Eutrophication and Geochemistry Drive Pelagic Calcite Precipitation in Lakes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experimental Setup
2.3. Open Water Dynamics
2.4. Measurements of Hydrochemical Parameters
2.5. Data Analysis
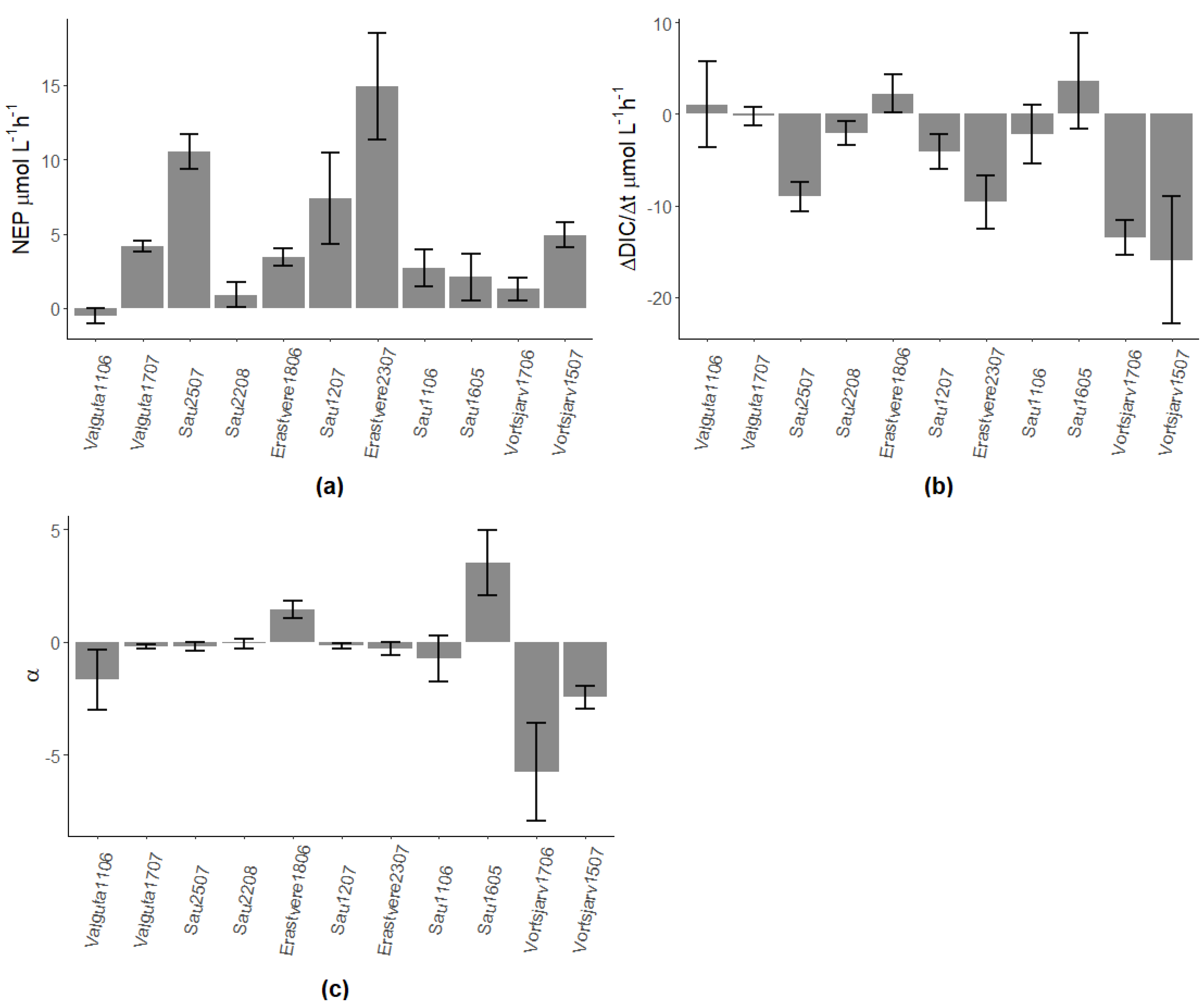
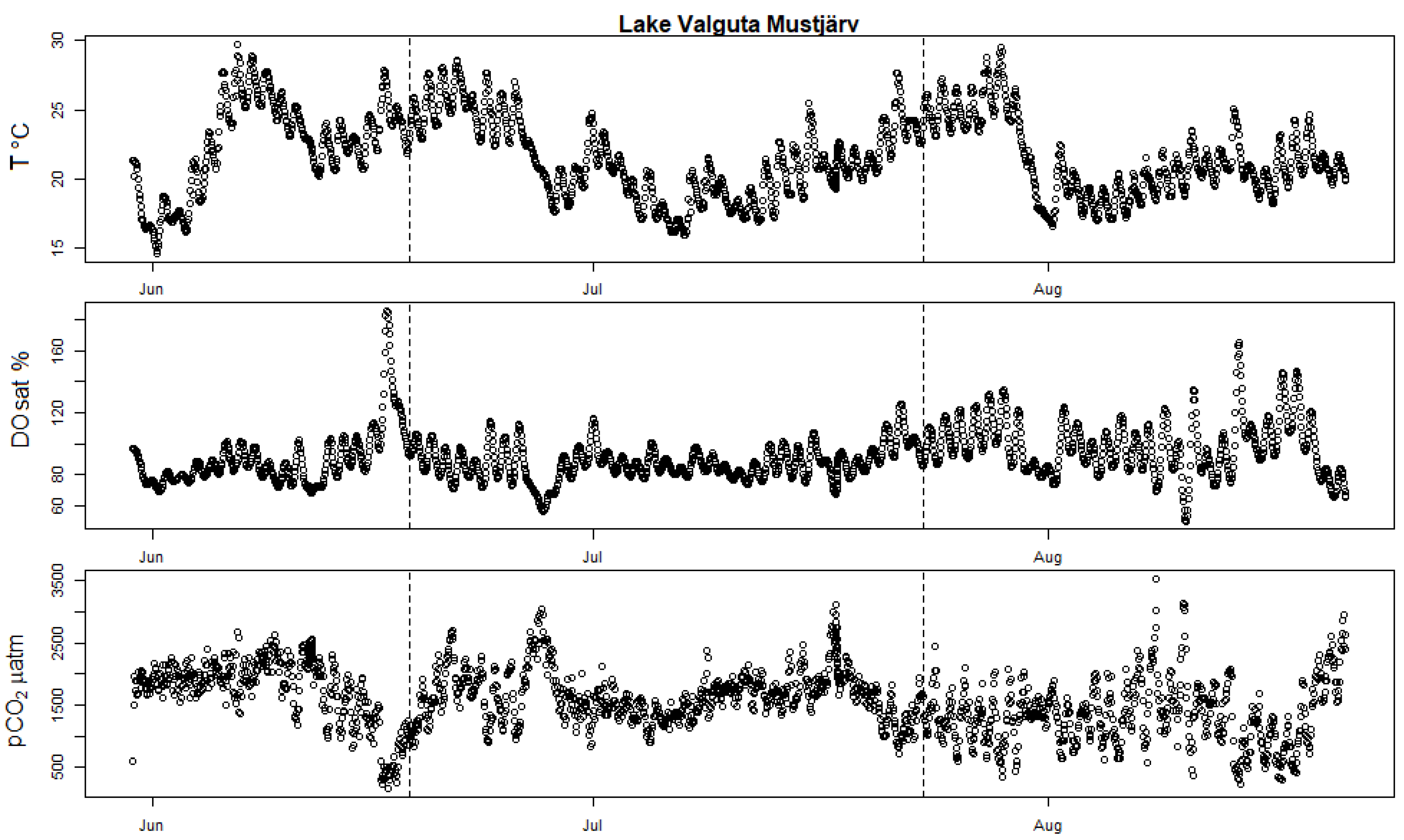
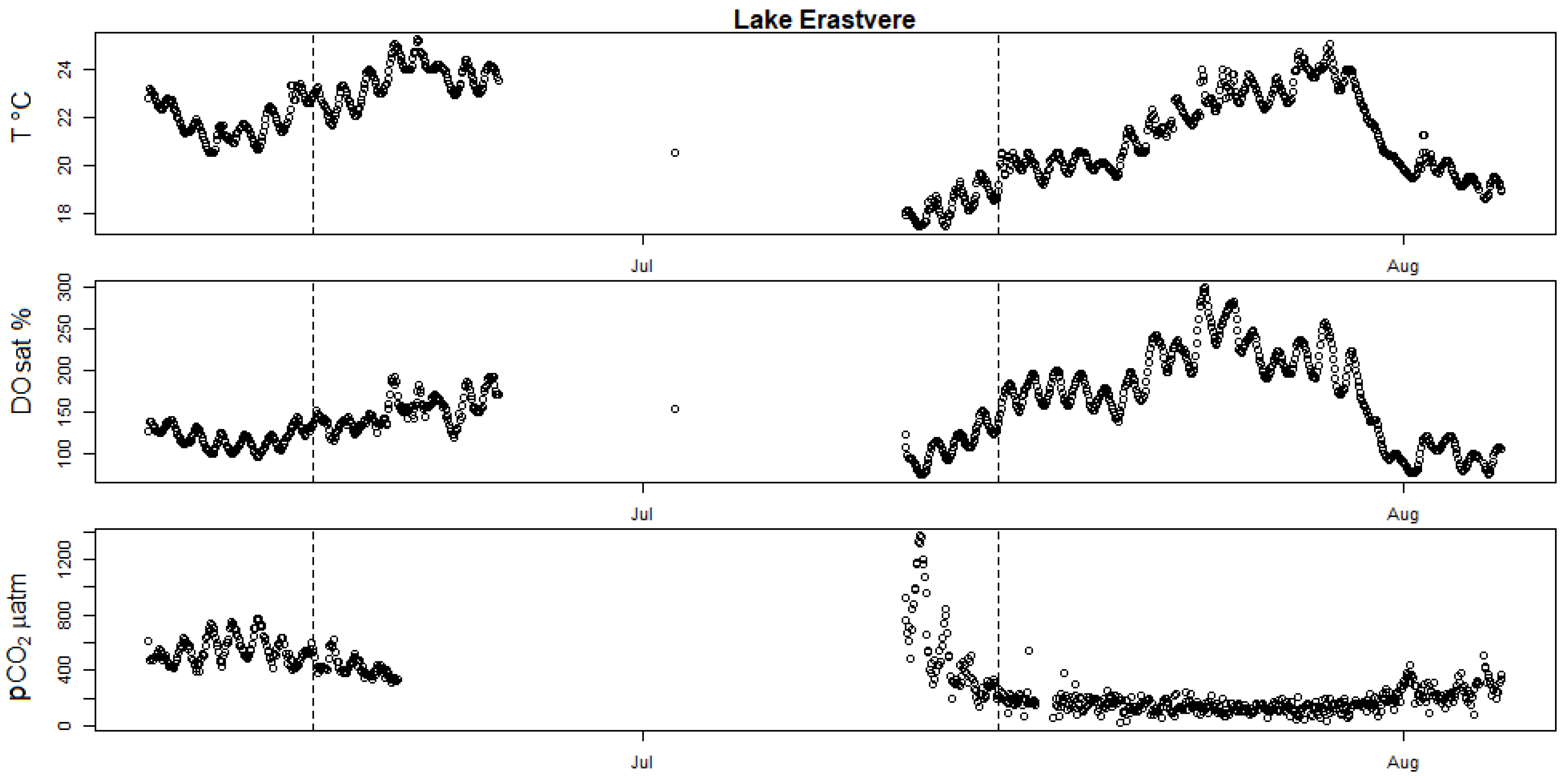
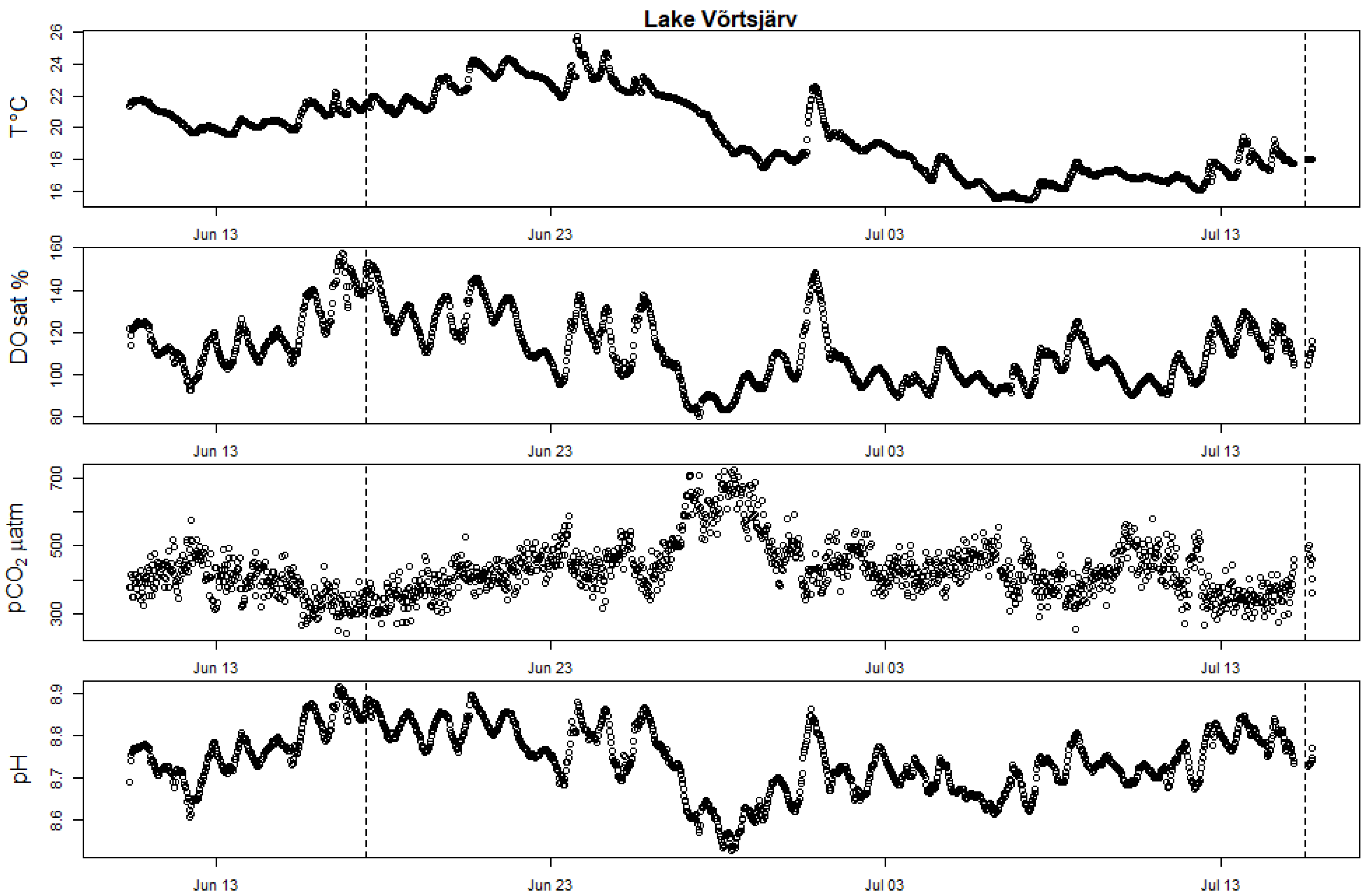
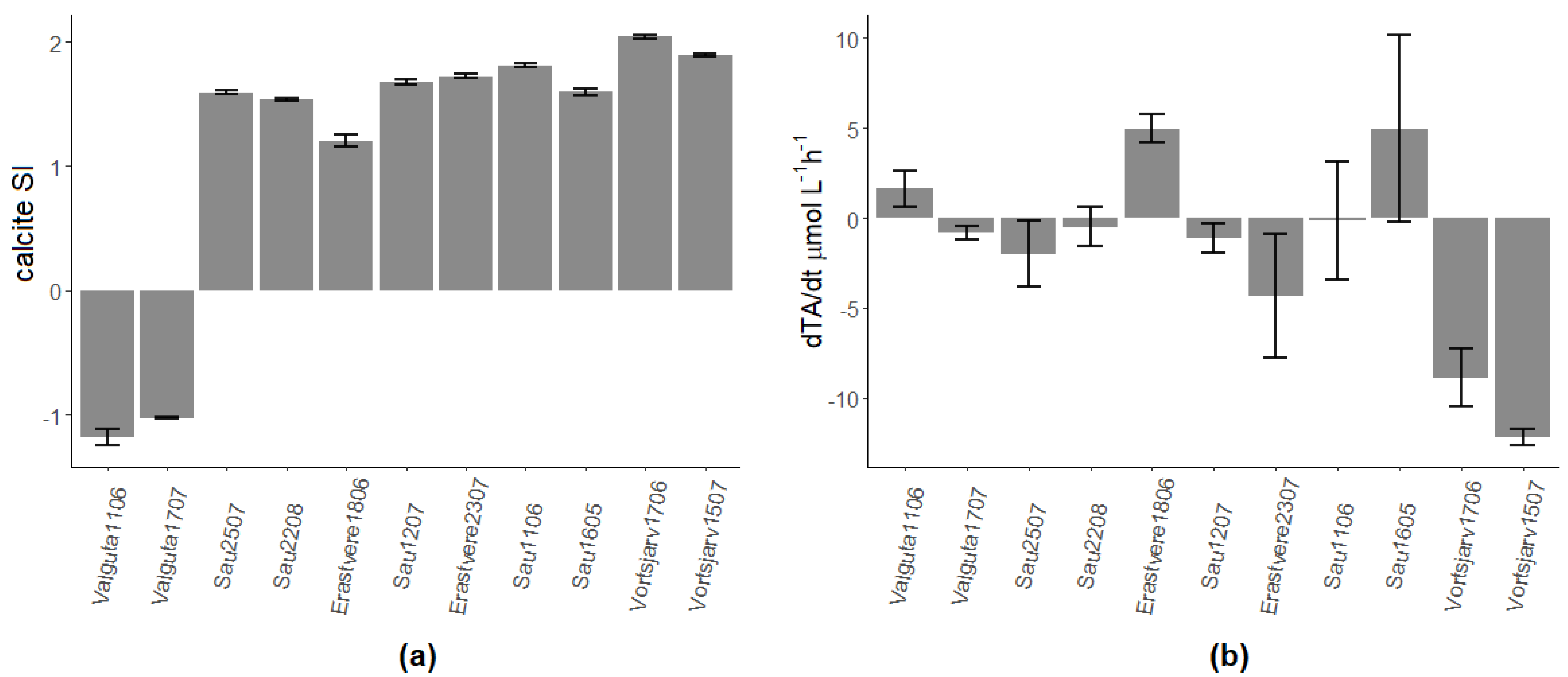
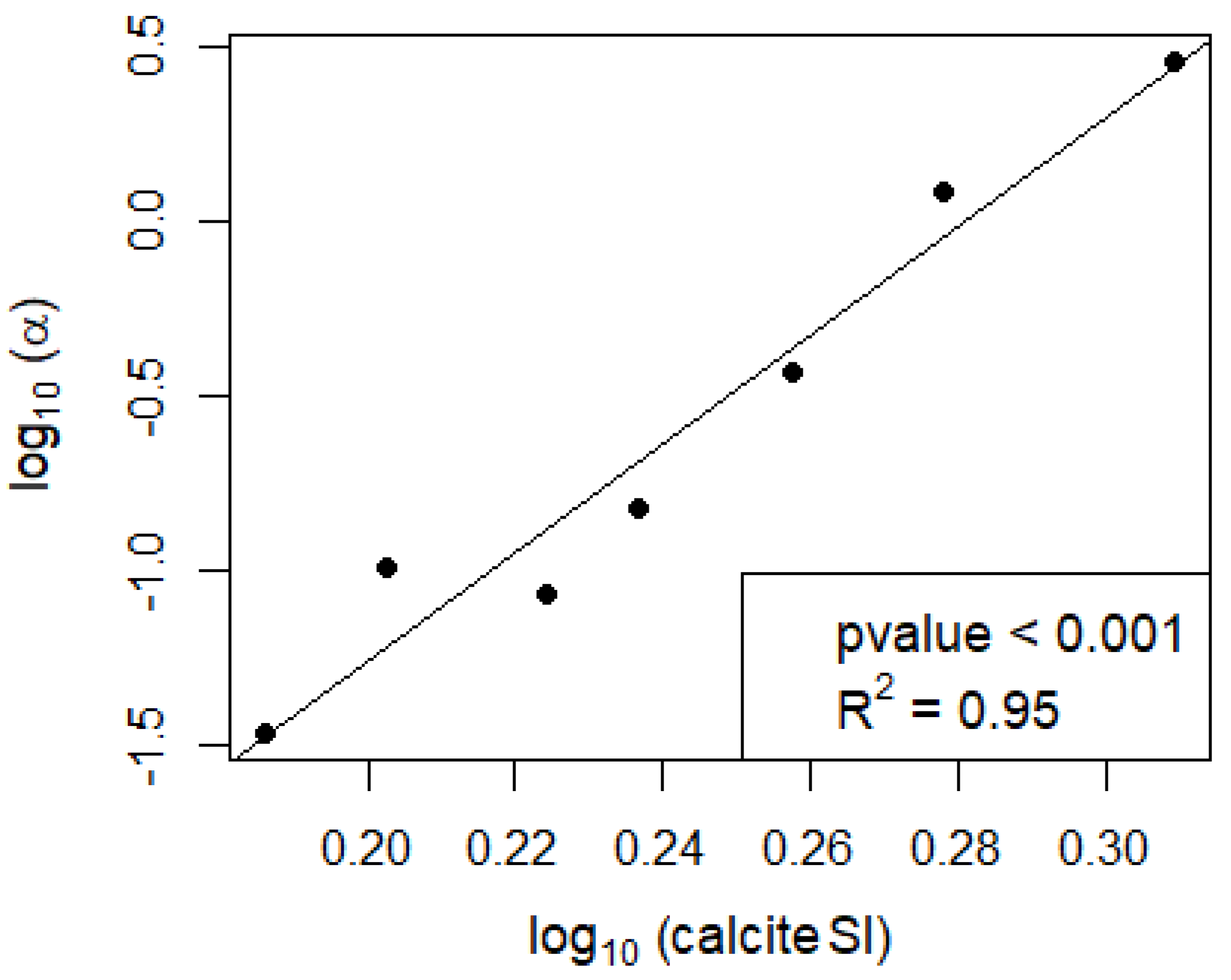
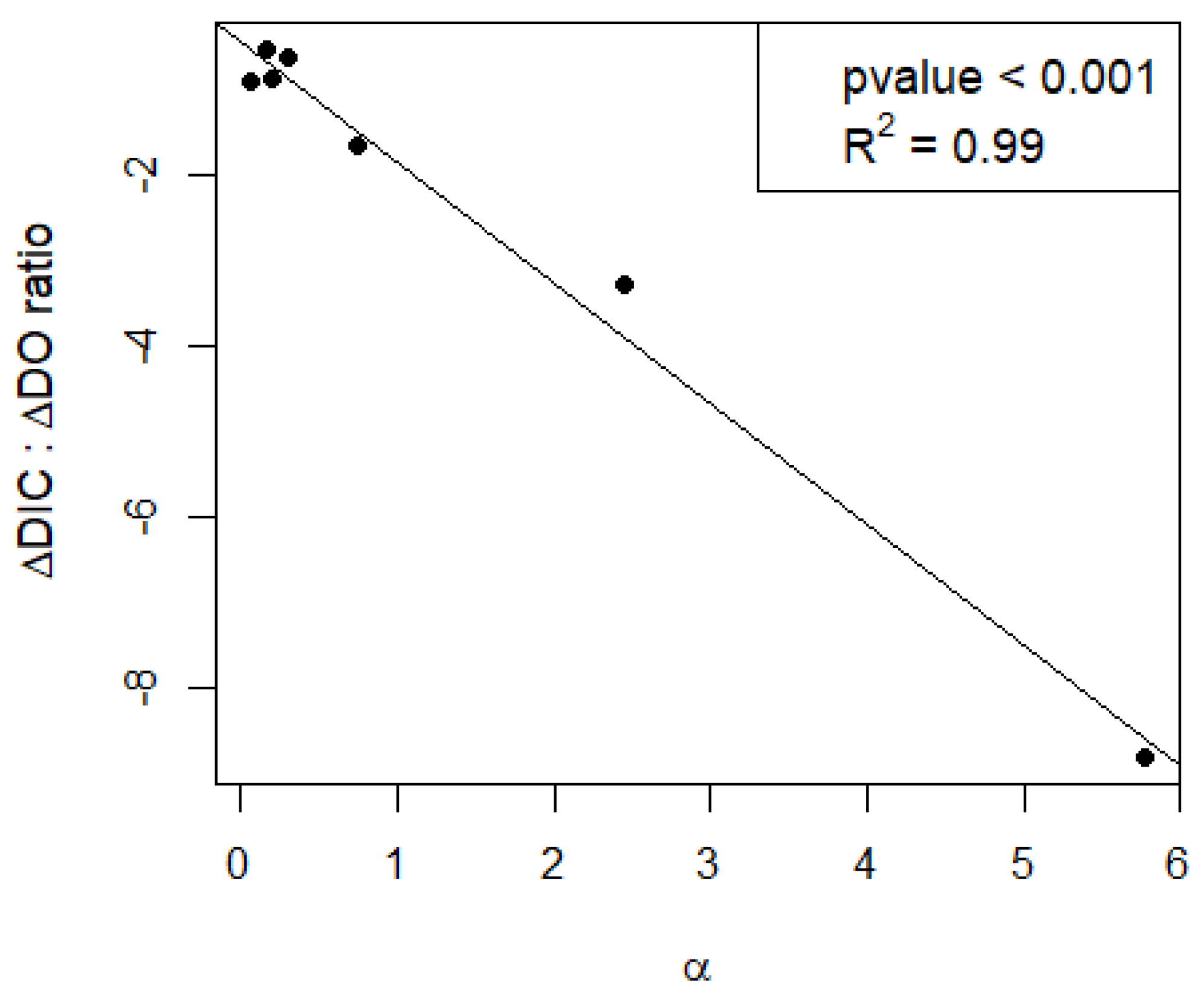
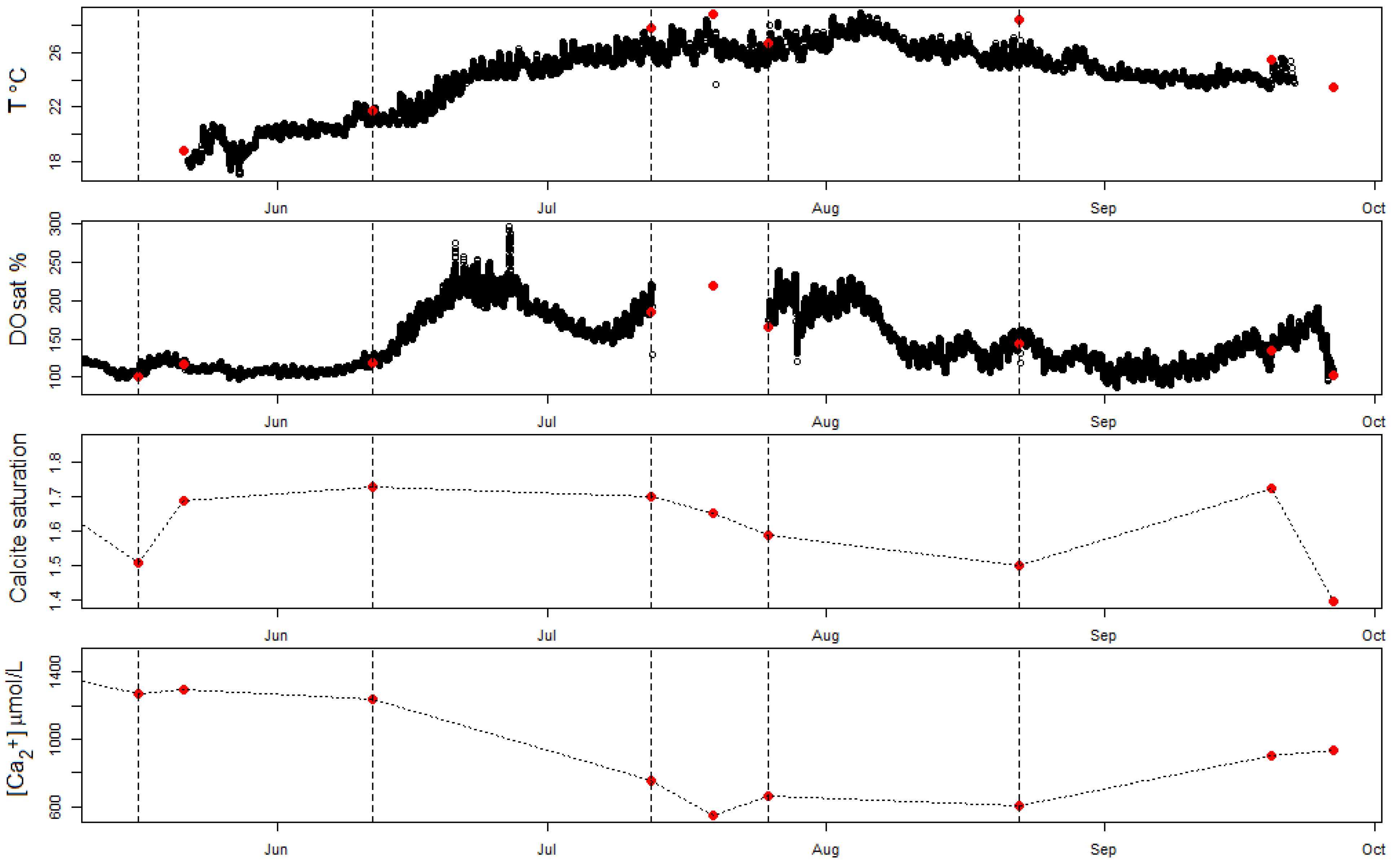
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Lake | Date | Alkalinity (meq/L) | Chlorophyll-a (µg/L) | TP (µg/L) | TN (mg/L) | DOC (mg/L) | DIC (µmol/L) | Ca2+ (µmol/L) |
|---|---|---|---|---|---|---|---|---|
| Valguta Mustjärv (EE) | 11 June 2019 | 0.52 | 14.0 | 641 | 2.0 | 42.1 | 696.6 | 234.7 |
| Valguta Mustjärv (EE) | 17 July 2019 | 0.67 | NA | 572 | 1.7 | 39.3 | 868.1 | 245.8 |
| Erastvere (EE) | 18 June 2019 | 1.48 | 10.5 | 43 | 0.9 | 12.6 | 1469.9 | 487.6 |
| Erastvere (EE) | 23 July 2019 | 1.79 | NA | 56 | 1.6 | 11.8 | 1568.4 | 491.3 |
| Võrtsjärv (EE) | 17 June 2019 | 3.23 | 26.1 | 23 | 1.1 | 12.0 | 3054.6 | 1066.3 |
| Võrtsjärv (EE) | 15 July 2019 | 4.08 | 22.3 | 36 | 1.4 | 12.4 | 3949.2 | 1091.9 |
