Identification, Abundance, and Chemical Characterization of Macro-, Meso-, and Microplastics in the Intertidal Zone Sediments of Two Selected Beaches in Sabah, Malaysia
Abstract
:1. Introduction
2. Materials and Methods
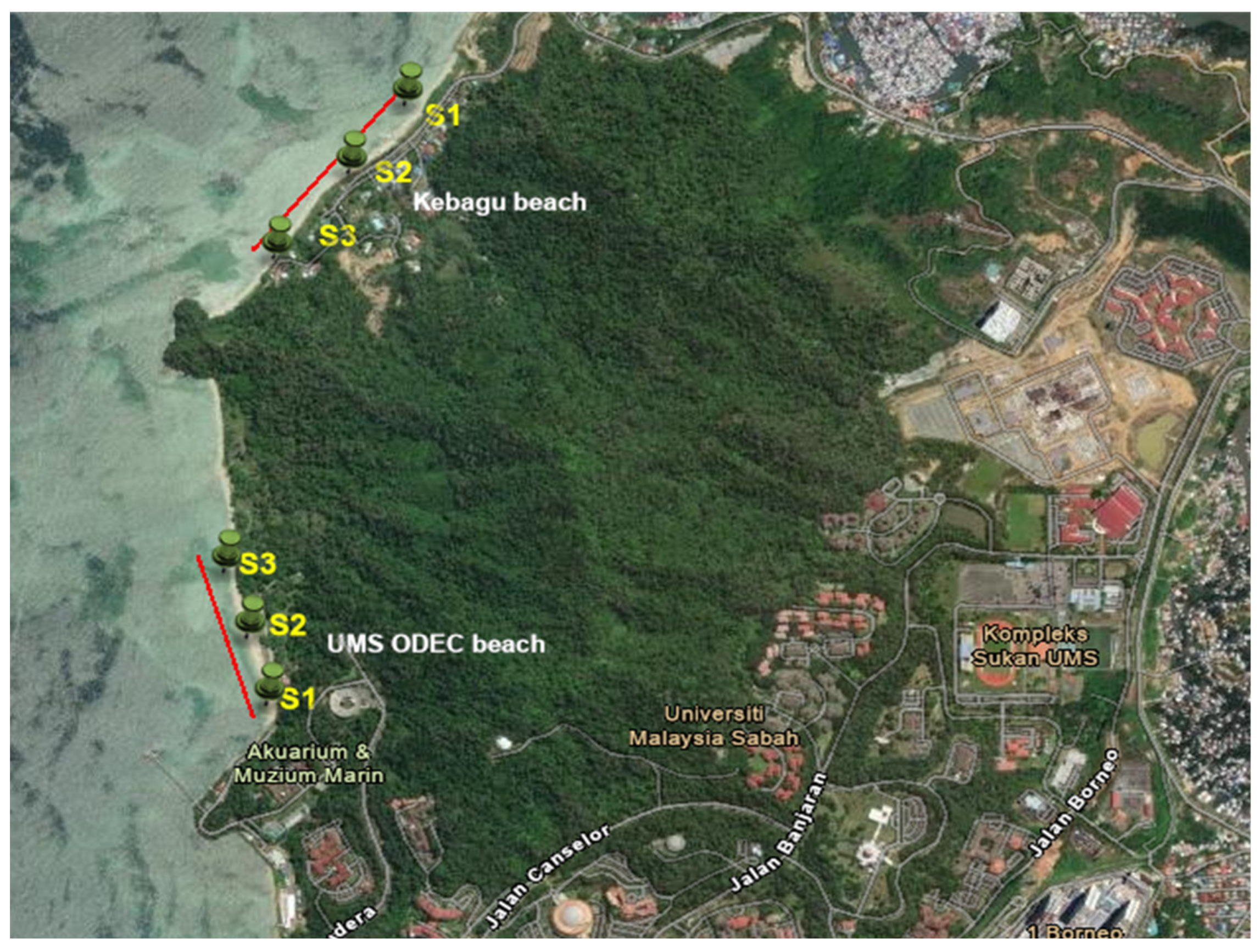
2.1. Study Area and Sampling Sites
2.2. Sample Collection and Sample Preparation
2.3. Identification of Polymer Types from Fourier-Transform Infrared (FTIR) and Surface Morphology Observation
2.4. Heavy Metal Analysis
3. Results and Discussion
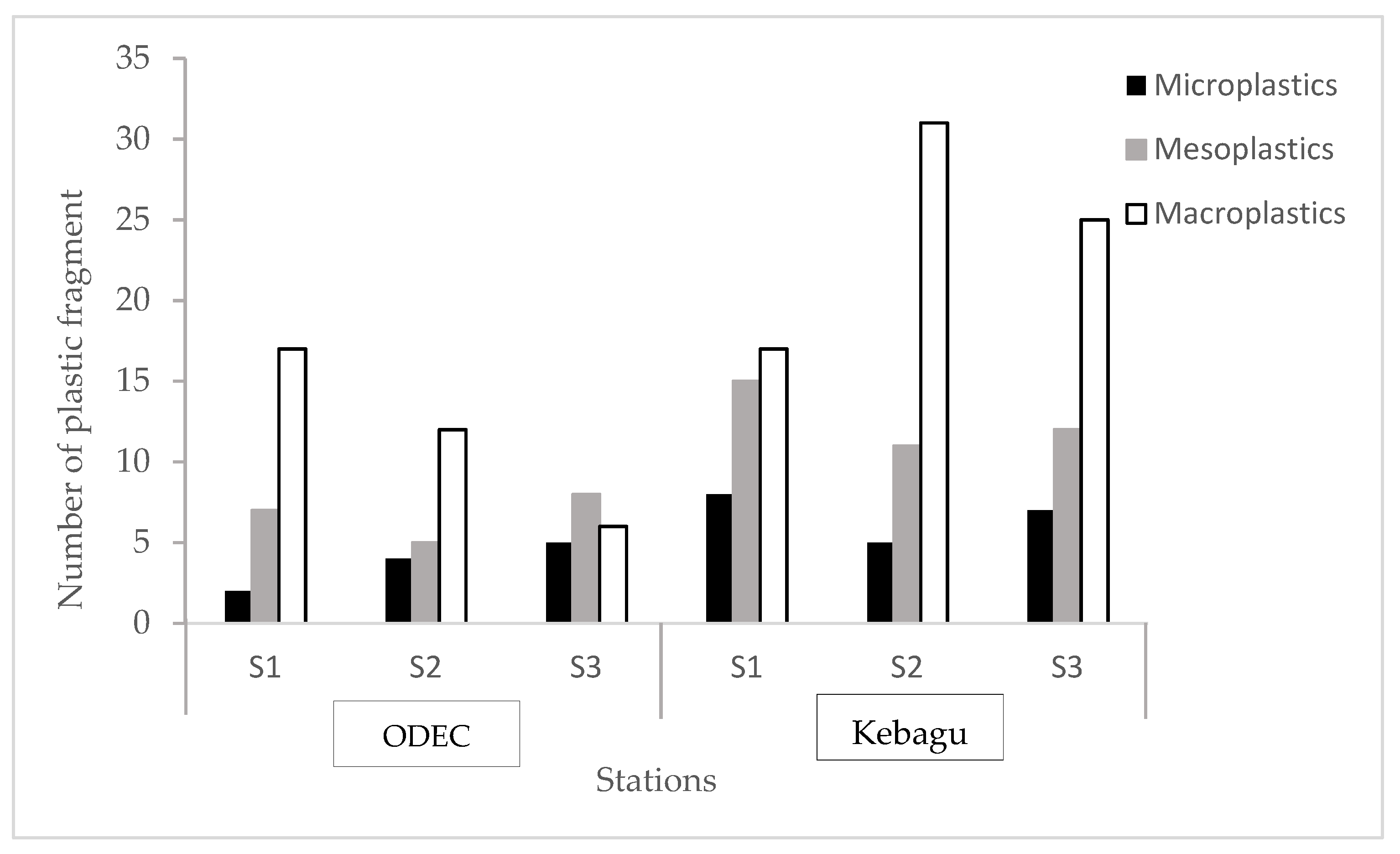
3.1. Weight and Quantity of Plastic Debris
3.2. Size Classification of Plastic Fragments and Particles
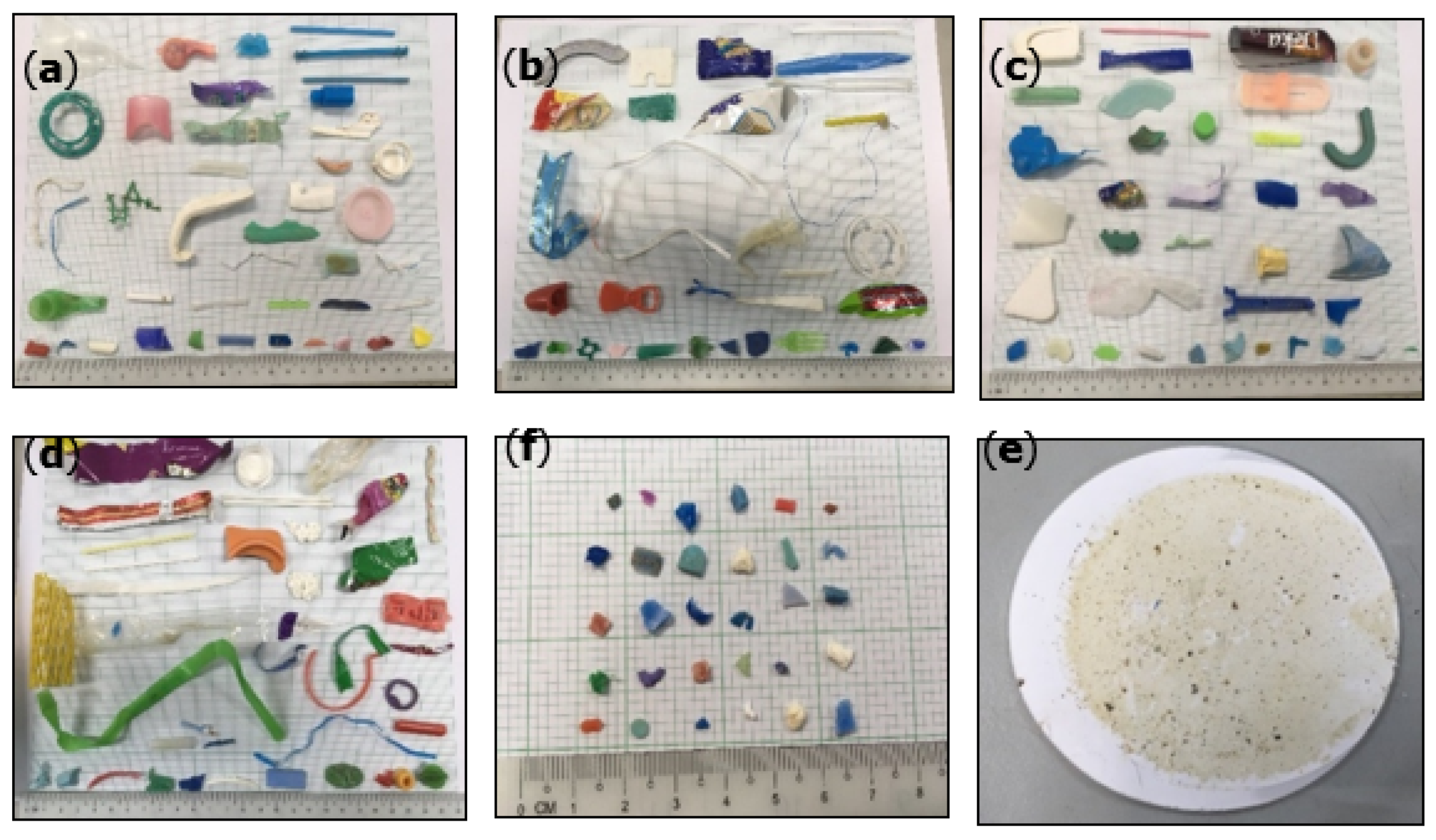
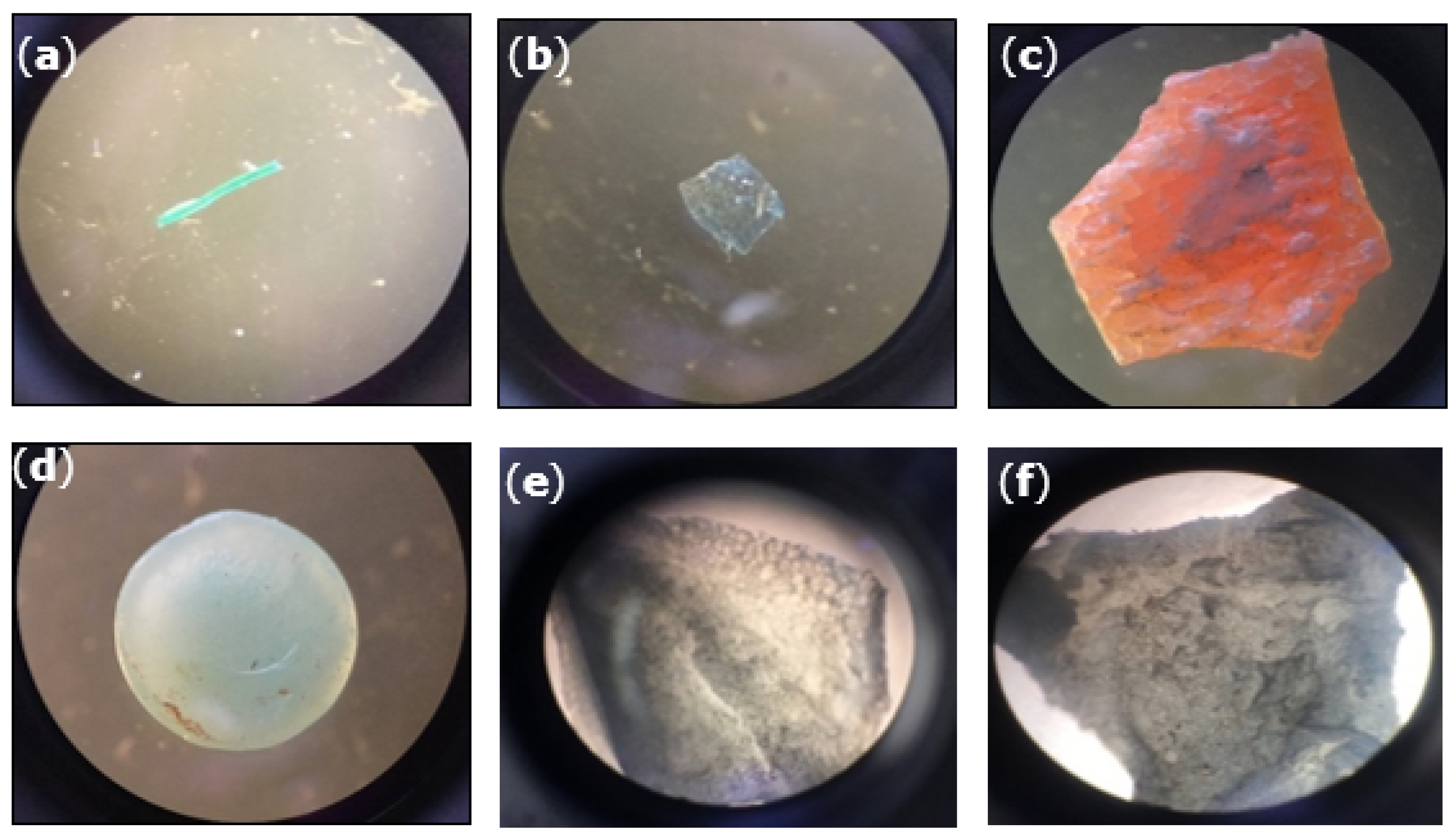
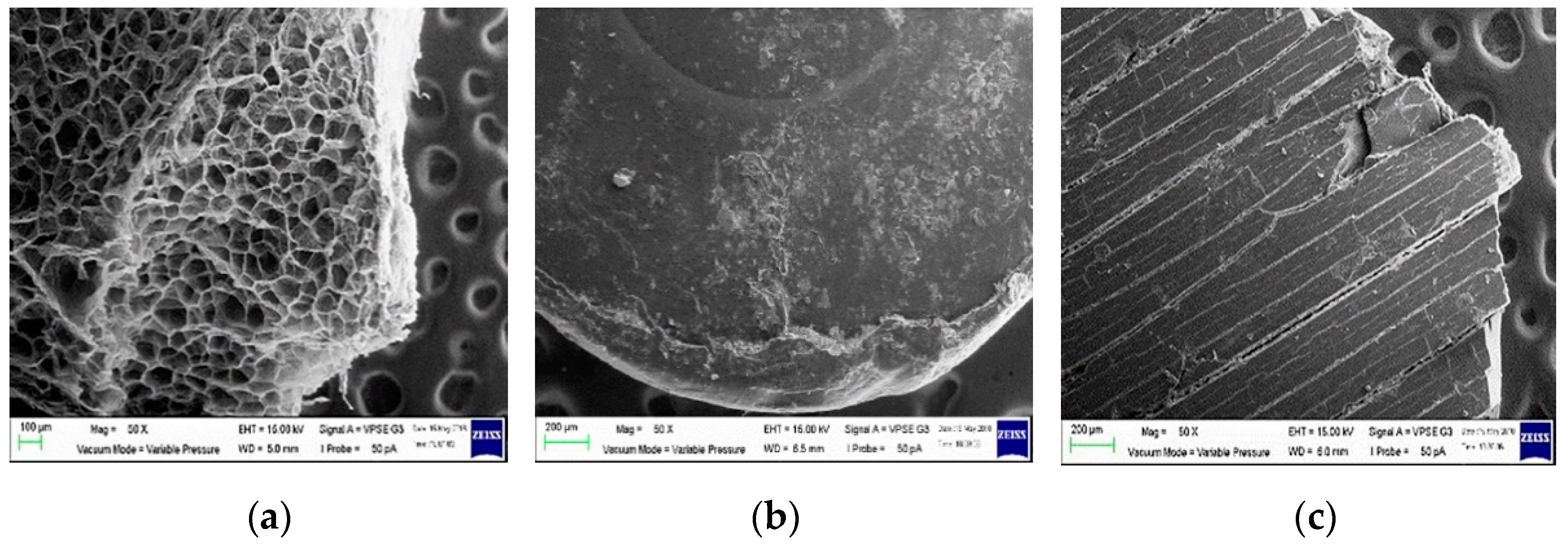
3.3. Observation of Plastic Fragments and Particles
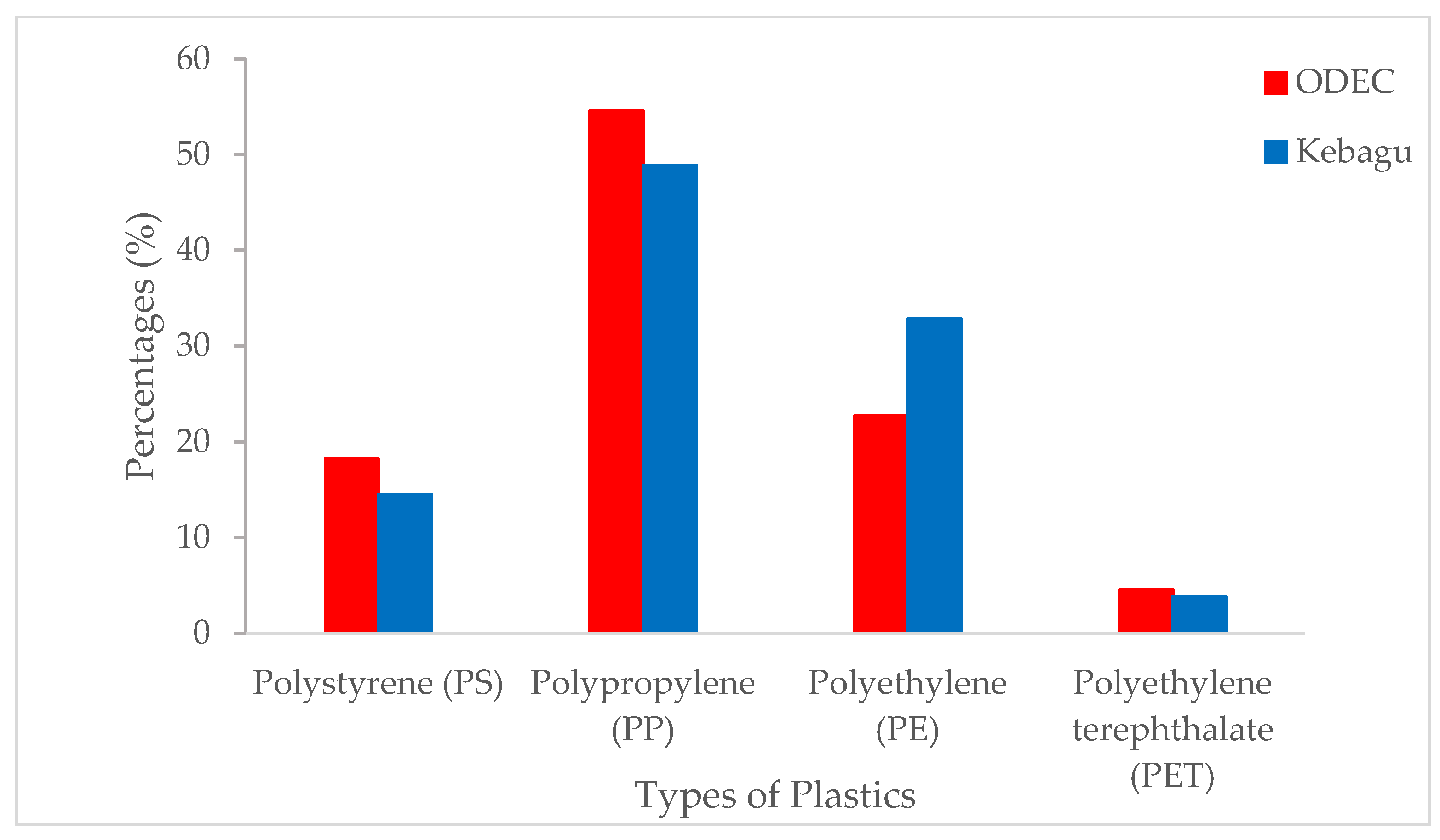
3.4. Identification of Plastic Polymers
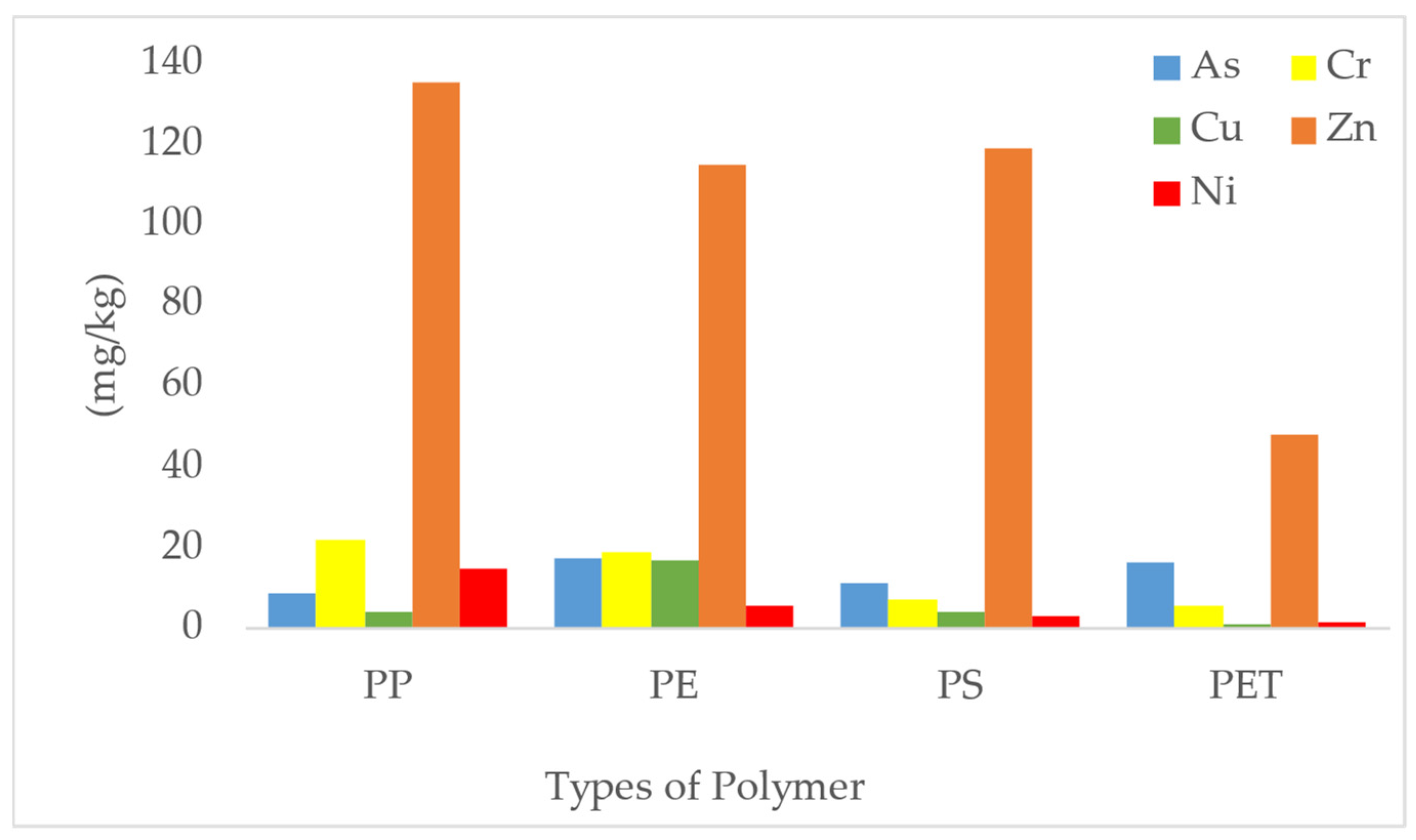
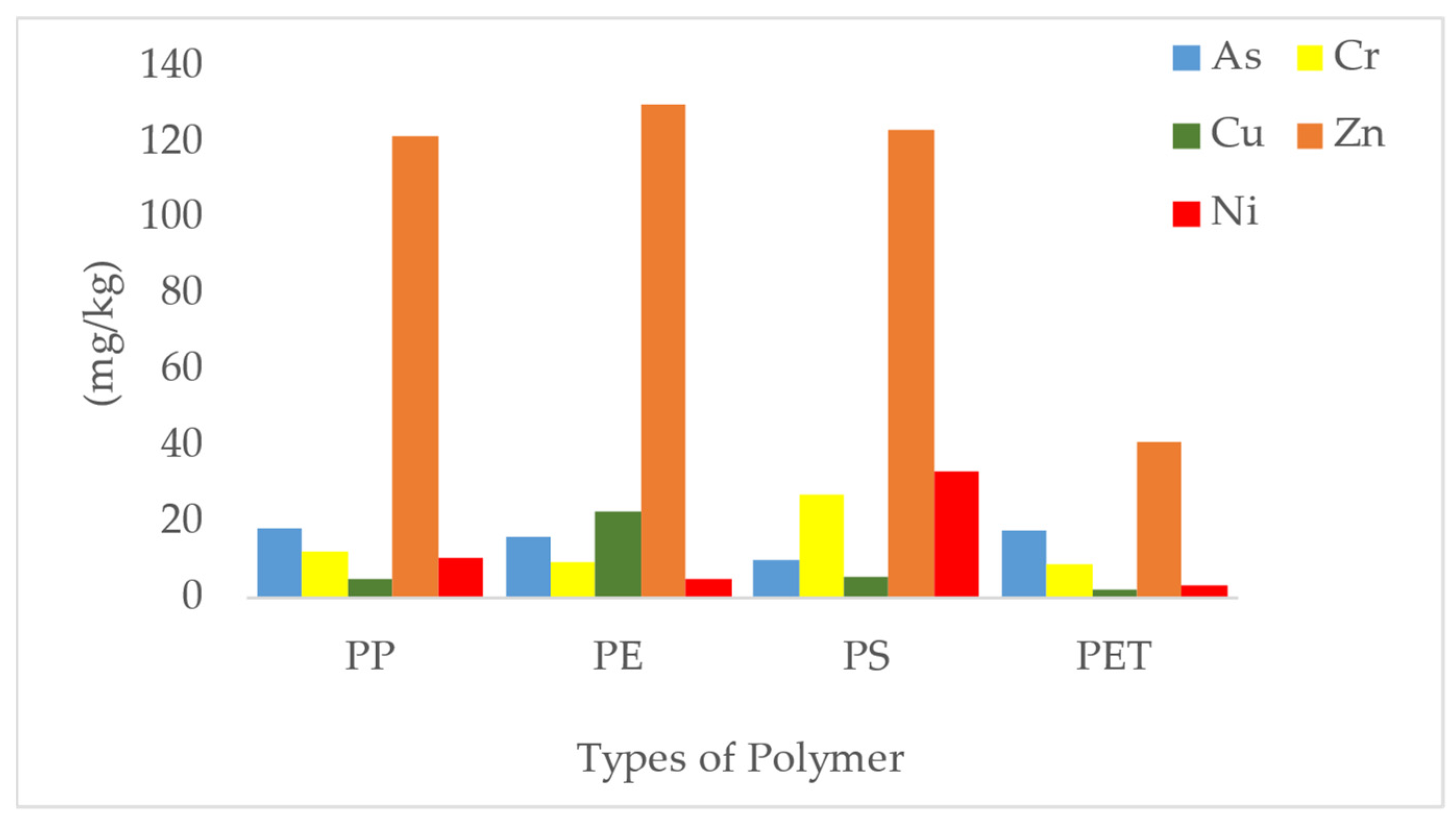
