1. Introduction
Despite their critical role in maintaining biodiversity and the essential socioeconomic services they provide, the importance of freshwaters and the need to treat them responsibly is often overlooked. Indeed, according to the Living Planet Index, there was an 84% decline in freshwater vertebrate populations 1970–2016, and almost one in three freshwater species is threatened with extinction [
1]. Increasing attention is now being paid to the restoration of ecosystems to help limit and mitigate the effects of climate change, to ensure the sustainable provision of essential ecosystem services, and to stem the loss of habitats and species. The United Nations has proclaimed 2021–2030 to be the Decade on Ecosystem Restoration [
2], with freshwater ecosystem conservation and restoration a major theme [
2].
In 2013, the River Restoration and Biodiversity Programme was established under the auspices of the International Union for the Conservation of Nature National Committee United Kingdom (IUCN NCUK [
3]). The aim of the Programme is to provide robust evidence for the biodiversity benefits of restoring rivers. The Programme defines river restoration as “the re-establishment of natural physical processes (e.g., variation of flow and sediment movement), features (e.g., sediment sizes and river shape) and physical habitats of a river system (including submerged, bank and floodplain areas)”. This approach uses nature-based solutions to restore natural processes to create a characteristic, self-sustaining, dynamic physical habitat that facilitates ecological recovery [
4,
5].
Understanding the response of biota to river restoration intervention is important when determining whether ecological objectives have been met, whether adaptive management is necessary, and to learn lessons for future projects [
6,
7]. It requires the collection of meaningful data to determine whether a project has been successful [
8,
9]. However, most projects have not implemented effective monitoring to assess the outcome of restoration [
7,
10], and there has been a reliance on subjective perceptions of success [
11]. If we are to understand and implement restoration measures effectively, their success must be rigorously evaluated [
5,
12].
The ongoing lack of research and monitoring following the recovery of degraded ecosystems means that theories about the trajectories of ecosystem change following restoration remain unclear [
13,
14]. Without confidence in the theoretical framework, developing generalised strategies for the evaluation of change following a restoration intervention is difficult. In short, we remain in a data gathering phase.
To address these gaps, in October 2018 as part of the IUCN (NCUK) River Restoration and Biodiversity Programme, a workshop of invited river restoration appraisal academics and practitioners was held. Its aim was to explore best practices and incorporate academic knowledge to develop a scientifically robust monitoring protocol for appraising the biodiversity benefits of restoration [
15]. This review builds on the findings of the workshop. The recommendations are intended to inform robust assessments of restoration effectiveness by both academics and practitioners worldwide.
2. History of Restoration Appraisal
In the 1990s, river restoration became established as an important method for the rehabilitation of river ecosystems [
16,
17,
18,
19]. Concurrent with this increase in restoration activity was the call for comprehensive monitoring, including flag-ship demonstration projects [
20] and well-structured monitoring and appraisal [
21] that take an ecosystem approach [
19]. Despite the recognition of the importance of monitoring change, restoration measures were often implemented on the assumption that the restoration of habitats would be followed by improvements in biodiversity. This approach is characterised by the phrase “if you build it, they will come”, otherwise known as the “Field of Dreams hypothesis” [
22]. The lack of appraisal meant that the success of restoration measures was poorly understood. A 2007 review found 89% of project contacts reported success but only 11% of the projects were considered successful due to a measurable ecological response [
23]. Similarly, in 2016, the UK National River Restoration Inventory compiled by the River Restoration Centre contained over 2800 completed projects with only 21% reporting any monitoring. Of the 179 projects added to this inventory in 2017, only 5% specifically reported any monitoring outcomes [
24]. A review of restoration work in California, USA, found that very few projects had been subjected to post-project evaluation resulting in a lost opportunity to learn and improve future design [
25]. This view was supported by others who also concluded that poorly planned appraisals can be misleading [
26].
Despite an increase in the number of published restoration appraisals [
27], there is still a noted lack of monitoring and appraisal [
12,
28]. There is also a lack of consideration of how to monitor project success effectively and the inherent difficulties in doing this [
29]. These appraisals are illustrated by poorly planned inappropriate monitoring strategies [
7,
30] and recommendations to undertake monitoring with more rigor to increase the likelihood of detecting actual changes in biodiversity [
5,
31], as well as linking hydromorphological changes to ecological responses [
32,
33].
