An Enhanced Electrocoagulation Process for the Removal of Fe and Mn from Municipal Wastewater Using Dielectrophoresis (DEP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Wastewater Samples
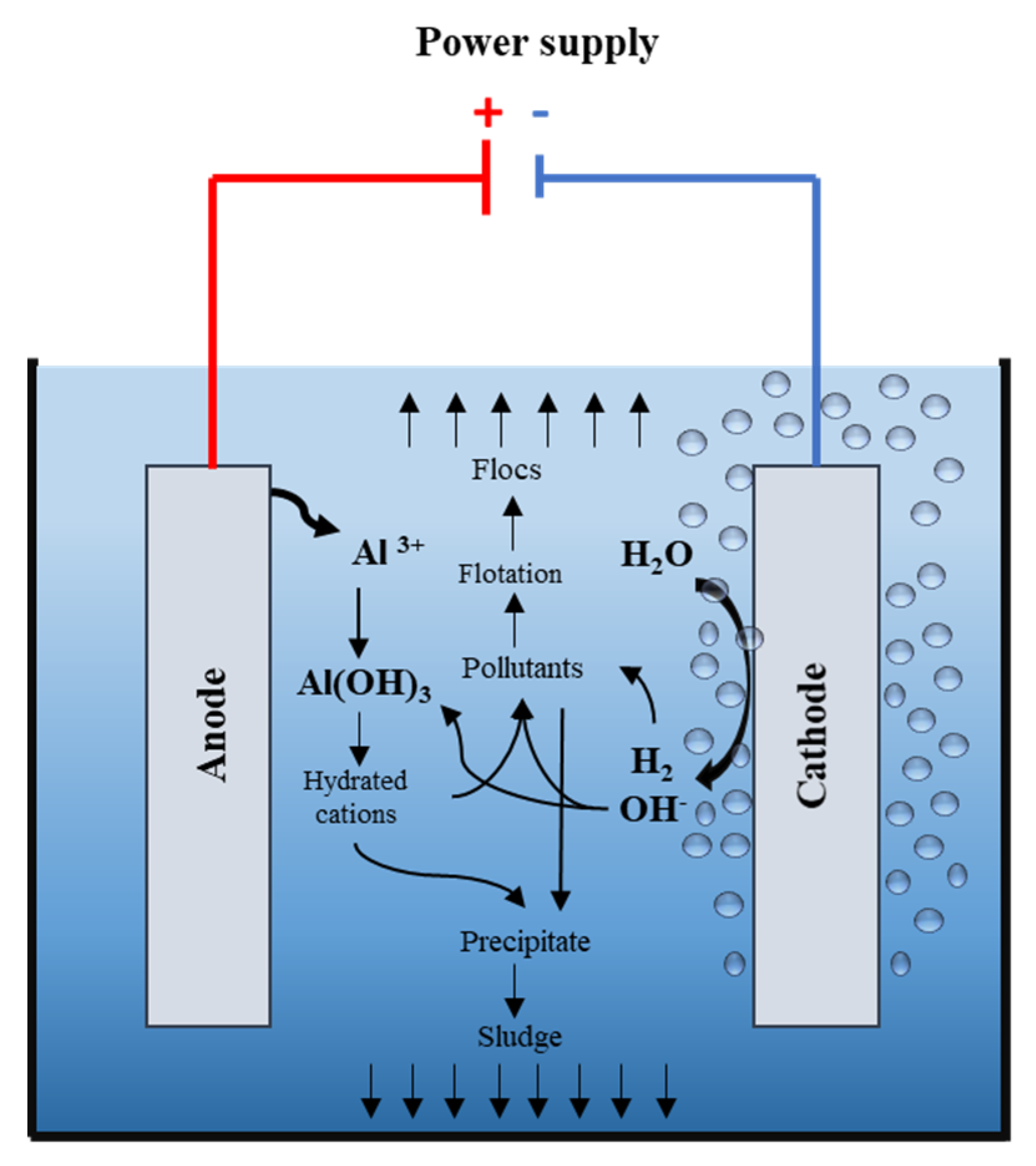
2.2. Experimental Setup
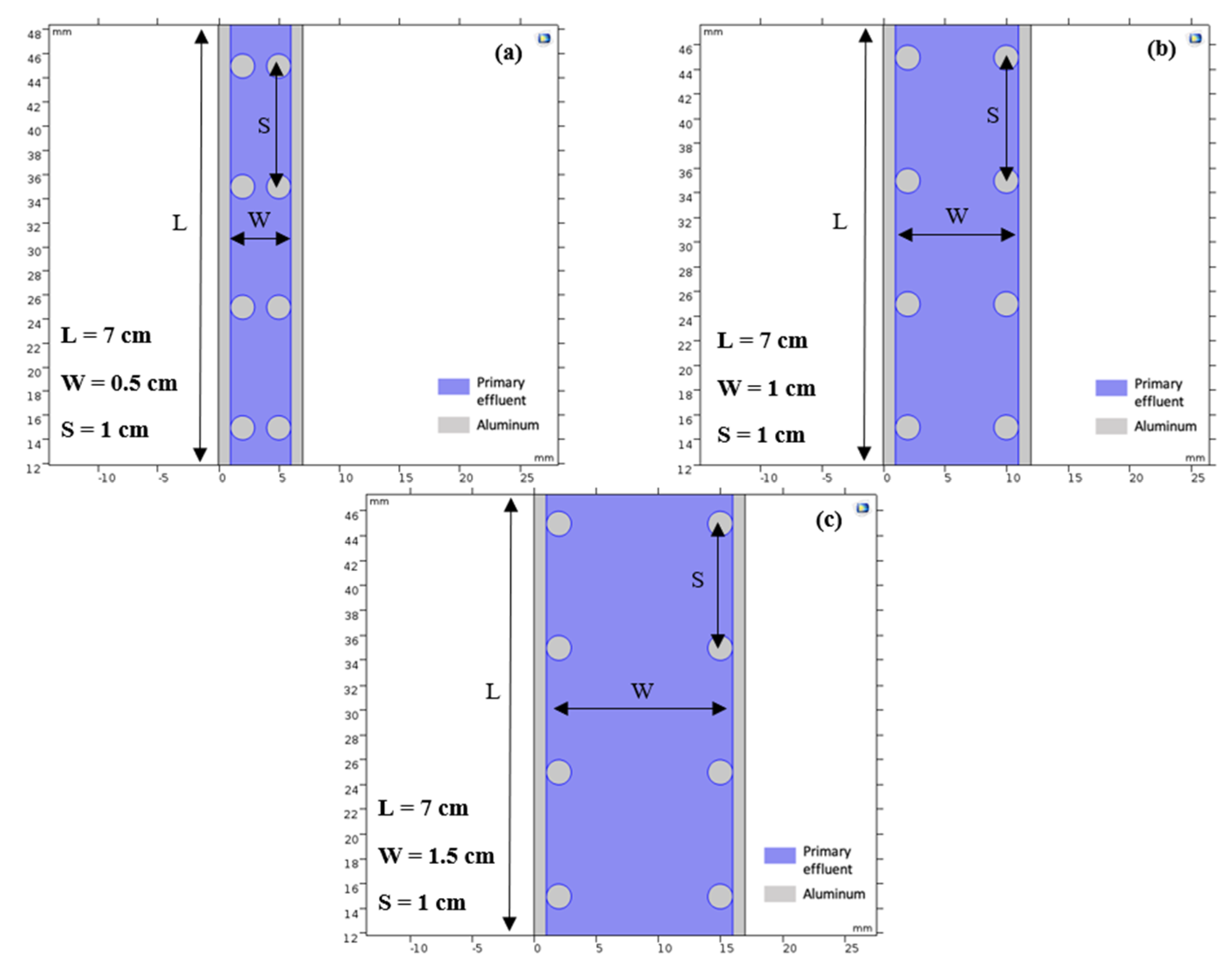
2.3. Numerical Simulation
2.4. Error Estimation
3. Results
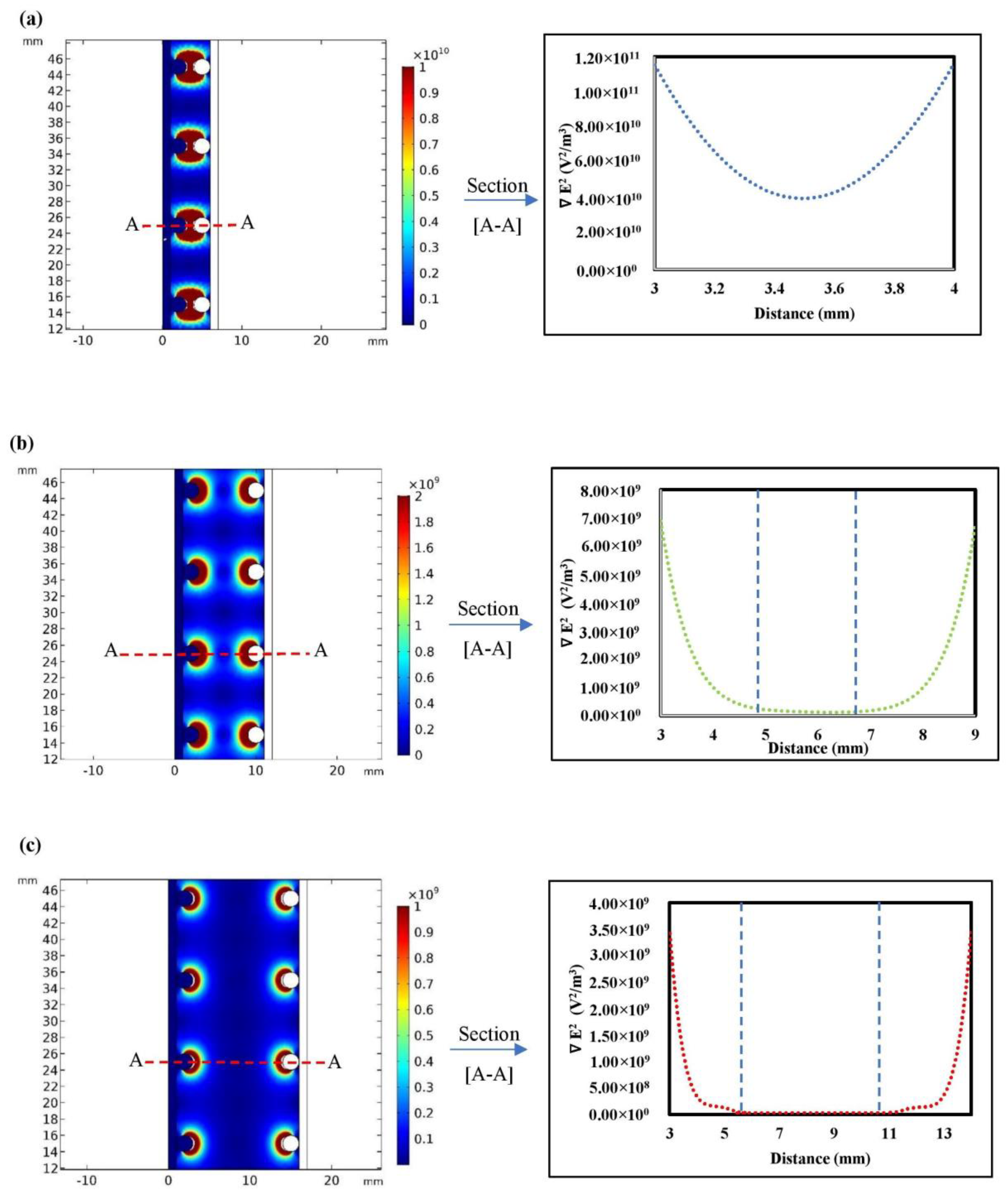
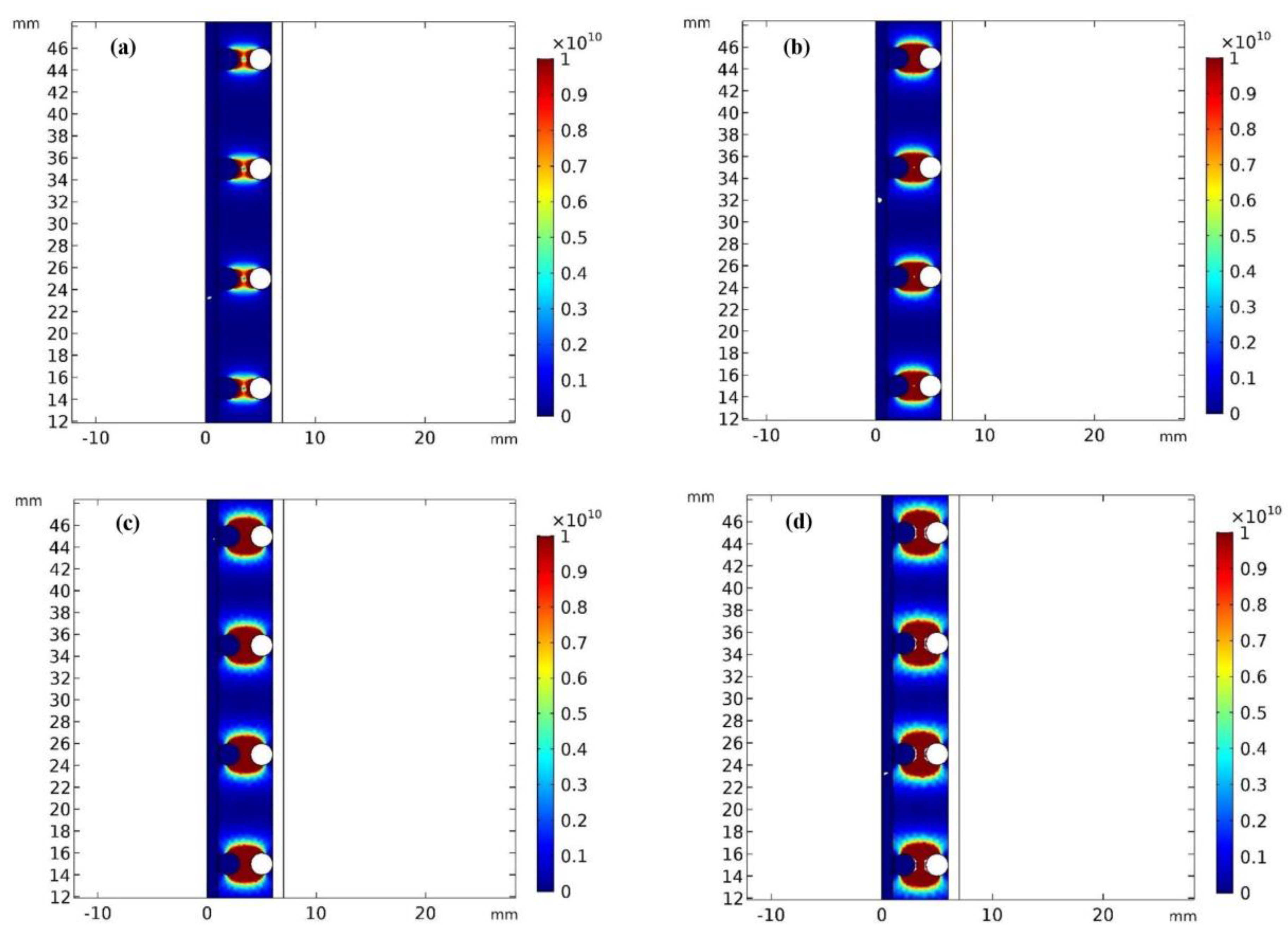
3.1. Numerical Simulation
3.1.1. Impact of the Electrode Spacing
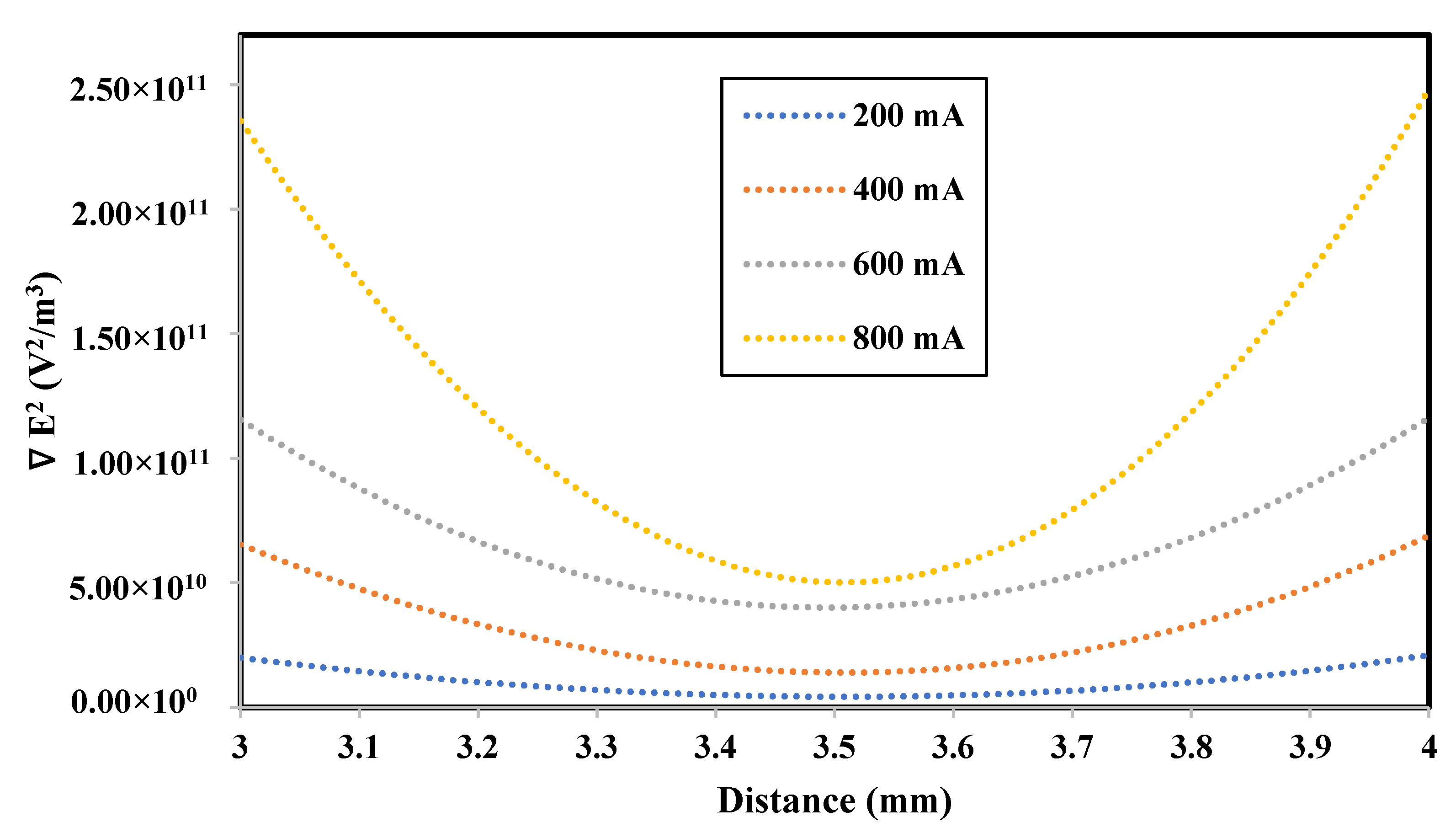
3.1.2. Impact of the Applied Current
3.2. Experimental Study
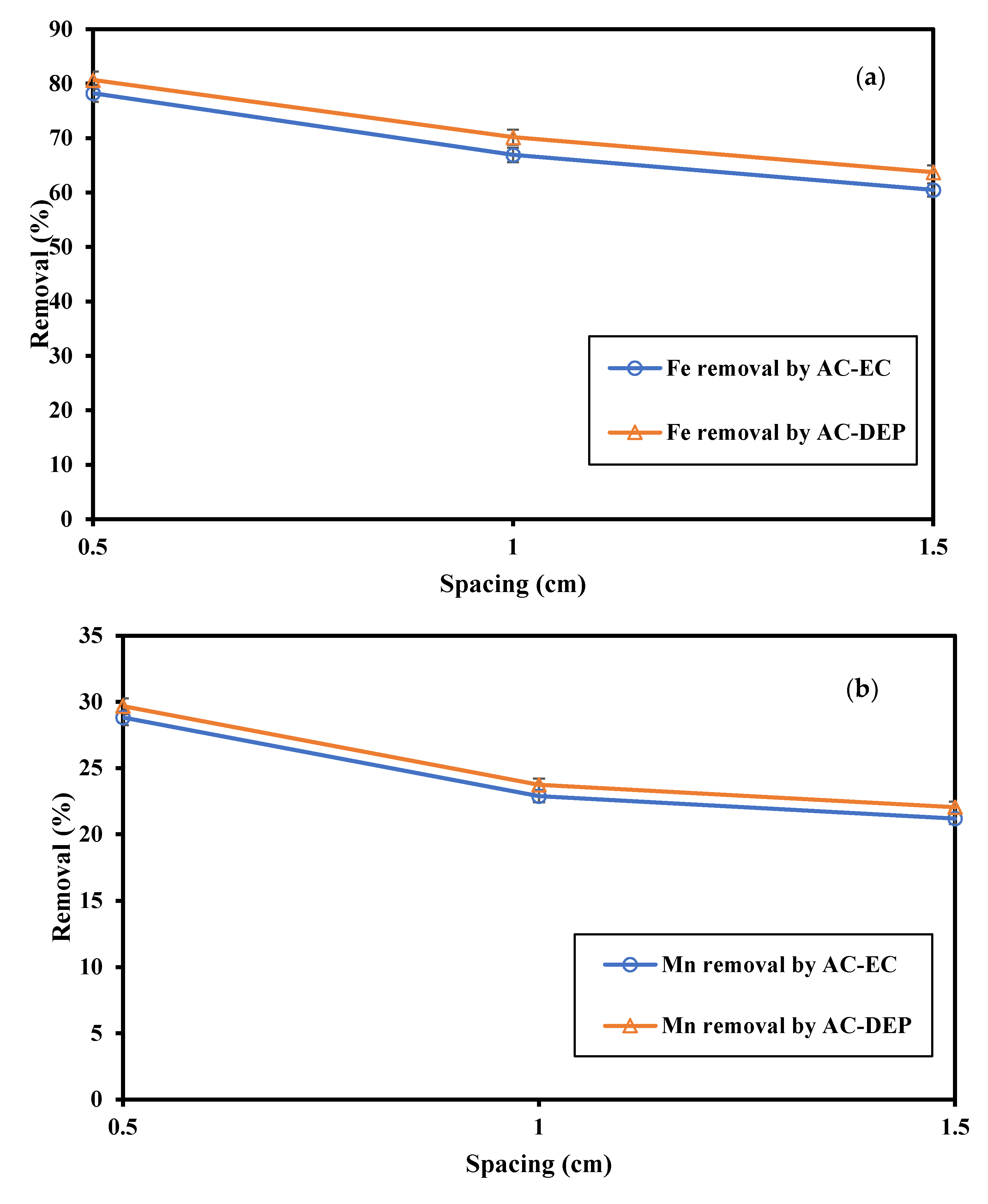
3.2.1. Impact of the Electrode Spacing
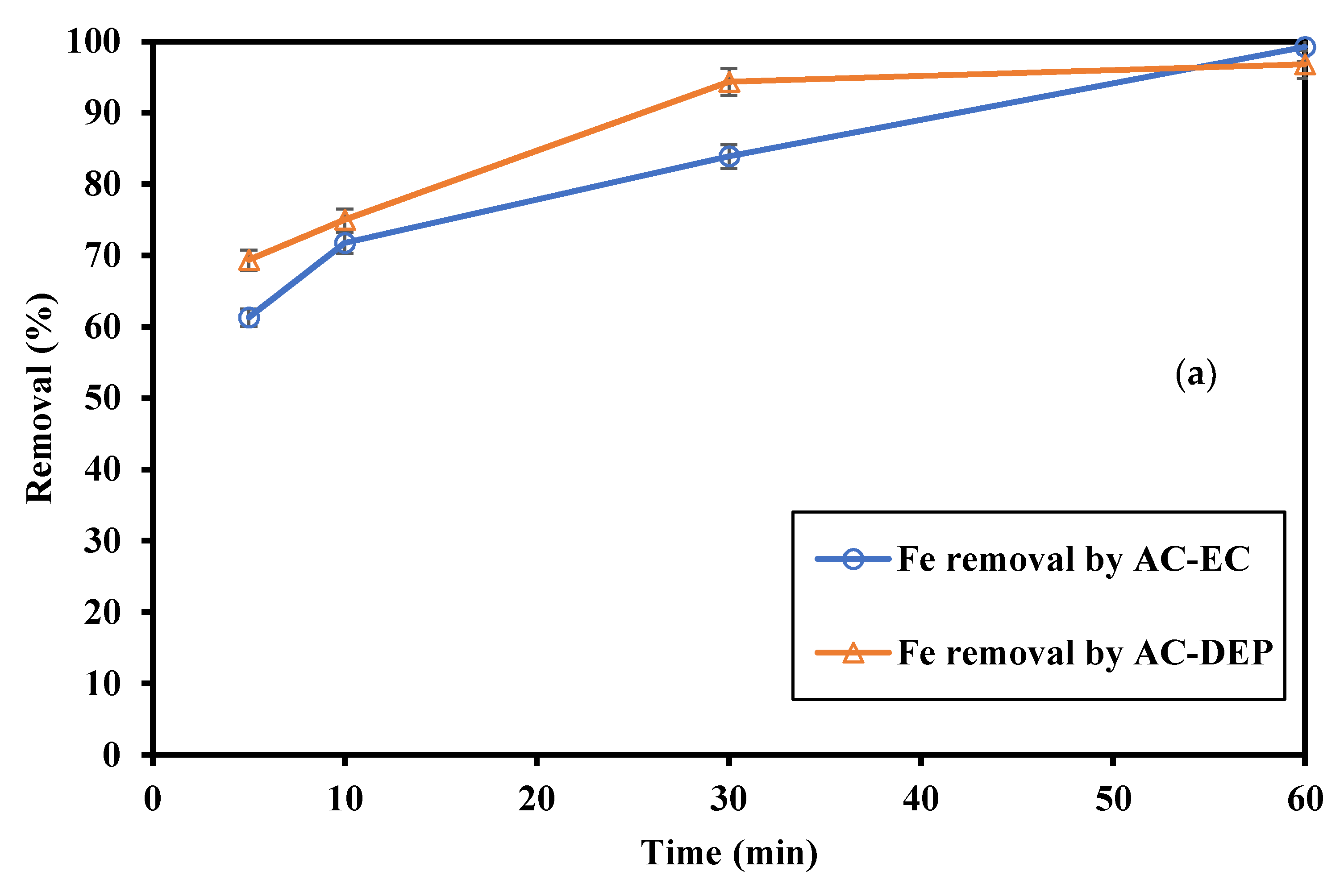
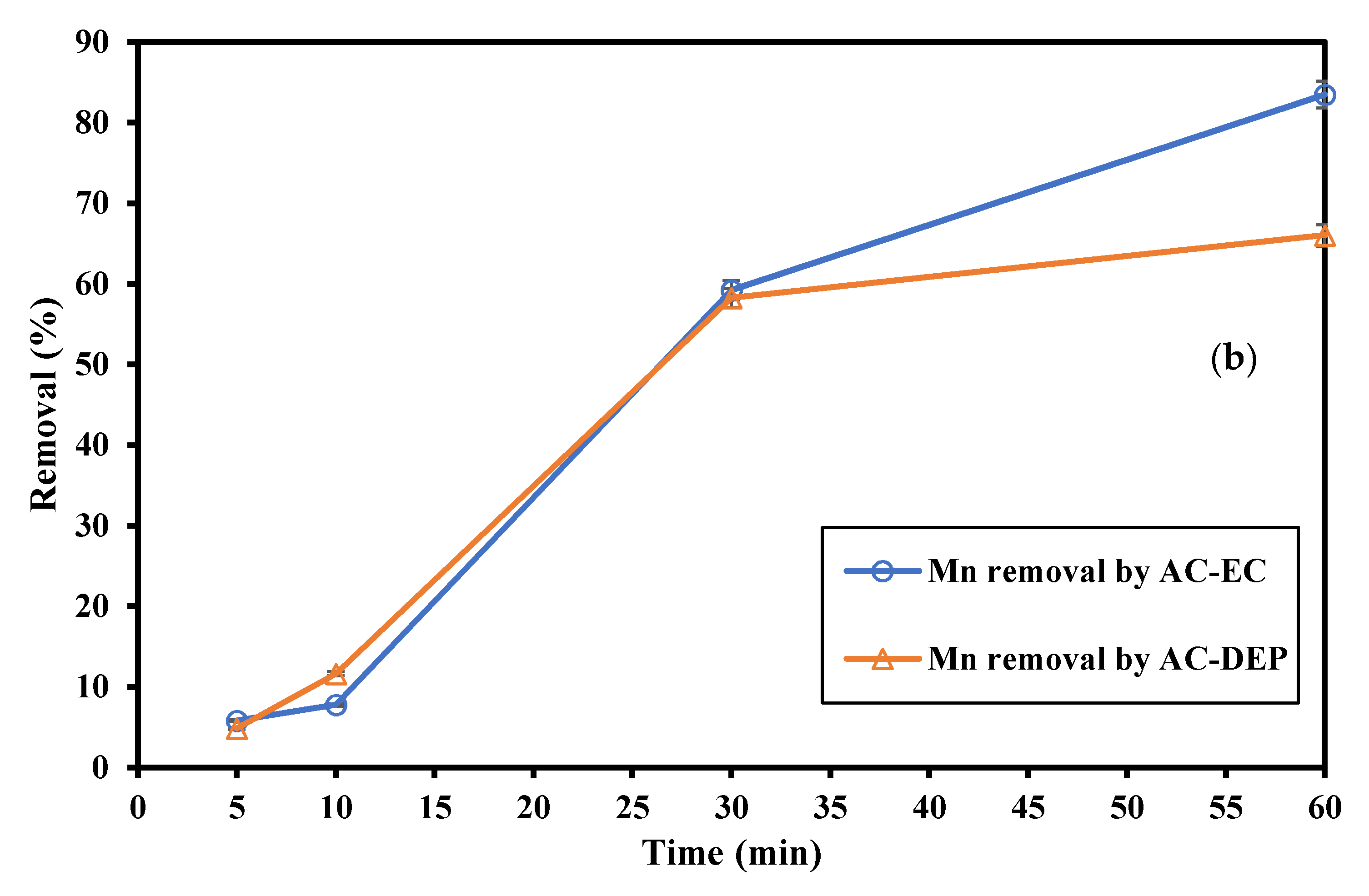
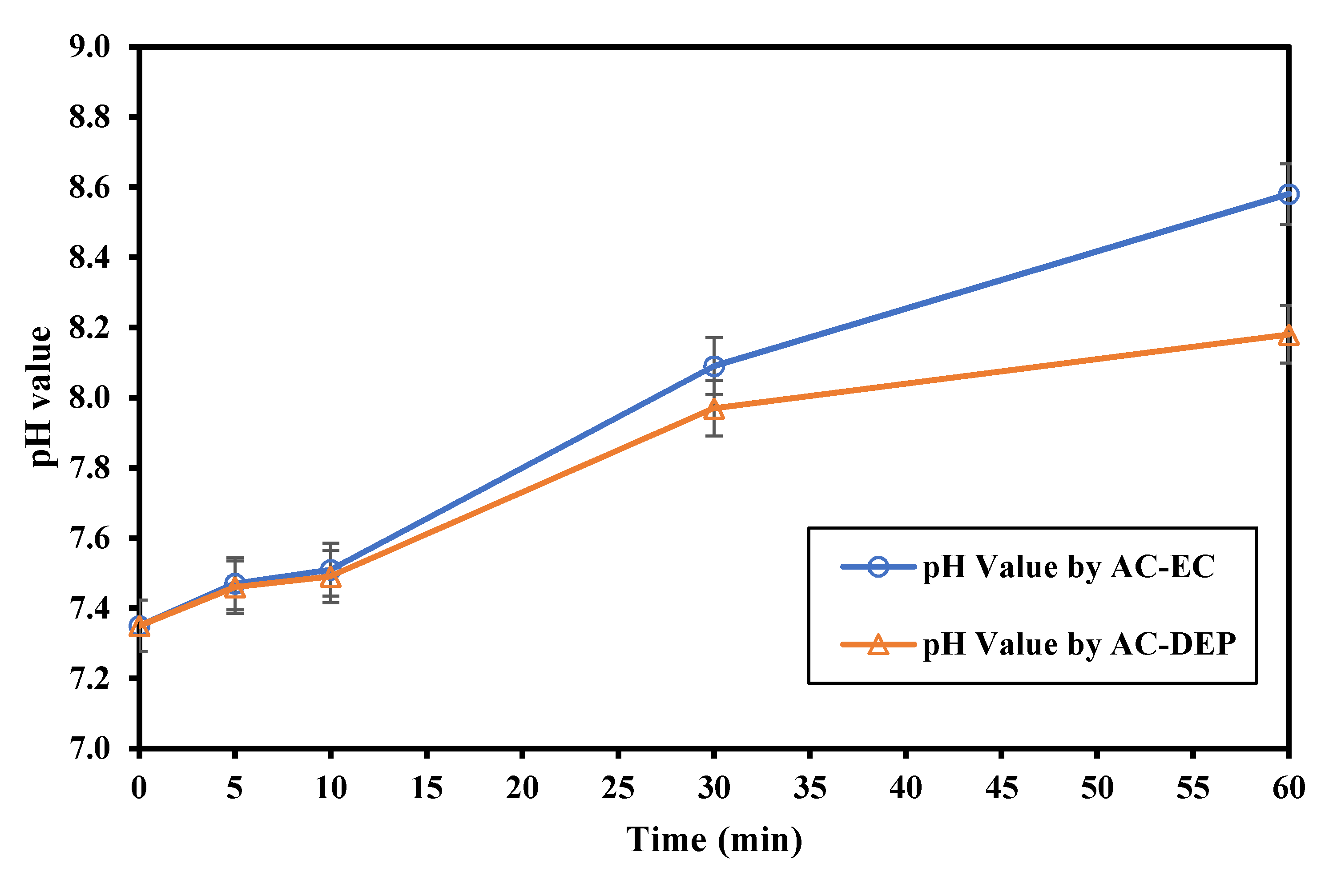
3.2.2. Impact of the Electrolysis Time
3.2.3. Impact of the Applied Current
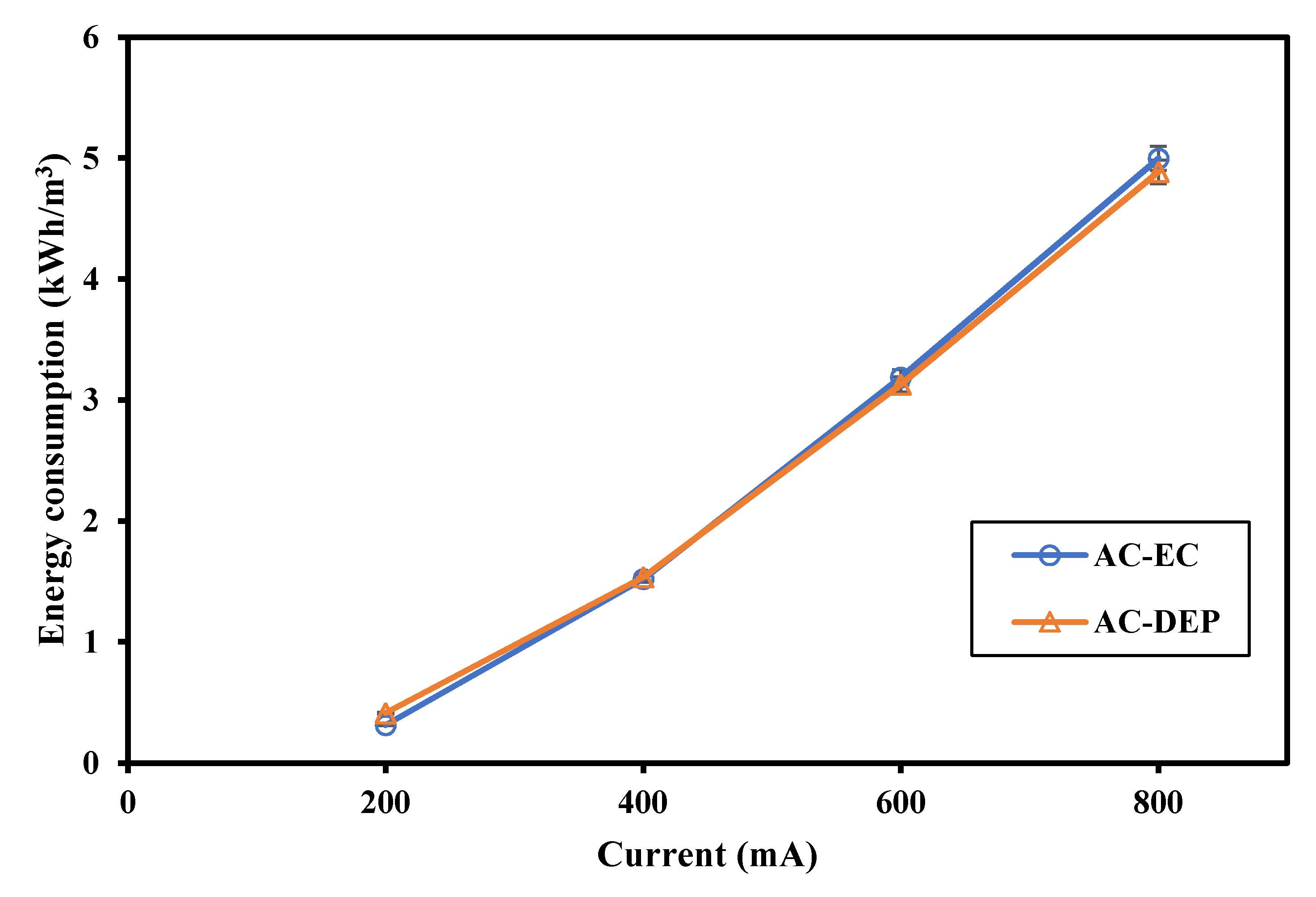
3.2.4. Energy Consumption
4. Conclusions
- As the electrolysis time increased, the removal of Fe and Mn increased. As the electrolysis time increased more ions were produced, and so higher removal efficiencies were expected. The maximum removal percentages of Fe and Mn were obtained at an electrolysis time of 60 min. The removal of Fe was 99.2% using AC-EC and 96.8% using AC-DEP. The removal of Mn was 83.5% and 66% using AC-EC and AC-DEP, respectively;
