Author Contributions
All authors listed have contributed substantially to the manuscript to be included as authors. Conceptualization, A.B. and H.M.; methodology, A.B. and H.M.; validation, A.B., H.M., G.L. and S.K.; formal analysis, A.B., H.M. and G.L.; investigation, A.B., H.M. and S.K.; resources, A.B., H.M. and S.K.; data curation, A.B., H.M. and S.K.; writing—original draft preparation, A.B., H.M. and G.L.; writing—review and editing, A.B., H.M., G.L. and S.K.; supervision, S.K.; project administration, H.M. and S.K.; funding acquisition, H.M. and S.K. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Study sites: Hydro Power Plants (HPP) Lavamünd (left), Schwabeck (middle) and Edling (right), situated at river Drava, Carinthia/Austria. Red circles indicate the entrances of the fish passes.
Figure 1.
Study sites: Hydro Power Plants (HPP) Lavamünd (left), Schwabeck (middle) and Edling (right), situated at river Drava, Carinthia/Austria. Red circles indicate the entrances of the fish passes.
Figure 2.
Austrian part of the river Drava with sites of the HPP, red squares indicate the runoff-river-stations with hydropeaking according to [
26], © Land Kärnten—KAGIS, BEV.
Figure 2.
Austrian part of the river Drava with sites of the HPP, red squares indicate the runoff-river-stations with hydropeaking according to [
26], © Land Kärnten—KAGIS, BEV.
Figure 3.
Entrance area fish pass of Edling with LED stripes and speakers.
Figure 3.
Entrance area fish pass of Edling with LED stripes and speakers.
Figure 4.
Means and variances of ascent rates across the different years; boxes indicate the inter-quartile range (IQR), black horizontal lines within the boxes show the median, whiskers show values within 1.5 × IQR anddots indicate outliers.
Figure 4.
Means and variances of ascent rates across the different years; boxes indicate the inter-quartile range (IQR), black horizontal lines within the boxes show the median, whiskers show values within 1.5 × IQR anddots indicate outliers.
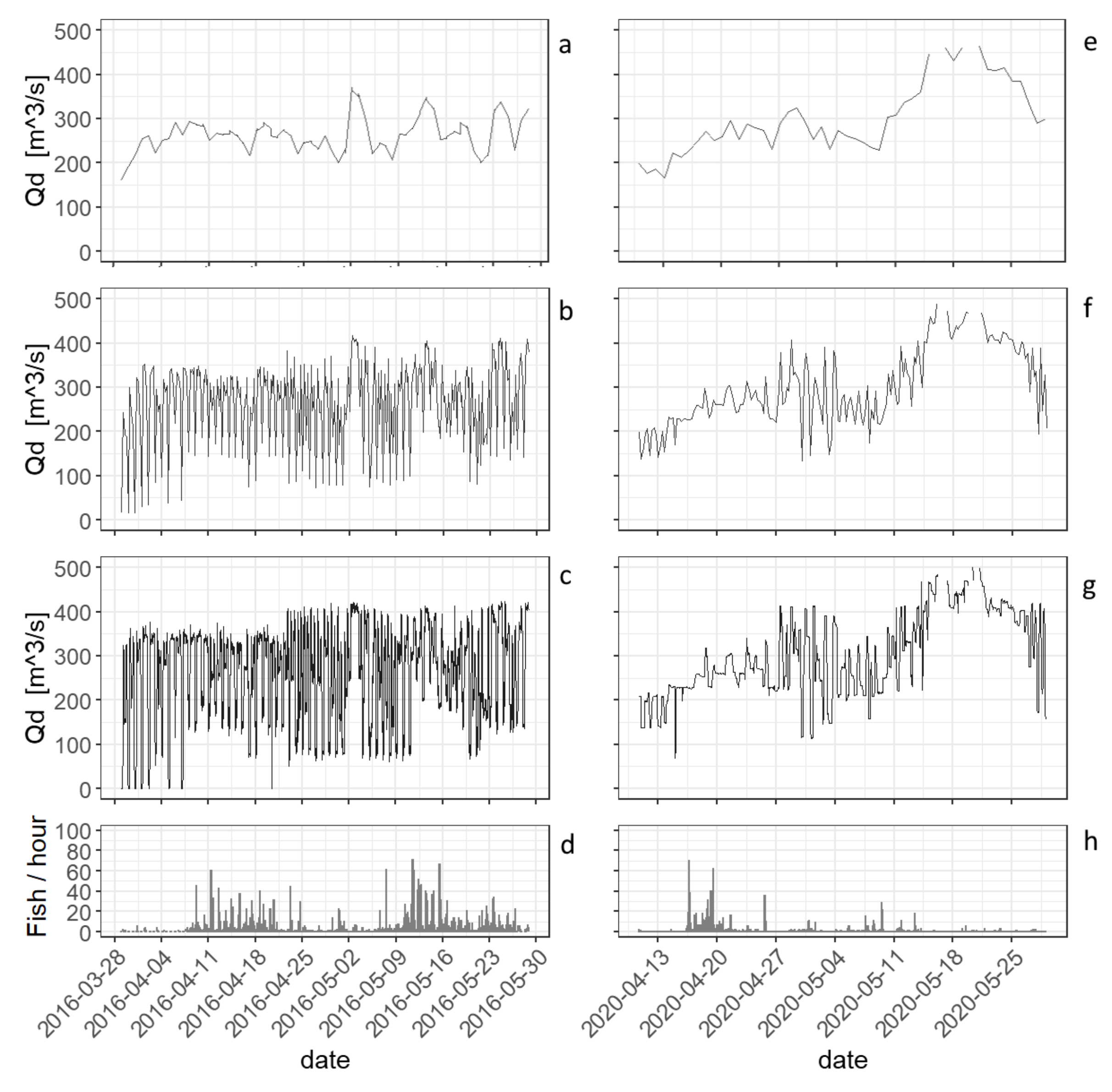
Figure 5.
