Development of a Fish-Based Multimetric Index for the Assessment of Lagoons’ Ecological Quality in Northern Greece
Abstract
:1. Introduction
2. Materials and Methods
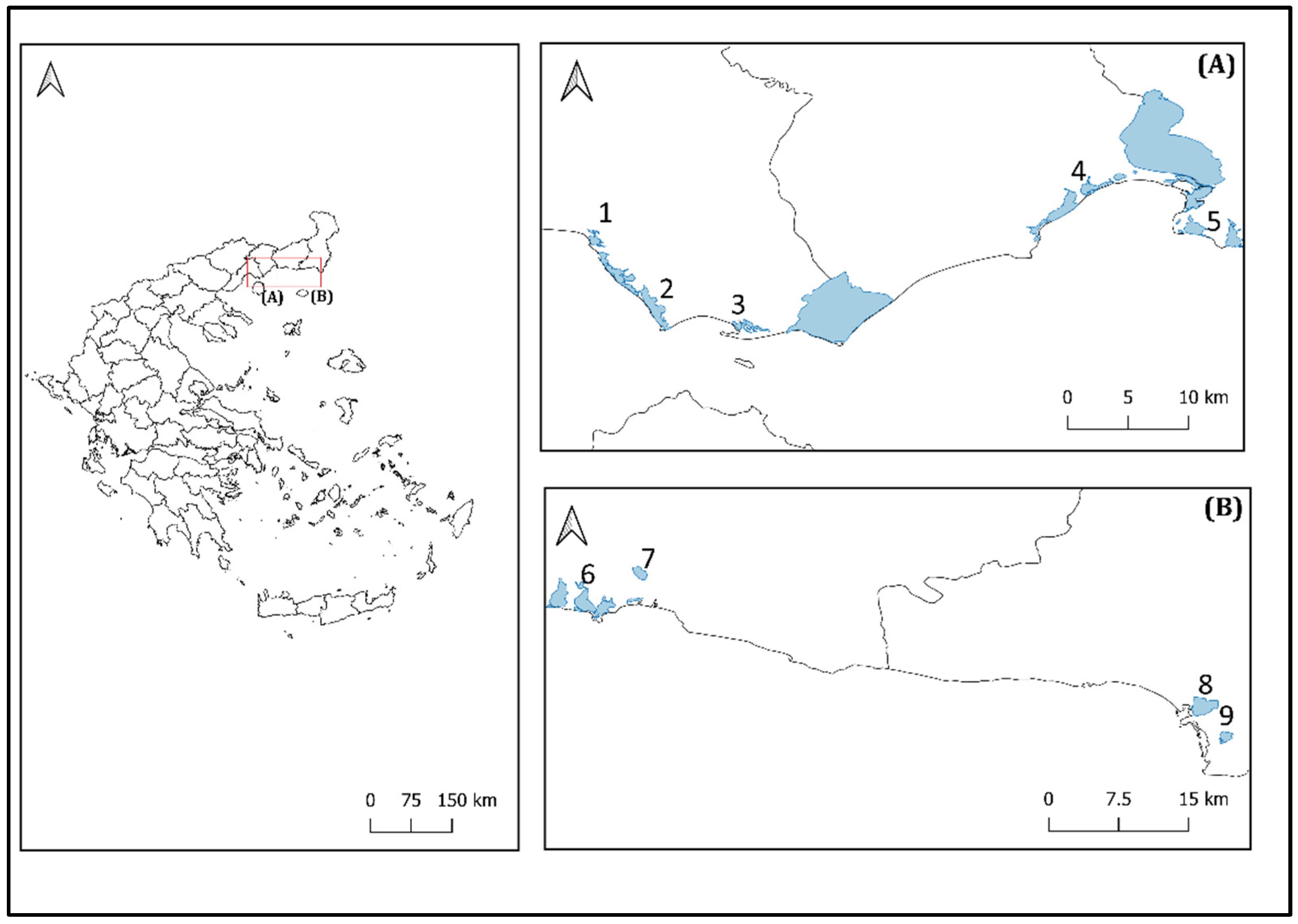
2.1. Study Area
2.2. Data Collection
2.3. Metrics Selection
2.4. Development of Lagoon Fish-Based Index (LFI)
3. Results
3.1. Metrics Selection and Development
3.2. Defining Class Limits
3.3. Index Application
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, M.; Hemingway, K.L. Fishes in Estuaries; Blackwell Science: Oxford, UK, 2002; p. 636. [Google Scholar]
- Koutrakis, E.T.; Kokkinakis, A.K.; Eleftheriadis, E.A.; Argyropoulou, M.D. Seasonal changes in distribution and abundance of the fish fauna in the two estuarine systems of Strymonikos Gulf (Macedonia, Greece). Belg. J. Zool. 2000, 130, 43–50. [Google Scholar]
- Franco, A.; Torricelli, P.; Franzoi, P. A habitat-specific fish-based approach to assess the ecological status of Mediterranean coastal lagoons. Mar. Pollut. Bull. 2009, 58, 1704–1717. [Google Scholar] [CrossRef] [PubMed]
- Lucena-Moya, P.; Pardo, I. An invertebrate multimetric index to classify the ecological status of small coastal lagoons in the Mediterranean ecoregion (MIBIIN). Mar. Freshw. Res. 2012, 63, 801–814. [Google Scholar] [CrossRef]
- Gamito, S.; Erzini, K. Trophic food web and ecosystem attributes of a water reservoir of the Ria Formosa (south Portugal). Ecol. Model. 2005, 181, 509–520. [Google Scholar] [CrossRef]
- Reizopoulou, S.; Nicolaidou, A. Index of size distribution (ISD): A method of quality assessment for coastal lagoons. Hydrobiologia 2007, 577, 141–149. [Google Scholar] [CrossRef]
- Deegan, L.A.; Finn, J.T.; Ayvazian, S.G.; Ryder-Kieffer, C.A.; Buonaccorsi, J. Development and validation of an estuarine biotic integrity index. Estuaries 1997, 20, 601–617. [Google Scholar] [CrossRef]
- Harrison, T.D.; Whitfield, A.K. A multi-metric fish index to assess the environmental condition of estuaries. J. Fish Biol. 2004, 65, 683–710. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02000L0060-20141120&from=EN (accessed on 10 February 2021).
- Borja, A.; Bald, J.; Franco, J.; Larreta, J.; Muxika, I.; Revilla, M.; Rodríguez, M.I.R.; Solaun, O.; Uriarte, A.; Valencia, V. Using multiple ecosystem components, in assessing ecological status in Spanish (Basque Country) Atlantic marine waters. Mar. Pollut. Bull. 2009, 59, 54–64. [Google Scholar] [CrossRef]
- Borja, Á. The European water framework directive: A challenge for nearshore, coastal and continental shelf research. Cont. Shelf Res. 2005, 25, 1768–1783. [Google Scholar] [CrossRef]
- Borja, A. The new European Marine strategy directive: Difficulties, opportunities, and challenges. Mar. Pollut. Bull. 2006, 52, 239–242. [Google Scholar] [CrossRef]
