Groundwater Nitrate Removal Performance of Selected Pseudomonas Strains Carrying nosZ Gene in Aerobic Granular Sequential Batch Reactors
Abstract
:1. Introduction
2. Materials and Methods
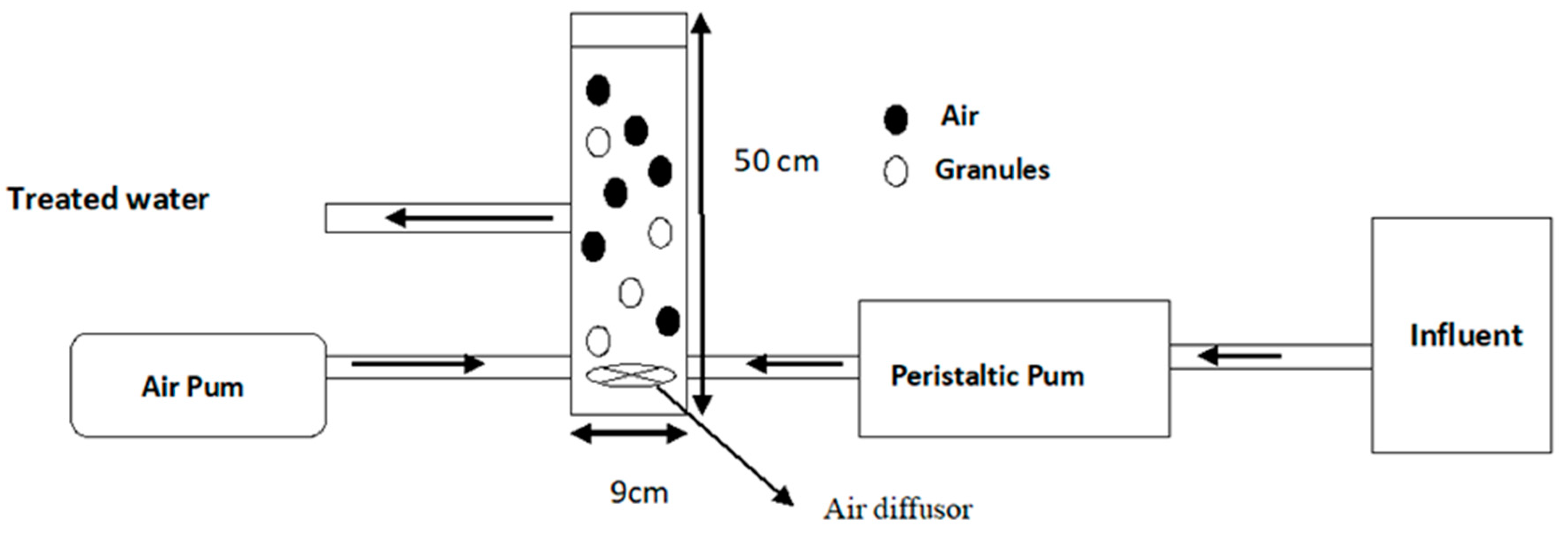
2.1. Design and Operating Conditions
2.2. Physical Determination
2.3. Chemical Determination
2.4. Mass Balance of Pollutants for All Bioreactors during the Operational Period
3. Results and Discussion