- As the distance between the electrodes decreased, the Fe and Mn removal increased. This was due to the decrease in the electrical resistance in the solution. A lower resistance would increase the dissolution of coagulants from the electrodes, thus increasing the removal efficiency of pollutants in the system. The maximum Fe and Mn removal was observed at an electrode distance of 0.5 cm;
- As the applied current increased, the removal efficiency increased. The production of Al3+ ions increased as the current increased due to the enhanced dissolution of the aluminum electrodes. Therefore, the amount of trapped heavy metal increased due to the increased amount of coagulants. The maximum Fe and Mn removal percentage was obtained at an applied current of 800 mA;
- The applied current had the highest impact on the removal efficiency of Fe and Mn ions when using AC-EC and AC-DEP. As the applied current increased from 200 mA to 800 mA using AC-EC, the removal efficiencies of Fe and Mn increased by 56% and 60%, respectively. Similarly, as the applied current increased from 200 mA to 800 mA using AC-DEP, the removal efficiencies of Fe and Mn increased by 66% and 63%, respectively;
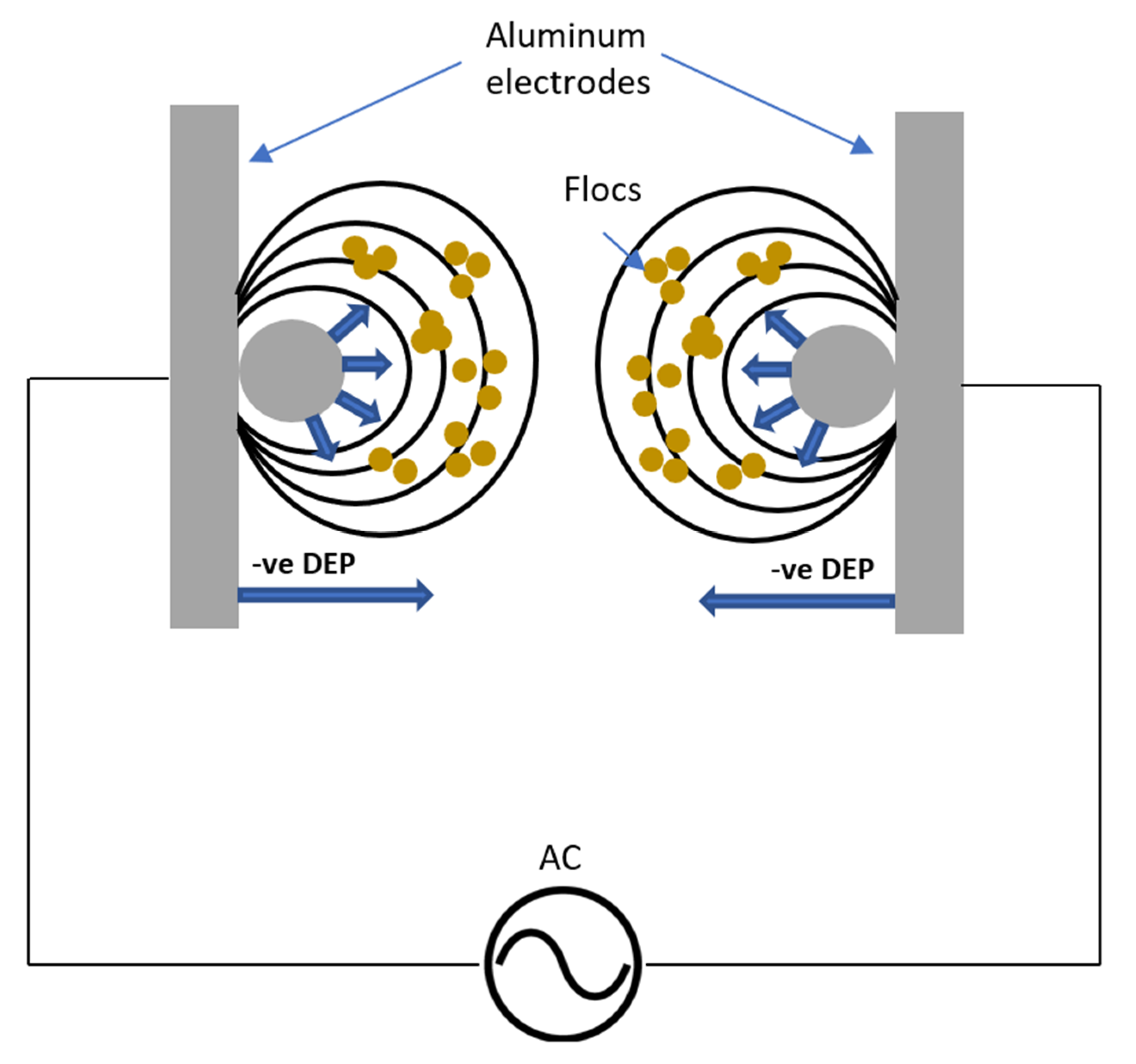
- The main advantage of using the new DEP-inducing electrodes lies not only in the enhanced removal efficiency, but also in the amount of consumed Al in the EC process. The aluminum content increased as the applied current increased using both AC-EC and AC-DEP configurations. However, the aluminum content obtained using the AC-DEP configuration was