Removal of Cyanide and Other Nitrogen-Based Compounds from Gold Mine Effluents Using Moving Bed Biofilm Reactor (MBBR)
Abstract
:1. Introduction
1.1. Mining Operation in Ghana
1.2. Mining Operation in Canada
2. Materials and Methods
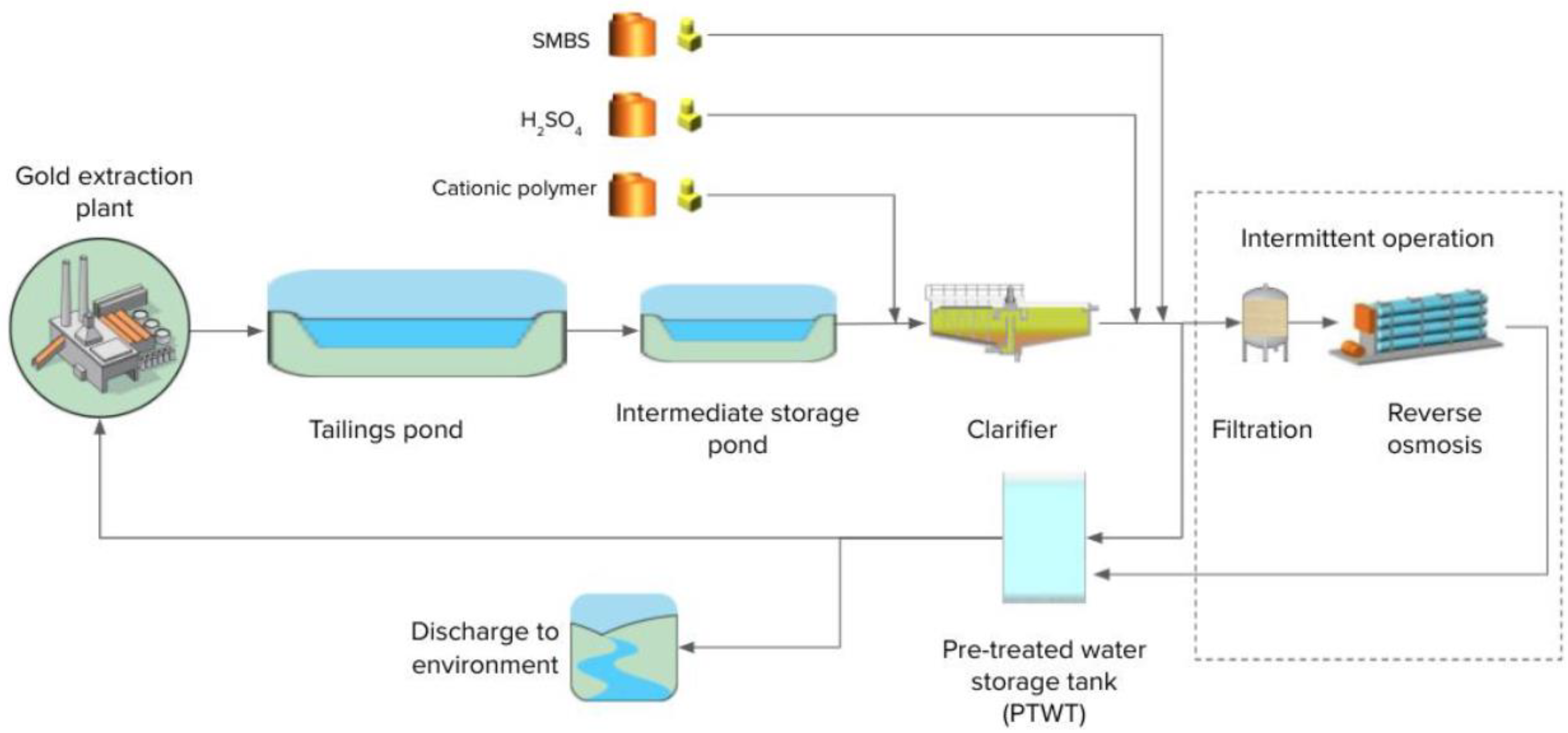
2.1. Pilot Plant in Ghana
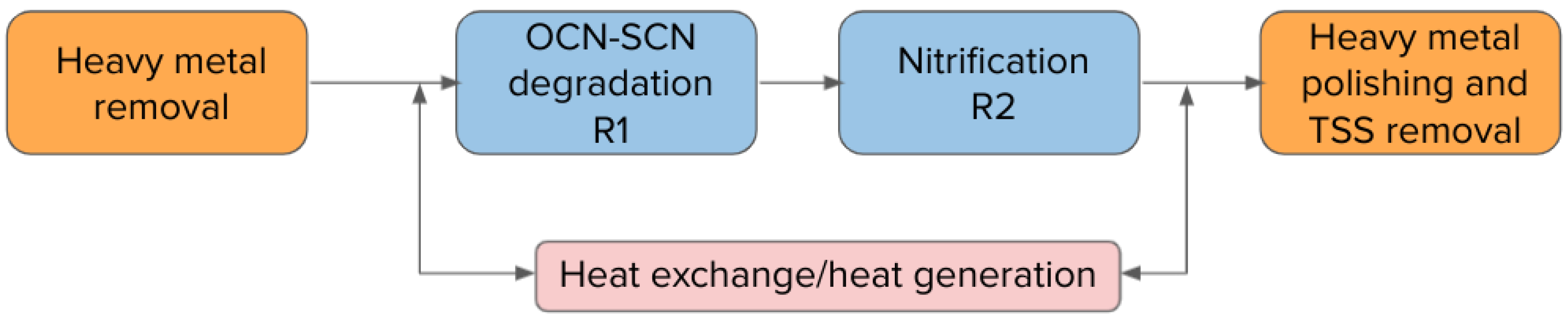
2.2. Full-Scale Plant in Canada
3. Results
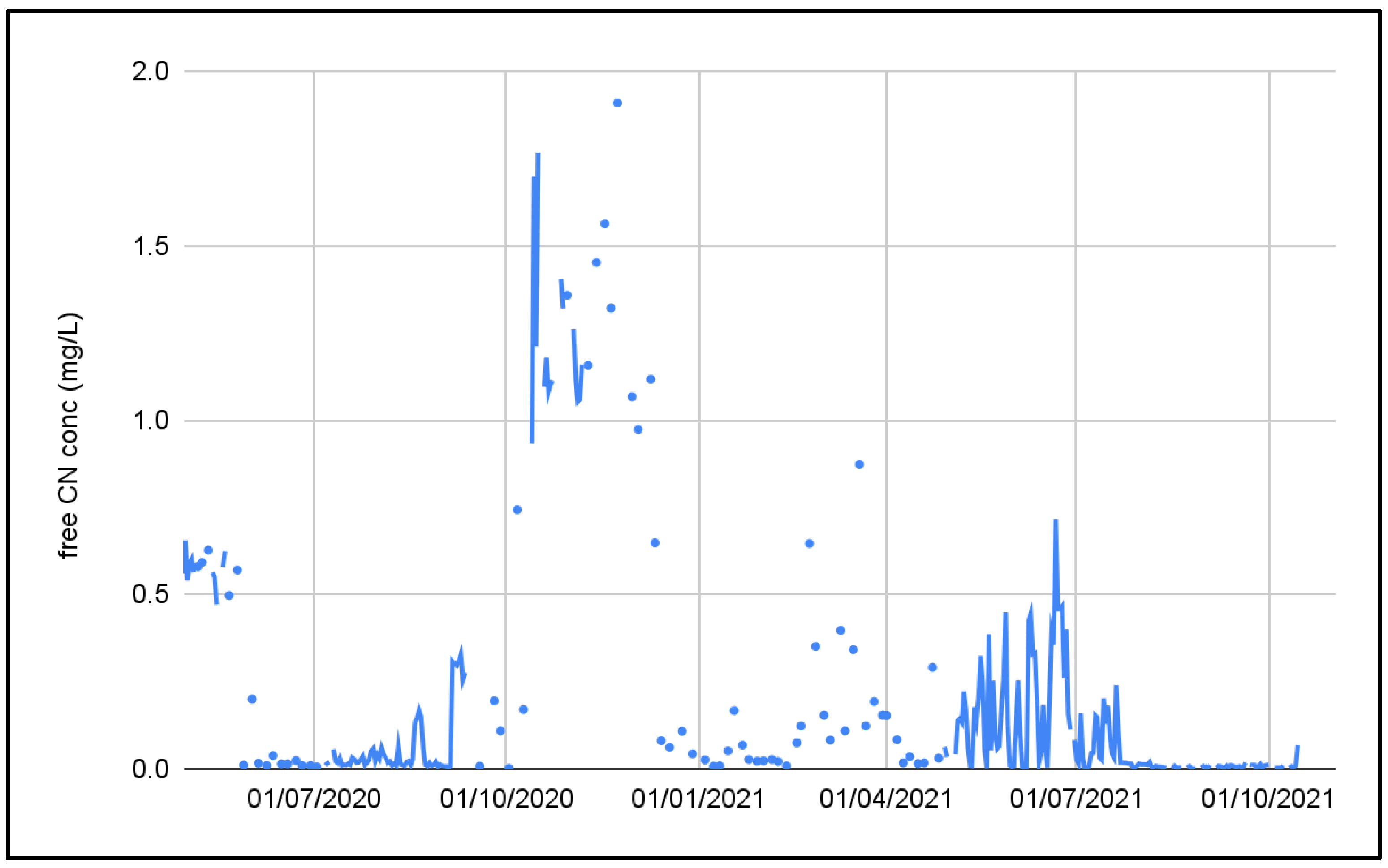
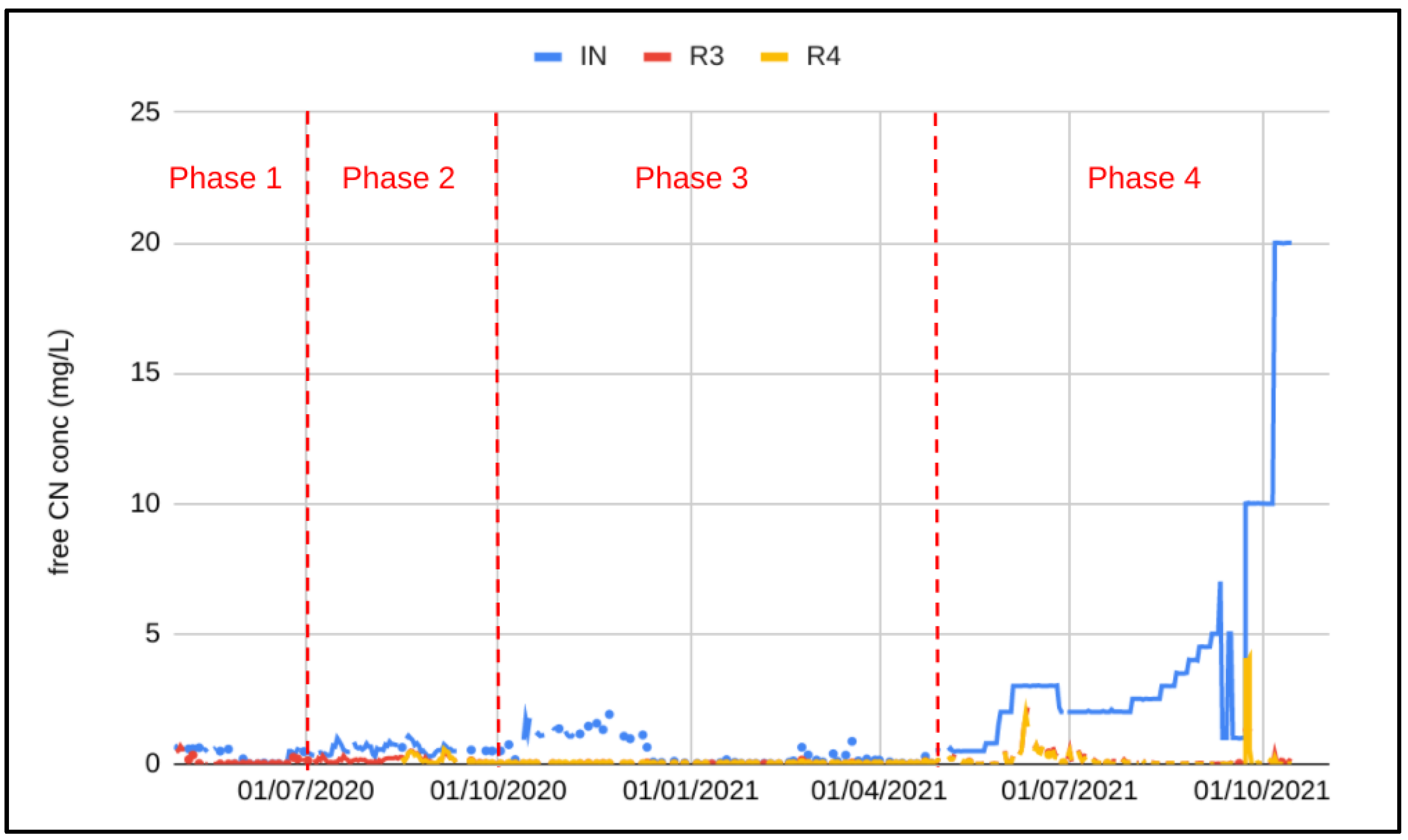
3.1. Ghana Pilot Plant Results
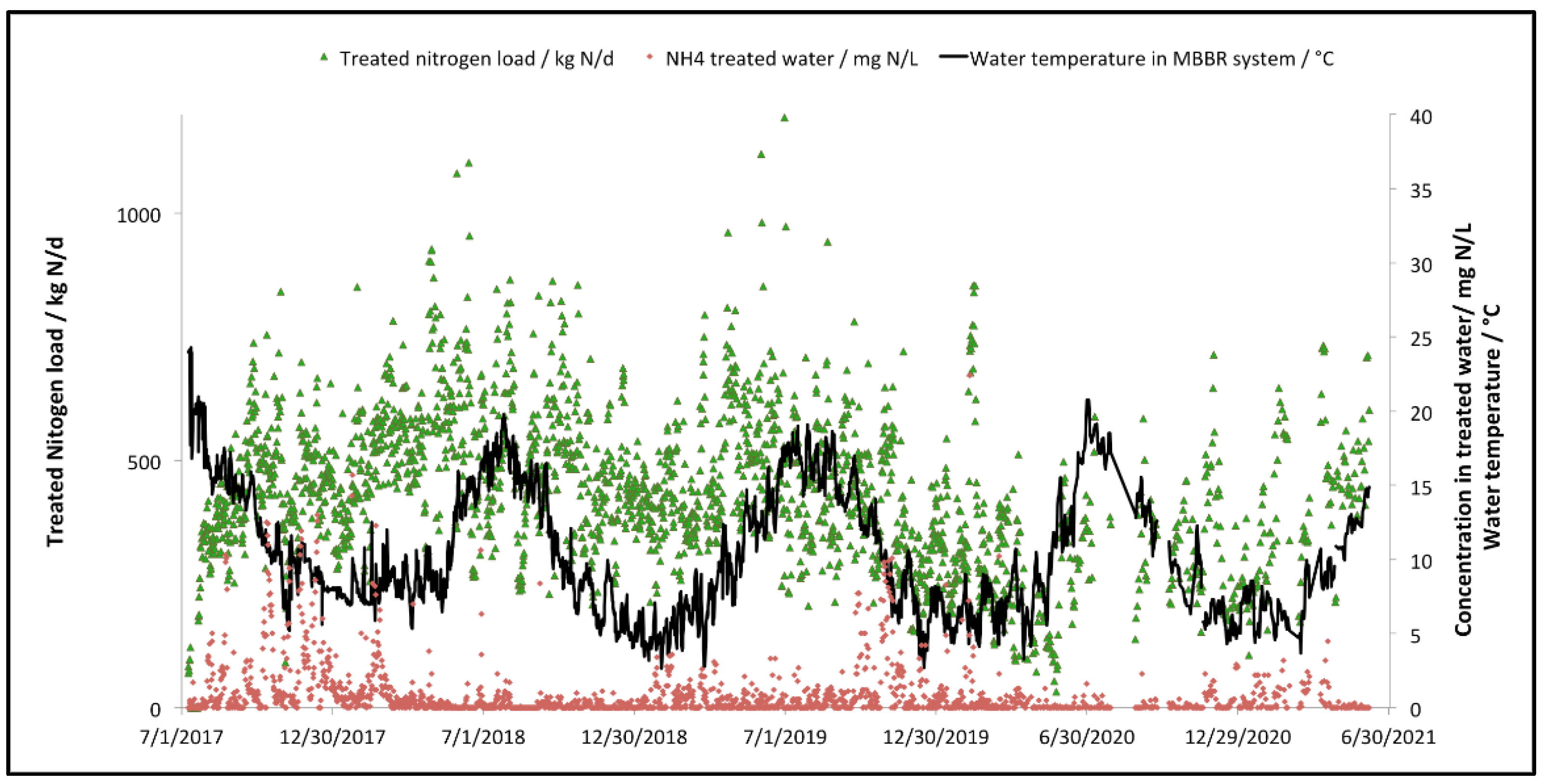
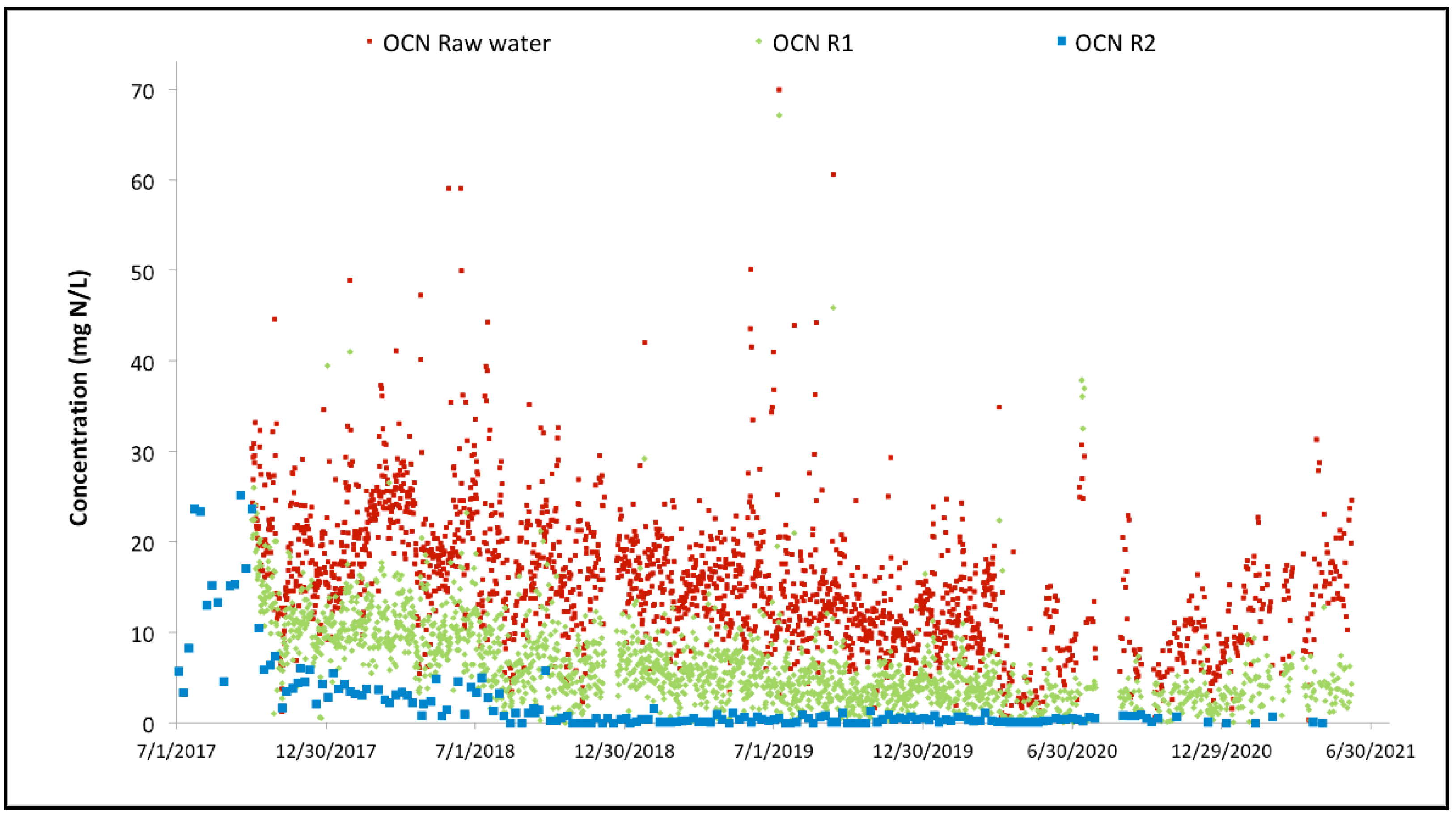
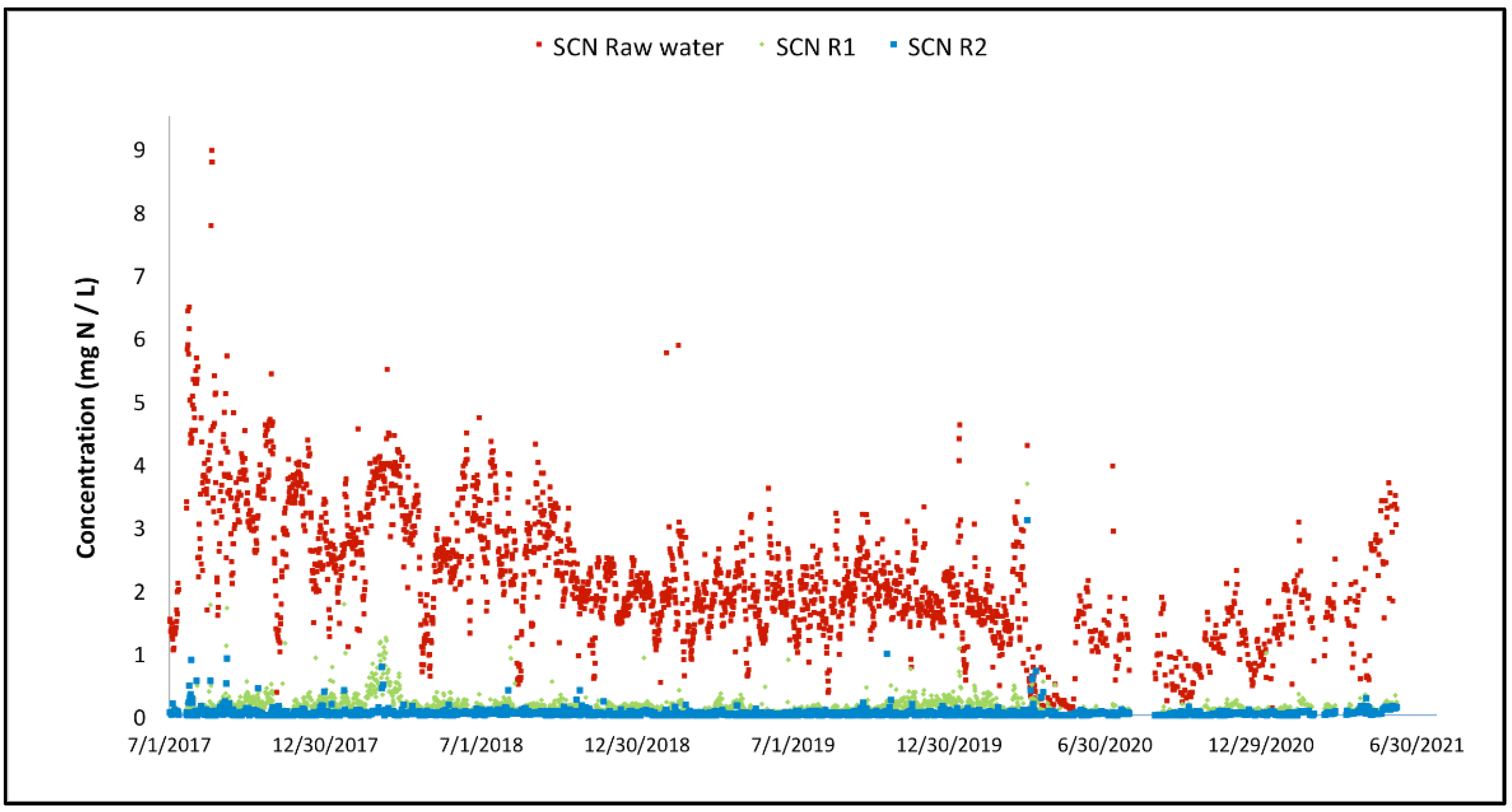