Diurnal variation of the discharge of the Drava at the HPP Lavamünd for different time intervals ((a,e): 24-h data; (b,f): 6-h data; (c,g): 1-h data) with ascent rates; ((d,h): 1-h data).
Figure 5.
Diurnal variation of the discharge of the Drava at the HPP Lavamünd for different time intervals ((a,e): 24-h data; (b,f): 6-h data; (c,g): 1-h data) with ascent rates; ((d,h): 1-h data).
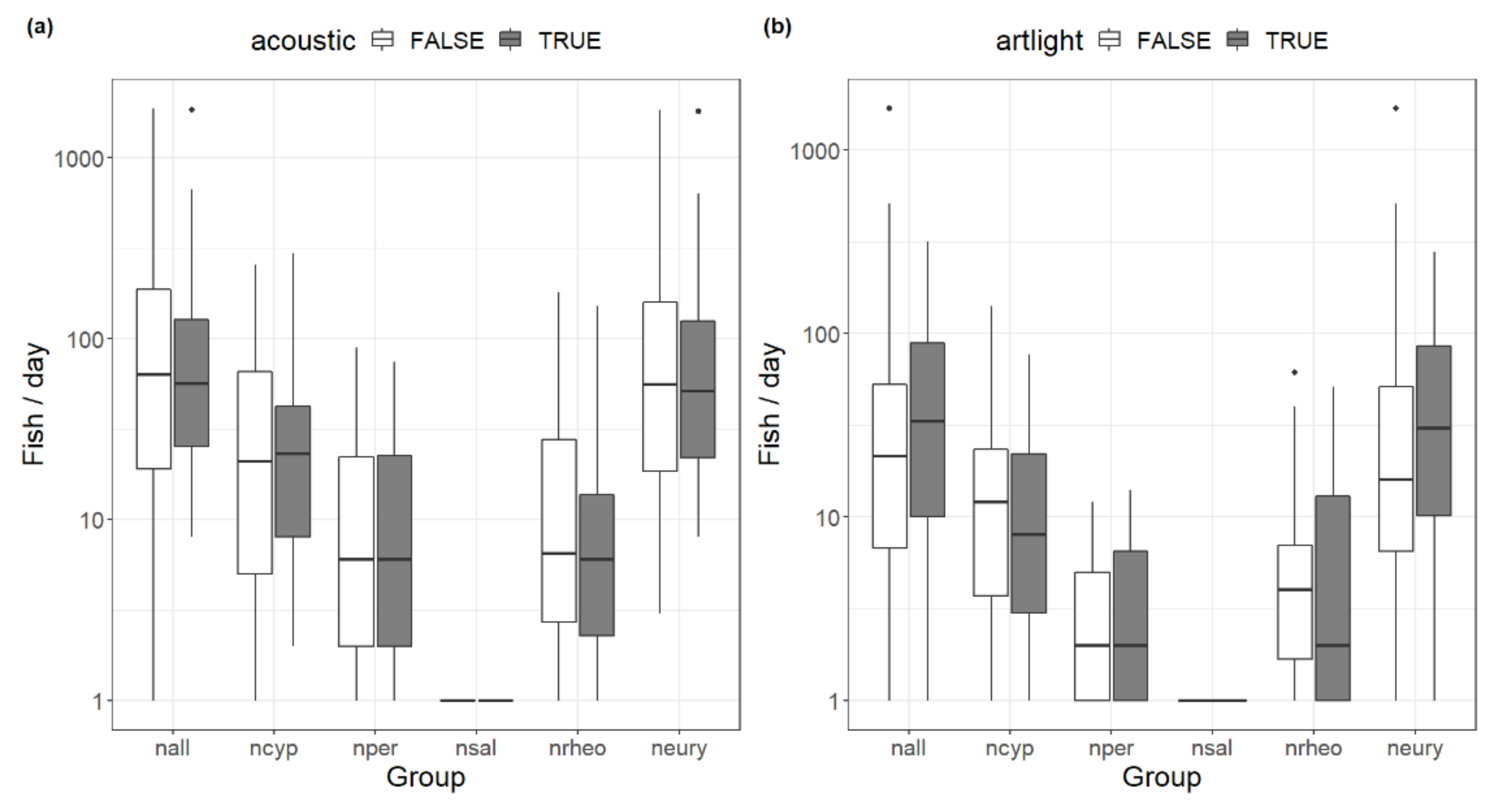
Figure 6.
