef] [PubMed]
- Zoroufchi Benis, K.; Mcphedran, K.N.; Soltan, J. Selenium removal from water using adsorbents: A critical review. J. Hazard. Mater. 2022, 424, 127603. [Google Scholar] [CrossRef] [PubMed]
- Alhashimi, H.A.; Aktas, C.B. Life cycle environmental and economic performance of biochar compared with activated carbon: A meta-analysis. Resour. Conserv. Recycl. 2017, 118, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Sun, J.; Chen, J.; Zhang, J.; Wang, Y.; Wang, J.; Zhang, H. Adsorption of enrofloxacin on acid/alkali-modified corn stalk biochar. Spectrosc. Lett. 2019, 52, 367–375. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Hassani, A.; Bhatnagar, A. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J. Hazard. Mater. 2020, 381, 120742. [Google Scholar] [CrossRef]
- Jia, L.; Yu, Y.; Li, Z.; Qin, S.; Guo, J.; Zhang, Y.; Wang, J.; Zhang, J.; Fan, B.; Jin, Y. Study on the Hg0 removal characteristics and synergistic mechanism of iron-based modified biochar doped with multiple metals. Bioresour. Technol. 2021, 332, 125086. [Google Scholar] [CrossRef]
- Nham, N.T.; Tahtamouni, T.M.A.; Nguyen, T.D.; Huong, P.T.; Jitae, K.; Viet, N.M.; Noi, N.V.; Phuong, N.M.; Anh, N.T.H. Synthesis of iron modified ricestraw biochar toward arsenic from groundwater. Mater. Res. Express 2019, 6, 115528. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Liu, S.; Liu, H.; Zeng, G.; Tan, X.; Yang, C.; Ding, Y.; Yan, Z.; Cai, X. Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem. Eng. J. 2017, 314, 223–231. [Google Scholar] [CrossRef]
- Cha, J.S.; Jang, S.; Lam, S.S.; Kim, H.; Kim, Y.; Jeon, B.; Park, Y. Performance of CO2 and Fe-modified lignin char on arsenic (V) removal from water. Chemosphere 2021, 279, 130521. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Soltan, J.; Mcphedran, K.N. Electrochemically modified adsorbents for treatment of aqueous arsenic: Pore diffusion in modified biomass vs. biochar. Chem. Eng. J. 2021, 423, 130061. [Google Scholar] [CrossRef]
- Khan, Z.H.; Gao, M.; Wu, J.; Bi, R.; Mehmood, C.T.; Song, Z. Mechanism of As (III) removal properties of biochar-supported molybdenum-disulfide/iron-oxide system. Environ. Pollut. 2021, 287, 117600. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Zoroufchi Benis, K.; Shakouri, M.; Mcphedran, K.; Soltan, J. Enhanced arsenate removal by Fe-impregnated canola straw: Assessment of XANES solid-phase speciation, impacts of solution properties, sorption mechanisms, and Evolutionary Polynomial Regression (EPR) models. Environ. Sci. Pollut. Res. 2021, 28, 12659–12676. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, Y.; Xie, X.; Gan, Y.; Su, C.; Pi, K.; Finfrock, Y.Z.; Liu, P. The dual role of oxygen in redox-mediated removal of aqueous arsenic (III/V) by Fe-modified biochar. Bioresour. Technol. 2021, 340, 125674. [Google Scholar] [CrossRef]
- Lata, S.; Prabhakar, R.; Adak, A.; Samadder, S.R. As (V) removal using biochar produced from anagricultural waste and prediction of removal efficiency using multiple regression analysis. Environ. Sci. Pollut. Res. 2019, 26, 32175–32188. [Google Scholar] [CrossRef]
- Wongrod, S.; Simon, S.; van Hullebusch, E.D.; Lens, P.N.L.; Guibaud, G. Changes of sewage sludge digestate-derived biochar properties after chemical treatments and influence on As (III and V) and Cd (II) sorption. Int. Biodeterior. Biodegrad. 2018, 135, 96–102. [Google Scholar] [CrossRef]
- Liu, S.; Huang, B.; Chai, L.; Liu, Y.; Zeng, G.; Wang, X.; Zeng, W.; Shang, M.; Deng, J.; Zhou, Z. Enhancement of As (V) adsorption from aqueous solution by a magnetic chitosan/biochar composite. RSC Adv. 2017, 7, 10891–10900. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Gao, B. Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Mosa, A.; Zimmerman, A.R.; Ma, L.Q.; Harris, W.G.; Migliaccio, K.W. Manganese oxide-modified biochars: Preparation, characterization, and sorption of arsenate and lead. Bioresour. Technol. 2015, 181, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar pyrolytically produced from municipal solid wastes for aqueous As (V) removal: Adsorption property and its improvement with KOH activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As (III) and As (V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Creamer, A.E.; He, F. