Estrogen Disrupting Pesticides in Nebraska Groundwater: Trends between Pesticide-contaminated Water and Estrogen-related Cancers in An Ecological Observational Study
Abstract
1. Introduction
2. Materials and Methods
Data Analysis
3. Results
3.1. Characteristics of Estrogen Disrupting Pesticides from Quality-assessed Agrichemical Contaminant for Nebraska Groundwater Database (1 January 1974–31 December 2012)
3.2. Characteristics of Estrogen-Related Cancer from State Profile of National Cancer Institute (January 2013–December 2017) and County Level Confounders from County Health Rankings (2010)
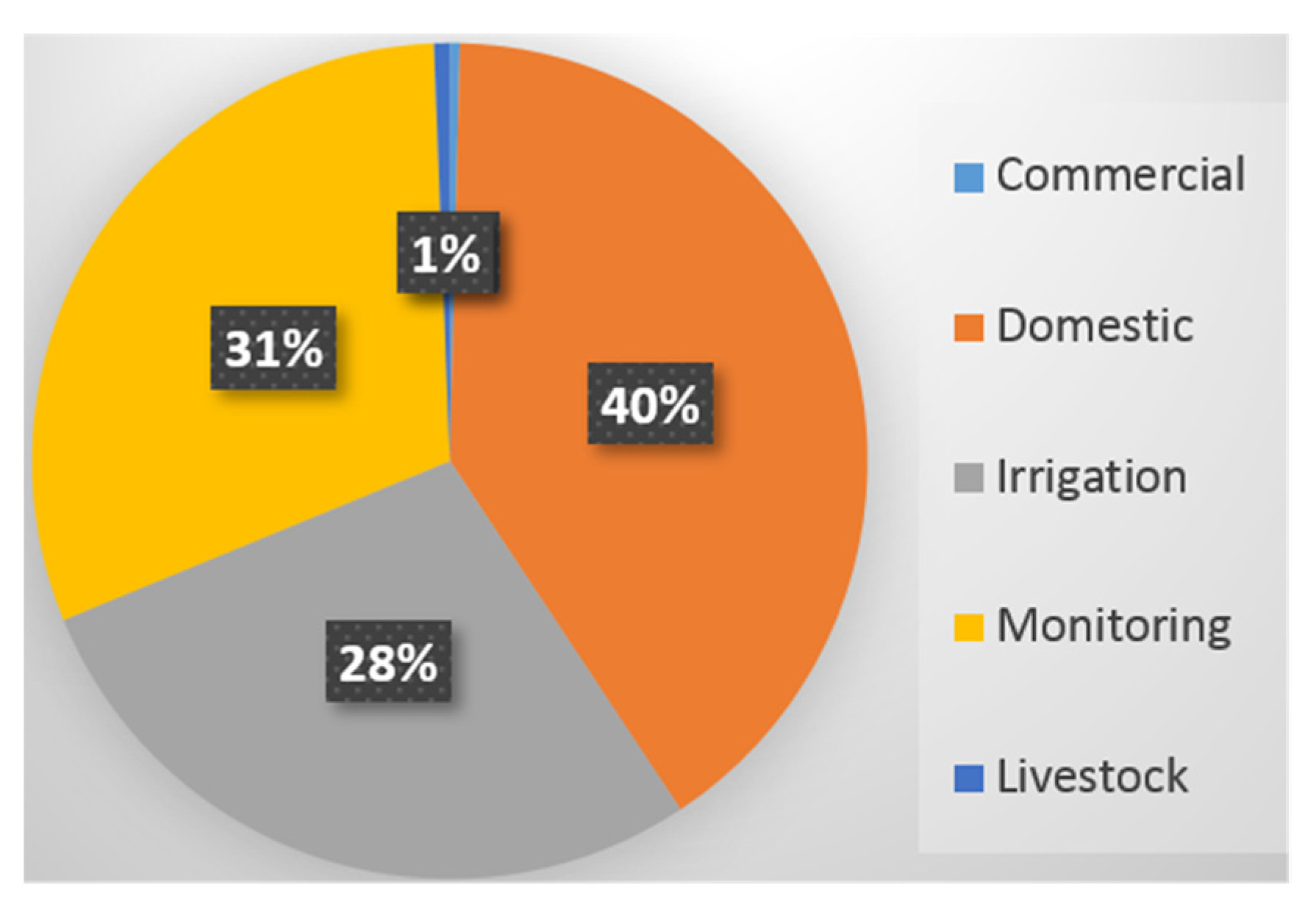
3.3. Ecological Distribution of Estrogen Disrupting Pesticides
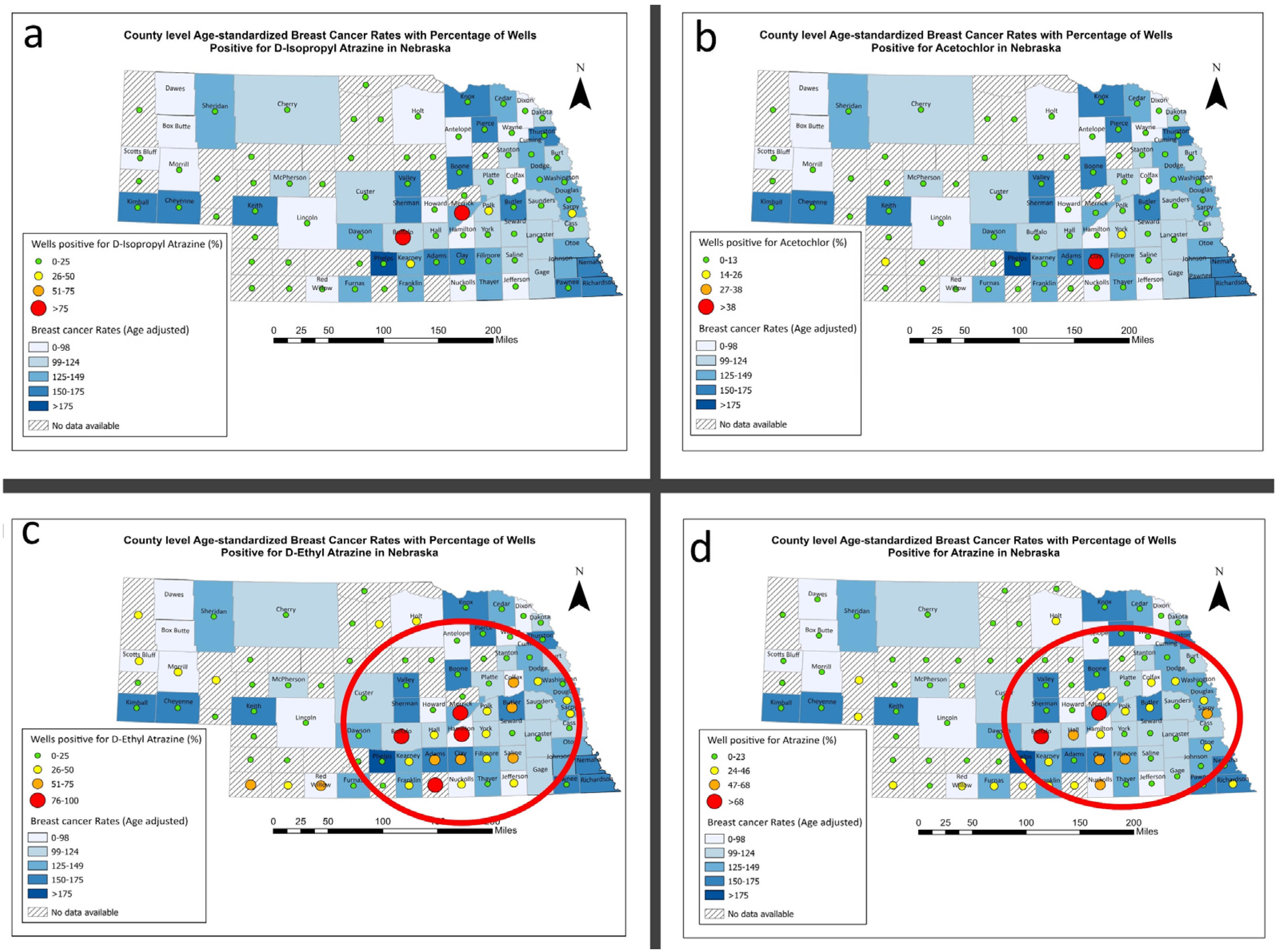
