Hydrochemistry of the Lhasa River, Tibetan Plateau: Spatiotemporal Variations of Major Ions Compositions and Controlling Factors Using Multivariate Statistical Approaches
Abstract
:1. Introduction
2. Study Area
3. Materials and Methods
3.1. Sampling and Analyzing
3.2. Statistical Analysis
4. Results
4.1. General Hydrochemistry
4.2. Seasonal Variations of Hydrochemistry
4.3. Spatial Distribution of Major Ions
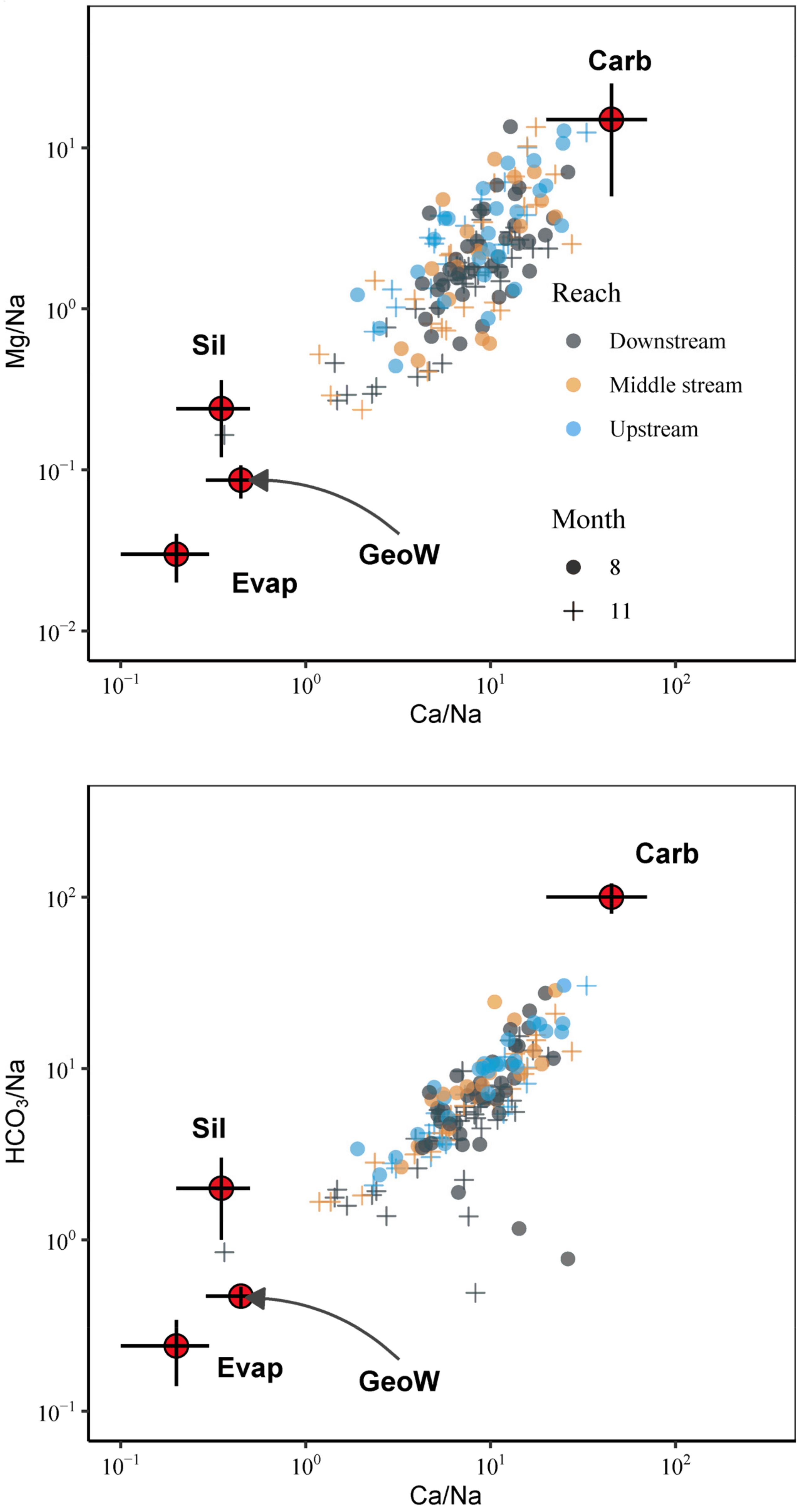
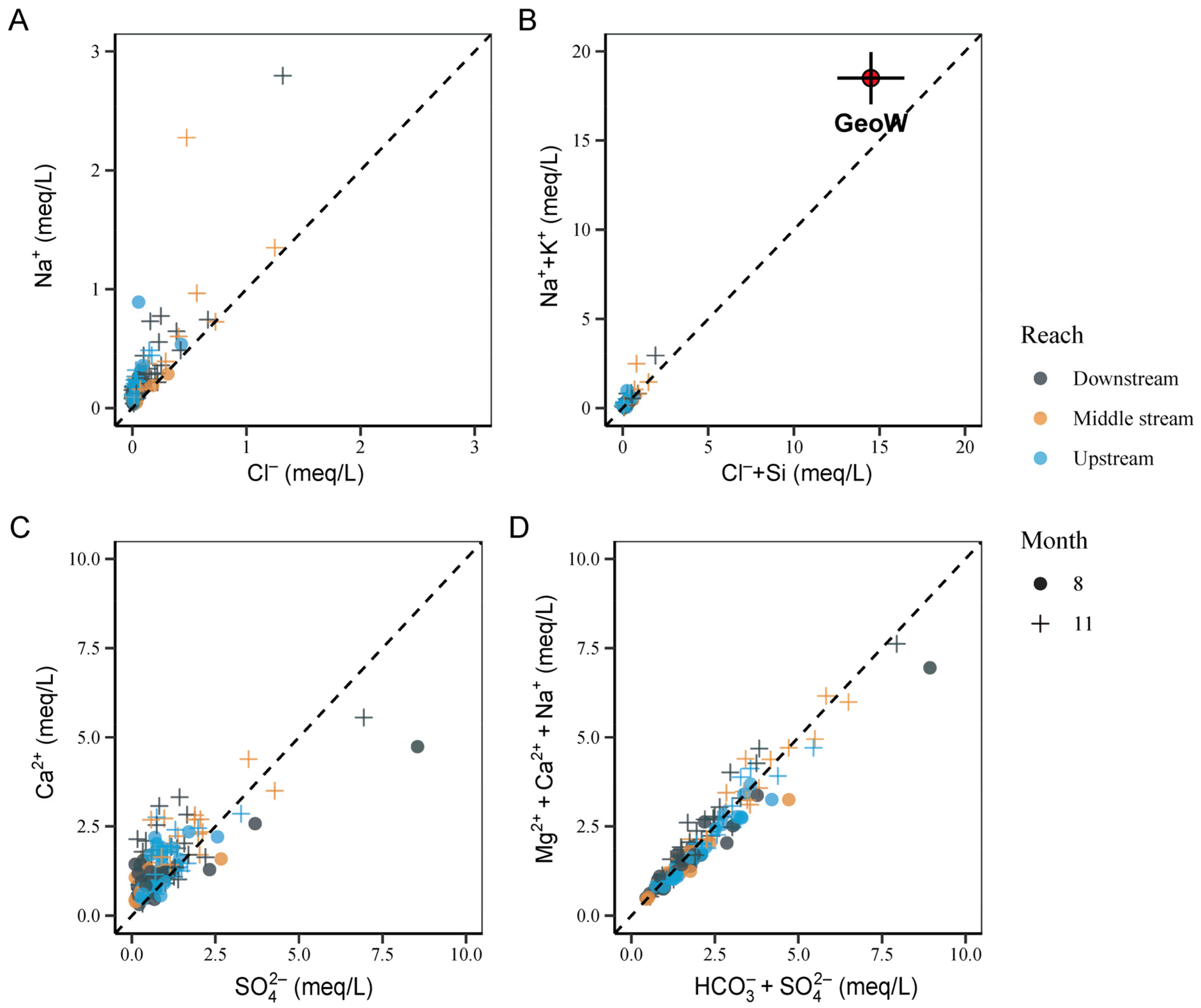
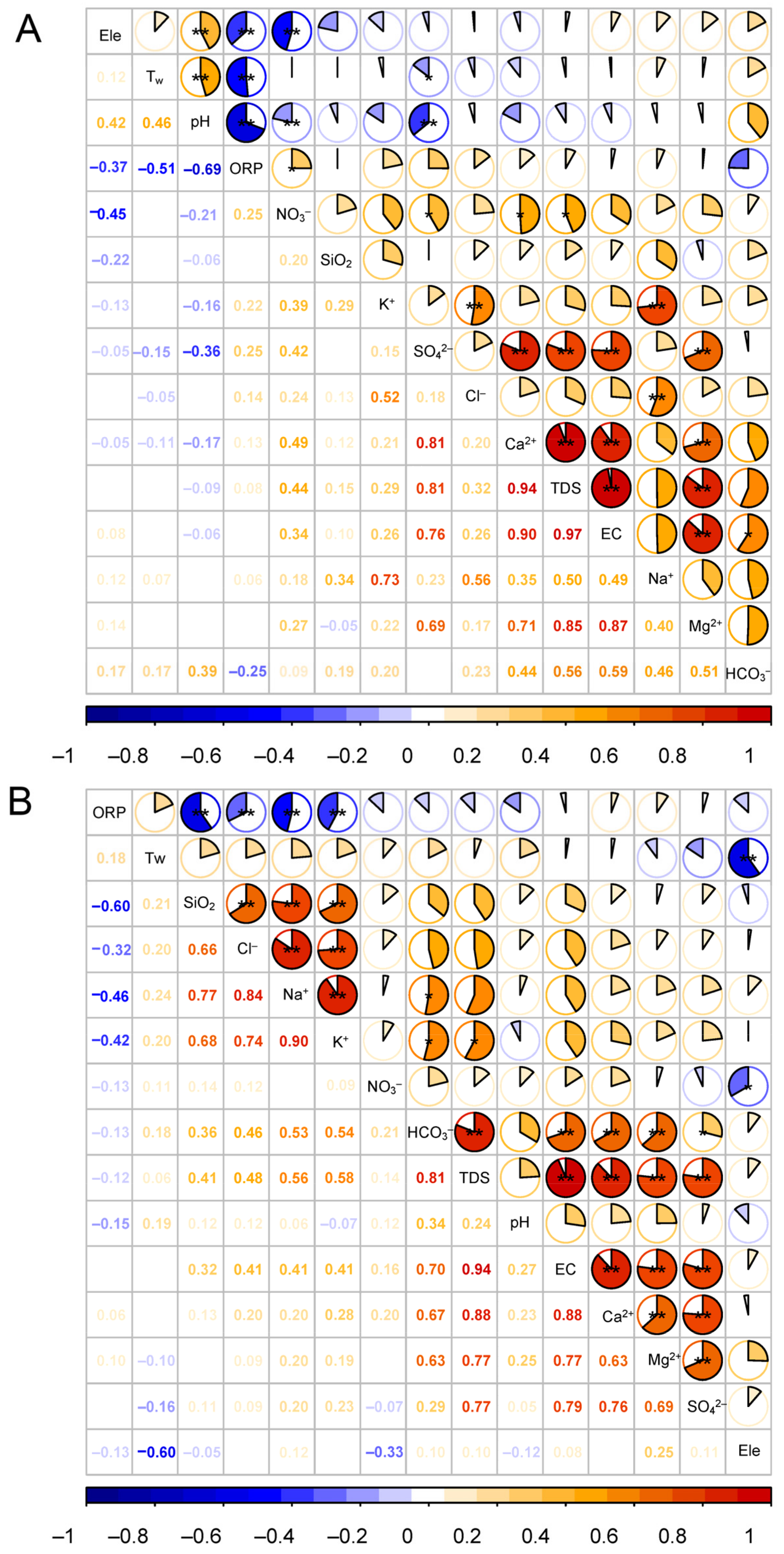
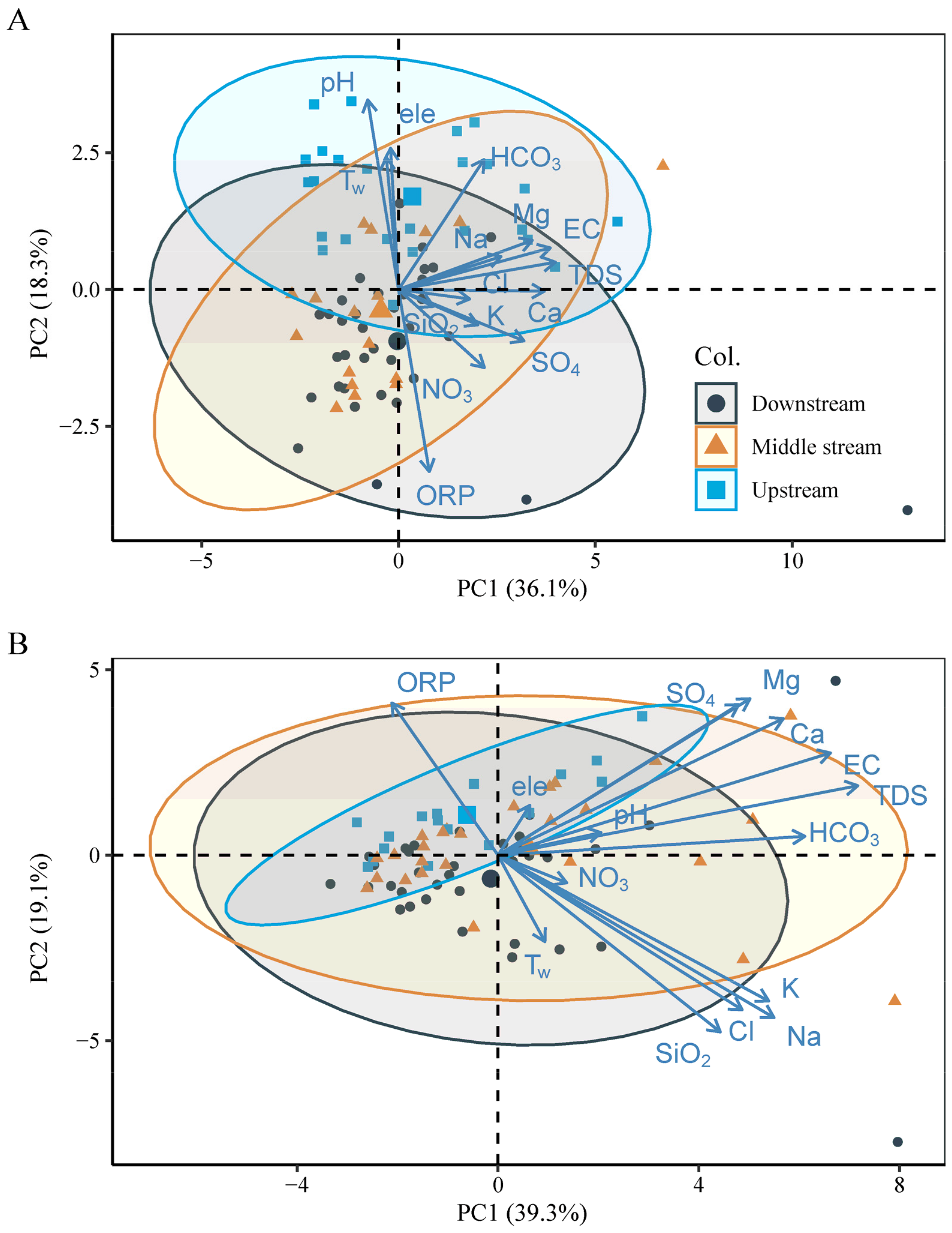
5. Discussion
5.1. Mechanisms Controlling the Major-Ion Chemistry
5.2. Multivariate Statistical Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vega, M.; Pardo, R.; Barrado, E.; Deban, L. Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res. 1998, 32, 3581–3592. [Google Scholar] [CrossRef]
- Meybeck, M.; Helmer, R. The quality of rivers from pristine stage to global pollution. Glob. Planet. Chang. 1989, 75, 283–309. [Google Scholar] [CrossRef]
- Hren, M.T.; Chamberlain, C.P.; Hilley, G.E.; Blisniuk, P.M.; Bookhagen, B. Major ion chemistry of the Yarlung Tsangpo-Brahmaputra river: Chemical weathering, erosion, and CO2 consumption in the southern Tibetan plateau and eastern syntaxis of the Himalaya. Geochim. Cosmochim. Acta 2007, 71, 2907–2935. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, A.K.; Rice, K.C.; Bricker, O.P.; Kennedy, M.M.; Anderson, R.T. Use of geochemical mass balance modelling to evaluate the role of weathering in determining stream chemistry in five mid-Atlantic watersheds on different lithologies. Hydrol. Process. 1997, 11, 719–744. [Google Scholar] [CrossRef]
- Qu, B.; Zhang, Y.; Kang, S.; Sillanpaa, M. Water chemistry of the southern Tibetan Plateau: An assessment of the Yarlung Tsangpo river basin. Environ. Earth Sci. 2017, 76. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Li, S.Y.; Xu, Z.F.; Wang, H.; Wang, J.H.; Zhang, Q.F. Geochemistry of the upper Han River basin, China 3: Anthropogenic inputs and chemical weathering to the dissolved load. Chem. Geol. 2009, 264, 89–95. [Google Scholar] [CrossRef]
- Pant, R.R.; Zhang, F.; Rehman, F.U.; Wang, G.; Ye, M.; Zeng, C.; Tang, H. Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal. Sci. Total Environ. 2018, 622, 770–782. [Google Scholar] [CrossRef]
- Da Silva, A.M.M.; Sacomani, L.B. Using chemical and physical parameters to define the quality of Pardo River water (Botucatu-SP-Brazil). Water Res. 2001, 35, 1609–1616. [Google Scholar] [CrossRef]
- Galy, A.; France-Lanord, C. Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem. Geol. 1999, 159, 31–60. [Google Scholar] [CrossRef]
- Sarin, M.M.; Krishnaswami, S.; Dilli, K.; Somayajulu, B.L.K.; Moore, W.S. Major ion chemistry of the Ganga-Brahmaputra river system: Weathering processes and fluxes to the Bay of Bengal. Geochim. Cosmochim. Acta 1989, 53, 997–1009. [Google Scholar] [CrossRef]