- Karr, J.R.; Fausch, K.D.; Angermeier, P.L.; Yant, P.R.; Schlosser, I.J. Assessing biological integrity in running waters: A method and its rationale. Ill. Nat. Hist. Surv. Spec. Publ. 1986, 5, 28. [Google Scholar]
- Harris, J.H.; Silveira, R. Large-scale assessments of river health using an index of biotic integrity with low-diversity fish communities. Freshw. Biol. 1999, 41, 235–252. [Google Scholar] [CrossRef]
- Simon, T. The use of biological criteria as a tool for water resource management. Environ. Sci. Policy 2000, 3, 43–49. [Google Scholar] [CrossRef]
- Roset, N.; Grenouillet, G.; Goffaux, D.; Pont, D.; Kestemont, P. A review of existing fish assemblage indicators and methodologies. Fish. Manag. Ecol. 2007, 14, 393–405. [Google Scholar] [CrossRef]
- Dennison, W.C.; Orth, R.; Moore, K.A.; Stevenson, J.C.; Carter, V.; Kollar, S.; Bergstrom, P.W.; Batiuk, R.A. Assessing water quality with submersed aquatic vegetation. BioScience 1993, 43, 86–94. [Google Scholar] [CrossRef]
- Ballesteros, E.; Torras, X.; Pinedo, S.; García, M.; Mangialajo, L.; de Torres, M. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European water framework directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Van Dolah, R.; Hyland, J.; Holland, A.; Rosen, J.; Snoots, T. A benthic index of biological integrity for assessing habitat quality in estuaries of the southeastern USA. Mar. Environ. Res. 1999, 48, 269–283. [Google Scholar] [CrossRef]
- Borja, A.; Miles, A.; Occhipinti-Ambrogi, A.; Berg, T. Current status of macroinvertebrate methods used for assessing the quality of European marine waters: Implementing the water framework directive. Hydrobiologia 2009, 633, 181–196. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Elliott, M. Fishes as indicators of environmental and ecological changes within estuaries: A review of progress and some suggestions for the future. J. Fish Biol. 2002, 61, 229–250. [Google Scholar] [CrossRef]
- Pérez-Domínguez, R.; Maci, S.; Courrat, A.; Lepage, M.; Borja, A.; Uriarte, A.; Neto, J.M.; Cabral, H.; Raykov, V.; Franco, A.; et al. Current developments on fish-based indices to assess ecological-quality status of estuaries and lagoons. Ecol. Indic. 2012, 23, 34–45. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Fausch, K.D.; Lyons, J.; Karr, J.R.; Angermeier, P.L. Fish communities as indicators of environmental degradation. Am. Fish. Soc. Symp. 1990, 8, 123–144. [Google Scholar]
- Breine, J.J.; Maes, J.; Quataert, P.; Bergh, E.V.D.; Simoens, I.; van Thuyne, G.; Belpaire, C. A fish-based assessment tool for the ecological quality of the brackish Schelde estuary in Flanders (Belgium). Hydrobiologia 2007, 575, 141–159. [Google Scholar] [CrossRef]