Boxplots of artificial stimuli acoustic (a) and light (b), at the fish pass of HPP Edling sorted by investigated groups; boxes indicate the inter-quartile range (IQR), black horizontal line within the boxes show the medians, whiskers show values within 1.5 × IQR and dots indicate outliers.
Figure 6.
Boxplots of artificial stimuli acoustic (a) and light (b), at the fish pass of HPP Edling sorted by investigated groups; boxes indicate the inter-quartile range (IQR), black horizontal line within the boxes show the medians, whiskers show values within 1.5 × IQR and dots indicate outliers.
Table 1.
Overview of the fish passes at the three study sites, Lavamünd, Schwabeck and Edling, situated at river Drava, Carinthia/Austria.
Table 1.
Overview of the fish passes at the three study sites, Lavamünd, Schwabeck and Edling, situated at river Drava, Carinthia/Austria.
| | Lavamünd | Schwabeck | Edling |
|---|
| Total drop height | 10 m | 20 m | 22 m |
| Total no. of pools | 74 | 158 | 148 |
| No. of resting pools | 14 | 22 | 24 |
| Drop height btw. pools | 13 cm | 13 cm | 13 cm |
| Pool dimension (l × w) | 3.0 m × 2.2 m | 3.0 m × 2.2 m | 3.0 m × 2.2 m |
| Slot width | 40 cm | 40 cm | 40 cm |
| Total length | 300 m | 550 m | 650 m |
| Distance to obstacle | 110 m | 120 m | 150 m |
Table 2.
Monitoring periods at the study sites Lavamünd, Schwabeck and Edling.
Table 2.
Monitoring periods at the study sites Lavamünd, Schwabeck and Edling.
| | Lavamünd | Schwabeck | Edling |
|---|
| Period 1 | 26 August–5 December 2015 | 8 April–26 May 2016 | 9 April–18 June 2019 |
| Period 2 | 29 March–8 November 2016 | 5 July–9 September 2016 | 17 September–4 November 2019 |
| Period 3 | | 3 October–6 December 2016 | 10 April–23 June 2020 |
| Period 4 | | 21 March–9 June 2017 | |
Table 3.
Specification of the temperature sensors for recording the water temperature at all three study sites.
Table 3.
Specification of the temperature sensors for recording the water temperature at all three study sites.
| | HOBO U22 Specifications |
|---|
| Operation range | −40 °C to 50 °C in water |
| Accuracy | ±0.21 °C from 0 °C to 50 °C |
| Resolution | 0.02 °C at 25 °C |
| Response time | 5 min in water |
| Waterproof | To 120 m |
Table 4.
Weekly pattern of the artificial key stimuli of light and acoustics at the fish pass at HPP Edling.
Table 4.
Weekly pattern of the artificial key stimuli of light and acoustics at the fish pass at HPP Edling.
| Day of Week | Stimulus | Day of Week | Stimulus |
|---|
| Monday | Acoustics | Thursday | Acoustics |
| Tuesday | Light | Friday | Light |
| Wednesday | - | Saturday/Sunday | - |
Table 5.
Observed numbers of fish species from FishCam recording (count and percent of total) and their occurrence according to the Natural guideline and electrofishing downstream from the study area. [
27].
Table 5.
Observed numbers of fish species from FishCam recording (count and percent of total) and their occurrence according to the Natural guideline and electrofishing downstream from the study area. [
27].