- Stallard, R.F.; Edmond, J.M. Geochemistry of the Amazon 1. Precipitation chemistry and the marine contribution to the dissolved-load at the time of peak discharge. J. Geophys. Res. 1981, 86, 9844–9858. [Google Scholar] [CrossRef]
- Huang, X.; Sillanpaa, M.; Gjessing, E.T.; Vogt, R.D. Water quality in the Tibetan Plateau: Major ions and trace elements in the headwaters of four major Asian rivers. Sci. Total Environ. 2009, 407, 6242–6254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yao, Z.; Liu, Z.; Wang, R.; Wu, S. Hydrochemistry and its controlling factors of rivers in the source region of the Yangtze River on the Tibetan Plateau. J. Geochem. Explor. 2015, 155, 76–83. [Google Scholar] [CrossRef]
- Huang, X.; Sillanpaa, M.; Gjessing, E.T.; Peraniemi, S.; Vogt, R.D. Water quality in the southern Tibetan Plateau: Chemical evaluation of the Yarlung Tsangpo (Brahmaputra). River Res. Appl. 2011, 27, 113–121. [Google Scholar] [CrossRef]
- Jiang, L.; Yao, Z.; Wang, R.; Liu, Z.; Wang, L.; Wu, S. Hydrochemistry of the middle and upper reaches of the Yarlung Tsangpo River system: Weathering processes and CO2 consumption. Environ. Earth Sci. 2015, 74, 2369–2379. [Google Scholar] [CrossRef]
- Pritchard, H.D. Asia’s shrinking glaciers protect large populations from drought stress. Nature 2019, 569, 649–654. [Google Scholar] [CrossRef]
- Yao, T.; Xue, Y.; Chen, D.; Chen, F.; Thompson, L.; Cui, P.; Koike, T.; Lau, W.K.M.; Lettenmaier, D.; Mosbrugger, V.; et al. Recent third pole’s rapid warming accompanies cryospheric melt and water cycle intensification and interactions between monsoon and environment: Multidisciplinary approach with observations, modeling, and analysis. Bull. Am. Meteorol. Soc. 2019, 100, 423–444. [Google Scholar] [CrossRef]
- Han, P.F.; Long, D.; Han, Z.Y.; Du, M.D.; Dai, L.Y.; Hao, X.H. Improved understanding of snowmelt runoff from the headwaters of China’s Yangtze River using remotely sensed snow products and hydrological modeling. Remote Sens. Environ. 2019, 224, 44–59. [Google Scholar] [CrossRef]
- Hu, M.H.; Stallard, R.F.; Edmond, J.M. Major ion chemistry of some large Chinese rivers. Nature 1982, 298, 550–553. [Google Scholar] [CrossRef]
- Immerzeel, W.W.; van Beek, L.P.H.; Bierkens, M.F.P. Climate change will affect the Asian Water Towers. Science 2010, 328, 1382–1385. [Google Scholar] [CrossRef]
- Barnett, T.P.; Adam, J.C.; Lettenmaier, D.P. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 2005, 438, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Bolch, T.; Kulkarni, A.; Kaab, A.; Huggel, C.; Paul, F.; Cogley, J.G.; Frey, H.; Kargel, J.S.; Fujita, K.; Scheel, M.; et al. The state and fate of Himalayan glaciers. Science 2012, 336, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Lutz, A.F.; Immerzeel, W.W.; Shrestha, A.B.; Bierkens, M.F.P. Consistent increase in High Asia’s runoff due to increasing glacier melt and precipitation. Nat. Clim. Chang. 2014, 4, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.E.; Hannah, D.M.; Milner, A.M.; Soulsby, C.; Hodson, A.J.; Brewer, M.J. Water source dynamics in a glacierized alpine river basin (Taillon-Gabiétous, French Pyrénées). Water Resour. Res. 2006, 42, W08404. [Google Scholar] [CrossRef]
