Abstract
Occupational enrichment (OE) is directed at introducing variations in the tank water so that fish can exercise as they do in the wild. Two trials were carried out to test the effects of randomly fired underwater currents (RFC) on rainbow trout (Oncorhynchus mykiss) maintained in tanks in a recirculation system, using 1226 trout distributed in two independent trials. In Trial 1, fingerling trout (n = 6 tanks, n = 40 fish per tank) were classified into two groups based on low (13%) or high (30%) coefficient of variation in live weight (CV), and exposed to RFC or no currents (controls). In Trial 2, adult trout (n = 12 tanks, n = 20 fish per tank) were either exposed to RFC or to a constant current (controls) from two submerged pumps. Both trials lasted four weeks. No significant differences in growth were observed between treatments in either trial. In Trial 1, RFC fish maintained a similar CV throughout the trial, while CV decreased in controls. Also, in Trial 1, plasma cortisol levels were higher and creatine phosphokinase (CPK) levels lower in tanks with a low initial CV. In Trial 2, the CV was lower in RFC trout, where cortisol levels were also significantly lower and triglycerides significantly higher. The results suggest that OE using RFC can have positive effects by helping to reduce stress levels, and provides fish with biologically meaningful environmental enrichment related to the natural history of the species.
1. Introduction
Rainbow trout (Oncorhynchus mykiss) are native to tributaries of the Pacific Ocean in Asia and North America, and have been introduced worldwide for sport fishing and commercial aquaculture [1]. Currently, they are one of the most largely farmed fish species worldwide (>848,000 t y), the biggest in the European (185,316 t) region and the second most common species produced in Spain (18,955 t) [2]. Salmonids evolved in natural systems with considerable environmental complexity and, as such, can exhibit numerous behavioral options in response to environmental challenges [3]. However, environmental complexity is almost totally absent in many artificial rearing environments, such as aquaculture tanks, ponds and raceways [4]. Additionally, these intensive systems are characterized by a high stocking density with high material and energy inputs, and possible negative impacts on water quality such as eutrophication and oxygen depletion [5]. However, in aquaculture, important efforts are devoted to developing efficient large-scale production while upholding fish welfare [6]. One way of ensuring animal welfare is by using environmental enrichment, a set of techniques used to modify the environment to be suitable and satisfactory for animal behavior by introducing artefacts or improving the breeding system [7]. Environmental enrichment can be classified into several types, e.g., physical enrichment, social enrichment, sensory enrichment and occupational enrichment [8].
Occupational enrichment (OE)’s purpose it to reduce physical and physiological monotony by introducing variation in the tank and providing opportunities for fish to exercise and perform preferred behaviors [9]. In this sense, OE can be a useful tool, based on the creation of a dynamic, complex and interactive environment that allows physical and cognitive challenges similar to those an animal would experience in nature [10,11]. Many recent studies have shown that environmental enrichment can reduce fish aggression and anxiety-like behaviors, increase behavioral flexibility and improve spatial learning and memory [8]. Some studies have been performed to enrich the culture environment for salmon in cages [12] and cod [13,14], but this is not a normal commercial practice. Most of the enrichment studies in salmonids have focused on how different structures can affect behavior related to increasing survivability after restocking, i.e., exploratory behavior, spatial leaning abilities, predation and anti-predatory behaviors [15,16]. Some authors have reported positive effects of enrichment, such as improved foraging ability in Atlantic salmon [15,17], reduced response to stress in Atlantic salmon [18] and improved recovery from stressful procedures in rainbow trout [19].
Few studies have assayed other types of enrichment in fish, such as tactile, auditory or visual elements, which would help avoid the possible negative consequences of a structural enrichment, such as the tendency for some territorial fish to defend the new resource [20], or logistical and health problems associated with adding foreign structures or objects into cages. Some types of structures may be difficult to use on a commercial level since they impede movement or get soiled by fouling. To our knowledge, Rodewald et al. [17] is one of the few studies that used OE in salmonids, by varying water currents randomly over time. Those authors found that enrichment improved the foraging behavior of Atlantic salmon parr, but since several types of enrichment were used at once (water current, current direction, water depth and shade), it is hard to isolate the effect of OE alone. Nordgreen et al. [12] found that Atlantic salmon parr often swam in front of water inlets (from a pump), underlining their possible use as enrichment. Both Rodewald et al. [17] and Nordgreen et al. [12] focused on the behavior of the fish in an enriched environment but did not consider physiological indicators of fish welfare. Thus, the aim of the present study was to analyze the effects of randomly fired water currents on physiological stress parameters and growth of rainbow trout.
2. Materials and Methods
The present study was carried out in accordance with the EU Directive 2010/63/EU for animal experiments and the Spanish guidelines for the care and use of animals in research [21].
2.1. General Study Conditions
Our study used 1226 trout distributed in two independent trials. The first trial tested the RFC program in fingerling trout (n = 677) and the second in adult trout (n = 549). Rainbow trout were obtained from Fuente Campillo fish farm located in Cifuentes, Guadalajara, Spain (40.7904 N, −2.1681 W), and transported live to the experimental aquaculture farm of the College of Forestry Engineering, Polytechnic University of Madrid in Madrid, Spain (40.4508 N, −3.7210 W). This experimental fish farm is located on a small slope divided into terraces, where the different raceways for fish housing are located and which have a capacity of 5.16 m3 each (Figure 1). The terraced design allows the water flow to go downwards to be distributed through the different tanks by means of channels. The tanks have a continuous flow of fresh water from a subway well. The tanks are outdoors, so the fish in the two trials were subjected to the natural photoperiod. The average water quality values for all tanks used were: ammonia 0.0034 ± 0.0022 ppm NH3-N, ammonium 0.36 ± 0.22 ppm NH4+-N, nitrite 0.27 ± 0.095 ppm NO2-N, alkalinity 130.66 ± 44.95 ppm CaCO3, carbon dioxide 22.66 ± 16.16 ppm CO2, dissolved oxygen 7.26 ± 1.10 ppm O2, chloride 26.66 ± 6.10 ppm Cl−, hardness 169.66 ± 22.35 ppm CaCO3 and pH 7.63 ± 0.23.