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests. J. Hazard. Mater. 2017, 322, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.A.; Zhu, J.; Muhammad, N.; Sheng, T.; Xu, X. Effect of synthesis methods on magnetic kans grass biochar for enhanced As (III, V) adsorption from aqueous solutions. Biomass Bioenergy Biomass Bioenergy 2014, 71, 299–310. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, C.; Srinivasakannan, C.; Wang, X. Waste walnut shell valorization to iron loaded biochar and its application to arsenic removal. Resour. Effic. Technol. 2017, 3, 29–36. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, W.; Wang, D.; Huang, Q.; Song, Z.; Chau, H.W. Arsenic removal in aqueous solution by a novel fe-mn modified biochar composite: Characterization and mechanism. Ecotoxicol. Environ. Saf. 2017, 144, 514–521. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Xionghui, J.; Ma, L. Efficient arsenate removal by magnetite-modified water hyacinth biochar. Environ. Pollut. 2016, 216, 575–583. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Zimmerman, A.R.; Cao, X. Sorption of arsenic onto Ni/Fe Layered Double Hydroxide (LDH)-biochar composites. RSC Adv. 2016, 6, 17792–17799. [Google Scholar] [CrossRef]
- Liang, T.; Li, L.; Zhu, C.; Liu, X.; Li, H.; Su, Q.; Ye, J.; Geng, B.; Tian, Y.; Sardar, M.F.; et al. Adsorption of As (V) by the novel and efficient adsorbent cerium-manganese modified biochar. Water 2020, 12, 2720. [Google Scholar] [CrossRef]
- Zhu, S.; Qu, T.; Irshad, M.K.; Shang, J. Simultaneous removal of Cd (II) and As (III) from co-contaminated aqueous solution by α-FeOOH modified biochar. Biochar 2020, 2, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhao, J.; Zhao, N.; Yang, X.; Chen, C.; Shang, J. Goethite modified biochar as a multifunctional amendment for cationic Cd (II), anionic As (III), roxarsone, and phosphorus in soil and water. J. Clean. Prod. 2020, 247, 119579. [Google Scholar] [CrossRef]
- Chunhui, L.; Jin, T.; Puli, Z.; Bin, Z.; Duo, B.; Xuebin, L. Simultaneous removal of fluoride and arsenic in geothermal water in tibet using modified yak dung biochar as an adsorbent. R. Soc. Open Sci. 2018, 5, 181266. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Xue, Y.; Long, L.; Zeng, Y.; Hu, X. Adsorptive removal of As (V) by crawfish shell biochar: Batch and column tests. Environ. Sci. Pollut. Res. 2018, 25, 34674–34683. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Gao, B.; Pullammanappallil, P.; Ding, W.; Zimmerman, A.R. Biochar from anaerobically digested sugarcane bagasse. Bioresour. Technol. 2010, 101, 8868–8872. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, D.; Wang, M.; Gong, T.; Sun, M.; Yang, T. A scientometric review of biochar preparation research from 2006 to 2019. Biochar 2021, 3, 283–298. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard-Nielsen, H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012, 46, 73–79. [Google Scholar] [CrossRef]
- Bruun, E.W.; Hauggaard-Nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenergy 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Jiang, Y.; Nan, H.; Yu, Y.; Jiang, J. Unraveling sorption of nickel from aqueous solution by KMnO4 and KOH-modified peanut shell biochar: Implicit mechanism. Chemosphere 2019, 214, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; Mcbride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef]
- Higashikawa, F.S.; Conz, R.F.; Colzato, M.; Cerri, C.E.P.; Alleoni, L.R.F. Effects of feedstock type and slow pyrolysis temperature in the production of biochars on the removal of cadmium and nickel from water. J. Clean. Prod. 2016, 137, 965–972. [Google Scholar] [CrossRef]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Yang, X.; Shaheen, S.M.; Wang, J.; Hou, D.; Ok, Y.S.; Wang, S.; Wang, H.; Rinklebe, J. Elucidating the redox-driven dynamic interactions between arsenic and iron-impregnated biochar in a paddy soil using geochemical and spectroscopic techniques. J. Hazard. Mater. 2022, 422, 126808. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Jeon, E.; Kim, D.; Tsang, D.C.W.; Alessi, D.S.; Kwon, E.E.; Baek, K. Effect of dissolved organic carbon from sludge, rice straw and spent coffee ground biochar on the mobility of arsenic in soil. Sci. Total Environ. 2018, 636, 1241–1248. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Motalebi Damuchali, A.; Soltan, J.; Mcphedran, K.N. Treatment of aqueous arsenic—A review of biochar modification methods. Sci. Total Environ. 2020, 739, 139750. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K. Recent advancements in biochar preparation, feedstocks, modification, characterization and future applications. Environ. Technol. Rev. 2019, 8, 47–64. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefin. 2022, 12, 925–947. [Google Scholar] [CrossRef]