much lower than with the AC-EC configuration. The aluminum content using AC-DEP was almost 42% less than the AC-EC at an applied current of 800 mA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eyvaz, M.; Gürbulak, E.; Kara, S.; Yüksel, E. Preventing of cathode passivation/deposition in electrochemical treatment methods–a case study on winery wastewater with electrocoagulation. In Modern Electrochemical Methods in Nano, Surface and Corrosion Science; IntechOpen: London, UK, 2014. [Google Scholar]
- Uduman, N.; Bourniquel, V.; Danquah, M.K.; Hoadley, A.F.A. A parametric study of electrocoagulation as a recovery process of marine microalgae for biodiesel production. Chem. Eng. J. 2011, 174, 249–257. [Google Scholar] [CrossRef]
- Heidmann, I.; Calmano, W. Removal of Zn(II), Cu(II), Ni(II), Ag(I) and Cr(VI) present in aqueous solutions by aluminium electrocoagulation. J. Hazard. Mater. 2008, 152, 934–941. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Akbal, F.; Camci, S. Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 2011, 269, 214. [Google Scholar] [CrossRef]
- Al Aji, B.; Yavuz, Y.; Koparal, A.S. Electrocoagulation of heavy metals containing model wastewater using monopolar iron electrodes. Sep. Purif. Technol. 2012, 86, 248–254. [Google Scholar] [CrossRef]
- Shafaei, A.; Rezaie, M.; Nikazar, M. Evaluation of Mn2+ and Co2+ removal by electrocoagulation: A case study. Chem. Eng. Process. Process Intensif. 2011, 50, 1115–1121. [Google Scholar] [CrossRef]
