Kinetics, Isotherms and Adsorption–Desorption Behavior of Phosphorus from Aqueous Solution Using Zirconium–Iron and Iron Modified Biosolid Biochars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of BC
2.3. Synthesis of Zr–BC, Zr–FeBC and Fe–BC
2.4. Batch Adsorption Setup: pH, Kinetics and Isotherms
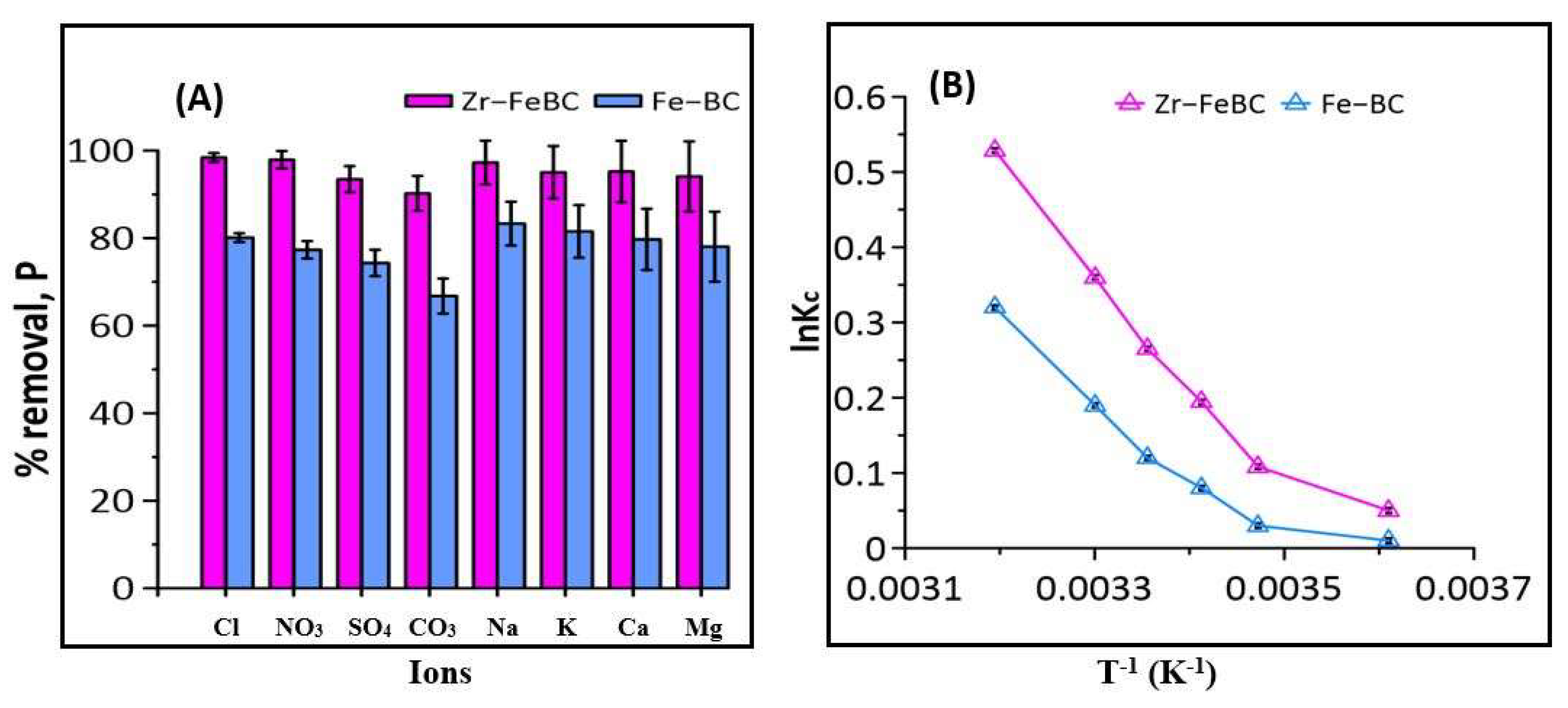
2.5. Effect of Major Anions and Cations on P Adsorption
2.6. Effect of Ionic Strength and Thermodynamics Studies
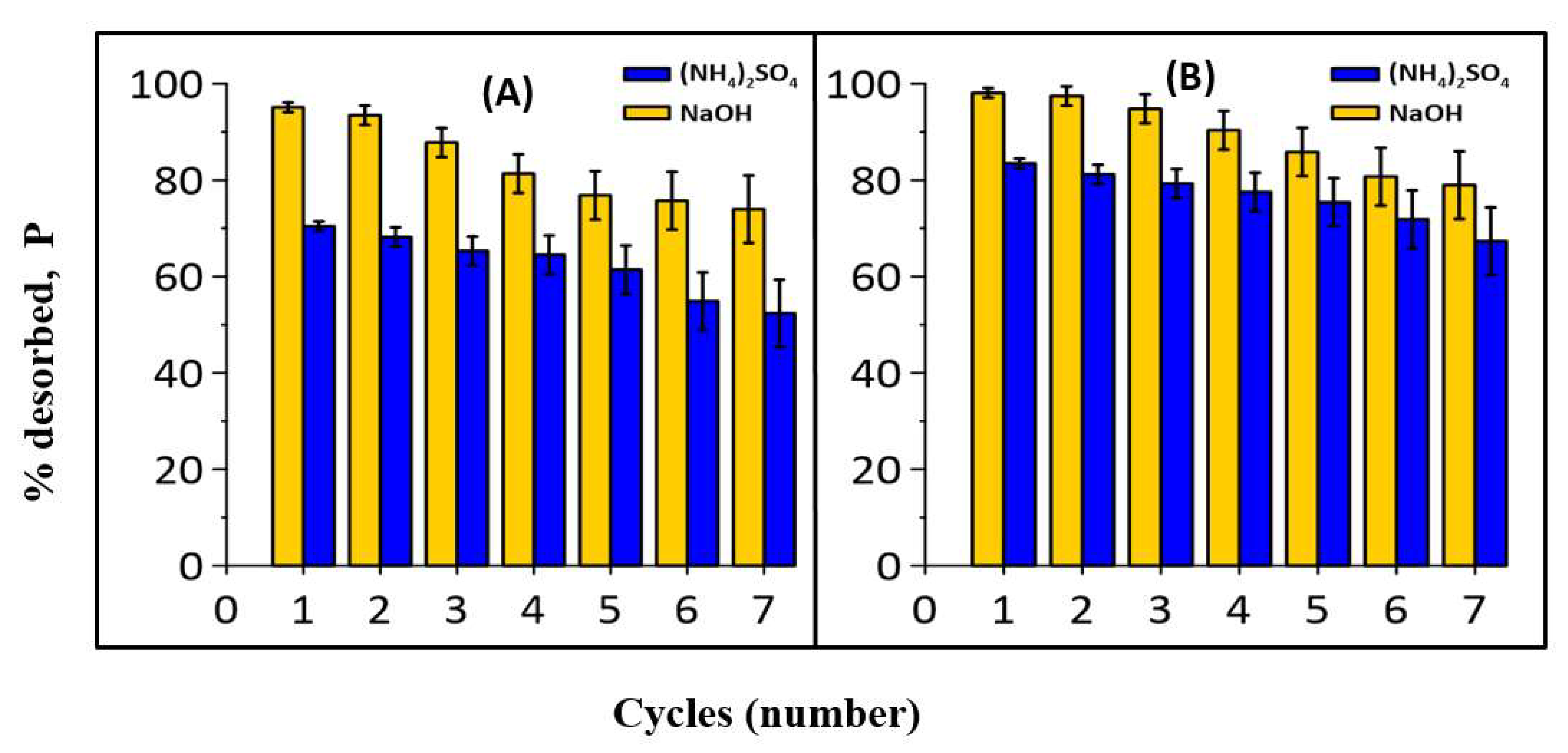
2.7. Desorption Study
3. Results and Discussion
3.1. Batch Experiments: P adsorption on Biochars
3.1.1. Influence of pH
3.1.2. Adsorption Kinetics and Fitted Model
3.1.3. Influence of Initial P Concentration and Isotherms Study
3.1.4. Effect of Biochar Concentration and Ionic Strength
3.1.5. Effect of Co-Existing Ions and Thermodynamic Study
3.2. Desorption and Reusability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, J.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total Value of Phosphorus Recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; da Silva, M.L.C.P. An investigation of phosphate adsorption from aqueous solution onto hydrous niobium oxide prepared by co-precipitation method. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 191–196. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Li, D. Adsorptive removal of phosphate from water using mesoporous materials: A review. J. Environ. Manag. 2017, 193, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Kunhikrishnan, A.; Rahman, A.; Lamb, D.; Bolan, N.S.; Saggar, S.; Surapaneni, A.; Chen, C. Rare earth elements (REE) for the removal and recovery of phosphorus: A review. Chemosphere 2022, 286, 131661. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.; Wang, K.; Ding, K.; Sha, M.; Cao, Y.; Kong, F.; Wang, S. Recovery of phosphorus from eutrophic water using nano zero-valent iron-modified biochar and its utilization. Chemosphere 2021, 284, 131391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.-Y.; Yu, J.-X.; Li, H.-X.; Chi, R.-A. Removal of phosphate from aqueous solution by ferrihydrite/bagasse composite prepared through in situ precipitation method. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125144. [Google Scholar] [CrossRef]
- Yang, G.; Wang, D.; Yang, Q.; Zhao, J.; Liu, Y.; Wang, Q.; Zeng, G.; Li, X.; Li, H. Effect of acetate to glycerol ratio on enhanced biological phosphorus removal. Chemosphere 2018, 196, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Vanotti, M.; Dube, P.; Szogi, A.; González, M.C.G. Recovery of ammonia and phosphate minerals from swine wastewater using gas-permeable membranes. Water Res. 2017, 112, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhang, D.; Li, J.; Guo, G.; Tang, S. Phosphate recovery from swine wastewater using plant ash in chemical crystallization. J. Clean. Prod. 2017, 168, 338–345. [Google Scholar] [CrossRef]
