Abstract
Natural and anthropogenic pressures in inland waters induce molecular response mechanisms in organisms as a defense against such multiple stressors. We studied, for the first time, the expression of the stress proteins, heat shock proteins (HSP) and mitogen-activated proteins kinase (MAPK), in a Daphnia magna natural population as a response to environmental changes in a heavily modified water body (Lake Koronia, Northern Greece). In parallel, the water physicochemical parameters, nutrients’ concentration and phytoplankton abundance were measured. Our results showed fluctuations of the proteins’ levels (HSP70, HSP90, phospho-p38 MAPK, phospho-p44/42 MAPK) providing evidence of their expression in situ. HSP70 showed an increasing tendency while for HSP90, no tendency was recorded. The MAPKs’ members followed a reverse pattern compared to each other. The differential expression of HSP and MAPK members indicates that D. magna in Lake Koronia experienced stressors such as increasing temperature, salinity and increased nutrient concentrations, high pH values and variations in phytoplankton abundance that triggered their activation. These in situ findings suggest that HSP and MAPK expression patterns have the potential to be used as biomarkers of stress factors in D. magna, for effective biomonitoring and setting ecological restoration targets.
1. Introduction
Organisms are continuously exposed to multiple natural and anthropogenic stressors [1] which drive the worldwide change on biodiversity and ecosystem’s structure and function [2,3]. Human-induced ecological changes (e.g., declining biodiversity and water quality, nutrient enrichment) have degraded most parts of the world’s inland waters [3,4]. In inland waters, cladocerans represent an important part of the food web as they are the most significant primary grazers and the primary food for planktivorous fish [5]. Due to their great ability to graze down the available phytoplankton biomass, Daphnia species are characterized as “key” organisms [6] and are a central force in biomanipulation for restoration of water bodies [5,7]. Furthermore, Daphnia spp. influence nutrient cycles affecting noxious cyanobacterial blooms [4,8]. As an animal with a high P-content in biomass, it tends to assimilate N and P at relatively low ratios and excretes N and P in high ratios, thus reducing the competitive advantage for N2-fixing cyanobacteria. By its fecal sedimentation it can further reduce the competitive advantage for cyanobacteria assemblage affecting biomanipulation success [4]. On the other hand, cyanobacteria have the potential to reduce somatic growth rate and reproductive rate of Daphnia while increasing mortality because of their poor palatability, feeding inhibition, low nutritional value, and potential toxicity [4]. Several Daphnia species are used as model organisms [9,10] and as biomarkers of water quality and environmental health [11], based on their high sensitivity to a wide range of pollutants [12] and their adaptive responses to environmental changes [11].
Phenotypic and genetic responses of Daphnia to stressors are well-studied with a rich data availability [11]. It is well known that Daphnia respond adaptively to abiotic (e.g., temperature, oxygen, pH) [13] and biological stressors (quality and quantity of phytoplankton, predation) [14,15] modifying their morphology, life history characteristics and behavior [9,14]. Additionally, it should be noted that species of the genus Daphnia (usually Daphnia magna) are widely used in standard acute and chronic toxicity tests, through monitoring their immobility and reproduction, in order to assess the chemicals’ effects [16,17,18,19]. On the other hand, the information of molecular defense mechanisms of Daphnia against stressors is limited [20,21,22,23].
One of the most well-studied molecular defense mechanisms against stress is the expression of heat shock proteins (HSPs) which have been found in numerous taxonomic groups with a great variety in their expression patterns [13,24,25]. Under a great diversity of stressors (temperature extremes, pH extremes, anoxia, hypoxia, heavy metal exposure, organic pollutants), the induction of HSPs benefits several organisms by enhancing their ability to recover from environmental stress [26]. Among HSP members, HSP70 and HSP90 are two of the most prominent heat shock proteins playing essential roles in protein folding, membrane translocation, degradation of misfolded proteins and other regulatory processes as molecular chaperones [27]. Despite the fact that changes in HSP profile have been already described in Daphnia spp. (e.g., [20,21,23]), the information is still restricted compared to other aquatic organisms.
Moreover, as a response to these extracellular stressors, the mitogen-activated protein kinase (MAPK) signaling pathway can be triggered [28,29]. The activation of MAPK members plays a central role to crucial cellular processes and physiological functions including cell differentiation, transformation, and survival in many organisms [29,30,31]. More specific, the ERK (p44/42 MAPK) protein is known for its cell growth and differentiation regulatory function while p38 MAPK is involved in apoptosis, inflammation or differentiation [32,33]. The activation of MAPK pathways has been investigated recently in two Daphnia species only as a response to exposure to polystyrene nanoplastics in laboratory populations [31,34], so the knowledge of the activation of MAPK pathway is still limited. Due to their critical role in the development of stress resistance and adaptation to the environment [35], HSPs and MAPKs can be used as biomarkers for determining the physiological condition, to quantify variable stress [26].
The primary aim of the present study was to assess the expression of stress proteins of Daphnia magna as a response to environmental changes in a temporary and heavily modified water body like Lake Koronia (Northern Greece). We hypothesized that changes in the lake’s environmental parameters will be expressed as differences in D. magna responses to the examined. In order to test that, we examined the expression of several molecular biomarkers (HSPs and MAPKs) and their relationships with water physicochemical parameters and phytoplankton abundance. For better understanding of the behavior of D. magna, along with the molecular biomarkers, we also examined its contribution in the zooplankton community structure in Lake Koronia. To the best of our knowledge, this is the first study that uses members of HSP and MAPK families in a natural population of D. magna attempting to correlate them with environmental parameters. The knowledge of molecular response mechanisms in species of the genus Daphnia will allow the development of more realistic predictions of the effects of anthropogenic pressures on inland waters, leading to a deeper understanding of their function; this may provide a useful warning ‘signpost’ for planning more effective actions in inland water biomonitoring and biomanipulation during restoration.
2. Materials and Methods
2.1. Study Area
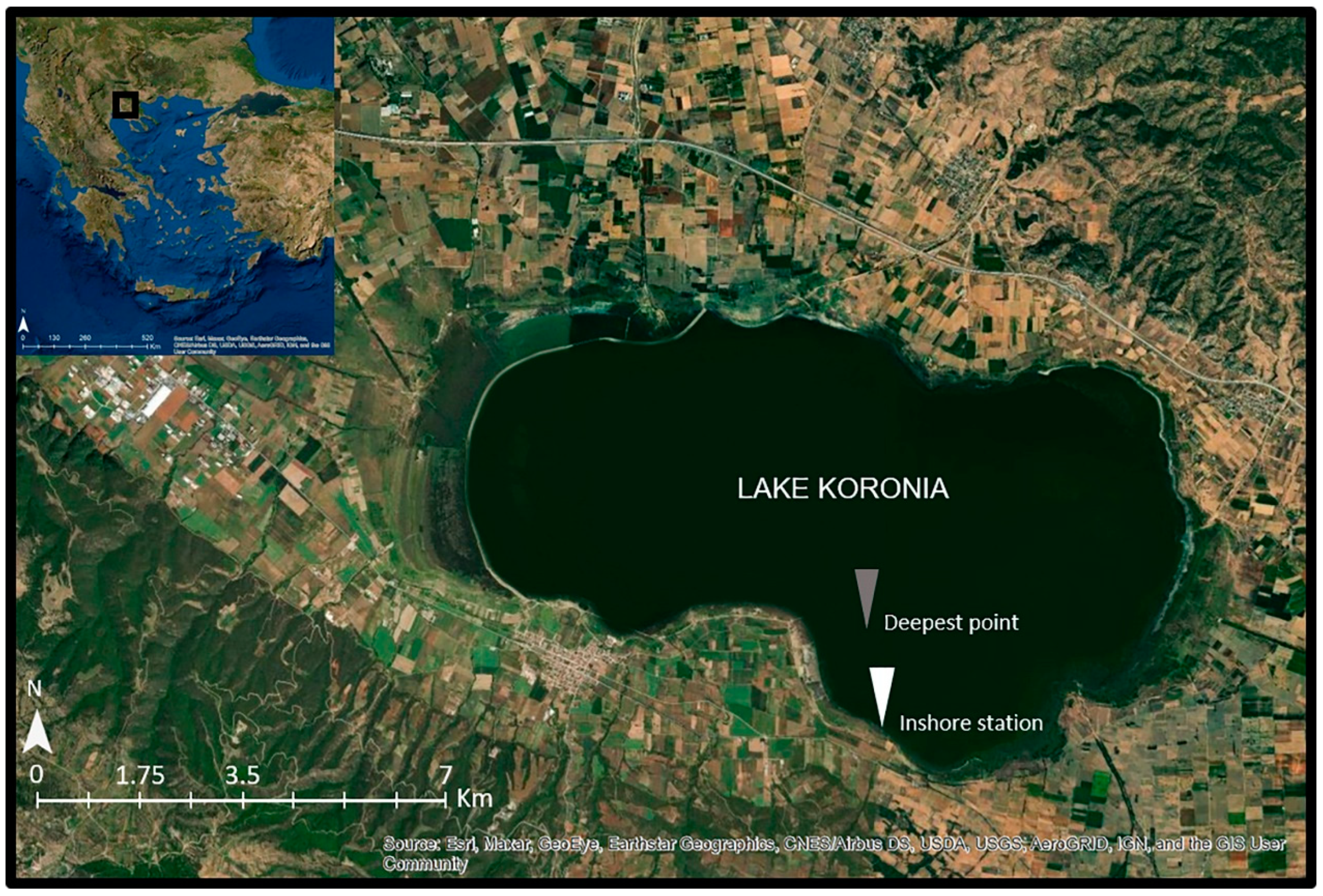
Lake Koronia (Figure 1) is a temporary, very shallow (maximum depth < 2 m), brackish (salinity range 4–7.1 ppt), hypertrophic, highly polluted and heavily modified water body [36] located in North Greece near the city of Thessaloniki (23°04′–23°14′ E, 40°7′–40°43′ N, approximate altitude: 75 m a.s.l., surface area: 29 km2). It is covered by the Directives 79/409/EEC and 92/43/EC, the RAMSAR Convention and is part of the National Wetland Park of Lakes Koronia-Volvi and Macedonian Temp [37].