- Koutrakis, E.T.; Conides, A.; Parpoura, A.C.; van Ham, E.H.; Katselis, G.; Koutsikopoulos, C. Lagoon fisheries resources in hellas. In State of Hellenic Fisheries; Papaconstantinou, C., Zenetos, A., Vassilopoulou, V., Tserpes, G., Eds.; HCMR Publications: Anavyssos, Greece, 2007; pp. 223–233. [Google Scholar]
- Orfanidis, S.; Papathanasiou, V.; Mittas, N.; Theodosiou, T.; Ramfos, A.; Tsioli, S.; Kosmidou, M.; Kafas, A.; Mystikou, A.; Papadimitriou, A. Further improvement, validation, and application of CymoSkew biotic index for the ecological status assessment of the Greek coastal and transitional waters. Ecol. Indic. 2020, 118, 106727. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. Ecological evaluation of transitional and coastal waters: A marine benthic macrophytes-based model. Mediterr. Mar. Sci. 2001, 2, 45. [Google Scholar] [CrossRef]
- Papathanasiou, V.; Orfanidis, S. Anthropogenic eutrophication affects the body size of Cymodocea nodosa in the North Aegean Sea: A long-term, scale-based approach. Mar. Pollut. Bull. 2018, 134, 38–48. [Google Scholar] [CrossRef]
- Koutrakis, E.; Sylaios, G.; Kamidis, N.; Markou, D.; Sapounidis, A. Fish fauna recovery in a newly re-flooded Mediterranean coastal lagoon. Estuar. Coast. Shelf Sci. 2009, 83, 505–515. [Google Scholar] [CrossRef]
- Koutrakis, E.; Emfietzis, G.; Sylaios, G.; Zoidou, M.; Katsiapi, M.; Moustaka-Gouni, M. Massive fish mortality in Ismarida Lake, Greece: Identification of drivers contributing to the fish kill event. Mediterr. Mar. Sci. 2016, 17, 280. [Google Scholar] [CrossRef] [Green Version]
- Zoidou, M.; Sylaios, G. Ecological risk assessment of heavy metals in the sediments of a Mediterranean lagoon complex. J. Environ. Health Sci. Eng. 2021, 1–15. [Google Scholar] [CrossRef]
- Orfanidis, S.; Stamatis, N.; Parasyri, A.; Mente, M.S.; Zerveas, S.; Pigada, P.; Papadimitriou, A.; Paschou, M.; Nisiforou, O.; Papazi, A.; et al. Solving nuisance cyanobacteria eutrophication through biotechnology. Appl. Sci. 2019, 9, 2566. [Google Scholar] [CrossRef] [Green Version]
- Gulland, J.A. Catch per unit effort as a measure of abundance. Rapports et procès-verbaux des reunions. Comm. Int. Pour L’explor. Sci. Mer Méditerr. 1964, 155, 8–14. [Google Scholar]
- Maraisand, J.; Baird, D. Seasonal abundance distribution and catch per unit effort of fishes in the Swartkops Estuary. S. Afr. J. Zool. 1980, 15, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Koutrakis, E.T.; Sinis, A.I.; Economidis, P.S. Seasonal occurrence, abundance and size distribution of grey mullet fry (Pisces, Mugilidae) in the Porto-Lagos Lagoon and Lake Vistonis (Aegean Sea, Greece). Isr. J. Aquac. Bamidgeh 1994, 46, 182–196. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase. Available online: www.fishbase.org (accessed on 10 February 2021).
