The Use of Phytoplankton in the Assessment of Water Quality in the Lower Section of Poland’s Largest River
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dokulil, M.T. Phytoplankton of the River Danube: Composition, seasonality and long-term dynamics. In The Danube River Basin Handbook Environmental Chemistry; Liska, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 411–428. [Google Scholar]
- Wu, N.; Schmalz, B.; Fohrer, N. Study Progress in Riverine Phytoplankton and its Use as Bio-Indicator—A Review. Austin J. Hydrol. 2014, 1, 9. [Google Scholar]
- Tockner, K.; Uehlinger, U.; Robinson, C.T. Rivers of Europe, 1st ed.; Academic Press: London, UK, 2008; p. 728. [Google Scholar]
- Stoyneva, M.P. Shallows of the lower Danube as additional sources of potamoplankton. Hydrobiologia 1994, 289, 171–178. [Google Scholar] [CrossRef]
- Descy, J.-P.; Darchambeau, F.; Lambert, T.; Stoyneva-Gaertner, M.P.; Bouillon, S.; Borges, A.V. Phytoplankton dynamics in the Congo River. Freshw. Biol. 2017, 62, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Kentzer, A.; Dembowska, E.; Giziński, A.; Napiórkowski, P. Influence of the Włocławek Reservoir on hydrochemistry and plankton of a large, lowland river (the Lower Vistula River, Poland). Ecol. Eng. 2010, 36, 1747–1753. [Google Scholar] [CrossRef]
- Dembowska, E. Phytoplankton species diversity of the Lower Vistula from Wyszogród to Toruń. Oceanol. Hydrobiol. Stud. 2009, 38, 63–74. [Google Scholar] [CrossRef]
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; Official Journal of the European Union: Luxemberg, 2000; Volume 164, p. 19. [Google Scholar]
- European Commission. Report from the Commission to the European Parliament and the Council on the Implementation of the Water Framework Directive (2000/60/EC) River Basin Management Plans; Official Journal of the European Union: Luxemberg, 2012; p. 14. [Google Scholar]
- Poikane, S.; Kelly, M.; Cantonati, M. Benthic algal assessment of ecological status in European lakes and rivers: Challenges and opportunities. Sci. Total Environ. 2016, 568, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Masouras, A.; Karaouzas, I.; Dimitriou, E.; Tsirtsis, G.; Smeti, E. Benthic Diatoms in River Biomonitoring—Present and Future Perspectives within the Water Framework Directive. Water 2021, 13, 478. [Google Scholar] [CrossRef]
- Journal of Laws of the Republic of Poland. Rozporządzenie Ministra Środowiska z dnia 22 października 2014r. w sprawie sposobu klasyfikacji jednolitych części wód powierzchniowych oraz środowiskowych norm jakości dla substancji priorytetowych 2014; 1482. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20140001482 (accessed on 26 November 2021).
- Głogowska, B. A geographical and hydrological profile of the study area. In Hydrobiology of the Lower Vistula River between Wyszogród and Toruń. An Assessment of the Influence of the Włocławek Dam on the Structure and Functions of the River Ecosystem, Part II; Giziński, A., Ed.; AUNC Limnol. Pap: Cary, NC, USA, 2000; Volume 21, pp. 11–21. Available online: https://link.springer.com/article/10.2478/s11756-013-0263-6 (accessed on 26 November 2021).
- Makowski, J. The lower Vistula River and its bunds. The historical development and the current state and maintenance during major floods. Part Two: The section from Toruń to Biała Góra; Instytut Budownictwa Wodnego PAN Biblioteka Naukowa Hydrotechnika: Gdańsk, Poland, 1998; Volume 27, p. 150. [Google Scholar]
- Cyberski, J.; Grześ, M.; Gutry-Korycka, M.; Nachlik, E.; Kundzewicz, Z.W. History of floods on the River Vistula. Hydrol. Sci. J. 2006, 51, 799–817. [Google Scholar] [CrossRef]
- Lyche Solheim, A.; Globevnik, L.; Austnes, K.; Kristensen, P.; Moe, S.J.; Persson, J.; Phillips, G.; Poikane, S.; van de Bund, W.; Birk, S. A new broad typology for rivers and lakes in Europe: Development and application for large-scale environmental assessments. Sci. Total Environ. 2019, 697, 134043. [Google Scholar] [CrossRef]
- Ettl, H. Xanthophyceae. In Süßwasserflora von Mitteleuropa Bd. 3; Ettl, H., Gerloff, J., Heynig, H., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1978; p. 530. [Google Scholar]
- Ettl, H. Chlorophyta I: Phytomonadina. In Süβwasserflora von Mitteleuropa Bd. 9; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1983; p. 807. [Google Scholar]
- Hindák, F. Colour Atlas of Cyanophytes; Veda Publishing House of the Slovak Academy of Sciences: Bratislava, Slovakia, 2008; p. 253. [Google Scholar]
- Javornickỳ, P. Taxonomic notes on some freshwater planktonic Cryptophyceae based on light microscopy. Hydrobiologia 2003, 50, 271–283. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil: Oscillatoriales. In Süβwasserflora von Mitteleuropa, Bd. 19/2; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2007; p. 759. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales. In Süβwasserflora von Mitteleuropa, Bd. 1/19; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2008; p. 548. [Google Scholar]