- Sewu, D.D.; Jung, H.; Kim, S.S.; Lee, D.S.; Woo, S.H. Decolorization of cationic and anionic dye-laden wastewater by steam-activated biochar produced at an industrial-scale from spent mushroom substrate. Bioresour. Technol. 2019, 277, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Bardestani, R.; Kaliaguine, S. Steam activation and mild air oxidation of vacuum pyrolysis biochar. Biomass Bioenergy 2018, 108, 101–112. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Maleki, A.; Shahmoradi, B.; Daraei, H.; Mahvi, A.H.; Barati, A.H.; Eslami, A. Elimination of arsenic contamination from water using chemically modified wheat straw. Desalin. Water Treat. 2013, 51, 2306–2316. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Motalebi Damuchali, A.; Mcphedran, K.N.; Soltan, J. Treatment of aqueous arsenic—A review of biosorbent preparation methods. J. Environ. Manag. 2020, 273, 111126. [Google Scholar] [CrossRef]
- Lima, I.; Ro, K.; Reddy, G.; Boykin, D.; Klasson, K. Efficacy of chicken litter and wood biochars and their activated counterparts in heavy metal clean up from wastewater. Agriculture 2015, 5, 806–825. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Imran, M.; Abbas, G.; Shahid, M.; Iqbal, M.; Naeem, M.A.; Murtaza, B.; Amjad, M.; Shah, N.S.; Ul Haq Khan, Z.; et al. A new biochar from cotton stalks for As (V) removal from aqueous solutions: Its improvement with H3PO4 and KOH. Environ. Geochem. Health 2020, 42, 2519–2534. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Wang, L. Enhanced hexavalent chromium (Cr(VI)) removal from aqueous solution by Fe–Mn oxide-modified cattail biochar: Adsorption characteristics and mechanism. Chem. Ecol. 2020, 36, 138–154. [Google Scholar] [CrossRef]
- Lin, S.; Lu, D.; Liu, Z. Removal of arsenic contaminants with magnetic γ-Fe2O3 nanoparticles. Chem. Eng. J. 2012, 211–212, 46–52. [Google Scholar] [CrossRef]
- He, R.; Peng, Z.; Lyu, H.; Huang, H.; Nan, Q.; Tang, J. Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci. Total Environ. 2018, 612, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, M.; Lin, X.; Huang, P.; Huang, Y. Synthesis of magnetic wheat straw for arsenic adsorption. J. Hazard. Mater. 2011, 193, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yang, B.; Wang, B.; Duan, S.; Chen, Z.; Ma, W. Novel dendrimerlike magnetic biosorbent based on modified orange peel waste: Adsorption–reduction behavior of arsenic. ACS Sustain. Chem. Eng. 2017, 5, 9692–9700. [Google Scholar] [CrossRef]
- Wen, T.; Wang, J.; Yu, S.; Chen, Z.; Hayat, T.; Wang, X. Magnetic porous carbonaceous material produced from tea waste for efficient removal of As (V), Cr (VI), Humic Acid, and Dyes. ACS Sustain. Chem. Eng. 2017, 5, 4371–4380. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, L.; Shih, K.; Chen, J.P. Yttrium-doped iron oxide magnetic adsorbent for enhancement in arsenic removal and ease in separation after applications. J. Colloid Interface Sci. 2018, 521, 252–260. [Google Scholar] [CrossRef]
- Dhoble, R.M.; Maddigapu, P.R.; Bhole, A.G.; Rayalu, S. Development of Bark-based Magnetic Iron Oxide Particle (BMIOP), a bio-adsorbent for removal of arsenic (III) from water. Environ. Sci. Pollut. Res. 2018, 25, 19657–19674. [Google Scholar] [CrossRef]