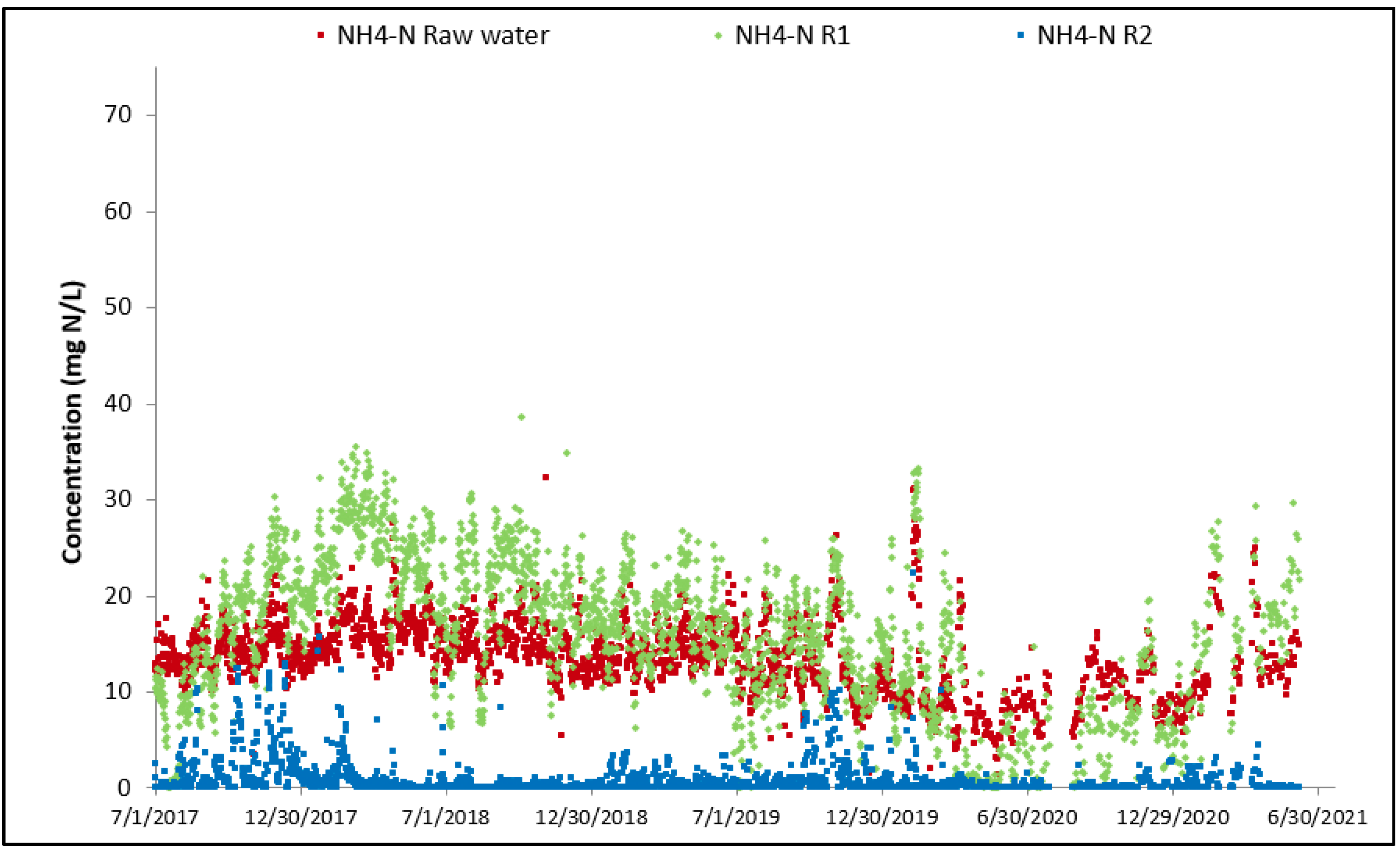
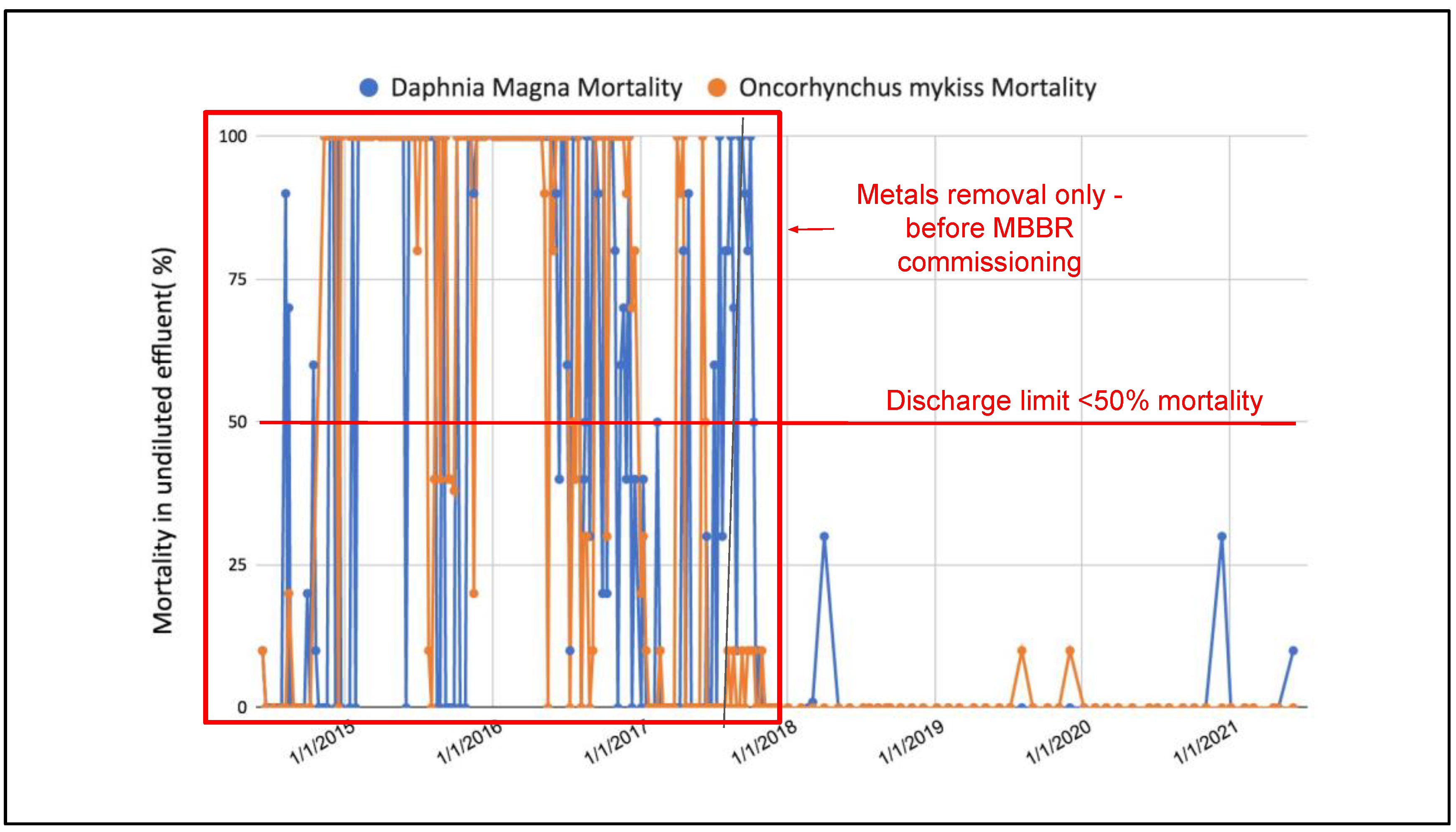
3.2. Full-Scale Plant Results—Canada
- Cyanide species removal: 146 kg N/d
- Nitrification reactor (treated nitrogen load): 462 kg N/d
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, G. Toxicity of cyanide in fishes: An overview. Univers. J. Pharm. 2013, 2, 23–26. [Google Scholar]
- Laliberté, M. Reducing the toxicity of a gold mine effluent using biological reactors and precipitation. Min. Metall. Explor. 2015, 32, 1–5. [Google Scholar] [CrossRef]