- Komárek, J.; Fott, B.; Chlorophyceae (Grünalgen), O. Chlorococcales. In Die Binnengewässer. Das Phytoplankton des Süβwassers, Systematik und Biologie, 7 Teil.1; Hälfte, E., Ed.; Schweizerbart′sche Verlagsbuchhandlung: Stuttgart, Germany, 1983; p. 1044. [Google Scholar]
- Komárek, J.; Komárkowa, J. Diversity of Aphanizomenon–like cyanobacteria. Diverzita sinic z okruhu rodu Aphanizomenon. Czech Phycol. Olomouc 2006, 6, 1–32. [Google Scholar]
- Komárek, J.; Zapomelova, E. Planktic morphospecies of the cyanobacterial genus Anabaena = subg. Dolichosperum—1. Part: Coiled types. Fottea 2007, 7, 1–31. [Google Scholar]
- Komárek, J.; Zapomelova, E. Planktic morphospecies of the cyanobacterial genus Anabaena = subg. Dolichosperum—2. Part: Straight types. Fottea 2008, 8, 1–14. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süsswasserflora von Mitteleuropa Bd. 2/1; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa, Bd. 2/2; Ettl, H., Gärtner, G., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1988; p. 796. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süsswasserflora von Mitteleuropa, Bd. 2/3; Ettl, H., Gärtner, G., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1991; p. 578. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae, Ktitische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis. In Süsswasserflora von Mitteleuropa, Bd. 2/4; Gustav Fischer Verlag: Stuttgart, Germany, 1991; p. 437. [Google Scholar]
- Popovskỳ, J.; Pfiester, L.A. Dinophyceae (Dinoflagellida). In Süβwasserflora von Mitteleuropa, Bd. 6; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1990; p. 272. [Google Scholar]
- Růžička, J. Die Desmidiaceen Mitteleuropas, Bd. 1/1; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1977; p. 336. [Google Scholar]
- Starmach, K.; Chrysophyta, I. Chrysophyceae—Złotowiciowce oraz wiciowce bezbarwne—Zooflagellata wolnożyjące. In Flora słodkowodna Polski, T. 5; Starmach, K., Siemińska, J., Eds.; Państwowe Wydawnictwo Naukowe: Warszawa, Poland; Kraków, Poland, 1968; p. 598. [Google Scholar]
- Starmach, K. Cryptophyceae—Kryptofity, Dinophyceae—Dinofity, Raphiophyceae—Rafidofity. In Flora słodkowodna Polski, T. 4; Starmach, K., Siemińska, J., Eds.; Państwowe Wydawnictwo Naukowe: Warszawa, Poland; Kraków, Poland, 1974; p. 520. [Google Scholar]
- Starmach, K. Euglenophyta—Eugleniny. In Flora słodkowodna Polski, T. 3; Starmach, K., Siemińska, J., Eds.; Państwowe Wydawnictwo Naukowe: Warszawa, Poland; Kraków, Poland, 1983; p. 594. [Google Scholar]
- Wołowski, K. Taxonomic and Environmental studies on Euglenophytes of the Kraków-Częstochowa Upland (Southern Poland). Fragm. Florist. Geobot. 1998, 6, 192. [Google Scholar]
- Wołowski, K.; Hindák, F. Atlas of Euglenophytes; Veda, Publishing House of the Slovak Academy of Sciences: Bratislava, Slovakia, 2003; p. 135. [Google Scholar]
- Utermöhl, H. Zur Vervollkommung der quantitativen Phytoplankton—Methodik. Mitt. Internat. Verein. Limnol. 1958, 9, 5–46. [Google Scholar]
- Hillenbrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef] [Green Version]
- Napiórkowska-Krzebietke, A.; Kobos, J. Assessment of the cell biovolume of phytoplankton widespread in coastal and inland water bodies. Water Res. 2016, 104, 532–546. [Google Scholar] [CrossRef]
- Holmes, R.W.; Norris, R.; Smayda, T.; Wood, E.J.F. Collection, fixation, identification and enumeration of phytoplankton standing stock. In Recommended Procedures for Measuring the Productivity of Plankton Standing Stock and Related Oceanic Properties; Anon, Ed.; National Academy of Sciences: Washington, DC, USA, 1969; pp. 17–46. [Google Scholar]
- Elser, J.J.; Carpenter, S.R. Predation-driven dynamics of zooplankton and phytoplankton communities in a whole-lake experiment. Oecologia 1988, 76, 148–154. [Google Scholar] [CrossRef]
- Nusch, E.A. Comparison of different methods for chlorophyll and phaeopigment. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1980, 14, 14–16. [Google Scholar]
- Picińska-Fałtynowicz, J.; Błachuta, J.; Pasztaleniec, A. Fitoplankton w rzekach i zbiornikach zaporowych. In Podręcznik do Monitoringu Elementów Biologicznych i Klasyfikacji Stanu Ekologicznego wód Powierzchniowych; Kolada, A., Ed.; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2020; pp. 23–43. [Google Scholar]
- European Commission. Carrying forward the Common Implementation Strategy for the Water Framework Directive-Progress and Work Programme for 2003/2004; Official Journal of the European Union: Luxemberg, 2003; p. 46. [Google Scholar]
- Journal of Laws of the Republic of Poland. Rozporządzenie Ministra Infrastruktury z dnia 25 czerwca 2021 r. w sprawie klasyfikacji stanu ekologicznego, potencjału ekologicznego i stanu chemicznego oraz sposobu klasyfikacji stanu jednolitych części wód powierzchniowych, a także środowiskowych norm jakości dla substancji priorytetowych. 2021; p. 1475. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001475 (accessed on 26 November 2021).