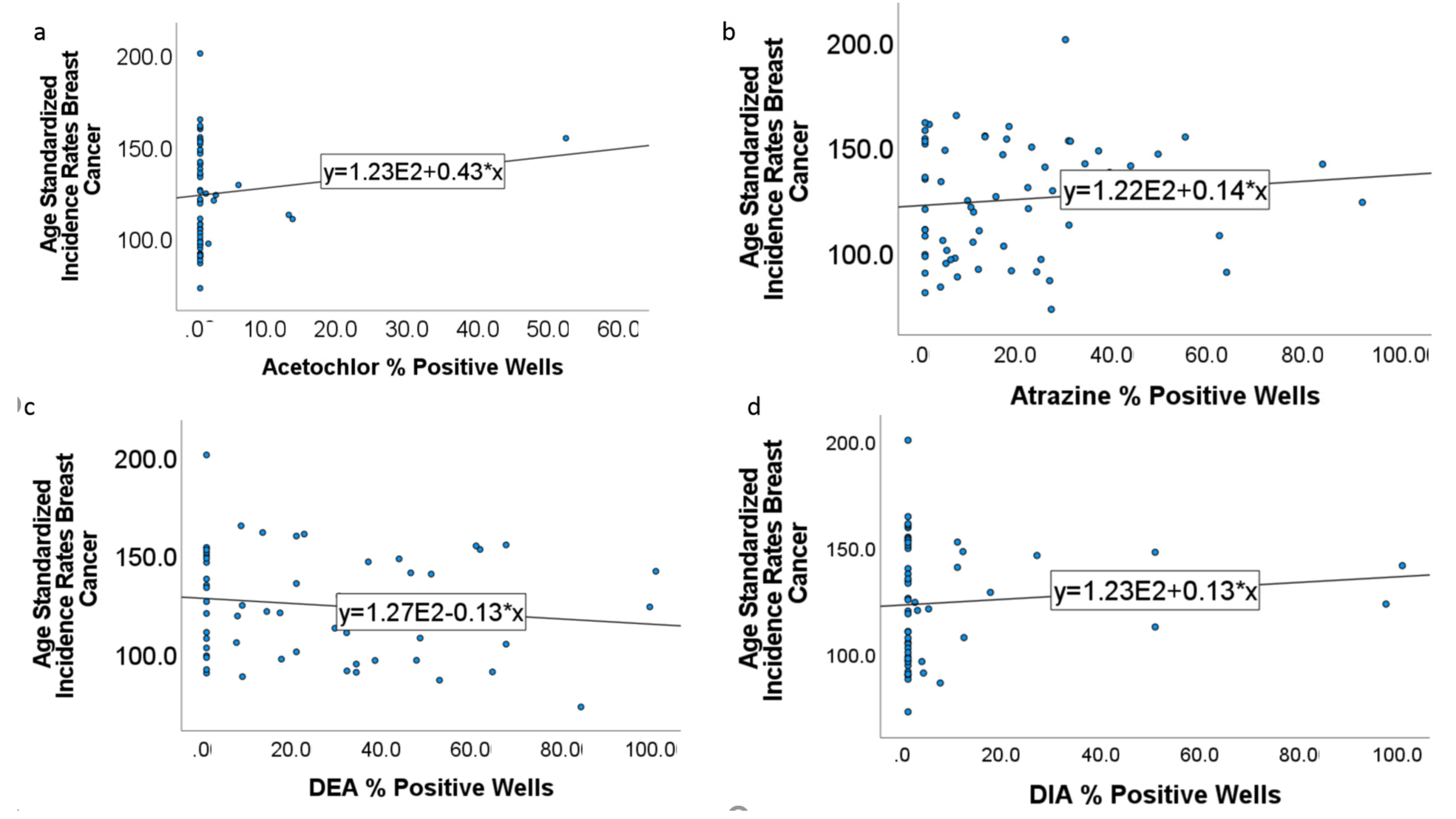
3.4. Breast Cancer and Estrogen Disrupting Pesticides
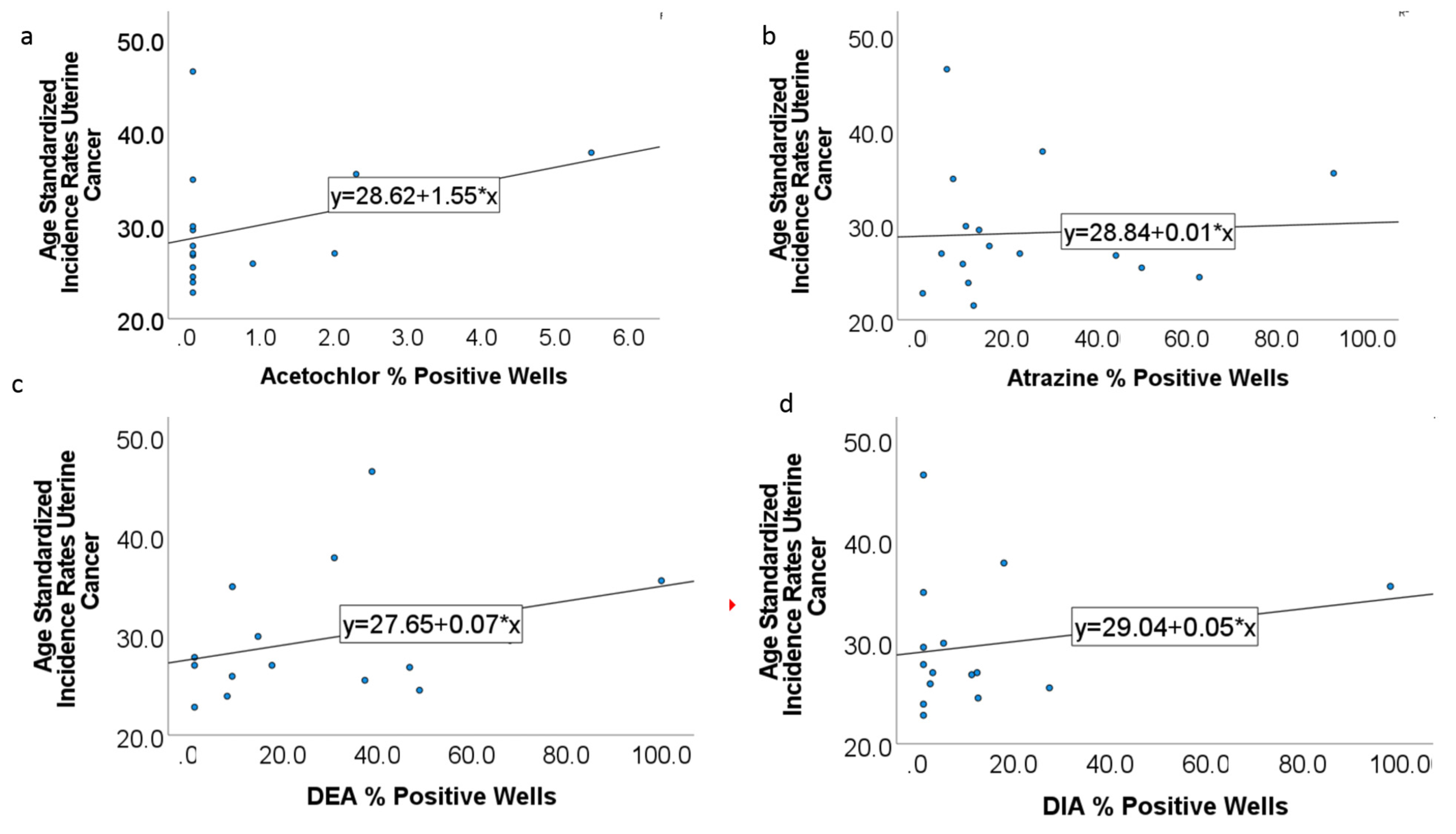
3.5. Uterine Cancer and Estrogen Disrupting Pesticides
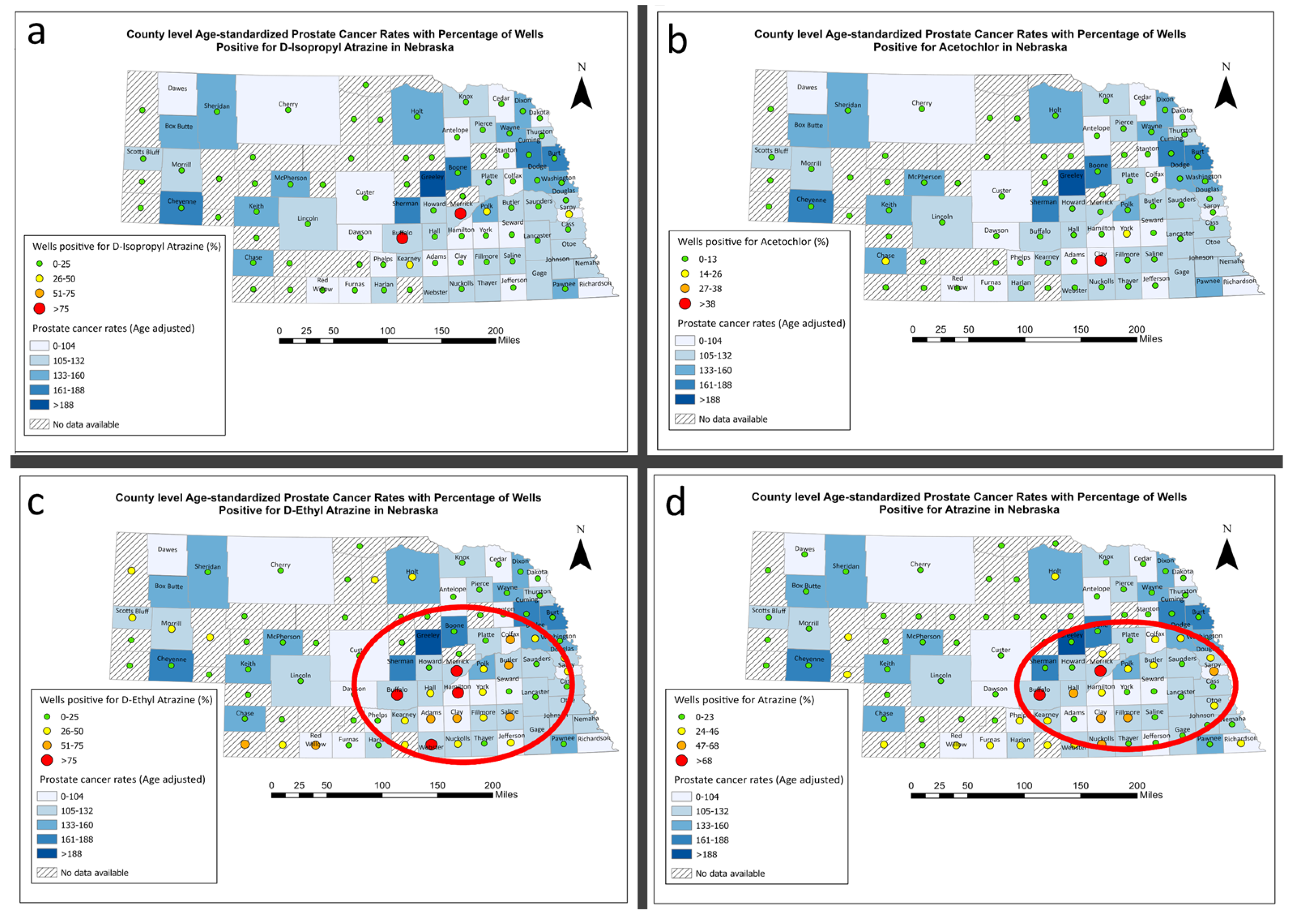
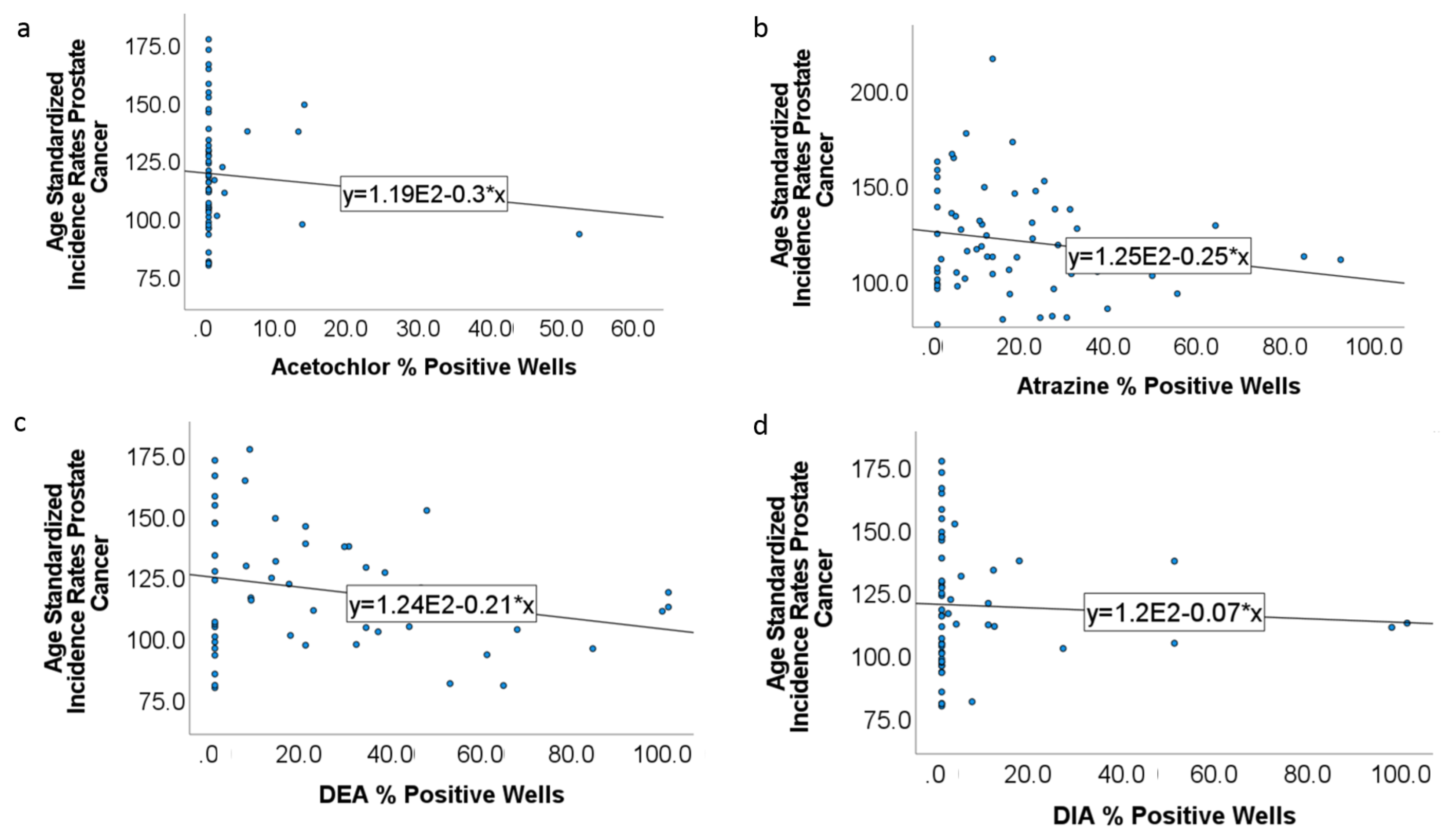
3.6. Prostate Cancer and Estrogen Disrupting Pesticides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Nisbett, N.; Pretty, J.; Robinson, S.; Toulmin, C.; Whiteley, R. The future of the global food system. R. Soc. 2010. [Google Scholar] [CrossRef]
- United States Environemental Protection Agency, DDT—A Brief History and Status. 2017. Available online: https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status (accessed on 23 December 2020).