- Bui, T.H.; Hong, S.P.; Yoon, J. Development of nanoscale zirconium molybdate embedded anion exchange resin for selective removal of phosphate. Water Res. 2018, 134, 22–31. [Google Scholar] [CrossRef]
- Wang, F.; Fu, R.; Lv, H.; Zhu, G.; Lu, B.; Zhou, Z.; Wu, X.; Chen, H. Phosphate Recovery from Swine Wastewater by a Struvite Precipitation Electrolyzer. Sci. Rep. 2019, 9, 8893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Lin, J.; He, S.; Wang, X.; Zhang, H.; Zhan, Y. Removal of phosphate from aqueous solution by a novel Mg (OH) 2/ZrO2 composite: Adsorption behavior and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 301–314. [Google Scholar] [CrossRef]
- Nagoya, S.; Nakamichi, S.; Kawase, Y. Mechanisms of phosphate removal from aqueous solution by zero-valent iron: A novel kinetic model for electrostatic adsorption, surface complexation and precipitation of phosphate under oxic conditions. Sep. Purif. Technol. 2019, 218, 120–129. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, D.; Kong, Z.; He, L.; Guan, Q.; Ning, P. Strong Immobilization of Phosphate in Wastewater onto the Surface of MgO-Modified Industrial Hemp-Stem-Driven Biochar by Flowerlike Crystallization. Ind. Eng. Chem. Res. 2020, 59, 14578–14586. [Google Scholar] [CrossRef]
- Su, Y.; Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res. 2013, 47, 5018–5026. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Maschio, L.J.; Coppio, L.D.S.C.; Thim, G.P.; Da Silva, M.L.C.P. Adsorption of phosphate from aqueous solution by hydrous zirconium oxide. Environ. Technol. 2012, 33, 1345–1351. [Google Scholar] [CrossRef]
- Du, W.; Li, Y.; Xu, X.; Shang, Y.; Gao, B.; Yue, Q. Selective removal of phosphate by dual Zr and La hydroxide/cellulose-based bio-composites. J. Colloid Interface Sci. 2019, 533, 692–699. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Abou Oualid, H.; Hsini, A.; El Ibrahimi, B.; Laabd, M.; El Ouardi, M.; Giácoman-Vallejos, G.; Gamero-Melo, P. Synthesis of zirconium-modified Merlinoite from fly ash for enhanced removal of phosphate in aqueous medium: Experimental studies supported by Monte Carlo/SA simulations. Chem. Eng. J. 2020, 404, 126600. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Tang, H.; Jin, Z.; Mao, Y.; Huang, T. Removal of phosphate from wastewater by modified bentonite entrapped in Ca-alginate beads. J. Environ. Manag. 2020, 260, 110130. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Removal of phosphate anions using the modified chitosan beads: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, M.; Wang, W.; Han, R. Phosphate Adsorption from Solution by Zirconium-Loaded Carbon Nanotubes in Batch Mode. J. Chem. Eng. Data 2019, 64, 2849–2858. [Google Scholar] [CrossRef]
