Behavioral Parameters of Planarians (Girardia tigrina) as Fast Screening, Integrative and Cumulative Biomarkers of Environmental Contamination: Preliminary Results
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Water Analysis
2.3. Planarians
Development and Design Test of Behavioral Responses
2.4. Statistical Analysis
3. Results
3.1. Sampling Sites
3.2. Feeding Rate
3.3. Planarian Locomotor Velocity (pLMV)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Companhia Nacional de Abastecimento (CONAB). Acompanhamento Safra Brasileira Grãos, v. 7—Safra 2019/20, n. 8—Oitavo Levantamento; Conab: Brasília, Brazil, 2020. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 10 June 2020).
- Companhia Nacional de Abastecimento (CONAB). Acompanhamento Safra Brasileira de Cana de Açúcar, v. 7—Safra 2019/20, n. 1—Primeiro Levantamento; Conab: Brasília, Brazil, 2020. Available online: https://www.conab.gov.br/info-agro/safras/cana/boletim-da-safra-de-cana-de-acucar (accessed on 10 June 2020).
- Tschoeke, P.H.; Oliveira, E.E.; Dalcin, M.S.; Silveira-Tschoeke, M.C.A.; Sarmento, R.A.; Santos, G.R. Botanical and synthetic pesticides alter the flower visitation rates of pollinator bees in Neotropical melon fields. Environ. Pollut. 2019, 251, 591–599. [Google Scholar] [CrossRef]
- De Lima, C.H.D.O.; Sarmento, R.A.; Pereira, P.S.; Ribeiro, A.V.; Souza, D.J.; Picanço, M.C. Economic injury levels and sequential sampling plans for control decision-making systems of Bemisia tabacibiotype B adults in watermelon crops. Pest. Manag. Sci. 2019, 75, 998–1005. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, L.; Ren, Y.; Wang, X. Rice black-streaked dwarf virus: From multiparty interactions among plant–virus–vector to intermittent epidemics. Mol. Plant. Pathol. 2020, 21, 1007–1019. [Google Scholar] [CrossRef]
- Sikes, B.A.; Bufford, J.L.; Hulme, P.E.; Cooper, J.A.; Johnston, P.R.; Duncan, R.P. Import volumes and biosecurity interventions shape the arrival rate of fungal pathogens. PLoS Biol. 2018, 16, e2006025. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. The history and current status of glyphosate. Pest. Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Wesseler, J. Perspective: Regulation of pest and disease control strategies and why (many) economists are concerned. Pest. Manag. Sci. 2018, 75, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, J.E.; Gibson, K.A.; Seiber, J.N. Pesticides and Related Toxicants in the Atmosphere. Residue Rev. 2018, 247, 147–196. [Google Scholar] [CrossRef]
- Béranger, R.; Billoir, E.; Nuckols, J.R.; Blain, J.; Millet, M.; Bayle, M.-L.; Combourieu, B.; Philip, T.; Schüz, J.; Fervers, B. Agricultural and domestic pesticides in house dust from different agricultural areas in France. Environ. Sci. Pollut. Res. 2019, 26, 19632–19645. [Google Scholar] [CrossRef] [PubMed]
- Saaristo, M.; Brodin, T.; Balshine, S.; Bertram, M.G.; Brooks, B.W.; Ehlman, S.M.; McCallum, E.S.; Sih, A.; Sundin, J.; Wong, B.B.M.; et al. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. R. Soc. B Boil. Sci. 2018, 285, 20181297. [Google Scholar] [CrossRef]
- Gunarathna, S.; Gunawardana, B.; Jayaweera, M.; Manatunge, J.; Zoysa, K. Glyphosate and AMPA of agricultural soil, surface water, groundwater and sediments in areas prevalent with chronic kidney disease of unknown etiology, Sri Lanka. J. Environ. Sci. Heal. Part. B 2018, 53, 729–737. [Google Scholar] [CrossRef]
- Santos, L.H.; Freixa, A.; Insa, S.; Acuña, V.; Sanchís, J.; Farré, M.; Sabater, S.; Barceló, D.; Rodríguez-Mozaz, S. Impact of fullerenes in the bioaccumulation and biotransformation of venlafaxine, diuron and triclosan in river biofilms. Environ. Res. 2019, 169, 377–386. [Google Scholar] [CrossRef]
