Water Resource Recovery Facilities (WRRFs): The Case Study of Palermo University (Italy)
Abstract
:1. Introduction
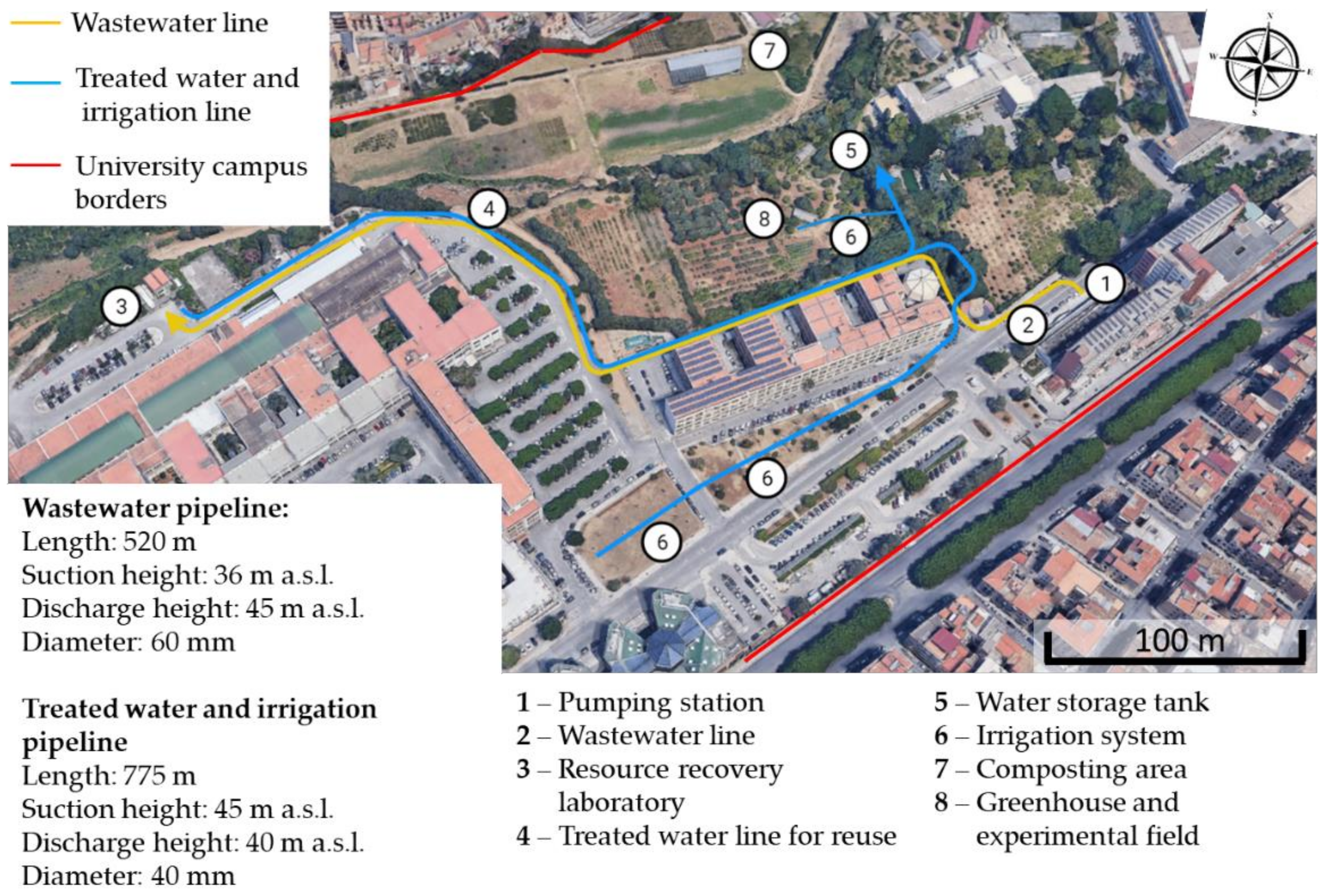
2. The Case Study of Palermo University
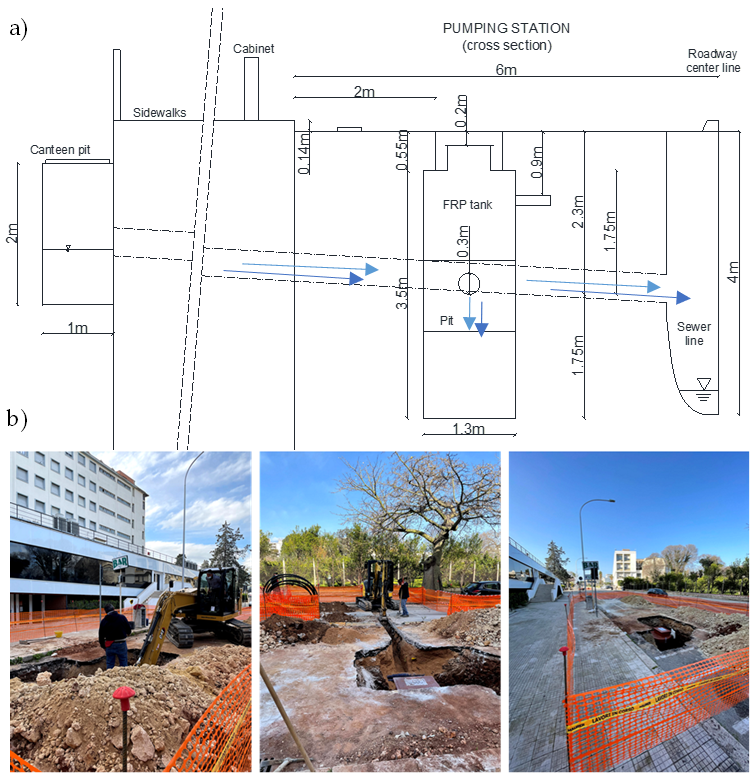
2.1. The Pumping Station
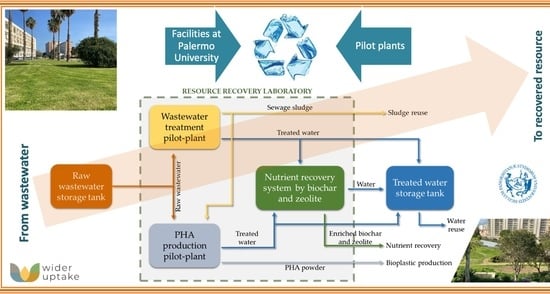
2.2. Resource Recovery Laboratory
- Water for reuse in green areas and experimental land;
- Sewage sludge to be composted as a soil improver;
- PHA powder for bioplastics production;
- Biochar and zeolite enriched in N and P as soil improver.