- Xi, H.; Li, Q.; Yang, Y.; Zhang, J.; Guo, F.; Wang, X.; Xu, S.; Ruan, S. Highly effective removal of phosphate from complex water environment with porous Zr-bentonite alginate hydrogel beads: Facile synthesis and adsorption behavior study. Appl. Clay Sci. 2021, 201, 105919. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, X.; Zhang, F.; Huang, Y.; Pan, J. A novel Fe–La-doped hierarchical porous silica magnetic adsorbent for phosphate removal. RSC Adv. 2016, 6, 87808–87819. [Google Scholar] [CrossRef]
- Gypser, S.; Hirsch, F.; Schleicher, A.; Freese, D. Impact of crystalline and amorphous iron- and aluminum hydroxides on mechanisms of phosphate adsorption and desorption. J. Environ. Sci. 2018, 70, 175–189. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; He, F.; Tang, J.; Li, D.; Zhu, Y.; Zhang, Y. Effective phosphate adsorption by Zr/Al-pillared montmorillonite: Insight into equilibrium, kinetics and thermodynamics. Appl. Clay Sci. 2015, 104, 252–260. [Google Scholar] [CrossRef]
- Ogata, F.; Iijima, S.; Toda, M.; Otani, M.; Nakamura, T.; Kawasaki, N. Characterization and Phosphate Adsorption Capability of Novel Nickel–Aluminum–Zirconium Complex Hydroxide. Chem. Pharm. Bull. 2020, 68, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, P.; Sirajudheen, P.; Nikitha, M.R.; Meenakshi, S. Removal of phosphate and nitrate via a zinc ferrite@activated carbon hybrid composite under batch experiments: Study of isotherm and kinetic equilibriums. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100378. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, R.; Wang, L.; Xue, K.; Chen, J. Strong adsorption of phosphate from aqueous solution by zirconium-loaded Ca-montmorillonite. Appl. Clay Sci. 2020, 192, 105638. [Google Scholar] [CrossRef]
- Van Tuyen, T.; Phuong, N.T.M.; Le Phuong, H.; Nguyen, T.V.; Ha, L.; Vu, X.H.; Pham, T.; Nguyen, T.N.; Quang, N.; Nguyen, X. Phosphate Adsorption by Silver Nanoparticles-Loaded Activated Carbon derived from Tea Residue. Sci. Rep. 2020, 10, 3634. [Google Scholar]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef]