- Li, D.; Luo, H.; Hu, T.; Shao, D.; Cui, Y.; Khan, S.; Luo, Y. Identification of the roles of climate factors, engineering construction, and agricultural practices in vegetation dynamics in the Lhasa River Basin, Tibetan Plateau. Remote Sens. 2020, 12, 1883. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Liu, W. Major hydrogeochernical processes in the two reservoirs of the Yangbajing geothermal field, Tibet, China. J. Volcanol. Geotherm. Res. 2007, 166, 255–268. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Liu, W. Hydrogeochemistry and environmental impact of geothermal waters from Yangyi of Tibet, China. J. Volcanol. Geotherm. Res. 2009, 180, 9–20. [Google Scholar] [CrossRef]
- Makokha, G.O.; Wang, L.; Zhou, J.; Li, X.; Wang, A.; Wang, G.; Kuria, D. Quantitative drought monitoring in a typical cold river basin over Tibetan Plateau: An integration of meteorological, agricultural and hydrological droughts. J. Hydrol. 2016, 543, 782–795. [Google Scholar] [CrossRef]
- Lin, L.; Gao, M.; Liu, J.; Wang, J.; Wang, S.; Chen, X.; Liu, H. Understanding the effects of climate warming on streamflow and active groundwater storage in an alpine catchment: The upper Lhasa River. Hydrol. Earth Syst. Sci. 2020, 24, 1145–1157. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Xie, J.; Gong, T.; Wang, H.; Xie, Y. Impacts of winter warming and permafrost degradation on water variability, upper Lhasa River, Tibet. Quat. Int. 2011, 244, 178–184. [Google Scholar] [CrossRef]
- You, Q.L.; Kang, S.C.; Wu, Y.H.; Yan, Y.P. Climate change over the Yarlung Zangbo river basin during 1961-2005. J. Geogr. Sci. 2007, 17, 409–420. [Google Scholar] [CrossRef]
- Huang, X.; Sillanpaa, M.; Gjessing, E.T.; Peraniemi, S.; Vogt, R.D. Environmental impact of mining activities on the surface water quality in Tibet: Gyama valley. Sci. Total Environ. 2010, 408, 4177–4184. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.X.; Zhao, Y.S.; Zhang, F.R.; Liu, J.J.; Huang, X. Spatiotemporal variability of heavy metals and identification of potential source tracers in the surface water of the Lhasa River basin. Environ. Sci. Pollut. Res. 2019, 26, 7442–7452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cai, W.; Li, Y.; Geng, T.; Zhang, Z.; Lv, Y.; Zhao, M.; Liu, J. Ion chemistry of groundwater and the possible controls within Lhasa River Basin, SW Tibetan Plateau. Arab. J. Geosci. 2018, 11, 510. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Wang, M.; Li, Y.; Shi, M.; Zhang, H.; Ma, Y. Hydrochemical characteristics and possible controls in the groundwater of the Yarlung Zangbo River Valley, China. Environ. Earth Sci. 2019, 78, 76. [Google Scholar] [CrossRef]