| Species | FishCam | Guideline | Downstream | Percent | Species | FishCam | Guideline | Downstream | Percent |
|---|
| Alburnus alburnus | 23,095 | t | t | 57.46 | Leuciscus aspius | 3 | | | 0.01 |
| Alburnoides bipunctatus | 4918 | t | t | 12.24 | Salvelinus fontinalis | 2 | | | 0 |
| Rutilus rutilus | 3299 | t | t | 8.21 | Barbatula barbatula | 2 | r | | 0 |
| Perca fluviatilis | 3018 | t | t | 7.51 | Leuciscus idus | 2 | | | 0 |
| Chondrostoma nasus | 1414 | l | l | 3.52 | Gymnocephalus schraetser | 2 | | | 0 |
| Abramis brama | 1065 | t | t | 2.65 | Lepomis gibbosus | 2 | | | 0 |
| Squalius cephalus | 816 | l | l | 2.03 | Anguilla anguilla | 1 | | | 0 |
| Blicca bjoerkna | 599 | | | 1.49 | Rutilus pigus | 1 | | | 0 |
| Unknown | 356 | | | 0.89 | Carassius carassius | 1 | r | | 0 |
| Cyprinidae unknown | 286 | | | 0.71 | Sander lucioperca | 1 | | | 0 |
| Gobio gobio | 211 | t | t | 0.52 | Ballerus sapa | 1 | | | 0 |
| Leuciscus leuciscus | 193 | t | | 0.48 | Hucho hucho | 0 | l | | - |
| Oncorhynchus mykiss | 185 | | | 0.46 | Telestes souffia | 0 | t | | - |
| Salmonidae unknown | 179 | | | 0.45 | Romanogobio kesslerii | 0 | r | | - |
| Scardinius erythroph. | 168 | r | | 0.42 | Vimba vimba | 0 | r | | - |
| Barbus barbus | 67 | l | l | 0.17 | Alburnus mento | 0 | r | | - |
| Salmo trutta fario | 57 | r | r | 0.14 | Barbus balcanicus | 0 | r | | - |
| Thymallus thymallus | 47 | t | | 0.12 | Cobitis elongatoides | 0 | r | | - |
| Esox lucius | 45 | t | t | 0.11 | Acipenser ruthenus | 0 | r | | - |
| Salmo trutta lacustris | 42 | | | 0.1 | Zingel streber | 0 | r | | - |
| Gymnocephalus cernua | 30 | | | 0.07 | Romanogobio vladykovi | 0 | r | | - |
| Cottus gobio | 28 | r | | 0.07 | Zingel zingel | 0 | r | | - |
| Silurus glanis | 13 | t | | 0.03 | TOTAL no. observed | 40,194 | | | |
| Carassius gibelio | 12 | | | 0.03 | | | | | |
| Tinca tinca | 11 | r | | 0.03 | Lead species (l) | 3 | 4 | 3 | |
| Lota lota | 10 | t | t | 0.02 | Typical species (t) | 12 | 13 | 8 | |
| Eudontomyzon mariae | 8 | t | | 0.02 | Rare species (r) | 7 | 16 | 1 | |
| Cyprinus carpio | 4 | r | | 0.01 | Not mentioned (-) | 14 | - | - | |
Table 6.
Predictor variables for the statistical analyses with survey intervals and study site.
Table 6.
Predictor variables for the statistical analyses with survey intervals and study site.
| Variable | Survey Interval | HPP Site |
|---|
| Drava discharge (Qd) | 15 min | All sites |
| Fishpass discharge (Qf) | 15 min | All sites |
| Guiding flow (Qa) | 15 min | All sites |
| Season-adjusted watertemp. (tres) | 1 h | All sites |
| Watertemperature difference fish pass/Drava (tdiff) | 1 h | All sites |
| Season (seas.) | - | All sites |
| Study location (Site) | - | All sites |
| Year | - | All sites |
| Artificial light | 1 day | Edling |
| Acoustic | 1 day | Edling |
Table 7.
Statistical summary over the independent variable discharges fish passes (Qf), discharge Drava (Qd), attraction flow (Qa), residuals of the water temperature Drava (tres), difference in water temperature fish passes–Drava (tdiff) and ascent rate of all observed fish (nall)—1-h data.
Table 7.
Statistical summary over the independent variable discharges fish passes (Qf), discharge Drava (Qd), attraction flow (Qa), residuals of the water temperature Drava (tres), difference in water temperature fish passes–Drava (tdiff) and ascent rate of all observed fish (nall)—1-h data.
| | Qf [m3/s] | Qd [m3/s] | Qa [%] | tres [°C] | tdiff [°C] | nall [-] |
|---|
| Min. | 0.08 | 0.1 | 0.025 | −5.32 | −2.38 | 0 |
| Mean | 0.38 | 274.2 | 4.66 | 0.16 | 0.096 | 2.18 |
| Median | 0.40 | 278.5 | 0.14 | 0.38 | 0.001 | 0 |
| Max. | 0.49 | 932.8 | 441 | 5.08 | 3.64 | 981 |
| SD | 0.059 | 140.433 | 24.984 | 1.768 | 0.410 | 15.51 |
Table 8.
Regression models for the group of all individuals, 1-h data and 24-h data with incident rate ratios (IRR), confidence intervals (CI) for the frequentist models and credibility intervals (CI) for the Bayesian models. Glmm = frequentist approach, brm = Bayesian approach, Qd = discharge Drava, Qf = discharge fish pass, Qa = attraction flow, tres = residuals of the water temperature and tdiff = difference in water temperature btw. Fish pass and Drava, SB = Schwabeck and ED = Edling; p-values < 0.05 indicating significant parameters.