- Rahman, A.; Lamb, D.; Rahman, M.M.; Bahar, M.; Sanderson, P.; Abbasi, S.; Bari, A.F.; Naidu, R. Removal of arsenate from contaminated waters by novel zirconium and zirconium-iron modified biochar. J. Hazard. Mater. 2021, 409, 124488. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.K.; Biswas, B.; Naidu, R.; Rahman, M.M. Mechanistic insights of hexavalent chromium remediation by halloysite-supported copper nanoclusters. J. Hazard. Mater. 2022, 421, 126812. [Google Scholar] [CrossRef]
- Deb, A.K.; Biswas, B.; Goswami, N.; Hilder, E.F.; Naidu, R.; Rahman, M.M. Synthesis of environmentally benign ultra-small copper nanoclusters-halloysite composites and their catalytic performance on contrasting azo dyes. Appl. Surf. Sci. 2021, 546, 149122. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef] [Green Version]
- Wendling, L.A.; Blomberg, P.; Sarlin, T.; Priha, O.; Arnold, M. Phosphorus sorption and recovery using mineral-based materials: Sorption mechanisms and potential phytoavailability. Appl. Geochem. 2013, 37, 157–169. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef]
- Muhammad, N.; Nafees, M.; Khan, M.H.; Ge, L.; Lisak, G. Effect of biochars on bioaccumulation and human health risks of potentially toxic elements in wheat (Triticum aestivum L.) cultivated on industrially contaminated soil. Environ. Pollut. 2020, 260, 113887. [Google Scholar] [CrossRef]
- Micháleková-Richveisová, B.; Frišták, V.; Pipíška, M.; Duriska, L.; Moreno-Jiménez, E.; Soja, G. Iron-impregnated biochars as effective phosphate sorption materials. Environ. Sci. Pollut. Res. 2017, 24, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gang, D.D.; Sun, P.; Lian, Q.; Yao, H. Goethite dispersed corn straw-derived biochar for phosphate recovery from synthetic urine and its potential as a slow-release fertilizer. Chemosphere 2021, 262, 127861. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Wang, X.; Ji, X.; Peng, B.; Tan, C.; Huang, X. Phosphate reclaim from simulated and real eutrophic water by magnetic biochar derived from water hyacinth. J. Environ. Manag. 2017, 187, 212–219. [Google Scholar] [CrossRef]
- Jack, J.; Huggins, T.M.; Huang, Y.; Fang, Y.; Ren, Z.J. Production of magnetic biochar from waste-derived fungal biomass for phosphorus removal and recovery. J. Clean. Prod. 2019, 224, 100–106. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B. Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Liao, T.; Li, T.; Su, X.; Yu, X.; Song, H.; Zhu, Y.; Zhang, Y. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal. Bioresour. Technol. 2018, 263, 207–213. [Google Scholar] [CrossRef]