- Sahu, U.K.; Sahu, S.; Mahapatra, S.S.; Patel, R.K. Synthesis and characterization of magnetic bio-adsorbent developed from aegle marmelos leaves for removal of As (V) from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 946–958. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, H.; Xia, M.; Lei, W.; Wang, F. The single/co-adsorption characteristics and microscopic adsorption mechanism of biochar-montmorillonite composite adsorbent for pharmaceutical emerging organic contaminant atenolol and lead ions. Ecotoxicol. Environ. Saf. 2020, 187, 109763. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Fang, J.; Zhang, M.; Chen, H.; Zhou, Y.; Creamer, A.E.; Sun, Y.; Yang, L. Characterization and environmental applications of clay–biochar composites. Chem. Eng. J. 2014, 242, 136–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Zimmerman, A.R.; Chen, H.; Hu, X.; Gao, B. Mechanisms and adsorption capacities of hydrogen peroxide modified ball milled biochar for the removal of methylene blue from aqueous solutions. Bioresour. Technol. 2021, 337, 125432. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Hu, X. Effects of wet and dry ball milling on the physicochemical properties of sawdust derived-biochar. Instrum. Sci. Technol. 2020, 48, 287–300. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A green method for production of nanobiochar by ball milling- optimization and characterization. J. Clean. Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Yan, T.; Qiao, J.; Cao, H. Adsorption of Arsenic, Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: Adsorption mechanism and depleted adsorbent utilization. Chemosphere 2016, 164, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Tan, F.; Zhang, C.; Jiang, X.; Chen, Z.; Li, H.; Zheng, Y.; Li, Q.; Wang, Y. ZnCl2—Activated biochar from biogas residue facilitates aqueous As (III) removal. Appl. Surf. Sci. 2016, 377, 361–369. [Google Scholar] [CrossRef]
- Lin, L.; Song, Z.; Huang, Y.; Khan, Z.H.; Qiu, W. Removal and oxidation of arsenic from aqueous solution by biochar impregnated with Fe-Mn oxides. Water Air Soil Pollut. 2019, 230, 105. [Google Scholar] [CrossRef]
- Yoon, K.; Cho, D.; Tsang, D.C.W.; Bolan, N.; Rinklebe, J.; Song, H. Fabrication of engineered biochar from paper mill sludge and its application into removal of arsenic and cadmium in acidic water. Bioresour. Technol. 2017, 246, 69–75. [Google Scholar] [CrossRef]
- Low, Y.W.; Yee, K.F. A review on lignocellulosic biomass waste into biochar-derived catalyst: Current conversion techniques, sustainable applications and challenge. Biomass Bioenergy 2021, 154, 106245. [Google Scholar] [CrossRef]
- Cruz, G.J.F.; Mondal, D.; Rimaycuna, J.; Soukup, K.; Gómez, M.M.; Solis, J.L.; Lang, J. Agrowaste derived biochars impregnated with ZnO for removal of arsenic and lead in water. J. Environ. Chem. Eng. 2020, 8, 103800. [Google Scholar] [CrossRef]
- Amen, R.; Bashir, H.; Bibi, I.; Shaheen, S.M.; Niazi, N.K.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G.; et al. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- Zhong, L.S.; Hu, J.S.; Liang, H.P.; Cao, A.M.; Song, W.G.; Wan, L.J. Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Van Vinh, N.; Zafar, M.; Behera, S.K.; Park, H.S. Arsenic (III) removal from aqueous solution by raw and zinc-loaded pine cone biochar: Equilibrium, kinetics, and thermodynamics studies. Int. J. Environ. Sci. Technol. 2015, 12, 1283–1294. [Google Scholar] [CrossRef] [Green Version]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Comparing the adsorption mechanism of Cd by rice straw pristine and KOH-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 11875–11883. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Yoon, T.; Chae, C.; Sun, Y.; Zhao, X.; Kung, H.H.; Lee, J.K. Bottom-up in situ formation of Fe3O4 nanocrystals in a porous carbon foam for lithium-ion battery anodes. J. Mater. Chem. 2011, 21, 17325. [Google Scholar] [CrossRef]
- Anstey, A.; Vivekanandhan, S.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Oxidative acid treatment and characterization of new biocarbon from sustainable miscanthus biomass. Sci. Total Environ. 2016, 550, 241–247. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Limousy, L.; Jeguirim, M. New insights on the structural evolution of biomass char upon pyrolysis as revealed by the raman spectroscopy and elemental analysis. Carbon 2017, 119, 519–521. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Keown, D.M.; Li, X.; Hayashi, J.; Li, C. Evolution of biomass char structure during oxidation in O2 as revealed with ft-raman spectroscopy. Fuel Process. Technol. 2008, 89, 1429–1435. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Faye, M.C.A.S.; Zhang, Y. Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation. Carbon 2018, 130, 730–740. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Ling, P.; Zhang, X.; Xu, K.; He, L.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Raman spectroscopy of biochar from the pyrolysis of three typical chinese biomasses: A novel method for rapidly evaluating the biochar property. Energy 2020, 202, 117644. [Google Scholar] [CrossRef]
