Forested Riparian Buffers Change the Taxonomic and Functional Composition of Stream Invertebrate Communities in Agricultural Catchments
Abstract
:1. Introduction
2. Materials and Methods
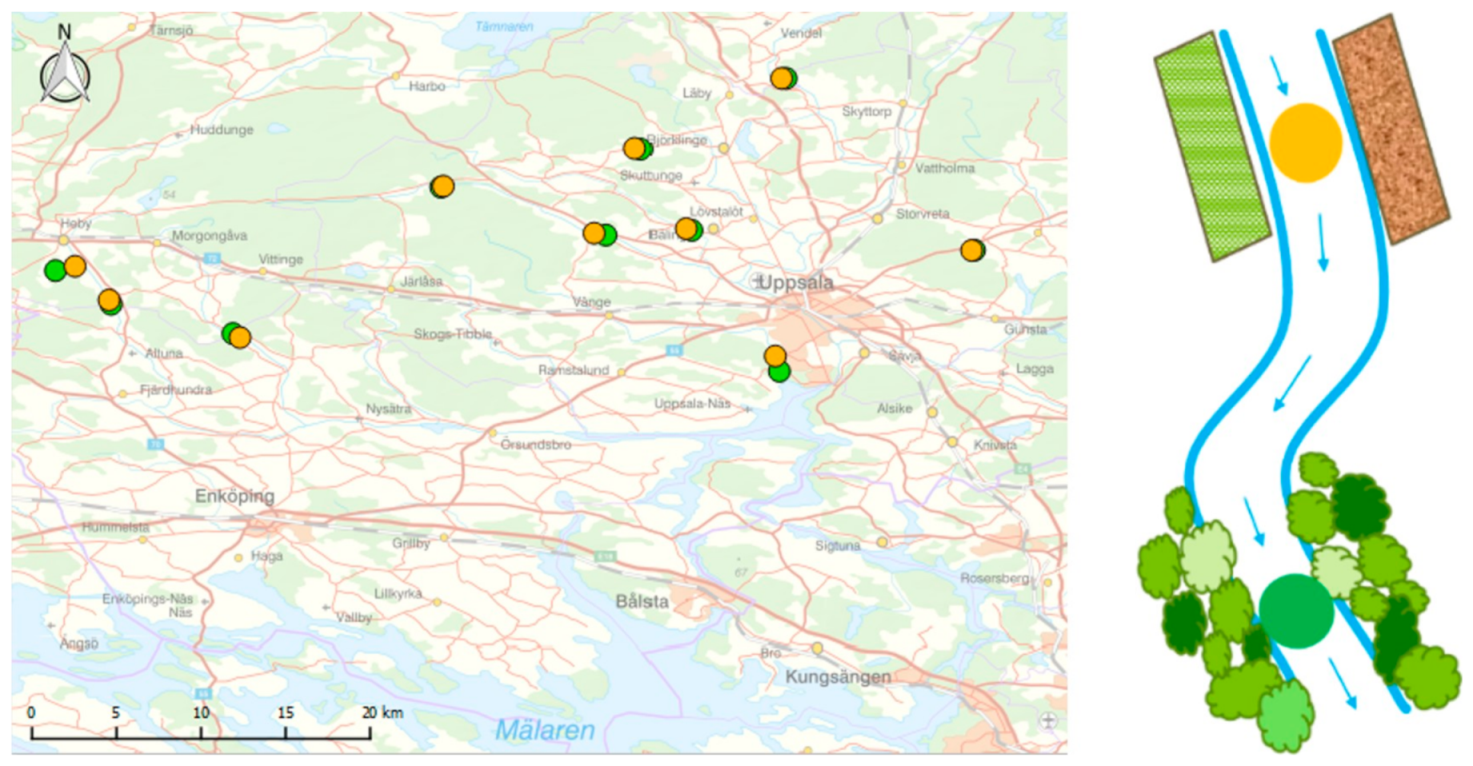
2.1. Study Sites
2.2. Sampling Methods
2.2.1. Environmental Variables
2.2.2. Macroinvertebrate Communities
2.3. Functional Traits Selection
- (1)
- body size (in cm): ≤0.25, 0.25–0.5, 0.5–1, 1–2, 2–4, 4–8, >8;
- (2)
- life cycle duration: ≤1 year, >1 year;
- (3)
- potential number of cycles per year: <1 (semivoltine), 1 (monovoltine), >1 (polyvoltine);
- (4)
- dispersal: aquatic passive, aquatic active, aerial passive, aerial active;
- (5)
- respiration: tegument, gill, plastron, spiracle;
- (6)
- feeding: deposit feeder, shredder, scraper, filter feeder, predator, parasite.