| Sau Reservoir (ES) | 16 May 2018 | 2.57 | NA | NA | NA | 20.5 | 2540.1 | 1312.1 |
| Sau Reservoir (ES) | 11 June 2018 | 2.45 | 20.1 | 59 | 6.2 | NA | 2393.8 | 1260.3 |
| Sau Reservoir (ES) | 12 July 2018 | 1.52 | 4.9 | 36 | 4.3 | NA | 1412.1 | 711.1 |
| Sau Reservoir (ES) | 25 July 2018 | 1.19 | 28.6 | 73 | 3.7 | NA | 1047.3 | 602.8 |
| Sau Reservoir (ES) | 22 August 2018 | 1.19 | 32.0 | 39 | 3.0 | NA | 1099.6 | 607.7 |
References
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, R.; Müller, R.A.; Clow, D.; Verpoorter, C.; Raymond, P.; Tranvik, L.J.; Sobek, S. Organic carbon burial in global lakes and reservoirs. Nat. Commun. 2017, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, C.M.; Prairie, Y.T. Prevalence of heterotrophy and atmospheric CO2 emissions from aquatic ecosystems. Ecosystems 2005, 8, 862–870. [Google Scholar] [CrossRef]
- Del Giorgio, P.A.; Cole, J.J.; Caraco, N.F.; Peters, R.H. Linking planktonic biomass and metabolism to net gas fluxes in northern temperate lakes. Ecology 1999, 80, 1422–1431. [Google Scholar] [CrossRef]
- Larsen, S.; Andersen, T.; Hessen, D.O. The pCO2 in boreal lakes: Organic carbon as a universal predictor? Glob. Biogeochem. Cycles 2011, 25. [Google Scholar] [CrossRef]
- Marcé, R.; Obrador, B.; Morguí, J.-A.; Riera, J.L.; López, P.; Joan, A. Carbonate weathering as a driver of CO2 supersaturation in lakes. Nat. Geosci. 2015, 8, 107–111. [Google Scholar] [CrossRef]