- Sapounidis, A.; Koutrakis, E.; Leonardos, I. Fish-based river integrity index: A first attempt in developing a water quality index for the assessment of the Greek rivers. Ecohydrol. Hydrobiol. 2019, 19, 620–628. [Google Scholar] [CrossRef]
- Oberdorff, T.; Hughes, R.M. Modification of an index of biotic integrity based on fish assemblages to characterize rivers of the Seine-Normandie basin, France. Hydrobiologia 1992, 228, 117–130. [Google Scholar] [CrossRef]
- Oberdorff, T.; Pont, D.; Hugueny, B.; Porcher, J.-P. Development and validation of a fish-based index for the assessment of ‘river health’ in France. Freshw. Biol. 2002, 47, 1720–1734. [Google Scholar] [CrossRef]
- Moyle, P.; Randall, P. Biotic Integrity of Watesheds. Sierra Nevada Ecosystem Project: Final Report to Congress; University of California: Davis, CA, USA, 1996; Volume 2, pp. 975–985. [Google Scholar]
- Belpaire, C.; Smolders, R.; Auweele, I.V.; Ercken, D.; Breine, J.; van Thuyne, G.; Ollevier, F. An index of biotic integrity characterizing fish populations and the ecological quality of Flandrian water bodies. Hydrobiologia 2000, 434, 17–33. [Google Scholar] [CrossRef]
- Meng, L.; Orphanides, C.D.; Powell, J.C. Use of a fish index to assess habitat quality in Narragansett Bay, Rhode Island. Trans. Am. Fish. Soc. 2002, 131, 731–742. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M.; Blanco-Garrido, F.; Prenda, J. Assessing the ecological status in species-poor systems: A fish-based index for Mediterranean Rivers (Guadiana River, SW Spain). Ecol. Indic. 2010, 10, 1152–1161. [Google Scholar] [CrossRef] [Green Version]
- Harrison, T.D.; Kelly, F.L. Development of an estuarine multi-metric fish index and its application to Irish transitional waters. Ecol. Indic. 2013, 34, 494–506. [Google Scholar] [CrossRef]
- Cooper, M.J.; Lamberti, G.A.; Moerke, A.H.; Ruetz, C.R.; Wilcox, D.A.; Brady, V.J.; Brown, T.N.; Ciborowski, J.J.H.; Gathman, J.P.; Grabas, G.P.; et al. An expanded fish-based index of biotic integrity for Great Lakes coastal wetlands. Environ. Monit. Assess. 2018, 190, 1–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzetti, M.; Schulz, U.H. An index of biotic integrity based on fish assemblages for subtropical streams in southern Brazil. Hydrobiologia 2004, 529, 133–144. [Google Scholar] [CrossRef]
- Breine, J.; Simoens, I.; Goethals, P.; Quataert, P.; Ercken, D.; van Liefferinghe, C.; Belpaire, C. A fish-based index of biotic integrity for upstream brooks in Flanders (Belgium). Hydrobiologia 2004, 522, 133–148. [Google Scholar] [CrossRef]
- Lyons, J. A fish-based index of biotic integrity to assess intermittent headwater streams in Wisconsin, USA. Environ. Monit. Assess. 2006, 122, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Gorley, R.N. PRIMER v7. User Manual/Tutorial; PRIMER-e: Plymouth, UK, 2015. [Google Scholar]