- Qin, H.; Gao, B.; He, L.; Hu, X.; Dong, L.; Sanjay, D.; Dong, A.; Sun, Z.; Wan, W. Hydrogeochemical characteristics and controlling factors of the Lhasa River under the influence of anthropogenic activities. Water 2019, 11, 948. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Tian, P.; Luo, H.; Hu, T.; Dong, B.; Cui, Y.; Khan, S.; Luo, Y. Impacts of land use and land cover changes on regional climate in the Lhasa River basin, Tibetan Plateau. Sci. Total Environ. 2020, 742, 140570. [Google Scholar] [CrossRef]
- Rao, W.; Chen, X.; Meredith, K.T.; Tan, H.; Gao, M.; Liu, J. Water uptake of riparian plants in the lower Lhasa River Basin, South Tibetan Plateau using stable water isotopes. Hydrol. Process. 2020, 34, 3492–3505. [Google Scholar] [CrossRef]
- Podila, S.P.; Penumaka, R.; Cherukuri, I. Hydrochemistry of groundwater from Chevella Watershed, Telangana State, India. J. Geol. Soc. India 2019, 94, 501–506. [Google Scholar] [CrossRef]
- Song, C.L.; Wang, G.X.; Mao, T.X.; Huang, K.W.; Sun, X.Y.; Hu, Z.Y.; Chang, R.Y.; Chen, X.P.; Raymond, P.A. Spatiotemporal variability and sources of DIC in permafrost catchments of the Yangtze River Source Region: Insights from stable carbon isotope and water chemistry. Water Resour. Res. 2020, 56, 22. [Google Scholar] [CrossRef]
- Alberto, W.D.; Del Pilar, D.M.; Valeria, A.M.; Fabiana, P.S.; Cecilia, H.A.; De Los Angeles, B.M. Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquia River basin (Cordoba-Argentina). Water Res. 2001, 35, 2881–2894. [Google Scholar] [CrossRef]
- Diamantini, E.; Lutz, S.R.; Mallucci, S.; Majone, B.; Merz, R.; Bellin, A. Driver detection of water quality trends in three large European river basins. Sci. Total Environ. 2018, 612, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Guggenmos, M.R.; Daughney, C.J.; Jackson, B.M.; Morgenstern, U. Regional-scale identification of groundwater-surface water interaction using hydrochemistry and multivariate statistical methods, Wairarapa Valley, New Zealand. Hydrol. Earth Syst. Sci. 2011, 15, 3383–3398. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.P.; Malik, A.; Mohan, D.; Sinha, S. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—A case study. Water Res. 2004, 38, 3980–3992. [Google Scholar] [CrossRef]
- Bengraine, K.; Marhaba, T.F. Using principal component analysis to monitor spatial and temporal changes in water quality. J. Hazard. Mater. 2003, 100, 179–195. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Yeh, T.C.J.; Zhen, P.; Wang, L.; Shi, L. Using multivariate statistical techniques and geochemical modeling to identify factors controlling the evolution of groundwater chemistry in a typical transitional area between Taihang Mountains and North China Plain. Hydrol. Process. 2020, 34, 1888–1905. [Google Scholar] [CrossRef]
- Nyenje, P.M.; Foppen, J.W.; Uhlenbrook, S.; Lutterodt, G. Using hydrochemical tracers to assess impacts of unsewered urban catchments on hydrochemistry and nutrients in groundwater. Hydrol. Process. 2014, 28, 5860–5878. [Google Scholar] [CrossRef]