- McDonald, C.P.; Stets, E.G.; Striegl, R.G.; Butman, D. Inorganic carbon loading as a primary driver of dissolved carbon dioxide concentrations in the lakes and reservoirs of the contiguous United States. Glob. Biogeochem. Cycles 2013, 27, 285–295. [Google Scholar] [CrossRef]
- Müller, B.; Meyer, J.S.; Gächter, R. Alkalinity regulation in calcium carbonate-buffered lakes. Limnol. Oceanogr. 2016, 61, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Nõges, P.; Cremona, F.; Laas, A.; Martma, T.; Rõõm, E.I.; Toming, K.; Viik, M.; Vilbaste, S.; Nõges, T. Role of a productive lake in carbon sequestration within a calcareous catchment. Sci. Total Environ. 2016, 550, 225–230. [Google Scholar] [CrossRef]
- Fuchs, A.; Selmeczy, G.B.; Kasprzak, P.; Padisák, J.; Casper, P. Coincidence of sedimentation peaks with diatom blooms, wind, and calcite precipitation measured in high resolution by a multi-trap. Hydrobiologia 2016, 763, 329–344. [Google Scholar] [CrossRef]
- Trapote, M.C.; Vegas-Vilarrúbia, T.; López, P.; Puche, E.; Gomà, J.; Buchaca, T.; Cañellas-Boltà, N.; Safont, E.; Corella, J.P.; Rull, V. Modern sedimentary analogues and integrated monitoring to understand varve formation in the Mediterranean Lake Montcortès (Central Pyrenees, Spain). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 496, 292–304. [Google Scholar] [CrossRef] [Green Version]