- Hanay, Ö.; Hasar, H. Effect of anions on removing Cu2+, Mn2+ and Zn2+ in electrocoagulation process using aluminum electrodes. J. Hazard. Mater. 2011, 189, 572–576. [Google Scholar] [CrossRef]
- Shafaei, A.; Rezayee, M.; Arami, M.; Nikazar, M. Removal of Mn2+ ions from synthetic wastewater by electrocoagulation process. Desalination 2010, 260, 23–28. [Google Scholar] [CrossRef]
- Das, D.; Nandi, B.K. Simultaneous removal of fluoride and Fe (II) ions from drinking water by electrocoagulation. J. Environ. Chem. Eng. 2020, 8, 103643. [Google Scholar] [CrossRef]
- Doggaz, A.; Attour, A.; Le Page Mostefa, M.; Tlili, M.; Lapicque, F. Iron removal from waters by electrocoagulation: Investigations of the various physicochemical phenomena involved. Sep. Purif. Technol. 2018, 203, 217–225. [Google Scholar] [CrossRef]
- Hashim, K.S.; Shaw, A.; Al Khaddar, R.; Pedrola, M.O.; Phipps, D. Iron removal, energy consumption and operating cost of electrocoagulation of drinking water using a new flow column reactor. J. Environ. Manag. 2017, 189, 98–108. [Google Scholar] [CrossRef]
- Das, D.; Nandi, B.K. Removal of Fe (II) ions from drinking water using Electrocoagulation (EC) process: Parametric optimization and kinetic study. J. Environ. Chem. Eng. 2019, 7, 103116. [Google Scholar] [CrossRef]
- Alkhatib, A.M.; Hawari, A.H.; Hafiz, M.A.; Benamor, A. A novel cylindrical electrode configuration for inducing dielectrophoretic forces during electrocoagulation. J. Water Process Eng. 2020, 35, 101195. [Google Scholar] [CrossRef]