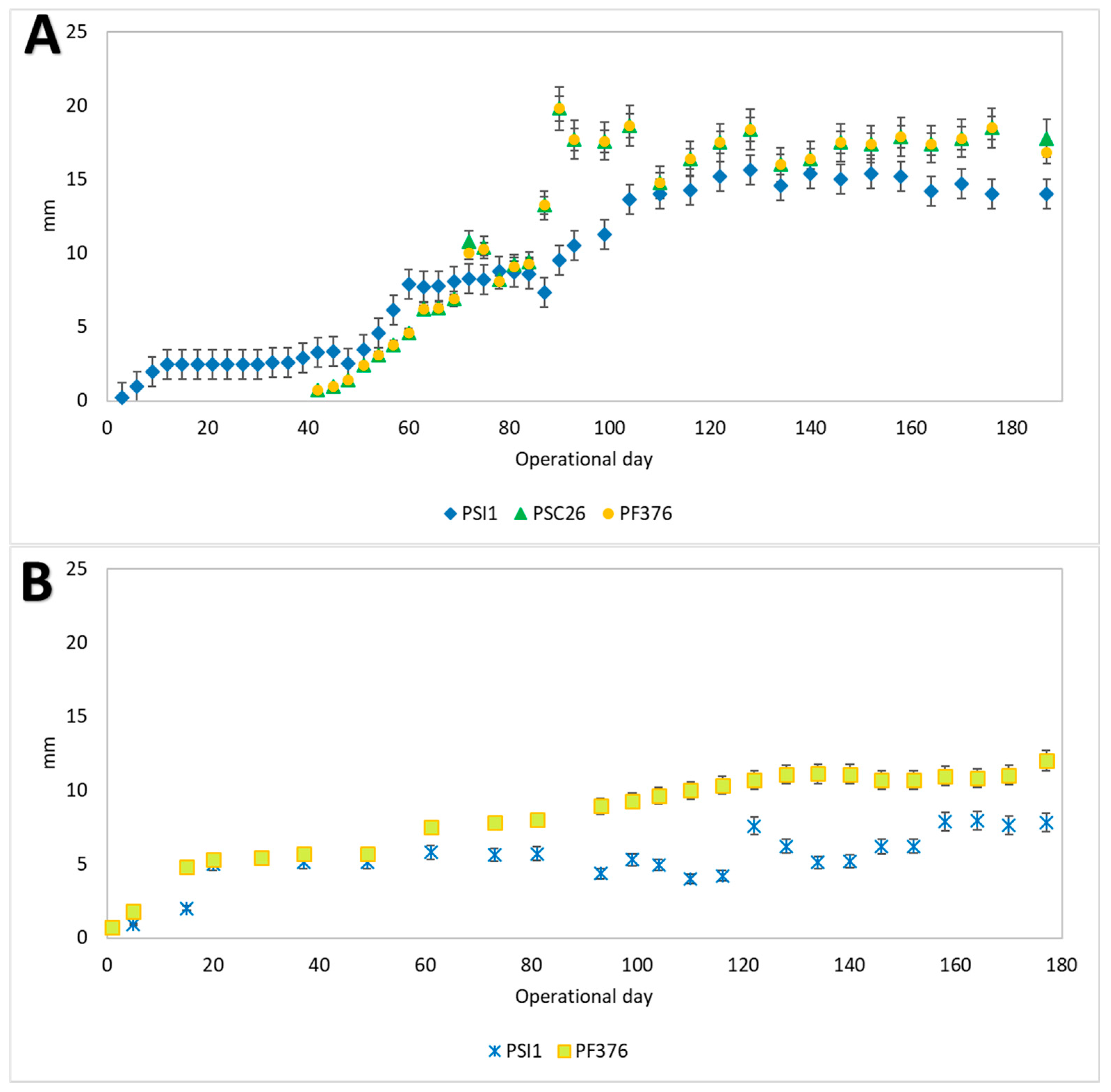
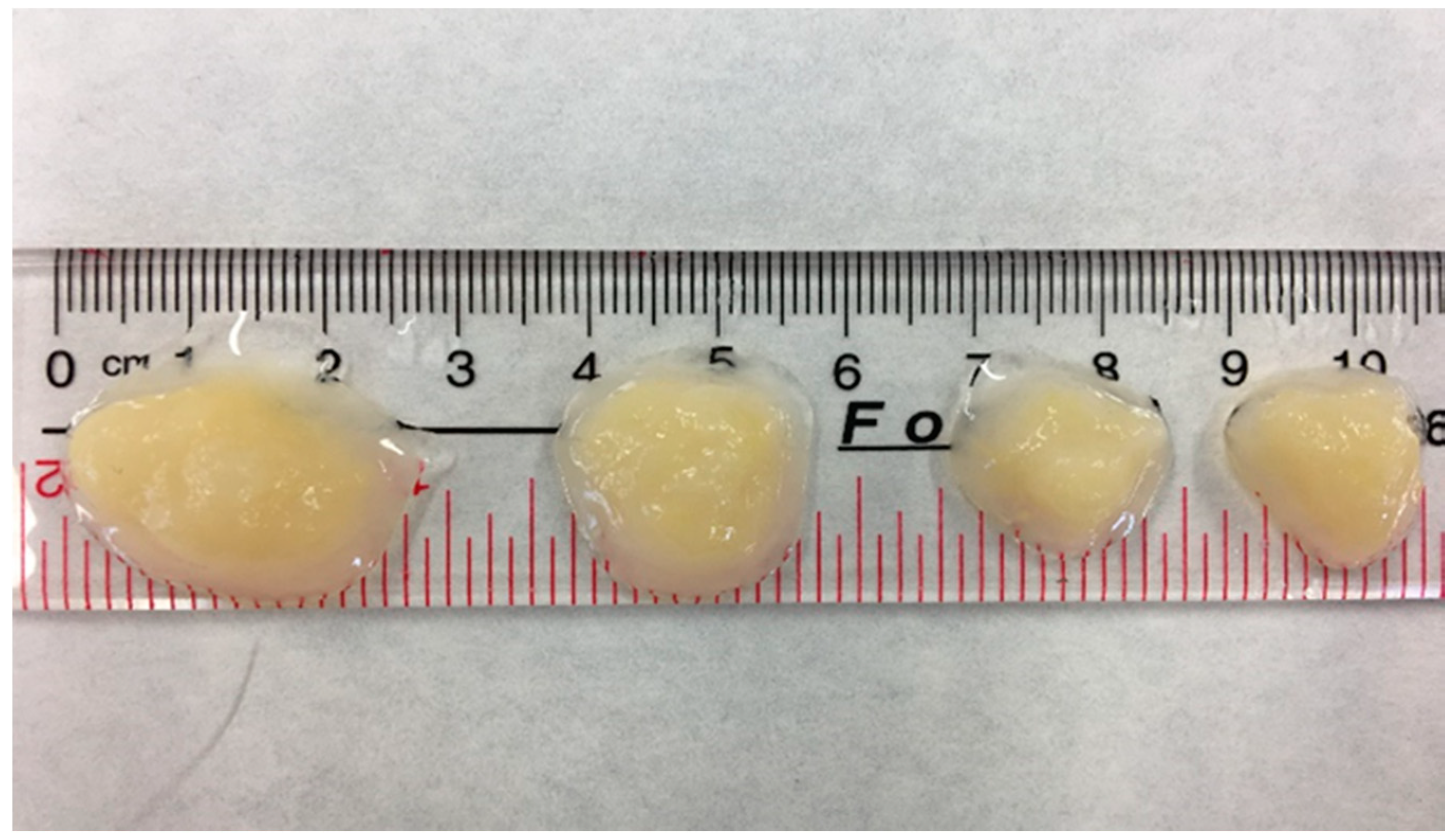
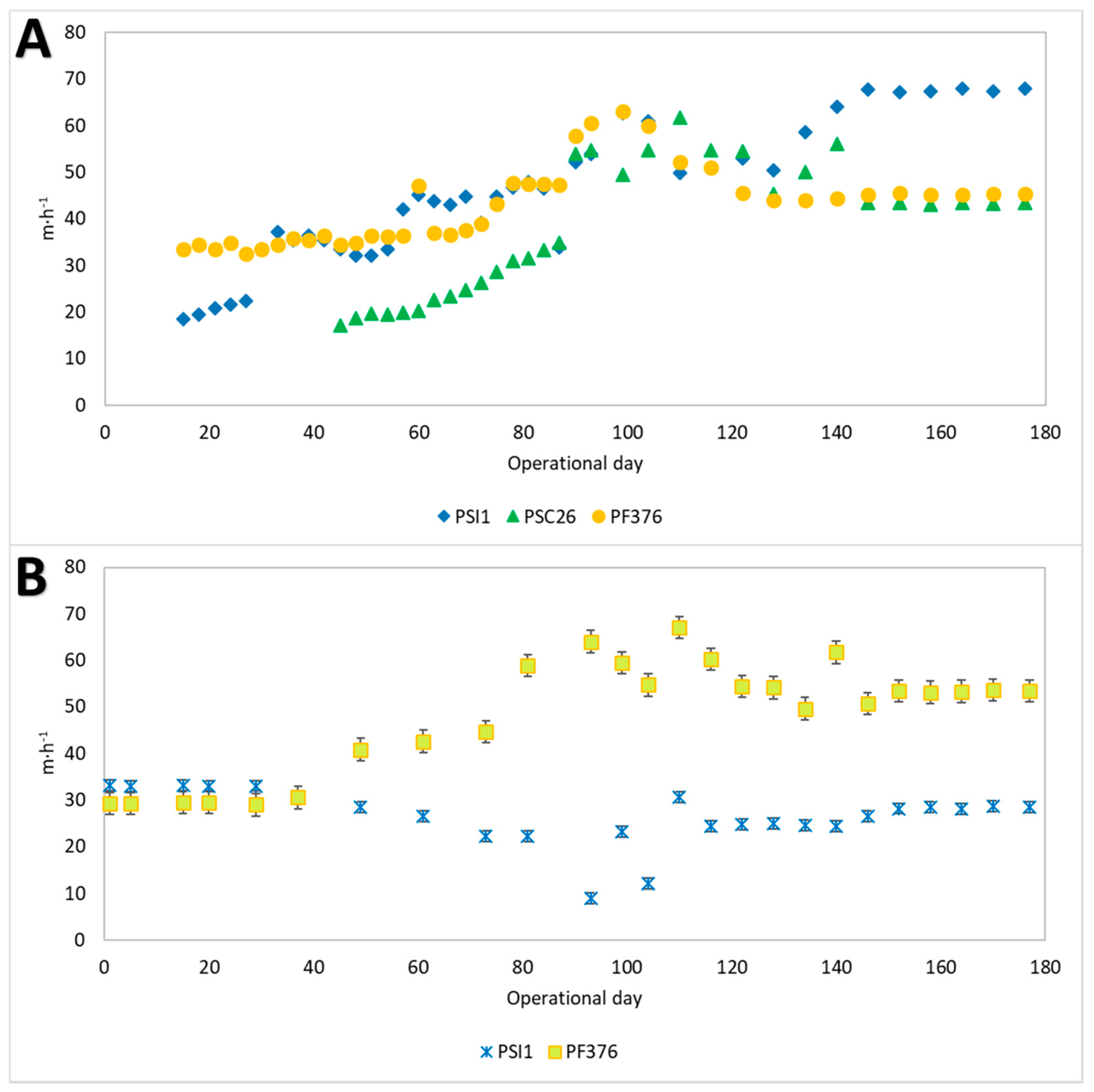
3.1. Granulation Processes and Bioreactor Start-Up
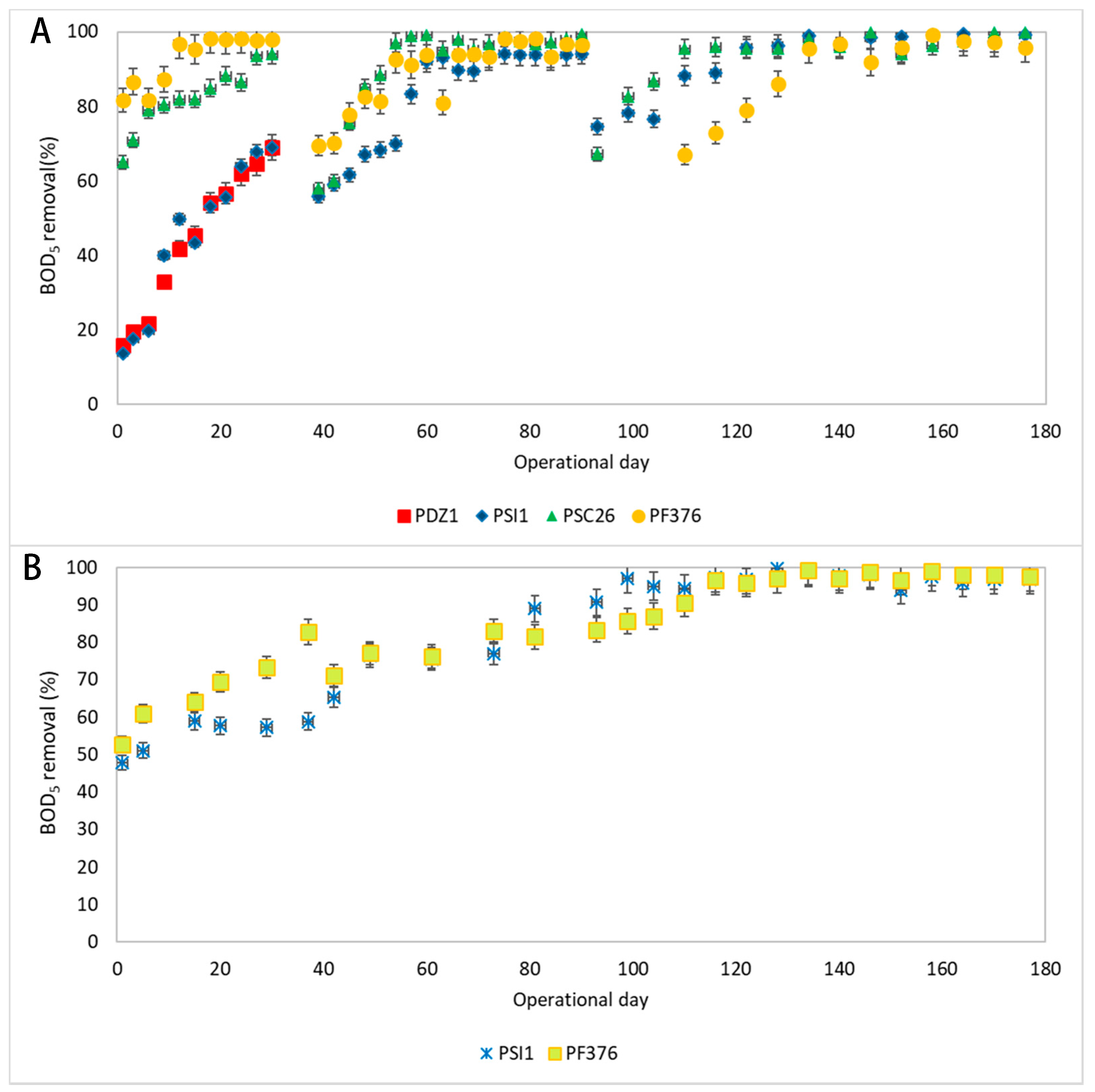
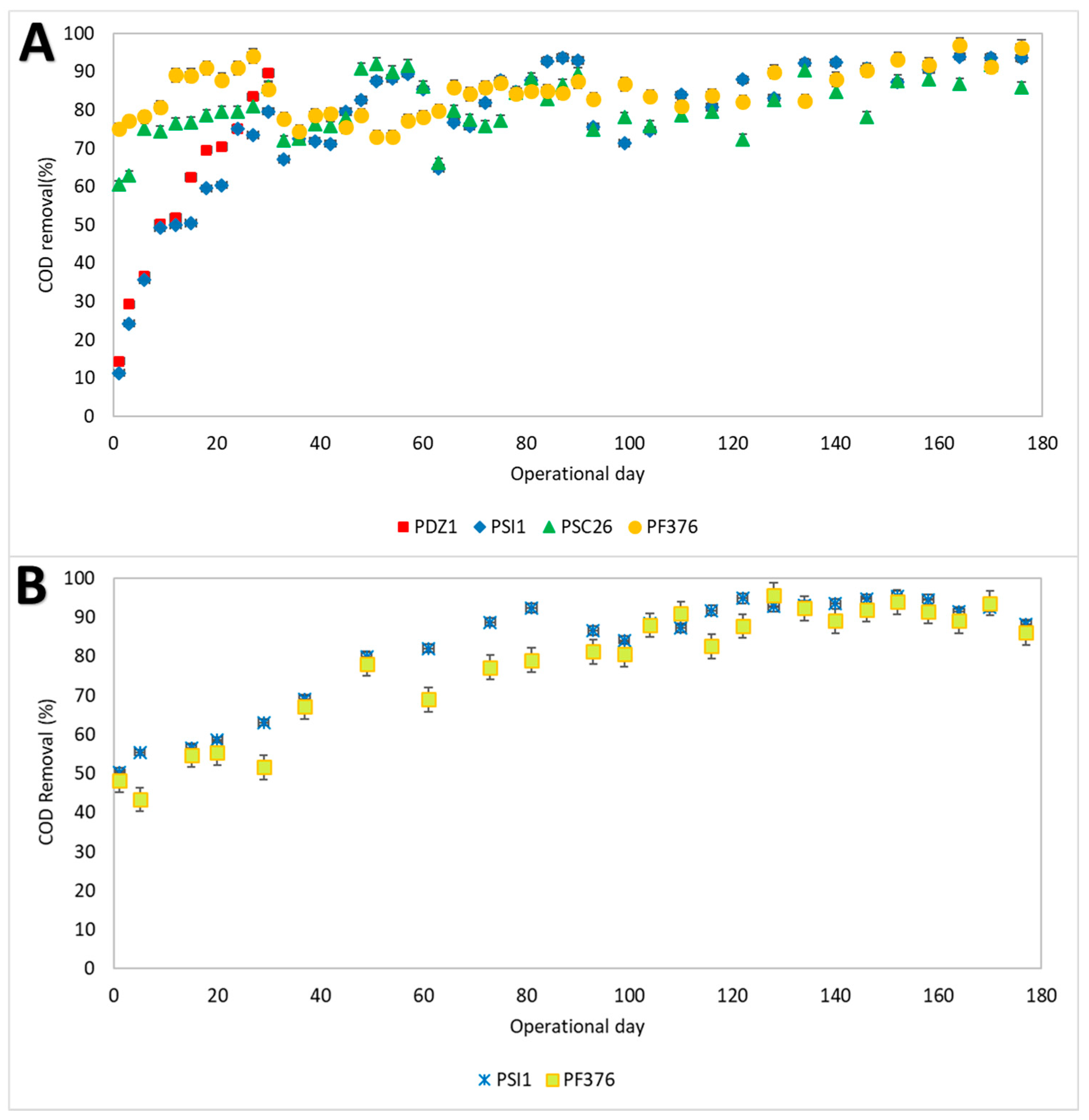
3.2. BOD5 and COD Degradation
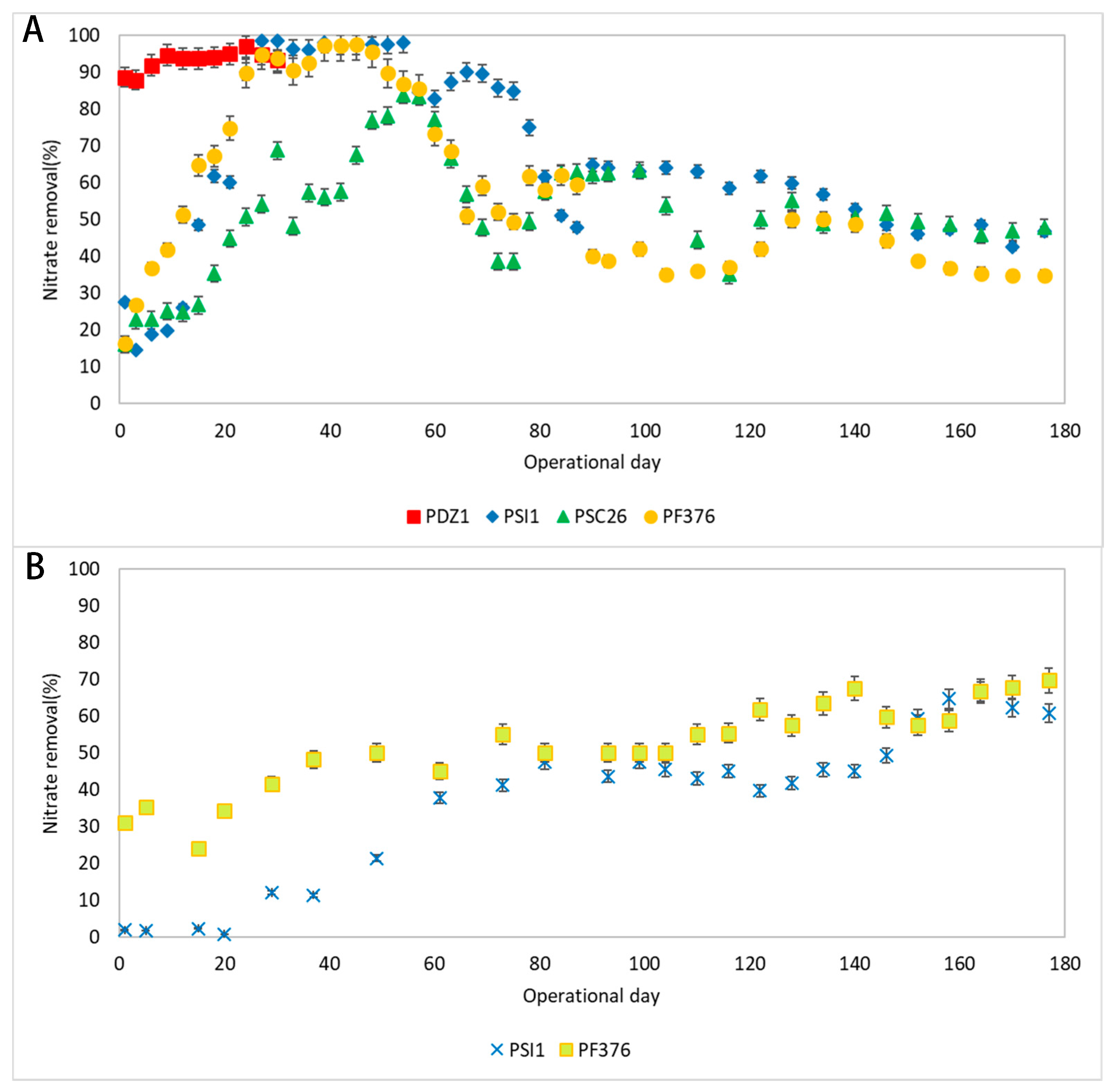
3.3. Nitrate Removal Performance
3.4. Nitrogen Mass Balance over the Period of Operation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mohseni-Bandpi, A.; Elliott, D.J.; Zazouli, M.A. Biological