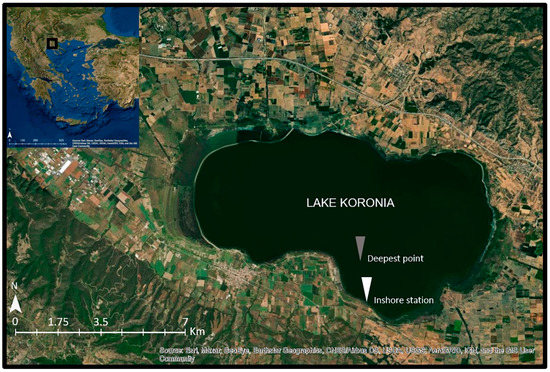
Figure 1.
Map of Lake Koronia showing the location of the sampling station at the deepest point (gray point) and the inshore station (white point).
In Lake Koronia, during the last decades, many dramatic changes and events have taken place. Due to anthropogenic pressures, i.e., unsustainable water management, agricultural runoff, industrial wastewaters and sewage effluents, and changes in climatic conditions (extended drought periods), a dramatic shrinkage of the surface area, water volume and depth, as well as water quality deterioration [37] have occurred. The lake has dried up many times during warm periods (1995, 2002, 2007, 2009 and 2014), while bird and fish kills have occurred several times in Lake Koronia due to hypertrophic conditions and blooms of known toxic prymnesiophytes and cyanobacteria [4,38]. These environmental issues and water management problems are in detail described by Moustaka-Gouni et al. [36,39], Michaloudi et al. [37,38], Malamataris et al. [40], Kolokytha and Malamataris [41]. Moreover, studies reported the range of nutrients and heavy metals in fish tissues [42], and water as well as sediments that may act as possible sink and source of these chemicals for the water column [43,44,45,46]. Recently, a study using biochemical and molecular biomarkers in fish tissues also indicates that Lake Koronia is highly affected by anthropogenic pressures [47].
2.2. Sample Selection in Field and Analysis
Six samplings were conducted on a monthly basis during the period from March to September 2019 (no sampling was performed in August due to lake’s low water level). Water samples were collected from the water column, including the surface layer, with a 2 L Niskin sampler (50 cm height) at the deepest point of the lake (Figure 1, gray point). In September, the samples were collected from an inshore station (Figure 1, white point) due to the shallowness of the lake. Each time, one water sample was collected for the three different analyses; one sample for phytoplankton analysis, one sample for zooplankton analysis, and one water sample for the analysis of nutrients (further separated in two sub-samples; one sub-sample was filtered while the other was not).
2.2.1. Water Physicochemical Parameters, Phytoplankton and Zooplankton Analysis
Water parameters such as temperature (°C), pH, salinity (ppt) and conductivity (μS cm−1) were measured in situ and transparency was measured with a Secchi disk. Water samples were also collected for analysis of nutrients (nitrogen and phosphorus). Samples were kept cool in the field and during their transportation to the laboratory and analyzed according to the standard methods [48,49]. Nitrates, nitrites, ammonium and orthophosphates were determined after the filtration of water samples through 0.45 μm membranes. Total nitrogen and acid hydrolysable phosphorus were determined in unfiltered samples. Nitrite was determined by employing colorimetric method (4500-NO2-B), nitrate by cadmium reaction method (4500-NO3-E), ammonium by phenate method (4500-NH3-F) and total nitrogen after digestion of unfiltered sample by persulfate method (4500-N-C). Regarding P species, soluble reactive phosphorus was determined colorimetrically by ascorbic method and total phosphorus after digestion of unfiltered sample with sulfuric acid/ammonium persulfate (4500-P-E) [49]. The N-species determined will be reported here as nitrogen and will be referred to as Dissolved Inorganic Nitrogen (N-NO3, N-NO2, N-NH4), total nitrogen (TN) and Total Organic Nitrogen (TON as the difference of total nitrogen minus dissolved inorganic nitrogen). The P-forms discussed here are Soluble Reactive Phosphorus (SRP) and acid hydrolysable phosphorus, expressed as total P (TP).
Phytoplankton composite samples were collected from the whole water column and preserved with Lugol’s solution. Phytoplankton counts (cells, filaments, colonies) were performed under a light inverted microscope (Nikon SE 2000) as in Michaloudi et al. [37].
For the zooplankton analysis, at least 30 L of the whole water column were filtered through a plankton net with a mesh size of 50 μm and the quantitative samples were preserved in 4% formalin. Zooplankton samples were examined under a light microscope (Leitz Laborlux S) and abundance estimation (ind L−1) was performed following the method of Bottrell et al. [50], Downing and Rigler [51] and Taggart [52]. For dry biomass (μg L−1) estimations individual dry weight data or length-dry weight relationships were used according to literature (e.g., [53,54,55,56]).
2.2.2. Daphnia magna Western Blot Analysis
In order to investigate the molecular biomarkers, qualitative samples were collected with vertical and horizontal hauls using plankton nets (50 and 100 μm mesh size) and maximum sampling effort was made during their collection. In situ, samples were separated instantly in several 50 mL falcons and were flash-frozen in dry ice, thereby avoiding interference by handling (time between separation and freezing less than a 1 min). Upon their transportation to the laboratory, all falcons were stored at −80 °C. Isolation of female Daphnia individuals was performed under a stereoscope and a microscope, and isolated Daphnia individuals (pool of ~40 female individuals) were transferred to 1.5 mL microfuge tubes (without water) and stored at −80 °C for later immunoblot analysis.
For the extraction of total protein, frozen D. magna individuals were homogenized on liquid ice with mortar and pestle. The whole extracts were diluted with a plastic pestle in 3 volumes of RIPA buffer (0.15 M NaCl, 1% deoxycholic sodium salt, 1% Triton X-100, 0.1% sodium dodecyl sulphate (SDS), 0.01 M Tris, pH 7.2) containing general protease inhibitors (200 μM leupeptin, 10 μΜ trans-epoxy succinyl-L-leucylamido-(4-guanidino) butane (E64), 5 mM dithiothreitol (DTT), 1 mΜ phenyl methyl sulfonyl fluoride (PMSF), 1% v/v Triton X-100, 50 μg mL−1 pepstatin). The homogenates were centrifuged at 10,000× g for 10 min at 4 °C and the supernatants were separated into new tubes; 10 μL of supernatants were used for the determination of protein concentration (BioRad protein assay) and the remaining supernatants were mixed with 0.50 volumes of SDS/PAGE sample buffer (330 mM Tris–HCl, 13% v/v glycerol, 133 mM DTT, 10% w/v SDS, 0.2% w/v bromophenol blue), boiled for 6 min and then frozen at −4° C.
Equivalent amounts of protein (50 μg) were separated on 10% (w/v) acrylamide and 0.275% (w/v) bisacrylamide slab gels and transferred electrophoretically to a nitrocellulose membrane (0.45 μm, Schleicher & Schuell, Keene, NH, USA). Non-specific binding sites on the membranes were blocked with 5% (w/v) non-fat milk in Tris buffered saline-Tween 20 (TBST) (20 mM Tris–HCl, 137 mM NaCl, 0.1% (v/v) Tween 20, pH 7.5) for 30 min at room temperature. Afterwards, the appropriate primary antibodies were left to react with the blocked proteins overnight at 4 °C. The antibodies used were as follows: polyclonal rabbit anti-heat shock protein, 70 kDa (Cat. No. 4872, Cell Signaling, Beverly, MA, USA), polyclonal rabbit anti-heat shock protein, 90 kDa (Cat. No. 4874, Cell Signaling, Beverly, MA, USA), monoclonal rabbit anti-phospho p44/42 MAPK (Thr202/Tyr204) (Cat. No. 4370, Cell Signaling, Beverly, MA, USA) and polyclonal rabbit anti-phospho-p38 MAP kinase (Thr180/Tyr182) (Cat. No. 9211, Cell Signaling, Beverly, MA, USA). Subsequently, the blots washed in TBST (3 times, 5 min each), were incubated with a secondary antibody (anti-rabbit IgG, HRP-linked Antibody (Cat. No. 7074, Cell Signaling, Beverly, MA, USA)) and washed again in TBST (3 times, 5 min each). The developed blots were visualized (bands) by following enhanced chemiluminescence procedures (Cell Signaling, Beverly, MA, USA) with exposure to Fuji Medical X-ray films. Finally, the blots were quantified (Image Studio Lite, LI-COR Biosciences, Lincoln, NE, USA). Ponceau staining has been applied in order to check equal loading of gels according to Romero-Calvo et al. [57] (data is not shown).
2.3. Statistical Analysis
In order to estimate changes in the expression of HSPs and MAPKs in D. magna during the study period, one-way analysis of variance (ANOVA) was performed for significance at the 5% level and post-hoc comparisons were performed using the Bonferroni test. Prior to the analysis, HSPs and MAPKs’ data were tested for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test) and ANOVA assumptions were met. Correlation between molecular biomarkers of D. magna and environmental parameters were assessed using the non-parametric Spearman correlation analysis due to non-linear relationship between variables and/or non-normal distribution of most environmental parameters. The above analyses were performed using the SPSS program (SPSS Statistics 25).
3. Results
3.1. Environmental Parameters
The values of the water physicochemical parameters and phytoplankton measured during the present study are given in Table 1. Temperature ranged from 12 to 27.8 °C and pH from 8.77 to 10.15. Salinity and conductivity increased during the study period with the highest values being recorded in September, 7.1 ppt and 12,160 μS cm−1, respectively. The maximum recorded depth was 1.3 m, declining further on, and finally the lake almost dried up (maximum depth 0.3 m) in September. Phytoplankton abundance ranged from 320.96 × 103 cell mL−1 to 2265.30 × 103 cell mL−1 and transparency was very low reaching its lowest value in September. High abundances of known harmful phytoplankton species (e.g., Prymnesium parvum, Anabaenopsis elenkinii) and oscillatorialean cyanobacteria (e.g., Pseudanabaena limnetica) were recorded during the study period. All nutrients of nitrogen and phosphorus (N-NO2−, N-NH4+, TN, TON, SRP and TP) reached their maximum values in July, except for N-NO3− which reached its peak in May.