3. Towards More Effective Appraisal of River Restoration
Discussions at the 2018 IUCN (NCUK) workshop concentrated on developing recommendations to complement existing guidance and established best practices. Specifically, all restoration activity should be undertaken within an integrated project framework. This framework should ensure there are sufficient baseline data to characterise the current status, identify causes of degradation, plan effective solutions, and set restoration targets [
12,
34,
35].
Once restoration targets have been established they should be turned into clear SMART (Specific, Measurable, Achievable, Realistic, and Time-bound (e.g., [
36]) objectives that are linked to project goals and predicted outcomes [
17,
21]. A lack of defined objectives and success criteria or end points against which to measure success has also been identified as a problem in river restoration appraisal [
12,
29].
The development of target outcomes or ‘expectations’, therefore, requires either historical information about the system that is to be restored, information from ecologically similar but undisturbed ‘reference’ sites, or expert opinion based on empirical and/or theoretical models [
26]. Quantified expectations allow the testing of scientific hypotheses about how systems respond to restoration [
37,
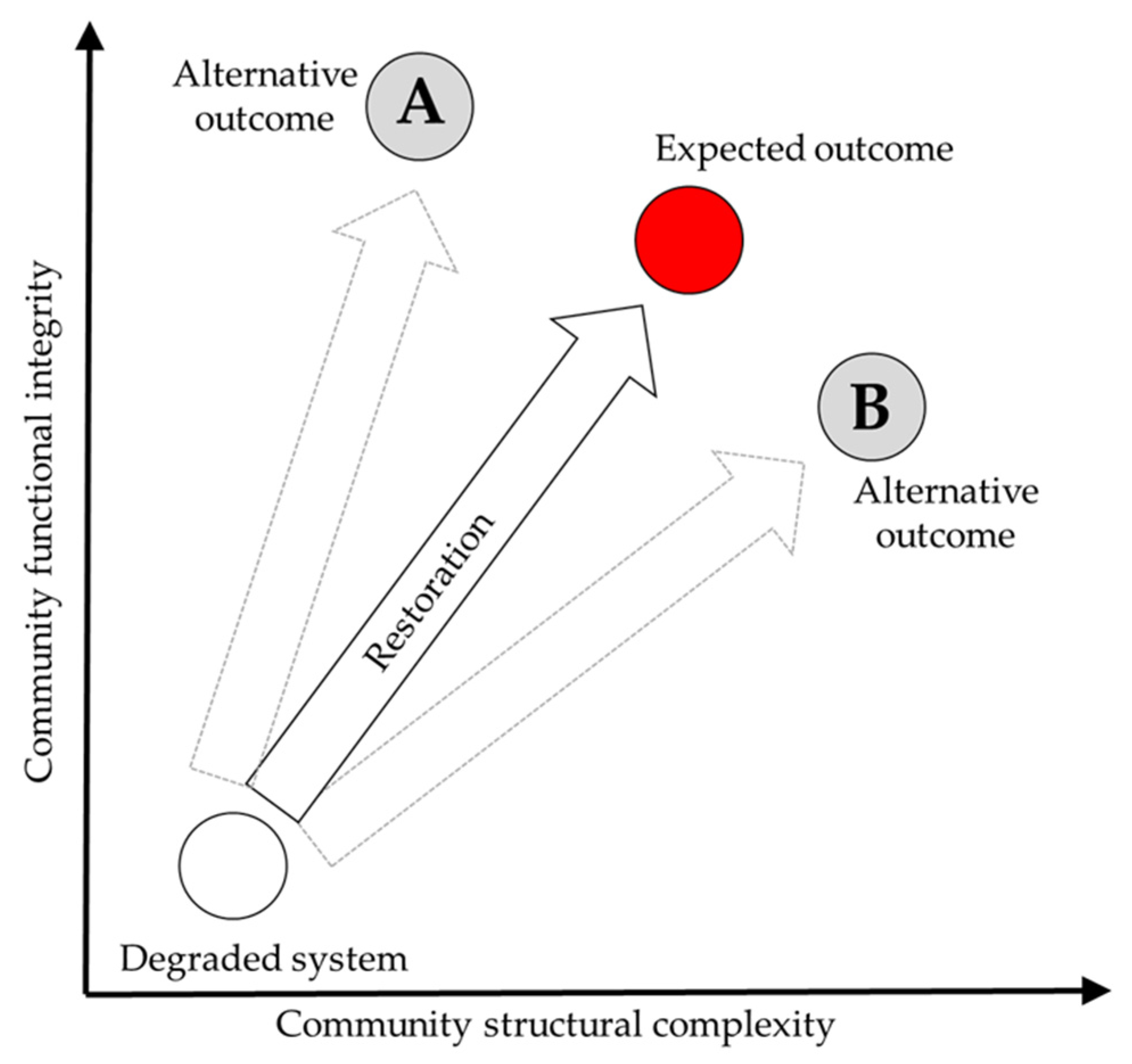
38]. The expectations must be set in the context of catchment condition and processes and should consider restoring both structural complexity and functional integrity [
39] (
Figure 1). Setting expectations or targets in a catchment context allows the interactions with other anthropogenic stressors to be considered [
32,
40,
41]. Expectations should also consider projected future changes within the catchments of restored rivers including climate change and likely land use changes such as urban development.
Exactly what is monitored to support the appraisal of restoration outcomes will depend on the impetus for a scheme and the scale of the restoration [
5]. However, it should follow an integrated ecosystem approach [
18] that includes geomorphological, habitat, and biota assessments [
4,
5,
17]. Where practicable, assessments points should be colocated [
29] to help understand biotic responses to habitat change.
Legitimacy of restoration appraisal requires the scientific review and public dissemination of findings [
5], yet appraisals are often poorly documented [
34]. There are two main dissemination routes: publications in peer-journals and less formal forums. Journal publication ensures scientific robustness and knowledge sharing. Less formal routes such as the EU-RiverWiki [
42], (an online tool used for sharing information on river restoration projects) allow wider dissemination and the sharing of less detailed studies, which can contribute to the weight of evidence of restoration success. To ensure the field of river restoration continues to advance, results should be shared regardless of whether they are positive, negative, or ambiguous [