- Habashi, F. Kinetics and Mechanism of Gold and Silver Dissolution in Cyanide Solution; Montana College of Mineral Science and Technology: Butte, MT, Canada, 1967; pp. 1–42. [Google Scholar]
- Rusten, B.; Hellström, B.G.; Hellström, F.; Sehested, O.; Skjelfoss, E.; Svendsen, B. Pilot testing and preliminary design of moving bed biofilm reactors for nitrogen removal at the FREVAR wastewater treatment plant. Water Sci. Technol. 2000, 41, 13–20. [Google Scholar] [CrossRef]
- CCME. Recommandations Canadiennes Pour la Qualité des Eaux: Protection de la vie Aquatique. 2010. Available online: https://ccme.ca/fr/priorites-actuelles/recommandations-canadiennes-pour-la-qualit-de-lenvironnement (accessed on 27 November 2021).
- Camargo, J.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]

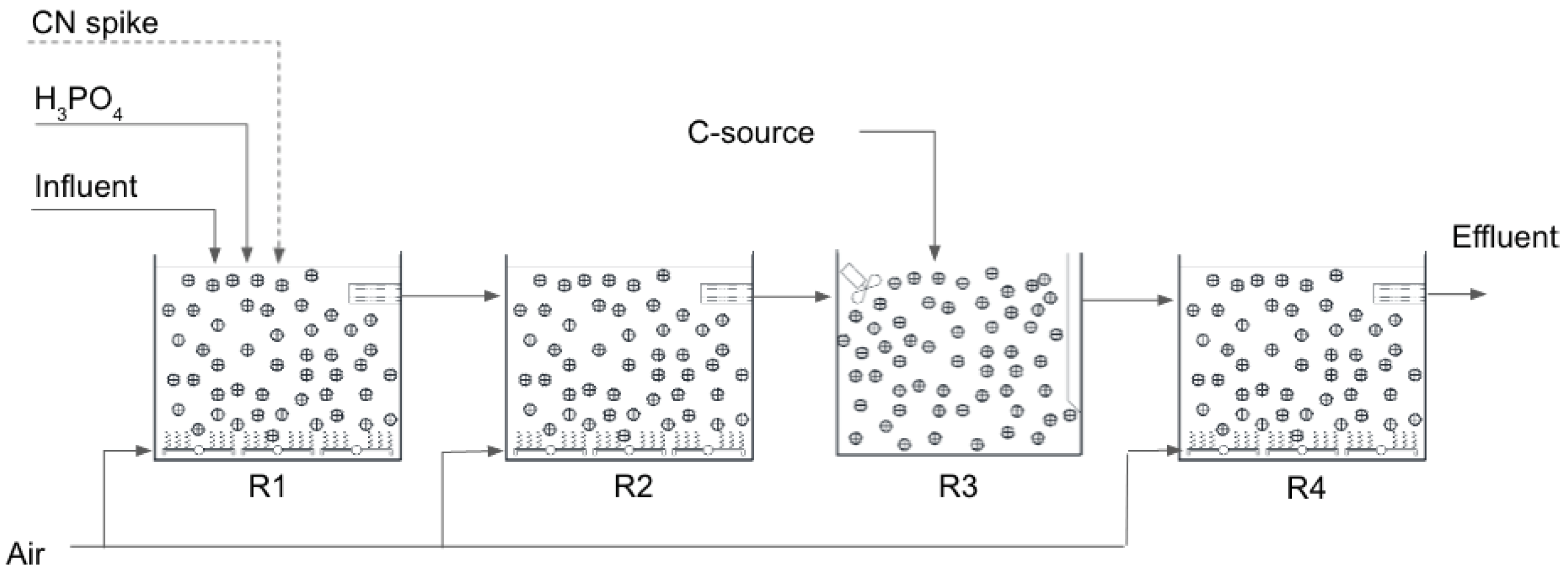

| Under aerobic conditions, the following reactions occur: |
| (1) conversion of cyanide to cyanate: CN− + → OCN− (2) conversion of cyanate and thiocyanate to ammonia-nitrogen: OCN− + 3H2O → + OH− SCN− + 2O2 + 3H2O → + + H+ + (3) conversion of ammonia-nitrogen to nitrate: + → + 2H+ + H2O + → |
| Under anoxic conditions, the following reaction occurs: |
| + Organic Matter → N2 + CO2 + H2O |
| R1 | R2 | R3 | R4 | |
|---|---|---|---|---|