3.5. Heavy Metal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nizetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci. Processes Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, P.; Verma, A.; Jha, P.K.; Singh, P.; Gupta, P.K.; Chandra, R.; Prasad, P.V.V. Effect of Physical Characteristics and Hydrodynamic Conditions on Transport and Deposition of Microplastics in Riverine Ecosystem. Water 2021, 13, 2710. [Google Scholar] [CrossRef]
- Andrady, A.L. Assessment of environmental biodegradation of synthetic polymers: A review. J. Macromol. Sci. Part C Polym. Rev. 1994, 34, 25–76. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ernst, T.; Popp, R.; Van Eldik, R. Quantification of heavy metals for the recycling of waste plastics from elctrotechnical applications. Talanta 2000, 53, 347–357. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut.Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Kang, H.; Kwon, O.Y.; Shim, W.J. Potential threat of microplastics to zooplanktivores in the surface waters of the southern sea of Korea. Arch. Environ. Contam. Toxicol. 2015, 69, 340–351. [Google Scholar] [CrossRef]
- Rosevelt, C.; Huertos, M.L.; Garza, C.; Nevins, H.M. Marine debris in central California: Quantifying type and abundance of beach litter in Monterey Bay, CA. Mar. Pollut. Bull. 2013, 71, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Ryan, P.G.; Moore, C.J.; Van Franeker, J.A.; Moloney, C.L. Monitoring the abundance of plastic debris in the marine environment. Philos. Trans. R. Soc. B 2009, 364, 1999–2012. [Google Scholar] [CrossRef] [Green Version]
- Frias, J.P.G.L.; Sobral, P.; Ferreira, A.M. Organic pollutants in microplastic from two beaches of the Portuguese coast. Mar. Pol. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Mar. Pol. Bull. 2013, 77, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Noro, J.; Kawase, A.; Fujinami, M.; Oguma, K. Digestion of plastic materials for the determination of toxic metals with a microwave oven for household use. Anal. Sci. 2006, 22, 225–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauziah, S.H.; Liyana, I.A.; Agamuthu, P. Plastic debris in the coastal environment: The invincible threat? Abundance of buried plastic debris on Malaysian beaches. Waste Manag. Res. 2015, 33, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Peng, G.; Su, L.; Gao, Y.; Gao, L.; Li, D. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef]