- Ulrich, E.M.; Tenbrook, P.L.; McMillan, L.M.; Wang, Q.; Lao, W. Enantiomer-specific measurements of current-use pesticides in aquatic systems. Environ. Toxicol. Chem. 2017, 37, 99–106. [Google Scholar] [CrossRef]
- Bhateria, R.; Jain, D. Water quality assessment of lake water: A review. Sustain. Water Resour. Manag. 2016, 2, 161–173. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, F.; Li, F.; Chen, G.; Yang, G.; Wang, J.; Du, K.; Liu, S.; Li, Z. Ammonia nitrogen sources and pollution along soil profiles in an in-situ leaching rare earth ore. Environ. Pollut. 2020, 267, 115449. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.; Sarneel, J.M.; Willers, B.J.; Roelofs, J.G.; Verhoeven, J.T.; Lamers, L.P. Interacting effects of sulphate pollution, sulphide toxicity and eutrophication on vegetation development in fens: A mesocosm experiment. Environ. Pollut. 2009, 157, 2072–2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.; Cai, Z.; Chen, X. Environmental losses and driving forces of nitrogen flow in two agricultural towns of Hebei province during 1997–2017. Environ. Pollut. 2020, 264, 114636. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 103–125. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B., Cl). Curr. Opin. Plant. Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Zhu, Y.; Costa, M. Metals and molecular carcinogenesis. Carcinogenesis 2020, 41. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total. Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Loppi, S.; Monaci, F.; Paoli, L.; Vannini, A.; Sorbo, S.; Maresca, V.; Fusaro, L.; Karam, E.A.; Lentini, M.; et al. In-field and in-vitro study of the moss Leptodictyum riparium as bioindicator of toxic metal pollution in the aquatic environment: Ultrastructural damage, oxidative stress and HSP70 induction. PLoS ONE 2018, 13, e0195717. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, L.P.R.; Dornelas, A.S.P.; Vieira, M.M.; Ferreira, J.S.D.J.; Sarmento, R.A.; Cavallini, G.S. Comparative ecotoxicological evaluation of peracetic acid and the active chlorine of calcium hypochlorite: Use of Dugesia tigrina as a bioindicator of environmental pollution. Chemosphere 2019, 233, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Peakall, D.B.; Walker, C.H. The role of biomarkers in environmental assessment (3). Vertebrates. Ecotoxicology 1994, 3, 173–179. [Google Scholar] [CrossRef]
- Hyne, R.V.; Maher, W.A. Invertebrate biomarkers: Links to toxicosis that predict population decline. Ecotoxicol. Environ. Saf. 2003, 54, 366–374. [Google Scholar] [CrossRef]
- Vila-Farré, M.; Rink, J.C. The Ecology of Freshwater Planarians. Methods Mol. Biol. 2018, 1774, 173–205. [Google Scholar] [CrossRef]
- Reddien, P.W.; Alvarado, A.S. Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 2004, 20, 725–757. [Google Scholar] [CrossRef]
- Sheiman, I.M.; Zubina, E.V.; Kreshchenko, N.D. Regulation of the Feeding Behavior of the Planarian Dugesia (Girardia) tigrina. J. Evol. Biochem. Physiol. 2002, 38, 414–418. [Google Scholar] [CrossRef]
- Ivankovic, M.; Haneckova, R.; Thommen, A.; Grohme, M.A.; Vila-Farré, M.; Werner, S.; Rink, J.C. Model systems for regeneration: Planarians. Development 2019, 146, dev167684. [Google Scholar] [CrossRef]
- Saraiva, A.S.; Sarmento, R.A.; Golovko, O.; Randak, T.; Pestana, J.L.T.; Soares, A.M.V.M. Lethal and sub-lethal effects of cyproconazole on freshwater organisms: A case study with Chironomus riparius and Dugesia tigrina. Environ. Sci. Pollut. Res. 2018, 25, 12169–12176. [Google Scholar] [CrossRef] [PubMed]
- Dornelas, A.S.; Sarmento, R.A.; Saraiva, A.S.; Barbosa, R.S.; Vieira, M.M.; Gravato, C.; Soares, A.M. Effects of two biopesticides and salt on behaviour, regeneration and sexual reproduction of the freshwater planarian Girardia tigrina. J. Hazard. Mater. 2021, 404, 124089. [Google Scholar] [CrossRef]