- (7)
- substratum preferences: boulders/cobbles/pebbles, gravel, sand, silt, mud, macrophytes, microphytes, twigs/roots, organic detritus/litter (hereafter CPOM);
- (8)
- flow: null (<5 cm/s), slow (5–25 cm/s), medium (25–50 cm/s), fast (>50 cm/s).
2.4. Statistical Analysis
2.4.1. Hypothesis 1
2.4.2. Hypotheses 2, 3a and 3b
2.4.3. Hypothesis 4
3. Results
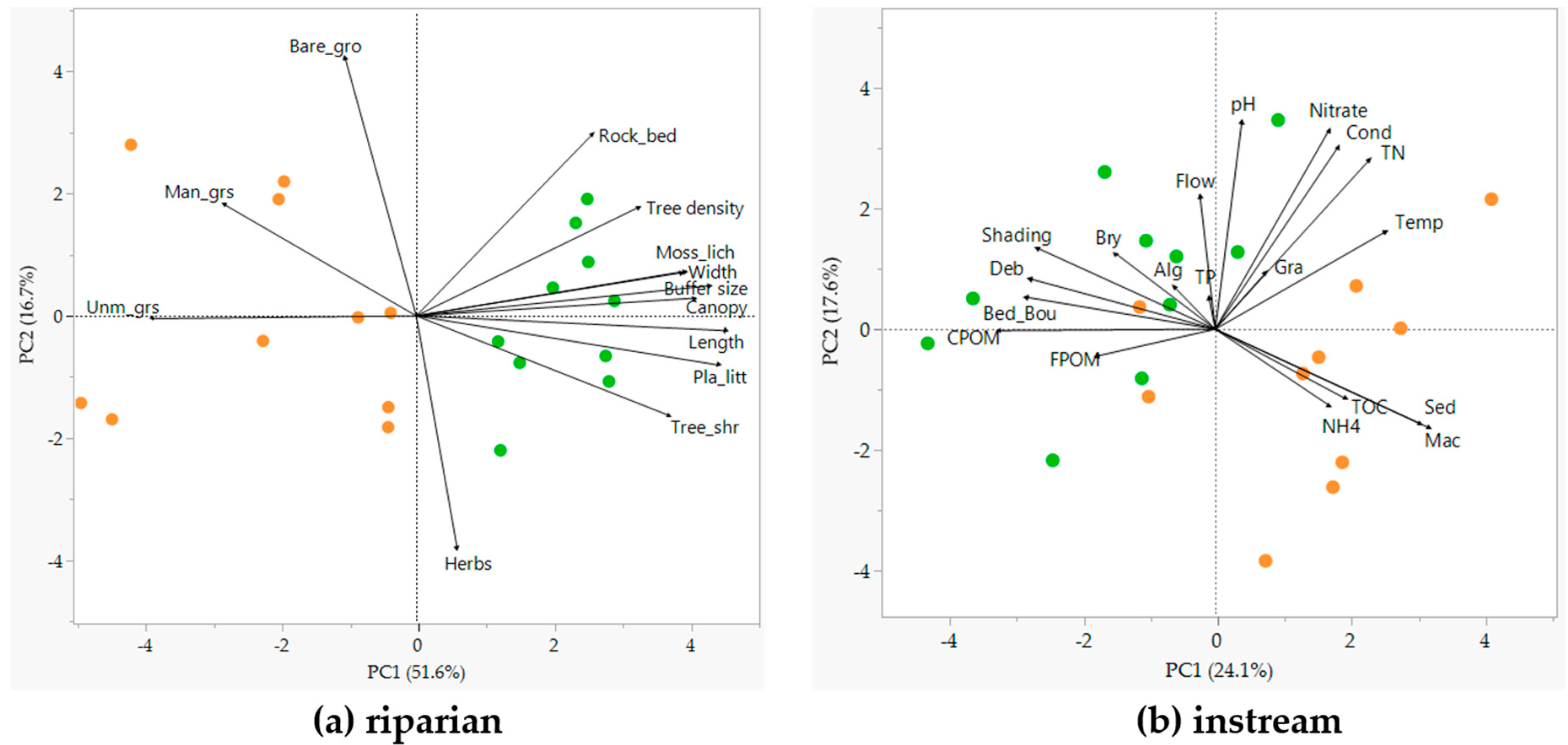
3.1. Riparian and Instream Habitats (Hypothesis 1)
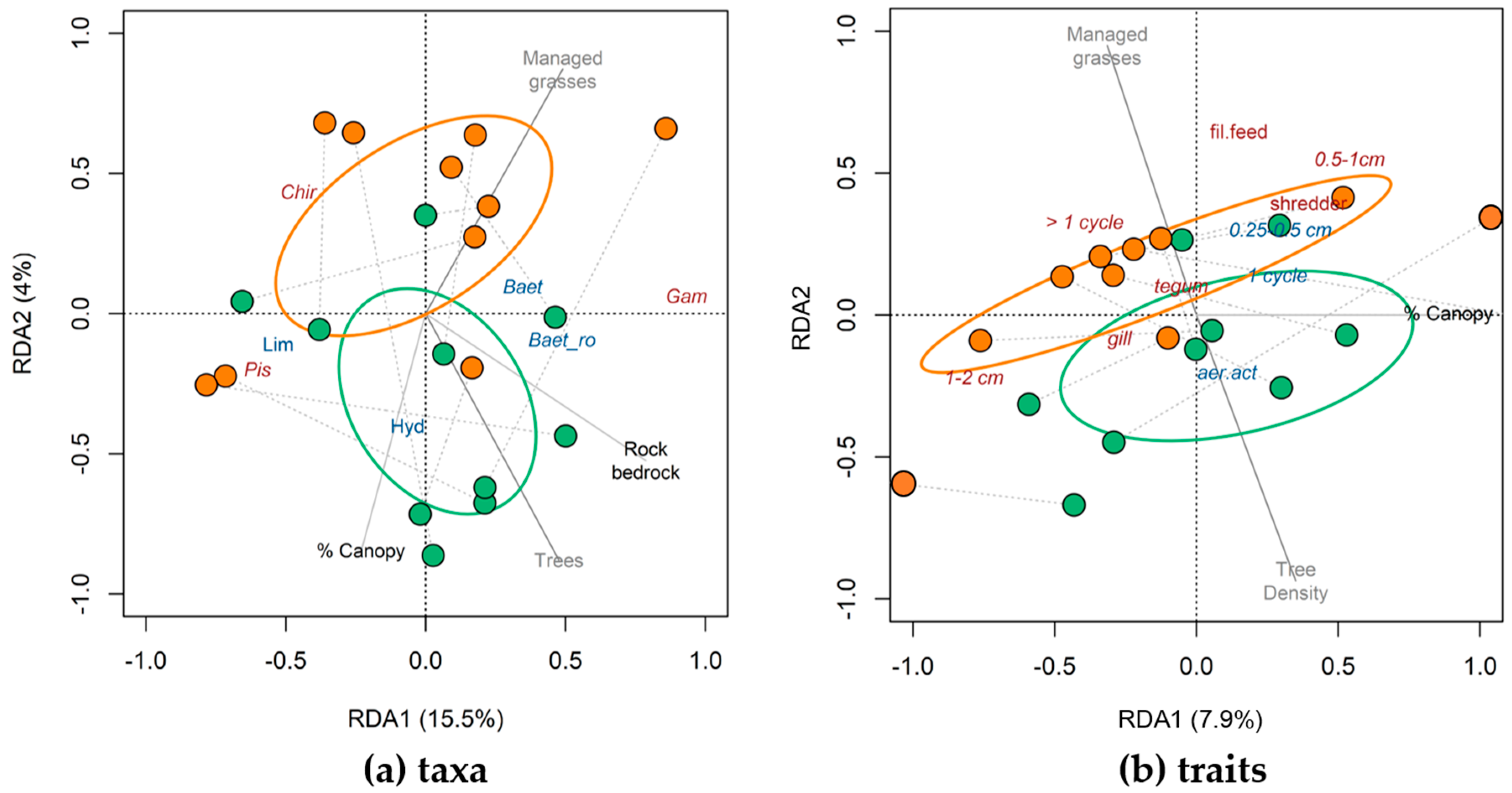
3.2. Taxonomic and Trait Differences Between Study Reaches (Hypotheses 2, 3a and 3b)
3.3. Taxonomic and Trait Responses to Environmental Variables (Hypothesis 4)
4. Discussion
4.1. Changes in Riparian and Instream Habitats
4.2. Taxonomic and Trait Changes in Buffered and Unbuffered Reaches
4.3. Taxonomic and Trait Response to Environmental Variables
4.4. Implications for Cross-Ecosystem Connectivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naiman, R.J.; Décamps, H. The ecology of interfaces: Riparian Zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Abarca, M.R.; Santos-Martin, F.; Martin-Lopez, B.; Sanchez-Montoya, M.M.; Suarez Alonso, M.L. Exploring the capacity of water framework directive indices to assess ecosystem services in fluvial and riparian systems: Towards a second implementation phase. Environ. Manag. 2016, 57, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Naiman, R.J.; Décamps, H.; McClain, M.; Likens, G. Biophysical connectivity and riparian functions. In Riparia: Ecology, Conservation, and Management of Streamside Communities; Academic Press: Burlington, NJ, USA, 2005; pp. 159–187. [Google Scholar]
- Naiman, R.J.; Decamps, H.; Pollock, M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Burdon, F.J.; Ramberg, E.; Sargac, J.; Forio, M.A.E.; de Saeyer, N.; Mutinova, P.T.; Moe, T.F.; Pavelescu, M.O.; Dinu, V.; Cazacu, C.; et al. Assessing the Benefits of Forested Riparian Zones: A Qualitative Index of Riparian Integrity Is Positively Associated with Ecological Status in European Streams. Water 2020, 12, 1178. [Google Scholar] [CrossRef] [Green Version]
- Bjelke, U.; Boberg, J.; Oliva, J.; Tattersdill, K.; McKie, B.G. Dieback of riparian alder caused by the Phytophthora alni complex: Projected consequences for stream ecosystems. Freshw. Biol. 2016, 61, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Wallace, J.B.; Judy, S.L.E.; Meyer, L.; Webster, J.R. Effects of resource limitation on a detrital-based ecosystem. Ecol. Monogr. 1999, 69, 409–442. [Google Scholar] [CrossRef]