- Paganelli, D.; Forni, G.; Marchini, A.; Mazziotti, C.; Occhipinti-Ambrogi, A. Critical appraisal on the identification of reference conditions for the evaluation of ecological quality status along the Emilia-Romagna coast (Italy) using M-AMBI. Mar. Pollut. Bull. 2011, 62, 1725–1735. [Google Scholar] [CrossRef]
- Borja, Á.; Dauer, D.M.; Grémare, A. The importance of setting targets and reference conditions in assessing marine ecosystem quality. Ecol. Indic. 2012, 12, 1–7. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: Hoboken, NJ, USA, 1999; 663p. [Google Scholar]
- Romero, J.; Martínez-Crego, B.; Alcoverro, T.; Pérez, M. A multivariate index based on the seagrass Posidonia oceanica (POMI) to assess ecological status of coastal waters under the water framework directive (WFD). Mar. Pollut. Bull. 2007, 55, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Pérez-Ruzafa, A.; Drouineau, H.; Franzoi, P.; Koutrakis, E.; Lepage, M.; Verdiell-Cubedo, D.; Bouchoucha, M.; López-Capel, A.; Riccato, F.; et al. Assessment of fish assemblages in coastal lagoon habitats: Effect of sampling method. Estuar. Coast. Shelf Sci. 2012, 112, 115–125. [Google Scholar] [CrossRef]
- Delpech, C.; Courrat, A.; Pasquaud, S.; Lobry, J.; le Pape, O.; Nicolas, D.; Boët, P.; Girardin, M.; Lepage, M. Development of a fish-based index to assess the ecological quality of transitional waters: The case of French estuaries. Mar. Pollut. Bull. 2010, 60, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.; McLusky, D. The need for definitions in understanding estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, A.K. Biology and Ecology of Fishes in Southern African Estuaries; J.L.B. Smith Institute of Ichthyology: Grahamstown, South Africa, 1998; p. 244. [Google Scholar]
- Selleslagh, J.; Lobry, J.; Amara, R.; Brylinski, J.-M.; Boët, P. Trophic functioning of coastal ecosystems along an anthropogenic pressure gradient: A French case study with emphasis on a small and low impacted estuary. Estuar. Coast. Shelf Sci. 2012, 112, 73–85. [Google Scholar] [CrossRef]
- Selleslagh, J.; Amara, R. Are estuarine fish opportunistic feeders? The case of a low anthropized nursery ground (the Canche Estuary, France). Chesap. Sci. 2014, 38, 252–267. [Google Scholar] [CrossRef]

| Candidate Metrics | Abbreviation | Response to Environmental Stress | |
|---|---|---|---|
| Fish fauna composition | |||
| 1 | Total number of species | TotNSp | ↓ |
| 2 | Number of families | Nfam | ↓ |
| 3 | Number of species comprising 90% of total individuals | 90%TotInd | ↓ |
| 4 | Relative abundance of all species | Rab | ↓ |
| 5 | H’ (Shannon–Wiener index) | H’ind | ↓ |
| 6 | J’ (Pielou’s evenness) | J’ind | ↓ |
| 7 | 1-_λ’ (Simpson diversity index) | 1-_λ’ | ↓ |
| 8 | Number of Resident species | Nres | ↓ |
| 9 | Residents (% species) | %ResSp | ↓ |
| 10 | Number of migrant species | Nmig | ↓ |
| 11 | Migrants (% species) | %MigrSp | ↓ |
| 12 | Number of Straggler species | Nstr | ↑ |
| 13 | Stragglers (% species) | %StrgSp | ↑ |
| 14 | Number of Benthic species (Soleidae, Gobiidae, etc.) | NBenthSp | ↓ |
| 15 | (Pelagic+Benthopelagic)/Benthic | ↑ | |