Table 8.
Regression models for the group of all individuals, 1-h data and 24-h data with incident rate ratios (IRR), confidence intervals (CI) for the frequentist models and credibility intervals (CI) for the Bayesian models. Glmm = frequentist approach, brm = Bayesian approach, Qd = discharge Drava, Qf = discharge fish pass, Qa = attraction flow, tres = residuals of the water temperature and tdiff = difference in water temperature btw. Fish pass and Drava, SB = Schwabeck and ED = Edling; p-values < 0.05 indicating significant parameters.
| All Fish | 1-h Glmm | 1-h Brm | 24-h Glmm | 24-h Brm |
|---|
| Predictors | IRR | CI (95%) | p-Value | IRR | CI (95%) | IRR | CI (95%) | p-Value | IRR | CI (95%) |
|---|
| (Intercept) | 0.36 | 0.09–1.39 | 0.137 | 0.29 | 0.03–2.39 | 30.93 | 3.96–241.85 | 0.001 | 19.03 | 0.41–294.58 |
| Qd | 0.99 | 0.97–1.00 | 0.055 | 0.99 | 0.97–1.00 | 0.85 | 0.80–0.91 | <0.001 | 0.85 | 0.80–0.91 |
| Qf | 0.94 | 0.90–0.98 | 0.003 | 0.94 | 0.90–0.98 | 0.93 | 0.80–1.09 | 0.371 | 0.92 | 0.79–1.08 |
| Qa | 1 | 0.99–1.00 | <0.001 | 1 | 0.99–1.00 | 0.58 | 0.01–26.48 | 0.781 | 0.74 | 0.02–40.05 |
| tres | 1.63 | 1.57–1.69 | <0.001 | 1.63 | 1.57–1.69 | 1.48 | 1.34–1.63 | <0.001 | 1.49 | 1.35–1.65 |
| tdiff | 2.97 | 2.61–3.39 | <0.001 | 2.96 | 2.60–3.39 | 1.69 | 1.03–2.78 | 0.04 | 1.65 | 1.01–2.66 |
| Site: SB | 1.89 | 1.61–2.23 | <0.001 | 1.9 | 1.62–2.24 | 1.8 | 1.12–2.89 | 0.014 | 1.81 | 1.14–2.93 |
| Site: ED | 3.88 | 0.51–29.18 | 0.188 | 4.17 | 0.07–218.60 | 6.47 | 0.80–52.46 | 0.08 | 8.43 | 0.15–980.87 |
| Seas.: Spring | 3.59 | 3.07–4.19 | <0.001 | 3.59 | 3.09–4.21 | 5.12 | 3.38–7.77 | <0.001 | 5.32 | 3.52–8.13 |
| Seas.: Summer | 1.94 | 1.65–2.29 | <0.001 | 1.94 | 1.65–2.30 | 3.92 | 2.52–6.09 | <0.001 | 4.02 | 2.58–6.32 |
| Seas.: Winter | 0.04 | 0.01–0.09 | <0.001 | 0.03 | 0.01–0.08 | 0.04 | 0.01–0.16 | <0.001 | 0.04 | 0.01–0.18 |
| Ran. Eff. | | | | | | | | | | |
| SD Intercept | 1.26 | | | 1.94 | | 1.30 | | | 2.04 | |
| Observations | 18,456 | 18,456 | 775 | 775 |
Table 9.
Regression models for the flow preference guilds rheophilic (rheo.) and eurytopic w/o Bream (eury.). Side by side, 1-h data and 24-h data, frequentist models and all study sites.
Table 9.
Regression models for the flow preference guilds rheophilic (rheo.) and eurytopic w/o Bream (eury.). Side by side, 1-h data and 24-h data, frequentist models and all study sites.