- Hagen, E.M.; McTammany, M.E.; Webster, J.R.; Benfield, E.F. Shifts in allochthonous input and autochthonous production in streams along an agricultural land-use gradient. Hydrobiologia 2010, 655, 61–77. [Google Scholar] [CrossRef]
- Ledesma, J.L.J.; Futter, M.N.; Blackburn, M.; Lidman, F.; Grabs, T.; Sponseller, R.A.; Laudon, H.; Bishop, K.H.; Köhler, S.J. Towards an improved conceptualization of riparian zones in Boreal forest headwaters. Ecosystems 2017, 21, 297–315. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, D. The influence of riparian vegetation on macroinvertebrate community structure and functional organization in 6 new Guinea streams. Hydrobiologia 1994, 294, 65–85. [Google Scholar] [CrossRef]
- Arnaiz, O.L.; Wilson, A.L.; Watts, R.J.; Stevens, M.M. Influence of riparian condition on aquatic macroinvertebrate communities in an agricultural catchment in south-eastern Australia. Ecol. Res. 2010, 26, 123–131. [Google Scholar] [CrossRef]
- Verberk, W.C.; Bilton, D.T. Respiratory control in aquatic insects dictates their vulnerability to global warming. Biol. Lett. 2013, 9, 20130473. [Google Scholar] [CrossRef]
- Burdon, F.J.; McIntosh, A.R.; Harding, J.S. Habitat loss drives threshold response of benthic invertebrate communities to deposited sediment in agricultural streams. Ecol. Appl. 2013, 23, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.; Merritt, R.W. Impacts of livestock grazing activities on stream insect communities and the riverine environment. Am. Entomol. 1999, 45, 13–29. [Google Scholar] [CrossRef] [Green Version]
- McKie, B.G.; Sandin, L.; Carlson, P.E.; Johnson, R.K. Species traits reveal effects of land use, season and habitat on the potential subsidy of stream invertebrates to terrestrial food webs. Aquat. Sci. 2018, 80, 15. [Google Scholar] [CrossRef] [Green Version]
- Verberk, W.C.E.P.; Siepel, H.; Esselink, H. Life-history strategies in freshwater macroinvertebrates. Freshw. Biol. 2008, 53, 1722–1738. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and Riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef] [Green Version]
- Wooster, D.E.; DeBano, S.J. Effect of woody riparian patches in croplands on stream macroinvertebrates. Arch. Hydrobiol. 2006, 165, 241–268. [Google Scholar] [CrossRef]
- Lind, L.; Hasselquist, E.M.; Laudon, H. Towards ecologically functional riparian zones: A meta-analysis to develop guidelines for protecting ecosystem functions and biodiversity in agricultural landscapes. J Environ. Manag. 2019, 249, 109391. [Google Scholar] [CrossRef]
- Mander, Ü.; Hayakawa, Y.; Kuusemets, V. Purification processes, ecological functions, planning and design of riparian buffer zones in agricultural watersheds. Ecol. Eng. 2005, 24, 421–432. [Google Scholar] [CrossRef]