- Bouwman, H.; Van den Berg, H.; Kylin, H. DDT and malaria prevention: Addressing the paradox. Environ. Health Perspect. 2011, 119, 744–747. [Google Scholar] [CrossRef]
- Eve, L.; Fervers, B.; Le Romancer, M.; Etienne-Selloum, N. Exposure to Endocrine Disrupting Chemicals and Risk of Breast Cancer. Int. J. Mol. Sci. 2020, 21, 9139. [Google Scholar] [CrossRef] [PubMed]
- Fischer Scientifc, Evolution of Chemical Pesticides. 2016. Available online: https://www.fishersci.com/us/en/scientific-products/publications/lab-reporter/2016/issue-4/the-evolution-chemical-pesticides.html (accessed on 23 December 2020).
- Mnif, W.; Hassine, A.I.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention, Agency for Toxic Substances and Disease Registry. Toxicological Profile for Malathion. 2003. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp154.pdf (accessed on 23 December 2020).
- Peillex, C.; Pelletier, M. The impact and toxicity of glyphosate and glyphosate-based herbicides on health and immunity. J. Immunotoxicol. 2020, 17, 163–174. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Exner, M.E.; Spalding, R.F.; Harrell, D.M. Development of a quality-assessed agrichemical database for monitoring anthropogenic impacts on ground-water quality. Environmental monitoring and assessment 2005, 107, 249–257. [Google Scholar] [CrossRef]
- Mesnage, R.; Phedonos, A.; Biserni, M.; Arno, M.; Balu, S.; Corton, J.C.; Ugarte, R.; Antoniou, M.N. Evaluation of estrogen receptor alpha activation by glyphosate-based herbicide constituents. Food Chem. Toxicol. 2017, 108, 30–42. [Google Scholar] [CrossRef]
- Florian, C.P.; Mansfield, S.R.; Schroeder, J.R. Differences in GPR30 regulation by chlorotriazine herbicides in human breast cells. Biochem. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Pestana, D.; Teixeira, D.; Faria, A.; Domingues, V.; Monteiro, R.; Calhau, C. Effects of environmental organochlorine pesticides on human breast cancer: Putative involvement on invasive cell ability. Environ. Toxicol. 2015, 30, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Fedak, K.M.; Bernal, A.; Capshaw, Z.A.; Gross, S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 2015, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.A.; Goyal, A.; Terry, M.B. Alcohol Intake and Breast Cancer Risk: Weighing the Overall Evidence. Curr. Breast Cancer Rep. 2013, 5, 208–221. [Google Scholar] [CrossRef]
- de Menezes, R.F.; Bergmann, A.; Thuler, L.C. Alcohol consumption and risk of cancer: A systematic literature review. Asian Pac. J. Cancer Prev. 2013, 14, 4965–4972. [Google Scholar] [CrossRef]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. (Phila) 2016, 9, 895–905. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef]
- Smith, G.L.; Lopez-Olivo, M.A.; Advani, P.G.; Ning, M.S.; Geng, Y.; Giordano, S.H.; Volk, R.J. Financial Burdens of Cancer Treatment: A Systematic Review of Risk Factors and Outcomes. J. Natl. Compr. Canc. Netw. 2019, 17, 1184–1192. [Google Scholar] [CrossRef]
- Jia, H.; Muennig, P.; Lubetkin, E.; Gold, M.R. Predicting geographical variations in behavioural risk factors: An analysis of physical and mental healthy days. J. Epidemiol. Community Health 2004, 58, 150–155. [Google Scholar] [CrossRef]
- St-Pierre, A.P.; Shikon, V.; Schneider, D.C. Count data in biology—Data transformation or model reformation? Ecol. Evol. 2018, 8, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Donath, C.; Gräßel, E.; Baier, D.; Pfeiffer, C.; Bleich, S.; Hillemacher, T. Predictors of binge drinking in adolescents: Ultimate and distal factors—A representative study. BMC Public Health 2012, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Berry, W.D.; Feldman, S.; Stanley Feldman, D. Multiple Regression in Practice; Sage: Thousand Oaks, CA, USA, 1985. [Google Scholar]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiol. (SunnyvaleCalif.) 2016, 6. [Google Scholar] [CrossRef]
- Database, Q.-A.A.C.; Water, F.N.G. 2019 Nebraska Groundwater. Quality Monitoring Report. 2019. Available online: http://dee.ne.gov/Publica.nsf/Pages/WAT333 (accessed on 23 December 2020).
- Burow, K.R.; Shelton, J.L.; Dubrovsky, N.M. Occurrence of Nitrate and Pesticides in Ground Water Beneath Three Agricultural Land-Use Settings in the Eastern San Joaquin Valley, California, 1993–1995; U.S. Department of the Interior, U.S. Geological Survey: Washington, DC, USA, 1998; Volume 97.