Table 1.
Physicochemical parameters and phytoplankton abundance measured in the water column from Lake Koronia during the study period (March to September 2019).
3.2. Daphnia magna and Zooplankton Community Structure
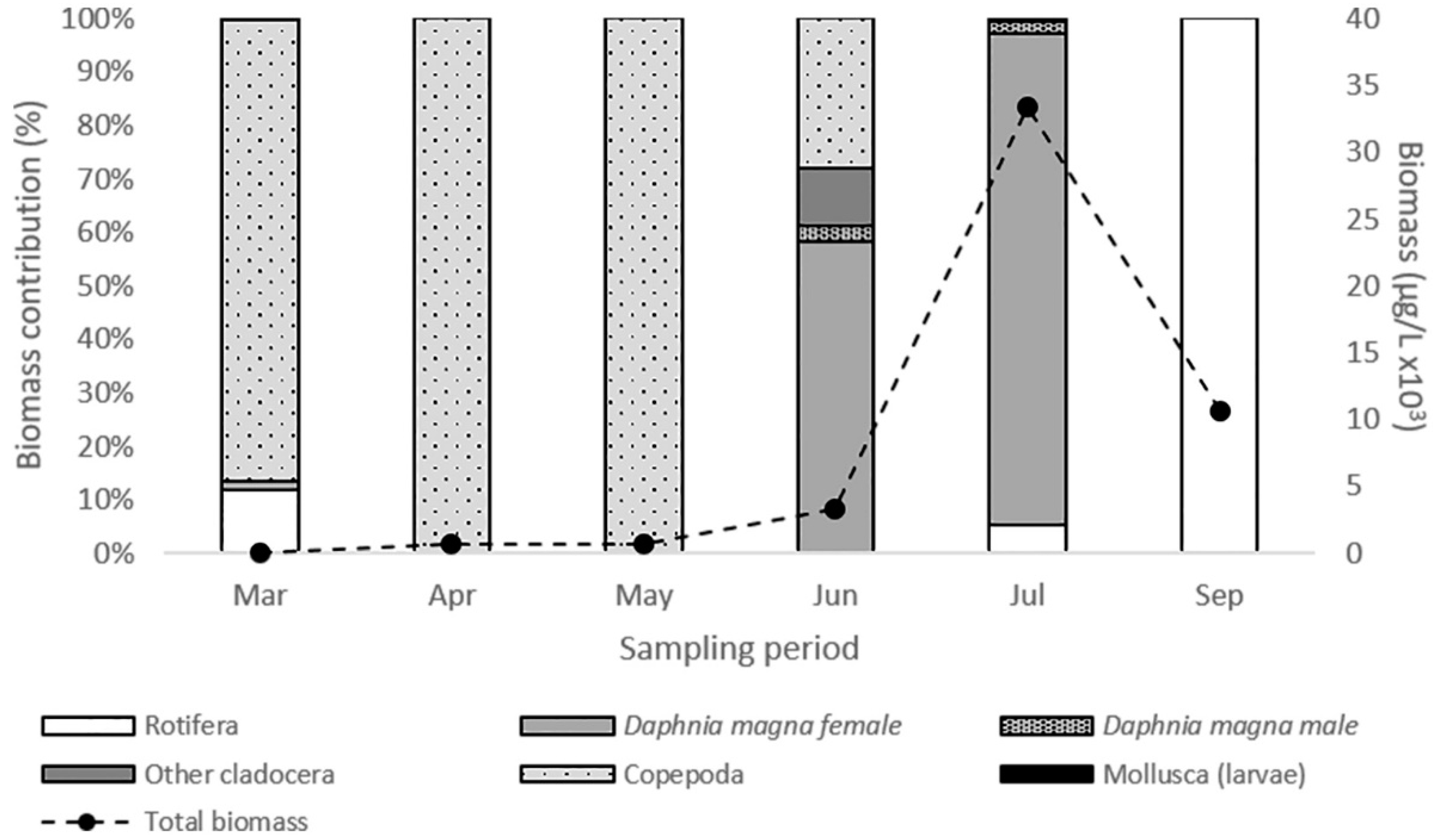
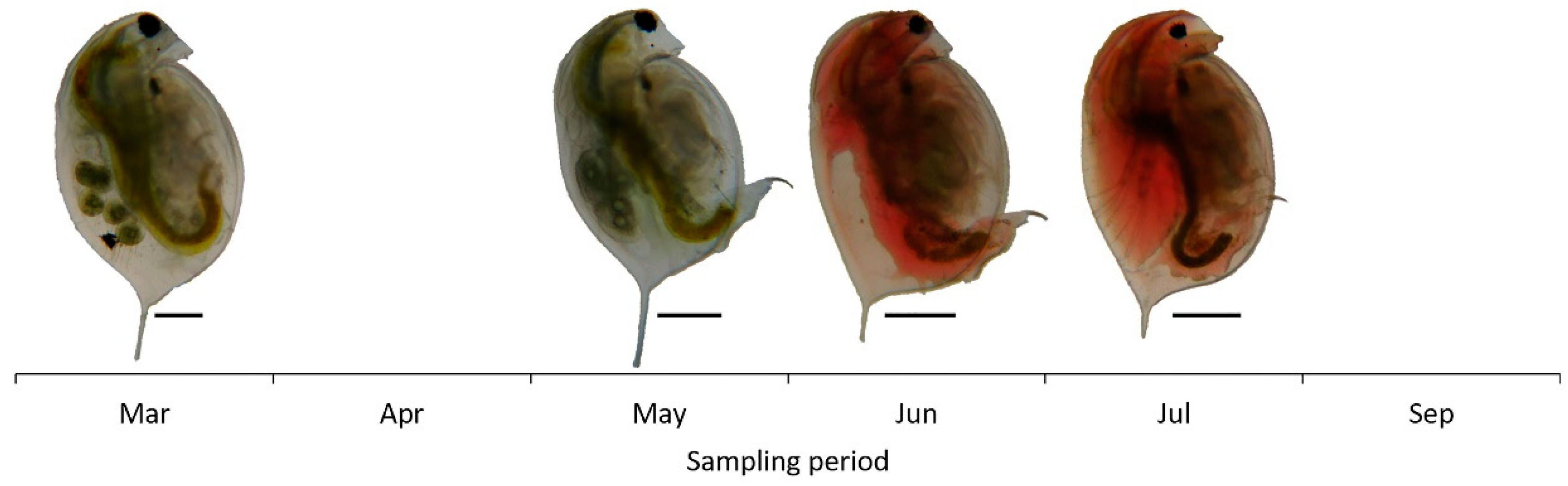
The zooplankton community biomass increased until July, declined further on and ranged from 39.07 μg L−1 (March) to 33.4 × 103 μg L−1 (July) (Figure 2). D. magna biomass ranged from 0.57 μg L−1 to 31.45 × 103 μg L−1 and shaped the total zooplankton biomass variations. Both female and male Daphnia individuals were recorded (Figure 2). However, females were dominant during the whole period while males’ contribution to total Daphnia biomass was only 4.8% and 2.6% in June and July, respectively (Table 2). During the peak of its population D. magna consisted mainly of female individuals >1300 μm in body length (Table 2). Especially, in July, smaller individuals were not recorded and female individuals with body size 1901–2100 μm (26.4%) and 2101–2300 μm (24.5%) were dominant. Throughout the study period, we observed a change in color of D. magna individuals (Figure 3). Almost all individuals appeared with reddish coloration in June and July compared to beige coloration of individuals in March and May.
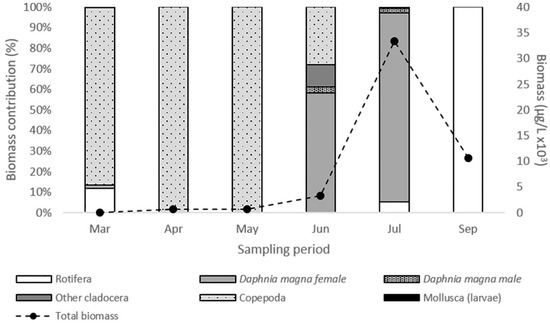
Figure 2.
Biomass contribution of the main groups of the zooplankton community during the study period.

Table 2.
Contribution (%) of the body size (μm) of Daphnia magna (females (F) and males (M)) during the study period.
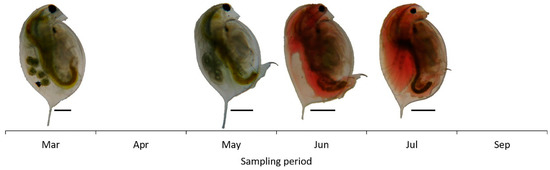
Figure 3.
Microphotographs of individuals of Daphnia magna showing color variation during the study period. In May, Daphnia individuals were present only in the qualitative samples due to maximum sampling effort (scale bar: 0.5 mm).