- Rose, T.J.; Schefe, C.; Weng, Z.; Rose, M.T.; van Zwieten, L.; Liu, L.; Rose, A.L. Phosphorus speciation and bioavailability in diverse biochars. Plant Soil 2019, 443, 233–244. [Google Scholar] [CrossRef]
- Wang, D.; Root, R.A.; Chorover, J. Biochar-templated surface precipitation and inner-sphere complexation effectively removes arsenic from acid mine drainage. Environ. Sci. Pollut. Res. 2021, 28, 45519–45533. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, X.; Feng, Y.; Ashraf, M.A.; Liu, Y.; Su, C.; Qian, K.; Liu, P. As (III) and As (V) removal mechanisms by fe-modified biochar characterized using synchrotron-based X-ray absorption spectroscopy and confocal micro-X-ray fluorescence imaging. Bioresour. Technol. 2020, 304, 122978. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Shaheen, S.M.; Rinklebe, J.; Wang, H.; Murtaza, B.; Islam, E.; Farrakh Nawaz, M.; et al. Arsenic removal by japanese oak wood biochar in aqueous solutions and well water: Investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci. Total Environ. 2018, 621, 1642–1651. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, P.; Wang, Y.; Finfrock, Y.Z.; Xie, X.; Su, C.; Liu, N.; Yang, Y.; Xu, Y. Distribution and speciation of iron in fe-modified biochars and its application in removal of As (V), As (III), Cr (VI), and Hg (II): An X-ray absorption study. J. Hazard. Mater. 2020, 384, 121342. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.; Xu, C.; Liu, P.; Lv, J.; Liu, Y.; Wang, Q. Removal mechanisms of aqueous Cr (VI) using apple wood biochar: A spectroscopic study. J. Hazard. Mater. 2020, 384, 121371. [Google Scholar] [CrossRef] [PubMed]
- Munira, S.; Dynes, J.J.; Islam, M.; Khan, F.; Adesanya, T.; Regier, T.Z.; Spokas, K.A.; Farenhorst, A. Farenhorst, A. Relative proportions of organic carbon functional groups in biochars as influenced by spectral data collection and processing. Chemosphere 2021, 283, 131023. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ptacek, C.J.; Blowes, D.W.; Finfrock, Y.Z.; Steinepreis, M.; Budimir, F. A method for redox mapping by confocal micro-X-ray fluorescence imaging: Using chromium species in a biochar particle as an example. Anal. Chem. 2019, 91, 5142–5149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lin, Y.; Lin, Y.; Yang, D.; Zheng, H. Adsorption behaviors of paper mill sludge biochar to remove Cu, Zn and As in wastewater. Environ. Technol. Innov. 2021, 23, 101616. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Ghani, W.A.W.A.; Idris, A.; Ahmad, M.B. Hydrogel biochar composite for arsenic removal from wastewater. Desalin. Water Treat. 2014, 57, 3674–3688. [Google Scholar] [CrossRef]
- Abid, M.; Niazi, N.K.; Bibi, I.; Farooqi, A.; Ok, Y.S.; Kunhikrishnan, A.; Ali, F.; Ali, S.; Igalavithana, A.D.; Arshad, M. Arsenic (V) biosorption by charred orange peel in aqueous environments. Int. J. Phytoremediat. 2015, 18, 442–449. [Google Scholar] [CrossRef]
- Guo, H.; Stüben, D.; Berner, Z. Removal of Arsenic from Aqueous Solution by Natural Siderite and Hematite. Appl. Geochem. 2007, 22, 1039–1051. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Lin, L.; Khan, Z.; Qiu, W.; Song, Z. Synthesis and characterization of novel Fe-Mn-Ce ternary oxide–biochar composites as highly efficient adsorbents for As (III) removal from aqueous solutions. Materials 2018, 11, 2445. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, J.; Nguyen, N.; Le, T.; Nguyen, N.; Chang, C. Specifically designed magnetic biochar from waste wood for arsenic removal. Sustain. Environ. Res. 2021, 31, 29. [Google Scholar] [CrossRef]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Rehman, M.Z.U.; Al-Wabel, M.I. A Critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Ghosh, S.; Prabhakar, R.; Samadder, S.R. Performance of γ-aluminium oxide nanoparticles for arsenic removal from groundwater. Clean Technol. Environ. Policy 2019, 21, 121–138. [Google Scholar] [CrossRef]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As (III) and Cd (II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Tuutijärvi, T.; Repo, E.; Vahala, R.; Sillanpää, M.; Chen, G. Effect of competing anions on arsenate adsorption onto maghemite nanoparticles. Chin. J. Chem. Eng. 2012, 20, 505–514. [Google Scholar] [CrossRef]