- Ren, Z.; Shao, L.; Zhang, G. Adsorption of Phosphate from Aqueous Solution Using an Iron–Zirconium Binary Oxide Sorbent. Water, Air, Soil Pollut. 2012, 223, 4221–4231. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, G.; Chen, J.P. Adsorptive removal of arsenic from water by an iron–zirconium binary oxide adsorbent. J. Colloid Interface Sci. 2011, 358, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dou, X.; Li, J. Antimony(V) removal from water by iron-zirconium bimetal oxide: Performance and mechanism. J. Environ. Sci. 2012, 24, 1197–1203. [Google Scholar] [CrossRef]
- Rahman, A.; Rahman, M.M.; Bahar, M.; Sanderson, P.; Lamb, D. Antimonate sequestration from aqueous solution using zirconium, iron and zirconium-iron modified biochars. Sci. Rep. 2021, 11, 8113. [Google Scholar] [CrossRef]
- Elias, M.; Wellner, A.; Goldin-Azulay, K.; Chabriere, E.; Vorholt, J.A.; Erb, T.; Tawfik, D.S. The molecular basis of phosphate discrimination in arsenate-rich environments. Nat. Cell Biol. 2012, 491, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Rahman, M.M.; Bahar, M.; Sanderson, P.; Lamb, D. Transformation of Antimonate at the Biochar–Solution Interface. ACS ES&T Water 2021, 1, 2029–2036. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chuang, Y.-H.; Chen, T.-Y.; Tian, Y.; Li, H.; Wang, M.-K.; Zhang, W. Mechanism of Arsenic Adsorption on Magnetite Nanoparticles from Water: Thermodynamic and Spectroscopic Studies. Environ. Sci. Technol. 2015, 49, 7726–7734. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Saqib, Z.; Nawaz, M.F.; Shaheen, S.M.; Wang, H.; Tsang, D.; Bundschuh, J.; et al. Exploring the arsenic removal potential of various biosorbents from water. Environ. Int. 2019, 123, 567–579. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, Z.; Sun, Y.; Cao, X.; Hou, D.; Alessi, D.S.; Ok, Y.S.; Tsang, D.C. Unraveling iron speciation on Fe-biochar with distinct arsenic removal mechanisms and depth distributions of As and Fe. Chem. Eng. J. 2021, 425, 131489. [Google Scholar] [CrossRef]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.-C.; Lo, I.M.C. Selective Phosphate Removal from Water and Wastewater using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol. 2020, 54, 50–66. [Google Scholar] [CrossRef]
- Van Truong, T.; Kim, D.-J. Phosphate removal using thermally regenerated Al adsorbent from drinking water treatment sludge. Environ. Res. 2021, 196, 110877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, F.; Yu, Z.; Wei, J.; Yang, Z.; Ma, C.; Li, Z.; Xu, Z.; Zeng, G. Performance of magnetic zirconium-iron oxide nanoparticle in the removal of phosphate from aqueous solution. Appl. Surf. Sci. 2017, 396, 1783–1792. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, M.; Mortimer, R.J.G.; Pan, G. Enhanced Phosphorus Locking by Novel Lanthanum/Aluminum–Hydroxide Composite: Implications for Eutrophication Control. Environ. Sci. Technol. 2017, 51, 3418–3425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Sun, X.; Yin, C.; Hu, C. Removal of phosphate by mesoporous ZrO2. J. Hazard. Mater. 2008, 151, 616–622. [Google Scholar] [CrossRef]