Figure 1.
Different aspects of the adaptation and operation of the tanks for the study: (a) overview of the complete raceway before the study; (b) adaptation of the tank sections; (c) image of the raceway with separations with tanks; (d) underwater image of one of the water pumps used for generating under water currents; and (e) image of one tank.
2.2. Trial 1: RFC Enrichment Program in Fingerling Rainbow Trout
In Trial 1, 677 fingerlings from the commercial farm were used. Fish were collected at the commercial farm and transferred to two fiberglass tanks, each equipped with oxygenation equipment inside the transport vehicle. Both tanks were filled with water from the fish raceways of origin. During transport, the stagnant water was saturated with oxygen through an oxygen diffuser connected by rubber tubes to a liquid oxygen canister. Fish were not sedated during transport and journey time for both trials did not exceed 2 h (130 km). Upon arrival at the experimental farm, approximately 350 fish were housed in each of two raceways for one week to allow them to adapt to the new environment. At the end of the week, they were then fasted for 24 h and weighed individually to separate them into smaller groups. To do that, we placed approximately 30 trout at a time into a 30-L container and anesthetized them using eugenol (1 g/L, pH 7.0). After 2 min, each trout was weighed and their length was measured (fork length). After having separated each raceway into six tanks each, each trout was placed into tanks according to size ranges from less than 10 g to more 30 g, in 5 g intervals. Eleven days later, they were re-stocked into their final tanks (n = 12) in groups of 40 fish each. That was performed in such a way as to form two groups with a high and low coefficient of variation (CV) in live weight (n = 6 tanks with a high CV and n = 6 with a low CV).
The final tanks were set up by separating the original raceway into six separate sections with the same water volume (0.86 m3) using stainless steel separators, allowing the passage of water from one section to another. Two weeks after placement into tanks, and one day before turning on water currents, all trout were weighed individually, but no blood samples were taken since fish were quite small. The next day, two water pumps were placed in half the tanks (1082 EHEIM marine stream on +5000 with a mobilization flow capacity of 5000 liters/h/3600 = 1.39 liters/s) at medium height in opposing corners to move water towards the centre of the tank. The pumps in the RFC tanks were connected to an Arduino UNO computer and programmed as in Trial 1 (see above). The average total weight (±SEM) of fish per tank at the beginning of the trial was 696.41 g ± 6.67, for a stocking density of 0.81 kg/m3. The control tanks had no pumps nor artificial currents. Fish remained in this way for four weeks, being fed twice a day at 3% body weight with a commercial feed (Biomar, the same as the source farm, with 42% crude protein, 23% fat, 4.1% ash and 2.0% crude fibre) and kept at a natural photoperiod. This trial was carried out between 19 April and 18 May 2017 (sunrise/sunset approximately 7 h 30 to 21 h 00 or for 13 h 30 min of daylight). Water temperature was measured every half hour and averaged 18.64 ± 0.03 °C. After four weeks (29 days), all fish were weighed and measured and four fish were blood sampled per tank.
2.3. Trial 2: RFC Enrichment Program in Adult Rainbow Trout
For this trial, we used 549 adult trout, also from the same commercial farm as Trial 1. The transport and logistic conditions of the fish were similar to those of Trial 1. Upon arrival at the experimental farm, the fish were housed in groups of approximately 250 for one week to allow them to adapt to the raceway (total volume 5.16 m 3 each). At the end of the week, fish were then fasted for 24 h and weighed individually to separate them into smaller groups. To do that, we placed 10–15 trout at a time into a 30-L container and anesthetized them using eugenol (1 g/L, pH 7.0). After 2 min, trout were weighed individually and placed into tanks according to size ranges, from 150 g to 450 g, in intervals of 25 g. One week later, they were re-stocked into their final tanks (n = 12) in groups of 20 fish each. That was performed in such a way as to keep a constant coefficient of variation in live weight (CV) in all tanks. The final tanks were set up by separating the original raceway into six separate sections with the same water volume (0.86 m3) using stainless steel separators, allowing the passage of water from one section to another. The average (±SEM) total weight of fish per tank was 5955 g ± 32.1, so the stocking density was 6.9 kg/m3.
Two weeks after placement into tanks, and one day before turning on water currents, a sample of four trout were taken from each tank (n = 12), weighed individually and blood sampled (see description below). The next day two water pumps were placed in all tanks (1082 EHEIM marine stream on +5000) at medium height in opposing corners to move water towards the center of the tank. The pumps in the RFC groups were connected to an Arduino UNO computer which was programmed to run an infinite loop in the sequence: random target pump (A or B), randomly choose functioning time (10, 20, 30, 40, 50 or 60 min) and, after one hour, restart the sequence. The pumps in the control groups were kept on constantly. The fish remained in their tanks for six weeks, being fed twice a day at 1.5% body weight with a commercial feed (Biomar, same as the source farm, with 42% crude protein, 23% fat, 4.1% ash and 2.0% crude fibre) and kept at a natural photoperiod. This trial was carried out between 21 April and 18 May 2016 (sunrise/sunset 7 h 30 to 21 h 00 or for 13 h 30 min of daylight). Water temperature was measured every half hour and averaged 17.02 ± 0.03 °C. After four weeks (27 days), fish were weighed individually and four fish were blood sampled per tank.