3.3. Protein Expression
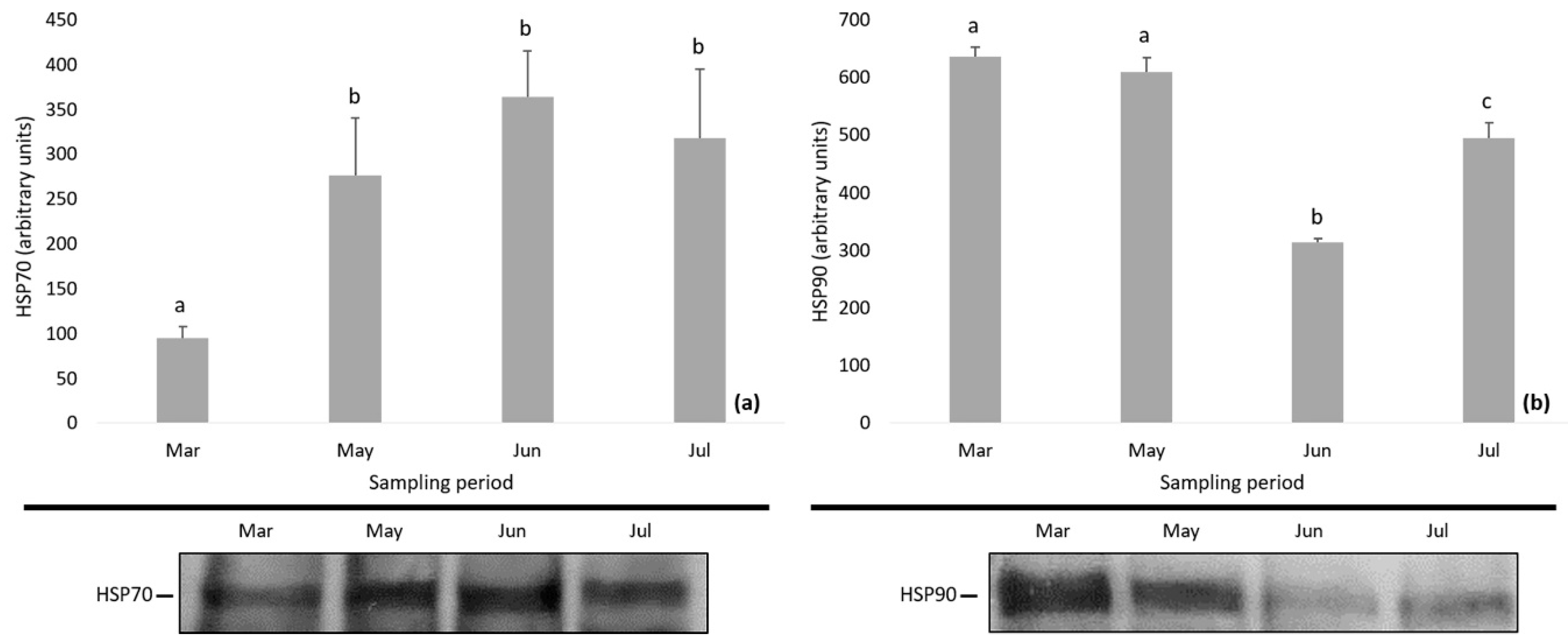
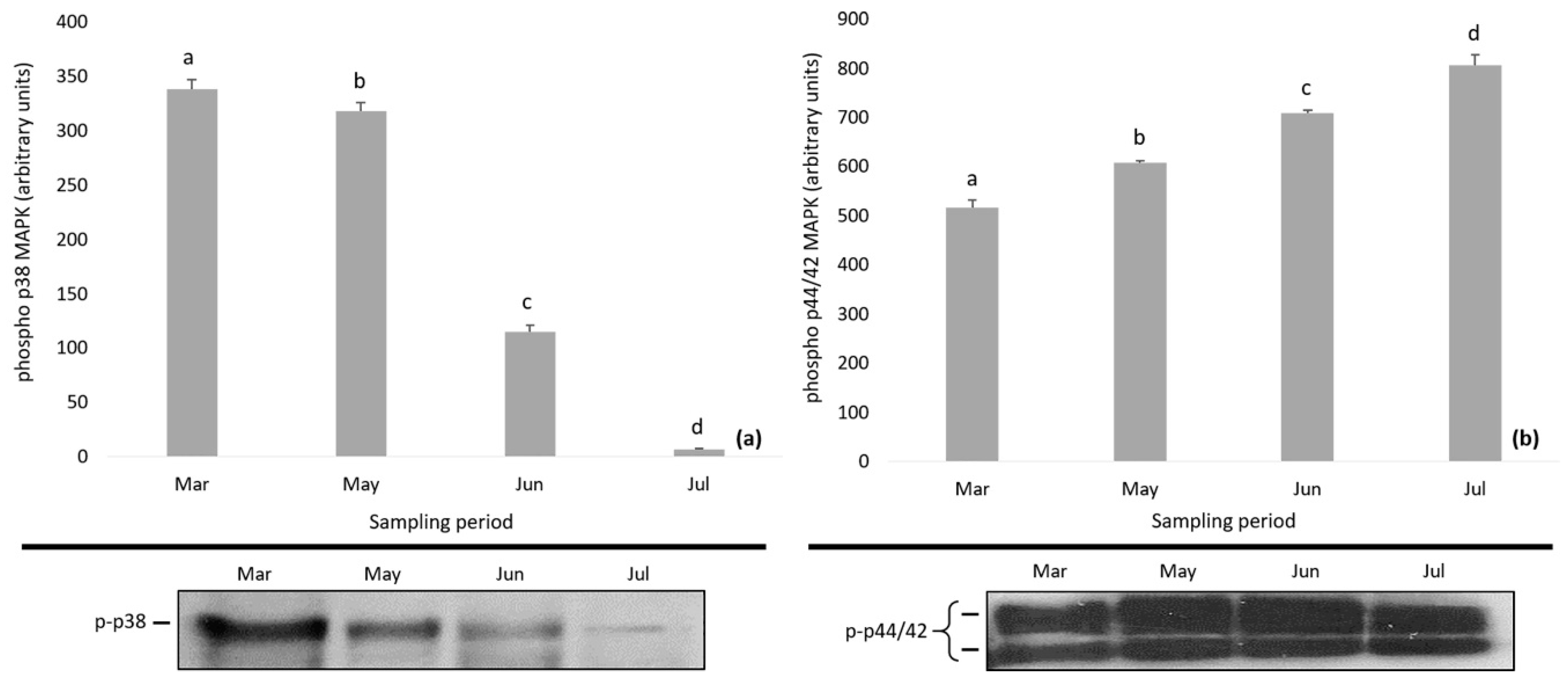
The levels of HSPs and MAPKs in D. magna during the study period are depicted in Figure 4 and Figure 5. Specifically, the expression of HSP70 showed an increasing tendency and the levels of HSP70 were found to be significantly lower in March compared to the following months (one-way ANOVA followed by Bonferroni post hoc test; p < 0.01) (Figure 4a). The expression of HSP90 decreased till June followed by an increase, while Bonferroni test differentiated the HSP90 levels among samplings (one-way ANOVA followed by Bonferroni post hoc test; p < 0.01) (Figure 4b).
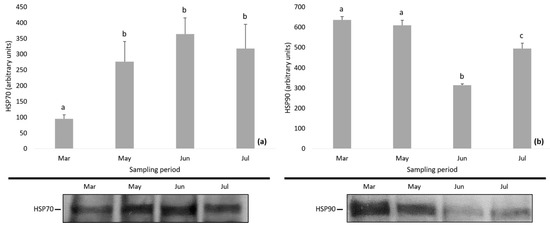
Figure 4.
Expression of (a) HSP70 and (b) HSP90 in Daphnia magna during the study period. Values represent means ± SD; n = 3 technical replicates and different letter (a, b, c) denotes significant (p < 0.05) difference between months.
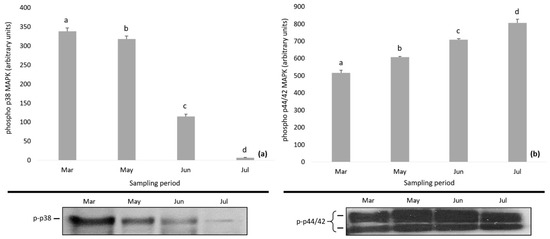
Figure 5.
Phosphorylation of (a) p38 MAPK and (b) p44/42 MAPK in Daphnia magna during the study period. Values represent means ± SD; n = 3 technical replicates and different letter (a, b, c, d) denotes significant (p < 0.05) difference between months.
During the study period, the phosphorylation of p38 MAPK and p44/42 MAPK followed a reverse pattern, i.e., decreasing levels of phospho p38 MAPK (p-p38 MAPK) and increasing levels of phospho p44/42 MAPK (p-p44/42 MAPK) (Figure 5). Statistically significant differences were found in the expression of phospho p38 MAPK and phospho p44/42 MAPK (one-way mixed ANOVA; p < 0.01), and Bonferroni test differentiated the months of the sampling period.
3.4. Interrelationships: Molecural Biomarkers and Environmental Parameters
The correlations between the molecular biomarkers and the water physicochemical parameters and phytoplankton abundance are presented in Table 3. Significant interrelations (Spearman’s rho; p < 0.01) were found between HSP70 and HSP90, and phospho p38 MAPK and phospho p44/42 MAPK. Additionally, HSP70 and HSP90 significantly correlated with phytoplankton abundance and phospho p38 MAPK and phospho p44/42 MAPK correlated negatively and positively, respectively, with temperature, conductivity, salinity, NO2−, NH4+, and TP (Table 2).

Table 3.
Spearman’s rank correlation results of molecular biomarkers, water physicochemical parameters and phytoplankton abundance. Significant correlation at the 0.01 level (** bold italics). Abbreviations according to Table 1.
4. Discussion
Changes in environmental conditions and ecological processes of inland waters induced by natural and anthropogenic activities is a highly complex issue [2,44]. In a heavily modified water body such as Lake Koronia, the study of species of the genus Daphnia can be very useful in order to understand the mechanisms of adaptation of organisms to disadvantageous environmental parameters in inland waters, since zooplankton community structure is highly affected by the anthropogenic activities [58]. Cladocerans are among the most important biological entities that contribute to food web complexity [59], while as non-selective filter feeders, can only reduce overall filtration rates under harmful phytoplankton blooms by ingesting less food [4]. Despite the fact that there are many studies describing phenotypic and genetic changes of Daphnia spp. to environmental stressors [10,11], information on the expression of specific stress response pathways such as HSPs and MAPKs is still restricted. In the present study, we tested the hypothesis that changes in environmental parameters will have an impact on the expression of molecular biomarkers in D. magna. In order to figure out the potential effects of environmental parameters on Daphnia, we also examined Daphnia’s changes in the zooplankton community structure.