- Helena, B.; Pardo, R.; Vega, M.; Barrado, E.; Fernandez, J.M.; Fernandez, L. Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res. 2000, 34, 807–816. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Meybeck, M. Global occurrence of major elements in rivers. Treatise Geochem. 2003, 5, 207–223. [Google Scholar] [CrossRef]
- Chinese Standards for Drinking Water Quality; GB 5749; Ministry of Public Health of China: Beijing, China, 2006.
- WHO. Guidelines for Drinking-Water Quality; WHO Chronicle; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Singh, S.K.; Sarin, M.M.; France-Lanord, C. Chemical erosion in the eastern Himalaya: Major ion composition of the Brahmaputra and delta C-13 of dissolved inorganic carbon. Geochim. Cosmochim. Acta 2005, 69, 3573–3588. [Google Scholar] [CrossRef]
- Blum, J.D.; Gazis, C.A.; Jacobson, A.D.; Chamberlain, C.P. Carbonate versus silicate weathering in the Raikhot watershed within the high Himalayan crystalline series. Geology 1998, 26, 411–414. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Ma, H.; Wang, L.; Martín, J.D. Identification of the hydrogeochemical processes and assessment of groundwater quality using classic integrated geochemical methods in the Southeastern part of Ordos basin, China. Environ. Pollut. 2016, 218, 879–888. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Gao, Z.; Wang, M.; Liu, M.; Wang, S.; Wang, Z. Hydrochemistry and relationship between groundwater and surface water in the middle and lower reaches of Lhasa River basin. J. Shandong Univ. Sci. Technol. Nat. Sci. 2020, 39, 10–20, (In Chinese with English abstract). [Google Scholar]
- Quade, J.; English, N.; DeCelles, P.G. Silicate versus carbonate weathering in the Himalaya: A comparison of the Arun and Seti River watersheds. Chem. Geol. 2003, 202, 275–296. [Google Scholar] [CrossRef]
- Tsering, T.; Abdel Wahed, M.S.M.; Iftekhar, S.; Sillanpää, M. Major ion chemistry of the Teesta River in Sikkim Himalaya, India: Chemical weathering and assessment of water quality. J. Hydrol. Reg. Stud. 2019, 24, 100612. [Google Scholar] [CrossRef]
- Zhao, P.; Ji, D.; Jian, J. A new geochemical model of the Yangbajing geothermal field, Tibet. Proceedings World Geothermal Congress, Kyushu-Tohoku, Japan, 28 May–10 June 2000; pp. 2007–2012. [Google Scholar]
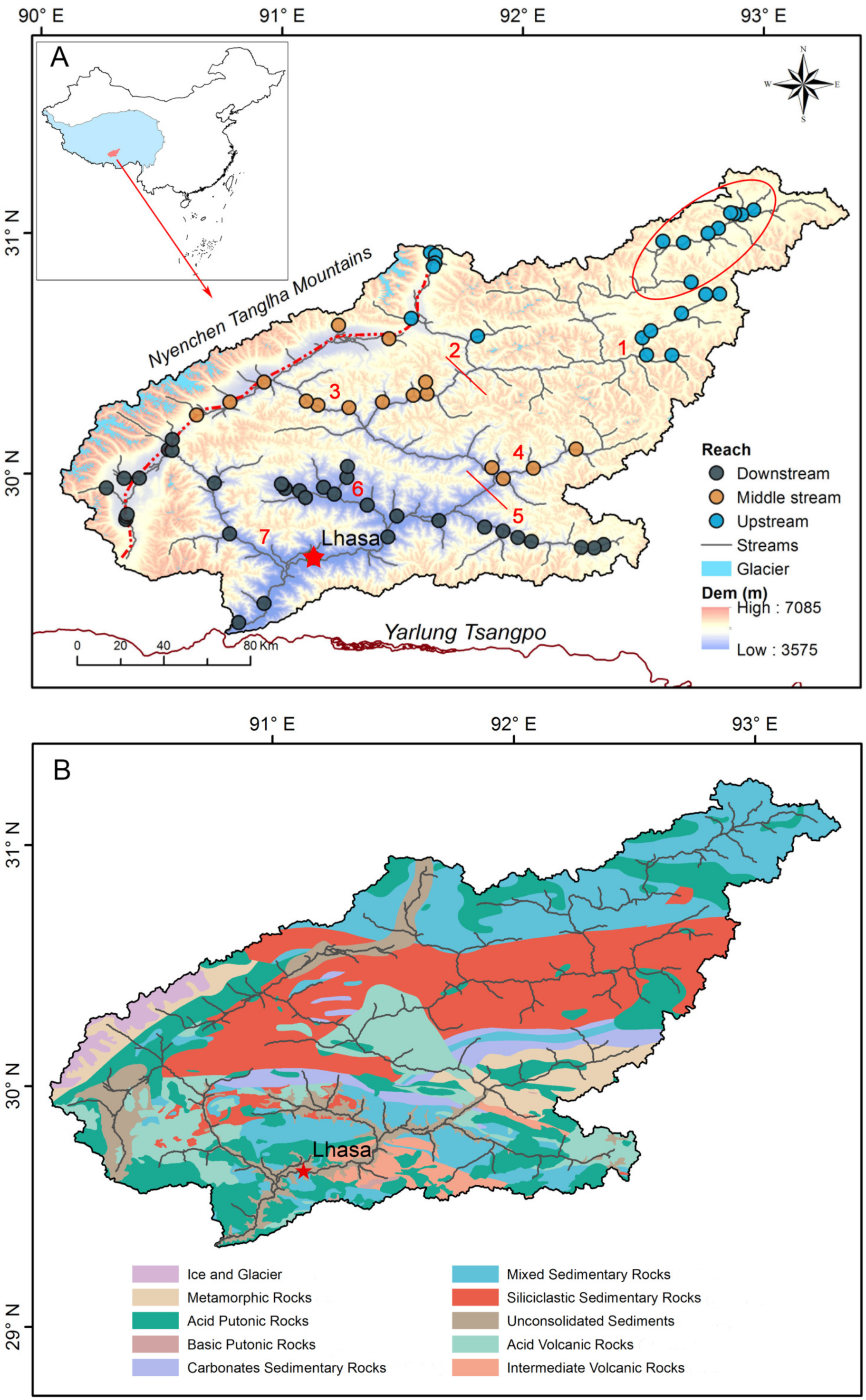
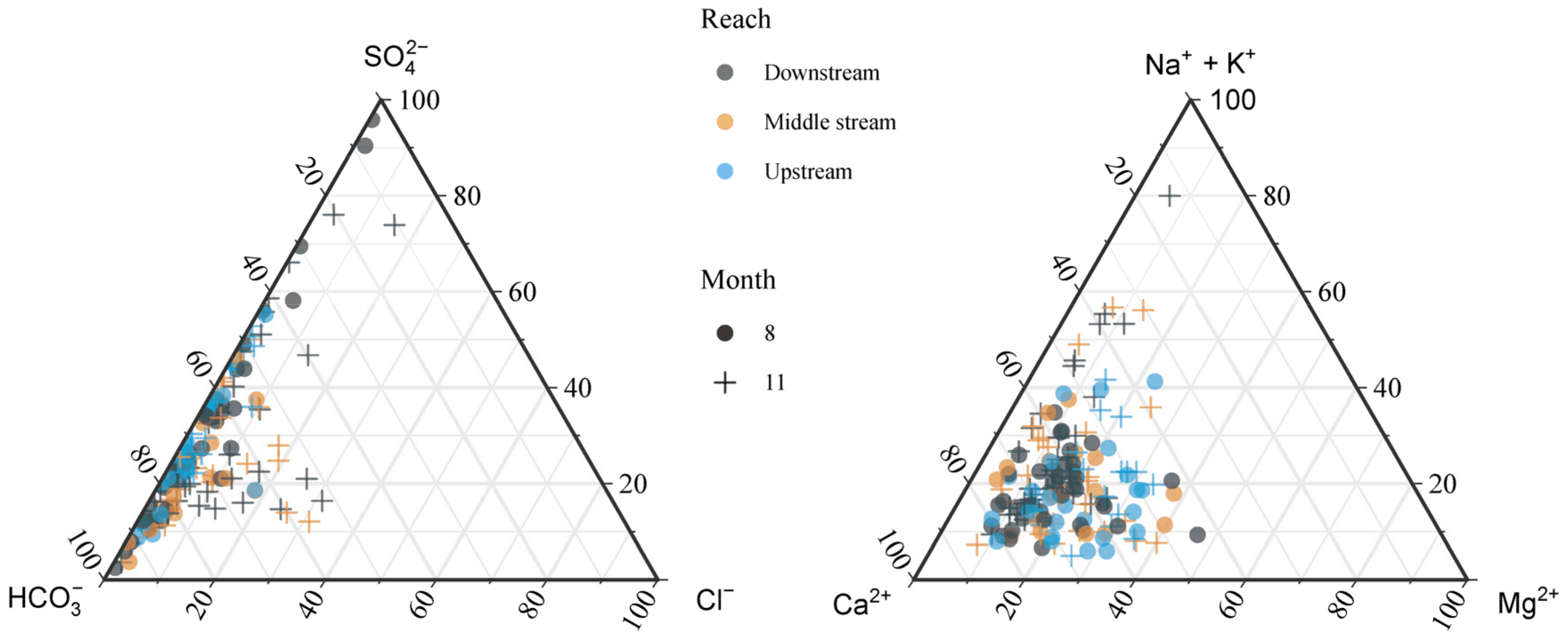
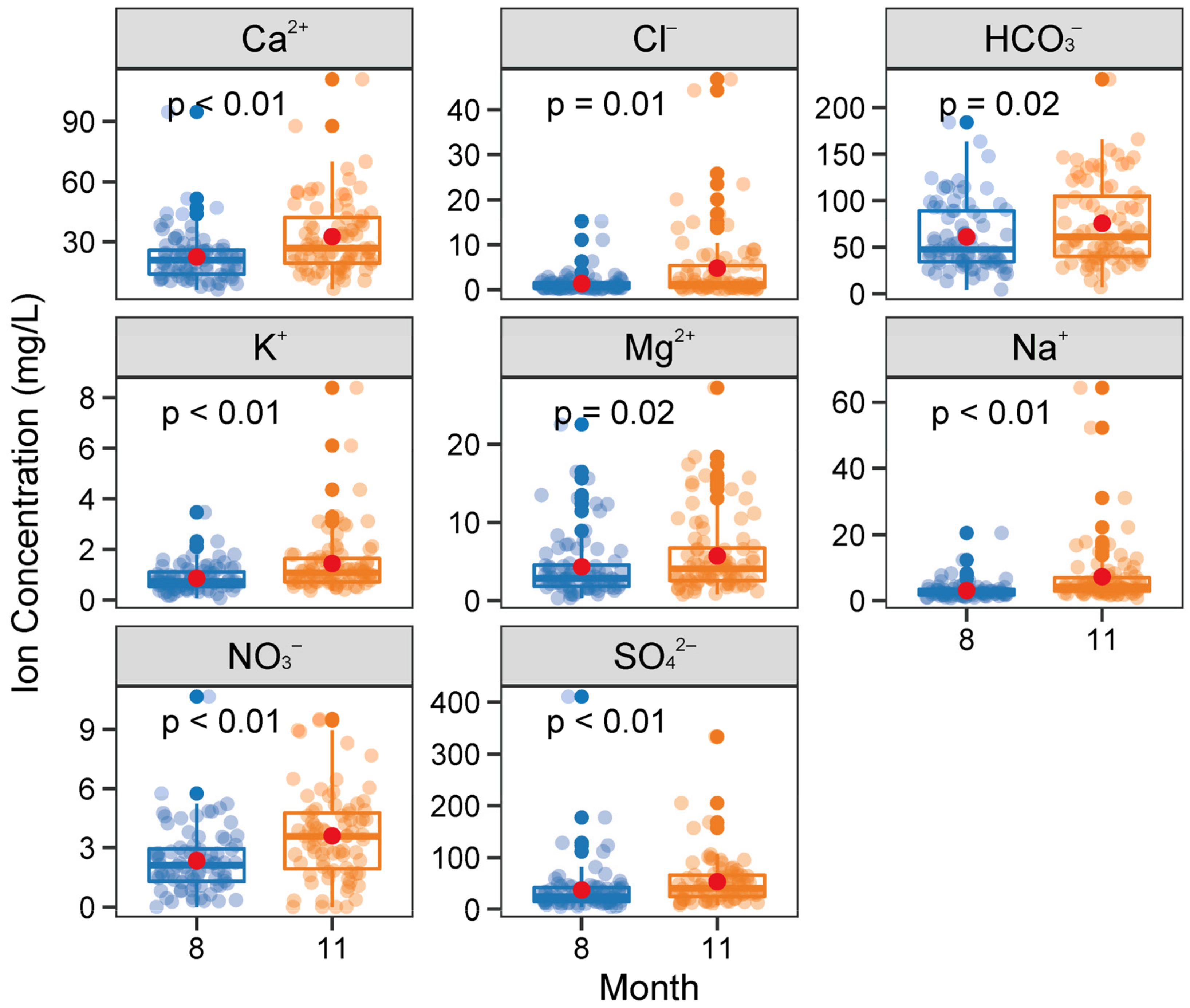
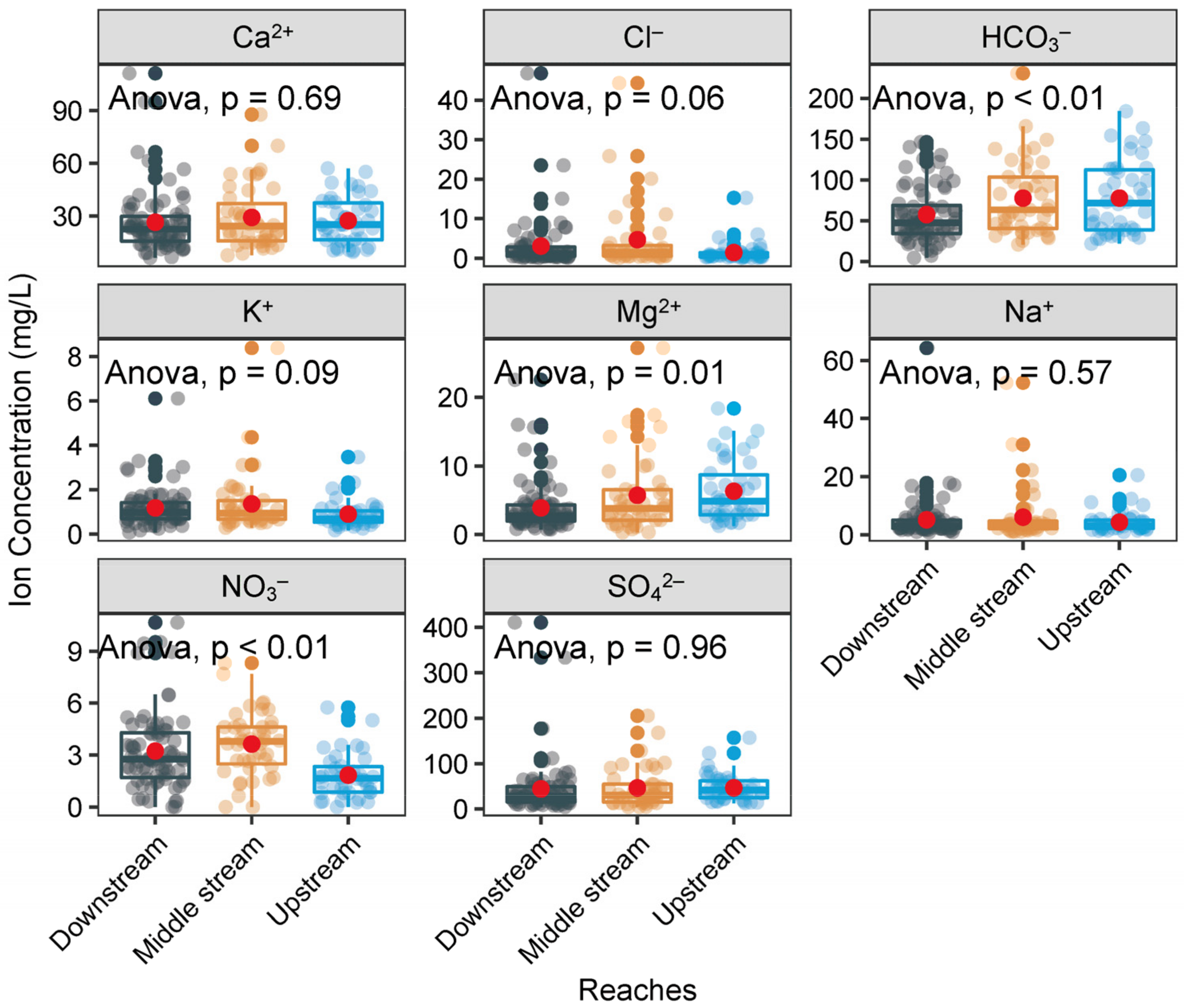
| Sites | Statistics | pH | Tw | ORP | EC | TDS | Na+ | K+ | Ca2+ | Mg2+ | Cl− | NO3− | SO42− | HCO3− | SiO2 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upstream | Mean | 8.3 | 9.9 | 133 | 202.4 | 171.6 | 4.3 | 0.9 | 27.4 | 6.3 | 1.4 | 1.8 | 46.5 | 77.7 | 5.2 | This study |
| Min | 7.3 | 0.5 | −9.4 | 75 | 65.2 | 1 | 0.2 | 9.7 | 1.3 | 0.1 | B.D. | 13.1 | 22 | 0.3 | ||
| Max | 9.4 | 19 | 289 | 420 | 379.3 | 20.5 | 3.5 | 57.1 | 18.4 | 15.3 | 5.8 | 156.8 | 184.2 | 13.4 | ||
| SE | 0.1 | 1 | 15.1 | 14.3 | 12.4 | 0.6 | 0.1 | 2 | 0.7 | 0.4 | 0.2 | 4.6 | 6.8 | 0.4 | ||
| Middle stream | Mean | 8.1 | 7.6 | 174.1 | 193.8 | 181.9 | 6 | 1.4 | 29.3 | 5.7 | 4.7 | 3.6 | 46.4 | 77.7 | 7.1 | |