- Papiernik, S.K.; Spalding, R.F. Atrazine, deethylatrazine, and deisopropylatrazine persistence measured in groundwater in situ under low-oxygen conditions. J. Agric. Food Chem. 1998, 46, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Fenga, C. Occupational exposure and risk of breast cancer. Biomed. Rep. 2016, 4, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Zadnik, V.; Krajc, M. Epidemiological trends of hormone-related cancers in Slovenia. Arh. Za Hig. Rada I Toksikol. 2016, 67, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, M.; Kern, P.; Bonefeld-Jørgensen, E.C. Serum levels of environmental pollutants is a risk factor for breast cancer in Inuit: A case control study. Environ. Health 2017, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency, Pesticides Industry Sales and Usage 2013. Available online: https://www.epa.gov/pesticides/pesticides-industry-sales-and-usage-2008-2012-market-estimates (accessed on 23 December 2020).
- Romieu, I.; Hernandez-Avila, M.; Lazcano-Ponce, E.; Weber, J.P.; Dewailly, E. Breast cancer, lactation history, and serum organochlorines. Am. J. Epidemiol. 2000, 152, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Falck, F., Jr.; Ricci, A., Jr.; Wolff, M.S.; Godbold, J.; Deckers, P. Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Arch. Environ. Health 1992, 47, 143–146. [Google Scholar]
- Güttes, S.; Failing, K.; Neumann, K.; Kleinstein, J.; Georgii, S.; Brunn, H. Chlororganic pesticides and polychlorinated biphenyls in breast tissue of women with benign and malignant breast disease. Arch. Environ. Contam. Toxicol. 1998, 35, 140–147. [Google Scholar] [CrossRef]
- Nebraska. Nebraska Economy. 2017. Available online: https://www.netstate.com/economy/ne_economy.htm#:~:text=Corn%20is%20Nebraska's%20most%20important,beans%2C%20sugar%20beets%20and%20potatoes (accessed on 23 December 2020).
- Institute of Agriculture and Natural Resources (UNL). Ag Health Study Examines Pesticide Exposure. 2000. Available online: https://cropwatch.unl.edu/ag-health-study-examines-pesticide-exposure-diseases (accessed on 23 December 2020).
- Rhoades, M.G.; Meza, J.L.; Beseler, C.L.; Shea, P.J.; Kahle, A.; Vose, J.M.; Eskridge, K.M.; Spalding, R.F. Atrazine and nitrate in public drinking water supplies and non-Hodgkin lymphoma in Nebraska, USA. Environ. Health Insights 2013, 7, EHI.S10629. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Colt, J.S.; Heineman, E.F.; McComb, R.; Weisenburger, D.D.; Lijinsky, W.; Ward, M.H. Agricultural pesticide use and risk of glioma in Nebraska, United States. Occup Environ. Med. 2005, 62, 786–792. [Google Scholar] [CrossRef] [PubMed]
- De Roos, A.J.; Zahm, S.H.; Cantor, K.P.; Weisenburger, D.D.; Holmes, F.F.; Burmeister, L.F.; Blair, A. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphoma among men. Occup Environ. Med. 2003, 60, E11. [Google Scholar] [CrossRef] [PubMed]
- American Land Brokers, Important Regions of Nebraska. 2019. Available online: https://www.americanlandbroker.com/states/nebraska/articles/nebraska-regions (accessed on 23 December 2020).
- U.S. Geological Survey, Water Quality in the Central Nebraska Basins Nebraska, 1992–1995. 1998. Available online: https://pubs.usgs.gov/circ/circ1163/circ1163.pdf (accessed on 23 December 2020).
- Hopenhayn-Rich, C.; Stump, M.; Browning, S. Regional assessment of atrazine exposure and incidence of breast and ovarian cancers in Kentucky. Arch. Environ. Contam. Toxicol. 2002, 42, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.K.; Yang, R. Regression analysis of pesticide use and breast cancer incidence in California Latinas. J. Environ. Health 2006, 68, 15–22. [Google Scholar]
- Muir, K.; Rattanamongkolgul, S.; Smallman-Raynor, M.; Thomas, M.; Downer, S.; Jenkinson, C. Breast cancer incidence and its possible spatial association with pesticide application in two counties of England. Public Health 2004, 118, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, L.; Fu, Z. Oral exposure to atrazine modulates hormone synthesis and the transcription of steroidogenic genes in male peripubertal mice. Gen. Comp. Endocrinol. 2013, 184, 120–127. [Google Scholar] [CrossRef]
- Fan, W.; Yanase, T.; Morinaga, H.; Gondo, S.; Okabe, T.; Nomura, M.; Komatsu, T.; Morohashi, K.-I.; Hayes, T.B.; Takayanagi, R. Atrazine-induced aromatase expression is SF-1 dependent: Implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ. Health Perspect. 2007, 115, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, J.W.; Swenberg, J.A.; Weiss, N.; Brusick, D.; Eldridge, J.C.; Stevens, J.T.; Handa, R.J.; Hovey, R.C.; Plant, T.M.; Pastoor, T.P. Atrazine and breast cancer: A framework assessment of the toxicological and epidemiological evidence. Toxicol. Sci. 2011, 123, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention, Agency for Toxic Substances and Disease Registry. Interaction Profile for: Atrazine, Deethylatrazine, Diazinon, Nitrate, and Simazine. 2006. Available online: https://www.atsdr.cdc.gov/interactionprofiles/ip-10/ip10.pdf (accessed on 23 December 2020).
