Spatial Distributions of Nitrogen and Phosphorus in Surface Sediments in Intertidal Flats of the Yellow River Delta, China
Abstract
1. Introduction
2. Materials and Methods
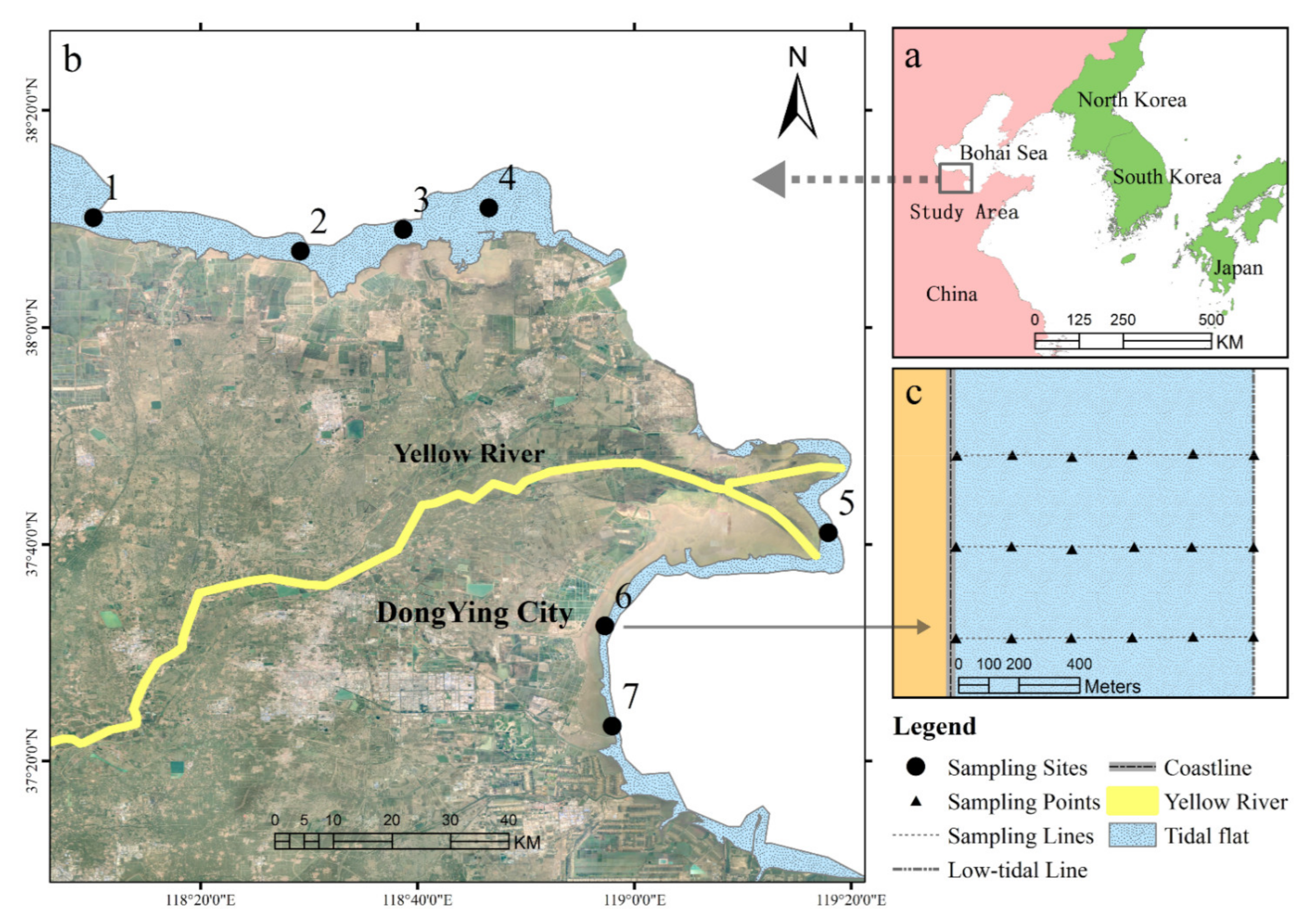
2.1. Study Area
2.2. Sample Collection
2.3. Characteristics and Analyses of the Sediments
2.4. Statistical Analyses