- Alvarenga, L.A.; Mello, C.R.D.; Colombo, A.; Cuartas, L.A. Hydrologic impacts due to the changes in riparian buffer in a headwater watershed. Cerne 2017, 23, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Stutter, M.I.; Chardon, W.J.; Kronvang, B. Riparian buffer strips as a multifunctional management tool in agricultural landscapes: Introduction. J. Environ. Qual. 2012, 41, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Tabacchi, E.; Lambs, L.; Guilloy, H.; Planty-Tabacchi, A.-M.; Muller, E.; Decamps, H. Impacts of riparian vegetation on hydrological processes. Hydrol. Process. 2000, 14, 2959–2976. [Google Scholar] [CrossRef]
- Burrell, T.; O’Brien, J.; Graham, E.; Simon, K.; Harding, J.; McIntosh, A. Riparian shading mitigates stream eutrophication in agricultural catchments. Freshw. Sci. 2014, 33, 73–84. [Google Scholar] [CrossRef]
- Johnson, R.K.; Almlöf, K. Adapting boreal streams to climate change: Effects of riparian vegetation on water temperature and biological assemblages. Freshw. Sci. 2016, 35, 984–997. [Google Scholar] [CrossRef]
- Amy, J.; Robertson, A.I. Relationships between livestock management and the ecological condition of riparian habitats along an Australian floodplain river. J. Appl. Ecol. 2001, 38, 63–75. [Google Scholar] [CrossRef]
- Cole, L.J.; Stockan, J.; Helliwell, R. Managing riparian buffer strips to optimise ecosystem services: A. review. Agric. Ecosyst. Environ. 2020, 296, 106891. [Google Scholar] [CrossRef]
- Angeler, D.G.; Allen, C.R.; Birgé, H.E.; Drakare, S.; McKie, B.G.; Johnson, R.K. Assessing and managing freshwater ecosystems vulnerable to environmental change. Ambio 2014, 43, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.K. Freshwater Biomonitoring Using Individuals Organisms, Populations, and Species Assemblages of Benthic Macroinvertebrates; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1993; pp. 40–158. [Google Scholar]
- Menezes, S.; Baird, D.J.; Soares, A.M.V.M. Beyond taxonomy: A review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. J. Appl. Ecol. 2010, 47, 711–719. [Google Scholar] [CrossRef]
- Rosenberg, D.M.; Resh, V.H. Freshwater Biomonitoring and Benthic Macroinvertebrates; Springer: New York, NY, USA, 1993. [Google Scholar]
- Woodward, G.; Gessner, M.O.; Giller, P.S.; Gulis, V.; Hladyz, S.; Lecerf, A.; Malmqvist, B.; McKie, B.G.; Tiegs, S.D.; Cariss, H.; et al. Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 2012, 336, 1438–1440. [Google Scholar] [CrossRef] [Green Version]