| Operating regime | Aerobic | Aerobic | Anoxic | Aerobic |
| Hydraulic retention time | 1.3–1.7 h | 1.3–1.7 h | 1.3–3.5 h | 1.3–3.5 h |
| Chemical dosing | Phosphoric acid for cell synthesis | Carbon source (molasse or ethanol) |
| R1 | R2 | |
|---|---|---|
| Operating regime | Aerobic | Aerobic |
| Hydraulic retention time | 0.5–3.4 h | 0.4–6 h |
| Chemical dosing | Hexametaphosphate solution for cell synthesis & NaOH for pH control | NaOH for pH control |
| Min | Average | 95%ile | Discharge Limit | ||
|---|---|---|---|---|---|
| pH | 6.73 | 7.73 | 8.39 | 6–9 | |
| EC | µS/cm | 953 | 1394 | 1735 | 1500 |
| Turbidity | NTU | 0.78 | 23 | 49 | 75 |
| SCOD tot | mg/L | 4 | 62.6 | 117 | 250 |
| TSS | mg/L | 1 | 18 | 42 | 50 |
| SO4 | mg/L | 99 | 230 | 329 | 300 |
| Cu | mg/L | 0.01 | 0.3 | 0.57 | 5 |
| NH4-N | mg/L | 0.01 | 4 | 9.5 | 1 |
| NO2-N | mg/L | 23 | 40.6 | 56 | - |
| NO3-N | mg/L | 18.8 | 33.2 | 49 | 50 |
| free CN | mg/L | 0.001 | 0.2 | 1.11 | 0.2 |
| Min | Average | 95%ile | ||
|---|---|---|---|---|
| Water temperature (in MBBR) | °C | 2.8 | 10.5 | 17.7 |
| Alkalinity-CaCO3 | mg/L | 33 | 104 | 140 |
| NH4-N | mg/L | 1.6 | 13.6 | 19.4 |
| NO2-N | mg/L | 0.2 | 3 | 5.1 |
| NO3-N | mg/L | 8.5 | 39.1 | 58 |
| OCN-N | mg/L | 0.2 | 15.5 | 27.5 |