11,
13,
21,
29,
34,
43]. The importance of engaging project stakeholders at this stage should not be overlooked as this can be critical to promoting buy-in for future projects.
4. Monitoring Strategies
There are two broad types of monitoring for restoration: confirmatory and investigative. Confirmatory monitoring is a process of confirming ecological expectations, for example, the presence of xylophagous (feeding on or boring into wood) invertebrates following the addition of large woody material. Confirmatory monitoring is simple but cannot answer fundamental ecological questions or make predictions; this capability is the domain of investigative monitoring, which is more complex and involves the collection of data on several related variables to answer questions associated with the outcome of habitat restoration.
Evaluation of change should be achieved through the collection of high quality information. “High quality” in this case means representative data gathered with sufficient replication over appropriate time and spatial scales relevant to the indicator or process, i.e., physical, biological, or ecological. To cover the range of scales required it is likely that a combination of different types of data and monitoring will be required [
44]. The information gathered must be able to account for the multiple influences on plausible recovery trajectories.
Before any data are collected, the factors causing variability within the system need to be identified and defined, and suitable indicators of interest must be determined. This will provide insight into the sampling effort required to gain a significant result—in statistical parlance, power analysis [
45]. This is of particular importance in river restoration projects where the restoration “signal” may be difficult to discern from the “noise” of uncontrolled confounding factors such as land use changes, climate change, interannual variation, natural disturbance, erratic events (e.g., La Nina), and observer-variability.
Both natural and anthropogenic stressors have been shown to generate contrasting patterns in the overall dissimilarity of species assemblages, species turnover, and assemblage nestedness (i.e., beta diversity factors; [
46]). For example, the increasing intensity of stress has been shown to reduce variability in the occurrence of subsets of sensitive species, particularly through anthropogenic stressors [
46].
Rivers exhibit gradients of change in hydromorphological and ecological attributes over their course, which poses a potential problem for locating suitable control or reference sites to determine the changes resulting from restoration intervention. This limitation can be reduced through the use of a paired river design and multiple controls in similar habitats, including neighbouring rivers [
47,
48,
49] and, for example [
50].