- Hawari, A.H.; Alkhatib, A.M.; Das, P.; Thaher, M.; Benamor, A. Effect of the induced dielectrophoretic force on harvesting of marine microalgae (Tetraselmis sp.) in electrocoagulation. J. Environ. Manag. 2020, 260, 110106. [Google Scholar] [CrossRef]
- Hawari, A.H.; Alkhatib, A.M.; Hafiz, M.; Das, P. A novel electrocoagulation electrode configuration for the removal of total organic carbon from primary treated municipal wastewater. Environ. Sci. Pollut. Res. 2020, 27, 23888–23898. [Google Scholar] [CrossRef] [PubMed]
- Hawari, A.H.; Larbi, B.; Alkhatib, A.; Yasir, A.T.; Du, F.; Baune, M.; Thöming, J. Impact of aeration rate and dielectrophoretic force on fouling suppression in submerged membrane bioreactors. Chem. Eng. Process. Intensif. 2019, 142, 107565. [Google Scholar] [CrossRef]
- Hawari, A.H.; Du, F.; Baune, M.; Thöming, J. A fouling suppression system in submerged membrane bioreactors using dielectrophoretic forces. J. Environ. Sci. 2015, 29, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Hawari, A.H.; Larbi, B.; Ltaief, A.; Pesch, G.R.; Baune, M.; Thöming, J. Fouling suppression in submerged membrane bioreactors by obstacle dielectrophoresis. J. Memb. Sci. 2018, 549, 466–473. [Google Scholar] [CrossRef]
- Du, F.; Hawari, A.; Baune, M.; Thöming, J. Dielectrophoretically intensified cross-flow membrane filtration. J. Memb. Sci. 2009, 336, 71–78. [Google Scholar] [CrossRef]