| Min | 6.8 | 0.1 | 53 | 58 | 43.2 | 1 | 0.3 | 7.8 | 0.3 | 0.1 | B.D. | 4.6 | 21 | 2.9 | ||
| Max | 8.6 | 17.6 | 267 | 553 | 500.7 | 52.3 | 8.4 | 87.7 | 27.2 | 44.3 | 8.3 | 205.2 | 230.6 | 20.3 | ||
| SE | 0.1 | 0.9 | 10.2 | 17.3 | 17.7 | 1.4 | 0.2 | 2.7 | 0.9 | 1.3 | 0.3 | 6.8 | 7 | 0.6 | ||
| Downstream | Mean | 7.9 | 10.3 | 153.6 | 174.6 | 152.9 | 5.1 | 1.2 | 26.5 | 3.9 | 3.1 | 3.2 | 44.3 | 57.6 | 8 | |
| Min | 5.5 | 0.3 | 19 | 35 | 39.8 | 0.8 | 0.1 | 6.3 | 0.8 | 0.1 | B.D. | 4.7 | 4.6 | 2.2 | ||
| Max | 8.7 | 19.7 | 295 | 664 | 582.6 | 64.3 | 6.1 | 111.1 | 22.5 | 46.8 | 10.7 | 410.4 | 146.4 | 36.3 | ||
| SE | 0.1 | 0.6 | 9.4 | 134.7 | 11 | 0.9 | 0.1 | 2.1 | 0.4 | 0.7 | 0.3 | 7.1 | 4 | 0.5 | ||
| Lhasa River | Mean | 8.1 | 9.4 | 153.7 | 187 | 165.6 | 5.2 | 1.2 | 27.5 | 5 | 3.1 | 3 | 45.4 | 68.3 | 7 | |
| Min | 5.5 | 0.1 | −9.4 | 35 | 39.8 | 0.8 | 0.1 | 6.3 | 0.3 | 0.1 | B.D. | 4.6 | 4.6 | 0.3 | ||
| Max | 9.4 | 19.7 | 295 | 664 | 582.6 | 64.3 | 8.4 | 111.1 | 27.2 | 46.8 | 10.7 | 410.4 | 230.6 | 36.3 | ||
| SE | 0 | 0.4 | 6.6 | 8.4 | 7.7 | 0.6 | 0.1 | 1.3 | 0.4 | 0.5 | 0.2 | 4 | 3.3 | 0.3 | ||
| Yarlung Tsangpo | Mean | - | - | - | - | 112 | 3 | 1 | 21 | 4 | 5 | 0 | 27 | 47 | 4 | [13] |
| Mekong River | Mean | - | - | - | - | 302 | 12 | 1 | 49 | 14 | 14 | 0 | 69 | 138 | 4 | [13] |
| Source of the Yangtze River | Mean | 8 | - | - | - | 778 | 157.7 | 5.5 | 53.4 | 22.9 | 233.7 | 1.3 | 114.9 | 188.5 a | - | [16] |
| Gandaki, Nepal | Mean | 8.3 | - | - | 530 | 269 | 12.4 | 3.5 | 39.7 | 13.9 | 16 | 1.8 | 49.4 | 130.2 | 7.2 | [8] |
| Global River | Mean | 8 | - | - | - | 120 | 6.3 | 2.3 | 15 | 4.1 | 7.8 | 1 | 11.2 | 58.4 | 16.4 | [51,52] |
| CSS | Mean | 6.5−9.5 | - | - | - | 1000 | 200 | - | - | - | 250 | 50 | 250 | - | - | [53] |
| WHO | Mean | 6-8.5 | - | - | - | 1000 | 200 | 100 | 100 | 50 | 250 | 50 | 250 | 600 | - | [54] |
| Tributaries | Statistics | Tw | pH | EC | ORP | Na+ | K+ | Ca2+ | Mg2+ | Cl− | NO3− | SO42− | HCO3− | TDS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duilong Qu | Mean | 7.6 | 8.1 | 158.9 | 102.1 | 8.3 | 1.5 | 21.5 | 2.8 | 5.6 | 3.7 | 35.4 | 52.1 | 141.5 |
| SE | 1.1 | 0.1 | 14.8 | 7.4 | 2.6 | 0.2 | 1.5 | 0.4 | 2.1 | 0.4 | 5.8 | 5.6 | 14.5 | |
| Min | 0.3 | 7.3 | 59 | 19 | 1 | 0.7 | 7.8 | 0.3 | 0.3 | 0.5 | 5.3 | 7.3 | 43.2 | |
| Max | 18.8 | 8.7 | 379 | 160 | 64.3 | 6.1 | 40.5 | 7.9 | 46.8 | 9.5 | 111.6 | 144 | 391 | |
| Mai Qu | Mean | 9.4 | 8.3 | 215.2 | 150.6 | 3.8 | 0.9 | 29.8 | 8.3 | 1.4 | 1.9 | 51.9 | 85.6 | 188.7 |
| Se | 1.8 | 0.1 | 20.9 | 26.3 | 0.8 | 0.1 | 3.4 | 1.3 | 0.5 | 0.3 | 5.4 | 12.5 | 19.7 | |
| Min | 0.5 | 7.9 | 92 | 4.7 | 1 | 0.4 | 10.6 | 2.9 | 0.2 | 0.8 | 17.1 | 31.5 | 75 | |
| Max | 18 | 9.4 | 320 | 275 | 11.2 | 2.1 | 55.1 | 15.1 | 6 | 3.6 | 95.2 | 163.5 | 319.7 | |
| Mozhu Qu | Mean | 8.2 | 7.5 | 122.3 | 190.4 | 2.5 | 0.7 | 19.9 | 2.8 | 0.8 | 2.2 | 42.1 | 31.2 | 108.4 |
| Se | 0.5 | 0.2 | 18.8 | 20.8 | 0.4 | 0.1 | 3.7 | 0.4 | 0.3 | 0.2 | 10.5 | 5.2 | 15.9 | |