Pre-restoration data are also important, because they allow the assessment of whether a restoration project has been successful against a baseline [
51]; understanding variability in the data improves confidence in assessments of success [
32,
52]. Whilst the pre-intervention data should cover as long a period as possible [
43], the actual number of years over which it is gathered is often arbitrarily set at three. This 3-year period is a compromise between understanding the current condition of the site, the resources (both money and time) available [
53], and statistical criteria. In many cases, there is simply no or very little pre-restoration data.
To ensure a true representation of the background natural variability in river condition (i.e., controls), multiple sites, sampled at the same time across similar habitats and/or rivers, could assist in understanding existing or underlying attributes. As well as enabling the success of an individual project to be evaluated, the information gathered also contributes to the development of wider understanding, i.e., to the building of a theoretical framework [
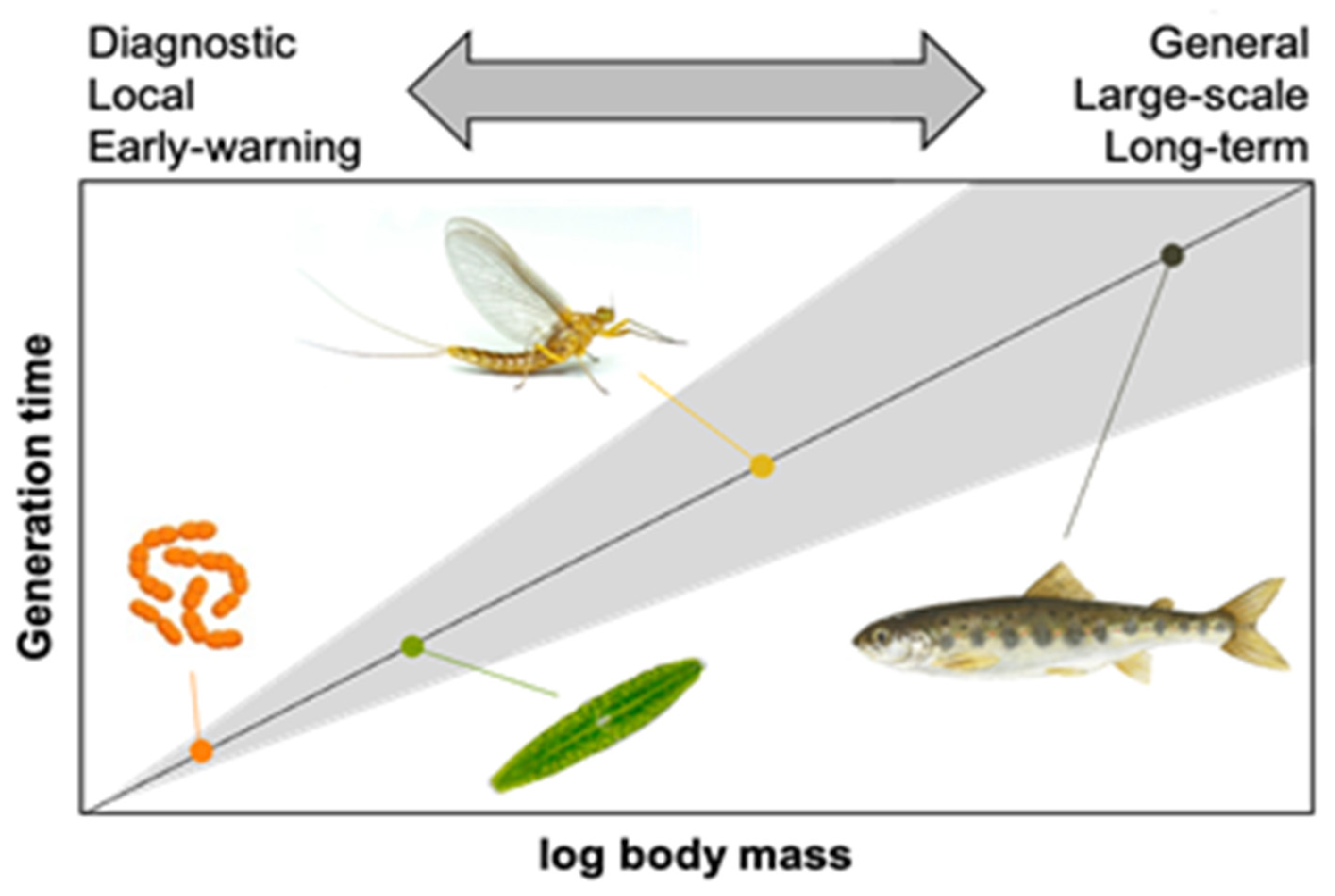
45]. Following restoration, the frequency and duration of monitoring should be consistent with the life histories of the target organisms and the timescale for exhibiting responses (i.e., the lag time). The magnitude and duration of responses vary from rapidly responding algae, through moderately responsive fish, to slowly responding riparian trees [
54],
Figure 2 [
55].