- Bij de Vaate, A.; Jazdzewski, K.; Ketelaars, H.A.M.; Gollasch, S.; Van der Velde, G. Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can. J. Fish. Aquat. Sci. 2002, 59, 1159–1174. [Google Scholar] [CrossRef]
- Romanowski, J. Vistula River Valley as the Ecological Corridor for Mammals. Pol. J. Ecol. 2007, 55, 805–819. [Google Scholar]
- Mysiak, M. Zmiany jakości wód rzecznych w Polsce w dwudziestopięcioleciu 1964–1990. Ochr. Srodowiska 1994, 16, 9–10. [Google Scholar]
- Wałęga, A.; Chmielowski, K.; Satora, S. Water and wastewater management condition in Poland regarding Water Framework Directive implementation. Infrastr. Ecol. Rural Areas 2009, 4, 57–72. [Google Scholar]
- Kentzer, A. Influence of the Włocławek Reservoir on the quality of waters in the Bay of Gdańsk. Limnol. Pap. 2009, 4, 9–14. [Google Scholar] [CrossRef]
- Kowalkowski, T.; Pastuszek, M.; Igras, J.; Buszewski, B. Differences in emission of nitrogen and phosphorus in to the Vistula and Oder basins in 1995–2008. Natural and anthropogenic causes (MONERIS model). J. Mar. Syst. 2012, 89, 48–60. [Google Scholar] [CrossRef]