- Obst, M.; Wehrli, B.; Dittrich, M. CaCO3 nucleation by cyanobacteria: Laboratory evidence for a passive, surface-induced mechanism. Geobiology 2009, 7, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, M.; Obst, M. Are picoplankton responsible for calcite precipitation in lakes? Ambio 2004, 33, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, M.; Kurz, P.; Wehrli, B. The Role of Autotrophic Picocyanobacteria in Calcite Precipitation in an Oligotrophic Lake. Geomicrobiol. J. 2004, 21, 45–53. [Google Scholar] [CrossRef]
- Strong, A.E.; Eadie, B.J. Satellite observations of calcium carbonate precipitation in the Great Lakes. Limnol. Ocean. 1978, 23, 877–887. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems: A global problem. Environ. Sci. Pollut. Res. Int. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. ECOLOGY: Controlling Eutrophication: Nitrogen and Phosphorus. Sci. Am. Assoc. Adv. Sci. 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Stets, E.G.; Striegl, R.G.; Aiken, G.R.; Rosenberry, D.O.; Winter, T.C. Hydrologic support of carbon dioxide flux revealed by whole-lake carbon budgets. J. Geophys. Res. 2009, 114, 1–14. [Google Scholar] [CrossRef]
- Perga, M.-E.; Maberly, S.C.; Jenny, J.-P.; Alric, B.; Pignol, C.; Naffrechoux, E. A century of human-driven changes in the carbon dioxide concentration of lakes. Glob. Biogeochem. Cycles 2016, 30, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Prairie, Y.T.; Montes, C.; Cole, J.J.; Striegl, R.; Melack, J.; Downing, J.A. CO2 emissions from saline lakes: A global estimate of a surprisingly large flux. J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Marcé, R.; Laas, A.; Obrador, B. The relevance of pelagic calcification in the global carbon budget of lakes and reservoirs. Limnetica. in press.
- Lu, W.; Wang, S.; Yeager, K.M.; Liu, F.; Huang, Q.; Yang, Y.; Xiang, P.; Lü, Y.; Liu, C. Importance of considered organic vs. inorganic source of carbon to lakes for calculating net effect on landscape C budgets. J. Geophys. Res. Biogeosci. 2018, 123, 1302–1317. [Google Scholar] [CrossRef]
- Megard, R.O. Planktonic photosynthesis and the environment of carbonate deposition in lakes. SIL Commun. 1953–1996 1968, 17, 94. [Google Scholar] [CrossRef]
- McConnaughey, T. Calcification in Chara corallina: CO2 hydroxylation generates protons for bicarbonate assimilation. Limnol. Oceanogr. 1991, 36, 619–628. [Google Scholar] [CrossRef]
- McConnaughey, T.A.; Whelan, J.F. Calcification generates protons for nutrient and bicarbonate uptake. Earth Sci. Rev. 1997, 42, 95–117. [Google Scholar] [CrossRef]