2.4. Blood Parameters
Trout were captured with dip nets, from which they were removed and immediately electrically stunned beside the tanks using a V shaped stunner that provided an electric current at 0.4 A, 90 V for 3 s. The process of catching the fish and stunning them took less than 2 min. After that, fish were blood sampled and then killed by severing the spinal cord. Blood samples (approximately 0.5–1.0 mL) were taken from the caudal vein and divided into two Eppendorf tubes, one with sodium fluoride (NaF) for the determination of glucose and lactate dehydrogenase (LDH) and another with ethylenediaminetetraacetic acid (EDTA) as an anticoagulant for cortisol, triglycerides, creatine phosphokinase (CPK), albumin and total proteins. Both tubes were centrifuged at 6000 rpm for 10 min to remove the plasma, and immediately stored at 4 °C until analysis. Cortisol was measured by enzyme immunoassay using a commercial Cortisol EIA well kit (Radim Ibérica S.A., Barcelona, Spain). Plasma concentrations of glucose and triglycerides were determined using reflectance spectrophotometry (Reflotron® System, Roche Diagnostics, Indianapolis, IN, USA). The CPK levels were measured using a Roche/Hitachi 717 Chemistry Analyzer (Roche Diagnostics, S.L., Sant Cugat del Valles, Spain) with Boehringer Mannheim reagents. To evaluate the plasma concentration of albumin and LDH, we used spectrophotometric liquid chemistry techniques (Bromocresol green, lactate) with commercial kits (Randox Diagnostic, London, UK). Total plasma proteins were quantified by refractometry (ATAGo T2-Ne®, Atago, Tokyo, Japan). After slaughter, each fish was weighed and weight gain was calculated as (slaughter weight (g)–initial weight (g)).
2.5. Statistical Analysis
The data were analyzed using SAS software ver. 9.0 (Statistical Analysis System Institute Inc., Cary, NC, USA). A prior analysis of the normality and homogeneity of variance of all variables was performed using the Shapiro–Wilks test with the UNIVARIATE procedure and Bartlett’s test with the ANOVA procedure for residues. We used the GLM procedure of SAS, with current treatment (no currents or RFC for Trial 1; constantly on or RFC for Trial 2) and live weight CV (in Trial 1 only) as the fixed effects. For slaughter weight, we included initial weight in the model as covariate. The Bonferroni test was used for mean comparison (p < 0.05). All averages are reported with the standard error of the mean (±SEM).
3. Results
3.1. Trial 1: RFC Enrichment Program in Fingerling Rainbow Trout
The average initial weight of trout when they first arrived was 15.7 g ± 0.12 (CV = 30.2%, range 2–31 g). After sorting, the tanks with a low initial CV had an average of 13.9% CV, and those with a high initial CV, 30.8% CV. In terms of production indices, there were no initial significant differences in live weight (initial weight for controls 17.7 ± 1.79; 17.1 ± 1.62 for currents; p = 0.1298) nor in final slaughter weight (see Table 1). For high CV trout, mortality was significantly higher but the final CV was lower than initial values. There was a significant interaction between CV type and RFC, since the final CV of high CV fish was lower in the RFC tanks and the final CV of low CV fish was higher in RFC tanks. As a result, all fish in RFC tanks had a similar final CV (Figure 2), while the final CV of fish in the control tanks was still significantly different. The haematological data at the end of the trial are shown in Table 2. The concentrations of plasma cortisol were significantly higher and CPK was lower in tanks with low initial CV. There was a tendency for an interaction between the CV and RFC for triglycerides, total proteins and albumin. In controls (no current), low CV fish had higher total proteins and albumin than high fish. For RFC tanks, however, the levels of plasma, total proteins and albumin presented no significant differences (Figure 2). The remaining haematological parameters were not significantly different among treatments.
3.2. Trial 2: RFC Enrichment Program in Adult Rainbow Trout
The average initial weight of trout when they first arrived to the experimental installations was 272.6 g ± 1.81 (CV = 15.6%, range 163–429 g). In terms of production indices, there were no initial differences in live weight among treatments (initial weight ± SEM for controls 296.37 ± 11.7; and for currents 299.13 ± 11.9; p = 0.4585) nor in final slaughter weight (Table 3). The initial values of the haematological parameters were not significantly different among fish at the beginning of the trials for cortisol (14.3 ± 2.53 controls; 10.9 ± 2.47 currents; p = 0.0610), glucose (125.2 ± 8.45 controls; 120.8 ± 7.68 currents; p = 0.4392) or triglycerides (255.6 ± 26.05 controls; 251.8 ± 20.29 currents; p = 0.8153), but were significantly different for lactate (2.18 ± 0.09 controls; 2.48 ± 0.19 currents; p = 0.0032), CPK (850.8 ± 378.3 controls; 394.2 ± 280.4 currents; p = 0.0586), total proteins (2.89 ± 0.14 controls; 2.73 ± 0.11 currents; p = 0.0701) and albumin (1.24 ± 0.07 controls; 1.16 ± 0.05 currents; p = 0.0466). The differences among treatments regarding haematological data at the end of the trial are shown in Table 1. Cortisol levels were significantly lower and triglycerides significantly higher in the blood plasma of fish in tanks with randomly fired currents.

Table 1.
Mean (±SEM) growth data of rainbow trout from Trial 1 at the end of the trial for high CV and low CV groups and controls and trout in tanks with randomly fired currents (RFC), including the significance of the main effects and their interaction.
Table 1.