- Merritt, R.W.; Cummins, K.W. An Introduction to The Aquatic Insects of North America; Kendall Hunt Publishing Company: Dubuque, IO, USA, 2008. [Google Scholar]
- Verdonschot, R.C.M.; Kail, J.; McKie, B.G.; Verdonschot, P.F.M. The role of benthic microhabitats in determining the effects of hydromorphological river restoration on macroinvertebrates. Hydrobiologia 2016, 769, 5566. [Google Scholar] [CrossRef] [Green Version]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Tachet, H.; Richoux, P.; Usseglio-Polatera, P. Invertébrés d’eau douce. Systématique, Biologie, Écologie; CNRS Éditions: Paris, France, 2010. [Google Scholar]
- Chevene, F.; Doleadec, S.; Chessel, D. A fuzzy coding approach for the analysis of long-term ecological data. Freshw. Biol. 1994, 31, 295–309. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.G.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field—Methodology matters! Funct. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER V5: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2001. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology With R.; Springer International Publishing: Cham, Switzerland, 2011. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; Oksanen, M. Vegan: Community Ecology Package. Version 1.15-4; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Dray, S.; Legendre, P.; Peres-Neto, P.R. Spatial modelling: A comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol. Model. 2006, 196, 483–493. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E.; Tatham, R.L. Multivariate Data Analysis, 7th ed.; Pearson: New York, NY, USA, 2010. [Google Scholar]
- Turunen, J.; Elbrecht, V.; Steinke, D.; Aroviita, J. Riparian forests can mitigate warming and ecological degradation of agricultural headwater streams. Freshw. Biol. 2021, 66, 785–798. [Google Scholar] [CrossRef]
- Forio, M.A.E.; De Troyer, N.; Lock, K.; Witing, F.; Baert, L.; Saeyer, N.D.; Rîșnoveanu, G.; Popescu, C.; Burdon, F.J.; Kupilas, B.; et al. Small patches of riparian woody vegetation enhance biodiversity of invertebrates. Water 2020, 12, 3070. [Google Scholar] [CrossRef]
- Johnson, R.K.; Angeler, D.G.; Hallstan, S.; Sandin, L.; McKie, B.G. Decomposing multiple pressure effects on invertebrate assemblages of boreal streams. Ecol. Indic. 2017, 77, 293–303. [Google Scholar] [CrossRef]
- Muenz, T.K.; Golladay, S.W.; Vellidis, G.; Smith, L.L. Stream buffer effectiveness in an agriculturally influenced area, southwestern Georgia: Responses of water quality, macroinvertebrates, and amphibians. J. Environ. Qual. 2006, 35, 1924–1938. [Google Scholar] [CrossRef] [Green Version]