- Anh, T.T.; Sandhya, B. Microplastics pollution with heavy metals in the aquaculture zone of the Chao Phraya River Estuary, Thailand. Mar. Pollut. Bull. 2020, 161, 111747. [Google Scholar]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Andrady, A.L. Plastics and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Cooper, D.A.; Corcoran, P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 2010, 60, 650–654. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and retention of microplastics by the Shore Crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, K.N.; Karapanagioti, K. Surface properties of beached plastic pellets. Mar. Environ. Res. 2012, 81, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Hanke, G.; Maes, T. Global distribution, composition and abundance of marine litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin, Germany, 2015; pp. 29–56. [Google Scholar]
- Andrady, A.L.M.; Bergmann, L.; Gutow, M.; Klages. Persistence of Plastic Litter in the Oceans, In Marine Anthropogenic Litter; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 57–72. [Google Scholar]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; Vom saal, F.S. Our plastic age. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasile, C.; Pascu, M. Practical Guide to Polyethylene; Rapra Technology Limited: Shrewsburg, UK, 2005. [Google Scholar]
- Anthony, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar]
- Tripathi, D.; Ebrary, I. Practical Guide to Polypropylene; Rapra Technology Limited: Shrewsburg, UK, 2002. [Google Scholar]
- Rochman, C.M.; Hentschel, B.T.; Teh, S.J. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS ONE 2014, 9, e85433. [Google Scholar] [CrossRef] [Green Version]
- Rehbein, W.; Lange, I.; Pazdzior, D. Substitution of Zinc Stearate in Cold Extrusion Processes. J. Tribol. Lubr. Appl. Liq. Gaseous Fuels Combust. Eng. 2015, 54, 343–356. [Google Scholar]
- Jones, A. Choosing antimicrobial additives for plastics. Plast. Add. Com. 2009, 11, 26–28. [Google Scholar] [CrossRef]
- Hoffmann, L.; Grinderslev, M.; Helweg, C.; Rasmussen, J.O. Massestrømsanalyse af Chrom og Chromforbindelser; Miljøprojekt Nr. 738; Miljøstyrelsen: Copenhagen, Denmark, 2002. [Google Scholar]