Mean (±SEM) growth data of rainbow trout from Trial 1 at the end of the trial for high CV and low CV groups and controls and trout in tanks with randomly fired currents (RFC), including the significance of the main effects and their interaction.
| Variables | p Value | ||||||
|---|---|---|---|---|---|---|---|
| High CV (n = 240) | Low CV (n = 240) | Control (n = 240) | RFC (n = 240) | CV | RFC | CV × RFC | |
| Slaughter weight (g) | 30.1 ± 0.45 | 30.0 ± 0.44 | 29.5 ± 0.50 | 30.6 ± 0.50 | 0.92 | 0.15 | 0.32 |
| Fork length (cm) | 12.5 ± 0.32 | 13.1 ± 0.32 | 12.2 ± 0.36 | 13.3 ± 0.36 | 0.20 | 0.05 | 0.17 |
| Mortality (%) | 3.75 ± 0.83 | 0.83 ± 0.83 | 1.67 ± 0.83 | 2.92 ± 0.83 | 0.03 | 0.32 | 0.32 |
| Initial CV | 0.31 ± 0.01 | 0.14 ± 0.01 | 0.23 ± 0.01 | 0.21 ± 0.01 | <0.001 | 0.22 | 0.55 |
| Final CV | 0.25 ± 0.01 | 0.16 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.002 | 0.93 | 0.01 |
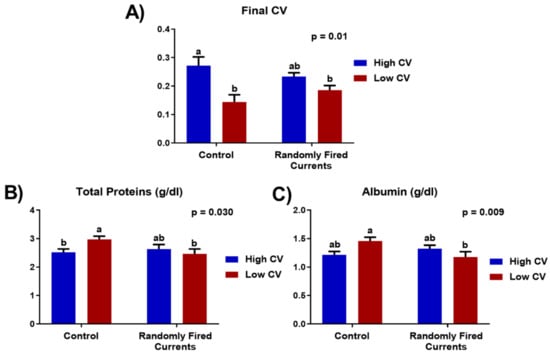
Figure 2.
Interaction between CV and occupational enrichment (randomly fired currents) for final CV (A) and plasma concentrations of total proteins (B) and albumin (C) in Trial 1. Data presented as means ± SEM. Different letters indicate significant differences due to the interaction between current treatment and live weight CV (p < 0.05).
Figure 2.
Interaction between CV and occupational enrichment (randomly fired currents) for final CV (A) and plasma concentrations of total proteins (B) and albumin (C) in Trial 1. Data presented as means ± SEM. Different letters indicate significant differences due to the interaction between current treatment and live weight CV (p < 0.05).
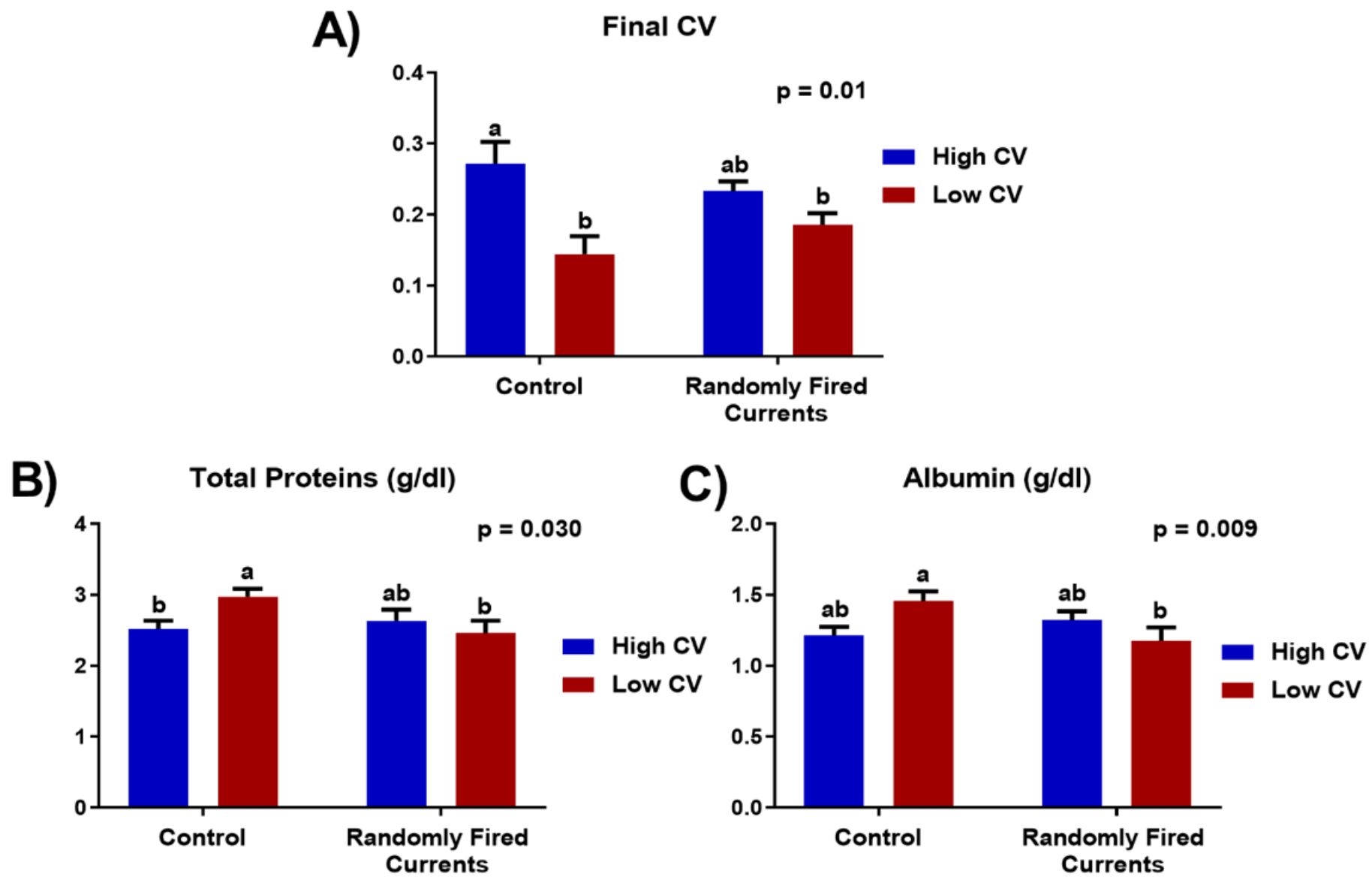

Table 2.