nitrate removal processes from drinking water supply-a review. J. Environ. Health Sci. Eng. 2013, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, F.; Sarrafzadeh, M.-H.; Ebrahimi, S.; Oh, H.-M. Nitrate removal from drinking water with a focus on biological methods: A review. Environ. Sci. Pollut. Res. 2019, 26, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Martinez, M.; Muñoz-Palazon, B.; Robles-Arenas, V.M.; Gonzalez-Martinez, A.; Gonzalez-Lopez, J. Biological nitrate removal from groundwater by an aerobic granular technology to supply drinking water at pilot-scale. J. Water Process. Eng. 2021, 40, 101786. [Google Scholar] [CrossRef]
- Coss, A.; Cantor, K.P.; Reif, J.S.; Lynch, C.F.; Ward, M.H. Pancreatic Cancer and Drinking Water and Dietary Sources of Nitrate and Nitrite. Am. J. Epidemiol. 2004, 159, 693–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brender, J.D.; Weyer, P.J.; Romitti, P.A.; Mohanty, B.P.; Shinde, M.U.; Vuong, A.M.; Huber, J.C., Jr. Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the national birth defects prevention study. Environ. Health Perspect. 2013, 121, 1083–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Environment Agency (EEA). Groundwater Nitrate. Available online: www.eea.europa.eu/: (accessed on 4 December 2020).