3. Results
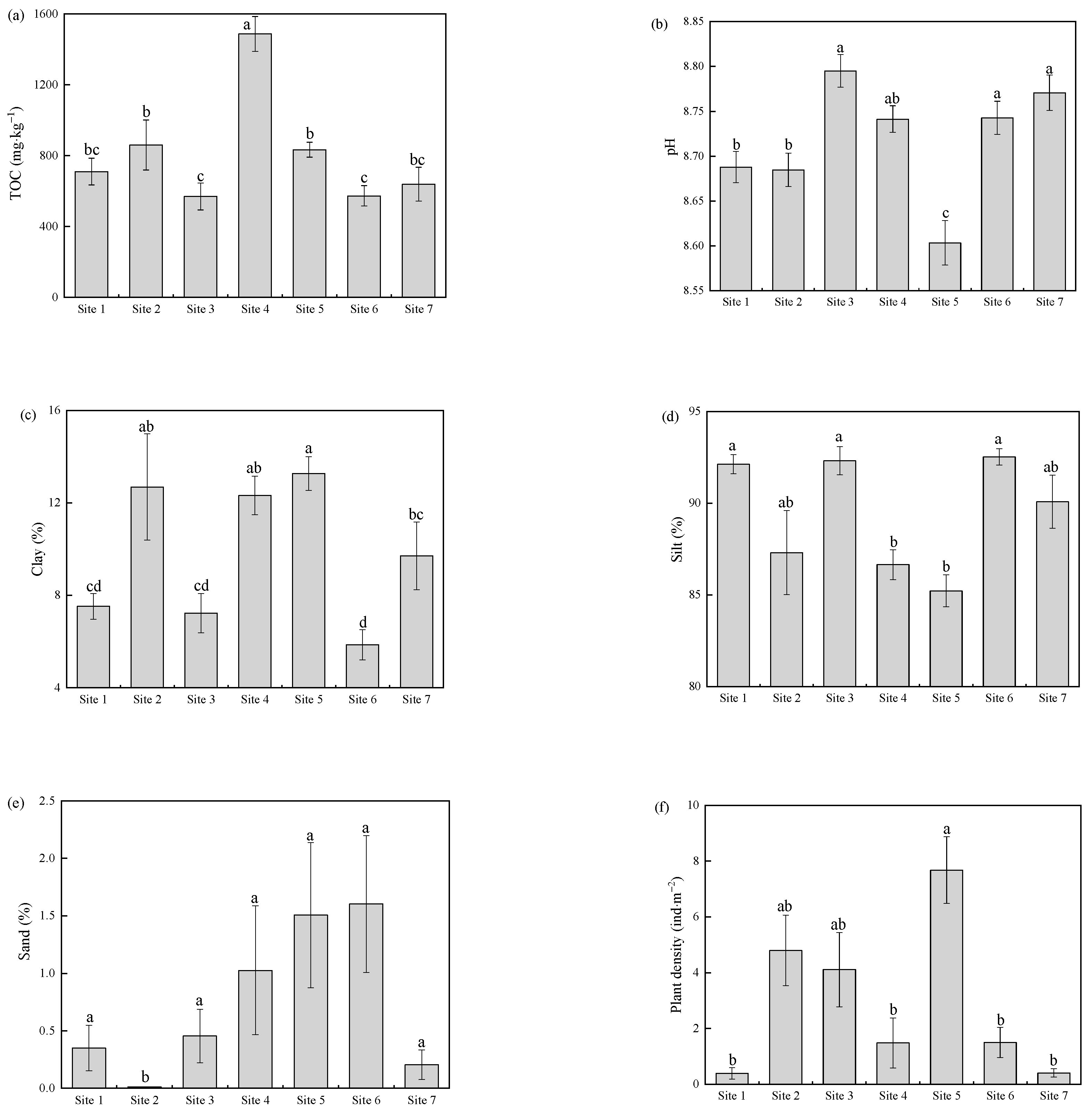
3.1. The Physicochemical Characterization and Plant Distribution Characteristics of the Sediments