| | Rheo. 1-h Glmm | Eury. 1-h Glmm | Rheo. 24-h Glmm | Eury. 24-h Glmm |
|---|
| Predictors | IRR | CI (95%) | p-Value | IRR | CI (95%) | p-Value | IRR | CI (95%) | p-Value | IRR | CI (95%) | p-Value |
|---|
| (Intercept) | 0.39 | 0.10–1.62 | 0.195 | 0.09 | 0.03–0.30 | <0.001 | 20.5 | 1.66–253.38 | 0.019 | 5.71 | 0.88–36.85 | 0.067 |
| Qd | 0.95 | 0.93–0.97 | <0.001 | 0.98 | 0.97–1.00 | 0.026 | 0.82 | 0.76–0.89 | <0.001 | 0.85 | 0.80–0.90 | <0.001 |
| Qf | 0.97 | 0.91–1.04 | 0.424 | 0.91 | 0.87–0.95 | <0.001 | 1 | 0.81–1.23 | 0.979 | 0.98 | 0.85–1.12 | 0.72 |
| Qa | 0.98 | 0.97–0.98 | <0.001 | 1 | 1.00–1.00 | 0.043 | 0.99 | 0.01–68.33 | 0.996 | 0.02 | 0.00–0.69 | 0.03 |
| tres | 1.38 | 1.29–1.47 | <0.001 | 1.82 | 1.75–1.89 | <0.001 | 1.2 | 1.06–1.37 | 0.005 | 1.77 | 1.63–1.92 | <0.001 |
| tdiff | 1.4 | 1.15–1.70 | 0.001 | 2.49 | 2.19–2.84 | <0.001 | 0.79 | 0.46–1.33 | 0.37 | 2.3 | 1.47–3.59 | <0.001 |
| Site: SB | 0.71 | 0.53–0.95 | 0.02 | 1.43 | 1.19–1.71 | <0.001 | 0.69 | 0.36–1.30 | 0.245 | 1.49 | 0.94–2.34 | 0.088 |
| Site: ED | 0.89 | 0.14–5.49 | 0.9 | 9.12 | 1.48–56.12 | 0.017 | 1.77 | 0.36–8.57 | 0.481 | 10.72 | 1.58–72.60 | 0.015 |
| Seas.: Spring | 2.85 | 2.24–3.63 | <0.001 | 1.88 | 1.63–2.17 | <0.001 | 3.35 | 2.17–5.17 | <0.001 | 2.97 | 2.08–4.23 | <0.001 |
| Seas.: Summer | 0.65 | 0.50–0.83 | 0.001 | 1.77 | 1.49–2.10 | <0.001 | 1.12 | 0.70–1.79 | 0.644 | 3.29 | 2.18–4.96 | <0.001 |
| Seas.: Winter | 0.08 | 0.03–0.23 | <0.001 | 0.03 | 0.00–0.21 | <0.001 | 0.06 | 0.01–0.27 | <0.001 | 0.03 | 0.00–0.28 | 0.002 |
| Ran. Eff. | | | | | | | | | | | | |
| SD Intercept | 1.01 | | 1.02 | 0.67 | | 1.09 |
| Observations | 18,456 | 18,456 | 775 | 775 |
Table 10.
Regression models for the families Salmonidae (sal.), Cyprinidae (cyp.) and Percidae (perc.); 1-h data and all study sites.
Table 10.
Regression models for the families Salmonidae (sal.), Cyprinidae (cyp.) and Percidae (perc.); 1-h data and all study sites.
| | Sal. 1-h Glmm | Cyp. 1-h Glmm | Perc. 1-h Glmm |
|---|
| Predictors | IRR | CI (95%) | p-Value | IRR | CI (95%) | p-Value | IRR | CI (95%) | p-Value |
|---|
| (Intercept) | 0 | 0.00–0.01 | <0.001 | 0.36 | 0.11–1.17 | 0.09 | 0.05 | 0.01–0.31 | 0.001 |
| Qd | 0.99 | 0.97–1.02 | 0.577 | 0.96 | 0.94–0.98 | <0.001 | 1.01 | 0.99–1.04 | 0.165 |
| Qf | 1.08 | 1.00–1.17 | 0.059 | 0.95 | 0.90–1.00 | 0.06 | 0.88 | 0.83–0.93 | <0.001 |
| Qa | 0.98 | 0.97–0.99 | <0.001 | 0.99 | 0.99–0.99 | <0.001 | 1 | 0.99–1.00 | 0.044 |
| tres | 1.31 | 1.21–1.43 | <0.001 | 1.47 | 1.41–1.54 | <0.001 | 2.19 | 2.04–2.35 | <0.001 |
| tdiff | 0.87 | 0.61–1.25 | 0.465 | 1.53 | 1.32–1.78 | <0.001 | 2.58 | 2.13–3.13 | <0.001 |
| Site: SB | 6.8 | 4.71–9.81 | <0.001 | 0.67 | 0.54–0.84 | <0.001 | 1.56 | 1.21–2.02 | 0.001 |
| Site: ED | 1.26 | 0.53–3.02 | 0.602 | 3.02 | 0.61–15.04 | 0.178 | 5.27 | 0.41–68.27 | 0.204 |
| Seas.: Spring | 1.09 | 0.79–1.51 | 0.606 | 3.74 | 3.13–4.46 | <0.001 | 0.39 | 0.32–0.49 | <0.001 |
| Seas.: Summer | 1.55 | 1.12–2.15 | 0.008 | 0.87 | 0.72–1.07 | 0.184 | 1.5 | 1.18–1.91 | 0.001 |
| Seas.: Winter | 0.29 | 0.07–1.25 | 0.096 | 0.04 | 0.01–0.14 | <0.001 | 0 | 0.00–Inf | 0.988 |
| Ran. Eff. | | | | | | | | | |
| SD Intercept | 0.17 | 0.79 | 2.02 |
| Observations | 18,456 | 18,456 | 18,456 |
Table 11.