- Johansson, C.L.; Paul, N.A.; de Nys, R.; Roberts, D.A. Simultaneous biosorption of selenium, arsenic and molybdenum with modified algal-based biochars. J. Environ. Manag. 2016, 165, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A Review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2015, 46, 406–433. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K.; Pratt, A.R. Arsenic Arsenic removal from aqueous solutions by mixed magnetite–maghemite nanoparticles. Environ. Earth Sci. 2011, 64, 411–423. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Lou, S.; Sillanpää, M. Removal of arsenic (V) by magnetic nanoparticle activated microfibrillated cellulose. Chem. Eng. J. 2015, 260, 886–894. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Rathke, S.J.; Laird, D.A. Arsenic sorption on zero-valent iron-biochar complexes. Water Res. 2018, 137, 153–163. [Google Scholar] [CrossRef]
- Wongrod, S.; Simon, S.; van Hullebusch, E.D.; Lens, P.N.L.; Guibaud, G. Assessing arsenic redox state evolution in solution and solid phase during As (III) sorption onto chemically-treated sewage sludge digestate biochars. Bioresour. Technol. 2019, 275, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, J.; Qian, W.; Xu, R. Arsenate adsorption from aqueous solution onto Fe(III)-modified crop straw biochars. Environ. Eng. Sci. 2015, 32, 922–929. [Google Scholar] [CrossRef]
- Alchouron, J.; Navarathna, C.; Chludil, H.D.; Dewage, N.B.; Perez, F.; Hassan, E.B.; Pittman, C.U., Jr.; Vega, A.S.; Mlsna, T.E. Assessing south american guadua chacoensis bamboo biochar and Fe3O4 nanoparticle dispersed analogues for aqueous arsenic (V) remediation. Sci. Total Environ. 2020, 706, 135943. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Usman, A.R.A.; Hussain, Q.; Al-Farraj, A.S.F.; Tsang, Y.F.; Bundschuh, J.; Al-Wabel, M.I. Fabrication and evaluation of silica embedded and zerovalent iron composited biochars for arsenate removal from water. Environ. Pollut. 2020, 266, 115256. [Google Scholar] [CrossRef] [PubMed]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 2014, 133, 309–314. [Google Scholar] [CrossRef]
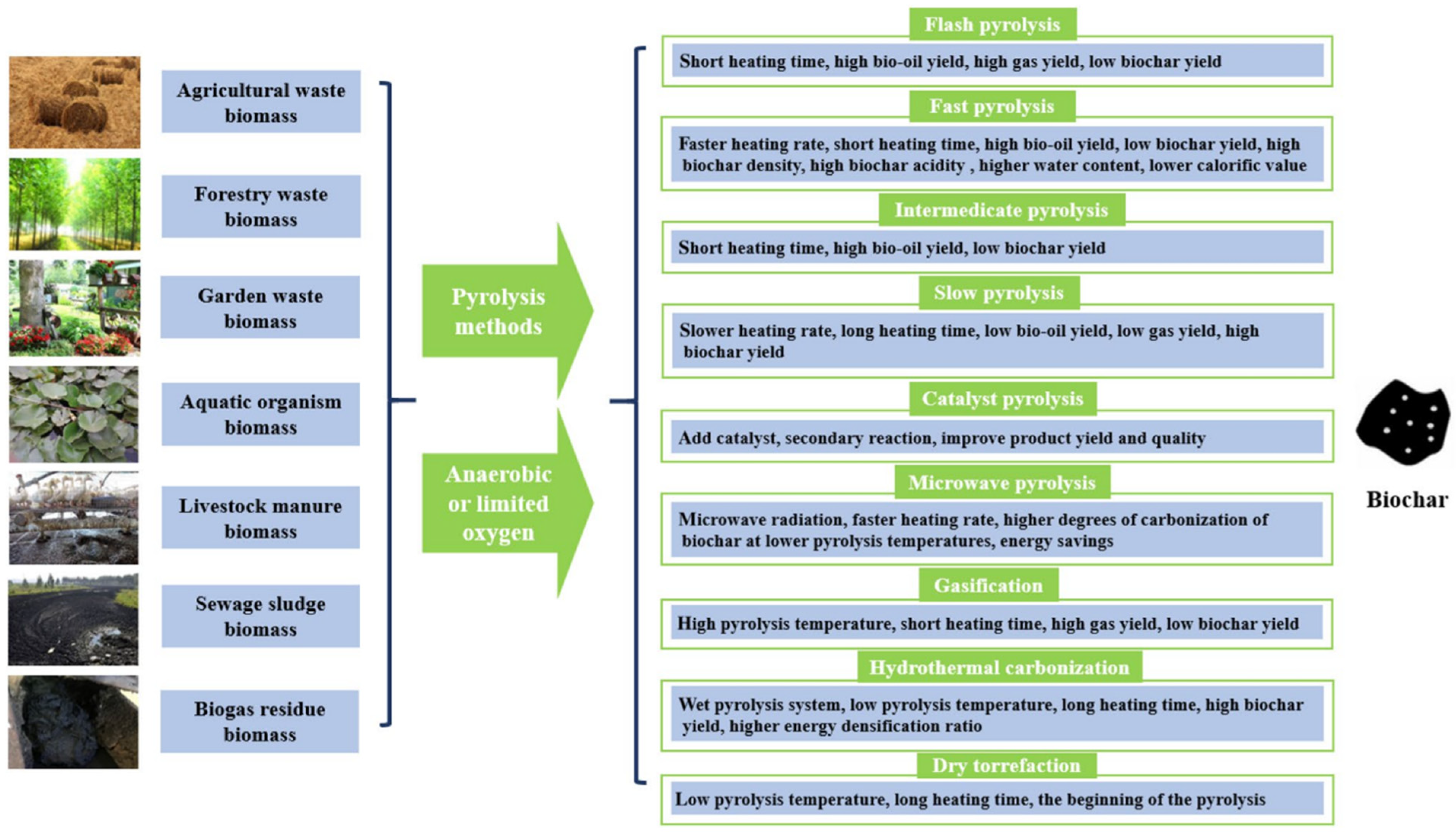
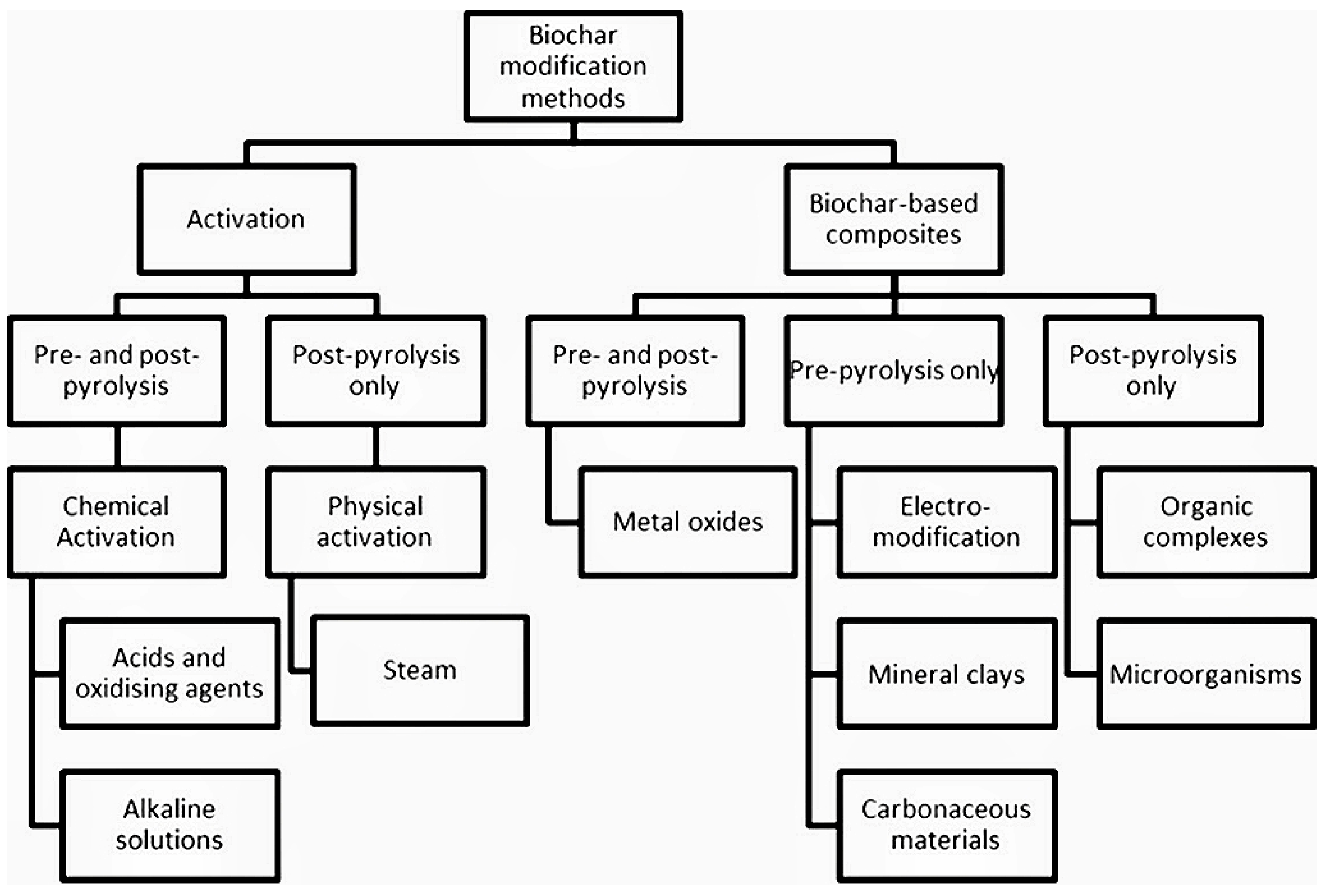
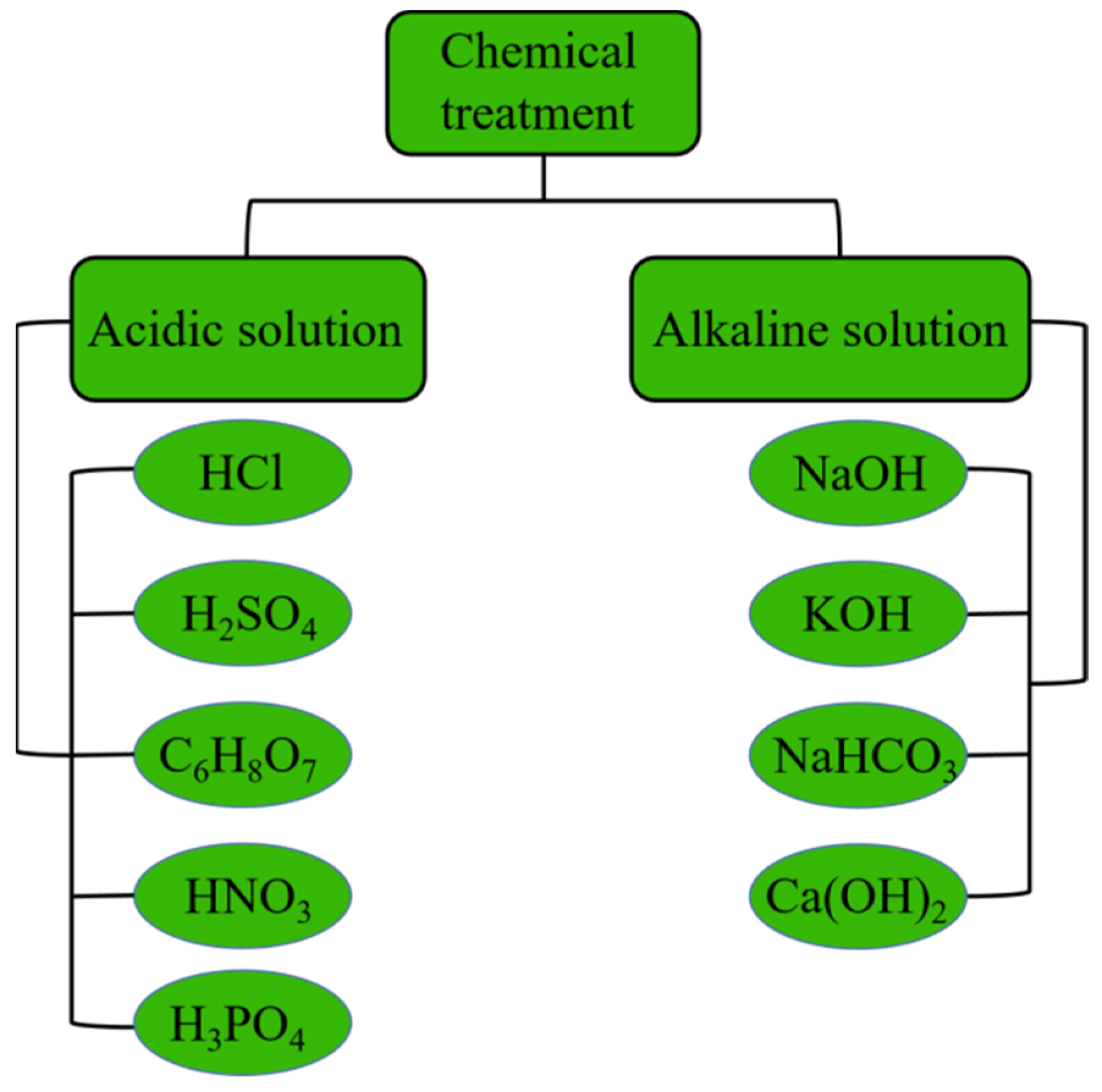
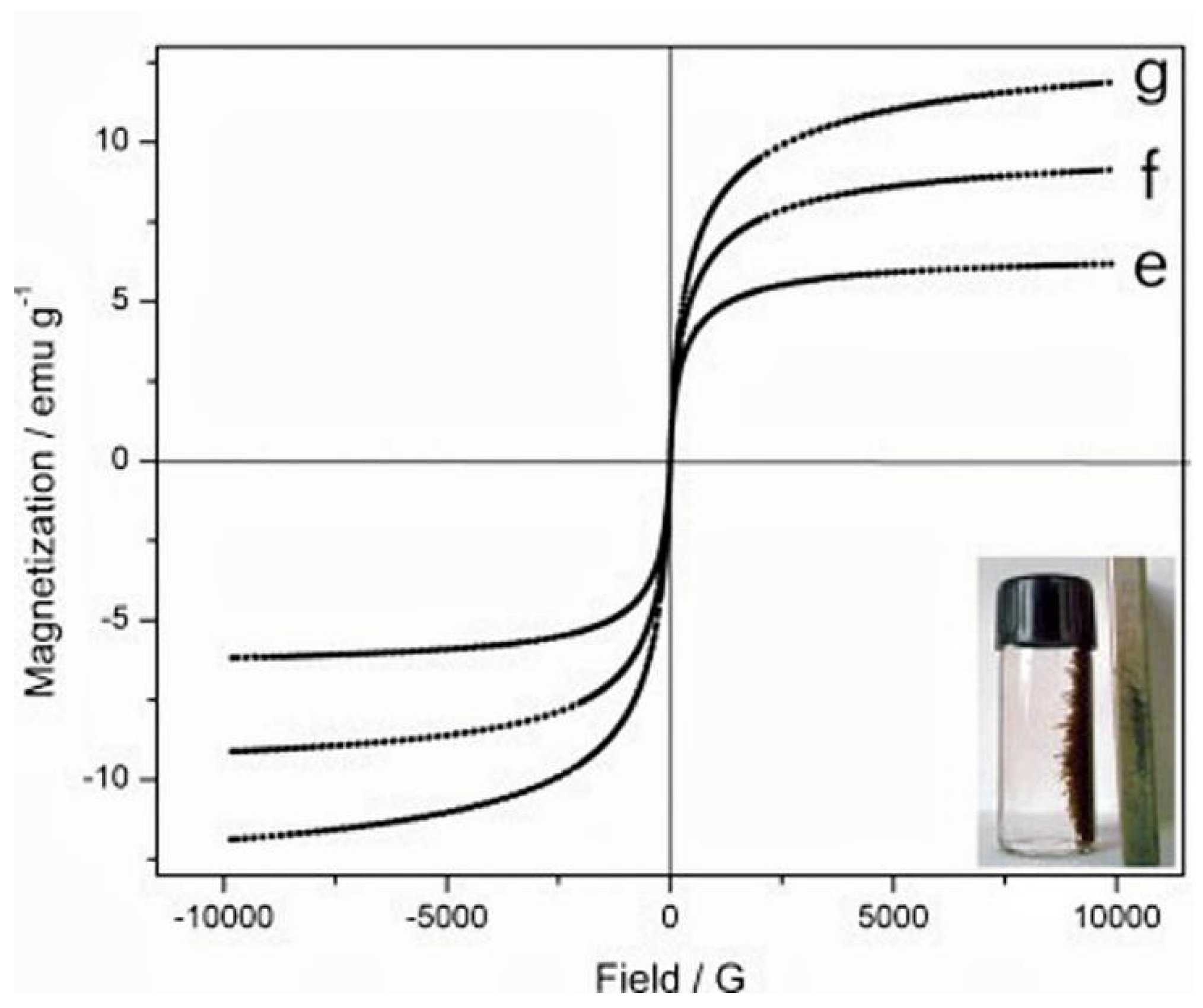
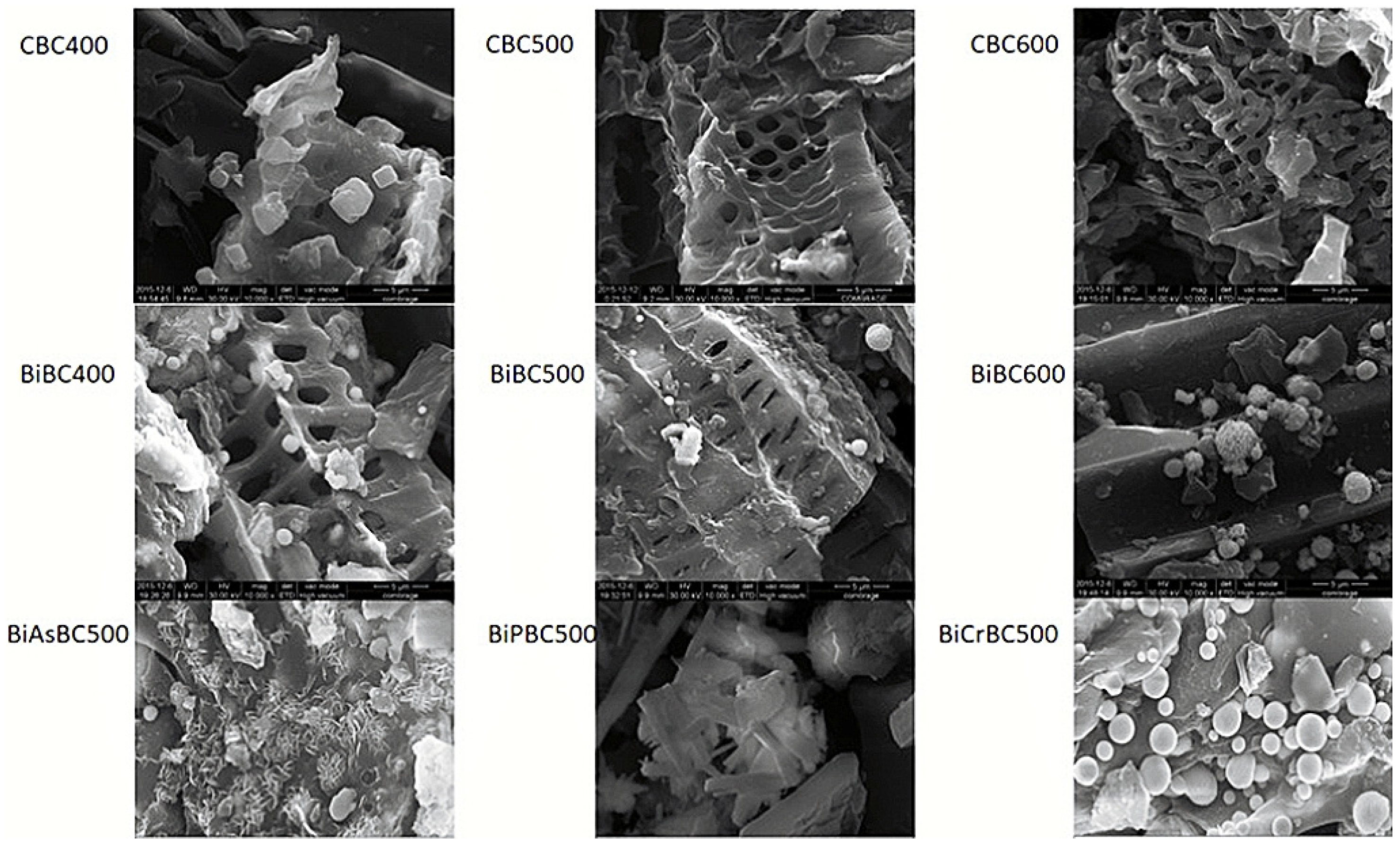
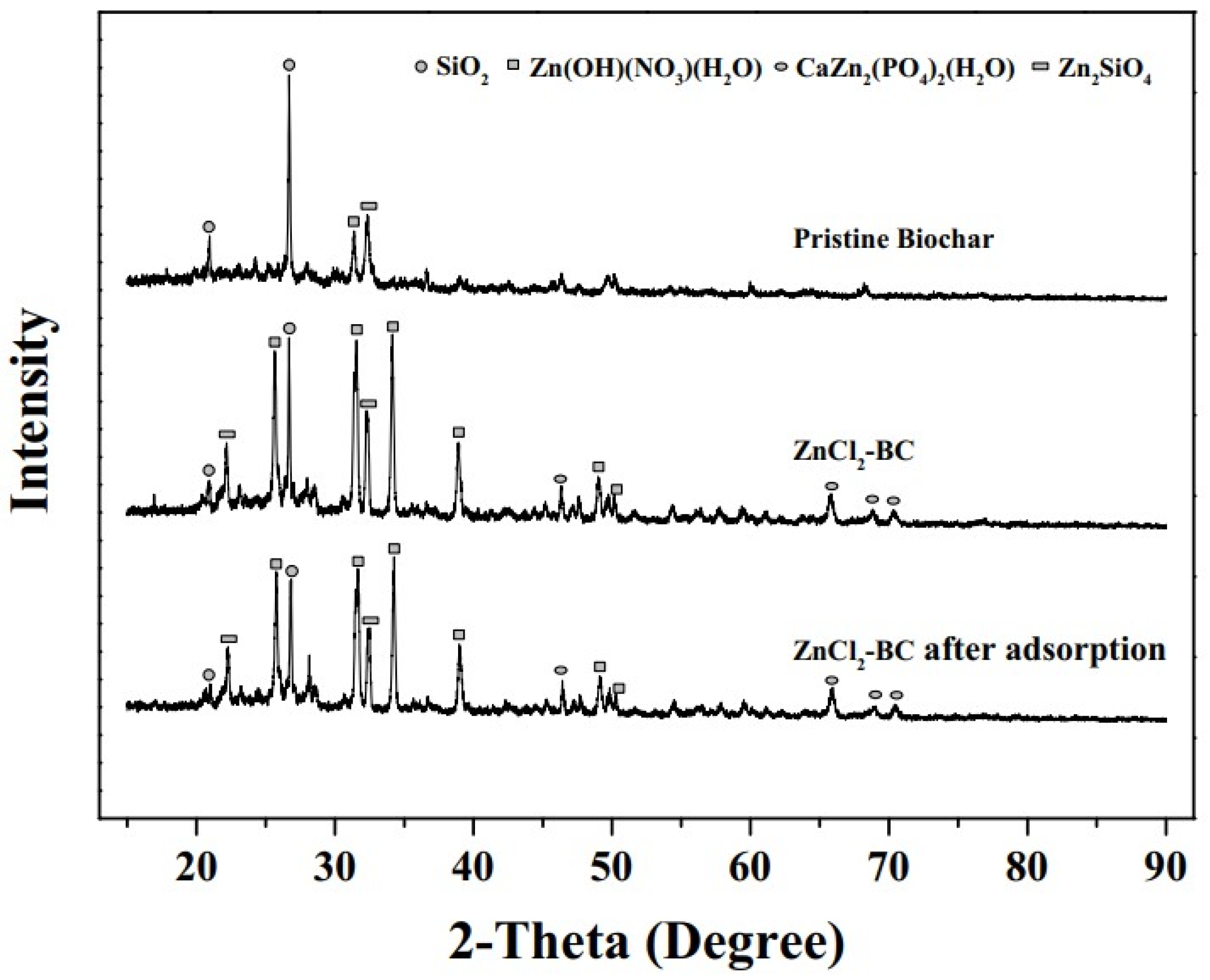
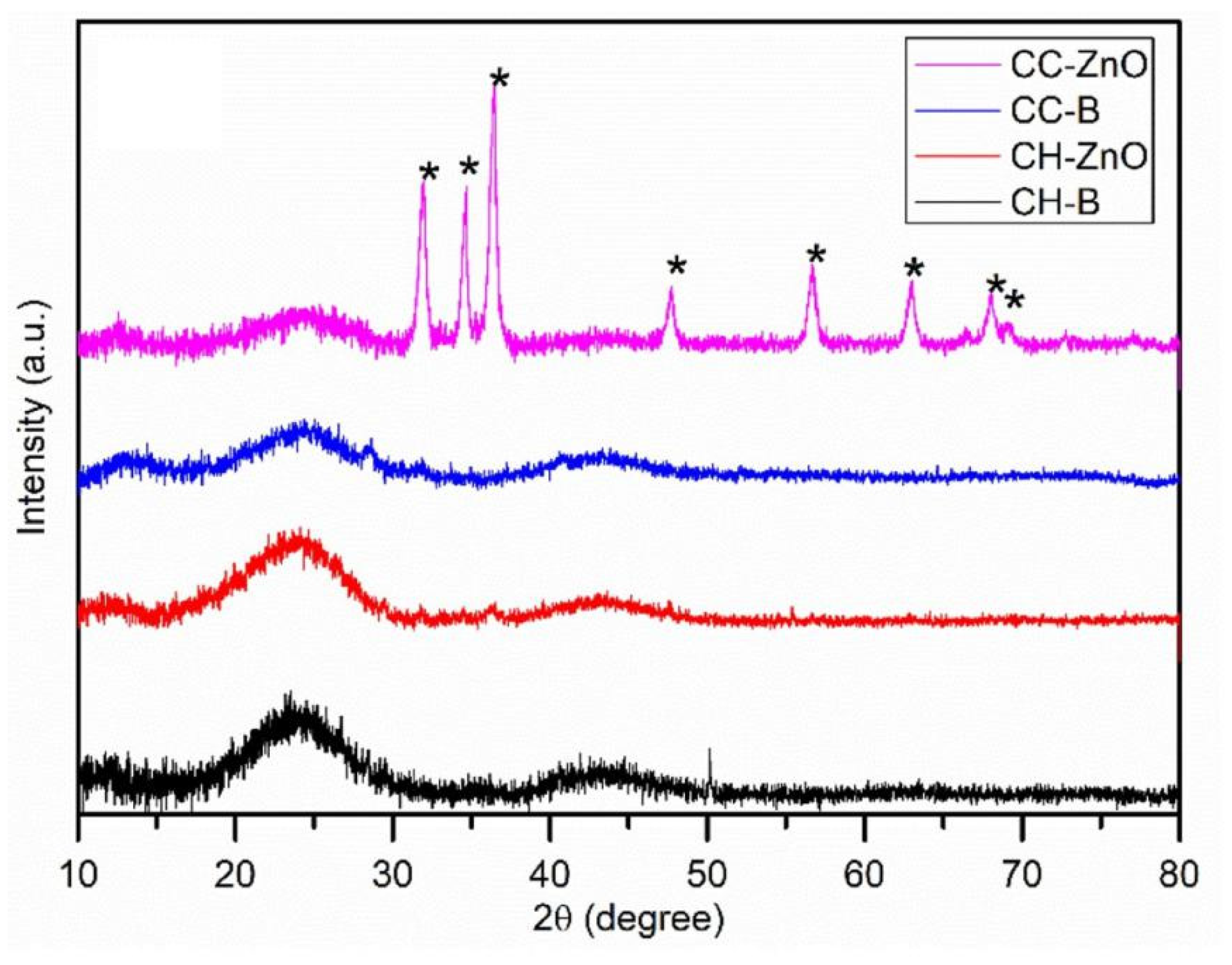

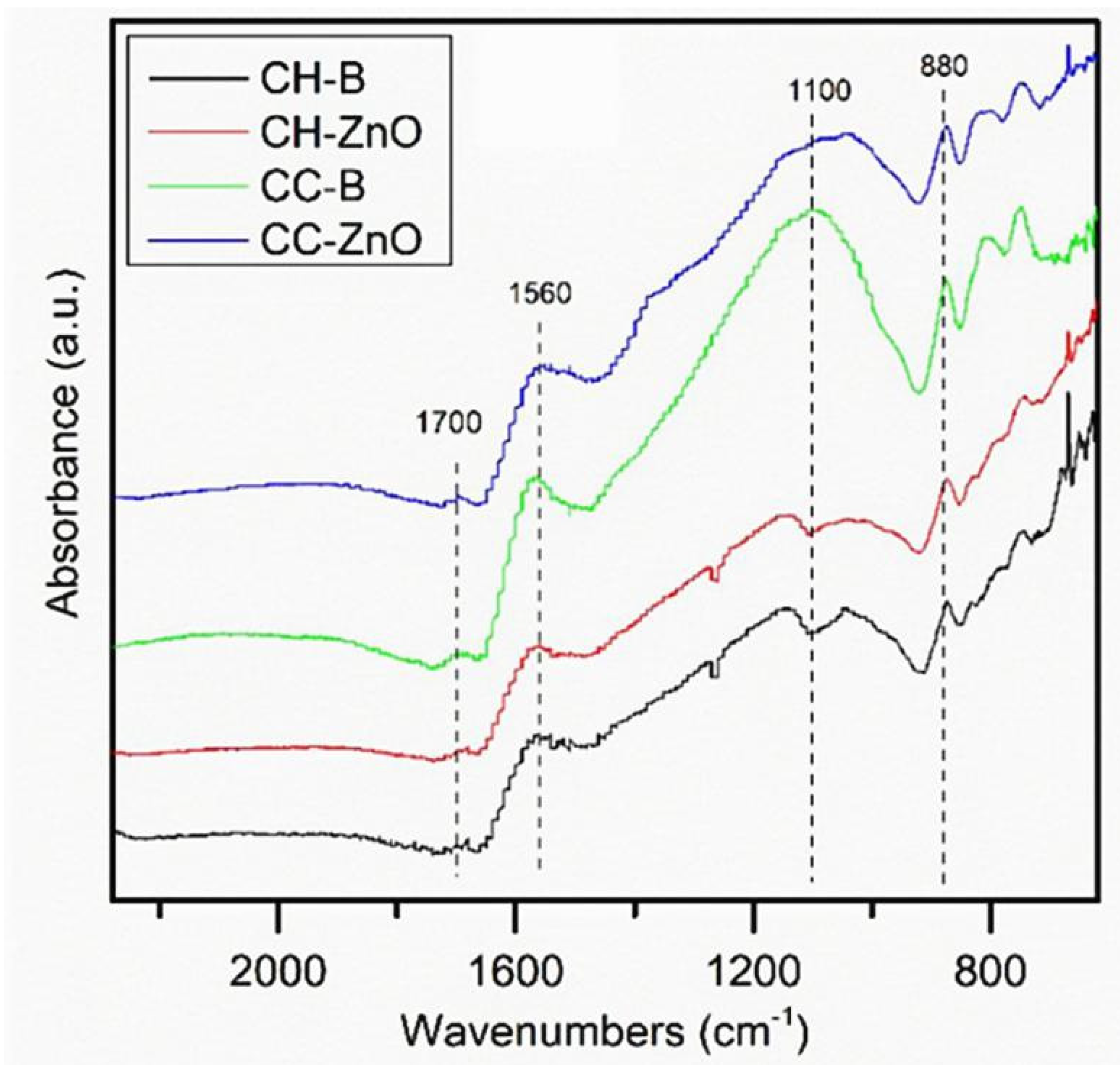
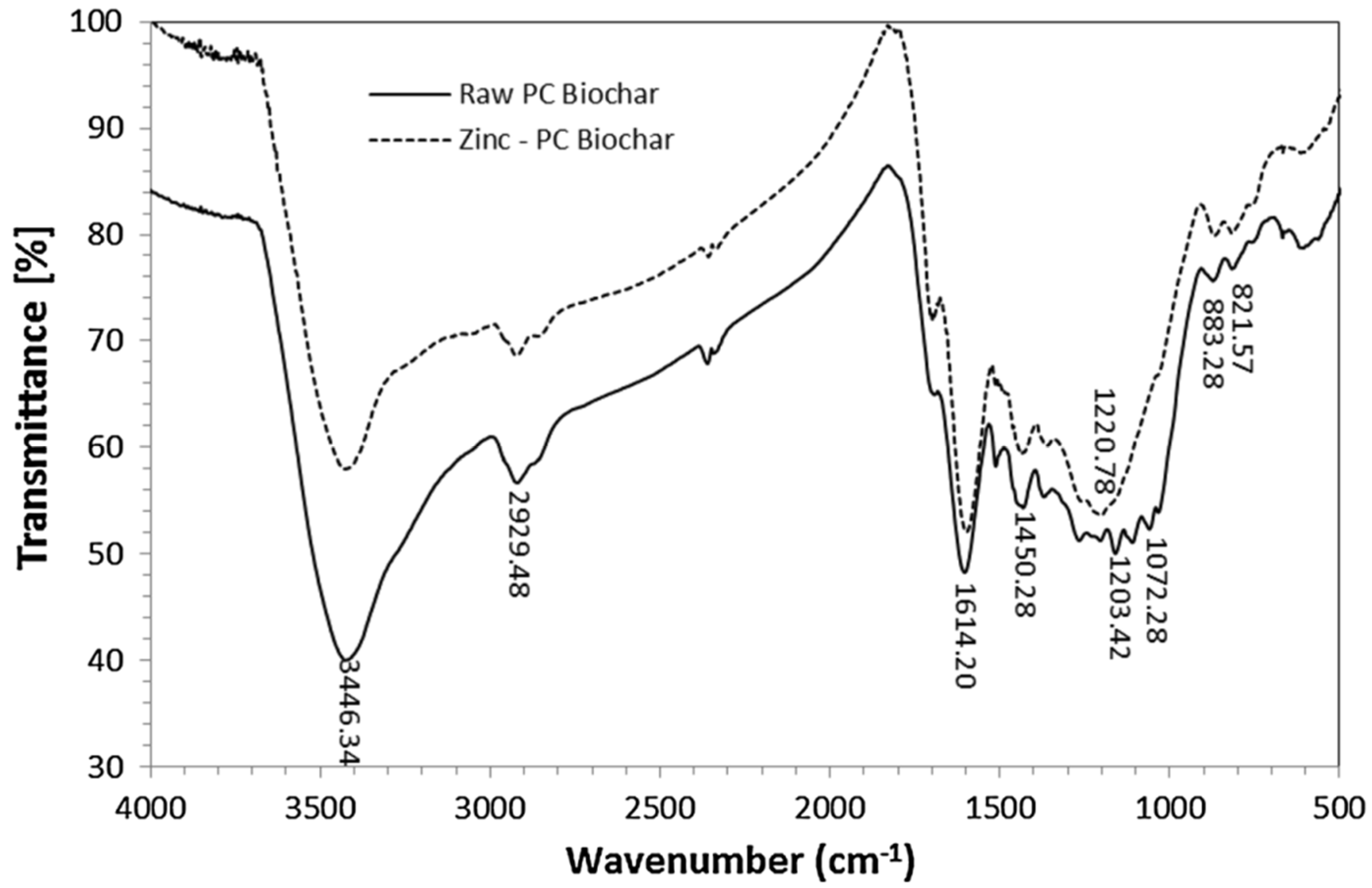
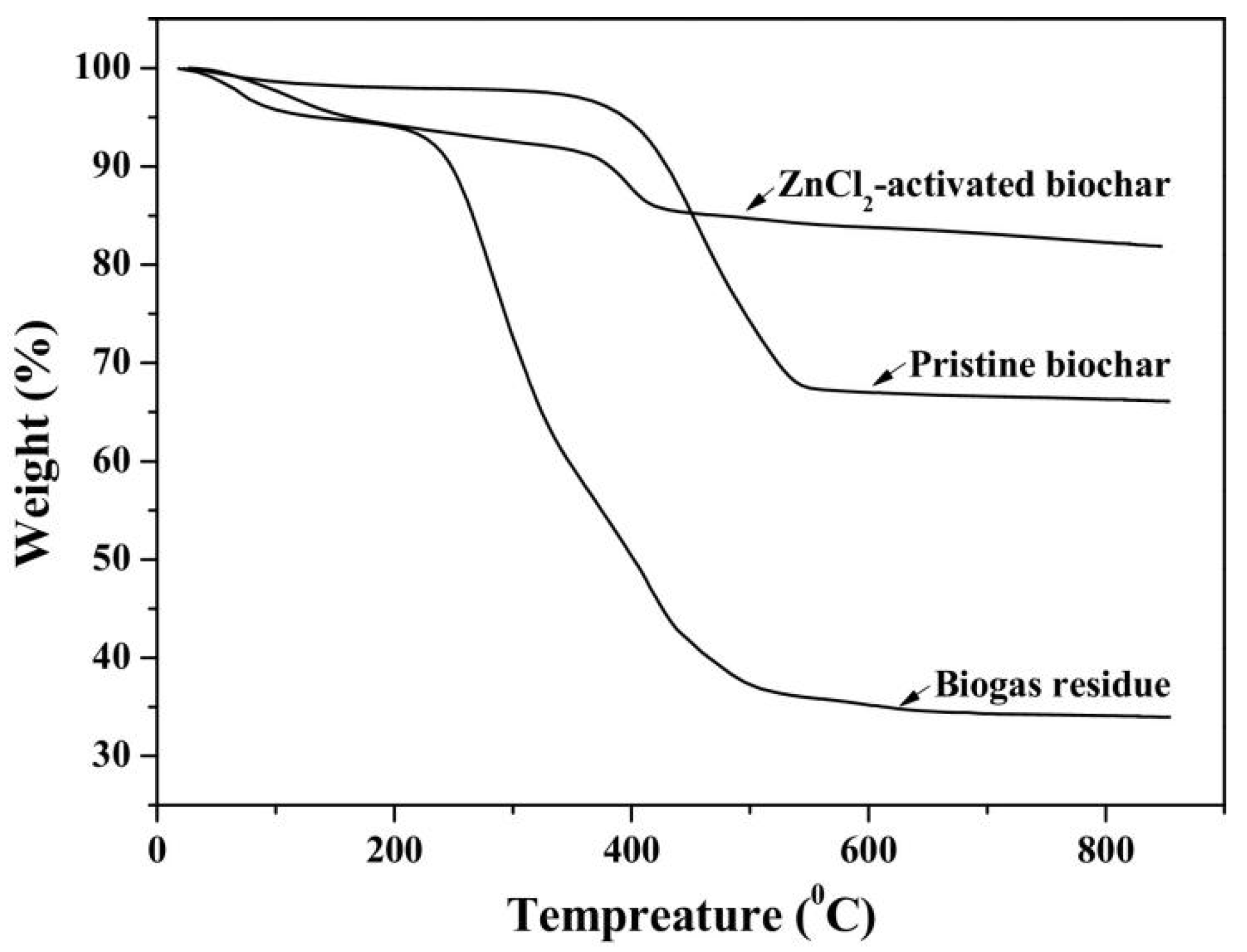
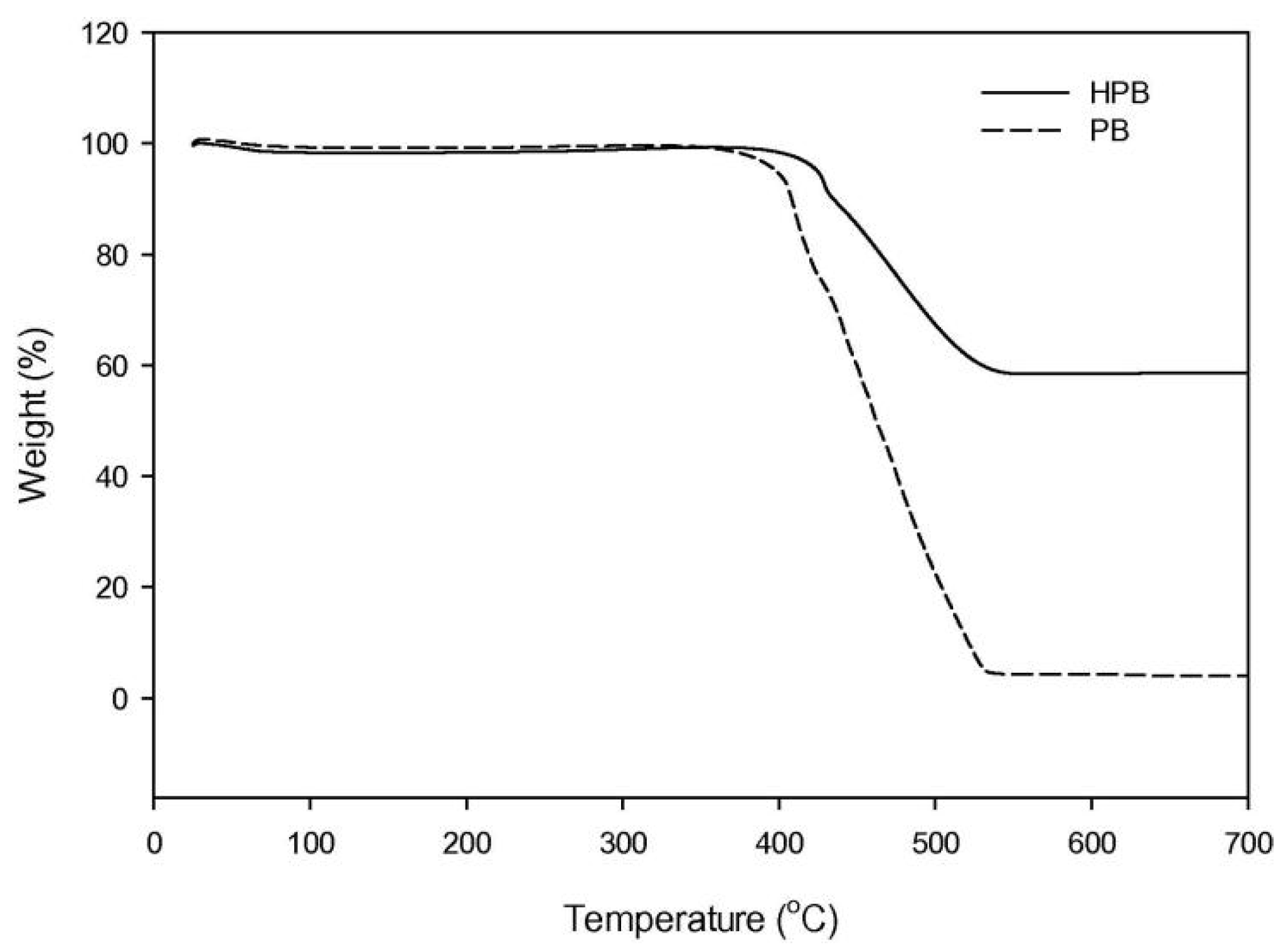

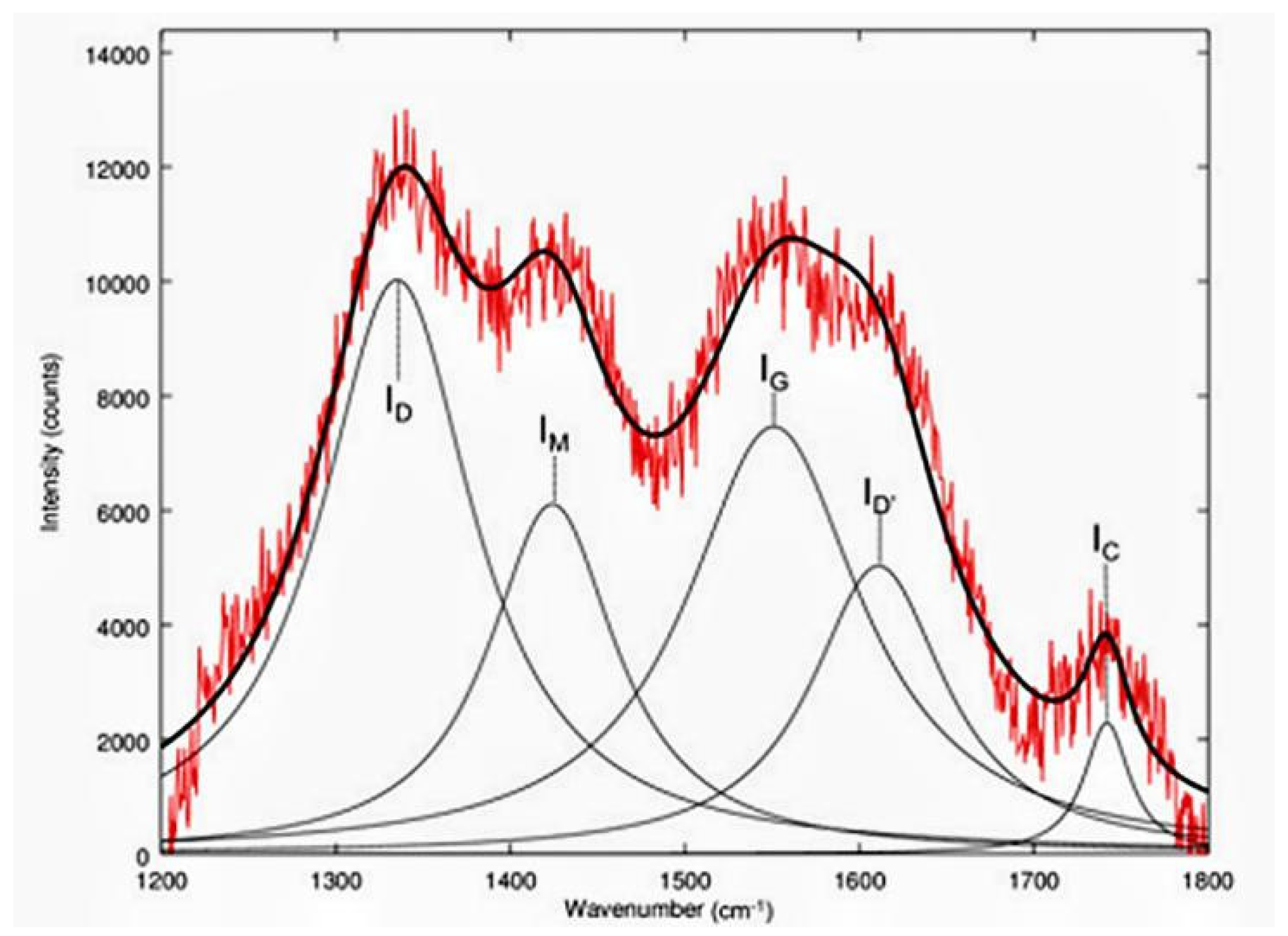

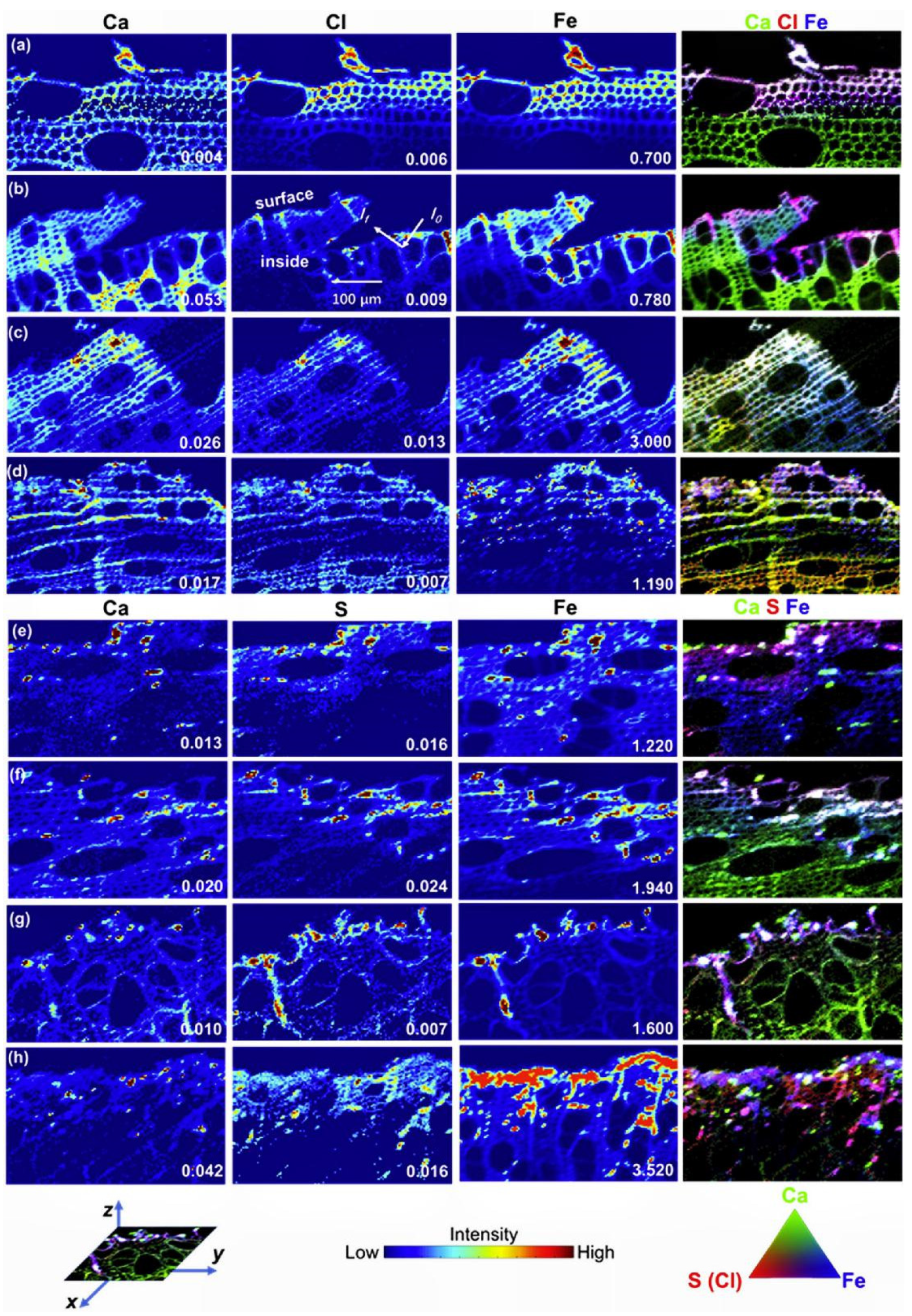
| Number | Feedstock of Biochar | Modified Material/Method | Arsenic Species | Max.Adsorption Capacity | Reference |
|---|---|---|---|---|---|
| 1 | Rice straw | FeSO4·7H2O and FeCl3·6H2O | As (V) | 26.9 mg/g | Nham et al. (2019) [39] |
| 2 | Chestnut shell | Gelatin, FeCl3 and FeCl2 | As (V) | 45.8 mg/g | Zhou et al. (2017) [40] |
| 3 | Kraft lignin | FeOx, CO2 | As (V) | 28.2 mg/g | Cha et al. (2021) [41] |
| 4 | Canola straw | Electrochemical modification | As (V) | 922 µg/g | Benis et al. (2021) [42] |
| 5 | Corn straw | FeOx, MoS2 | As (III) | 27 mg/g | Khan et al. (2021) [43] |
| 6 | Hickory chips | Fe(NO3)3·9H2O | As (V) | 2.16 mg/g | Hu et al. (2015) [44] |
| 7 | Canola straw | FeCl3 | As (V) | 5.5 mg/g | Benis et al. (2021) [45] |
| 8 | Poplar wood particles | FeCl3 | As (III)/As (V) | 48.6 /122.0 mg/g | Feng et al. (2021) [46] |
| 9 | Banana pith | Ferric nitrate nonahydrate | As (V) | 120.91 μg/g | Lata et al. (2019) [47] |
| 10 | Sewage sludge digestate | KOH | As (V) | 8.5 μmol/g | Wongrod et al. (2018) [48] |
| 11 | Rice straw, Chitosan | Fe2+, Fe3+ | As (V) | 17.876 mg/g | Liu et al. (2017) [49] |