Taking the aspects outlined above into account when monitoring will increase the likelihood that data of adequate quality will be collected to summarize restoration outcome with sufficient precision. The Before–After–Control–Impact (BACI) monitoring design [
47,
48] coupled with stratified random sampling [
56], is often appropriate, especially when data need to be gathered over a long period to inform future generalised monitoring strategies [
56]. Variations of the BACI design exist but have inherent assumptions which must be recognised. For example, in the absence of controls, Before–After studies must collect information for long enough before and after restoration to allow the quantification of natural variability (e.g., high and low flows); this has become known as the “intensively monitored watershed” [
49]. In the absence of “Before” information, assumptions have been made that Control and Impact sites were of comparable condition before any restoration intervention. To address this assumption extensive post-treatment (EPT; [
49]) monitoring has been undertaken to attempt to adequately quantify spatial variability. A related alternative design is the Before–After–Gradient (BAG) approach. This allows determination of the spatial (i.e., lateral and longitudinal) and temporal extent of change associated with restoration and improves statistical power over control–impact and BACI designs by incorporating distance as an independent variable in the analysis [
57,
58]. The purpose of the controls used in a monitoring programme must be considered. A positive control (often described in experimental design as the treatment control) represents the best example of what the restoration work is trying to achieve, providing some indication of how a restoration site is changing to meet an ideal. A negative control represents the pre-restoration state of a site and provides a baseline against which change in a restored site can be compared. In the absence of control sites, establishing whether any observed changes are due to restoration or other environmental changes is difficult [
59]. However, control site selection will unavoidably entail compromise, and understanding the aspects that differ from the restoration site is essential in order to interpret BACI results.
Monitoring strategy—recommendations:Follow a Before–After–Control–Impact (BACI) approach. Controls can be positive (the best example of what the restoration work is trying to achieve), negative (the state of a restoration site before the restoration intervention), or a combination of both. Base evaluations on representative data gathered over an appropriate length of time and at spatial scales relevant to the change of interest (i.e., physical, biological, and ecological). Identify factors causing variability in the system (catchment context) to help identify suitable indicators of interest; this includes consideration of multiple pressures. Collect pre-restoration data to quantify baseline variability and ensure that appropriate target outcomes are set. Three years of pre-intervention data is a pragmatic choice, but multiple control sites can help give an understanding of background spatial variability. Ensure post-restoration monitoring design is appropriate for the the life histories of the target organisms.
|
6. Analysis
Biological data can be analysed and summarized using both taxonomic (e.g., species richness) and trait-based (e.g., functional richness) approaches. The consideration of functional traits (inherent biological characteristics) is often ignored in post-project appraisals [
102], yet, it could help elucidate the mechanistic understanding of biotic responses to restoration [
100,
102], Studies and reviews have shown that the effect of restoration on community structure, traits, and functional indicators can be more pronounced than the effect on taxonomic composition or diversity [
80,
102]. Whereas others have found that functional outcomes are harder to achieve, e.g., [
9], take longer, or that neither were achieved [
103].