- Saraiva, A.S.; Sarmento, R.A.; Gravato, C.; Rodrigues, A.C.; Campos, D.; Simão, F.C.; Soares, A.M. Strategies of cellular energy allocation to cope with paraquat-induced oxidative stress: Chironomids vs Planarians and the importance of using different species. Sci. Total. Environ. 2020, 741, 140443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, B.; Yi, H.; Zhao, B. Mortality and antioxidant responses in the planarian (Dugesia japonica) after exposure to copper. Toxicol. Ind. Heal. 2012, 30, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Guecheva, T.; Henriques, J.A.; Erdtmann, B. Genotoxic effects of copper sulphate in freshwater planarian in vivo, studied with the single-cell gel test (comet assay). Mutat. Res. Toxicol. Environ. Mutagen. 2001, 497, 19–27. [Google Scholar] [CrossRef]
- Calevro, F.; Filippi, C.; Deri, P.; Albertosi, C.; Batistoni, R. Toxic effects of aluminium, chromium and cadmium in intact and regenerating freshwater planarians. Chemosphere 1998, 37, 651–659. [Google Scholar] [CrossRef]
- Agência Nacional de Águas (ANA). Guia Nacional de Coleta e Preservação de Amostras: Água, Sedimento, Comunidades Aquáticas e Efluentes Líquidos; Agência Nacional de Águas: Brasília, Brazil, 2011; p. 326. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). Field Sampling Manual; Department of Environmental Protection: Trenton, NJ, USA, 2005; p. 574. [Google Scholar]
- American Standards for Testing and Materials (ASTM). Standard Practice for Conducting Acute Toxicity Tests with Fishes, Macroinvertebrates and Amphibians; American Standards for Testing and Materials: Philadelphia, PA, USA, 1980; p. 1980. [Google Scholar]
- Mori, M.; Narahashi, M.; Hayashi, T.; Ishida, M.; Kumagai, N.; Sato, Y.; Bagherzadeh, R.; Agata, K.; Inoue, T. Calcium ions in the aquatic environment drive planarians to food. Zool. Lett. 2019, 5, 1–15. [Google Scholar] [CrossRef]
- Fraps, M. Studies on Respiration and Glycolysis in Planaria. I. Methods and Certain Basic Factors in Respiration. Physiol. Zool. 1930, 3, 242–270. [Google Scholar] [CrossRef]
- Conama—Conselho Nacional do Meio Ambiente (National Environment Council), Brazil—Resolution No 357, of 17 March 2005 Published in DOU No. 053, of 18 March 2005. pp. 58–63. Available online: https://moodle.ufsc.br/pluginfile.php/2292313/mod_resource/content/1/Conama%20357.pdf (accessed on 25 March 2021).
- Maltby, L.; Clayton, S.A.; Wood, R.M.; McLoughlin, N. Evaluation of the Gammarus pulex in situ feeding assay as a biomonitor of water quality: Robustness, responsiveness, and relevance. Environ. Toxicol. Chem. 2002, 21, 361–368. [Google Scholar] [CrossRef]
- Alonso, A.; Camargo, J.A. The freshwater planarian Polycelis felina as a sensitive species to assess the long-term toxicity of ammonia. Chemosphere 2011, 84, 533–537. [Google Scholar] [CrossRef]
- Hellou, J. Behavioural ecotoxicology, an “early warning” signal to assess environmental quality. Environ. Sci. Pollut. Res. 2010, 18, 1–11. [Google Scholar] [CrossRef]
- Nishimura, K.; Kitamura, Y.; Inoue, T.; Umesono, Y.; Sano, S.; Yoshimoto, K.; Inden, M.; Takata, K.; Taniguchi, T.; Shimohama, S.; et al. Reconstruction of dopaminergic neural network and locomotion function in planarian regenerates. Dev. Neurobiol. 2007, 67, 1059–1078. [Google Scholar] [CrossRef] [PubMed]
- Noreña, C.; Damborenea, C.; Brusa, F. Phylum Platyhelminthes. In Thorp and Covich’s Freshwater Invertebrates; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 181–203. [Google Scholar]