| 16 | Presence of Sentinel species | SentSp | ↓ |
| Feeding strategies | |||
| 17 | Number of Omnivores | NOmn | ↑ |
| 18 | Omnivores (% species) | %OmnSp | ↑ |
| 19 | Number of Carnivores | NCarn | ↑ |
| 20 | Carnivores (% species) | %CarnSp | ↑ |
| 21 | Number of benthic feeders | NBenth | ↓ |
| 22 | Benthic Feeders (% species) | %BenthSp. | ↓ |
| 23 | Piscivores (% species) | %PiscSp | ↓ |
| 24 | Dentrivores (% species) | %DentrSp | ↓ |
| 25 | Number of Trophic guilds | TrophGuil | ↓ |
| Family | Species | Trophic Guild | Life Cycle Category | Habitat | |
|---|---|---|---|---|---|
| 1 | Anguillidae | Anguilla anguilla (Linnaeus, 1758) | Carnivore | Diadromous | F, B, M |
| 2 | Argentinidae | Argentina sphyraena (Linnaeus, 1758) | Carnivore | Straggler | M |
| 3 | Atherinidae | Atherina boyeri (Risso, 1810) | Carnivore | Resident | B, M |
| 4 | Blenniidae | Salaria pavo (Risso, 1810) | Omnivore | Resident | B, M |
| 5 | Bothidae | Arnoglossus thori (Kyle, 1913) | Carnivore | Straggler | M |
| 6 | Callionymidae | Callionymus risso (Lesueur, 1814) | Carnivore | Migrant | M |
| 7 | Callionymus pusillus (Delaroche, 1809) | Carnivore | Migrant | M | |
| 8 | Clupeidae | Sardina pilchardus (Walbaum, 1792) | Carnivore | Straggler | M |
| 9 | Cyprinidae | Carassius gibelio (Linnaeus, 1758) | Omnivore | Straggler | F |
| 10 | Vimba melanops (Heckel, 1837) | Omnivore | Straggler | F | |
| 11 | Cyprinodontidae | Aphanius faschiatus (Valenciennes, 1821) | Omnivore | Resident | B |
| 12 | Gobiidae | Zosterisessor ophiocephalus (Pallas, 1814) | Carnivore | Resident | B |
| 13 | Engraulidae | Engraulis engrasicholus (Linnaeus, 1758) | Carnivore | Straggler | M |
| 14 | Gasterosteidae | Gasterosteus aculeatus (Linnaeus, 1758) | Carnivore | Migrant | F, B, M |
| 15 | Gobiidae | Gobius niger (Linnaeus, 1758) | Carnivore | Resident | B, M |
| 16 | Knipowitschia caucasica (Berg, 1916) | Carnivore | Migrant | F, B, M | |
| 17 | Pomatoschistus marmoratus (Risso, 1810) | Carnivore | Resident | F, B, M | |
| 18 | Pomatoschistus microps (Krøyer, 1838) | Carnivore | Resident | F, B, M | |
| 19 | Thorogobius ephipiatus (Lowe, 1839) | Carnivore | Straggler | M | |
| 20 | Labridae | Symphodus cinereus (Bonnaterre, 1788) | Carnivore | Straggler | M |
| 21 | Symphodus roissali (Risso, 1810) | Carnivore | Straggler | M | |
| 22 | Moronidae | Dicentrarchus labrax (Linnaeus, 1758) | Carnivore | Migrant | M, E |
| 23 | Mugilidae | Chelon labrosus (Risso, 1826) | Omnivore | Migrant | M, E |
| 24 | Liza ramada (Risso, 1826) | Omnivore | Migrant | M, E | |
| 25 | Liza saliens (Risso, 1810) | Omnivore | Migrant | M, E | |
| 26 | Mugil cephalus (Linnaeus, 1758) | Omnivore | Migrant | M, E | |
| 27 | Mullidae | Mullus barbatus (Linnaeus, 1758) | Carnivore | Straggler | M |
| 28 | Poeciliidae | Gambusia holbrooki (Baird and Girard, 1853) | Carnivore | Introduced | F, M |
| 29 | Scophthalmidae | Scophthalmus rhombus (Linnaeus, 1758) | Carnivore | Straggler | M |
| 30 | Soleidae | Microchirus variegatus (Donovan, 1808) | Carnivore | Migrant | M |