4.1. Daphnia magna in the Zooplankton Community
In order to figure out the potential effects of environmental parameters on Daphnia, we also examined Daphnia’s changes in the zooplankton community structure. The presence of D. magna in the zooplankton community of Lake Koronia has always been a factor affecting the rest of the members of the community. It competitively excludes other filter feeders, mainly rotifers [37,38,60], which is well expected as large-bodied D. magna have higher filtering rates compared to the small-sized rotifers [61]. This effect is more pronounced when Daphnia is recorded in high numbers, which at the same time, can affect Daphnia’s reproduction mode. Under favorable environmental conditions, Daphnia reproduce without males (asexual reproduction) [62,63]. The induction of sexual reproduction seems to be triggered in response to high population density (overcrowding) [62,64,65] which was also the case in our study. Even though Daphnia mainly consisted of female individuals, males were recorded during the highest density of its population.
Both biotic and abiotic factors have been recorded to affect D. magna in Lake Koronia. More specifically, high pH values (>10), and the collapse and re-introduction of fish populations have regulated the presence of D. magna in the community as well as the size of its members, respectively [37,60]. In pH values above 10, Daphnia individuals and its eggs and neonates show high mortality [66,67]. So, the very low biomass observed in spring (under detection level in April–May) and September could probably be associated with the high pH values (9.75–10.15) recorded in these periods. In addition, phytoplankton should also be considered as a major factor affecting Daphnia populations since it is well known that Daphnia is negatively affected by toxin-producing algae like Prymnesium parvum [68] and toxic or non-toxic cyanobacterial filaments [69]. The recorded high abundances of the latter throughout the period during which D. magna was absent actually confirm this trend. On the other hand, the extremely high biomass levels and the presence of large-bodied D. magna individuals recorded in summer (June–July) reflected a lower impact or absence of fish predation pressure during this period [4]. Although we lack quantitative fish data, it is well known that fish predation reduces zooplankton and shifts body size [70], an effect that has been identified in Lake Koronia following fish introduction events in 2003 [37]. Noteworthy, the general public witnessed a new mass fish kill in Koronia in September 2019, with most fish in advanced decomposition stage.
Along with the changes in the community contribution of D. magna, its individuals altered their color during the study period. In summer, the population comprised mainly of red individuals. D. magna individuals appear in red coloration through a strong induction of hemoglobin synthesis as a response to environmental hypoxia (low oxygen) [71] and an acclimation to warm temperatures [72,73]. This mechanism provides them with a survival advantage and concomitant tolerance to hypoxic conditions [71,74]. The red individuals recorded in Lake Koronia might demonstrate that D. magna experienced low oxygen conditions, decreasing further on with the rising of temperature. However, data for the oxygen levels were not recorded during the sampling period.
4.2. Changes in Daphnia magna Protein Expression
Under these environmental conditions, we searched for changes in the expression of members of the HSP family. As a response to various environmental pressures, elevated levels of HSPs are expected in order to protect organisms from their impact [13,23,25,35]. Our results showed elevated levels of HSP70 and fluctuations in HSP90 expression patterns. Similar to our results, many studies have found an up-regulation of HSP70 in Daphnia species under the effect of stressors such as warm temperature, hypoxia, heavy metals and fish predation [20,22,75,76,77,78]. However, others have reported, as a response to stressors, an insignificant effect [21,79,80] or a down-regulation pattern [35,78,81]. In our study, the significantly altered expression of HSPs, the elevated HSP70 and the variated HSP90 levels indicated that during the study period, D. magna in Lake Koronia experienced stress conditions (e.g., high pH, low oxygen, harmful phytoplankton, nutrient enrichment) that triggered the activation of these defense mechanisms.
During the study period in Lake Koronia, we recorded seasonal increase in temperature, salinity increase following decrease of lake water volume, and very high pH values, nutrient concentrations and phytoplankton abundances. Considering these environmental parameters, the expression of HSPs were correlated only with phytoplankton abundance. It is well known that food availability is an important parameter influencing Daphnia populations in the field [14], since phytoplankton quantity and quality affect their filtering and feeding rates [4,82], survival, growth and reproduction [83]. In Lake Koronia during this study, phytoplankton was a cocktail of abundant known toxic taxa, such as Anabaenopsis and Arthrospira species (Cyanobacteria), and the known as killer alga Prymnesium parvum. Harmful algal and cyanobacterial blooms have often been reported in the lake to contribute to a very high phytoplankton biomass which is indicative of a degradation in the lake’s water quality [36]. Recently, the effect of food availability has been examined on the expression of HSP90, setting food deprivation as a stressor upon an acute heat stress [81]. Klumpen et al. have shown continuous increased HSP90 levels in well-fed Daphnia populations, whereas starved animals showed an earlier maximum level followed by a decline [81]. In our case, the pattern of HSPs might have been affected by the phytoplankton abundance along with its bad quality (known toxic P. parvum and filamentous cyanobacteria unsuitable for consumption) and the increasing temperature. However, in natural populations, organisms are exposed to a plethora of stressors having interactive effects which are difficult to predict [15,84]. Hence, the impact of phytoplankton may interact with other environmental factors which often co-vary and might affect the HSP protein expression in order to protect D. magna from their impact.
Although organisms living in stressful habitats require frequent induction of HSPs, the synthesis of HSP is energetically costly [77]. Therefore, there are cases where in order to minimize their costs, those organisms probably down-regulate the heat-shock response, and depend mostly on constitutive HSP levels instead [24,77]. Thus, it is also possible that D. magna partly limits its costs for the expression of HSP90, probably investing more energy in other factors such as life-history traits (survival, growth, reproduction) and/or production of hemoglobin; the latter was evident during the summer period of our samplings when large-bodied and red-colored D. magna individuals were recorded in high biomass.
Along with the activation of HSPs under stress conditions, the mitogen-activated protein kinases (MAPKs) can be phosphorylated [29,85]. As one of the most thoroughly studied signal transduction pathways in many organisms, the activation of MAPK members has been confirmed to play a vital role in those pathways linking extracellular signals to intracellular processes [86]. Concerning MAPKs, the present results revealed a differential activation of MAPKs, indicating a reverse expression of phosphorylation levels of p38 MAPK and p44/42 MAPK. These findings suggest that the inhibition of phospho p38 and the activation of phospho p44/42 MAPK in D. magna might be probably related to the lake’s changing conditions acting as potential stressors. Each activated MAPK is involved in many biological processes by regulating vital signal cascades via phosphorylation of target proteins in response to a variety of factors such as thermal and oxidative stress, irradiation, chemicals and increased salinity [28,29,33]. In several marine zooplankton organisms (copepods and rotifers), the activation of MAPK members is associated with various environmental stressors (e.g., ultraviolet B radiation, metals, polystyrene nano-microplastics) [29,86,87,88,89,90]. Nevertheless, some stressors have been found not to affect the MAPK proteins, thus resulting in no significant changes in their profile [86,89,90]. However, our knowledge on how stressful factors influence the activation of MAPK members in Daphnia spp. is still limited. Only the effects of polystyrene nanoplastics on MAPK pathways of Daphnia is known, showing a dose-dependent increased MAPK protein expression in most cases [31,34] or a reverse expression [34].
A number of studies suggest that MAPK cascades are involved in the regulation of HSPs. Particularly, in mammalian [91,92,93] and fish tissues [94] as well as in aquatic invertebrates [95], a direct relationship between phosphorylation of p38 MAPK and induction of HSP70 has been shown, through the presence of p38 MAPK inhibitor. However, other studies have shown no correlation between the activation of HSP70 and the phosphorylation of p38 MAPK, either under the presence of p38 MAPK inhibitor [96] or not [28,97]; this pattern is in accordance with our results. In addition, the fact that in D. magna the increased phosphorylation levels of p44/42 MAPK were in parallel with the elevated HSP70 levels provides support of the induced activation of HSP70 via the phosphorylation of p44/42 MAPK, which agrees with many previous findings [92,98,99,100]. Still, in order to understand if the activation of MAPKs is essential for the adaptation of Daphnia to stressors through the induction of HSP70, further investigation is required.
In the present study, for the first time, we attempted to assess the expression of members of the MAPK family of D. magna as a response to environmental changes. Both MAPK members showed strong correlation with temperature, conductivity, NO2−, NH4+, and TP suggesting that these factors could transiently affect, positively or negatively, the expression patterns of MAPK-activating proteins in D. magna. It is long known that higher temperature [101,102], increasing salinity (and therefore conductivity) [103], high phosphorus [104] and high ammonia levels [105] might impact the survival, growth rate, fecundity and longevity of Daphnia populations. Hence, the phosphorylation pattern of p38 MAPK and p44/42 MAPK recorded in the present study reveal that these factors act as stressors that can trigger the activation of members of the MAPK family. However, in nature, the environmental and anthropogenic stressors usually interact in an additive, synergistic, or antagonistic manner [15]. Understanding their interactive effects is a huge challenge in studying the impacts of environmental stressors on organisms, since in most laboratory studies, the evaluated trade-offs are merely associated with one trait [15,106]. Contrary to the large body of studies concerning changes in behavior characteristics of Daphnia members responding to those stressors, to date there is no available information regarding the MAPK expression patterns. To shed some light into this, laboratory experiments are required to test whether and how those factors can affect the expression of MAPKs, as well as to reveal the possible extant of interactions among them.
5. Conclusions
In the present study, we provide evidence related to the in situ expression of stress protein levels (HSPs and MAPKs) in a natural population of D. magna, as fluctuations of protein levels were observed during the sampling period. These findings suggest that HSP and MAPK expression have the potential to be used as ecological biomarkers of stress factors in Daphnia. The differential response of HSP and MAPK members to stressors, remarks on a different mode of action for these molecules in the cellular defense process and signaling pathway, respectively, under the effect of changing conditions in the natural environment. In order to better understand the adaptation proteins’ levels in organisms when exposed to unstable environmental factors, insight into the organisms’ ability to cope with certain stressors and the concomitant trade-offs among them are required.