| 12 | Cottonwood | AlCl3·6H2O | As(V) | 17.41 mg/g | Zhang et al. (2013) [50] |
| 13 | Pinewood | γ-Fe2O3 | As(V) | 429 mg/kg | Wang et al. (2015) [51] |
| 14 | Loblolly pine | MnCl2·4H2O/KMnO4 | As(V) | 0.59 /0.91 g/kg | Wang et al. (2015) [52] |
| 15 | Municipal solid waste | KOH | As(V) | 30.98 mg/g | Jin et al. (2014) [53] |
| 16 | Empty fruit bunch/rice husk | Fe(III) | As(III)/As(V) | 31.4/31.4 mg/g | Samsuri et al. (2013) [54] |
| 17 | Pinewood | FeCl3·6H2O | As (V) | 124.5 g/kg | Wang et al. (2013) [55] |
| 18 | Kans grass | Fe3O4 | As(III)/As(V) | 2.004/3.132 mg/g | Baig et al. (2014) [56] |
| 19 | Waste walnut | FeCl3·6H2O | As(V) | 1.91 mg/g | Duan et al. (2017) [57] |
| 20 | Corn stem | KMnO4, Fe(NO3)3 | As(III) | 8.25 mg/g | Lin et al. (2017) [58] |
| 21 | Hyacinth | FeCl2, FeCl3 | As(V) | 7.41 mg/g | Zhang et al. (2016) [59] |
| 22 | Pine | Ni/Fe-LDH/Layered double hydroxides | As(V) | 1.56/4.38 g/kg | Wang et al. (2016) [60] |
| 23 | Wheat straws | CeCl3, KMnO4 | As(V) | 108.88 mg/g | Liang et al. (2020) [61] |
| 24 | Wheat straw | Fe(NO3)3, KOH | As(III) | 78.3 mg/g | Zhu et al. (2020) [62] |
| 25 | Wheat straw | Fe(NO3)3, KOH | As(III) | 65.3 mg/g | Zhu et al. (2020) [63] |
| 26 | Yak dung | FeCl2 | As(V) | 2.926 mg/g | Chunhui et al. (2018) [64] |
| 27 | Crayfish shell | ZnCl2 | As(V) | 17.2 mg/g | Yan et al. (2018) [65] |
| Feedstock | Pyrolysis Temp. (°C) | Ash (%) | pH | Element Content (%) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | |||||||
| Peanut shell | 300 | 1.24 ± 0.08 | 7.76 ± 0.06 | 68.27 | 3.85 | 25.89 | 1.91 | 3.14 | / | Ahmad et al. (2012) [72] |
| 500 | / | / | / | / | / | / | 0.7447 | 0.002651 | An et al. (2019) [73] | |
| 700 | 17.18 ± 0.25 | 10.57 ± 0.05 | 83.76 | 1.75 | 13.34 | 1.14 | 448.2 | 0.20 | Ahmad et al. (2012) [72] | |
| Soybean stover | 300 | 10.41 ± 0.52 | 7.27 ± 0.03 | 68.81 | 4.29 | 24.99 | 1.88 | 5.61 | / | Ahmad et al. (2012) [72] |
| 700 | 17.18 ± 0.25 | 11.32 ± 0.02 | 68.81 | 1.27 | 15.45 | 1.30 | 420.3 | 0.19 | ||
| Hardwood | 450 | 38.55 | 5.57 | 53.41 | 2.30 | 5.67 | 0.07 | 0.43 | 0.00036 | Chen et al. (2011) [74] |
| Corn straw | 600 | 60.19 | 9.54 | 35.88 | 1.64 | 1.86 | 0.43 | 13.08 | 0.014 | Chen et al. (2011) [74] |
| Dairy manures | 500 | 90 | 10 | 1.67 ± 0.4 | / | / | 0.04 ± 0.01 | 13 | / | Cao et al. (2010) [75] |
| Orange Peels | 300 | 1.57 | / | 69.3 | 4.51 | 22.2 | 2.36 | 32.3 | 0.0313 | Chen et al. (2010) [76] |
| 350 | 2.00 | / | 73.2 | 4.19 | 2.30 | 18.3 | 51.0 | 0.0098 | ||
| 400 | 2.10 | / | 71.7 | 3.48 | 20.8 | 1.92 | 34.0 | 0.0099 | ||
| 500 | 4.27 | / | 71.4 | 2.25 | 20.3 | 1.83 | 42.4 | 0.0191 | ||
| 600 | 4.04 | / | 77.8 | 1.97 | 14.4 | 1.80 | 7.78 | 0.0083 | ||
| 700 | 2.79 | / | 71.6 | 1.76 | 22.2 | 1.72 | 201 | 0.0350 | ||
| Chicken manure | 350 | 38.21 ± 2.75 | 8.21 ± 0.06 | 38.11 ± 0.46 | 3.40 ± 0.03 | 55.91 ± 4.06 | 2.59 ± 3.63 | / | / | Higashikawa et al. (2016) [77] |
| 650 | 48.76 ± 2.38 | 9.96 ± 0.02 | 32.56 ± 0.27 | 0.91 ± 0.10 | 65.08 ± 0.19 | 1.46 ± 0.18 | / | / | ||
| Sugarcane straw | 350 | 24.22 ± 2.07 | 8.67 ± 0.03 | 60.13 ± 5.96 | 2.44 ± 0.16 | 35.78 ± 7.07 | 1.66 ± 0.95 | / | / | |
| 650 | 13.32 ± 1.54 | 9.17 ± 0.05 | 69.37 ± 1.91 | 2.45 ± 0.95 | 26.69 ± 3.08 | 1.50 ± 2.11 | / | / | ||
| Rice husk | 350 | 40.44 ± 0.29 | 8.44 ± 0.08 | 32.79 ± 7.99 | 1.09 ± 0.35 | 66.09 ± 8.32 | 0.04 ± 0.02 | / | / | |
| 650 | 41.97 ± 0.27 | 8.72 ± 0.06 | 49.48 ± 1.70 | 1.47 ± 0.06 | 49.04 ± 1.75 | 0.02 ± 0.01 | / | / | ||
| Sawdust | 350 | 1.24 ± 0.22 | 7.59 ± 0.17 | 49.04 ± 1.75 | 3.94 ± 0.06 | 24.35 ± 0.40 | 0.10 ± 0.12 | / | / | |
| 650 | 1.19 ± 0.11 | 7.48 ± 0.02 | 84.60 ± 1.15 | 2.84 ± 0.39 | 12.35 ± 1.05 | 0.22 ± 0.29 | / | / | ||
| Cacao shell | 350 | 17 | 10.42 | 70 | 1.6 | / | 1.4 | 18.6 | 0.0147 | Hale et al. (2013) [78] |
| Corn cob | 400 | 13 | 8.97 | 66 | 2.6 | / | 0.6 | 36.4 | 0.0222 | |
| Platanus orientalis L. | 650 | 9.7 | 9.3 | 69.3 | 2.7 | / | 1.1 | / | / | Yang et al. (2022) [79] |
| Rice straw | 300 | / | 8.2 ± 0.01 | 53.5 ± 0.2 | 3.7 ± 0.1 | / | 1.5 ± 0.1 | / | / | Kim et al. (2018) [80] |
| 550 | / | 10.5 ± 0.1 | 59.8 ± 0.4 | 1.7 | / | 1.3 ± 0.1 | / | / | ||
| 700 | / | 10.3 ± 0.1 | 60.8 ± 0.4 | 1.2 ± 0.1 | / | 1.2 ± 0.1 | / | / | ||