- Quinn, J.M.; Cooper, A.B.; Davies-Colley, R.J.; Rutherford, J.C.; Williamson, R.B. Land use effects on habitat, water quality, periphyton, and benthic invertebrates in Waikato, New Zealand, hill-country streams. N Z. J. Mar. Freshw. Res. 1997, 31, 579–597. [Google Scholar] [CrossRef]
- Sponseller, R.A.; Benfield, E.F.; Valett, H.M. Relationships between land use, spatial scale and stream macroinvertebrate communities. Freshw. Biol. 2001, 46, 1409–1424. [Google Scholar] [CrossRef] [Green Version]
- Townsend, C.R.; Uhlmann, S.S.; Matthaei, C.D. Individual and combined responses of stream ecosystems to multiple stressors. J. Appl. Ecol. 2008, 45, 1810–1819. [Google Scholar] [CrossRef]
- Truchy, A.; Göthe, E.; Angeler, D.G.; Ecke, F.; Sponseller, R.A.; Bundschuh, M.; Johnson, R.K.; McKie, B.G. Partitioning spatial, environmental, and community drivers of ecosystem functioning. Landsc. Ecol. 2019, 34, 2371–2384. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, L.M.; Otto, C. Effects of population density and of presence of Gammarus pulex L. (Amphipoda) on the growth in larvae of Potamophylax cingulatus steph. (Trichoptera). Hydrobiologia 1977, 54, 109–112. [Google Scholar] [CrossRef]
- Power, M.E.; Dietrich, W.E. Food webs in river networks. Ecol. Res. 2002, 17, 451–471. [Google Scholar] [CrossRef]
- Burdon, F. Agriculture and Mining Contamination Contribute to a Productivity Gradient Driving Cross-Ecosystem Associations Between Stream Insects and Riparian Arachnids. In Contaminants and Ecological Subsidies: The Land-Water Interface; Kraus, J.M., Walters, D.M., Mills, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 61–90. [Google Scholar]
- Hall, L.W.; Killen, W.D.; Anderson, R.D.; Alden, R.W. The influence of physical habitat, pyrethroids, and metals on benthic community condition in an urban and residential stream in California. Hum. Ecol. Risk Assess. Int. J. 2009, 15, 526–553. [Google Scholar] [CrossRef]
- Graf, W.; Murphy, J.; Dahl, J.; Zamora-Muñoz, C.; López-Rodríguez, M.J. Distribution and Ecological Preferences of European Freshwater Species. Volume 1: Trichoptera; Schmidt-Kloiber, A., Hering, D., Eds.; Pensoft Publishers: Sofia, Bulgaria, 2008. [Google Scholar]
- Gullefors, B. Nattsländan Rhyacophila nubilas äggläggning (Trichoptera). [The egglaying of the caddisfly Rhyacophila nubila (Trichoptera)]. Entomol. Tidskr. 2014, 135, 147–151. [Google Scholar]
- Lenat, J.K.; Crawford, D.R. Effects of Land Use on The Water Quality and Aquatic Biota of Three Streams in The Piedmont Province of North Carolina Streams. Water-Resources Investigations Report 89-4007; U.S. Geological Survey: Reston, VA, USA, 1989.