- Hakeim, O.A.; Diab, H.A.; Adam, J. Preparation and characterization of UV curable encapsulated phthalocyanine blue pigment. Prog. Org. Coat. 2015, 84, 70–78. [Google Scholar] [CrossRef]
- Markarian, J. Plasticulture comes of age. Plast. Add. Com. 2005, 7, 16–19. [Google Scholar] [CrossRef]
- Cozar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández León, S.; Duarte, C.M. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [Green Version]
- Dennis, B.; Bernardo, D.; Filipa, P.; Isabel, C.; Joan, C.C. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar]
- Ashton, K.; Holmes, L.; Turner, A. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Karapanagioti, H.K.; Klontza, I. Testing phenanthrene distribution properties of virgin plastic pellets and plastic eroded pellets found on Lesvos island beaches (Greece). Mar. Environ. Res. 2008, 65, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef] [PubMed]
- Zahari, N.Z.; Fong, N.S.; Cleophas, F.N.; Rahim, S.A. The Potential of Pistia stratiotes in the Phytoremediation of Selected Heavy Metals from Simulated Wastewater. Int. J. Technol. 2021, 12, 613–624. [Google Scholar] [CrossRef]
- Jundong, W.; Jinping, P.; Zhi, T.; Yifan, G.; Zhiwei, Z.; Qiuqiang, C.; Liqi, C. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar]