- Inoue, T.; Hoshino, H.; Yamashita, T.; Shimoyama, S.; Agata, K. Planarian shows decision-making behavior in response to multiple stimuli by integrative brain function. Zool. Lett. 2015, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gensemer, R.W.; Gondek, J.C.; Rodriquez, P.H.; Arbildua, J.J.; Stubblefield, W.A.; Cardwell, A.S.; Santore, R.C.; Ryan, A.C.; Adams, W.J.; Nordheim, E. Evaluating the effects of pH, hardness, and dissolved organic carbon on the toxicity of aluminum to freshwater aquatic organisms under circumneutral conditions. Environ. Toxicol. Chem. 2017, 37, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Bundschuh, M.; Gergs, R.; Brühl, C.A.; Diehl, D.; Entling, M.H.; Fahse, L.; Frör, O.; Jungkunst, H.F.; Lorke, A.; et al. Review on environmental alterations propagating from aquatic to terrestrial ecosystems. Sci. Total. Environ. 2015, 538, 246–261. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kim, Y.-S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elements Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Ding, X.; Song, L.; Han, Y.; Wang, Y.; Tang, X.; Cui, G.; Xu, Z. Effects of Fe3+ on Acute Toxicity and Regeneration of Planarian (Dugesia japonica) at Different Temperatures. BioMed Res. Int. 2019, 2019, 8591631. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Me-tal induced Oxidative Damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Bossuyt, B.T.; Janssen, C.R. Copper toxicity to different field-collected cladoceran species: Intra- and inter-species sensitivity. Environ. Pollut. 2005, 136, 145–154. [Google Scholar] [CrossRef]
- Quintaneiro, C.; Ranville, J.; Nogueira, A. Effects of the essential metals copper and zinc in two freshwater detritivores species: Biochemical approach. Ecotoxicol. Environ. Saf. 2015, 118, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.C.E.P.; Bilton, D.T. Respiratory control in aquatic insects dictates their vulnerability to global warming. Biol. Lett. 2013, 9, 20130473. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.C.E.P.; Bilton, D.T.; Calosi, P.; Spicer, J.I. Oxygen supply in aquatic ectotherms: Partial pressure and solubility together explain biodiversity and size patterns. Ecology 2011, 92, 1565–1572. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Sokolov, E.P.; Haider, F. Mitochondrial Mechanisms Underlying Tolerance to Fluctuating Oxygen Conditions: Lessons from Hypoxia-Tolerant Organisms. Integr. Comp. Biol. 2019, 59, 938–952. [Google Scholar] [CrossRef]
- Portner, H.-O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef]
- Canesi, L. Pro-oxidant and antioxidant processes in aquatic invertebrates. Ann. N. Y. Acad. Sci. 2014, 1340, 1–7. [Google Scholar] [CrossRef]
- Lewallen, M.; Burggren, W. Metabolic physiology of the freshwater Planaria Girardia dorotocephela and Schmidtea mediterranea: Reproductive mode, specific dynamic action, and temperature. Am. J. Physiol. Integr. Comp. Physiol. 2020, 319, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, L.; Amatya, C.; de Saer, C.J.; Dalhoff, Z.; Eggerichs, M.R. Galantamine reverses scopolamine-induced behavioral alterations in Dugesia tigrina. Invertebr. Neurosci. 2014, 14, 91–101. [Google Scholar] [CrossRef]
- Da Silva, C.B.; Pott, A.; Elifio-Esposito, S.; Dalarmi, L.; Nascimento, K.F.D.; Burci, L.M.; de Oliveira, M.; Dias, J.D.F.G.; Zanin, S.M.W.; Miguel, O.G.; et al. Effect of Donepezil, Tacrine, Galantamine and Rivastigmine on Acetylcholinesterase Inhibition in Dugesia tigrina. Molecules 2016, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, D.W.; Loreau, M. Stability trophic cascades in food chains. R. Soc. Open Sci. 2018, 5, 180995. [Google Scholar] [CrossRef] [PubMed]
 = Water collection sites. Water collected in each site was used to expose adult planarians (G. tigrina) in the laboratory.
= Water collection sites. Water collected in each site was used to expose adult planarians (G. tigrina) in the laboratory.
 = Water collection sites. Water collected in each site was used to expose adult planarians (G. tigrina) in the laboratory.