- European Union (EU). Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources; European Union (EU): Brussels, Belgium, 1991. [Google Scholar]
- Vitoria, I.; Maraver, F.; Sanchez-Valverde, F.; Armijo, F. Nitrate concentrations in tap water in Spain. Gac. Sanit. 2015, 29, 217. [Google Scholar] [CrossRef] [Green Version]
- Eljamal, R.; Eljamal, O.; Maamoun, I.; Yilmaz, G.; Sugihara, Y. Enhancing the characteristics and reactivity of nZVI: Polymers effect and mechanisms. J. Mol. Liq. 2020, 315, 113714. [Google Scholar] [CrossRef]
- Martínez, J.; Ortiz, A.; Ortiz, I. State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Appl. Catal. B Environ. 2017, 207, 42–59. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, A.S.; Wang, A.; Condit, W.E.; Battelle, C.; Sorg, T.J.; Supply, W. Arsenic and Nitrate Removal from Drinking Water by Ion Exchange US EPA Demonstration Project at Vale. OR Final Performance Evaluation Report; National Risk Management Research Laboratory: Cincinnati, OH, USA, 2011.
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs. benefits. and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef] [Green Version]
- Epsztein, R.; Nir, O.; Lahav, O.; Green, M. Selective nitrate removal from groundwater using a hybrid nanofiltration–reverse osmosis filtration scheme. Chem. Eng. J. 2015, 279, 372–378. [Google Scholar] [CrossRef]
- Zeng, H.; Yin, C.; Zhang, J.; Li, D. Start-Up of a Biofilter in a Full-Scale Groundwater Treatment Plant for Iron and Manganese Removal. Int. J. Environ. Res. Public Health 2019, 16, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirsaheb, M.; Khosravi, T.; Sharafi, K.; Mouradi, M. Comparing operational cost and performance evaluation of electrodialysis and reverse osmosis systems in nitrate removal from drinking water in Golshahr. Mashhad. Desalin. Water Treat. 2015, 57, 5391–5397. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Panepinto, D.; Genon, G.; Borsarelli, A. Improvement of nitrogen removal in a large municipal wastewater plant. Chem. Eng. Trans. 2013, 34, 67–72. [Google Scholar]
- Corsino, S.F.; Capodici, M.; Di Pippo, F.; Tandoi, V.; Torregrossa, M. Comparison between kinetics of autochthonous marine bacteria in activated sludge and granular sludge systems at different salinity and SRTs. Water Res. 2019, 148, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.; Reddy, G.K.K. Aerobic granular sludge technology: Mechanisms of granulation and biotechnological applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- Rusanowska, P.; Cydzik-Kwiatkowska, A.; Świątczak, P.; Wojnowska-Baryła, I. Changes in extracellular polymeric substances (EPS) content and composition in aerobic granule size-fractions during reactor cycles at different organic loads. Bioresour. Technol. 2019, 272, 188–193. [Google Scholar] [CrossRef]
- Eljamal, R.; Kahraman, I.; Eljamal, O.; Thompson, I.P.; Maamoun, I.; Yilmaz, G. Impact of nZVI on the formation of aerobic granules. bacterial growth and nutrient removal using aerobic sequencing batch reactor. Environ. Technol. Innov. 2020, 19, 100911. [Google Scholar] [CrossRef]
- Lycus, P.; Soriano-Laguna, M.J.; Kjos, M.; Richardson, D.J.; Gates, A.J.; Milligan, D.A.; Frostegård, Å.; Bergaust, L.; Bakken, L.R. A bet-hedging strategy for denitrifying bacteria curtails their release of N2O. Proc. Natl. Acad. Sci. USA 2018, 115, 11820–11825. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Palazon, B.; Rodriguez-Sanchez, A.; Hurtado-Martinez, M.; Santana, F.; Gonzalez-Lopez, J.; Mack, L.; Gonzalez-Martinez, A. Polar Arctic Circle biomass enhances performance and stability of aerobic granular sludge systems operated under different temperatures. Bioresour. Technol. 2020, 300, 122650. [Google Scholar] [CrossRef]
- Wu, L.; Peng, L.; Wei, W.; Wang, D.; Ni, B.-J. Nitrous oxide production from wastewater treatment: The potential as energy resource rather than potent greenhouse gas. J. Hazard. Mater. 2020, 387, 121694. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Cao, S.; Li, B.; Wang, S.; Niu, M. Mechanisms and microbial structure of partial denitrification with high nitrite accumulation. Appl. Microbiol. Biotechnol. 2016, 100, 2011–2021. [Google Scholar] [CrossRef]