| Sampling Sites | Type of Beach | Items (m−2) | References |
|---|---|---|---|
| Kebagu beach | Fishing and recreational | 131 | Present work |
| UMS ODEC beach | Outdoor activities | 66 | Present work |
| Tanjung Aru beach | Recreational | 183 | [14] |
| Teluk Likas beach | Fishing and recreational | 239 | [14] |
| Changjiang estuary | Economic value (industry and aquaculture) | 231 | [15] |
| Choa Phraya River | Economic value (aquaculture) | 48 | [16] |
| Type of Polymer | Sampling Area | Concentrations of Metals (ug/g) | Reference | |||
|---|---|---|---|---|---|---|
| Ni | Cu | Zn | Pb | |||
| PP | Kebagu beach | 14 | 3 | 120 | - | Present work |
| PE | 3 | 20 | 129 | - | ||
| PP | UMS ODEC beach | 15 | 2 | 135 | - | |
| PE | 3 | 18 | 115 | - | ||
| PE | Beijing lake | 1.1 | 19.6 | 0.25 ± 0.1 | 219.7 | [38] |
| PP + PE | Beijing river sediment | <0.1 | 80.9–500.6 | 2414–14,815 | 38.2–131.1 | [43] |
| PP | Chao Phraya river estuary | <0.1 | - | 1-10 | - | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahari, N.Z.; Tuah, P.M.; Junaidi, M.R.; Mohd Ali, S.A. Identification, Abundance, and Chemical Characterization of Macro-, Meso-, and Microplastics in the Intertidal Zone Sediments of Two Selected Beaches in Sabah, Malaysia. Water 2022, 14, 1600. https://doi.org/10.3390/w14101600
Zahari NZ, Tuah PM, Junaidi MR, Mohd Ali SA. Identification, Abundance, and Chemical Characterization of Macro-, Meso-, and Microplastics in the Intertidal Zone Sediments of Two Selected Beaches in Sabah, Malaysia. Water. 2022; 14(10):1600. https://doi.org/10.3390/w14101600
Chicago/Turabian StyleZahari, Nur Zaida, Piakong Mohd Tuah, Muhammad Ramdi Junaidi, and Siti Aishah Mohd Ali. 2022. "Identification, Abundance, and Chemical Characterization of Macro-, Meso-, and Microplastics in the Intertidal Zone Sediments of Two Selected Beaches in Sabah, Malaysia" Water 14, no. 10: 1600. https://doi.org/10.3390/w14101600
APA StyleZahari, N. Z., Tuah, P. M., Junaidi, M. R., & Mohd Ali, S. A. (2022). Identification, Abundance, and Chemical Characterization of Macro-, Meso-, and Microplastics in the Intertidal Zone Sediments of Two Selected Beaches in Sabah, Malaysia. Water, 14(10), 1600. https://doi.org/10.3390/w14101600