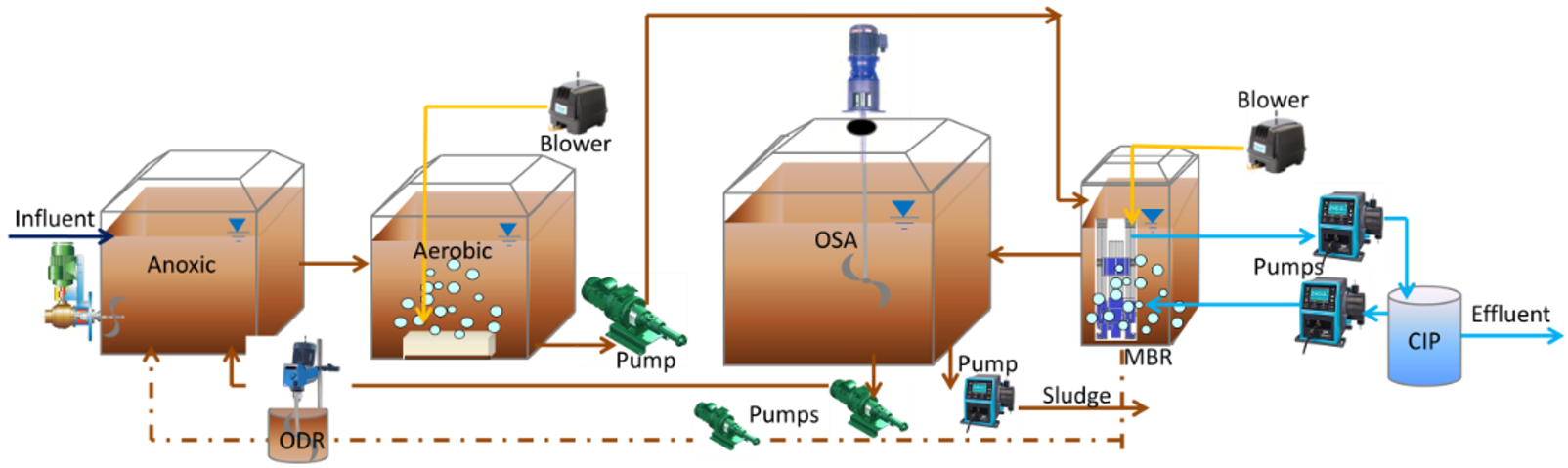
2.2.1. Sewage Sludge Reduction Technology
- (a)
- an anoxic reactor (V = 146 L) for denitrification, fed by the incoming raw wastewater, the recirculation system from the aerobic reactor and the recirculation system from the OSA reactor;
- (b)
- an aerobic reactor (V = 257 L) where the main processes for the removal of organic pollutants take place;
- (c)
- an oxygen depletion reactor (ODR) (V = 53 L) inserted in the recirculation system between the aerobic and anoxic reactors which allows to eliminate dissolved oxygen before entering the anoxic reactor;
- (d)
- a settler (V = 62 L) for solid–liquid separation;
- (e)
- an OSA interchange reactor (V = 477 L) in the sludge rewinding system to create the conditions that induce the metabolic splitting between anabolism and catabolism;
- (f)
- an ultrafiltration membrane system (V = 48 L) to increase the quality of the effluent for reuse, equipped with a clean-in-place (CIP) system.
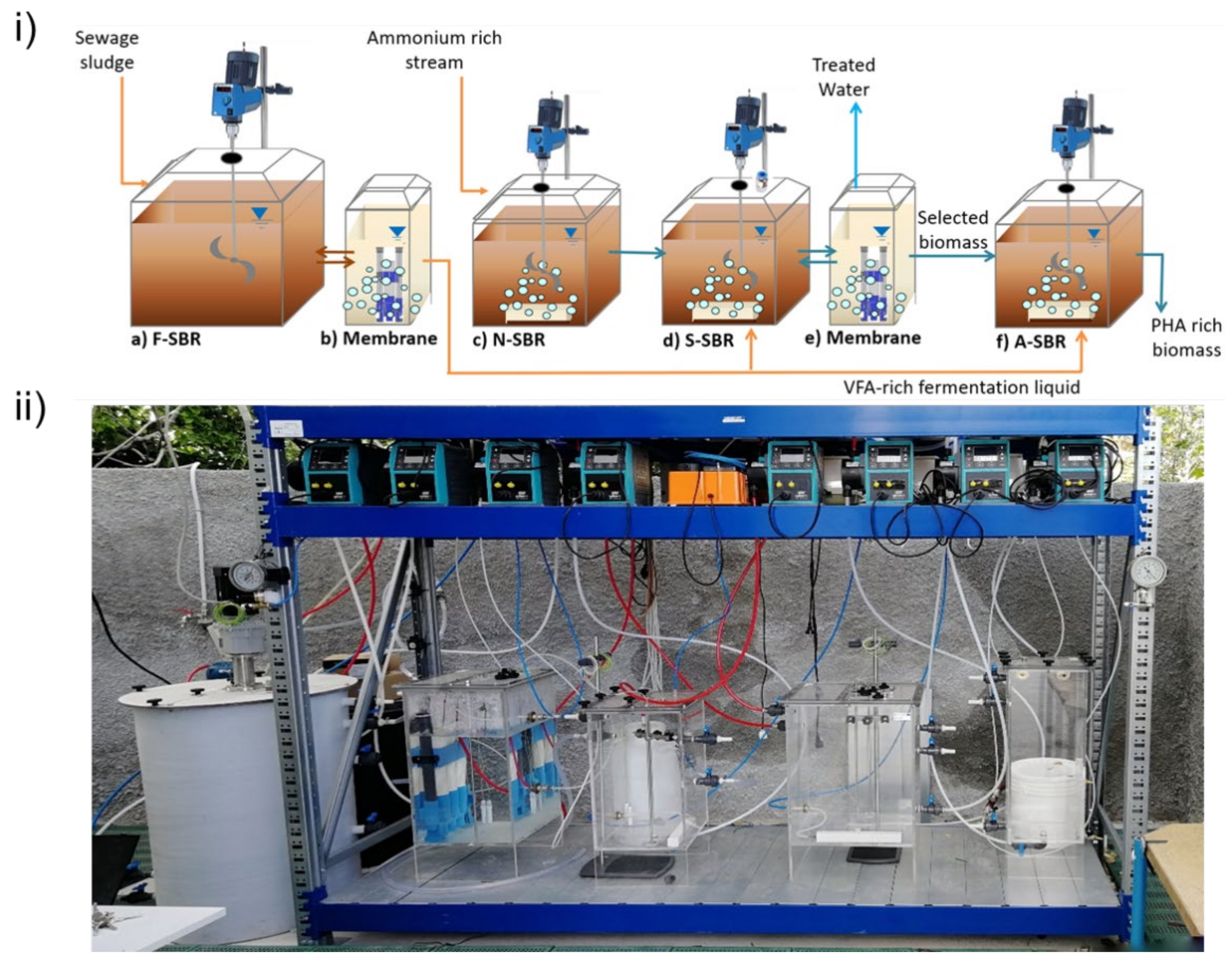
2.2.2. Biopolymers Production
- (a)
- F-SBR for the production of VFAs by sludge acidogenic fermentation;
- (b)
- Ultrafiltration membrane unit for solid/liquid separation to obtain an optimum fermentation liquid quality;
- (c)
- N-SBR for ammonium rich stream nitritation, to be used as electron acceptor in S-SBR famine phase;
- (d)
- S-SBR for the selection of a biomass with high PHA accumulation capacity through aerobic feast and anoxic famine cycles;
- (e)
- Ultrafiltration membrane unit for solid/liquid separation to enhance biomass selection;
- (f)
- A-SBR for fed-batch PHA accumulation using biomass form S-SBR and VFAs from F-SBR.