- Mohora, E.; Rončević, S.; Dalmacija, B.; Agbaba, J.; Watson, M.; Karlović, E.; Dalmacija, M. Removal of natural organic matter and arsenic from water by electrocoagulation/flotation continuous flow reactor. J. Hazard. Mater. 2012, 235–236, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Oladegaragoze, A.; Djafarzadeh, N. Decolorization of basic dye solutions by electrocoagulation: An investigation of the effect of operational parameters. J. Hazard. Mater. 2006, 129, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Shafaei, A.; Pajootan, E.; Nikazar, M.; Arami, M. Removal of Co (II) from aqueous solution by electrocoagulation process using aluminum electrodes. Desalination 2011, 279, 121–126. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L.; Bellakhal, N.; Belgaied, J.-E. Treatment of electroplating wastewater containing Cu2+, Zn2+ and Cr(VI) by electrocoagulation. J. Hazard. Mater. 2004, 112, 207–213. [Google Scholar] [CrossRef]
- Aoudj, S.; Khelifa, A.; Drouiche, N.; Hecini, M.; Hamitouche, H. Electrocoagulation process applied to wastewater containing dyes from textile industry. Chem. Eng. Process. Process Intensif. 2010, 49, 1176–1182. [Google Scholar]
- Gao, S.; Yang, J.; Tian, J.; Ma, F.; Tu, G.; Du, M. Electro-coagulation–flotation process for algae removal. J. Hazard. Mater. 2010, 177, 336–343. [Google Scholar] [CrossRef] [PubMed]




| Feed Water | Electrodes No | Electrodes Material | Initial Concentration (mg/L) | Electrolysis Time (min) | Current Density (mA/cm2) | Electrodes Spacing (cm) | Removal Percentage | Ref. |