Author Contributions
Conceptualization, E.M., E.A., M.M.-G. and M.D.; methodology, E.M., E.A., M.M.-G., D.V. and M.D.; validation, M.D. and A.K.; formal analysis, M.D. and A.K.; investigation, M.D. and A.K.; resources, E.M., E.A., M.M.-G. and D.V.; data curation, M.D., E.M., E.A., M.M-G., D.V. and A.K.; writing—original draft preparation, M.D., E.M., E.A., M.M.-G. and D.V.; writing—review and editing, M.D., E.M., E.A., M.M.-G., D.V. and A.K.; visualization, M.D.; supervision, E.M., E.A. and M.M.-G.; project administration, E.M., E.A. and M.M.-G.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Greece and the European Union (European Social Fund—ESF) through the Operational Program “Human Resources Development, Education and Lifelong Learning” in the context of the project “Strengthening Human Resources Research Potential via Doctorate Research” (MIS-5000432), implemented by the State Scholarships Foundation (IKY).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study is available in the current manuscript; raw data is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Smolders, R.; Baillieul, M.; Blust, R. Relationship between the Energy Status of Daphnia magna and Its Sensitivity to Environmental Stress. Aquat. Toxicol. 2005, 73, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.R.; Graham, M.D.; Vinebrooke, R.D.; Findlay, D.L.; Paterson, M.J.; Turner, M.A. Multiple Anthropogenic Stressors Cause Ecological Surprises in Boreal Lakes. Glob. Chang. Biol. 2006, 12, 2316–2322. [Google Scholar] [CrossRef]
- Paerl, H.W.; Valdes, L.M.; Peierls, B.L.; Adolf, J.E.; Harding, L.J.W. Anthropogenic and Climatic Influences on the Eutrophication of Large Estuarine Ecosystems. Limnol. Oceanogr. 2006, 51, 448–462. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Sommer, U. Effects of Harmful Blooms of Large-Sized and Colonial Cyanobacteria on Aquatic Food Webs. Water 2020, 12, 1587. [Google Scholar] [CrossRef]
- Lampert, W.; Sommer, U. Limnoecology: The Ecology of Lakes and Streams, 2nd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Lampert, W. Daphnia: Development of a Model Organism in Ecology and Evolution. Excell. Ecol. 2011, 21, 1–275. [Google Scholar]
- Tatarazako, N.; Oda, S. The Water Flea Daphnia magna (Crustacea, Cladocera) as a Test Species for Screening and Evaluation of Chemicals with Endocrine Disrupting Effects on Crustaceans. Ecotoxicology 2007, 16, 197–203. [Google Scholar] [CrossRef]
- Elser, J.J. The Pathway to Noxious Cyanobacteria Blooms in Lakes: The Food Web as the Final Turn. Freshw. Biol. 1999, 42, 537–543. [Google Scholar] [CrossRef]
- Lampert, W. Daphnia: Model Herbivore, Predator and Prey. Pol. J. Ecol. 2006, 54, 607–620. [Google Scholar]
- Miner, B.E.; De Meester, L.; Pfrender, M.E.; Lampert, W.; Hairston, N.G. Linking Genes to Communities and Ecosystems: Daphnia as an Ecogenomic Model. Proc. Biol. Sci. 2012, 279, 1873–1882. [Google Scholar] [CrossRef]
- Orsini, L.; Gilbert, D.; Podicheti, R.; Jansen, M.; Brown, J.B.; Solari, O.S.; Spanier, K.I.; Colbourne, J.K.; Rusch, D.B.; Decaestecker, E.; et al. Daphnia magna Transcriptome by RNA-Seq across 12 Environmental Stressors. Sci. Data 2016, 3, 160030. [Google Scholar] [CrossRef]
- Dalla Bona, M.; Di Leva, V.; De Liguoro, M. The Sensitivity of Daphnia magna and Daphnia curvirostris to 10 Veterinary Antibacterials and to Some of Their Binary Mixtures. Chemosphere 2014, 115, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, A.; Grzesiuk, M.; Kloc, M.; Pijanowska, J. Heat Shock Proteins in Daphnia Detected Using Commercial Antibodies: Description and Responsiveness to Thermal Stress. Chemoecology 2009, 19, 69. [Google Scholar] [CrossRef]
- Coors, A.; Hammers-Wirtz, M.; Ratte, H.T. Adaptation to Environmental Stress in Daphnia magna Simultaneously Exposed to a Xenobiotic. Chemosphere 2004, 56, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, I.; McLeod, A.M.; Colbourne, J.K.; Yan, N.D.; Cristescu, M.E. Synergistic Interactions of Biotic and Abiotic Environmental Stressors on Gene Expression. Genome 2015, 58, 99–109. [Google Scholar] [CrossRef]
- ISO 10706:2000. Water Quality—Determination of Long Term Toxicity of Substances to Daphnia Magna Straus (Cladocera, Crustacea); ISO: Genève, Switzerland, 2000. [Google Scholar]
- OECD 202: 2004. Guideline for Testing of Chemicals. Daphnia sp., Acute Immobilisation Test; OECD: Paris, France, 2004. [Google Scholar]
- ISO 6341:2012. Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test; ISO: Genève, Switzerland, 2012. [Google Scholar]
- OECD 211: 2012. Guidelines for the Testing of Chemicals. Daphnia magna Reproduction Test; OECD: Paris, France, 2012. [Google Scholar]
- Pijanowska, J.; Kloc, M. Daphnia Response to Predation Threat Involves Heat-Shock Proteins and the Actin and Tubulin Cytoskeleton. Genesis 2004, 38, 81–86. [Google Scholar] [CrossRef]
- Pauwels, K.; Stoks, R.; de Meester, L. Coping with Predator Stress: Interclonal Differences in Induction of Heat-Shock Proteins in the Water Flea Daphnia magna. J. Evol. Biol. 2005, 18, 867–872. [Google Scholar] [CrossRef]
- Mikulski, A.; Bernatowicz, P.; Grzesiuk, M.; Kloc, M.; Pijanowska, J. Differential Levels of Stress Proteins (HSPs) in Male and Female Daphnia magna in Response to Thermal Stress: A Consequence of Sex-Related Behavioral Differences? J. Chem. Ecol. 2011, 37, 670–676. [Google Scholar] [CrossRef]
- Mikulski, A.; Grzesiuk, M.; Rakowska, A.; Bernatowicz, P.; Pijanowska, J. Thermal Shock in Daphnia: Cost of Diel Vertical Migrations or Inhabiting Thermally-Unstable Waterbodies? Fundam. Appl. Limnol. 2017, 213–220. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The Evolutionary and Ecological Role of Heat Shock Proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Schumpert, C.A.; Anderson, C.; Dudycha, J.L.; Patel, R.C. Involvement of Daphnia pulicaria Sir2 in Regulating Stress Response and Lifespan. Aging 2016, 8, 402–417. [Google Scholar] [CrossRef][Green Version]
- Dahlhoff, E.P. Biochemical Indicators of Stress and Metabolism: Applications for Marine Ecological Studies. Annu. Rev. Physiol. 2004, 66, 183–207. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Duan, Y.; Chen, P.; Liu, P. The Roles of Heat Shock Proteins 70 and 90 in Exopalaemon carinicauda After WSSV and Vibrio anguillarum Challenges. J. Ocean Univ. China 2018, 17, 399–406. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Kentepozidou, E.; Feidantsis, K.; Roufidou, C.; Despoti, S.; Chatzifotis, S. Starvation and Re-Feeding Affect Hsp Expression, MAPK Activation and Antioxidant Enzymes Activity of European Sea Bass (Dicentrarchus labrax). Comp. Biochem. Physiol. A 2013, 165, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-M.; Jeong, C.-B.; Kim, M.-S.; Lee, J.-S.; Zhou, J.; Lee, Y.H.; Kim, D.-H.; Moon, E.; Kweon, H.-S.; Lee, S.-J.; et al. The Role of the P38-Activated Protein Kinase Signaling Pathway-Mediated Autophagy in Cadmium-Exposed Monogonont Rotifer. Aquat. Toxicol. 2018, 194, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Widmann, C.; Gibson, S.; Jarpe, M.B.; Johnson, G.L. Mitogen-Activated Protein Kinase: Conservation of a Three-Kinase Module from Yeast to Human. Physiol. Rev. 1999, 79, 143–180. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Jiao, Y.; Chen, Q.; Wu, D.; Yu, P.; Li, Y.; Cai, M.; Zhao, Y. Polystyrene Nanoplastic Induces ROS Production and Affects the MAPK-HIF-1/NFkB-Mediated Antioxidant System in Daphnia pulex. Aquat. Toxicol. 2020, 220, 105420. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Sounding the Alarm: Protein Kinase Cascades Activated by Stress and Inflammation. J. Biol. Chem. 1996, 271, 24313–24316. [Google Scholar] [CrossRef]
- Roux, P.P.; Blenis, J. ERK and P38 MAPK-Activated Protein Kinases: A Family of Protein Kinases with Diverse Biological Functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, R.; Hu, S.; Xiao, X.; Wu, J.; Wei, S.; Xiong, Y.; Ouyang, G. Investigating the Toxicities of Different Functionalized Polystyrene Nanoplastics on Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 180, 509–516. [Google Scholar] [CrossRef]