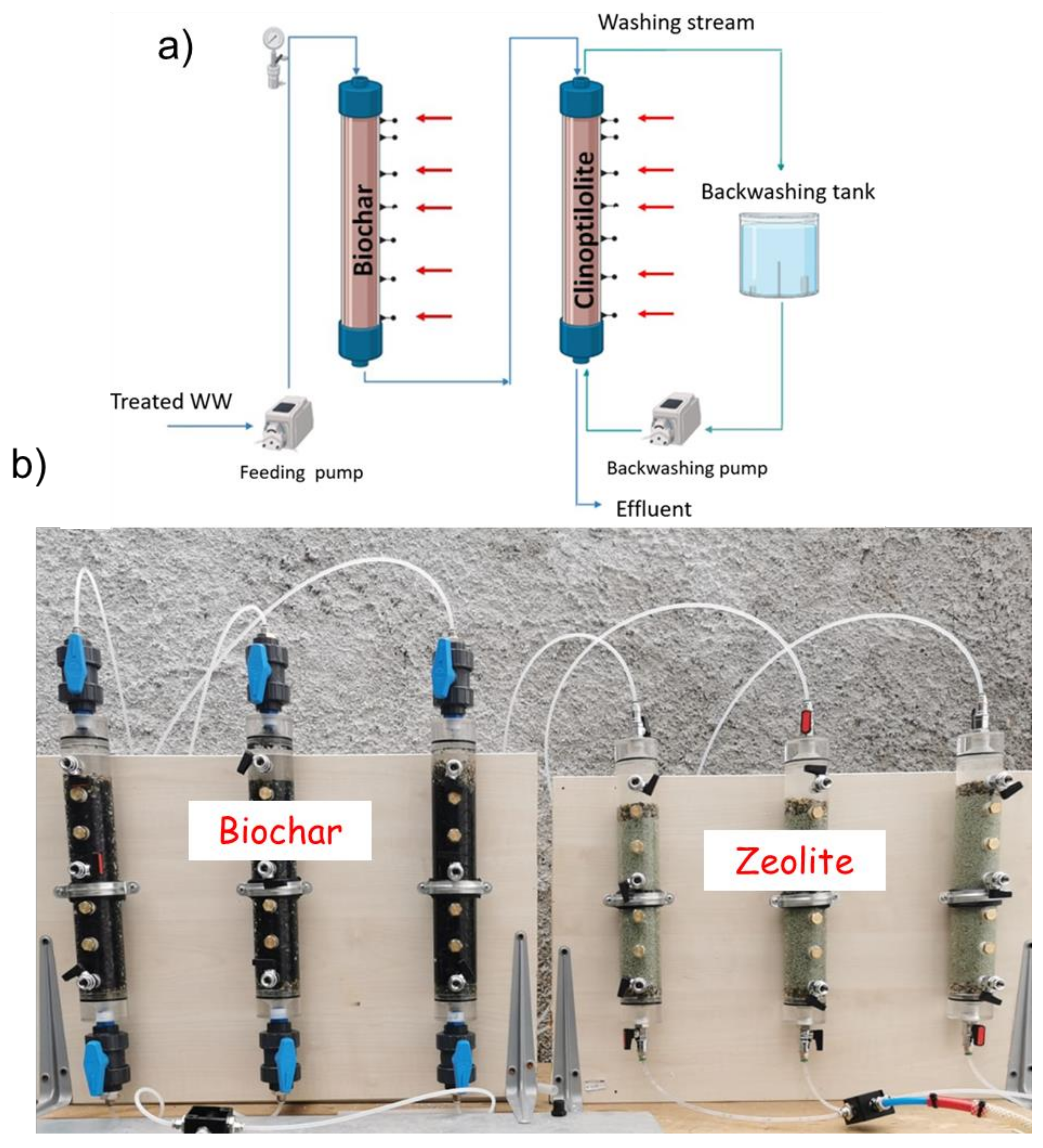
2.2.3. Nutrient Recovery by Biochar and Zeolite
2.3. Water Reuse (Treated Water Transport, Storage Tank, Irrigation Systems, Irrigation Area (Grass Area Building 6, Agriculture Area))
2.4. Sludge Composting (Composting Area, Composting Strategies, Compost Usage)
- (1)
- dried sludge (75%) and bulking agents (25%);
- (2)
- dried sludge (75%) and bulking agents (25%) amended with worms (Eisenia Foetida);
- (3)
- dried sludge (75%), bulking agents (12.5%) and zeolite (12.5%);
- (4)
- dried sludge (75%), bulking agents (12.5%) and biochar (12.5%).