- Andrade, G.; Esteban, E.; Velasco, L.; Lorite, M.J.; Bedmar, E.J. Isolation and identification of N2-fixing microorganisms from the rhizosphere of Capparis spinosa (L.). Plant Soil 1997, 197, 19–23. [Google Scholar] [CrossRef]
- Wragg, J.; Harrison, H.; West, J.M.; Yoshikawa, H. Comparison of microbiological influences on the transport properties of intact mudstone and sandstone and its relevance to the geological disposal of radioactive waste. Miner. Mag. 2012, 76, 3251–3259. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Jiang, Y.; Chang, Z.; Wang, J.; Song, X.; Huang, Z.; Chen, S.; Li, J. Denitrification characteristics and pathways of a facultative anaerobic denitrifying strain. Pseudomonas denitrificans G1. J. Biosci. Bioeng. 2020, 129, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Palazon, B.; Rodriguez-Sanchez, A.; Hurtado-Martinez, M.; Gonzalez-Lopez, J.; Pfetzing, P.; Gonzalez-Martinez, A. Performance and microbial community structure of aerobic granular bioreactors at different operational temperature. J. Water Process. Eng. 2020, 33, 101110. [Google Scholar] [CrossRef]
- Laguna, A.; Ouattara, A.; Gonzalez, R.O.; Baron, O.; Fama, G.; El Mamouni, R.; Macarie, H. A simple and low cost technique for determining the granulometry of upflow anaerobic sludge blanket reactor sludge. Water Sci. Technol. 1999, 40, 1–8. [Google Scholar] [CrossRef]
- American Public Health Association. APHA Standard Methods for the Examination of Water and Wastewatern, 21st ed.; APHA-AWWWA-WEF: Washington, DC, USA, 2012. [Google Scholar]
- González-Martínez, A.; Calderón, K.; Albuquerque, A.; Hontoria, E.; González-López, J.; Guisado, I.M.; Osorio, F. Biological and technical study of a partial-SHARON reactor at laboratory scale: Effect of hydraulic retention time. Bioprocess Biosyst. Eng. 2012, 36, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, A.; Muñoz-Palazon, B.; Hurtado-Martinez, M.; Mikola, A.; Gonzalez-Lopez, J.; Vahala, R.; Gonzalez-Martinez, A. Analysis of microbial communities involved in organic matter and nitrogen removal in a full-scale moving bed biofilm reactor located near the Polar Arctic Circle. Int. Biodeterior. Biodegrad. 2020, 146, 104830. [Google Scholar] [CrossRef]
- Wisniewski, K.; Kowalski, M.; Makinia, J. Modeling nitrous oxide production by a denitrifying-enhanced biologically phosphorus removing (EBPR) activated sludge in the presence of different carbon sources and electron acceptors. Water Res. 2018, 142, 55–64. [Google Scholar] [CrossRef]
- Directive, W.F. Water Framework Directive. J. Ref. OJL 2000, 327, 1–73. [Google Scholar]
- Tian, T.; Yu, H.-Q. Denitrification with non-organic electron donor for treating low C/N ratio wastewaters. Bioresour. Technol. 2020, 299, 122686. [Google Scholar] [CrossRef] [PubMed]
- Eljamal, O.; Jinno, K.; Hosokawa, T. Denitrification of secondary wastewater using sawdust. Mem. Fac. Eng. 2006, 66, 115–128. [Google Scholar]
- Mokete, R.; Eljamal, O.; Sugihara, Y. Exploration of the reactivity of nanoscale zero-valent iron (NZVI) associated nanoparticles in diverse experimental conditions. Chem. Eng. Process. Process. Intensif. 2020, 150, 107879. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Song, X. High-effective denitrification of low C/N wastewater by combined constructed wetland and biofilm-electrode reactor (CW–BER). Bioresour. Technol. 2016, 203, 245–251. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Zhou, L. Effect of carbon source. C/N ratio. nitrate and dissolved oxygen concentration on nitrite and ammonium production from denitrification process by Pseudomonas stutzeri D6. Bioresour. Technol. 2012, 104, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Feng, L.; Li, A.; Zhang, X.; Yang, J.; Ma, F. Ammonium assimilation: An important accessory during aerobic denitrification of Pseudomonas stutzeri T13. Bioresour. Technol. 2017, 234, 264–272. [Google Scholar] [CrossRef]