The two most common approaches to the analysis of biological community data collected from restoration programmes are model-based and algorithm-based. A model-based approach results in a statistical model, based on the observed data. With the potential to separate the signal from the noise (error), statistical approaches may be better suited to answering well-defined ecological questions. Model-based approaches (e.g., linear regression methods) provide the framework for prediction (i.e., for a unit increase in x there is a change in y) and can be used to quantify a priori expectations of restoration action (e.g., [
104]). An algorithm-based approach uses summary statistics (e.g., pair-wise measures of dissimilarity) generated from the observed data to look for patterns of association [
105]. Algorithm-based approaches (e.g., discriminant analysis) provide measurements of association (i.e., a community of type x is associated with a habitat of type y). Algorithm-based approaches can provide a framework for prediction when broad scale (spatial and/or temporal) information is available against which to compare collected data (e.g., RIVPACS; [
106]). Historically, these two methods were commonly split into multivariate (algorithm-based) and univariate (model-based) methods, but recent advances in the last decade have seen the application of model-based approaches to multivariate data, e.g., [
107]. Modelling observed data directly means important ecological relationships can be directly quantified and predicted [
105], the importance of which, at this early stage of understanding the biota’s response to restoration intervention, cannot be overstated.
Analysis—recommendations:Consult a statistician as early as possible, ideally at the project and appraisal planning stage. Identify which modelling approach (model-based or algorithm-based) will provide the appropriate answers to the project. Assess results in terms of both taxonomic and functional response.
|
7. Timescales of Recovery
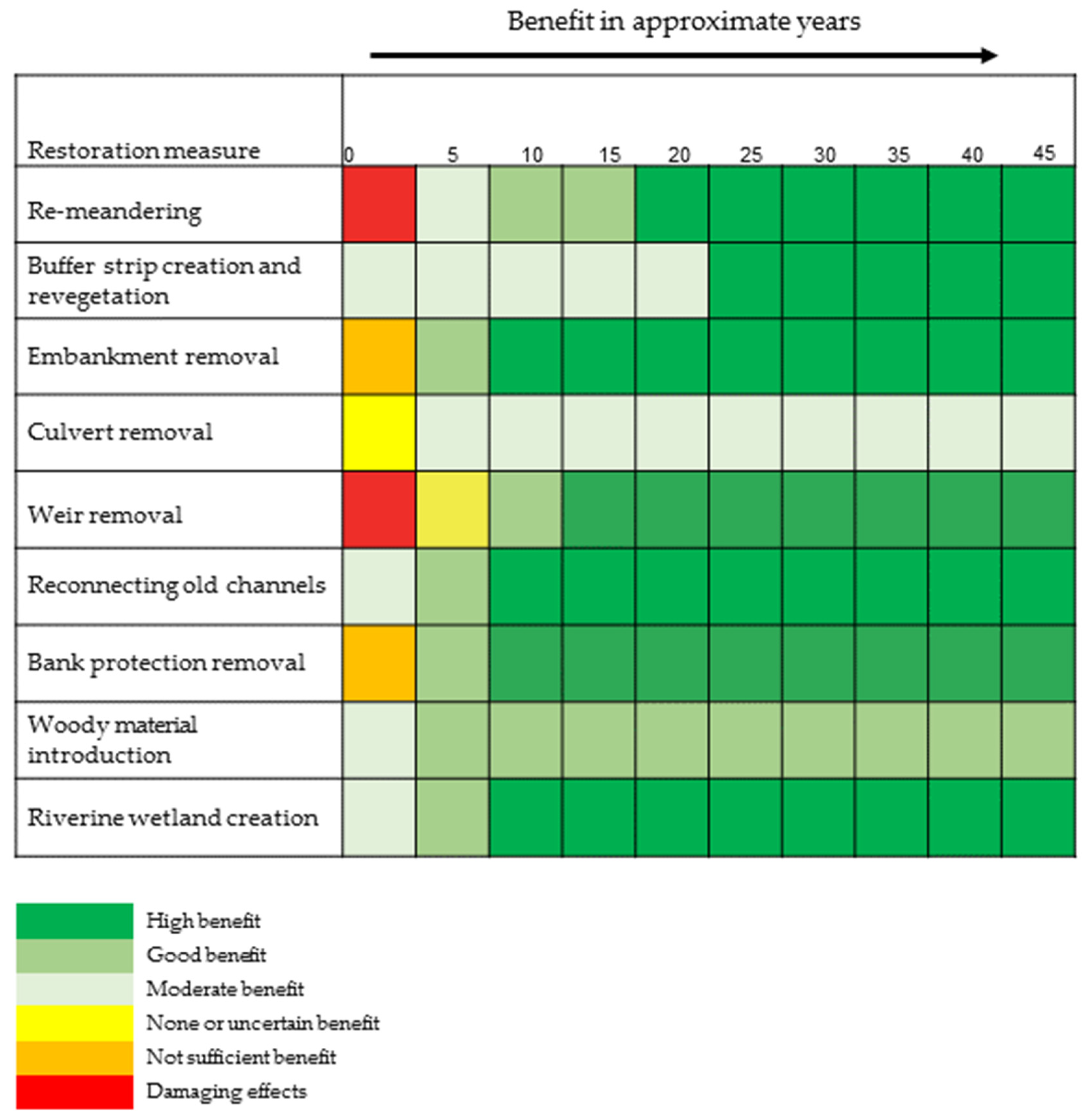
Post-restoration recovery time is influenced by a number of factors including the type of restoration activity (
Figure 3), morphological response [
108], sources of colonisers [
80,
109], spatial scale of restoration [
110], and the presence of other pressures [
40,
41,
111]. Understanding and anticipating these influences will help identify the appropriate temporal scale for monitoring [
13].
The majority of monitoring and appraisal studies take place over short timescales (<1–3 years), with some involving medium (up to 10 years), and a few much longer commitments (~20 years) [
80,
112]. The short-term nature of most monitoring rarely reflects the timescale of ecosystem recovery [
113,
114]. For example, habitat conditions are likely to temporarily deteriorate following restoration, generally associated with loss of plant cover, which may affect invertebrates and fish populations, which could affect results [
115]. This focus on short-term evaluation means that some relatively ineffective/unsustainable restoration methods may continue to be used [