3. Preliminary Results
3.1. Fermentation Batch Tests
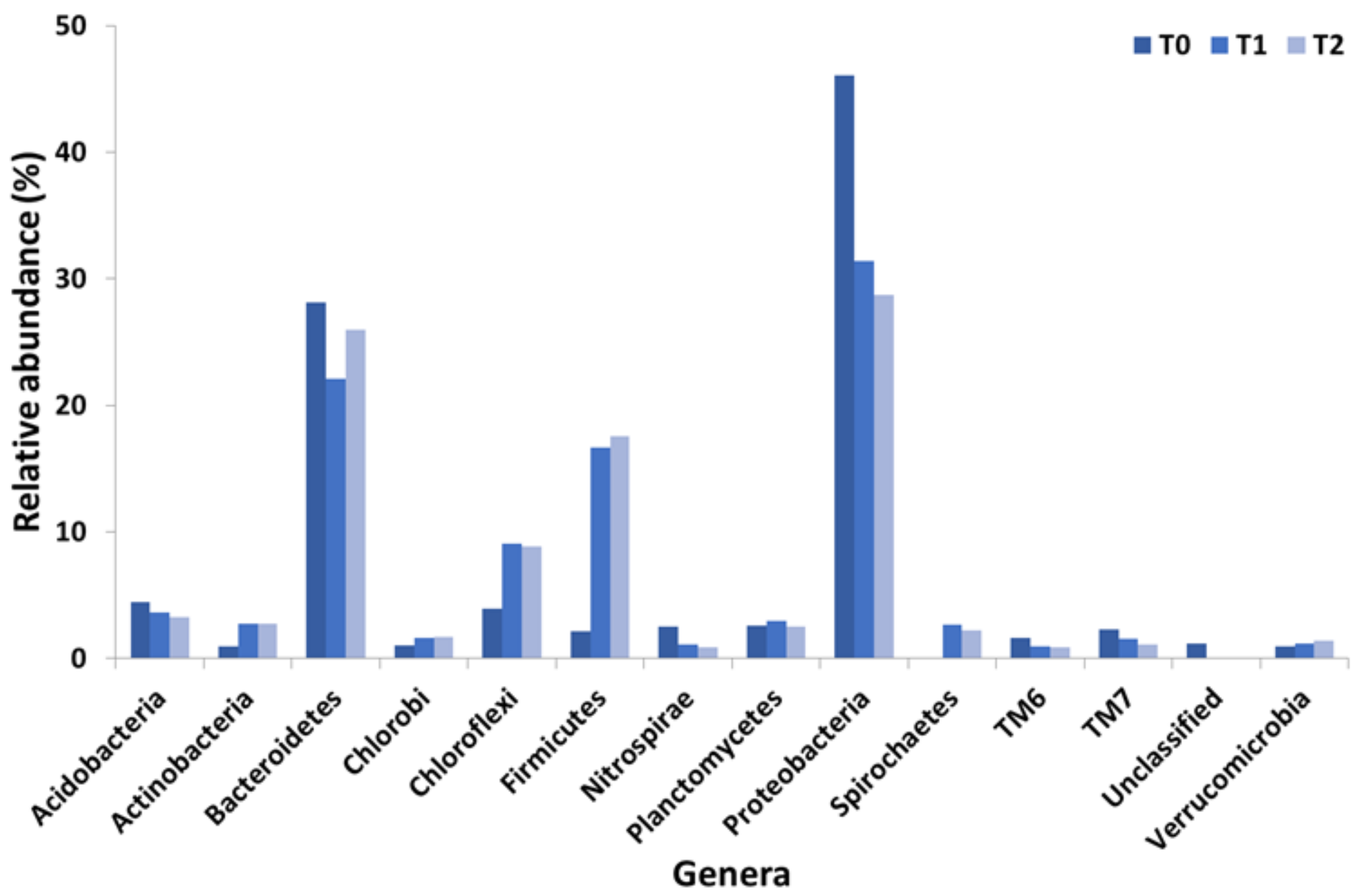
3.2. Metagenomic Results
- T1 and T2 reactors at the endpoint;
- The sludge recycle line of the real WWTP (the same used to inoculate T1 and T2 reactors) that was considered as the control condition (T0).
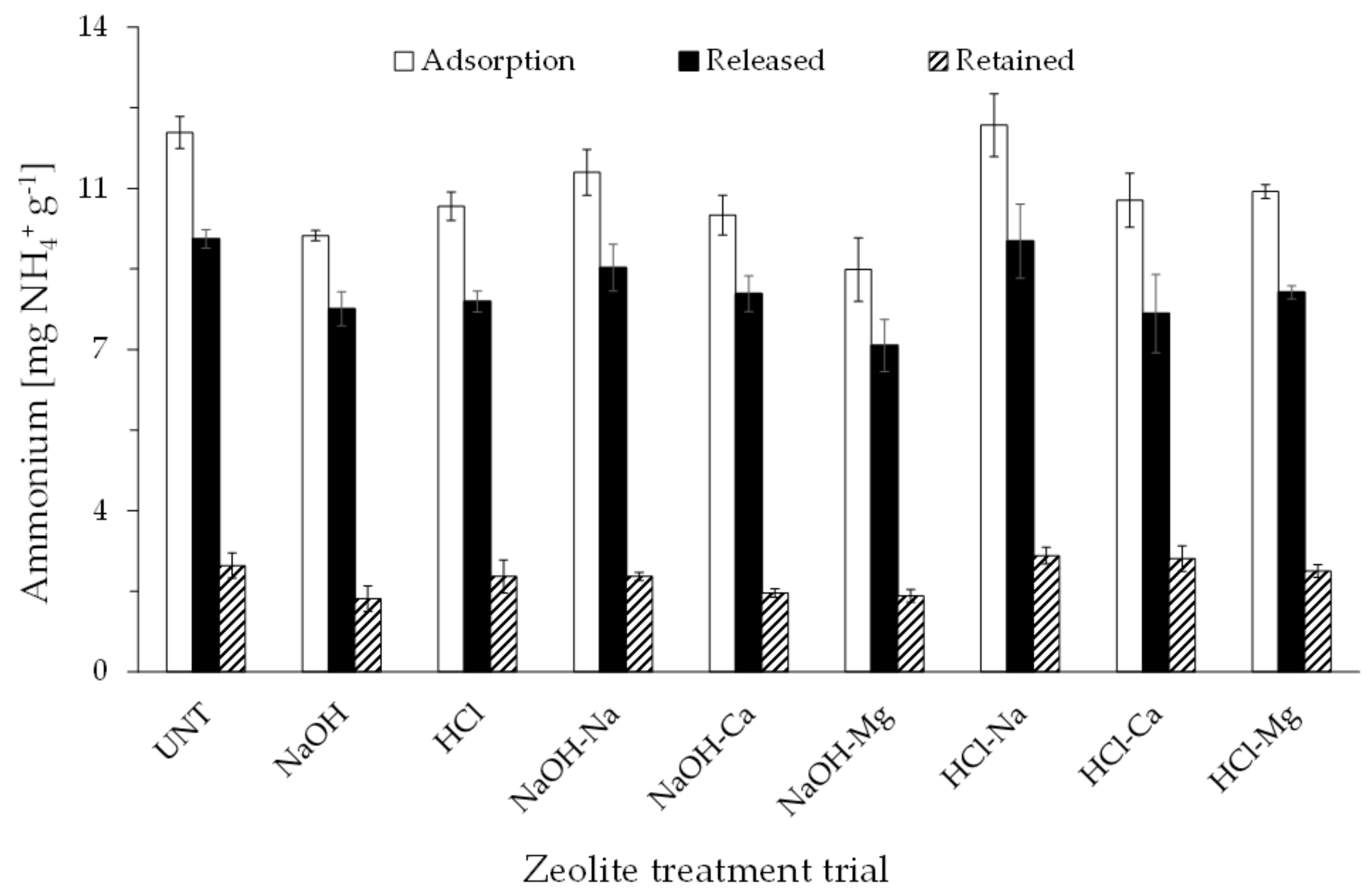
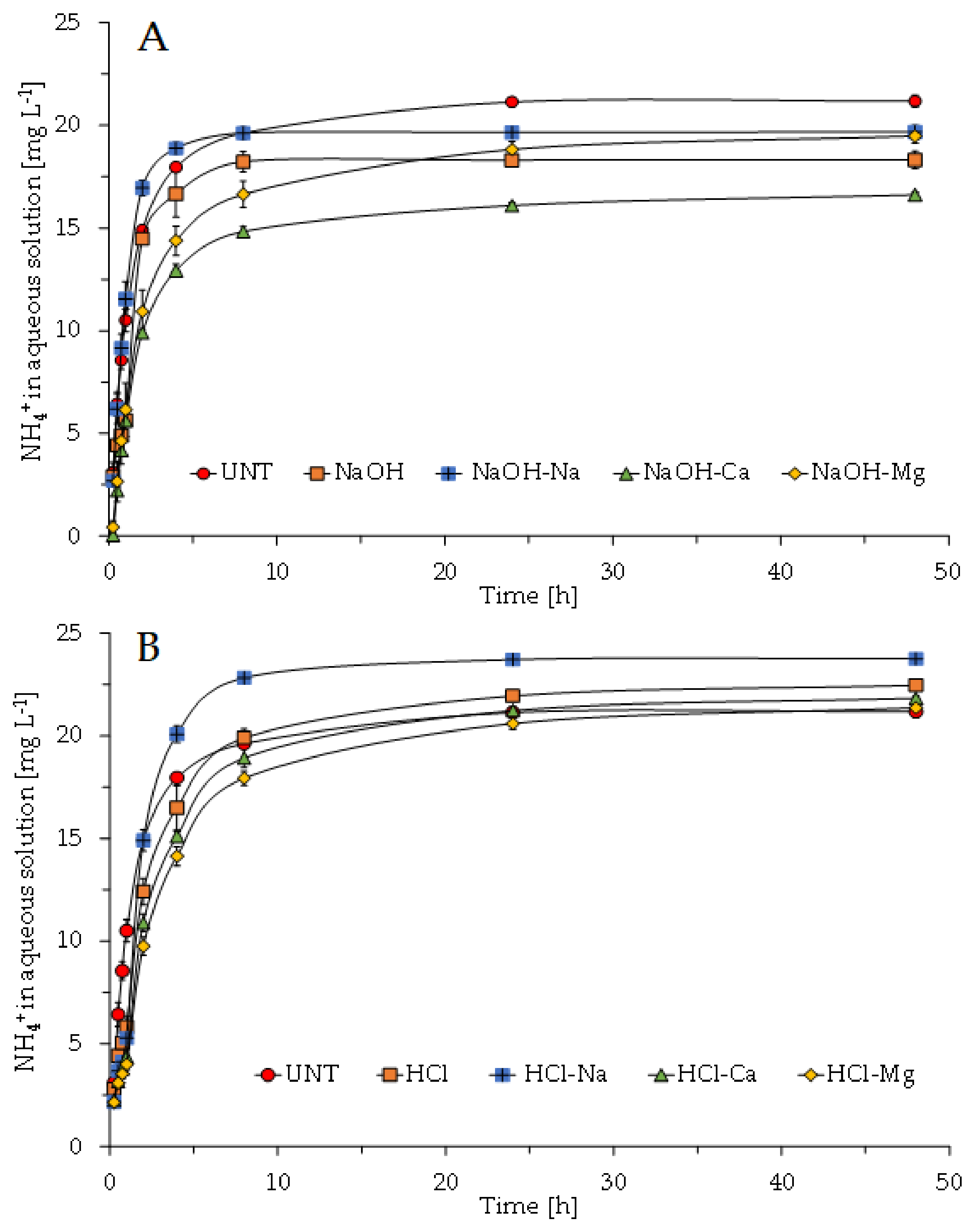
3.3. Adsorption Batch Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mannina, G.; Badalucco, L.; Barbara, L.; Cosenza, A.; Di Trapani, D.; Gallo, G.; Laudicina, V.; Marino, G.; Muscarella, S.; Presti, D.; et al. Enhancing a transition to a circular economy in the water sector: The eu project wider uptake. Water 2021, 13, 946. [Google Scholar] [CrossRef]
- Pearce, D.W.; Turner, R.K. Economics of Natural Resources and the Environment. Land Econ. 1990, 67, 272. [Google Scholar]
- Ilic, D.D.; Eriksson, O.; Ödlund, L.; Åberg, M. No zero burden assumption in a circular economy. J. Clean. Prod. 2018, 182, 352–362. [Google Scholar] [CrossRef]
- Salmenperä, H.; Pitkänen, K.; Kautto, P.; Saikku, L. Critical factors for enhancing the circular economy in waste management. J. Clean. Prod. 2021, 280, 124339. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Jan, E. The Circular Economy e A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Jawahir, I.S.; Bradley, R. Technological Elements of Circular Economy and the Principles of 6R-Based Closed-loop Material Flow in Sustainable Manufacturing. Procedia CIRP 2016, 40, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Tura, N.; Hanski, J.; Ahola, T.; Ståhle, M.; Piiparinen, S.; Valkokari, P. Unlocking circular business: A framework of barriers and drivers. J. Clean. Prod. 2019, 212, 90–98. [Google Scholar] [CrossRef]
- Kivimaa, P.; Boon, W.; Hyysalo, S.; Klerkx, L. Towards a typology of intermediaries in sustainability transitions: A systematic review and a research agenda. Res. Policy 2019, 48, 1062–1075. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Q.L.; Dong, Y.B.; He, Y.H.; Wang, L. Physicochemical properties and mechanism study of clinoptilolite modified by NaOH. Microporous Mesoporous Mater. 2015, 218, 174–179. [Google Scholar] [CrossRef]
- Kehrein, P.; Van Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A critical review of resource recovery from municipal wastewater treatment plants-market supply potentials, technologies and bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef] [Green Version]
- De Wit, M. The CIRCULARITY GAP Report. 2018. Available online: https://www.circle-economy.com (accessed on 15 July 2021).