|---|---|---|---|---|---|---|---|---|
| Metal plating | 3 sets | Anode: Fe Cathode: Al | Cu: 45 Cr: 44.5 Ni: 394 | 20 | 10 | 1 | Cu: 100% Cr: 100% Ni: 100% | [5] |
| Model | 3 sets | Anode: Fe Cathode: Fe | Mn:250 Zn:250 Cu:250 Ni: 250 | 50 | 25 | 0.3 | Mn: 72.6% Zn: 96% Cu: 96% Ni: 96% | [6] |
| Synthetic solution | 1 set | Anode: Al Cathode: Al | Mn: 22.5 | 60 | 6.2 | 1 | Mn: 39.6% | [7] |
| Synthetic solution | 2 sets | Anode: Al Cathode: Al | Cu: 50–200 Zn: 50–200 Mn: 50–200 | 35 | 15 | 0.5 | Cu:100% Zn: 100% Mn: 80–85% | [8] |
| Synthetic solution | 1 set | Anode: Al Cathode: Al | Mn: 100 | 30 | 6.2 | 1 | Mn: 75% | [9] |
| Drinking water | 1 set | Anode: Al Cathode: Al | Fe (II): 20 F: 10 | 60 | 4.31 | 1 | Fe (II): 100% F: 96% | [10] |
| Groundwater | 1 set | Anode: Al Cathode: Al | Fe (II): 25 | 20 | 10 | 1 | Fe (II): >97% | [11] |
| Drinking water | 2 sets | Anode: Al Cathode: Al | Fe (II): 20 | 20 | 1.5 | 0.5 | Fe (II): 98.5% | [12] |
| Drinking water | 1 set | Anode: Al Cathode: Al | Fe (II): 20 | 45 | 2 | 1 | Fe (II):98.6% | [13] |
| Municipal wastewater | 1 set | Anode: Al Cathode: Al | Fe (II): 0.124 Mn: 0.118 | 3060 | 22.86 | 0.5 | Fe (II): 94% Mn: 66% | Present study |
| Parameters | Value | Standard Method |
|---|---|---|
| Temperature (°C) | 22.5 | APHA.2550 Temp |
| pH | 7.35 | APHA 4500-H+ B. Electro-metric Method |
| Turbidity (NTU) | 158 | APHA 2130 B. Nephelometric Method |