Mean (±SEM) haematological parameters of fingerling rainbow trout from Trial 1 for low and high CV groups, and control or RFC groups at the end of the trial, including the significance of the main effects and their interaction.
Table 2.
Mean (±SEM) haematological parameters of fingerling rainbow trout from Trial 1 for low and high CV groups, and control or RFC groups at the end of the trial, including the significance of the main effects and their interaction.
| Variables | p Value | ||||||
|---|---|---|---|---|---|---|---|
| High CV (n = 40) | Low CV (n = 28) | Control (n = 34) | RFC (n = 34) | CV | RFC | CV × RFC | |
| Cortisol (ng/mL) | 0.76 ± 0.33 | 2.69 ± 0.41 | 1.85 ± 0.38 | 1.60 ± 0.36 | 0.001 | 0.64 | 0.53 |
| Glucose (mg/dL) | 72.5 ± 4.84 | 59.9 ± 5.38 | 67.8 ± 4.84 | 64.6 ± 5.38 | 0.09 | 0.66 | 0.22 |
| Triglycerides (mg/dL) | 138 ± 7.43 | 126 ± 8.26 | 141 ± 7.43 | 124 ± 8.26 | 0.29 | 0.13 | 0.05 |
| LDH (U/L) | 2250 ± 179 | 1960 ± 203 | 2143 ± 183 | 2067 ± 199 | 0.29 | 0.78 | 0.88 |
| CPK (U/L) | 1243 ± 147 | 684 ± 166 | 1020 ± 150 | 907 ± 163 | 0.015 | 0.61 | 0.57 |
| Total proteins (g/dL) | 2.57 ± 0.09 | 2.72 ± 0.10 | 2.75 ± 0.09 | 2.54 ± 0.10 | 0.32 | 0.16 | 0.030 |
| Albumin (g/dL) | 1.27 ± 0.05 | 1.32 ± 0.05 | 1.33 ± 0.05 | 1.25 ± 0.05 | 0.48 | 0.23 | 0.009 |

Table 3.
Means (±SEM) of production and haematological parameters of rainbow trout from Trial 2 with constant currents (controls) or randomly fired currents (RFC), including significance. Different letters on the same row indicate significant differences due to current treatment (p < 0.05).
Table 3.
Means (±SEM) of production and haematological parameters of rainbow trout from Trial 2 with constant currents (controls) or randomly fired currents (RFC), including significance. Different letters on the same row indicate significant differences due to current treatment (p < 0.05).
| Variables | Controls | RFC | p Value |
|---|---|---|---|
| Slaughter weight (g) | 370 ± 4.14 | 377 ± 3.45 | 0.17 |
| Mortality (%) | 5.8 ± 2.01 | 5.0 ± 1.29 | 0.73 |
| Final CV (%) | 11.4 ± 0.79 | 9.6 ± 0.31 | 0.05 |
| Cortisol (ng/mL) | 21.5 ± 0.88a | 16.0 ± 1.40b | 0.003 |
| Glucose (mg/dL) | 110 ± 1.18 | 110 ± 2.25 | 0.95 |
| Lactate (mmol/L) | 3.11 ± 0.11 | 3.51 ± 0.18 | 0.06 |
| Triglycerides (mg/dL) | 286 ± 16.7b | 347 ± 24.2a | 0.04 |
| CPK (U/L) | 1608 ± 227 | 1122 ± 156 | 0.09 |
| Total proteins (g/dL) | 3.89 ± 0.08 | 4.06 ± 0.08 | 0.13 |
| Albumin (g/dL) | 1.74 ± 0.05 | 1.83 ± 0.03 | 0.15 |
4. Discussion
The main objective of an environmental enrichment program is to provide elements with biological significance that are linked with the natural history of the species [22]. In the wild, pre-smolt salmonids live in rivers rich in hiding places, with varied bottom-substrate and water currents moving at different intensities, directions and speeds [12]. The randomly fired underwater currents used in the present study were meant to mimic, to a degree, this natural environment. The results suggest that OE with RFC did not appear to have important positive (or negative) effects on growth, although RFC trout tended to have a longer fork length compared to controls (Trial 1). Brockmark et al. [23] found that structural enrichment increased growth in Atlantic salmon, but their study lasted longer than ours. Possibly, if we had maintained the RFC for a longer period, we would have also seen greater differences in weight gain between the two treatments. Rodewald et al. [17] also found that feeding rates and growth were higher in salmon parr from enriched tanks. They included varying water currents, but the shifts in current were altered weekly, not every 10–50 min, as in the current study. As in our study, Roberts et al. [16] did not find differences in size between control and structurally enriched Atlantic salmon. The effects of enrichment on fish growth seem to vary depending on species and size [9]. The different findings in the literature may also be related to different coping styles of the fish in the tank and the proportion of proactive or reactive fish [24]. Although we did not assess behavior or coping styles in the current study, we did test whether the effects of OE varied with CV in live weight within the tank.