- Riebesell, U.; Zondervan, I.; Rost, B.; Tortell, P.D.; Zeebe, R.E.; Morel, F.M.M. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 2000, 407, 364–367. [Google Scholar] [CrossRef] [Green Version]
- Gattuso, J.-P.; Allemand, D.; Frankignoulle, M. Photosynthesis and Calcification at cellular, organismal and community levels in coral reefs: A review on interactions and control by carbonate chemistry. Integr. Comp. Biol. 1999, 39, 160–183. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Laas, A.; Marcé, R.; Obrador, B. Major effects of alkalinity on the relationship between metabolism and dissolved inorganic carbon dynamics in lakes. Ecosystems 2020, 23, 1566–1580. [Google Scholar] [CrossRef] [Green Version]
- Laas, A.; Cremona, F.; Meinson, P.; Rõõm, E.-I.; Nõges, T.; Nõges, P. Summer depth distribution profiles of dissolved CO2 and O2 in shallow temperate lakes reveal trophic state and lake type specific differences. Sci. Total Environ. 2016, 566–567, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Cremona, F.; Laas, A.; Arvola, L.; Pierson, D.; Nõges, P.; Nõges, T. Numerical exploration of the planktonic to benthic primary production ratios in lakes of the Baltic Sea catchment. Ecosystems 2016, 19, 1386–1400. [Google Scholar] [CrossRef]