- Thurman, E.M.; Scribner, E.A. A decade of measuring, monitoring, and studying the fate and transport of triazine herbicides and their degradation products in groundwater, surface water, reservoirs, and precipitation by the U.S. Geological Survey. Triazine Herbic. 2008, 50, 451–475. [Google Scholar]
- Rivero, J.; Henríquez-Hernández, L.A.; Luzardo, O.P.; Pestano, J.; Zumbado, M.; Boada, L.D.; Valerón, P.F. Differential gene expression pattern in human mammary epithelial cells induced by realistic organochlorine mixtures described in healthy women and in women diagnosed with breast cancer. Toxicol. Lett. 2016, 246, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Eldakroory, S.; Morsi, D.E.; Abdel-Rahman, R.; Roshdy, S.; Gouida, M.; Khashaba, E. Correlation between toxic organochlorine pesticides and breast cancer. Hum. Exp. Toxicol. 2017, 36, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Albert, A.; Herman, P.; Hamoir, E.; Gaspard, U.; Meurisse, M.; Plomteux, G. Breast cancer and serum organochlorine residues. Occup. Environ. Med. 2003, 60, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: From bench to treatment. Pharmacol. Rev. 2005, 57, 359–383. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Á.G.P.; López, M.I.R.; Casillas, T.Á.D.; León, J.A.A.; Mahjoub, O.; Prusty, A.K. Monitoring of organochlorine pesticides in blood of women with uterine cervix cancer. Environ. Pollut. 2017, 220, 853–862. [Google Scholar] [CrossRef]
- Rodríguez, Á.G.P.; López, M.I.R.; Casillas, T.A.D.; León, J.A.; Prusty, B.A.K.; Cervera, F.J.Á. Levels of persistent organic pollutants in breast milk of Maya women in Yucatan, Mexico. Environ. Monit. Assess. 2017, 189, 59. [Google Scholar] [CrossRef]
- Cohn, B.A.; Cirillo, P.M.; Terry, M.B. DDT and Breast Cancer: Prospective Study of Induction Time and Susceptibility Windows. Jnci J. Natl. Cancer Inst. 2019, 111, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Jaga, K.; Duvvi, H. Risk reduction for DDT toxicity and carcinogenesis through dietary modification. J. R. Soc. Promot. Health 2001, 121, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Takeda, M.; Kojima, S.; Tomiyama, N. Toxicity and Carcinogenicity of Dichlorodiphenyltrichloroethane (DDT). Toxicol. Res. 2016, 32, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Nelles, J.L.; Hu, W.Y.; Prins, G.S. Estrogen action and prostate cancer. Expert Rev. Endocrinol. Metab 2011, 6, 437–451. [Google Scholar] [CrossRef]
- Lee, H.H.; Chou, C.T.; Liang, W.Z.; Chen, W.C.; Wang, J.L.; Yeh, J.H.; Kuo, C.C.; Shieh, P.; Kuo, D.H.; Chen, F.A.; et al. Ca²⁺ Movement Induced by Deltamethrin in PC3 Human Prostate Cancer Cells. Chin. J. Physiol 2016, 59, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Tian, Y.; Du, Y.; Huang, L.; Chen, J.; Li, N.; Liu, W.; Liang, Z.; Zhao, L. Atrazine promotes RM1 prostate cancer cell proliferation by activating STAT3 signaling. Int. J. Oncol. 2016, 48, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.K.; Bernstein, L.; Pike, M.; Henderson, B.; Lobo, R.; Stanczyk, F.; Shimizu, H. 5-alpha-reductase activity and risk of prostate cancer among Japanese and U.S. white and black males. Lancet 1992, 339, 887–889. [Google Scholar] [CrossRef]
- Weihua, Z.; Lathe, R.; Warner, M.; Gustafsson, J.-Å. An endocrine pathway in the prostate, ERβ, AR, 5α-androstane-3β, 17β-diol, and CYP7B1, regulates prostate growth. Proc. Natl. Acad. Sci. USA 2002, 99, 13589–13594. [Google Scholar] [CrossRef] [PubMed]
| Pesticide | Well Types | Tested | |
|---|---|---|---|
| Negative | Positive | ||
| Atrazine | C | 15 (68) | 7 (32) |
| D | 1913 (92) | 167 (8) | |
| I | 939 (73) | 343 (27) | |
| Q | 323 (25) | 956 (75) | |
| S | 16 (62) | 10 (38) | |
| Acetochlor | C | 13 (100) | 0 |
| D | 178 (100) | 0 | |
| I | 479 (95) | 24 (5) | |
| Q | 963 (98) | 24 (2) | |
| S | 7 (100) | 0 | |
| Deethylatrazine | C | 16 (76) | 5 (24) |
| D | 171(91) | 17 (9) | |
| I | 449 (72) | 179 (28) | |
| Q | 284 (24) | 874 (75) | |
| S | 5 (100) | 0 | |
| Deisopropylatrazine | C | 18 (95) | 1 (5) |
| D | 179 (98) | 3 (2) | |
| I | 575 (98) | 13 (2) | |
| Q | 356 (34) | 698 (66) | |
| S | 4 (100) | 0 | |
| Variables | Mean | Median | Minimum | Maximum | Percentiles | |
|---|---|---|---|---|---|---|
| 25th | 75th | |||||
| Age Standardized Breast Cancer Incidence | 124.27 | 124.4 | 72.2 | 200.2 | 98.7 | 148.825 |
| Age Standardized Uterine Cancer Incidence | 29.175 | 27 | 21.5 | 46.5 | 24.75 | 33.65 |
| Age Standardized Prostate Cancer Incidence | 120.066 | 114.9 | 76.5 | 215.7 | 102.45 | 135.8 |