- Tran, D.N.H.; Kabiri, S.; Wang, L.; Losic, D. Engineered graphene–nanoparticle aerogel composites for efficient removal of phosphate from water. J. Mater. Chem. A 2015, 3, 6844–6852. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; da Silva, M.L.C.P. Adsorption kinetic, thermodynamic and desorption studies of phosphate onto hydrous niobium oxide prepared by reverse microemulsion method. Adsorption 2010, 16, 173–181. [Google Scholar] [CrossRef]
- Tang, Y.; Zong, E.; Wan, H.; Xu, Z.; Zheng, S.; Zhu, D. Zirconia functionalized SBA-15 as effective adsorbent for phosphate removal. Microporous Mesoporous Mater. 2012, 155, 192–200. [Google Scholar] [CrossRef]
- Vu, M.T.; Nguyen, L.N.; Johir, A.H.; Ngo, H.H.; Skidmore, C.; Fontana, A.; Galway, B.; Bustamante, H.; Nghiem, L.D. Phosphorus removal from aqueous solution by steel making slag—Mechanisms and performance optimisation. J. Clean. Prod. 2021, 284, 124753. [Google Scholar] [CrossRef]
- Oginni, O.; Yakaboylu, G.A.; Singh, K.; Sabolsky, E.M.; Unal-Tosun, G.; Jaisi, D.; Khanal, S.; Shah, A. Phosphorus adsorption behaviors of MgO modified biochars derived from waste woody biomass resources. J. Environ. Chem. Eng. 2020, 8, 103723. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X. Kinetics and Thermodynamics of Efficient Phosphorus Removal by a Composite Fiber. Appl. Sci. 2019, 9, 2220. [Google Scholar] [CrossRef] [Green Version]
- Guerra, A.A.A.M.; Campos, A.F.C.; de Lima, R.M.; Kern, C.; da Silva, F.G.; Gomide, G.; Depeyrot, J.; Amorim, A.K.B. Efficient uptake of phosphorus from water by core@shell bimagnetic nanoadsorbents. J. Environ. Chem. Eng. 2020, 8, 103888. [Google Scholar] [CrossRef]
- Salameh, Y.; Albadarin, A.; Allen, S.; Walker, G.; Ahmad, M. Arsenic(III,V) adsorption onto charred dolomite: Charring optimization and batch studies. Chem. Eng. J. 2015, 259, 663–671. [Google Scholar] [CrossRef]
- Aryee, A.A.; Dovi, E.; Shi, X.; Han, R.; Li, Z.; Qu, L. Zirconium and iminodiacetic acid modified magnetic peanut husk as a novel adsorbent for the sequestration of phosphates from solution: Characterization, equilibrium and kinetic study. Colloids Surfaces A: Physicochem. Eng. Asp. 2021, 615, 126260. [Google Scholar] [CrossRef]
- Goldberg, S.; Johnston, C. Mechanisms of Arsenic Adsorption on Amorphous Oxides Evaluated Using Macroscopic Measurements, Vibrational Spectroscopy, and Surface Complexation Modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef]