= Water collection sites. Water collected in each site was used to expose adult planarians (G. tigrina) in the laboratory.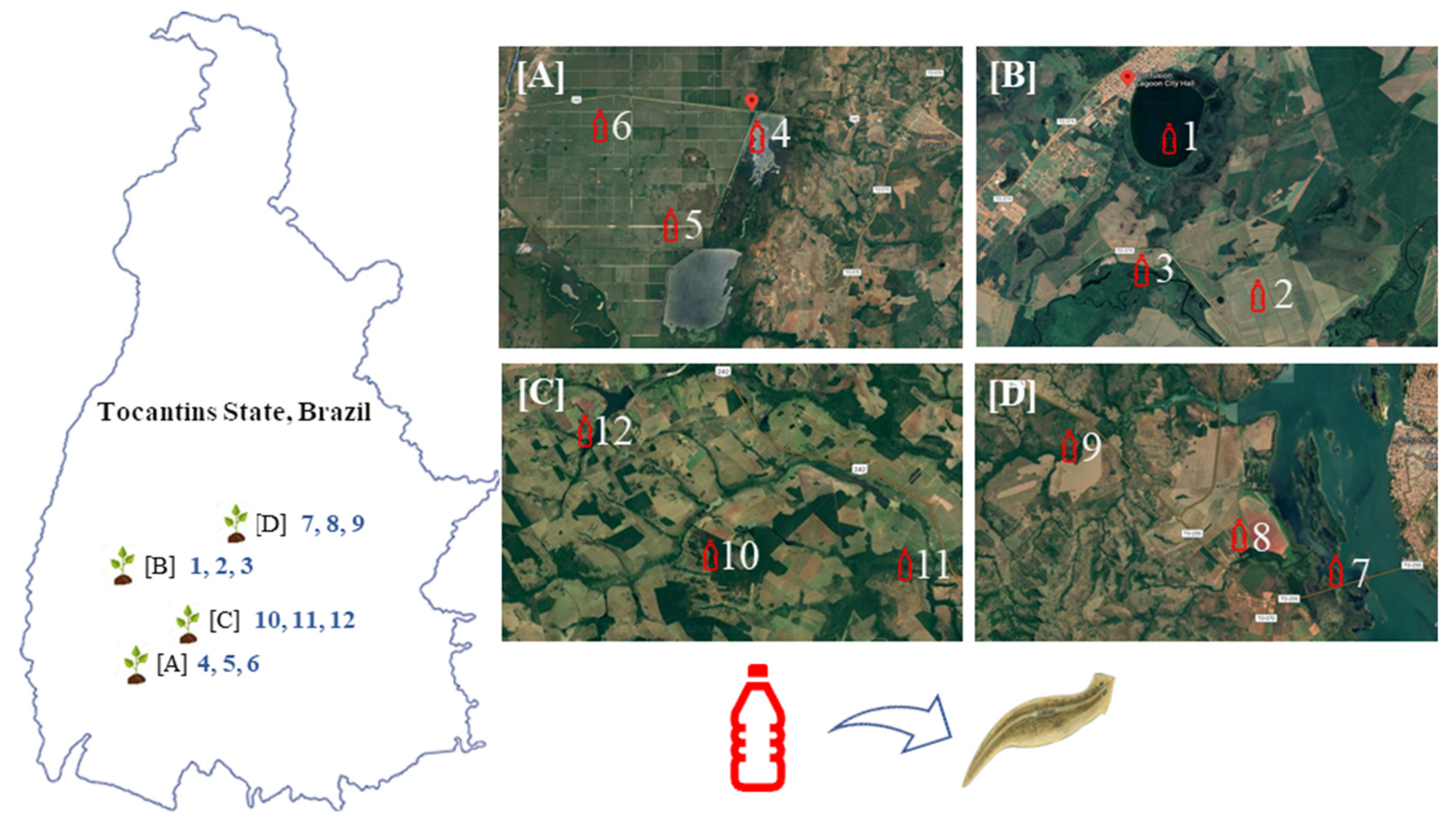
 very high concentrations,
very high concentrations,  high concentrations,
high concentrations,  above acceptable limits,
above acceptable limits,  acceptable limit.
acceptable limit.
 very high concentrations,
very high concentrations,  high concentrations,
high concentrations,  above acceptable limits,
above acceptable limits,  acceptable limit.