- Daigger, G.T. Evolving Urban Water and Residuals Management Paradigms: Water Reclamation and Reuse, Decentralization, and Resource Recovery. Water Environ. Res. 2009, 81, 809–823. [Google Scholar] [CrossRef]
- Bozkurt, H.; Gernaey, K.V.; Sin, G. Superstructure-based optimization tool for plant design and retrofitting. In Innovative Wastewater Treatment & Resource Recovery Technologies: Impacts on Energy, Economy and Environment; IWA Publishing: London, UK, 2017; pp. 581–597. ISBN 978-1-78040-787-6. [Google Scholar]
- Li, W.-W.; Yu, H.-Q.; Rittmann, B.E. Chemistry: Reuse water pollutants. Nature 2015, 528, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.; Hoek, V.; Der Fooij, H.; De Struker, A. Resources, Conservation and Recycling Wastewater as a resource: Strategies to recover resources from Amsterdam’s wastewater. Resour. Conserv. Recycl. 2016, 113, 53–64. [Google Scholar]
- Guest, J.S.; Skerlos, S.J.; Barnard, J.L.; Beck, M.B.; Daigger, G.T.; Hilger, H.; Jackson, S.J.; Karvazy, K.; Kelly, L.; Macpherson, L.; et al. A New Planning and Design Paradigm to Achieve Sustainable Resource Recovery from Wastewater. Environ. Sci. Technol. 2009, 43, 6126–6130. [Google Scholar] [CrossRef] [Green Version]
- Maryam, B.; Büyükgüngör, H. Wastewater reclamation and reuse trends in Turkey: Opportunities and challenges. J. Water Process. Eng. 2019, 30, 100501. [Google Scholar] [CrossRef]
- Council of the European Communities. Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. L 1986, 181, 6–12. [Google Scholar]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Torretta, V. What advanced treatments can be used to minimize the production of sewage sludge in WWTPs? Appl. Sci. 2019, 9, 2650. [Google Scholar] [CrossRef] [Green Version]
- Eurostat. Sewage Sludge Production and Disposal. 2020. Available online: https://ec.europa.eu/eurostat/web/products-datasets/-/env_ww_spd (accessed on 21 June 2021).
- Karlikanovaite-Balikçi, A.; Yağci, N. A review on promising strategy to decrease sludge production: Oxic-settling-anoxic/anaerobic process. Environ. Res. Technol. 2020, 3, 81–91. [Google Scholar] [CrossRef]
- Eurostat. Water Statistics. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Water_statistics (accessed on 21 June 2021).
- Council of the European Communities. Council Directive 91/271/EEC of 21 May 1991 concerning urban waste-water treatment. Off. J. L 1991, 135, 40–52. [Google Scholar]
- European Commission. A European Green Deal. 2020. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 21 June 2021).
- Sun, L.P.; Lin, Y.J.; Shi, C.Y.; Wang, S.Q.; Luo, W.X.; Wang, M. Effects of interchange ratio on sludge reduction and microbial community structures in an anaerobic/anoxic/oxic process with combined anaerobic side-stream reactor. Water Sci. Technol. 2020, 81, 1250–1263. [Google Scholar] [CrossRef]
- Semblante, G.U.; Hai, F.I.; Ngo, H.H.; Guo, W.; You, S.J.; Price, W.E.; Nghiem, L. Sludge cycling between aerobic, anoxic and anaerobic regimes to reduce sludge production during wastewater treatment: Performance, mechanisms, and implications. Bioresour. Technol. 2014, 155, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhou, Z.; Zheng, Y.; Jiang, J.; Huang, J.; Qiang, J.; An, Y.; Jiang, L.; Jiang, L.-M.; Wang, Z. Understanding mechanisms of sludge in situ reduction in anaerobic side-stream reactor coupled membrane bioreactors packed with carriers at different filling fractions. Bioresour. Technol. 2020, 316, 123925. [Google Scholar] [CrossRef]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Zabed, H.M.; Qi, X.H. Recent developments in Polyhydroxyalkanoates (PHAs) production—A review. Bioresour. Technol. 2020, 306, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon recovery from wastewater through bioconversion into biodegradable polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Russo, I.; Bolzonella, D.; Pavan, P.; Majone, M.; Valentino, F. An urban biorefinery for food waste and biological sludge conversion into polyhydroxyalkanoates and biogas. Water Res. 2020, 170, 115371. [Google Scholar] [CrossRef]
- Frison, N.; Katsou, E.; Malamis, S.; Oehmen, A.; Fatone, F. Development of a Novel Process Integrating the Treatment of Sludge Reject Water and the Production of Polyhydroxyalkanoates (PHAs). Environ. Sci. Technol. 2015, 49, 10877–10885. [Google Scholar] [CrossRef] [Green Version]
- Conca, V.; da Ros, C.; Valentino, F.; Eusebi, A.L.; Frison, N.; Fatone, F. Long-term validation of polyhydroxyalkanoates production potential from the sidestream of municipal wastewater treatment plant at pilot scale. Chem. Eng. J. 2020, 390, 124627. [Google Scholar] [CrossRef]