- Pastuszek, M.; Stalnacke, P.; Pawlikowski, K.; Witek, Z. Response of Polish rivers (Vistula, Oder) to reduce pressure from point sources and agriculture during the transition period (1988–2008). J. Mar. Syst. 2012, 94, 157–173. [Google Scholar] [CrossRef]
- Uherkovich, G. Über das Wisła–Phytoseston Zwischen Kraków und Tczew. Acta Hydrobiol. 1970, 12, 161–190. [Google Scholar]
- Praszkiewicz, A.; Spodniewska, I.; Węgleńska, T. Seston Wisły i zbiorników kaskady Wisły na odcinku od ujścia Sanu do Włocławka. In Ekologiczne Podstawy Zagospodarowania Wisły i jej Dorzecza; Kajak, Z., Ed.; Państwowe Wydawnictwo Naukowe: Warszawa, Poland; Łódź, Poland, 1983. [Google Scholar]
- Dembowska, E. Tentative assessment of the phytoplankton in the Vistula between Płock and Toruń (with the Włocławek Reservoir), Poland. AUNC Limnol. Pap. 2005, 24, 19–43. [Google Scholar]
- Baykal, T.; Açikgöz, İ.; Udoh, A.U.; Yildiz, K. Seasonal variations in phytoplankton composition and biomass in a small lowland river-lake system (Melen River, Turkey). Turk. J. Biol. 2011, 35, 485–501. [Google Scholar]
- Da Silva, I.G.; Pelicice, F.M.; Rodrigues, L.C. Loss of phytoplankton functional and taxonomic diversity induced by river regulation in a large tropical river. Hydrobiologia 2020, 847, 3471–3485. [Google Scholar] [CrossRef]
- Várbíró, G.; Ács, É.; Borics, G.; Érces, K.; Fehér, G.; Grigorszky, I.; Japport, T.; Kocsis, G.; Krasznai, E.; Nagy, K.; et al. Use of Self-Organizing Maps (SOM) for characterization of riverine phytoplankton associations in Hungary. Arch. Hydrobiol. 2007, 17, 383–394. [Google Scholar] [CrossRef]
- Stanković, I.; Vlahović, T.; Gligora Udovič, M.; Várbíró, G.; Borics, G. Phytoplankton functional and morpho-functional approach in large floodplain rivers. Hydrobiologia 2012, 698, 217–231. [Google Scholar] [CrossRef]
- Friedrich, G.; Pohlmann, M. Long-term plankton studies at the lower Rhine/Germany. Limnologica 2009, 39, 14–39. [Google Scholar] [CrossRef] [Green Version]