| Acetochlor % Positive Wells | 1.5412 | 0 | 0 | 51.61 | 0 | 0 |
| Atrazine % Positive Wells | 15.6111 | 9.0909 | 0 | 91.15 | 0 | 26.1449 |
| DEA % Positive Wells | 22.2408 | 12.5 | 0 | 100 | 0 | 37.5 |
| DIA % Positive Wells | 5.3684 | 0 | 0 | 100 | 0 | 0 |
| Physically unhealthy days | 2.8493 | 2.85 | 1.4 | 5.79 | 2.46 | 3.13 |
| % Adult Smoking | 18.0999 | 18.29 | 1.17 | 29.19 | 14.89 | 21.09 |
| % Adult Obesity | 28.876 | 28.8 | 26.2 | 32.4 | 28.1 | 29.6 |
| % Binge Drinking | 16.6403 | 16.725 | 6.09 | 27.87 | 13.94 | 19.63 |
| % Uninsured | 18.5387 | 18.3 | 9.1 | 33.2 | 13.55 | 21.85 |
| % Pesticide Positive Wells | Square Root of Percent Positive Wells for Atrazine | Square Root of Percent Positive Wells for Acetochlor | Square Root of Percent Positive Wells for DEA | Square Root of Percent Positive Wells for DIA | |
|---|---|---|---|---|---|
| Square Root of Percent Positive Wells for Atrazine | Pearson Correlation | 1 | 0.204 | 0.598 ** | 0.559 ** |
| p value (2-tailed) | 0.075 | 0.000 | 0.000 | ||
| Square Root of Percent Positive Wells for Acetochlor | Pearson Correlation | 0.204 | 1 | 0.257 * | 0.121 |
| p value (2-tailed) | 0.075 | 0.024 | 0.301 | ||
| Square Root of Percent Positive Wells for DEA | Pearson Correlation | 0.598 ** | 0.257 * | 1 | 0.472 ** |
| p value (2-tailed) | 0.000 | 0.024 | 0.000 | ||
| Square Root of Percent Positive Wells for DIA | Pearson Correlation | 0.559 ** | 0.121 | 0.472 ** | 1 |
| p value (2-tailed) | 0.000 | 0.301 | 0.000 | ||
| N | 77 | 75 | 77 | 77 | |
| Potential County Level Confounders | Age Standardized Breast Cancer Incidence | Age Standardized Uterine Cancer Incidence | Age Standardized Prostate Cancer Incidence | |
|---|---|---|---|---|
| Physically unhealthy days | Pearson Correlation | −0.205 | 0.518 * | −0.012 |
| p value (2-tailed) | 0.104 | 0.04 | 0.924 | |
| % Adult Smoking | Pearson Correlation | 0.043 | 0.048 | −0.055 |
| p value (2-tailed) | 0.741 | 0.866 | 0.67 | |
| % Adult Obesity | Pearson Correlation | −0.078 | 0.313 | −0.107 |
| p value (2-tailed) | 0.542 | 0.238 | 0.398 | |
| % Binge Drinking | Pearson Correlation | 0.033 | −0.585 * | −0.018 |
| p value (2-tailed) | 0.797 | 0.022 | 0.89 | |
| % Uninsured | Pearson Correlation | −0.008 | −0.231 | 0.387 ** |
| p value (2-tailed) | 0.952 | 0.388 | 0.001 | |
| Model | % Positive Wells (Square Root Transformation) | Potential Cofounders | Slope, β (p Values) | R2 |
|---|---|---|---|---|
| Breast cancer rates | ||||
| 1 | Physically unhealthy days | −10.30 (0.20) | 0.037 | |
| Atrazine | 0.48 (0.74) | |||
| Acetochlor | 0.94 (0.76) | |||
| 2 | Physically unhealthy days | −8.06 (0.32) | 0.079 | |
| DEA | −1.90 (0.16) | |||
| DIA | 2.10 (0.23) | |||
| Acetochlor | 1.84 (0.56) | |||
| Uterine cancer rates | ||||
| 1 | Physically unhealthy days | 13.31 (0.04) | 0.67 | |
| % Binge drinking | −0.53 (0.36) | |||
| Atrazine | 0.26 (0.70) | |||
| Acetochlor | 4.62 (0.03) | |||
| 2 | Physically unhealthy days | 15.46 (0.014) | 0.75 | |
| % Binge drinking | −0.31 (0.63) | |||
| DEA | 0.52 (0.39) | |||
| DIA | 0.36 (0.58) | |||
| Acetochlor | 4.01 (0.04) | |||
| Prostate cancer rates | ||||
| 1 | % uninsured | 1.36 (0.12) | 0.085 | |
| Atrazine | −1.38 (0.30) | |||
| Acetochlor | −0.64 (0.81) | |||
| 2 | % uninsured | 1.52 (0.09) | 0.10 | |
| DEA | −1.54 (0.22) | |||
| DIA | 1.18 (0.46) | |||
| Acetochlor | −0.65 (0.81) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
New-Aaron, M.; Naveed, Z.; Rogan, E.G. Estrogen Disrupting Pesticides in Nebraska Groundwater: Trends between Pesticide-contaminated Water and Estrogen-related Cancers in An Ecological Observational Study. Water 2021, 13, 790. https://doi.org/10.3390/w13060790
New-Aaron M, Naveed Z, Rogan EG. Estrogen Disrupting Pesticides in Nebraska Groundwater: Trends between Pesticide-contaminated Water and Estrogen-related Cancers in An Ecological Observational Study. Water. 2021; 13(6):790. https://doi.org/10.3390/w13060790
Chicago/Turabian StyleNew-Aaron, Moses, Zaeema Naveed, and Eleanor G. Rogan. 2021. "Estrogen Disrupting Pesticides in Nebraska Groundwater: Trends between Pesticide-contaminated Water and Estrogen-related Cancers in An Ecological Observational Study" Water 13, no. 6: 790. https://doi.org/10.3390/w13060790
APA StyleNew-Aaron, M., Naveed, Z., & Rogan, E. G. (2021). Estrogen Disrupting Pesticides in Nebraska Groundwater: Trends between Pesticide-contaminated Water and Estrogen-related Cancers in An Ecological Observational Study. Water, 13(6), 790. https://doi.org/10.3390/w13060790