| Granular sludge | 300 | / | 7.8 ± 0.1 | 53.5 ± 0.2 | 5.8 ± 0.1 | / | 9.0 | / | / | |
| 550 | / | 9.5 ± 0.1 | 48.0 ± 0.2 | 1.8 ± 0.1 | / | 6.9 | / | / | ||
| 700 | / | 9.7 ± 0.1 | 50.5 ± 0.4 | 1.0 ± 0.1 | / | 6.5 ± 0.1 | / | / | ||
| Adsorbent | Arsenic Species | Sorption Capacity Qmax (mg/g) | pH | Best Isotherm Model | Best Kinetic Model | Proposed Sorption Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Fe(III)-modified Crop Straw biochars | As(V) | 33.7 g/kg | 5 | Langmuir | / | Surface complexation | Pan et al. [153] |
| corn stem Biochar Impregnated with Fe-Mn Oxides | As(III) | 8.80 mg/g | 7 | Freundlich | Pseudo-second-order | Surface adsorption, oxidation | Lin et al. [109] |
| ZnCl-activated biogas residue biochar | As(III) | 27.67 mg/g | 7 | Freundlich | Pseudo-second-order | Ligand exchange, porous adsorption | Xia et al. [108] |
| FeCl3 treated corn straw biochar | As(V) | 6.80 mg/g | 6 | Freundlich | Pseudo-second-order | Electrostatic attraction, precipitation | He et al. [94] |
| Hematite modified Pinus taeda biochar | As(V) | 429 mg/kg | 7 | Langmuir | Elovich | Electrostatic attractions | Wang et al. [51] |
| Fe-impregnated hickory chips biochar | As(V) | 2.16 mg/g | / | Temkin | / | Surface complexation | Hu et al. [44] |
| Fe-coated empty fruit bunch biochar | As(III) | 31. 4 mg/g | 8 | Langmuir | / | Surface complexation, interactions with FeOH and FeOH2+ groups | Samsuri et al. [54] |
| As(V) | 15.2 mg/g | 5 | |||||
| Fe-coated rice husk biochar | As(III) | 30.7 mg/g | 9 | ||||
| As(V) | 16.0 mg/g | 6 | |||||
| Biochar-supported molybdenum-disulfide/iron-oxide system | As(III) | 30.9 mg/ g | 4 | Freundlich | Pseudo-second-order | Ligand exchange, the coexistence of SO4•− and •OH | Khan et al. [43] |
| Magnetic chitosan/biochar composite | As(V) | 17.876 mg/ g | 5 | Langmuir | Pseudo-second-order | Electrostatic attraction and the increased adsorption sites that iron provided | Liu et al. [49] |
| KOH activated municipal solid wastes biochar | As(V) | 30.98 mg/g | / | Langmuir | Pseudo-second-order | Surface complexation, π–π electron donor–acceptor interaction | Jin et al. [53] |
| α-FeOOH modified wheat straw biochar | As(III) | 78.3 mg/g | Langmuir | Pseudo-second-order | Co-precipitation, ion exchange | Zhu et al. [62] | |
| Fe3O4 nanoparticle-covered Hybrid bamboo biochar | As(V) | 868 mg/g | / | Langmuir | Pseudo-second-order | Electrostatic attraction, precipitation | Alchouron et al. [154] |
| Pinewood supported nZVI (nZVI/BC) | As(V) | 124.5 mg/g | 4.1 | Freundlich | Pseudo-frst order | Surface complexation | Wang et al. [55] |
| Zero valentnano iron activated date palm biochar | As(V) | 26.52 mg/g | 2–6 | Langmuir | Pseudo frst-order | Electrostatic interactions, surface complexation and diffusion | Ahmad et al. [155] |
| FeOx CaCO3 + paper mill sludge biochar | As(V) | 23.1 mg/g | 8.6 | Langmuir | Pseudo-second-order | Electrostatic attraction, co-precipitation | Yoon et al. [110] |
| Fe0 + red oak & switch grass biochar | As(III) | 7.92–15.58 mg/g | 7.0–7.5 | Langmuir | Pseudo second order | Surface adsorption, co-precipitation | Bakshi et al. [150] |
| Fe-composite Rice husk biochar | As(V) | 0.760 mg/g | 7 | Freundlich | Pseudo second order | Precipitation, electrostatic attraction | Agrafioti et al. [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Yu, F.; Han, C.; Houda, C.; Hao, M.; Wang, Q. Research Progress on Adsorption of Arsenic from Water by Modified Biochar and Its Mechanism: A Review. Water 2022, 14, 1691. https://doi.org/10.3390/w14111691
Sun Y, Yu F, Han C, Houda C, Hao M, Wang Q. Research Progress on Adsorption of Arsenic from Water by Modified Biochar and Its Mechanism: A Review. Water. 2022; 14(11):1691. https://doi.org/10.3390/w14111691
Chicago/Turabian StyleSun, Yongchang, Fangxin Yu, Caohui Han, Chouarfa Houda, Mingge Hao, and Qiongyao Wang. 2022. "Research Progress on Adsorption of Arsenic from Water by Modified Biochar and Its Mechanism: A Review" Water 14, no. 11: 1691. https://doi.org/10.3390/w14111691
APA StyleSun, Y., Yu, F., Han, C., Houda, C., Hao, M., & Wang, Q. (2022). Research Progress on Adsorption of Arsenic from Water by Modified Biochar and Its Mechanism: A Review. Water, 14(11), 1691. https://doi.org/10.3390/w14111691