| SCN-N | mg/L | 0.1 | 2.5 | 4 |
| Total treated nitrogen load | kg N/d | 1 | 436 | 696 |
| Total treated SCN-OCN load | kg N/d | 0 | 183 | 330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwofie, I.A.; Jogand, H.; De Ladurantaye-Noël, M.; Dale, C. Removal of Cyanide and Other Nitrogen-Based Compounds from Gold Mine Effluents Using Moving Bed Biofilm Reactor (MBBR). Water 2021, 13, 3370. https://doi.org/10.3390/w13233370
Kwofie IA, Jogand H, De Ladurantaye-Noël M, Dale C. Removal of Cyanide and Other Nitrogen-Based Compounds from Gold Mine Effluents Using Moving Bed Biofilm Reactor (MBBR). Water. 2021; 13(23):3370. https://doi.org/10.3390/w13233370
Chicago/Turabian StyleKwofie, Isaac Amoesih, Henri Jogand, Myriam De Ladurantaye-Noël, and Caroline Dale. 2021. "Removal of Cyanide and Other Nitrogen-Based Compounds from Gold Mine Effluents Using Moving Bed Biofilm Reactor (MBBR)" Water 13, no. 23: 3370. https://doi.org/10.3390/w13233370
APA StyleKwofie, I. A., Jogand, H., De Ladurantaye-Noël, M., & Dale, C. (2021). Removal of Cyanide and Other Nitrogen-Based Compounds from Gold Mine Effluents Using Moving Bed Biofilm Reactor (MBBR). Water, 13(23), 3370. https://doi.org/10.3390/w13233370