- Montiel-Jarillo, G.; Gea, T.; Artola, A.; Fuentes, J.; Carrera, J.; Suárez-Ojeda, M.E. Towards PHA Production from Wastes: The Bioconversion Potential of Different Activated Sludge and Food Industry Wastes into VFAs Through Acidogenic Fermentation. Waste Biomass Valorization 2021, 12, 6861–6873. [Google Scholar] [CrossRef]
- Xin, X.; He, J.; Li, L.; Qiu, W. Enzymes catalyzing pre-hydrolysis facilitated the anaerobic fermentation of waste activated sludge with acidogenic and microbiological perspectives. Bioresour. Technol. 2018, 250, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Zhang, J.; Liang, S. Nutrient recovery from wastewater: From technology to economy. Bioresour. Technol. Rep. 2020, 11, 100425. [Google Scholar] [CrossRef]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and Phosphorus Recovery from Wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Saliu, T.D.; Oladoja, N.A. Nutrient recovery from wastewater and reuse in agriculture: A review. Environ. Chem. Lett. 2021, 19, 2299–2316. [Google Scholar] [CrossRef]
- You, X.; Valderrama, C.; Cortina, J.L. Simultaneous recovery of ammonium and phosphate from simulated treated wastewater effluents by activated calcium and magnesium zeolites. J. Chem. Technol. Biotechnol. 2017, 92, 2400–2409. [Google Scholar] [CrossRef] [Green Version]
- Canellas, J.; Soares, A.; Jefferson, B. Removing Ammonia From Wastewater Using Natural and Synthetic Zeolites: A Batch Experiment; Cambridge Open Engage: Cambridge, UK, 2019; pp. 1–32. [Google Scholar] [CrossRef]
- Ramesh, K.; Reddy, D.D. Zeolites and Their Potential Uses in Agriculture. Adv. Agron. 2011, 113, 219–241. [Google Scholar]
- Steiner, C. Considerations in Biochar Characterization. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Soil Science Society of America, Inc.: Madison, WI, USA, 2011; pp. 87–100. [Google Scholar]
- Wang, Y.; Qiu, L.P.; Hu, M.F. Magnesium Ammonium Phosphate Crystallization: A Possible Way for Recovery of Phosphorus from Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 032032. [Google Scholar] [CrossRef]
- Nobaharan, K.; Novair, S.B.; Lajayer, B.A.; Van Hullebusch, E.D. Phosphorus removal from wastewater: The potential use of biochar and the key controlling factors. Water 2021, 13, 517. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Mckay, G.; Kochkodan, V.; Ali Atieh, M.; Al-Ansari, T. A state of the art review on phosphate removal from water by biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Visser, C.; Korving, L. PHARIO Stepping Stone to a Sustainable Value Chain for PHA Bioplastic Using Municipal Activated Sludge; STOWA Report: Amersfoort, The Netherlands, 2017. [Google Scholar]
- Presti, D.; Cosenza, A.; Capri, F.C.; Gallo, G.; Alduina, R.; Mannina, G. Influence of volatile solids and pH for the production of volatile fatty acids: Batch fermentation tests using sewage sludge. Accept. Bioresour. Technol. 2021, 342, 125853. [Google Scholar] [CrossRef]
- Cinà, P.; Bacci, G.; Arancio, W.; Gallo, G.; Fani, R.; Puglia, A.M.; Di Trapani, D.; Mannina, G. Assessment and characterization of the bacterial community structure in advanced activated sludge systems. Bioresour. Technol. 2019, 282, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Ma, F.; Wei, L.; Chua, H. Using rice straw fermentation liquor to produce bioflocculants during an anaerobic dry fermentation process. Bioresour. Technol. 2012, 113, 83–88. [Google Scholar] [CrossRef]
- Li, L.; He, J.; Wang, M.; Xin, X.; Xu, J.; Zhang, J. Efficient volatile fatty acids production from waste activated sludge after ferrate pretreatment with alkaline environment and the responding microbial community shift. ACS Sustain. Chem. Eng. 2018, 6, 16819–16827. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- Lv, X.; Chai, J.; Diao, Q.; Huang, W.; Zhuang, Y.; Zhang, N. The Signature Microbiota Drive Rumen Function Shifts in Goat Kids Introduced to Solid Diet Regimes. Microorganisms 2019, 7, 516. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Yang, C.; Guan, L.L.; Wang, J.; Xue, M.; Liu, J.X. Persistence of Cellulolytic Bacteria Fibrobacter and Treponema After Short-Term Corn Stover-Based Dietary Intervention Reveals the Potential to Improve Rumen Fibrolytic Function. Front. Microbiol. 2018, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Watari, T.; Mai, T.C.; Tanikawa, D.; Hirakata, Y.; Hatamoto, M.; Syutsubo, K.; Fukuda, M.; Nguyen, N.B.; Yamaguchi, T. Development of downflow hanging sponge (DHS) reactor as post treatment of existing combined anaerobic tank treating natural rubber processing wastewater. Water Sci. Technol. 2017, 75, 57–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscarella, S.M.; Badalucco, L.; Cano, B.; Laudicina, V.A.; Mannina, G. Ammonium adsorption, desorption and recovery by acid and alkaline treated zeolite. Bioresour. Technol. 2021, 341, 125812. [Google Scholar] [CrossRef]