- Rõõm, E.I.; Nõges, P.; Feldmann, T.; Tuvikene, L.; Kisand, A.; Teearu, H.; Nõges, T. Years are not brothers: Two-year comparison of greenhouse gas fluxes in large shallow Lake Võrtsjärv, Estonia. J. Hydrol. 2014, 519, 1594–1606. [Google Scholar] [CrossRef]
- Marcé, R.; Moreno-Ostos, E.; Armengol, J. The role of river inputs on the hypolimnetic chemistry of a productive reservoir: Implications for management of anoxia and total phosphorus internal loading. Lake Reserv. Manag. 2008, 24, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Marcé, R.; George, G.; Buscarinu, P.; Deidda, M.; Dunalska, J.; De Eyto, E.; Flaim, G.; Grossart, H.P.; Istvanovics, V.; Lenhardt, M.; et al. Automatic High Frequency Monitoring for Improved Lake and Reservoir Management. Environ. Sci. Technol. 2016, 50, 10780–10794. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Hansen, H.P.; Koroleff, F. Determination of nutrients. In Methods of Seawater Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1999; pp. 159–228. ISBN 9783527613984. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA; AWWA: Denver, CO, USA; WEF: Alexandria, VA, USA, 1999. [Google Scholar]
- CEN European Commitee for Standardization EN 1484 Water analysis—Guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC) Analyse. CEN Eur. Commitee Stand. 1997, 2007, 11.