| 31 | Solea solea (Linnaeus, 1758) | Carnivore | Migrant | M | |
| 32 | Sparidae | Dentex marocanus (Valenciennes, 1830) | Carnivore | Straggler | M |
| 33 | Diplodus annularis (Linnaeus, 1758) | Carnivore | Migrant | M | |
| 34 | Diplodus sargus (Linnaeus, 1758) | Omnivore | Migrant | M | |
| 35 | Lithognathus mormyrus (Linnaeus, 1758) | Carnivore | Migrant | M, E | |
| 36 | Liza aurata (Risso, 1810) | Omnivore | Migrant | M, E | |
| 37 | Sparus aurata (Linnaeus, 1758) | Carnivore | Migrant | M, E | |
| 38 | Syngnathidae | Syngnathus abaster (Risso, 1826) | Omnivore | Migrant | F, B, M |
| 39 | Syngnathus acus (Linnaeus, 1758) | Omnivore | Resident | B, M | |
| 40 | Syngnathus typhle (Linnaeus, 1758) | Omnivore | Resident | F, B, M |
| Metric | Abbreviation of the Metric | 1 | 3 | 5 | |
|---|---|---|---|---|---|
| 1 | Total Number of Species consisting the 90% of the total recorded | NSp90 | <2 | 2–4 | >4 |
| 2 | Relative Abundance | Rab | <77.6 | 77.6–879.69 | >879.69 |
| 3 | Shannon-Wiener Index | H’ind | <0.60 | 0.60–1.33 | >1.33 |
| 4 | Number of Residents | Nres | <3 | 3–5 | >5 |
| 5 | Number of Migrants | Nmig | >3 | 3–5 | <5 |
| 6 | Number of Stragglers | NStr | <2 | 2–4 | >4 |
| 7 | Presence of Sensitive Species | SentSp | Absent | Present | |
| 8 | Number of Omnivores | NOmn | >4 | 2–4 | <2 |
| Class | LFI | LFIEQR |
|---|---|---|
| High | ≥33.5–38 | ≥0.85–1.00 |
| Good | ≥27.5–<33.5 | ≥0.65–<0.85 |
| Moderate | ≥18.5–<27.5 | 0.35≥–<0.65 |
| Poor | ≥12.5–<18.5 | ≥0.15–<0.35 |
| Bad | 8–<12.5 | 0–<0.15 |
| Lagoon | LFI | Expert Judgment | CymoSkew [28] | EEI [29] | CymoSkewm [30] |
|---|---|---|---|---|---|
| Drana | Moderate | Moderate | Good | ||
| Monolimni | Good | Moderate | |||
| Keramoti | Poor | Poor | Moderate | Poor | |
| Ismarida | Moderate | Poor | Poor | ||
| Vassova | Moderate | Poor | Moderate | ||
| Agiasma | Moderate | Moderate | Good | Moderate | |
| Ptelea | Moderate | Moderate | Good | ||
| Xirolimni/Fanari | Poor | Moderate | High | Good | |
| Lafri | Moderate | Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapounidis, A.S.; Koutrakis, E.T. Development of a Fish-Based Multimetric Index for the Assessment of Lagoons’ Ecological Quality in Northern Greece. Water 2021, 13, 3008. https://doi.org/10.3390/w13213008
Sapounidis AS, Koutrakis ET. Development of a Fish-Based Multimetric Index for the Assessment of Lagoons’ Ecological Quality in Northern Greece. Water. 2021; 13(21):3008. https://doi.org/10.3390/w13213008
Chicago/Turabian StyleSapounidis, Argyrios S., and Emmanuil T. Koutrakis. 2021. "Development of a Fish-Based Multimetric Index for the Assessment of Lagoons’ Ecological Quality in Northern Greece" Water 13, no. 21: 3008. https://doi.org/10.3390/w13213008
APA StyleSapounidis, A. S., & Koutrakis, E. T. (2021). Development of a Fish-Based Multimetric Index for the Assessment of Lagoons’ Ecological Quality in Northern Greece. Water, 13(21), 3008. https://doi.org/10.3390/w13213008