34]. Long-term monitoring is strongly recommended, ideally for a minimum of 10 years [
29]; more rigorous and longer monitoring will increase the likelihood of accurately detecting changes [
31,
111]. When a pressing operational need to understand the effectiveness of restoration arises, perhaps to inform adaptive management and future restoration measures, shorter-term expectations or indications of restoration success may be incorporated in an appraisal strategy; these will necessitate repeated temporal monitoring and appraisal. In a meta-analysis of salmonid abundance to in-stream restoration structures, 45% of the 211 projects reviewed were monitored only once [
116]. For more accurate and reliable results, restoration evaluation should be a continual activity [
34], although it is recognised that the costs associated are significant.
Where long-term monitoring and assessment is undertaken it is important the interim targets are set and appraised. Recording and publicising interim activities and findings are essential to stimulate public interest, inform discussion, and inspire buy-in to related projects. Reporting on key sightings and monitoring activities is vital to maintain stakeholder support and can be as powerful in securing buy-in as the ‘final’ appraisal results; it can also be a powerful stimulus.
Temporal monitoring—recommendations:Always integrate hydroecological and hydromorphological assessments. Plan for long-term monitoring to allow meaningful knowledge on rates of river recovery following restoration. Plan an appraisal period that is long enough to illustrate the ecological response to restoration. The length of time needed will vary by group or species. Collect repeat information at similar times (ideally identical) in the year to avoid adding variability (i.e., error) to the data. Consider life history and seasonal trends when monitoring specific species and/or communities.
|
9. Key Messages/Conclusions
Including monitoring and appraisal in a restoration scheme will demonstrate project effectiveness, allow an adaptive management approach, identify needs for further restoration work, and contribute to the wider evidence base. Appraisals should aim to assess the relationships between physical changes and ecological responses; this will necessitate sequential monitoring from physical change to biological response, and will enable the telling of a clear story about the evidence basis of river restoration.
Such approaches will not be possible everywhere but should instead be directed to where they will provide the greatest evidence [
53], such as ‘flagship’ or demonstration restoration sites for detailed long-term (>5 years) monitoring [
5]. A flagship approach would provide the robust evidence needed to understand what restoration works where, and the knowledge gained could be incorporated in conservation practice [
156]. Understanding which restoration measures work in which catchment contexts will enable them to be applied more widely with greater confidence and reduce the need for further appraisal.