- Karadag, D.; Koc, Y.; Turan, M.; Armagan, B. Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite. J. Hazard. Mater. 2006, 136, 604–609. [Google Scholar] [CrossRef]
- Lebedynets, M.; Sprynskyy, M.; Sakhnyuk, I.; Zbytniewski, R.; Golembiewski, R.; Buszewski, B. Adsorption of ammonium ions onto a natural zeolite: Transcarpathian clinoptilolite. Adsorpt. Sci. Technol. 2004, 22, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Bolan, N.S.; Mowatt, C.; Adriano, D.C.; Blennerhassett, J.D. Removal of Ammonium Ions from Fellmongery Effluent by Zeolite. Commun. Soil Sci. Plant Anal. 2003, 34, 1861–1872. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lin, F.; Pang, W.-Q. Ammonium exchange in aqueous solution using Chinese natural clinoptilolite and modified zeolite. J. Hazard. Mater. 2007, 142, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Ho, Y.S.; Tang, X. Comparative sorption kinetic studies of ammonium onto zeolite. J. Hazard. Mater. 2006, 133, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Zeolite as a potential medium for ammonium recovery and second cheese whey treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Freni, G.; Mannina, G. Uncertainty in water quality modelling: The applicability of variance decomposition approach. J. Hydrol. 2010, 394, 324–333. [Google Scholar] [CrossRef]
- Mannina, G.; Ekama, G.A.; Capodici, M.; Cosenza, A.; Di Trapani, D.; Ødegaard, H. Moving bed membrane bioreactors for carbon and nutrient removal: The effect of C/N variation. Biochem. Eng. J. 2017, 125, 31–40. [Google Scholar] [CrossRef]
- Mannina, G.; Viviani, G. Separate and combined sewer systems: A long-term modelling approach. Water Sci. Technol. 2009, 60, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Villanueva, P.; Sauri, D. Wastewater Treatment Plants in Mediterranean Spain: An Exploration of Relations between Water Treatments, Water Reuse, and Governance. Water 2021, 13, 1710. [Google Scholar] [CrossRef]
- Cazurra, T. Water reuse of south Barcelona’s wastewater reclamation plant. Desalination 2008, 218, 43–51. [Google Scholar] [CrossRef]
- Teijon, G.; Candela, L.; Tamoh, K.; Molina-Díaz, A.; Fernandez-Alba, A.R. Occurrence of emerging contaminants, priority substances 683 (2008/105/CE) and heavy metals in treated wastewater and groundwater at Depurbaix facility (Barcelona, Spain). Sci. Total Environ. 2010, 408, 3584–3595. [Google Scholar] [CrossRef] [PubMed]




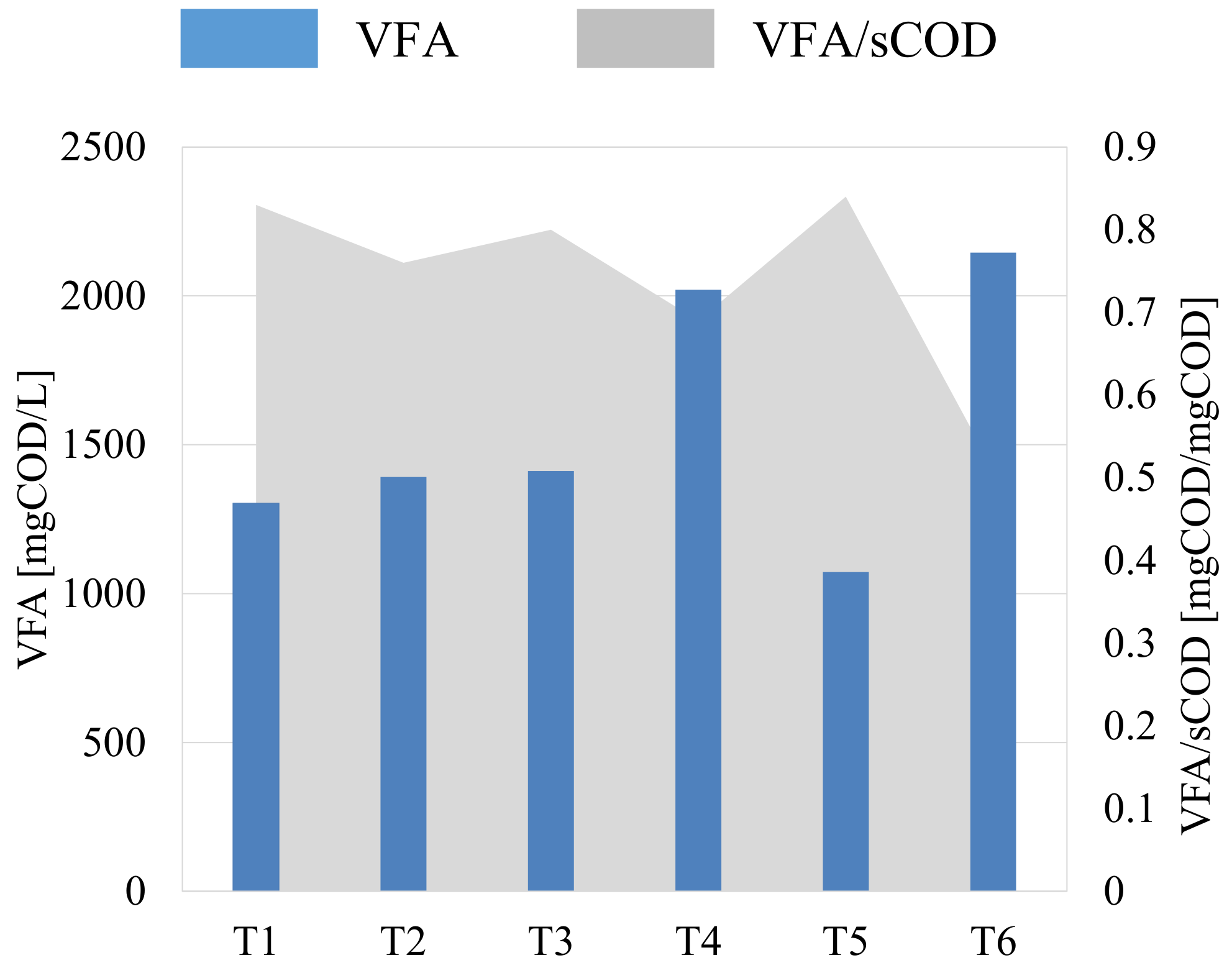

| Batch Test | Details |
|---|---|
| T1 | VSS = 4 g/L Uncontrolled pH |
| T2 | VSS = 5.9 g/L Initial pH = 8 |
| T3 | VSS = 5.9 g/L Uncontrolled pH |
| T4 | VSS = 5.9 g/L Initial pH = 10 |
| T5 | VSS = 2.8 g/L Uncontrolled pH |
| T6 | VSS = 5.9 g/L pH = 10 (continuously adjusted) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannina, G.; Alduina, R.; Badalucco, L.; Barbara, L.; Capri, F.C.; Cosenza, A.; Di Trapani, D.; Gallo, G.; Laudicina, V.A.; Muscarella, S.M.; et al. Water Resource Recovery Facilities (WRRFs): The Case Study of Palermo University (Italy). Water 2021, 13, 3413. https://doi.org/10.3390/w13233413
Mannina G, Alduina R, Badalucco L, Barbara L, Capri FC, Cosenza A, Di Trapani D, Gallo G, Laudicina VA, Muscarella SM, et al. Water Resource Recovery Facilities (WRRFs): The Case Study of Palermo University (Italy). Water. 2021; 13(23):3413. https://doi.org/10.3390/w13233413
Chicago/Turabian StyleMannina, Giorgio, Rosa Alduina, Luigi Badalucco, Lorenzo Barbara, Fanny Claire Capri, Alida Cosenza, Daniele Di Trapani, Giuseppe Gallo, Vito Armando Laudicina, Sofia Maria Muscarella, and et al. 2021. "Water Resource Recovery Facilities (WRRFs): The Case Study of Palermo University (Italy)" Water 13, no. 23: 3413. https://doi.org/10.3390/w13233413
APA StyleMannina, G., Alduina, R., Badalucco, L., Barbara, L., Capri, F. C., Cosenza, A., Di Trapani, D., Gallo, G., Laudicina, V. A., Muscarella, S. M., & Presti, D. (2021). Water Resource Recovery Facilities (WRRFs): The Case Study of Palermo University (Italy). Water, 13(23), 3413. https://doi.org/10.3390/w13233413