- Stone, M.L.; Whiles, M.R.; Webber, J.A.; Williard, K.W.; Reeve, J.D. Macroinvertebrate communities in agriculturally impacted southern Illinois streams: Patterns with riparian vegetation, water quality, and in-stream habitat quality. J. Environ. Qual. 2005, 34, 907–917. [Google Scholar] [CrossRef]
- Battin, J.; Wiley, M.W.; Ruckelshaus, M.H.; Palmer, R.N.; Korb, E.; Bartz, K.K.; Imaki, H. Projected impacts of climate change on salmon habitat restoration. Proc. Natl. Acad. Sci. USA 2007, 104, 6720–6725. [Google Scholar] [CrossRef] [Green Version]
- Broadmeadow, S.B.; Jones, J.G.; Langford, T.E.L.; Shaw, P.J.; Nisbet, T.R. The influence of riparian shade on lowland stream water temperatures in southern England and their viability for brown trout. River Res. Appl. 2011, 27, 226–237. [Google Scholar] [CrossRef]
- Kristensen, P.B.; Kristensen, E.A.; Riis, T.; Baisner, A.J.; Larsen, S.E.; Verdonschot, P.F.M.; Baattrup-Pedersen, A. Riparian forest as a management tool for moderating future thermal conditions of lowland temperate streams. Hydrol. Earth Syst. Sci. Discuss. 2013, 2013, 6081–6106. [Google Scholar]
- Delong, M.D.; Brusven, M.A. Macroinvertebrate community structure along the longitudinal gradient of an agriculturally impacted stream. Environ. Manag. 1998, 22, 445–457. [Google Scholar] [CrossRef]
- Poff, N.L. Landscape filters and species traits: Towards mechanistic understanding and prediction in stream ecology. J. N. Am. Benthol. Soc. 1997, 16, 391–409. [Google Scholar] [CrossRef]
- Heino, J. The importance of metacommunity ecology for environmental assessment research in the freshwater realm. Biol. Rev. 2013, 88, 166–178. [Google Scholar] [CrossRef]
- Burdon, F.J.; Reyes, M.; Alder, A.C.; Joss, A.; Ort, C.; Räsänen, K.; Jokela, J.; Eggen, R.I.L.; Stamm, C. Environmental context and magnitude of disturbance influence trait-mediated community responses to wastewater in streams. Ecol. Evol. 2016, 6, 3923–3939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the multiple meanings of β diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Woodward, G. Biodiversity, ecosystem functioning and food webs in fresh waters: Assembling the jigsaw puzzle. Freshw. Biol. 2009, 54, 2171–2187. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Raitif, J.; Plantegenest, M.; Agator, O.; Piscart, C.; Roussel, J.-M. Seasonal and spatial variations of stream insect emergence in an intensive agricultural landscape. Sci. Total. Environ. 2018, 644, 594–601. [Google Scholar] [CrossRef]
- Burdon, F.J.; Harding, J.S. The linkage between riparian predators and aquatic insects across a stream-resource spectrum. Freshw. Biol. 2008, 53, 330–346. [Google Scholar] [CrossRef]
- Carlson, P.E.; McKie, B.G.; Sandin, L.; Johnson, R.K. Strong land-use effects on the dispersal patterns of adult stream insects: Implications for transfers of aquatic subsidies to terrestrial consumers. Freshw. Biol. 2016, 61, 848–861. [Google Scholar] [CrossRef]
- Mutinova, P.T.; Kahlert, M.; Kupilas, B.; McKie, B.G.; Friberg, N.; Burdon, F.J. Benthic Diatom Communities in Urban Streams and the Role of Riparian Buffers. Water 2020, 12, 2799. [Google Scholar] [CrossRef]