- Lewis, E.; Wallace, D.; Allison, L.J. Program developed for CO2 system calculations. Carbon Dioxide Inf. Anal. Cent. 1998, 1–21. [Google Scholar] [CrossRef]
- Millero, F.J. The thermodynamics of the carbonate system in seawater. Geochim. Cosmochim. Acta 1979, 43, 1651–1661. [Google Scholar] [CrossRef]
- Mucci, A. The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure. Am. J. Sci. 1983, 283. [Google Scholar] [CrossRef]
- Hamilton, S.K.; Bruesewitz, D.A.; Horst, G.P.; Weed, D.B.; Sarnelle, O. Biogenic calcite–phosphorus precipitation as a negative feedback to lake eutrophication. Can. J. Fish. Aquat. Sci. 2009, 66, 343–350. [Google Scholar] [CrossRef]
- Khan, H. Calcite precipitation and pCO2 model. 2020. [Google Scholar] [CrossRef]
- Walsh, J.R.; Corman, J.R.; Munoz, S.E. Coupled long-term limnological data and sedimentary records reveal new control on water quality in a eutrophic lake. Limnol. Oceanogr. 2019, 64, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.H.; Ishikawa, K.; Sakai, Y.; Ishikawa, T.; Ichise, S.; Yamamoto, Y.; Kuo, T.C.; Park, H.D.; Yamamura, N.; Kumagai, M. Phytoplankton community reorganization driven by eutrophication and warming in Lake Biwa. Aquat. Sci. 2010, 72, 467–483. [Google Scholar] [CrossRef]
- Drake, T.W.; Tank, S.E.; Zhulidov, A.V.; Holmes, R.M.; Gurtovaya, T.; Spencer, R.G.M. Increasing Alkalinity Export from Large Russian Arctic Rivers. Environ. Sci. Technol. 2018, 52, 8302–8308. [Google Scholar] [CrossRef] [PubMed]
- Raymond, P.A.; Cole, J.J. Increase in the export of alkalinity from North America’s largest river. Science 2003, 301, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Gattuso, J.-P.; Frankignoulle, M.; Smith, S. V Measurement of community metabolism and significance in the coral reef CO2 source-sink debate. Proc. Natl. Acad. Sci. USA 1999, 96, 13017–13022. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A. Combined effects of photosynthesis and calcification on the partial pressure of carbon dioxide in seawater. J. Oceanogr. 1998, 54, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lerman, A.; Mackenzie, F.T. Carbonate minerals and the CO2-carbonic acid system. In Encyclopedia of Geochemistry; Encyclopedia of Earth Sciences Series; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Stabel, H.-H. Calcite precipitation in Lake Constance: Chemical equilibrium, sedimentation, and nucleation by algae. Limnol. Oceanogr. 1986, 31, 1081–1093. [Google Scholar] [CrossRef] [Green Version]
- Ridgwell, A.; Zeebe, R.E. The role of the global carbonate cycle in the regulation and evolution of the Earth system. Earth Planet. Sci. Lett. 2005, 234, 299–315. [Google Scholar] [CrossRef]
- Kalokora, O.J.; Buriyo, A.S.; Asplund, M.E.; Gullström, M.; Mtolera, M.S.P.; Björk, M. An experimental assessment of algal calcification as a potential source of atmospheric CO2. PLoS ONE 2020, 15, E0231971. [Google Scholar] [CrossRef] [PubMed]
- Obrador, B.; Pretus, J.L. Carbon and oxygen metabolism in a densely vegetated lagoon: Implications of spatial heterogeneity. Limnetica 2013, 32, 321–336. [Google Scholar]
- Vachon, D.; Sadro, S.; Bogard, M.J.; Lapierre, J.; Baulch, H.M.; Rusak, J.A.; Denfeld, B.A.; Laas, A.; Klaus, M.; Karlsson, J.; et al. Paired O2–CO2 measurements provide emergent insights into aquatic ecosystem function. Limnol. Oceanogr. Lett. 2020, 5, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Andersen, R.M.; Kragh, T.; Martinsen, T.; Kristensen, E.; Sand-Jensen, K. The carbon pump supports high primary production in a shallow lake. Aquat. Sci. 2019, 81. [Google Scholar] [CrossRef]
- Finlay, K.; Vogt, R.J.; Simpson, G.L.; Leavitt, P.R. Seasonality of pCO2 in a hard-water lake of the northern Great Plains: The legacy effects of climate and limnological conditions over 36 years. Limnol. Oceanogr. 2019, 64, S118–S129. [Google Scholar] [CrossRef] [Green Version]

| Lake | Trophic State | Mixing Regime | Area (Ha) | Mean Depth (m) | Maximum Depth (m) | Kd (m) | Watershed Size (km2) | Water Residence Time (y) |
|---|---|---|---|---|---|---|---|---|
| Valguta Mustjärv (EE) | hypertrophic | polymictic | 20.4 | <1 | 1 | 10.3 | 1.34 | source lake |
| Erastvere (EE) | hypertrophic | dimictic | 16.3 | 3.5 | 9.7 | 2.9 | 5.2 | 0.5 |
| Võrtsjärv (EE) | eutrophic | polymictic | 27000 | 2.8 | 6 | 2.7 | 3116 | 1 |
| Sau Reservoir (ES) | eutrophic | monomictic | 600 | 25.2 | 65 | 0.9 | 1522 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, H.; Laas, A.; Marcé, R.; Sepp, M.; Obrador, B. Eutrophication and Geochemistry Drive Pelagic Calcite Precipitation in Lakes. Water 2021, 13, 597. https://doi.org/10.3390/w13050597
Khan H, Laas A, Marcé R, Sepp M, Obrador B. Eutrophication and Geochemistry Drive Pelagic Calcite Precipitation in Lakes. Water. 2021; 13(5):597. https://doi.org/10.3390/w13050597
Chicago/Turabian StyleKhan, Hares, Alo Laas, Rafael Marcé, Margot Sepp, and Biel Obrador. 2021. "Eutrophication and Geochemistry Drive Pelagic Calcite Precipitation in Lakes" Water 13, no. 5: 597. https://doi.org/10.3390/w13050597