- Wu, B.; Fang, L.; Fortner, J.D.; Guan, X.; Lo, I.M. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La (OH) 3/Fe3O4 nanocomposites. Water Res. 2017, 126, 179–188. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Guo, J.; Zhang, L. Removal of phosphate from wastewater by lanthanum modified bioceramisite. J. Environ. Chem. Eng. 2021, 9, 106123. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Sun, Y.; Chen, Y.; Qian, J. La(OH)3 loaded magnetic mesoporous nanospheres with highly efficient phosphate removal properties and superior pH stability. Chem. Eng. J. 2019, 360, 342–348. [Google Scholar] [CrossRef]
- Ahmed, S.; Lo, I.M. Phosphate removal from river water using a highly efficient magnetically recyclable Fe3O4/La(OH)3 nanocomposite. Chemosphere 2020, 261, 128118. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Klementiev, K.; Hassenkam, T.; Qian, W.; Ai, J.; Hansen, H.C.B. High affinity lanthanum doped iron oxide nanosheets for phosphate removal. Chem. Eng. J. 2021, 422, 130009. [Google Scholar] [CrossRef]
- Shirazinezhad, M.; Faghihinezhad, M.; Baghdadi, M.; Ghanbari, M. Phosphate removal from municipal effluent by a porous MgO-expanded graphite composite as a novel adsorbent: Evaluation of seawater as a natural source of magnesium ions. J. Water Process. Eng. 2021, 43, 102232. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, D.; Zhang, H.; Lu, S.; Chen, L.; Yu, X. Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions. J. Hazard. Mater. 2010, 183, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.N. Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents. J. Hazard. Mater. 2010, 184, 538–546. [Google Scholar] [CrossRef]
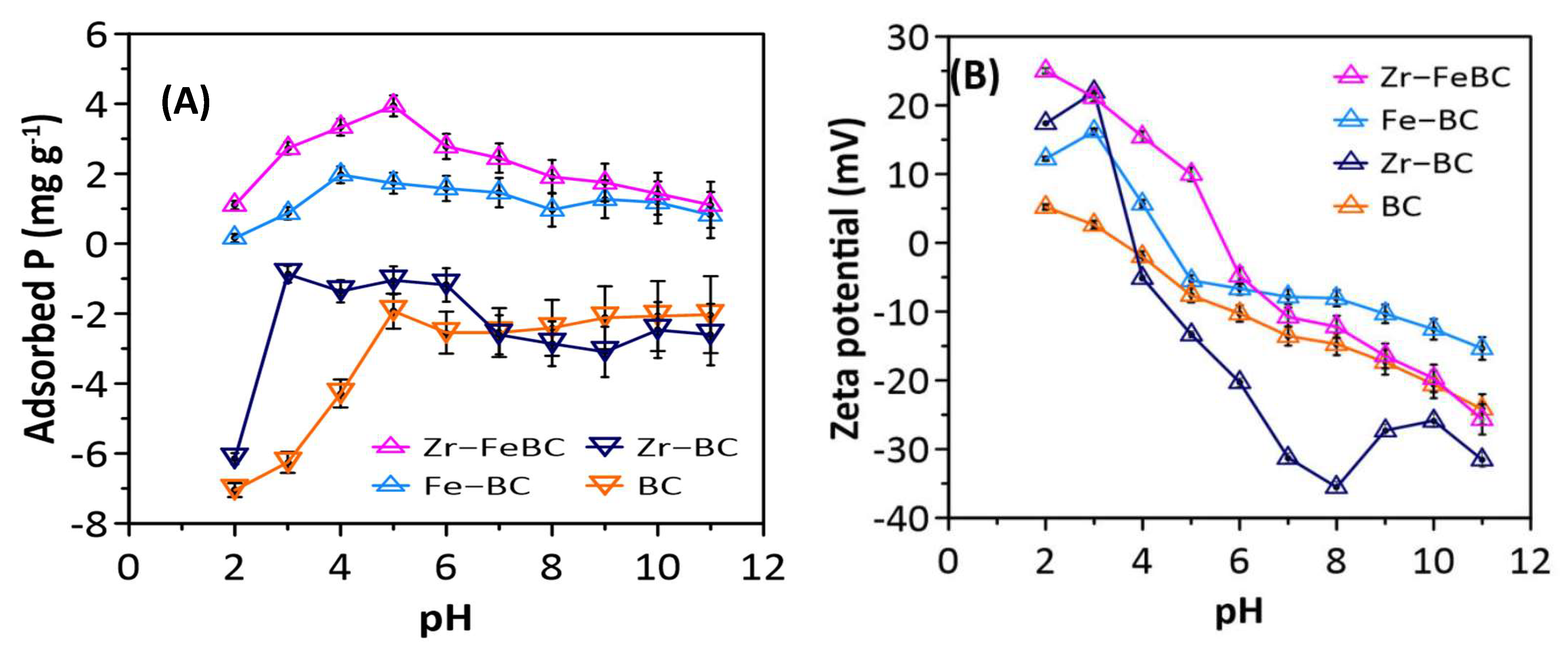
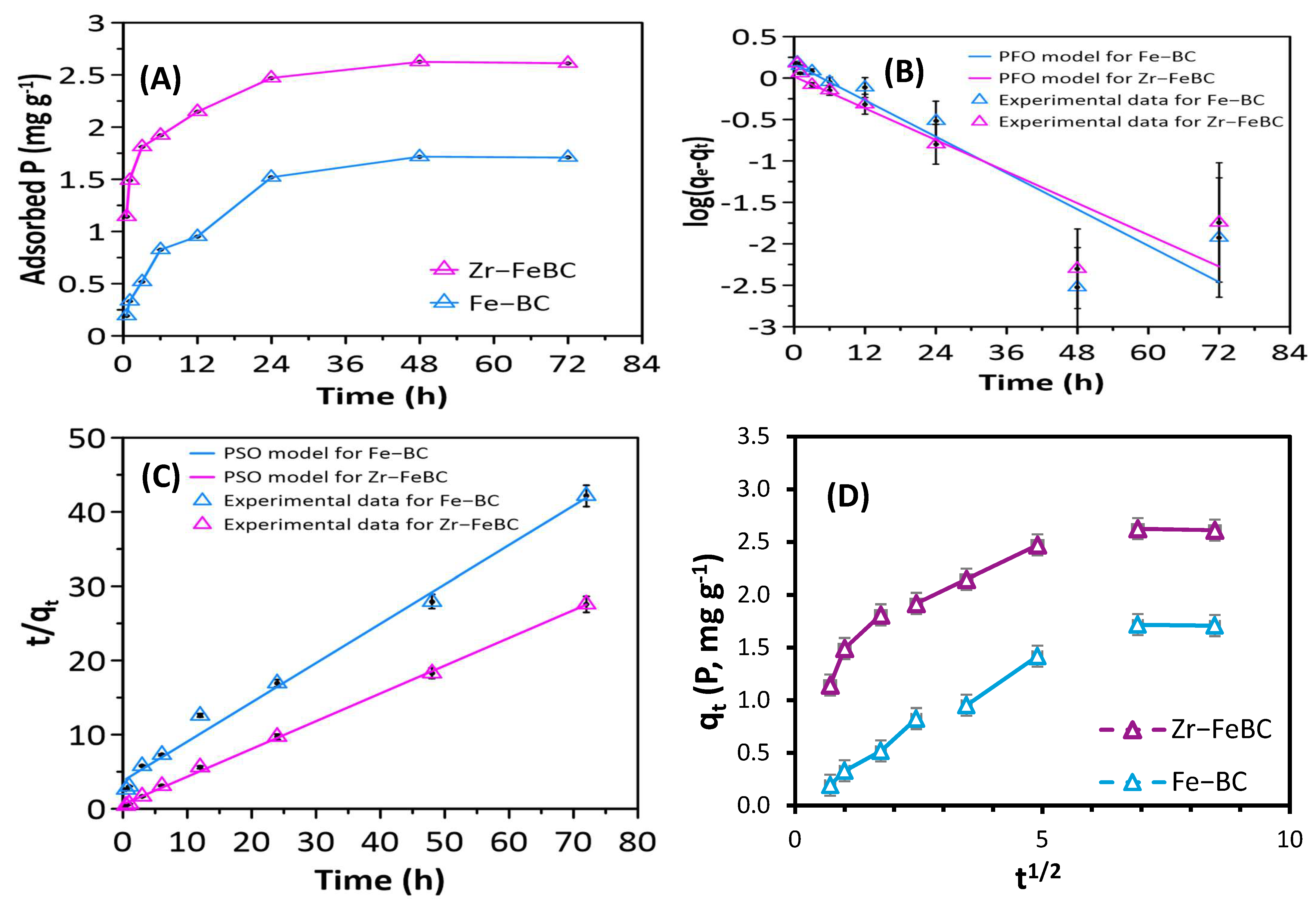

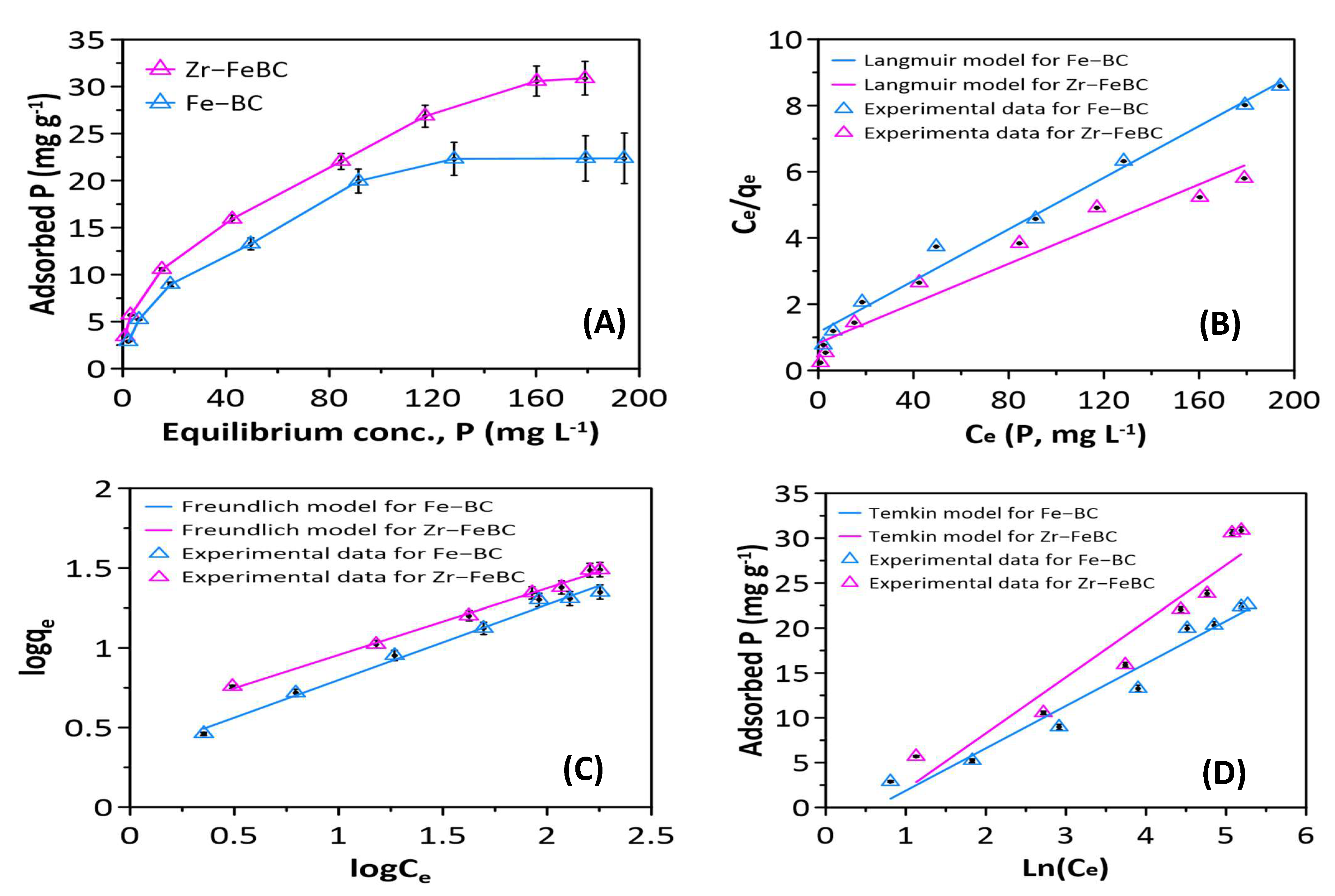
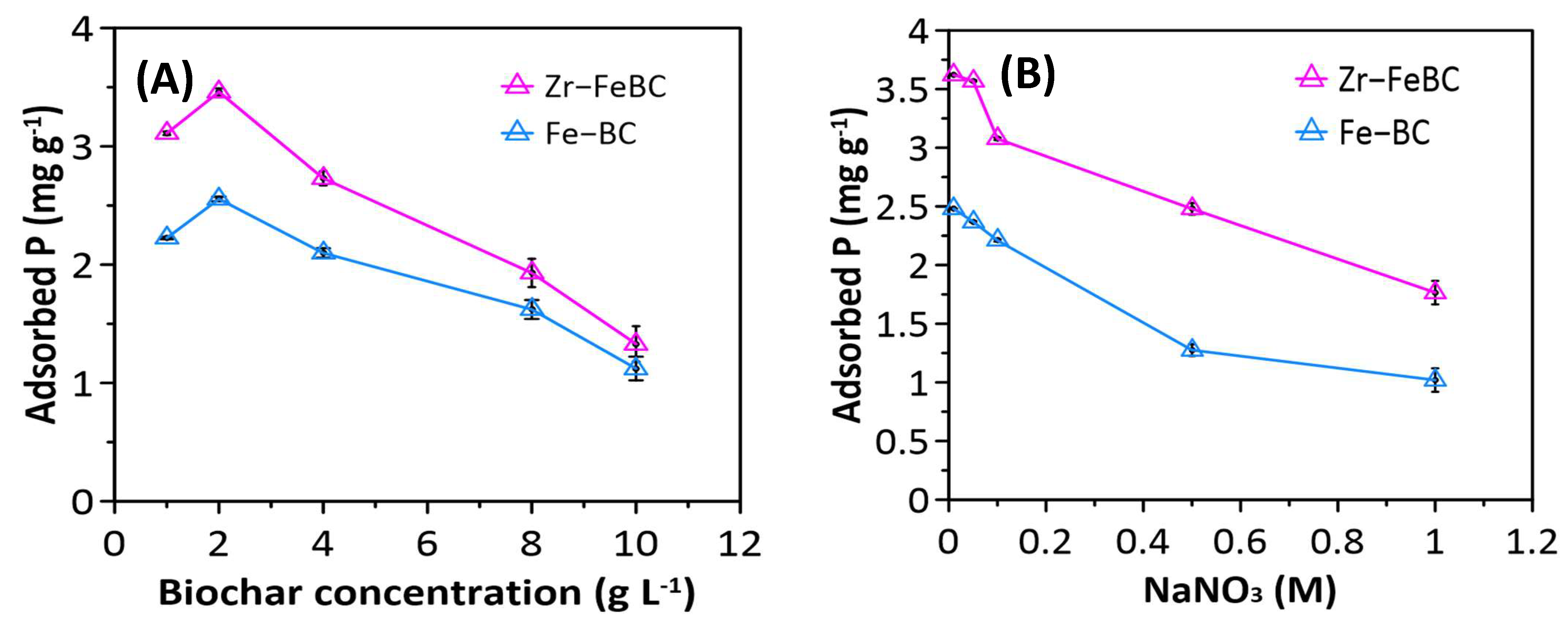
| Biochar | Specific Surface Area (BET), (m2/g) | Pore Volume (cm3/g) | Pore Size/ Diameter (nm) | Average Particle Size (nm) | pHPZC | EC (mS/cm) | pH | CEC (cmol(+)/kg) | |
|---|---|---|---|---|---|---|---|---|---|
| In H2O | In CaCl2 | ||||||||
| BC | 4.64 ± 0.1 | 0.006 ± 0.002 | 6.51 ± 1.41 | 1292 ± 76 | 3.6 ± 0.15 | 0.32 ± 0.09 | 7.12 ± 0.12 | 5.98 ± 0.13 | 8.1 ± 0.61 |
| Zr–BC | 75.85 ± 2.73 | 0.06 ± 0.005 | 3.37 ± 1.01 | 79 ± 10 | 3.8 ± 0.1 | 10.34 ± 1.15 | 8.97 ± 0.14 | 8.37 ± 0.15 | 6.24 ± 0.68 |
| Zr–FeBC | 25.51 ± 2.81 | 0.027 ± 0.003 | 3.92 ± 0.21 | 235 ± 32 | 5.7 ± 0.08 | 18.57 ± 1.6 | 5.64 ± 0.12 | 5.17 ± 0.11 | 5.63 ± 0.57 |