- Miyahara, M.; Kim, S.-W.; Fushinobu, S.; Takaki, K.; Yamada, T.; Watanabe, A.; Miyauchi, K.; Endo, G.; Wakagi, T.; Shoun, H. Potential of Aerobic Denitrification by Pseudomonas stutzeri TR2 To Reduce Nitrous Oxide Emissions from Wastewater Treatment Plants. Appl. Environ. Microbiol. 2010, 76, 4619–4625. [Google Scholar] [CrossRef] [Green Version]
- Vacková, L.; Srb, M.; Stloukal, R.; Wanner, J. Comparison of denitrification at low temperature using encapsulated Paracoccus denitrificans. Pseudomonas fluorescens and mixed culture. Bioresour. Technol. 2011, 102, 4661–4666. [Google Scholar] [CrossRef]
- Hallin, S.; Philippot, L.; Löffler, F.E.; Sanford, R.A.; Jones, C.M. Genomics and Ecology of Novel N2O-Reducing Microorganisms. Trends Microbiol. 2018, 26, 43–55. [Google Scholar] [CrossRef] [PubMed]

| Operational Day | Sodium Acetate | Sodium Nitrate | Methanol |
|---|---|---|---|
| (mg·L−1) | |||
| 0–30 | 900 | 127 | 300 |
| 31–60 | 400 | 127 | 300 |
| 61–90 | 300 | 100 | 300 |
| 91–180 | 200 | 100 | 200 |
| Strain | Phase | TNin | TNout | NO3−Nin | NO3−Nout | NO2−Nout | NO, N2O, N2 Gas | Rem Perform (%) | |
|---|---|---|---|---|---|---|---|---|---|
| P. fluorescens PSC26 | NaAc:NaNO3 | 900:127 | 130.75 | 88.32 | 130.75 | 88.27 | 0.05 | 42.44 | 32.45 |
| 400:127 | 130.75 | 23.50 | 130.75 | 23.50 | 0.00 | 107.25 | 82.02 | ||
| 300:100 | 102.95 | 53.28 | 102.95 | 53.25 | 0.03 | 49.67 | 48.25 | ||
| 200:100 | 102.95 | 37.80 | 102.95 | 37.79 | 0.00 | 65.16 | 63.29 | ||
| Average | 116.85 | 50.72 | 116.85 | 50.70 | 0.02 | 66.13 | |||
| St dev. | 16.05 | 27.85 | 16.05 | 27.83 | 0.02 | 29.00 | |||
| P. fluorescens 376 | NaAc:NaNO3 | 900:127 | 130.75 | 14.11 | 130.75 | 14.11 | 0.00 | 116.64 | 89.21 |
| 400:127 | 130.75 | 20.31 | 130.75 | 20.31 | 0.00 | 110.44 | 84.46 | ||
| 300:100 | 102.95 | 37.79 | 102.95 | 37.79 | 0.00 | 65.16 | 63.29 | ||
| 200:100 | 102.95 | 44.50 | 102.95 | 44.50 | 0.00 | 58.45 | 56.78 | ||
| CH3OH:NaNO3 | 300:100 | 102.95 | 63.51 | 102.95 | 63.51 | 0.00 | 39.44 | 38.31 | |
| 200:100 | 102.95 | 35.59 | 102.95 | 35.59 | 0.00 | 67.36 | 65.43 | ||
| Average | 112.22 | 35.97 | 112.22 | 35.97 | 0.00 | 76.25 | |||
| St dev. | 14.35 | 17.65 | 14.35 | 17.65 | 0.00 | 30.57 | |||
| P. stutzeri | NaAc:NaNO3 | 900:127 | 130.75 | 4.39 | 130.75 | 4.34 | 0.05 | 126.36 | 96.64 |
| 400:127 | 130.75 | 24.16 | 130.75 | 24.16 | 0.00 | 106.59 | 81.52 | ||
| 300:100 | 102.95 | 49.74 | 102.95 | 49.73 | 0.00 | 53.22 | 51.69 | ||
| 200:100 | 102.95 | 34.79 | 102.95 | 34.79 | 0.00 | 68.16 | 66.21 | ||
| CH3OH:NaNO3 | 300:100 | 102.95 | 34.79 | 102.95 | 34.79 | 0.00 | 68.16 | 66.21 | |
| 200:100 | 102.95 | 58.17 | 102.95 | 58.17 | 0.00 | 44.78 | 43.50 | ||
| Average | 112.22 | 34.34 | 112.22 | 34.33 | 0.01 | 77.88 | |||
| St dev. | 13.10 | 17.35 | 13.10 | 17.36 | 0.02 | 29.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurtado-Martinez, M.; Muñoz-Palazon, B.; Gonzalez-Martinez, A.; Manzanera, M.; Gonzalez-Lopez, J. Groundwater Nitrate Removal Performance of Selected Pseudomonas Strains Carrying nosZ Gene in Aerobic Granular Sequential Batch Reactors. Water 2021, 13, 1119. https://doi.org/10.3390/w13081119
Hurtado-Martinez M, Muñoz-Palazon B, Gonzalez-Martinez A, Manzanera M, Gonzalez-Lopez J. Groundwater Nitrate Removal Performance of Selected Pseudomonas Strains Carrying nosZ Gene in Aerobic Granular Sequential Batch Reactors. Water. 2021; 13(8):1119. https://doi.org/10.3390/w13081119
Chicago/Turabian StyleHurtado-Martinez, Miguel, Barbara Muñoz-Palazon, Alejandro Gonzalez-Martinez, Maximino Manzanera, and Jesus Gonzalez-Lopez. 2021. "Groundwater Nitrate Removal Performance of Selected Pseudomonas Strains Carrying nosZ Gene in Aerobic Granular Sequential Batch Reactors" Water 13, no. 8: 1119. https://doi.org/10.3390/w13081119
APA StyleHurtado-Martinez, M., Muñoz-Palazon, B., Gonzalez-Martinez, A., Manzanera, M., & Gonzalez-Lopez, J. (2021). Groundwater Nitrate Removal Performance of Selected Pseudomonas Strains Carrying nosZ Gene in Aerobic Granular Sequential Batch Reactors. Water, 13(8), 1119. https://doi.org/10.3390/w13081119