acceptable limit.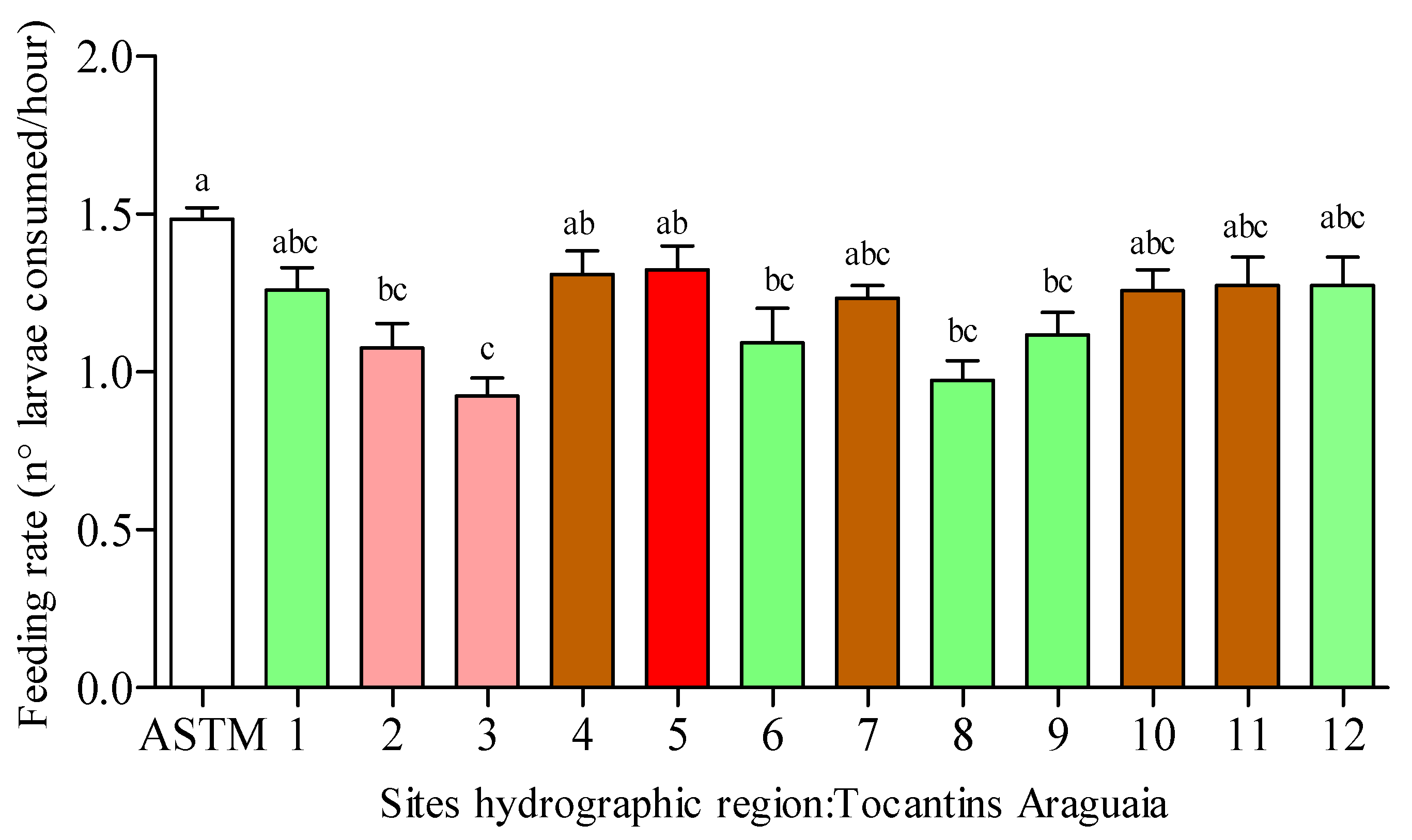
 very high concentrations,
very high concentrations,  high concentrations,
high concentrations,  above acceptable limits,
above acceptable limits,  acceptable limit.
acceptable limit.
 very high concentrations,
very high concentrations,  high concentrations,
high concentrations,  above acceptable limits,
above acceptable limits,  acceptable limit.