- Haap, T.; Köhler, H.-R. Cadmium Tolerance in Seven Daphnia magna Clones Is Associated with Reduced Hsp70 Baseline Levels and Induction. Aquat. Toxicol. 2009, 94, 131–137. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Michaloudi, E.; Kormas, K.A.; Katsiapi, M.; Vardaka, E.; Genitsaris, S. Plankton Changes as Critical Processes for Restoration Plans of Lakes Kastoria and Koronia. Eur. Water 2012, 40, 43–51. [Google Scholar]
- Michaloudi, E.; Moustaka-Gouni, M.; Pantelidakis, K.; Katsiapi, M.; Genitsaris, S. Plankton Succession in the Temporary Lake Koronia after Intermittent Dry-Out. Int. Rev. Hydrobiol. 2012, 97, 405–419. [Google Scholar] [CrossRef]
- Michaloudi, E.; Moustaka-Gouni, M.; Gkelis, S.; Pantelidakis, K. Plankton Community Structure during an Ecosystem Disruptive Algal Bloom of Prymnesium parvum. J. Plankton Res. 2009, 31, 301–309. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Cook, C.M.; Gkelis, S.; Michaloudi, E.; Pantlelidakis, K.; Pyrovetsi, M.; Lanaras, T. The Coincidence of a Prymnesium parvum Bloom and the Mass Kill of Birds and Fish in Lake Koronia. Harmful Algae News 2004, 26, 1–2. [Google Scholar]
- Malamataris, D.; Kolokytha, E.; Mylopoulos, I.; Loukas, A. Critical Review of Adaptation Strategies for the Restoration of Lake Koronia. Eur. Water 2017, 58, 203–208. [Google Scholar]
- Kolokytha, E.; Malamataris, D. Integrated Water Management Approach for Adaptation to Climate Change in Highly Water Stressed Basins. Water Resour. Manag. 2020, 34, 1173–1197. [Google Scholar] [CrossRef]
- Bobori, D.C.; Economidis, P.S. The Effect of Size, Sex and Season on the Accumulation of Heavy Metals in Perch (Perca fluviatilis L., Pisces: Cyprinidae) in Lake Koronia (Macedonia, Greece). Toxicol. Environ. Chem. 1996, 57, 103–121. [Google Scholar] [CrossRef]
- Kaiserli, A.; Voutsa, D.; Samara, C. Phosphorus Fractionation in Lake Sediments--Lakes Volvi and Koronia, N. Greece. Chemosphere 2002, 46, 1147–1155. [Google Scholar] [CrossRef]
- Petaloti, C.; Voutsa, D.; Samara, C.; Sofoniou, M.; Stratis, I.; Kouimtzis, T. Nutrient Dynamics in Shallow Lakes of Northern Greece. Environ. Sci. Pollut. Res. 2004, 11, 11. [Google Scholar] [CrossRef]
- Papadaki, E.; Voutsa, D. Arsenic Cycling in Lakes Volvi and Koronia, Northern Greece. Assessment of Arsenic Mobility in Sediments. Fresenius Environ. Bull. 2007, 16, 421–427. [Google Scholar]
- Anthemidis, A.; Zachariadis, G.; Voutsa, D.; Kouras, A.; Samara, C. Variation of Heavy Metal and Other Toxic Element Concentrations in Surface Waters of Macedonia. In Proceedings of the 1st Environmental Conference of Macedonia, Thessaloniki, Greece, 1 March 2002; pp. 104–109. [Google Scholar]
- Kaloyianni, M.; Feidantsis, K.; Nteli, I.; Stergiou, P.; Tsoulia, T.; Dimitriadi, A.; Antonopoulou, E.; Bobori, D. Biochemical and Molecular Responses of Cyprinids in Two Mediterranean Lacustrine Ecosystems: Opportunities for Ecological Assessment and Biomonitoring. Aquat. Toxicol. 2019, 211, 105–115. [Google Scholar] [CrossRef] [PubMed]
- ISO 5667-3:2018. Water Quality–Sampling-Part 3: Preservation and Handling of Water Samples; ISO: Genève, Switzerland, 2018. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Bottrell, H.H.; Duncan, A.; Gliwicz, Z.M.; Grygierek, E.; Herzig, A.; Hillbright-Ilkowska, A.; Kurasawa, H.; Larsson, P.; Weglenska, T.A. A Review of Some Problems in Zooplankton Production Studies. Nor. J. Zool. 1976, 24, 419–456. [Google Scholar]
- Downing, J.A.; Rigler, F.H. A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters; Blackwell Scientific Publications: Oxford, UK, 1984. [Google Scholar]
- Taggart, C.T. Hypolimnetic Aeration and Zooplankton Distribution: A Possible Limitation to the Restoration of Cold-Water Fish Production. Can. J. Fish. Aquat. Sci. 1984, 41, 191–198. [Google Scholar] [CrossRef]
- Herzig, A. Some Population Characteristics of Planktonic Crustaceans in Neusiedler See. Oecologia 1974, 15, 127–141. [Google Scholar] [CrossRef]
- Dumont, H.J.; Van de Velde, I.; Dumont, S. The Dry Weight Estimate of Biomass in a Selection of Cladocera, Copepoda and Rotifera from the Plankton, Periphyton and Benthos of Continental Waters. Oecologia 1975, 19, 75–97. [Google Scholar] [CrossRef]
- Zarfdjian, M.H. Seasonal Variations and Spatial Distribution of Planktic Invertebrates in Lake Volvi (Macedonia Greece). Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1989. [Google Scholar]
- Michaloudi, E. Dry Weights of the Zooplankton of Lake Mikri Prespa (Macedonia, Greece). Belg. J. Zool. 2005, 135, 223–227. [Google Scholar]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau Staining as a Loading Control Alternative to Actin in Western Blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef]
- Liu, X.; Dur, G.; Ban, S.; Sakai, Y.; Ohmae, S.; Morita, T. Planktivorous Fish Predation Masks Anthropogenic Disturbances on Decadal Trends in Zooplankton Biomass and Body Size Structure in Lake Biwa, Japan. Limnol. Oceanogr. 2020, 65, 667–682. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kim, S.-K.; Chang, K.-H.; Kim, M.-C.; La, G.-H.; Joo, G.-J.; Jeong, K.-S. Population Growth of the Cladoceran, Daphnia magna: A Quantitative Analysis of the Effects of Different Algal Food. PLoS ONE 2014, 9, e95591. [Google Scholar] [CrossRef]
- Michaloudi, E.; Kostecka, M. Zooplankton of Lake Koroneia (Macedonia, Greece). Biologia 2004, 59, 165–172. [Google Scholar]
- Gilbert, J.J. Suppression of Rotifer Populations by Daphnia: A Review of the Evidence, the Mechanisms, and the Effects on Zooplankton Community Structure1. Limnol. Oceanogr. 1988, 33, 1286–1303. [Google Scholar] [CrossRef]
- Ebert, D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2005. [Google Scholar]
- Decaestecker, E.; De Meester, L.; Mergeay, J. Cyclical Parthenogenesis in Daphnia: Sexual Versus Asexual Reproduction. In Lost Sex: The Evolutionary Biology of Parthenogenesis; Schön, I., Martens, K., Dijk, P., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 295–316. [Google Scholar] [CrossRef]
- Pijanowska, J.; Stolpe, G. Summer Diapause in Daphnia as a Reaction to the Presence of Fish. J. Plankton Res. 1996, 18, 1407–1412. [Google Scholar] [CrossRef][Green Version]
- Oda, S.; Tatarazako, N.; Watanabe, H.; Morita, M.; Iguchi, T. Production of Male Neonates in Daphnia magna (Cladocera, Crustacea) Exposed to Juvenile Hormones and Their Analogs. Chemosphere 2005, 61, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Mortensen, E.; Hansen, A.-M.; Jørgensen, T. Cascading Trophic Interactions from Fish to Bacteria and Nutrients after Reduced Sewage Loading: An 18-Year Study of a Shallow Hypertrophic Lake. Ecosystems 1998, 1, 250–267. [Google Scholar] [CrossRef]
- Ghazy, M.M.E.-D.; Habashy, M.M.; Mohammady, E.Y. Effects of PH on Survival, Growth and Reproduction Rates of the Crustacean. Aust. J. Basic Appl. Sci. 2011, 5, 1–10. [Google Scholar]
- Roelke, D.L.; Errera, R.M.; Riesling, R.; Brooks, B.W.; Grover, J.P.; Schwierzke, L.; Urena-Boeck, F.; Baker, J.; Pinckney, J.L. Effects of Nutrient Enrichment on Prymnesium parvum Population Dynamics and Toxicity: Results from Field Experiments, Lake Possum Kingdom, USA. Aquat. Microb. Ecol. 2007, 46, 16. [Google Scholar] [CrossRef]
- Luu, P.T.; Yen, T.T.H.; Thai, T.T.; Quang, N.X. Effects of Non-Toxic Filamentous Cyanobacteria Isolated from Tri an Reservoir on Daphnia. Acad. J. Biol. 2020, 42. [Google Scholar] [CrossRef]
- Brooks, J.L.; Dodson, S.I. Predation, Body Size, and Composition of Plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef]
- Pirow, R.; Bäumer, C.; Paul, R.J. Benefits of Haemoglobin in the Cladoceran Crustacean Daphnia magna. J. Exp. Biol. 2001, 204, 3425–3441. [Google Scholar]
- Gerke, P.; Börding, C.; Zeis, B.; Paul, R.J. Adaptive Haemoglobin Gene Control in Daphnia pulex at Different Oxygen and Temperature Conditions. Comp. Biochem. Physiol. A 2011, 159, 56–65. [Google Scholar] [CrossRef]
- Cambronero, M.C.; Zeis, B.; Orsini, L. Haemoglobin-mediated Response to Hyper-thermal Stress in the Keystone Species Daphnia magna. Evol. Appl. 2017, 11, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Gonoi, H. Horizontal Movement of Pale and Red Daphnia magna in Low Oxygen Concentration. Physiol. Zool. 1985, 58, 190–196. [Google Scholar] [CrossRef]