| Min | 4.3 | 5.5 | 35 | 83.4 | 0.8 | 0.1 | 6.3 | 1.7 | 0.1 | 0.3 | 10.5 | 4.6 | 39.8 | |
| Max | 11.4 | 8.4 | 325 | 295 | 7.6 | 1.2 | 56.6 | 8.4 | 5.1 | 4 | 177.1 | 98.7 | 255.1 | |
| Pengbo Qu | Mean | 14.3 | 8.3 | 208.4 | 138.6 | 3.8 | 1.1 | 34.8 | 4.1 | 3.1 | 4.1 | 25.7 | 95.9 | 180.2 |
| Se | 0.6 | 0.1 | 16.1 | 22.5 | 0.6 | 0.2 | 3.5 | 0.6 | 1 | 0.7 | 4.1 | 7.4 | 14.7 | |
| Min | 9.7 | 7.6 | 137 | 60.3 | 1.7 | 0.4 | 19.2 | 1.5 | 0.4 | B.D. | 4.7 | 46.4 | 109.7 | |
| Max | 19.7 | 8.6 | 354 | 281 | 11.2 | 3.0 | 66.4 | 10.6 | 15.1 | 9.4 | 68.6 | 146.4 | 327.2 | |
| Sang Qu | Mean | 6.2 | 8.2 | 300.7 | 156.4 | 8.5 | 1.7 | 45 | 7.6 | 8.4 | 3.3 | 78.7 | 109.7 | 271.9 |
| Se | 1.4 | 0.1 | 30.9 | 14.3 | 2.1 | 0.3 | 4.5 | 1.8 | 3.1 | 0.4 | 13.7 | 9.0 | 26.6 | |
| Min | 0.4 | 7.9 | 131 | 90.5 | 2.1 | 0.3 | 19.2 | 1.3 | 0.3 | 1.1 | 23.6 | 52.5 | 114.8 | |
| Max | 14.3 | 8.5 | 553 | 236 | 31 | 4.4 | 87.7 | 27.2 | 44.3 | 6 | 205.2 | 165.9 | 470.6 | |
| Source area | Mean | 9.8 | 8.3 | 163.2 | 137.2 | 3.9 | 0.7 | 21.4 | 5.4 | 0.7 | 1 | 42.8 | 57.4 | 137.5 |
| Se | 1.6 | 0.1 | 18.6 | 24.4 | 0.4 | 0.1 | 2.8 | 0.9 | 0.1 | 0.2 | 7.2 | 7.8 | 17.2 | |
| Min | 0.6 | 7.3 | 75 | −9.4 | 1.4 | 0.2 | 9.7 | 1.7 | 0.1 | B.D. | 13.1 | 22 | 65.2 | |
| Max | 19 | 9.4 | 365 | 289 | 7.4 | 1.2 | 57.1 | 18.4 | 1.5 | 2.6 | 156.8 | 133 | 379.3 | |
| Wululong Qu | Mean | 9.7 | 7.8 | 161.2 | 162.1 | 7.4 | 1.7 | 20.9 | 5.4 | 3 | 3.3 | 29.4 | 73.3 | 151.3 |
| Se | 1.5 | 0.1 | 24.3 | 16.8 | 3.1 | 0.5 | 3 | 1.4 | 1.2 | 0.4 | 6.9 | 14.0 | 27.9 | |
| Min | 0.1 | 6.8 | 55 | 53 | 1 | 0.2 | 8.8 | 0.3 | 0.2 | 0.6 | 4.6 | 21.5 | 46.8 | |
| Max | 17.6 | 8.6 | 420 | 263 | 52.3 | 8.4 | 54 | 15.7 | 16.9 | 5.8 | 98.3 | 230.6 | 500.7 | |
| Xuerong Qu | Mean | 8.9 | 7.9 | 150.9 | 179.7 | 1.9 | 0.7 | 23.8 | 4.3 | 0.7 | 1.6 | 40.9 | 52.6 | 131.3 |
| Se | 1.1 | 0.2 | 9.9 | 25.3 | 0.3 | 0.1 | 1.8 | 0.5 | 0.3 | 0.4 | 3.4 | 6 | 9.9 | |
| Min | 4.3 | 7 | 109.0 | 96.5 | 1.3 | 0.6 | 18.3 | 2.5 | 0.1 | B.D. | 27.3 | 35.5 | 94.3 | |
| Max | 12.2 | 8.4 | 188.0 | 267 | 3.6 | 1 | 32.9 | 6.5 | 2.5 | 3 | 54.9 | 83 | 172.1 |
| Variables | Rainy Season | Dry Season | ||||
|---|---|---|---|---|---|---|
| Upstream | Middle Stream | Downstream | Upstream | Middle Stream | Downstream | |
| HCO3−/Ca2+ | 1.02 ± 0.05 | 1.06 ± 0.1 | 0.82 ± 0.05 | 0.79 ± 0.05 | 0.82 ± 0.04 | 0.78 ± 0.06 |
| (HCO3− + SO42−)/Ca2+ | 1.66 ± 0.05 | 1.58 ± 0.12 | 1.48 ± 0.05 | 1.65 ± 0.05 | 1.46 ± 0.06 | 1.4 ± 0.07 |
| (HCO3− + SO42−)/(Ca2+ + Mg2+) | 1.22 ± 0.02 | 1.18 ± 0.04 | 1.16 ± 0.03 | 1.16 ± 0.03 | 1.12 ± 0.03 | 1.14 ±0.05 |
| (HCO3− + SO42−)/(Ca2+ + Mg2+ + Na+) | 1.11 ± 0.02 | 1.07 ± 0.04 | 1.06 ± 0.03 | 1.04 ± 0.03 | 0.98 ± 0.02 | 0.95 ± 0.02 |
| HCO3−/(HCO3−+SO42)− | 0.62 ± 0.03 | 0.67 ± 0.04 | 0.56 ± 0.04 | 0.48 ± 0.03 | 0.57 ± 0.03 | 0.56 ± 0.03 |
| (Ca2+ + Mg2+)/TZ+ | 0.9 ± 0.01 | 0.89 ± 0.01 | 0.9 ± 0.01 | 0.88 ± 0.02 | 0.87 ± 0.02 | 0.84 ± 0.02 |
| (HCO3− + SO42−)/TZ− | 0.97 ± 0.01 | 0.93 ± 0.01 | 0.95 ± 0.01 | 0.97 ± 0.0 | 0.92 ± 0.01 | 0.91 ± 0.01 |