acceptable limit.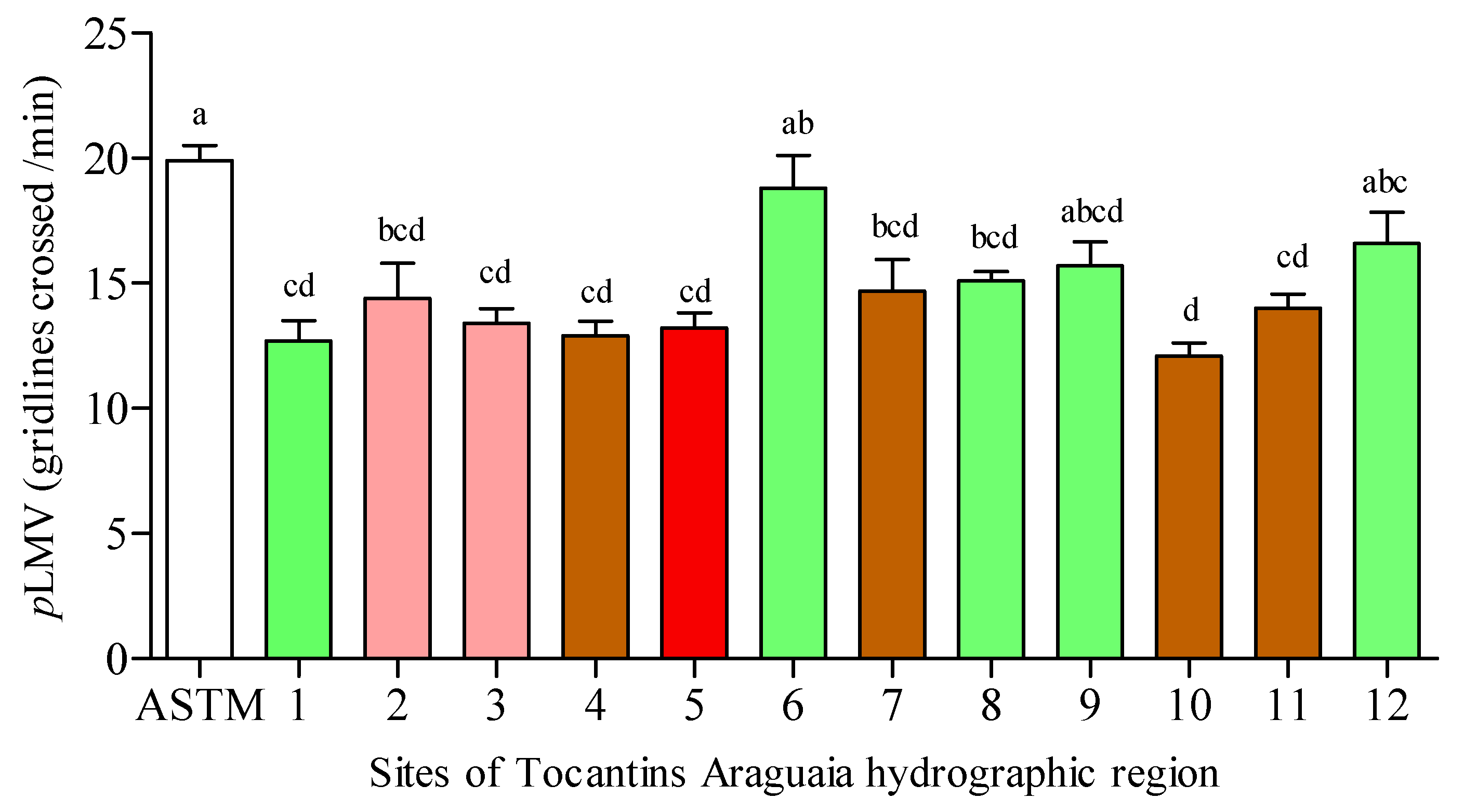
| Test | AL | LQ | Unit | Sites of Hydrographic Region Tocantins Araguaia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||||
| Biochemical oxygen demand | 5 | 0.2 | mg L−1 | ||||||||||||
| Dissolved oxygen | >5 | 0.1 | mg L−1 | 4.7 | 3.9 | 2.8 | |||||||||
| Turbidity | 100 | 0.21 | NTU | ||||||||||||
| Acidity/alkalinity | 6.0–9.0 | 0.1 | pH | 5.44 | |||||||||||
| Total dissolved solids | 500 | 0.05 | mg L−1 | ||||||||||||
| Cyanobacterial density | 50,000 | 1 | Cel mL−1 | ||||||||||||
| Dissolved aluminum | 0.1 | 0.004 | mg L−1 | 0.11 | 0.13 | 1.02 | 0.27 | 0.38 | |||||||
| Total barium | 0.7 | 0.005 | mg L−1 | ||||||||||||
| Total chloride | 250 | 0.5 | mg L−1 | ||||||||||||
| Total chlorine | 0.01 | 0.01 | mg L−1 | 0.04 | 0.07 | ||||||||||
| Dissolved iron | 0.3 | 0.04 | mg L−1 | 0.36 | 0.34 | 0.62 | 0.88 | ||||||||
| Total fluoride | 1.4 | 0.04 | mg L−1 | ||||||||||||
| Total manganese | 0.1 | 0.007 | mg L−1 | ||||||||||||
| Nitrates | 10 | 0.1 | mg L−1 | ||||||||||||
| Nitric nitrogen | 1 | 0.001 | mg L−1 | ||||||||||||
| Total sulfate | 250 | 0.11 | mg L−1 | ||||||||||||
| Total zinc | 0.18 | 0.007 | mg L−1 | 0.44 | |||||||||||
| Surfactants | NR | 0.001 | mg L−1 | 0.74 | 0.61 | 0.68 | 0.76 | 0.64 | 0.63 | 0.64 | 0.62 | 0.7 | 0.61 | 0.59 | <LQ |
 Very high concentrations;
Very high concentrations;  High concentrations;
High concentrations;  Above acceptable limits;
Above acceptable limits;  Acceptable limit (AL);
Acceptable limit (AL);  <Limit of Quantitation (LQ);
<Limit of Quantitation (LQ);  Limit not recorded. Sites of TAHR: Lagoa da Confusão (1–3); Formoso do Araguaia (4–6); Porto Nacional (7–9); Gurupi (10–12). The coloration for the results is expressed in comparison to the acceptable limit for surface waters (AL), these colors are associated with the graphs. AL: In agreement with the National Environment Council (Conselho Nacional do Meio Ambiente—CONAMA), BRAZIL-Resolution No 357, of 17 March, 2005 [46]. Provides for the classification of bodies of water and environmental guidelines for their classification, as well as establishing the conditions and standards for the discharge of effluents, and other measures.