In both trials, RFC had some effects on live weight CV. In Trial 1, the CV of RFC trout was lower than control fish who were in tanks with a constant current. In Trial 2, the final CV was significantly higher in the high CV tanks, but it decreased with respect to the initial value (from 31 to 25%). Mortality was also higher in high CV tanks, implying that the initial size distribution may have predisposed the group to health problems. In Trial 1, there was also a significant interaction for final CV, with different trends depending on the presence or absence of RFC. Overall, the RFC seemed to decrease CV in the high CV tanks and increase CV in the low CV tanks. Williams et al. [25] found that increased water flow reduces dominance problems in salmonids, since subordinate fish have more opportunities to feed. In our case, the increased randomness of currents may have facilitated a reduction in size disparity for the high CV fish. Pounder et al. [19] also reported that intraspecific variability in cortisol values is lower in rainbow trout from enriched tanks, which coincides with our results of lower intraspecific variability in live weight in RFC trout.
Regarding physiological indicators, RFC trout in Trial 2 had lower cortisol levels than controls (constant current). Similarly, fingerling trout in Trial 1 had lower cortisol, triglycerides and CPK than controls (no currents). The plasma cortisol levels of control fish in Trial 2 were below 22 ng/mL, which is within the normal range for trout (e.g., below or around 20 ng/mL; [26,27,28]), but was higher than the levels found in RFC fish. Pounder et al. [19] found no effects of structural enrichment on cortisol concentration in rainbow trout, while Näslund et al. [18] found no differences in post-stressed plasma cortisol levels in Atlantic salmon in enriched tanks with respect to barren ones. In addition, enrichment did not appear to have any effect on behavioral measures of anxiety (novel tank diving test) in rainbow trout [14]. However, Pounder et al. [19] reported a quicker recovery for enriched trout when exposed to stress. Structural enrichment such as shelter has been reported to reduce basal plasma cortisol concentration in Atlantic salmon [18] and in South American catfish [29]. Sánchez et al. [30] found that fish welfare (measured in terms of cortisol levels) was higher in sea bream fed on a regular schedule, compared to random feeding, which coincides with our findings. Jones et al. [31] found the opposite when the unpredictable factor was food. In that study, the salmon receiving food on an unpredictable feeding schedule had a higher incidence of dorsal fin erosion, but individual attacks were higher in the predictable feeding treatment. Although we did not consider individual animal behaviors, the similarity in production indices and lack of fin erosion suggest that the use of unpredictable currents was in no way detrimental.
In Trial 2, the levels of cortisol in the control fish may have affected the triglyceride levels, which were also lower, implying higher levels of stress and lower free fatty acids in blood (approximately 286 mg/dL triglycerides in controls, 347 mg/dL in tout with currents). The control levels are comparable to rainbow trout from the same source farm, but which in another study had been fasted for five days (350 mg/dL) [32]. Overall, however, most haematological parameters were similar among treatments, implying that RFC did not appear to have a great influence on fish physiology. Just taking into consideration the lower cortisol and higher triglyceride levels would suggest that firing the random current provided a beneficial psychological challenge [9,33] and that the rainbow trout seem to have reacted to that stimulus in a positive way.
In Trial 1, RFC also had little effect on blood plasma variables. The cortisol levels were quite low, but still within the range reported in other studies (1–20 ng/mL) [27]. Despite these low levels, on average, the low CV trout had higher levels than high CV. Possibly, there was more competition in the low CV fish in order to establish hierarchies, which raised cortisol levels [34]. However, contrary to what we might expect from stressed fish, the low CV trout had lower CPK levels, an enzyme related to tissue damage. Thus, only considering CPK, the high CV fish appear to have been more stressed, which may have been related to their higher mortality [35]. Total proteins and albumin were higher in low CV fish without currents compared to high CV fish without currents, but there were no significant differences in RFC tanks, again underlining the importance of group composition. Since albumin is entirely produced by the liver, its increase (and the increase in total proteins in plasma) could be attributed to protein synthesis required for an increased energy demand [36].
5. Conclusions
Our results suggest that OE with randomly fired underwater currents may have beneficial effects on rainbow trout, since they had a lower level of stress-related biochemical indicators. However, we found no evidence that RFC enrichment had an effect on weight gain. In any case, the importance of the use of RFC is that it provides fish with biologically meaningful environmental enrichment related to the natural history of the species. This type of enrichment may help the fish to better adapt to the captive environment of the raceways on the farm and, in any case, did not produce harmful effects. Along those lines, it is important to develop environmental enrichment strategies that are easy to implement at the commercial level and that do not pose a risk to the animals or the operators.
Author Contributions
Conceptualization, M.V. and J.D.l.F.; methodology, M.V., R.B.-P. and E.G.-d.C.; validation, R.B.-P., C.P. and F.T.; formal analysis, R.B.-P. and J.D.l.F.; investigation, M.V., G.C.M.-d.l.L., R.B.-P. and J.D.l.F.; resources, M.V..; data curation, R.B.-P. and J.D.l.F.; writing—original draft preparation, M.V., R.B.-P., G.C.M.-d.l.L.; writing—review and editing, M.V., G.C.M.-d.l.L.; visualization, G.C.M.-d.l.L.; supervision, M.V.; funding acquisition, M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the EU ANIHWA ERA-Net project entitled WIN-FISH (grant number ERA58-WIN-FISH).
Institutional Review Board Statement
All animal handling processes performed in this project were part of routine animal husbandry practices, and according to the Legislation for the Protection of Animals used for Scientific Purposes (EU Directive 2010-63-EU and Spanish RD 53/2013), no procedure was performed. Therefore, this project was exempted from an ethical review by the Ethical Committee of the Technical University of Madrid.