| Conductivity (mS/cm) | 2.20 | APHA 2510 B. Conductivity |
| Fe (mg/L) | 0.1240.001 | EPA Method 200.8 |
| Mn (mg/L) | 0.1180.001 | |
| Al (mg/L) | 0.0380.001 | |
| Zn (mg/L) | 0.0030.001 | |
| Ni (mg/L) | 0.0030.001 | |
| Cu (mg/L) | 0.0010.001 | |
| Cd | <LDL | |
| Cr | <LDL | |
| Pb | <LDL | |
| Co | <LDL |
| Element | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al (min) |
|---|---|---|---|---|---|---|---|---|---|
| Wt. % | 0.2 | 0.25 | 0.04 | 0.03 | 0.03 | - | 0.04 | 0.03 | 99.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almukdad, A.; Hawari, A.H.; Hafiz, M. An Enhanced Electrocoagulation Process for the Removal of Fe and Mn from Municipal Wastewater Using Dielectrophoresis (DEP). Water 2021, 13, 485. https://doi.org/10.3390/w13040485
Almukdad A, Hawari AH, Hafiz M. An Enhanced Electrocoagulation Process for the Removal of Fe and Mn from Municipal Wastewater Using Dielectrophoresis (DEP). Water. 2021; 13(4):485. https://doi.org/10.3390/w13040485
Chicago/Turabian StyleAlmukdad, Abdulkarim, Alaa H. Hawari, and MhdAmmar Hafiz. 2021. "An Enhanced Electrocoagulation Process for the Removal of Fe and Mn from Municipal Wastewater Using Dielectrophoresis (DEP)" Water 13, no. 4: 485. https://doi.org/10.3390/w13040485
APA StyleAlmukdad, A., Hawari, A. H., & Hafiz, M. (2021). An Enhanced Electrocoagulation Process for the Removal of Fe and Mn from Municipal Wastewater Using Dielectrophoresis (DEP). Water, 13(4), 485. https://doi.org/10.3390/w13040485