Regression models for the families Salmonidae (sal.), Cyprinidae (cyp.) and Percidae (per.); 24-h data and all study sites.
Table 11.
Regression models for the families Salmonidae (sal.), Cyprinidae (cyp.) and Percidae (per.); 24-h data and all study sites.
| | Sal. 24-h Glmm | Cyp. 24-h Glmm | Perc. 24-h Glmm |
|---|
| Predictors | IRR | CI (95%) | p-Value | IRR | CI (95%) | p-Value | IRR | CI (95%) | p-Value |
|---|
| (Intercept) | 1.36 | 0.24–7.84 | 0.727 | 5.06 | 0.52–48.91 | 0.162 | 4.6 | 0.38–55.15 | 0.229 |
| Qd | 0.84 | 0.77–0.92 | <0.001 | 0.82 | 0.76–0.89 | <0.001 | 0.9 | 0.83–0.97 | 0.008 |
| Qf | 1.04 | 0.88–1.22 | 0.656 | 1.1 | 0.91–1.33 | 0.318 | 0.87 | 0.74–1.04 | 0.128 |
| Qa | 0 | 0.00–0.10 | 0.002 | 0.76 | 0.01–51.62 | 0.899 | 0.08 | 0.00–4.84 | 0.226 |
| tres | 1.33 | 1.20–1.48 | <0.001 | 1.33 | 1.19–1.48 | <0.001 | 2.12 | 1.88–2.39 | <0.001 |
| tdiff | 1.46 | 0.72–2.97 | 0.299 | 0.66 | 0.40–1.08 | 0.095 | 3.61 | 1.87–6.95 | <0.001 |
| Site: SB | 4.88 | 2.90–8.20 | <0.001 | 0.87 | 0.49–1.54 | 0.63 | 1.67 | 0.94–2.97 | 0.079 |
| Site: ED | 0.97 | 0.43–2.21 | 0.946 | 7.96 | 1.51–41.97 | 0.014 | 4.95 | 0.38–65.04 | 0.224 |
| Seas.: Spring | 1.3 | 0.88–1.90 | 0.185 | 5.23 | 3.45–7.90 | <0.001 | 0.55 | 0.35–0.88 | 0.012 |
| Seas.: Summer | 2.26 | 1.46–3.49 | <0.001 | 1.47 | 0.92–2.33 | 0.103 | 2.76 | 1.71–4.47 | <0.001 |
| Seas.: Winter | 0.27 | 0.05–1.36 | 0.113 | 0.03 | 0.01–0.17 | <0.001 | 0 | 0.00–Inf | 0.995 |
| Ran. Eff. | | | | | | | | | |
| SD Intercept | 0.08 | 0.77 | 1.95 |
| Observations | 775 | 775 | 775 |
Table 12.
Regression models for the species Bleak (Alburnus alburnus), Nase (Chondrostoma nasus), Bream (Abramis brama) and Chub (Squalius cephalus); 1-h data and all study sites.
Table 12.
Regression models for the species Bleak (Alburnus alburnus), Nase (Chondrostoma nasus), Bream (Abramis brama) and Chub (Squalius cephalus); 1-h data and all study sites.
| | Bleak 1-h Glmm | Nase 1-h Glmm | Bream1-h Glmm | Chub 1-h Glmm |
|---|
| Predictors | IRR | CI | p-Value | IRR | CI | p-Value | IRR | CI | p-Value | IRR | CI | p-Value |
|---|
| (Intercept) | 0.11 | 0.02–0.67 | 0.016 | 0.64 | 0.01–52.16 | 0.842 | 0 | 0.00–0.03 | <0.001 | 0.02 | 0.01–0.09 | <0.001 |
| Qd | 0.99 | 0.97–1.02 | 0.539 | 0.62 | 0.53–0.74 | <0.001 | 0.93 | 0.90–0.96 | <0.001 | 0.95 | 0.92–0.99 | 0.004 |
| Qf | 0.88 | 0.82–0.93 | <0.001 | 1.19 | 0.90–1.57 | 0.228 | 0.93 | 0.85–1.03 | 0.149 | 0.94 | 0.84–1.05 | 0.245 |
| Qa | 1 | 1.00–1.01 | 0.492 | 0 | 0.00–0.00 | <0.001 | 1 | 0.99–1.00 | 0.41 | 0.98 | 0.97–0.99 | 0.002 |
| tres | 1.76 | 1.65–1.87 | <0.001 | 1.74 | 1.34–2.26 | <0.001 | 1.35 | 1.23–1.48 | <0.001 | 1.38 | 1.25–1.52 | <0.001 |
| tdiff | 4.76 | 3.74–6.06 | <0.001 | 7.22 | 3.25–16.05 | <0.001 | 3.59 | 2.73–4.72 | <0.001 | 2.07 | 1.51–2.84 | <0.001 |
| Site: SB | 2.66 | 2.05–3.44 | <0.001 | 0.05 | 0.02–0.14 | <0.001 | 2.91 | 1.84–4.61 | <0.001 | 1.05 | 0.64–1.73 | 0.845 |
| Site: ED | 7.12 | 0.54–93.32 | 0.135 | 0.01 | 0.00–1.55 | 0.071 | 27.4 | 1.37–549 | 0.03 | 1.85 | 0.75–4.55 | 0.181 |
| Seas.: Spring | 6.19 | 4.65–8.23 | <0.001 | 33.42 | 13.88–80.46 | <0.001 | 4.24 | 3.02–5.95 | <0.001 | 5.49 | 3.76–8.02 | <0.001 |
| Seas.: Summer | 4.4 | 3.28–5.90 | <0.001 | 1.28 | 0.45–3.59 | 0.645 | 2.34 | 1.46–3.76 | <0.001 | 2.43 | 1.50–3.93 | <0.001 |
| Seas.: Winter | 0 | 0.00–Inf | 0.985 | 11.27 | 1.21–104.88 | 0.033 | 0 | 0.00–Inf | 0.993 | 0 | 0.00–Inf | 0.993 |
| Ran. Eff. | | | | | | | | | | | | |
| SD Intercept | 2.04 | 7.38 | 2.70 | 0.20 |
| Observations | 18,456 | 18,456 | 18,456 | 18,456 |
Table 13.