Data Availability Statement
The data presented in this study are available on request from the corresponding author Genaro C. Miranda-de la Lama by contacting genaro@unizar.es.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Candiotto, A.; Bo, T.; Fenoglio, S. Biological and Ecological Data on an Established Rainbow Trout (Oncorhynchus Mykiss) Population in an Italian Stream. Fal 2011, 179, 67–76. [Google Scholar] [CrossRef]
- Tomás-Almenar, C.; Toledo-Solís, F.J.; Larrán, A.M.; de Mercado, E.; Alarcón, F.J.; Rico, D.; Martín-Diana, A.B.; Fernández, I. Effects and Safe Inclusion of Narbonne Vetch (Vicia narbonensis) in Rainbow Trout (Oncorhynchus mykiss) Diets: Towards a More Sustainable Aquaculture. Animals 2020, 10, 2175. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.G.; Moriarty, M. A Simple Modelling Tool for Assessing Interaction with Host and Local Infestation of Sea Lice from Salmonid Farms on Wild Salmonids Based on Processes Operating at Multiple Scales in Space and Time. Ecol. Model. 2021, 443, 109459. [Google Scholar] [CrossRef]
- Cogliati, K.M.; Herron, C.L.; Noakes, D.L.G.; Schreck, C.B. Reduced Stress Response in Juvenile Chinook Salmon Reared with Structure. Aquaculture 2019, 504, 96–101. [Google Scholar] [CrossRef]
- Li, F.; Sun, Z.; Qi, H.; Zhou, X.; Xu, C.; Wu, D.; Fang, F.; Feng, J.; Zhang, N. Effects of Rice-Fish Co-Culture on Oxygen Consumption in Intensive Aquaculture Pond. Rice Sci. 2019, 26, 50–59. [Google Scholar] [CrossRef]
- Frisk, M.; Høyland, M.; Zhang, L.; Vindas, M.A.; Øverli, Ø.; Johansen, I.B. Intensive Smolt Production Is Associated with Deviating Cardiac Morphology in Atlantic Salmon (Salmo salar L.). Aquaculture 2020, 529, 735615. [Google Scholar] [CrossRef]
- Favero Neto, J.; Giaquinto, P.C. Environmental Enrichment Techniques and Tryptophan Supplementation Used to Improve the Quality of Life and Animal Welfare of Nile Tilapia. Aquac. Rep. 2020, 17, 100354. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Q.; Xu, X.; Guo, H.; Zhang, X. Effects of Environmental Enrichment on the Welfare of Juvenile Black Rockfish Sebastes Schlegelii: Growth, Behavior and Physiology. Aquaculture 2020, 518, 734782. [Google Scholar] [CrossRef]
- Näslund, J.; Johnsson, J.I. Environmental Enrichment for Fish in Captive Environments: Effects of Physical Structures and Substrates. Fish Fish. 2016, 17, 1–30. [Google Scholar] [CrossRef]
- Baßmann, B.; Brenner, M.; Palm, H.W. Stress and Welfare of African Catfish (Clarias Gariepinus Burchell, 1822) in a Coupled Aquaponic System. Water 2017, 9, 504. [Google Scholar] [CrossRef]
- Tatemoto, P.; Bernardino, T.; Alves, L.; Cristina de Oliveira Souza, A.; Palme, R.; José Zanella, A. Environmental Enrichment for Pregnant Sows Modulates HPA-Axis and Behavior in the Offspring. Appl. Anim. Behav. Sci. 2019, 220, 104854. [Google Scholar] [CrossRef]
- Nordgreen, J.; Bjørge, M.H.; Janczak, A.M.; Hovland, A.L.; Moe, R.O.; Ranheim, B.; Horsberg, T.E. The Time Budget of Atlantic Salmon (Salmo salar) Held in Enriched Tanks. Appl. Anim. Behav. Sci. 2013, 144, 147–152. [Google Scholar] [CrossRef]
- Zimmermann, E.W.; Purchase, C.F.; Fleming, I.A. Reducing the Incidence of Net Cage Biting and the Expression of Escape-Related Behaviors in Atlantic Cod (Gadus morhua) with Feeding and Cage Enrichment. Appl. Anim. Behav. Sci. 2012, 141, 71–78. [Google Scholar] [CrossRef]
- Ahlbeck Bergendahl, I.; Salvanes, A.G.V.; Braithwaite, V.A. Determining the Effects of Duration and Recency of Exposure to Environmental Enrichment. Appl. Anim. Behav. Sci. 2016, 176, 163–169. [Google Scholar] [CrossRef]
- Brown, C.; Davidson, T.; Laland, K. Environmental Enrichment and Prior Experience of Live Prey Improve Foraging Behaviour in Hatchery-Reared Atlantic Salmon. J. Fish Biol. 2003, 63, 187–196. [Google Scholar] [CrossRef]
- Roberts, L.J.; Taylor, J.; Garcia de Leaniz, C. Environmental Enrichment Reduces Maladaptive Risk-Taking Behavior in Salmon Reared for Conservation. Biol. Conserv. 2011, 144, 1972–1979. [Google Scholar] [CrossRef]
- Rodewald, P.; Hyvärinen, P.; Hirvonen, H. Wild Origin and Enriched Environment Promote Foraging Rate and Learning to Forage on Natural Prey of Captive Reared Atlantic Salmon Parr. Ecol. Freshw. Fish 2011, 20, 569–579. [Google Scholar] [CrossRef]
- Näslund, J.; Rosengren, M.; Del Villar, D.; Gansel, L.; Norrgård, J.R.; Persson, L.; Winkowski, J.J.; Kvingedal, E. Hatchery Tank Enrichment Affects Cortisol Levels and Shelter-Seeking in Atlantic Salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2013, 70, 585–590. [Google Scholar] [CrossRef]