Limit not recorded. Sites of TAHR: Lagoa da Confusão (1–3); Formoso do Araguaia (4–6); Porto Nacional (7–9); Gurupi (10–12). The coloration for the results is expressed in comparison to the acceptable limit for surface waters (AL), these colors are associated with the graphs. AL: In agreement with the National Environment Council (Conselho Nacional do Meio Ambiente—CONAMA), BRAZIL-Resolution No 357, of 17 March, 2005 [46]. Provides for the classification of bodies of water and environmental guidelines for their classification, as well as establishing the conditions and standards for the discharge of effluents, and other measures.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, A.M.C.; Saraiva, A.d.S.; Gravato, C.; Soares, A.M.V.M.; Sarmento, R.A. Behavioral Parameters of Planarians (Girardia tigrina) as Fast Screening, Integrative and Cumulative Biomarkers of Environmental Contamination: Preliminary Results. Water 2021, 13, 1077. https://doi.org/10.3390/w13081077
López AMC, Saraiva AdS, Gravato C, Soares AMVM, Sarmento RA. Behavioral Parameters of Planarians (Girardia tigrina) as Fast Screening, Integrative and Cumulative Biomarkers of Environmental Contamination: Preliminary Results. Water. 2021; 13(8):1077. https://doi.org/10.3390/w13081077
Chicago/Turabian StyleLópez, Ana M. Córdova, Althiéris de Souza Saraiva, Carlos Gravato, Amadeu M. V. M. Soares, and Renato Almeida Sarmento. 2021. "Behavioral Parameters of Planarians (Girardia tigrina) as Fast Screening, Integrative and Cumulative Biomarkers of Environmental Contamination: Preliminary Results" Water 13, no. 8: 1077. https://doi.org/10.3390/w13081077
APA StyleLópez, A. M. C., Saraiva, A. d. S., Gravato, C., Soares, A. M. V. M., & Sarmento, R. A. (2021). Behavioral Parameters of Planarians (Girardia tigrina) as Fast Screening, Integrative and Cumulative Biomarkers of Environmental Contamination: Preliminary Results. Water, 13(8), 1077. https://doi.org/10.3390/w13081077