- Schumpert, C.; Handy, I.; Dudycha, J.L.; Patel, R.C. Relationship between Heat Shock Protein 70 Expression and Life Span in Daphnia. Mech. Ageing Dev. 2014, 139, 1–10. [Google Scholar] [CrossRef][Green Version]
- Lyu, K.; Wang, Q.; Li, Z.; Chen, R.; Zhu, C.; Liu, J.; Yang, Z. Age-Dependent Survival and Selected Gene Expression in Daphnia magna after Short-Term Exposure to Low Dissolved Oxygen. J. Plankton Res. 2015, 37, 66–74. [Google Scholar] [CrossRef][Green Version]
- Haap, T.; Schwarz, S.; Köhler, H.-R. Metallothionein and Hsp70 Trade-off against One Another in Daphnia magna Cross-Tolerance to Cadmium and Heat Stress. Aquat. Toxicol. 2016, 170, 112–119. [Google Scholar] [CrossRef]
- Wilczynski, W.; Dynak, P.; Babkiewicz, E.; Bernatowicz, P.; Leniowski, K.; Maszczyk, P. The Combined Effects of Hypoxia and Fish Kairomones on Several Physiological and Life History Traits of Daphnia. Freshw. Biol. 2019, 64, 2204–2220. [Google Scholar] [CrossRef]
- Pauwels, K.; Stoks, R.; Meester, L.D. Enhanced Anti-Predator Defence in the Presence of Food Stress in the Water Flea Daphnia magna. Funct. Ecol. 2010, 24, 322–329. [Google Scholar] [CrossRef]
- Effertz, C.; von Elert, E. Light Intensity Controls Anti-Predator Defences in Daphnia: The Suppression of Life-History Changes. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133250. [Google Scholar] [CrossRef]
- Klumpen, E.; Hoffschröer, N.; Zeis, B.; Gigengack, U.; Dohmen, E.; Paul, R.J. Reactive Oxygen Species (ROS) and the Heat Stress Response of Daphnia pulex: ROS-Mediated Activation of Hypoxia-Inducible Factor 1 (HIF-1) and Heat Shock Factor 1 (HSF-1) and the Clustered Expression of Stress Genes. Biol. Cell 2017, 109, 39–64. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Filtering Rates, Food Size Selection, and Feeding Rates in Cladocerans—Another Aspect of Interspecific Competition in Filter-Feeding Zooplankton. In Evolution and Ecology of Zooplankton Communities; Kerfoot, W.C., Ed.; The University Press of New England: Hanover, NH, USA, 1980; pp. 282–291. [Google Scholar]
- Sarpe, D.; de Senerpont Domis, L.N.; Declerck, S.A.J.; van Donk, E.; Ibelings, B.W. Food Quality Dominates the Impact of Food Quantity on Daphnia Life History: Possible Implications for Re-Oligotrophication. Inland Waters 2014, 4, 363–368. [Google Scholar] [CrossRef]
- Coors, A.; Meester, L.D. Synergistic, Antagonistic and Additive Effects of Multiple Stressors: Predation Threat, Parasitism and Pesticide Exposure in Daphnia magna. J. Appl. Ecol. 2008, 45, 1820–1828. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP Kinase Signalling Pathways in Cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-B.; Won, E.-J.; Kang, H.-M.; Lee, M.-C.; Hwang, D.-S.; Hwang, U.-K.; Zhou, B.; Souissi, S.; Lee, S.-J.; Lee, J.-S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-P38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-M.; Rhee, J.-S.; Lee, K.-W.; Kim, M.-J.; Shin, K.-H.; Lee, S.-J.; Lee, Y.-M.; Lee, J.-S. UV-B Radiation-Induced Oxidative Stress and P38 Signaling Pathway Involvement in the Benthic Copepod Tigriopus japonicus. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2015, 167, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Puthumana, J.; Kang, H.-M.; Lee, M.-C.; Jeong, C.-B.; Han, J.; Hwang, D.-S.; Kim, I.-C.; Lee, J.W.; Lee, J.-S. Adverse Effects of MWCNTs on Life Parameters, Antioxidant Systems, and Activation of MAPK Signaling Pathways in the Copepod Paracyclopina nana. Aquat. Toxicol. 2016, 179, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Puthumana, J.; Lee, S.-H.; Kang, H.-M.; Park, J.C.; Jeong, C.-B.; Han, J.; Hwang, D.-S.; Seo, J.S.; Park, H.G.; et al. BDE-47 Induces Oxidative Stress, Activates MAPK Signaling Pathway, and Elevates de Novo Lipogenesis in the Copepod Paracyclopina nana. Aquat. Toxicol. 2016, 181, 104–112. [Google Scholar] [CrossRef]
- Jeong, C.-B.; Kang, H.-M.; Lee, M.-C.; Kim, D.-H.; Han, J.; Hwang, D.-S.; Souissi, S.; Lee, S.-J.; Shin, K.-H.; Park, H.G.; et al. Adverse Effects of Microplastics and Oxidative Stress-Induced MAPK/Nrf2 Pathway-Mediated Defense Mechanisms in the Marine Copepod Paracyclopina nana. Sci. Rep. 2017, 7, 41323. [Google Scholar] [CrossRef]
- Uehara, T.; Kaneko, M.; Tanaka, S.; Okuma, Y.; Nomura, Y. Possible Involvement of P38 MAP Kinase in HSP70 Expression Induced by Hypoxia in Rat Primary Astrocytes. Brain Res. 1999, 823, 226–230. [Google Scholar] [CrossRef]
- Rafiee, P.; Theriot, M.E.; Nelson, V.M.; Heidemann, J.; Kanaa, Y.; Horowitz, S.A.; Rogaczewski, A.; Johnson, C.P.; Ali, I.; Shaker, R.; et al. Human Esophageal Microvascular Endothelial Cells Respond to Acidic PH Stress by PI3K/AKT and P38 MAPK-Regulated Induction of Hsp70 and Hsp27. Am. J. Physiol. Cell Physiol. 2006, 291, C931–C945. [Google Scholar] [CrossRef]
- Bironaite, D.; Brunk, U.; Venalis, A. Protective Induction of Hsp70 in Heat-Stressed Primary Myoblasts: Involvement of MAPKs. J. Cell. Biochem. 2013, 114, 2024–2031. [Google Scholar] [CrossRef]
- Feidantsis, K.; Pörtner, H.O.; Markou, T.; Lazou, A.; Michaelidis, B. Involvement of P38 MAPK in the Induction of Hsp70 during Acute Thermal Stress in Red Blood Cells of the Gilthead Sea Bream, Sparus aurata. J. Exp. Zool. Part Ecol. Genet. Physiol. 2012, 317, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kefaloyianni, E.; Gourgou, E.; Ferle, V.; Kotsakis, E.; Gaitanaki, C.; Beis, I. Acute Thermal Stress and Various Heavy Metals Induce Tissue-Specific pro- or Anti-Apoptotic Events via the P38-MAPK Signal Transduction Pathway in Mytilus galloprovincialis (Lam.). J. Exp. Biol. 2005, 208 Pt 23, 4427–4436. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Hamad, D.; Di Mari, J.; Suki, W.N.; Safirstein, R.; Watts, B.A.; Rouse, D. P38 Kinase Activity Is Essential for Osmotic Induction of MRNAs for HSP70 and Transporter for Organic Solute Betaine in Madin-Darby Canine Kidney Cells. J. Biol. Chem. 1998, 273, 1832–1837. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Chouri, E.; Feidantsis, K.; Lazou, A.; Chatzifotis, S. Effects of Partial Dietary Supplementation of Fish Meal with Soymeal on the Stress and Apoptosis Response in the Digestive System of Common Dentex (Dentex dentex). J. Biol. Res. Thessalon. 2017, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Escobar, M.D.C.; Souza, V.; Bucio, L.; Hernández, E.; Gómez-Quiroz, L.E.; Ruiz, M.C.G. MAPK Activation Is Involved in Cadmium-Induced Hsp70 Expression in HepG2 Cells. Toxicol. Mech. Methods 2009, 19, 503–509. [Google Scholar] [CrossRef]
- Goodman, R.; Lin-Ye, A.; Geddis, M.S.; Wickramaratne, P.J.; Hodge, S.E.; Pantazatos, S.P.; Blank, M.; Ambron, R.T. Extremely Low Frequency Electromagnetic Fields Activate the ERK Cascade, Increase Hsp70 Protein Levels and Promote Regeneration in Planaria. Int. J. Radiat. Biol. 2009, 85, 851–859. [Google Scholar] [CrossRef]
- Lim, S.; Kim, D.G.; Kim, S. ERK-Dependent Phosphorylation of the Linker and Substrate-Binding Domain of HSP70 Increases Folding Activity and Cell Proliferation. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Korpelainen, H. The Effects of Temperature and Photoperiod on Life History Parameters of Daphnia magna (Crustacea: Cladocera). Freshw. Biol. 1986, 16, 615–620. [Google Scholar] [CrossRef]
- Williams, P.J.; Dick, K.B.; Yampolsky, L.Y. Heat Tolerance, Temperature Acclimation, Acute Oxidative Damage and Canalization of Haemoglobin Expression in Daphnia. Evol. Ecol. 2012, 26, 591–609. [Google Scholar] [CrossRef]
- Al-Seria, M.H.M. Salinity Effects on Survival and Life History of Daphnia magna. Int. J. Aquat. Sci. 2019, 10, 19–26. [Google Scholar]
- Jeyasingh, P.D.; Weider, L.J. Phosphorus Availability Mediates Plasticity in Life-History Traits and Predator–Prey Interactions in Daphnia. Ecol. Lett. 2005, 8, 1021–1028. [Google Scholar] [CrossRef]
- Lyu, K.; Cao, H.; Chen, R.; Wang, Q.; Yang, Z. Combined Effects of Hypoxia and Ammonia to Daphnia similis Estimated with Life-History Traits. Environ. Sci. Pollut. Res. 2013, 20, 5379–5387. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, K.; Stoks, R.; Decaestecker, E.; De Meester, L. Evolution of Heat Shock Protein Expression in a Natural Population of Daphnia magna. Am. Nat. 2007, 170, 800–805. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).