- Descy, J.-P.; Leitao, M.; Everbecq, E.; Smitz, J.S.; Deliège, J.-F. Phytoplankton of the River Loire, France: A biodiversity and modelling study. J. Plankton Res. 2012, 34, 120–135. [Google Scholar] [CrossRef] [Green Version]
- Verasztó, C.S.; Kiss, K.T.; Sipkay, C.S.; Gimesi, L.; Vadadi-Fülöp, C.S.; Türei, D.; Hufnagel, L. Long-term dynamic patterns and diversity of phytoplankton communities in a large eutrophic river (the case of River Danube, Hungary). Appl. Ecol. Environ. Res. 2010, 8, 329–349. [Google Scholar] [CrossRef]
- Bilous, O.; Barinova, S.; Klochenko, P. Phytoplankton communities in ecological assessment of the Southern Bug River upper reaches (Ukraine). Ecohydrol. Hydrobiol. 2012, 12, 211–230. [Google Scholar] [CrossRef]
- Mischke, U.; Belkinova, D.; Birk, S.; Borics, G.; Garbea, R.; Hlúbiková, D.; Jekabsone, J.; Opatrilova, L.; Panek, P.; Picińska-Fałtynowicz, J.; et al. Intercalibration of the national classifications of ecological status for very large rivers in Europe. Biological quality element: Phytoplankton. In Joint Research Centre Technical Report; Poikane, S., Ed.; Publications Office of the European Union: Luxembourg, 2018; p. 174. [Google Scholar]
- Arle, J.; Mohaupt, V.; Kirst, I. Monitoring of Surface Waters in Germany under the Water Framework Directive—A Review of Approaches, Methods and Results. Water 2016, 8, 217. [Google Scholar] [CrossRef]
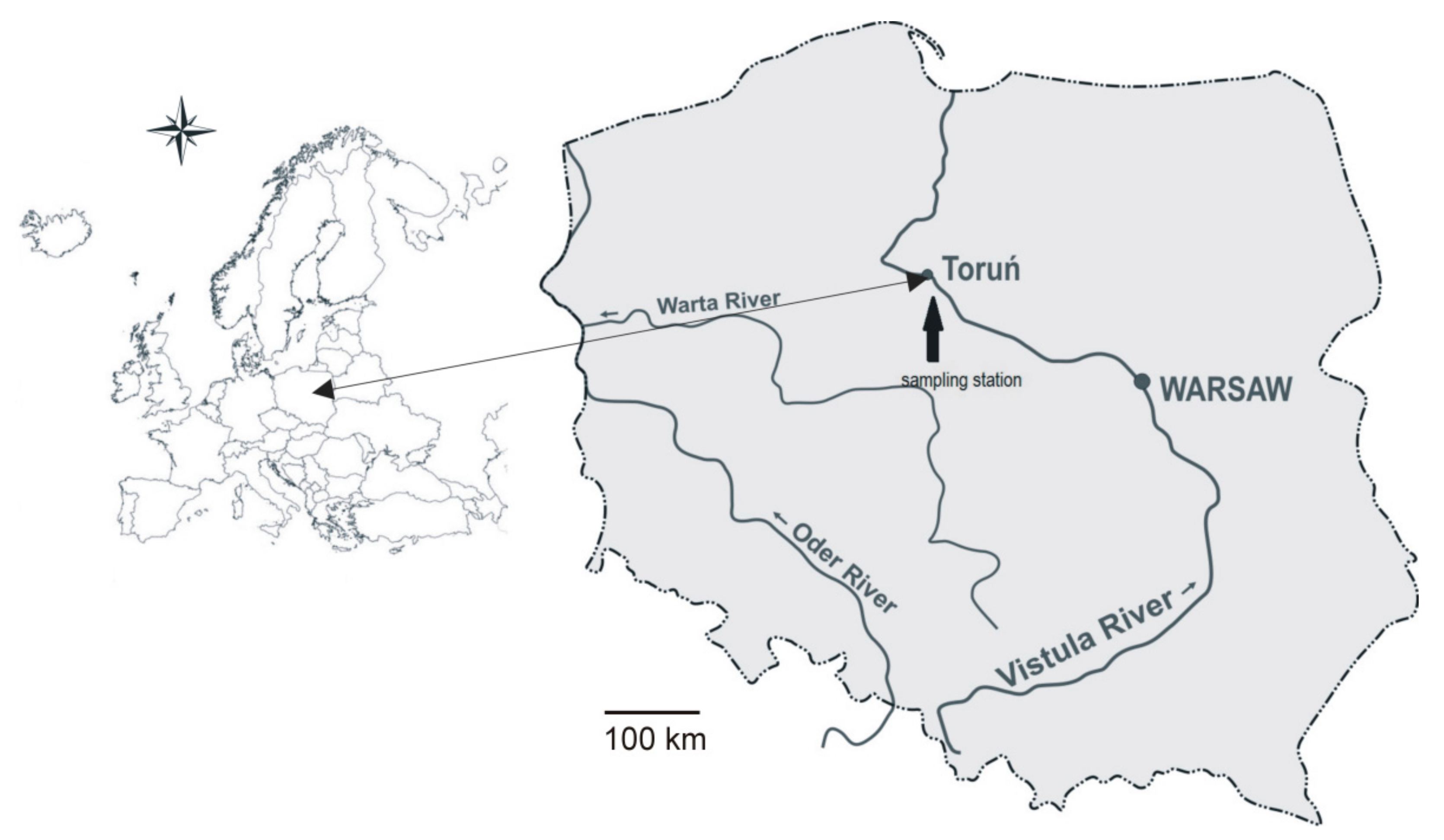
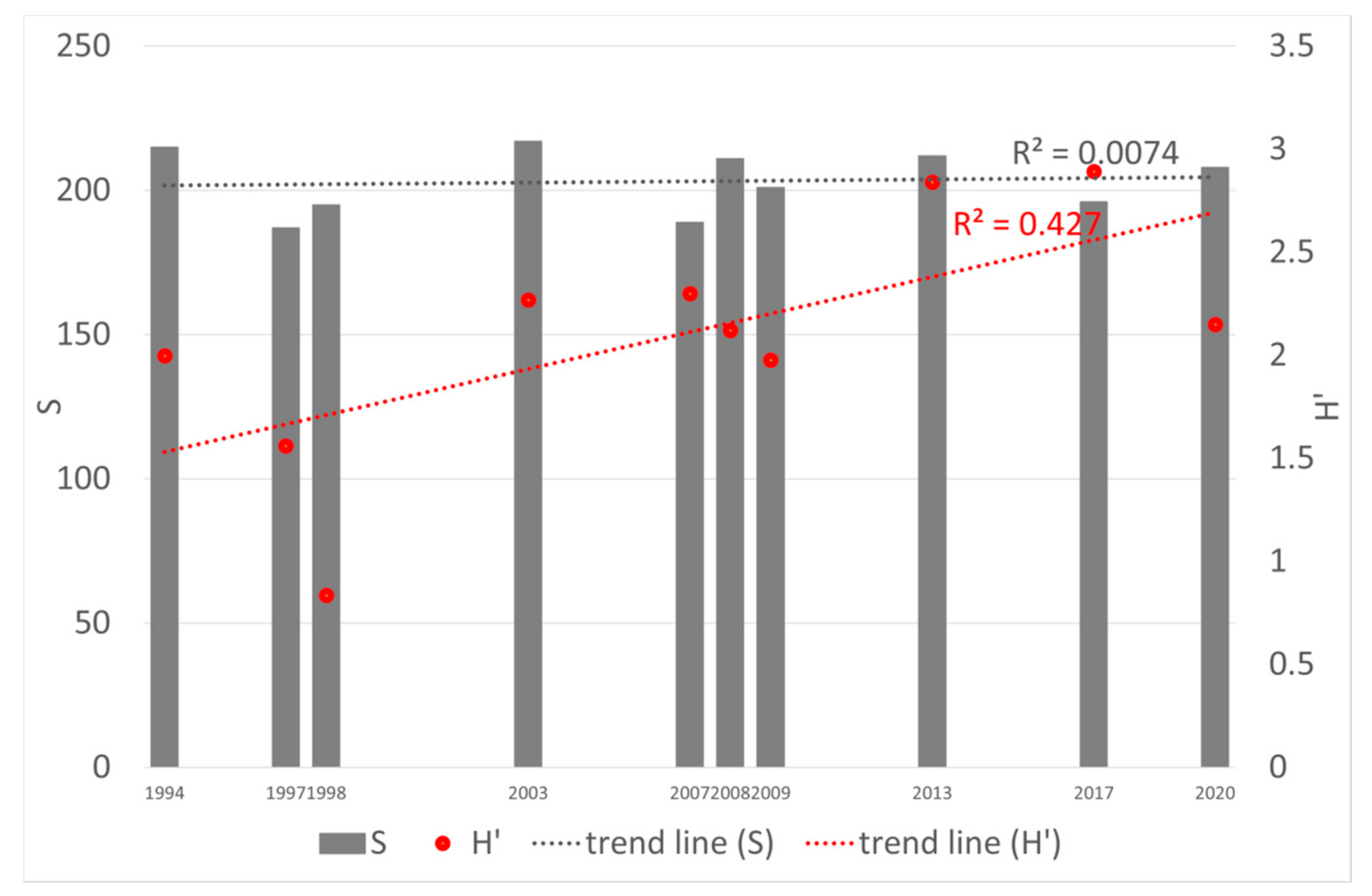
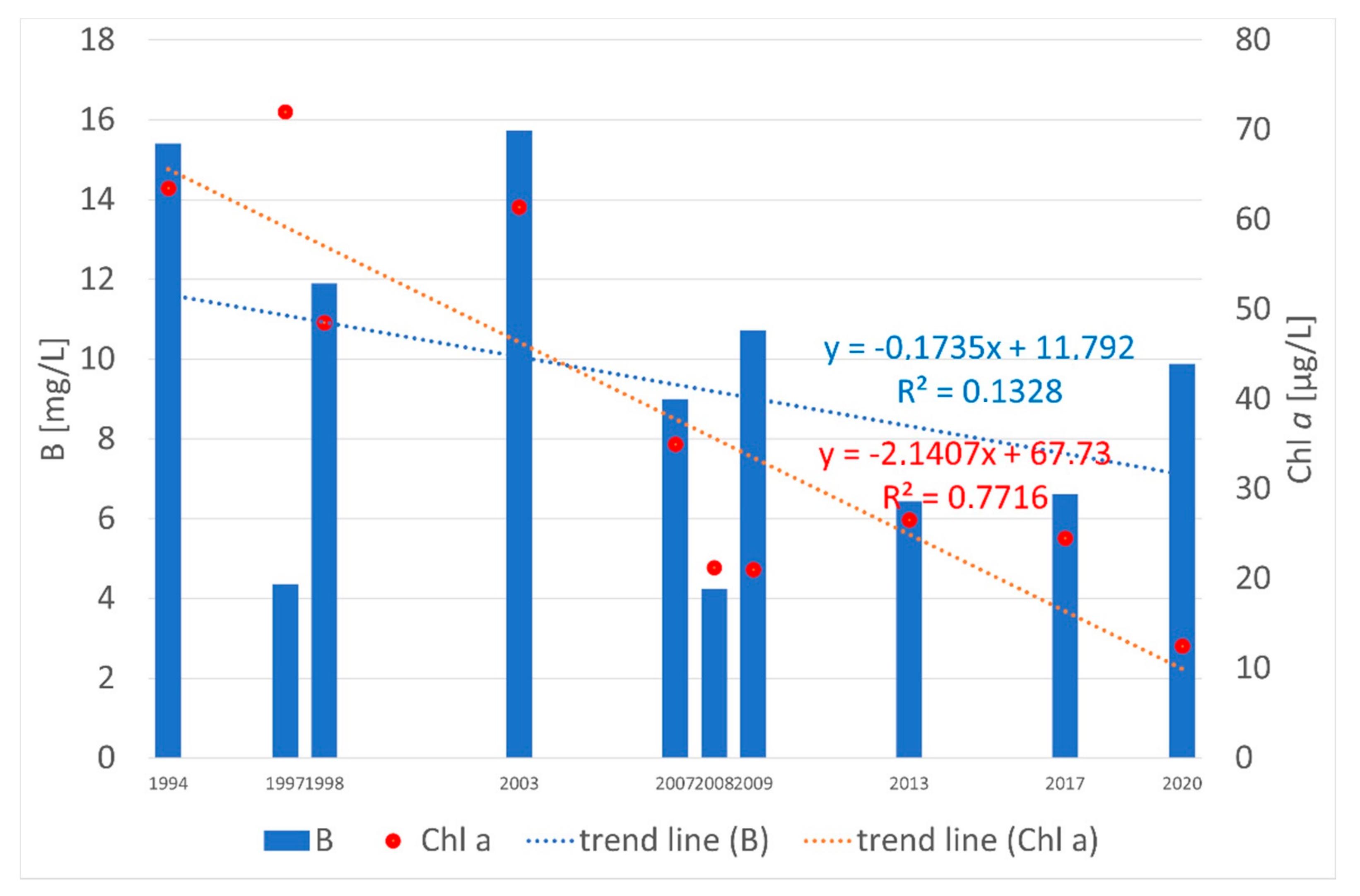
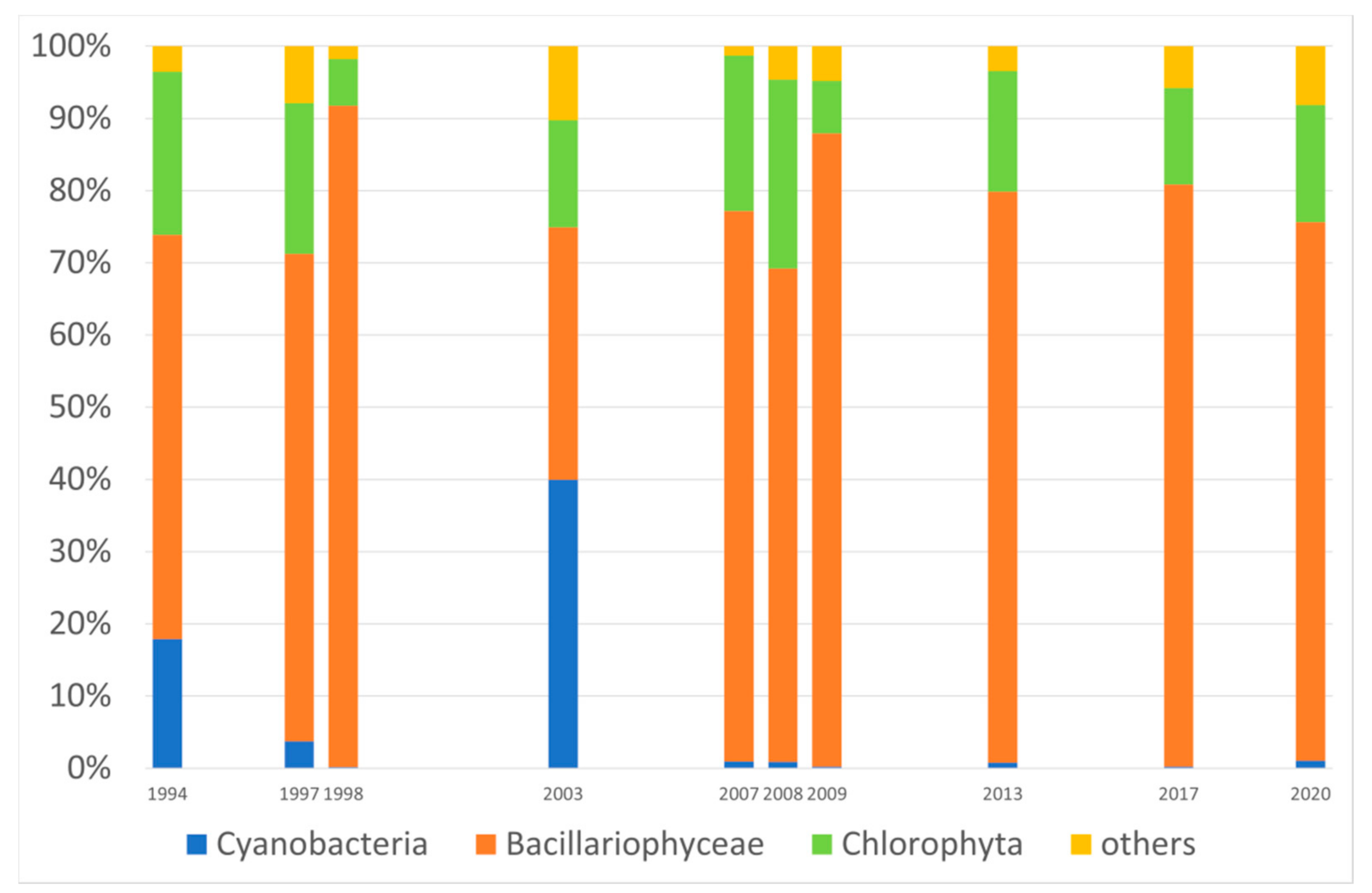

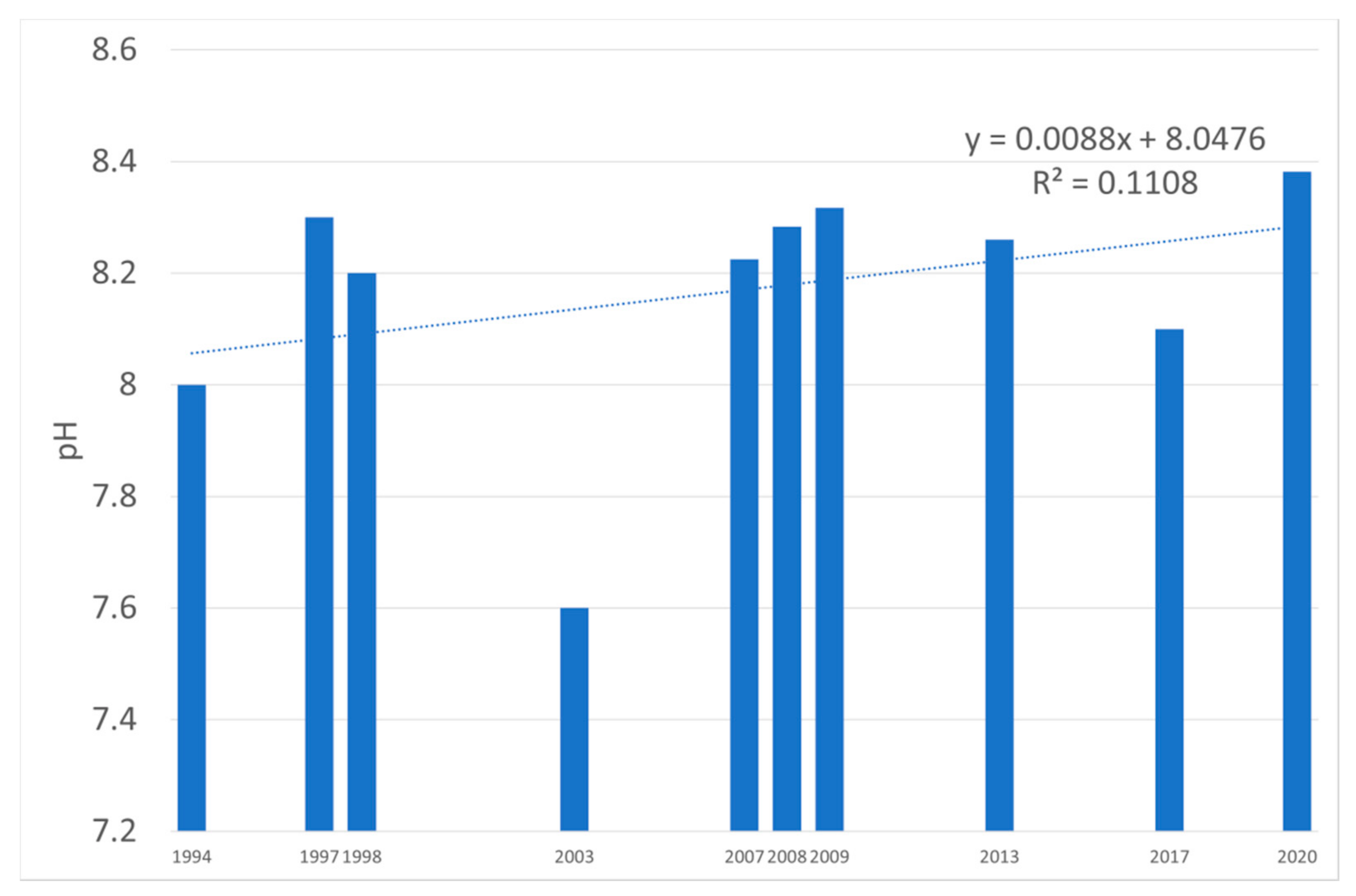
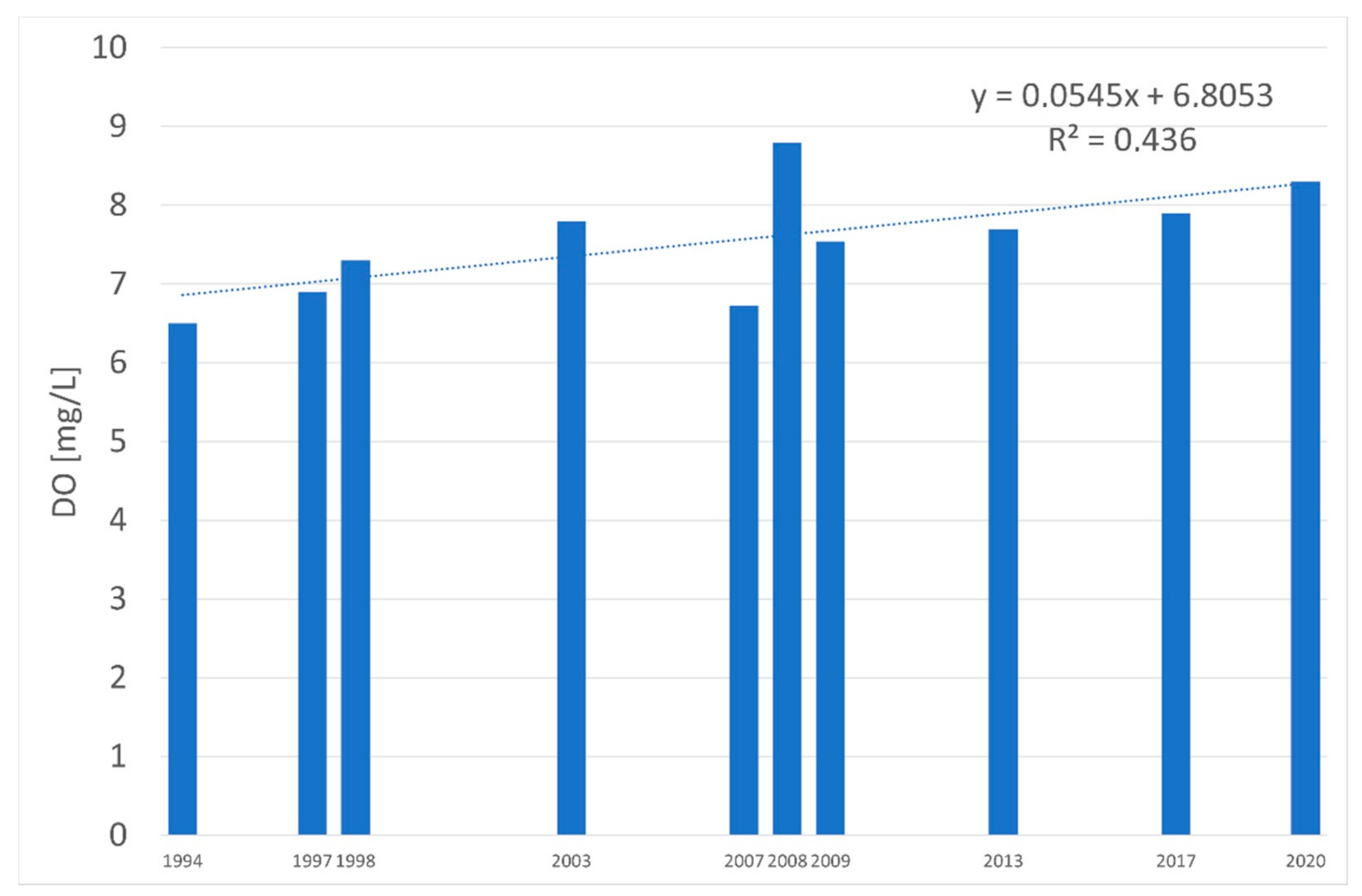
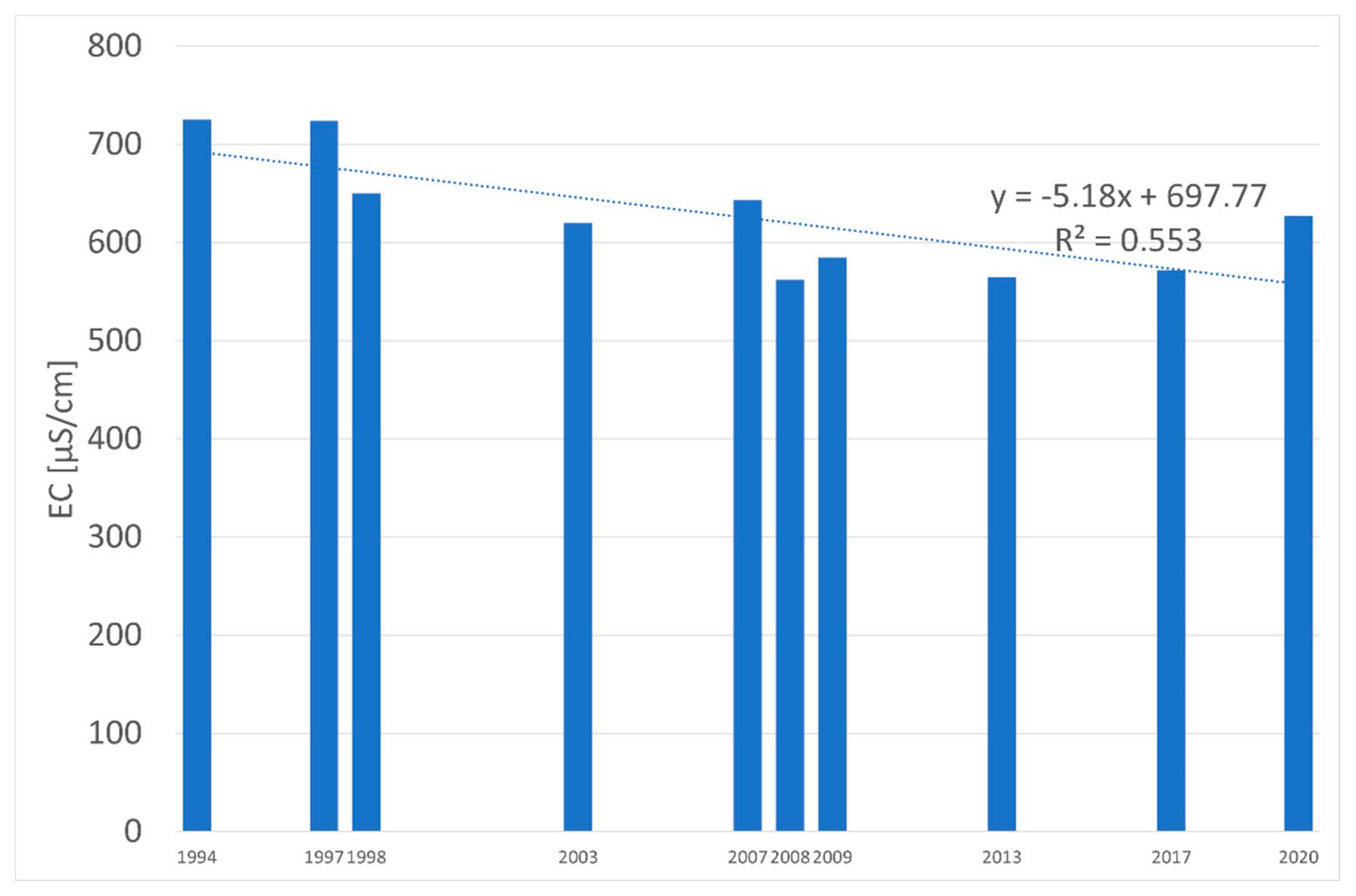
| Water Quality Class | Ecological Status/Potential | IFPL Values |
|---|---|---|
| First | high/maximal | ≥0.96 |
| Second | good | ≥0.79 |
| Third | moderate | ≥0.47 |
| Fourth | poor | ≥0.16 |
| Fifth | bad | <0.16 |
| Year | TP (mg/L) | P-PO4 (mg/L) | TN (mg/L) | Nmin. (mg/L) |
|---|---|---|---|---|
| 1994 | 0.26 | 0.13 | 3.0 | 1.88 |
| 1998 | 0.26 | 0.09 | 2.2 | 1.89 |
| 2008 | n.d. | 0.09 | n.d. | 0.93 |
| 2009 | n.d. | 0.05 | n.d. | 1.08 |
| 2017 | 0.14 | 0.05 | 3.6 | 1.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembowska, E.A. The Use of Phytoplankton in the Assessment of Water Quality in the Lower Section of Poland’s Largest River. Water 2021, 13, 3471. https://doi.org/10.3390/w13233471
Dembowska EA. The Use of Phytoplankton in the Assessment of Water Quality in the Lower Section of Poland’s Largest River. Water. 2021; 13(23):3471. https://doi.org/10.3390/w13233471
Chicago/Turabian StyleDembowska, Ewa Anna. 2021. "The Use of Phytoplankton in the Assessment of Water Quality in the Lower Section of Poland’s Largest River" Water 13, no. 23: 3471. https://doi.org/10.3390/w13233471
APA StyleDembowska, E. A. (2021). The Use of Phytoplankton in the Assessment of Water Quality in the Lower Section of Poland’s Largest River. Water, 13(23), 3471. https://doi.org/10.3390/w13233471