Regression models for the species Bleak (Alburnus alburnus), Nase (Chondrostoma nasus), Bream (Abramis brama) and Chub (Squalius cephalus); 24-h data and all study sites.
Table 13.
Regression models for the species Bleak (Alburnus alburnus), Nase (Chondrostoma nasus), Bream (Abramis brama) and Chub (Squalius cephalus); 24-h data and all study sites.
| | Bleak 24-h Glmm | Nase 24-h Glmm | Bream 24-h Glmm | Chub 24-h Glmm |
|---|
| Predictors | IRR | CI | p-Value | IRR | CI | p-Value | IRR | CI | p-Value | IRR | CI | p-Value |
|---|
| (Intercept) | 33.88 | 1.72–667.09 | 0.021 | 2513.7 | 0.20–3×106 | 0.103 | 0.33 | 0.01–17.04 | 0.58 | 0.57 | 0.08–4.32 | 0.588 |
| Qd | 0.86 | 0.78–0.96 | 0.007 | 0.44 | 0.25–0.79 | 0.005 | 0.75 | 0.66–0.87 | <0.001 | 1.02 | 0.95–1.10 | 0.567 |
| Qf | 0.76 | 0.59–0.98 | 0.034 | 1.09 | 0.58–2.06 | 0.79 | 1.04 | 0.77–1.39 | 0.818 | 0.88 | 0.74–1.05 | 0.168 |
| Qa | 0.98 | 0.00–519 | 0.994 | 0 | 0.00–0.47 | 0.044 | 0 | 0.00–2.29 | 0.083 | 9.65 | 0.15–613 | 0.285 |
| tres | 1.59 | 1.35–1.86 | <0.001 | 1.66 | 0.97–2.84 | 0.066 | 1.33 | 1.14–1.55 | <0.001 | 1.19 | 1.07–1.33 | 0.001 |
| tdiff | 2.65 | 1.14–6.20 | 0.024 | 505.69 | 6.90–3×103 | 0.004 | 3.31 | 1.62–6.78 | 0.001 | 1.34 | 0.90–1.98 | 0.146 |
| Site: SB | 1.99 | 0.95–4.16 | 0.068 | 0.04 | 0.01–0.23 | <0.001 | 2.84 | 1.10–7.37 | 0.032 | 0.96 | 0.51–1.80 | 0.905 |
| Site: ED | 11.17 | 0.81–153 | 0.071 | 0 | 0.00–0.13 | 0.009 | 33.91 | 1.31–881 | 0.034 | 2.64 | 1.23–5.66 | 0.013 |
| Seas.: Spring | 9.27 | 4.46–19.25 | <0.001 | 59.01 | 9.76–356.75 | <0.001 | 7.45 | 3.95–14.03 | <0.001 | 3.49 | 2.26–5.42 | <0.001 |
| Seas.: Summer | 9.4 | 4.38–20.21 | <0.001 | 6.41 | 0.65–62.91 | 0.111 | 6.5 | 2.91–14.50 | <0.001 | 1.77 | 0.99–3.15 | 0.053 |
| Seas.: Winter | 0 | 0.00–Inf | 0.993 | 15.4 | 0.39–605.60 | 0.144 | 0 | 0.00–Inf | 0.996 | 0 | 0.00–Inf | 0.996 |
| Ran. Eff. | | | | | | | | | | | | |
| SD Intercept | 1.97 | 5.13 | 3.09 | 0.11 |
| Observations | 775 | 775 | 775 | 775 |