| Fe–BC | 24.02 ± 2.17 | 0.019 ± 0.002 | 4.80 ± 0.46 | 249 ± 12 | 4.5 ± 0.09 | 20.54 ± 2.27 | 5.88 ± 0.13 | 5.45 ± 0.16 | 5.06 ± 0.46 |
| Biochar | qe-exp (mg g−1) | Pseudo First-Order | Pseudo Second-Order | Elovich | Intraparticle Diffusion | pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 (h−1) | qe-cal | R2 | k2 (g mg−1.h−1) | qe-cal (mg g−1) | h (mg g−1.h−1) | R2 | β (mg g−1) | α (mg g−1.h−1) | R2 | kid (g mg−1 h−1/2) | C (mg g−1) | R2 | |||
| Zr–FeBC | 2.63 | 0.07 | 0.93 | 0.83 | 0.24 | 2.67 | 1.68 | 0.99 | 1.48 | 0.28 | 0.98 | 0.17 | 1.38 | 0.85 | 5 |
| Fe–BC | 1.72 | 0.08 | 0.92 | 0.84 | 0.07 | 1.88 | 0.26 | 0.99 | 0.21 | 0.36 | 0.96 | 0.21 | 0.19 | 0.94 | 4 |
| Biochar | qexp (mg g−1) | Langmuir Model Parameters | Freundlich Model Parameters | Temkin Model Parameters | pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qcal (mg g−1) | qm (mg g−1) | KL (L mg−1) | RL | R2 | qcal (mg g−1) | KF (g mg−1.h−1) | 1/n | R2 | b (J mol−1) | A (L g−1) | R2 | |||
| Zr–FeBC | 30.89 | 28.89 | 33.33 | 0.04 | 0.1–0.75 | 0.95 | 30.16 | 3.45 | 0.42 | 0.99 | 392 | 0.51 | 0.92 | 5 |
| Fe–BC | 22.62 | 22.31 | 25.71 | 0.03 | 0.11–0.76 | 0.99 | 24.74 | 2.17 | 0.46 | 0.99 | 519 | 0.55 | 0.96 | 4 |
| Biochar | ∆G (kJ mol−1) | ∆H (kJ mol−1) | ∆S (kJ mol−1 K−1) | pH | |||||
|---|---|---|---|---|---|---|---|---|---|
| 277 K | 288 K | 293 K | 298 K | 303 K | 313 K | ||||
| Zr–FeBC | −0.67 | −1.11 | −1.79 | −2.31 | −2.77 | −3.38 | 26.53 | 0.25 | 5 |
| Fe–BC | −0.53 | −0.97 | −1.22 | −1.45 | −1.95 | −2.47 | 18.27 | 0.14 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Lamb, D.; Kunhikrishnan, A.; Rahman, M.M. Kinetics, Isotherms and Adsorption–Desorption Behavior of Phosphorus from Aqueous Solution Using Zirconium–Iron and Iron Modified Biosolid Biochars. Water 2021, 13, 3320. https://doi.org/10.3390/w13233320
Rahman MA, Lamb D, Kunhikrishnan A, Rahman MM. Kinetics, Isotherms and Adsorption–Desorption Behavior of Phosphorus from Aqueous Solution Using Zirconium–Iron and Iron Modified Biosolid Biochars. Water. 2021; 13(23):3320. https://doi.org/10.3390/w13233320
Chicago/Turabian StyleRahman, Md. Aminur, Dane Lamb, Anitha Kunhikrishnan, and Mohammad Mahmudur Rahman. 2021. "Kinetics, Isotherms and Adsorption–Desorption Behavior of Phosphorus from Aqueous Solution Using Zirconium–Iron and Iron Modified Biosolid Biochars" Water 13, no. 23: 3320. https://doi.org/10.3390/w13233320
APA StyleRahman, M. A., Lamb, D., Kunhikrishnan, A., & Rahman, M. M. (2021). Kinetics, Isotherms and Adsorption–Desorption Behavior of Phosphorus from Aqueous Solution Using Zirconium–Iron and Iron Modified Biosolid Biochars. Water, 13(23), 3320. https://doi.org/10.3390/w13233320