| Na+/Cl− | 8.52 ± 1.33 | 3.72 ± 0.76 | 5.95 ± 0.54 | 10.48 ± 1.84 | 5.09 ± 0.96 | 6.78 ± 1.45 |
| Si/(Na+ + K+) | 0.73 ± 0.16 | 0.76 ± 0.07 | 0.92 ± 0.07 | 0.37 ± 0.06 | 0.52 ± 0.06 | 0.63 ± 0.04 |
| Ca2+/SO42− | 1.88 ± 0.17 | 2.75 ± 0.53 | 2.41 ± 0.4 | 1.33 ± 0.17 | 1.93 ± 0.22 | 2.39 ± 0.47 |
| (Ca2+ + Mg2+)/(Na+ + K+) | 13.04 ± 1.62 | 10.61 ± 1.37 | 10.86 ± 0.89 | 11.08 ± 2.07 | 11.09 ± 1.5 | 7.76 ± 0.7 |
| Na+/(Na+ + Ca2+) a | 0.12 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.14 ± 0.02 | 0.14 ± 0.02 | 0.17 ± 0.02 |
| Cl−/(Cl− + HCO3−) a | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.02 | 0.1 ± 0.02 |
| Rainy Season | Dry Season | |||||||
|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 | |
| Elevation (ele) | 0.08 | 0.08 | 0.29 | −0.81 | 0.13 | 0.06 | −0.82 | −0.26 |
| Tw | −0.04 | −0.04 | 0.74 | 0.14 | −0.03 | 0.20 | 0.87 | 0.07 |
| TDS | 0.96 | 0.25 | 0.03 | 0.09 | 0.91 | 0.40 | 0.00 | 0.10 |
| pH | −0.10 | −0.01 | 0.83 | −0.26 | 0.21 | −0.01 | 0.04 | 0.74 |
| Cl | 0.13 | 0.75 | −0.11 | −0.01 | 0.17 | 0.85 | 0.11 | 0.02 |
| SiO2 | −0.04 | 0.40 | 0.15 | 0.52 | 0.05 | 0.86 | 0.02 | 0.18 |
| ORP | 0.05 | 0.13 | −0.83 | 0.19 | 0.19 | −0.61 | 0.44 | −0.41 |
| EC | 0.95 | 0.23 | 0.06 | 0.01 | 0.92 | 0.25 | 0.00 | 0.13 |
| NO3− | 0.38 | 0.14 | −0.10 | 0.72 | 0.05 | 0.06 | 0.23 | 0.65 |
| SO42− | 0.85 | −0.04 | −0.32 | 0.10 | 0.85 | 0.01 | −0.11 | −0.15 |
| HCO3− | 0.46 | 0.40 | 0.53 | −0.07 | 0.67 | 0.42 | 0.06 | 0.28 |
| Na+ | 0.30 | 0.87 | 0.07 | −0.01 | 0.22 | 0.94 | 0.03 | −0.05 |
| K+ | 0.10 | 0.83 | −0.12 | 0.24 | 0.26 | 0.89 | 0.09 | −0.11 |
| Ca2+ | 0.93 | 0.10 | −0.06 | 0.17 | 0.91 | 0.05 | 0.09 | 0.16 |
| Mg2+ | 0.89 | 0.15 | 0.06 | −0.11 | 0.87 | −0.01 | −0.16 | 0.08 |
| Eigenvalue | 5.42 | 2.75 | 1.96 | 1.24 | 5.9 | 2.86 | 1.87 | 1.14 |
| Explained variance % | 36.11 | 18.33 | 13.09 | 8.25 | 39.33 | 19.1 | 12.48 | 7.63 |
| Cumulative variance % | 36.11 | 54.44 | 67.53 | 75.78 | 39.33 | 58.43 | 70.91 | 78.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Kuang, X.; Feng, Y.; Hao, Y.; He, Q.; Zhou, H.; Chen, J.; Zou, Y.; Zheng, C. Hydrochemistry of the Lhasa River, Tibetan Plateau: Spatiotemporal Variations of Major Ions Compositions and Controlling Factors Using Multivariate Statistical Approaches. Water 2021, 13, 3660. https://doi.org/10.3390/w13243660
Zhu M, Kuang X, Feng Y, Hao Y, He Q, Zhou H, Chen J, Zou Y, Zheng C. Hydrochemistry of the Lhasa River, Tibetan Plateau: Spatiotemporal Variations of Major Ions Compositions and Controlling Factors Using Multivariate Statistical Approaches. Water. 2021; 13(24):3660. https://doi.org/10.3390/w13243660
Chicago/Turabian StyleZhu, Meizhuang, Xingxing Kuang, Yuqing Feng, Yinlei Hao, Qiule He, Hui Zhou, Jianxin Chen, Yiguang Zou, and Chunmiao Zheng. 2021. "Hydrochemistry of the Lhasa River, Tibetan Plateau: Spatiotemporal Variations of Major Ions Compositions and Controlling Factors Using Multivariate Statistical Approaches" Water 13, no. 24: 3660. https://doi.org/10.3390/w13243660