- Pounder, K.C.; Mitchell, J.L.; Thomson, J.S.; Pottinger, T.G.; Buckley, J.; Sneddon, L.U. Does Environmental Enrichment Promote Recovery from Stress in Rainbow Trout? Appl. Anim. Behav. Sci. 2016, 176, 136–142. [Google Scholar] [CrossRef]
- Barreto, R.E.; Carvalho, G.G.A.; Volpato, G.L. The Aggressive Behavior of Nile Tilapia Introduced into Novel Environments with Variation in Enrichment. Zoology 2011, 114, 53–57. [Google Scholar] [CrossRef]
- Ministerio de la Presidencia. Real Decreto 1201/2005, de 10 de Octubre, Sobre Protección de Los Animales Utilizados Para Experimentación y Otros Fines Científicos; BOE (Diario Oficial Boletín Oficial del Estado): Madrid, Spain, 2005; Volume 252, pp. 34367–34391. [Google Scholar]
- Yavuzcan Yildiz, H.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish Welfare in Aquaponic Systems: Its Relation to Water Quality with an Emphasis on Feed and Faeces—A Review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Brockmark, S.; Neregård, L.; Bohlin, T.; Björnsson, B.T.; Johnsson, J.I. Effects of Rearing Density and Structural Complexity on the Pre- and Postrelease Performance of Atlantic Salmon. Trans. Am. Fish. Soc. 2007, 136, 1453–1462. [Google Scholar] [CrossRef]
- Thomson, J.S.; Watts, P.C.; Pottinger, T.G.; Sneddon, L.U. Plasticity of Boldness in Rainbow Trout, Oncorhynchus Mykiss: Do Hunger and Predation Influence Risk-Taking Behaviour? Horm. Behav. 2012, 61, 750–757. [Google Scholar] [CrossRef]
- Williams, T.D.; Readman, G.D.; Owen, S.F. Key Issues Concerning Environmental Enrichment for Laboratory-Held Fish Species. Lab Anim 2009, 43, 107–120. [Google Scholar] [CrossRef]
- Davidson, G.W.; Davie, P.S.; Young, G.; Fowler, R.T. Physiological Responses of Rainbow Trout Oncorhynchus Mykiss to Crowding and Anesthesia with AQUI-STM. J. World Aquac. Soc. 2000, 31, 105–114. [Google Scholar] [CrossRef]
- Culbert, B.M.; Gilmour, K.M. Rapid Recovery of the Cortisol Response Following Social Subordination in Rainbow Trout. Physiol. Behav. 2016, 164, 306–313. [Google Scholar] [CrossRef]
- Bermejo-Poza, R.; De la Fuente, J.; Pérez, C.; Lauzurica, S.; González de Chávarri, E.; Diaz, M.; Villarroel, M. Reducing the Effect of Pre-Slaughter Fasting on the Stress Response of Rainbow Trout (Oncorhynchus mykiss). Anim. Welf. 2016, 25, 339–346. [Google Scholar] [CrossRef][Green Version]
- Barcellos, L.J.G.; Kreutz, L.C.; Quevedo, R.M.; da Rosa, J.G.S.; Koakoski, G.; Centenaro, L.; Pottker, E. Influence of Color Background and Shelter Availability on Jundiá (Rhamdia quelen) Stress Response. Aquaculture 2009, 288, 51–56. [Google Scholar] [CrossRef]
- Sánchez, J.A.; López-Olmeda, J.F.; Blanco-Vives, B.; Sánchez-Vázquez, F.J. Effects of Feeding Schedule on Locomotor Activity Rhythms and Stress Response in Sea Bream. Physiol. Behav. 2009, 98, 125–129. [Google Scholar] [CrossRef]
- Cañon Jones, H.A.; Noble, C.; Damsgård, B.; Pearce, G.P. Investigating the Influence of Predictable and Unpredictable Feed Delivery Schedules upon the Behaviour and Welfare of Atlantic Salmon Parr (Salmo salar) Using Social Network Analysis and Fin Damage. Appl. Anim. Behav. Sci. 2012, 138, 132–140. [Google Scholar] [CrossRef]
- Bermejo-Poza, R.; De la Fuente, J.; Pérez, C.; González de Chavarri, E.; Diaz, M.T.; Torrent, F.; Villarroel, M. Determination of Optimal Degree Days of Fasting before Slaughter in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2017, 473, 272–277. [Google Scholar] [CrossRef][Green Version]
- Hyvärinen, P.; Rodewald, P. Enriched Rearing Improves Survival of Hatchery-Reared Atlantic Salmon Smolts during Migration in the River Tornionjoki. Can. J. Fish. Aquat. Sci. 2013, 70, 1386–1395. [Google Scholar] [CrossRef]
- Jeffrey, J.D.; Gollock, M.J.; Gilmour, K.M. Social Stress Modulates the Cortisol Response to an Acute Stressor in Rainbow Trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2014, 196, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Peres, H.; Santos, S.; Oliva-Teles, A. Selected Plasma Biochemistry Parameters in Gilthead Seabream (Sparus aurata) Juveniles. J. Appl. Ichthyol. 2013, 29, 630–636. [Google Scholar] [CrossRef]
- Javed, M.; Ahmad, M.I.; Usmani, N.; Ahmad, M. Multiple Biomarker Responses (Serum Biochemistry, Oxidative Stress, Genotoxicity and Histopathology) in Channa Punctatus Exposed to Heavy Metal Loaded Waste Water. Sci. Rep. 2017, 7, 1675. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