- Popescu, C.; Oprina-Pavelescu, M.; Dinu, V.; Cazacu, C.; Burdon, F.J.; Forio, M.A.E.; Kupilas, B.; Friberg, N.; Goethals, P.; McKie, B.G.; et al. Riparian vegetation structure influences terrestrial invertebrate communities in an agricultural landscape. Water 2021, 13, 188. [Google Scholar] [CrossRef]
- Ramberg, E.; Burdon, F.J.; Sargac, J.; Kupilas, B.; Rîşnoveanu, G.; Lau, D.C.P.; Johnson, R.K.; McKie, B.G. The structure of riparian vegetation in agricultural landscapes influences spider communities and aquatic-terrestrial Linkages. Water 2020, 12, 2855. [Google Scholar] [CrossRef]
| 1. SPATIAL | 3. RIPARIAN | 4. INSTREAM | |
|---|---|---|---|
| Latitude | Riparian buffer size (m2) | Bankfull width (m) | Algae (%) |
| Longitude | Length (m) | Bankfull depth (cm) | Bedrock, boulders (%) |
| Principal Coordinates of Neighbourhood Matrix ((PCNM) | Width (m) | Channel width (m) | Bryophytes (%) |
| 2. CATCHMENT | Tree density (trees/m2) | Channel depth (cm) | Coarse particulate organic matter (CPOM %) |
| Elevation | Unmanaged grass (%) | Flow (m/s) | Large woody debris (%) |
| Catchment area (m2) | Herbs (%) | Temperature (°C) | Fine particulate organic matter (FPOM %) |
| Forest: broad-leafed, coniferous and mixed (%) | Moss/lichens (%) | Instream shading (%) | Gravel (%) |
| Arable land and crops (%) | Trees/shrubs (%) | pH | Macrophytes (%) |
| Pasture (%) | Rocks/bedrock (%) | Conductivity (mS/m2) | Fine sediment (%) |
| Natural areas (%) | Plant litter (%) | Total organic carbon (mg/l) | |
| Urban and industrial (%) | Bare ground (%) | NH4 (µg/l) | |
| Water (%) | Managed grass (%) | NO3–N (µg/l) | |
| Wetlands (%) | Total N (µg/l) | ||
| Other (%) | Total P (µg/l) | ||
| Environmental Variables | Taxa | Traits | ||||
|---|---|---|---|---|---|---|
| Adj R2 | F | p | Adj R2 | F | p | |
| Spatial | ||||||
| PCNM1 | 0.11 | 1.75 | 0.044 | |||
| PCNM9 | 0.03 | 1.65 | 0.089 | 0.13 | 2.42 | 0.062 |
| PCNM10 | 0.06 | 2.24 | 0.066 | |||
| Catchment | ||||||
| Catchment area (m2) | 0.08 | 1.61 | 0.081 | 0.19 | 2.03 | 0.089 |
| Riparian | ||||||
| Canopy (%) | 0.03 | 1.55 | 0.094 | 0.08 | 2.65 | 0.034 |
| Rock/bedrock (%) | 0.07 | 1.86 | 0.041 | |||
| Instream | ||||||
| Nitrate | 0.09 | 2.96 | 0.002 | 0.24 | 3.39 | 0.018 |
| TP | 0.30 | 2.29 | 0.054 | |||
| Algae (%) | 0.16 | 2.49 | 0.004 | 0.13 | 3.9 | 0.009 |
| Gravel (%) | 0.04 | 1.72 | 0.047 | 0.15 | 4.35 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargac, J.; Johnson, R.K.; Burdon, F.J.; Truchy, A.; Rîşnoveanu, G.; Goethals, P.; McKie, B.G. Forested Riparian Buffers Change the Taxonomic and Functional Composition of Stream Invertebrate Communities in Agricultural Catchments. Water 2021, 13, 1028. https://doi.org/10.3390/w13081028
Sargac J, Johnson RK, Burdon FJ, Truchy A, Rîşnoveanu G, Goethals P, McKie BG. Forested Riparian Buffers Change the Taxonomic and Functional Composition of Stream Invertebrate Communities in Agricultural Catchments. Water. 2021; 13(8):1028. https://doi.org/10.3390/w13081028
Chicago/Turabian StyleSargac, Jasmina, Richard K. Johnson, Francis J. Burdon, Amélie Truchy, Geta Rîşnoveanu, Peter Goethals, and Brendan G. McKie. 2021. "Forested Riparian Buffers Change the Taxonomic and Functional Composition of Stream Invertebrate Communities in Agricultural Catchments" Water 13, no. 8: 1028. https://doi.org/10.3390/w13081028