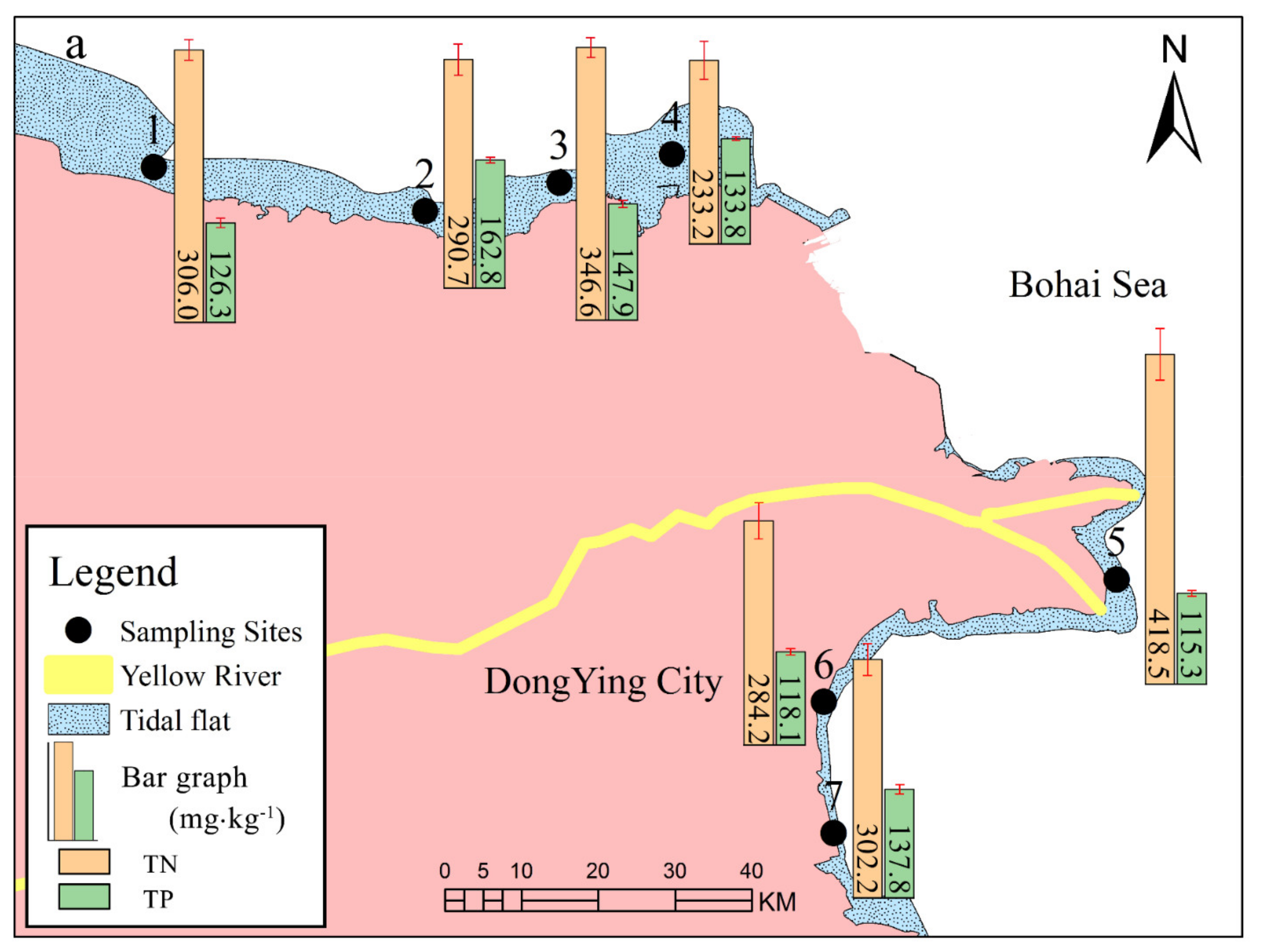
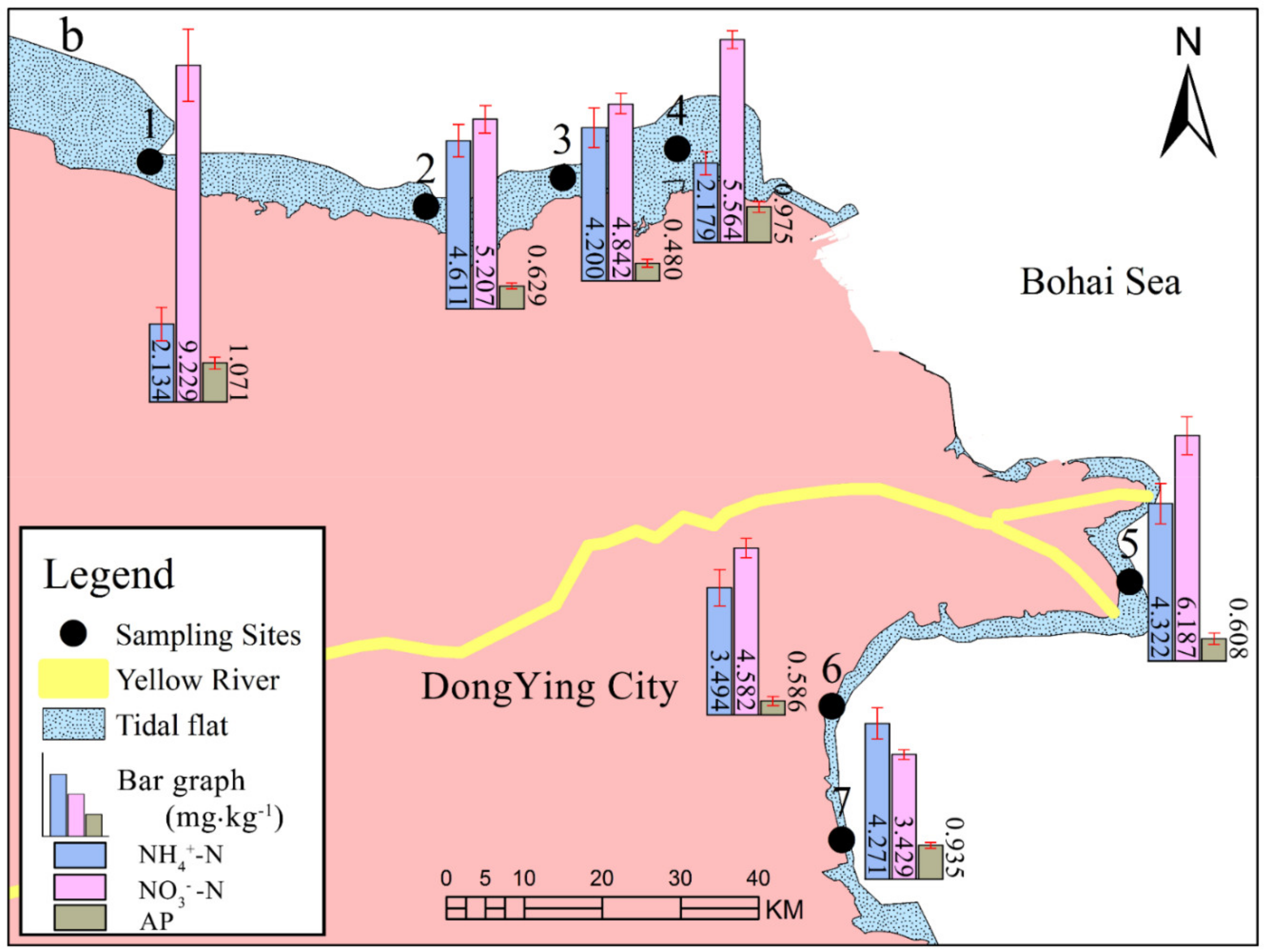
3.2. Nitrogen (N) and Phosphorus (P) Fractions of the Sediments at Different Locations
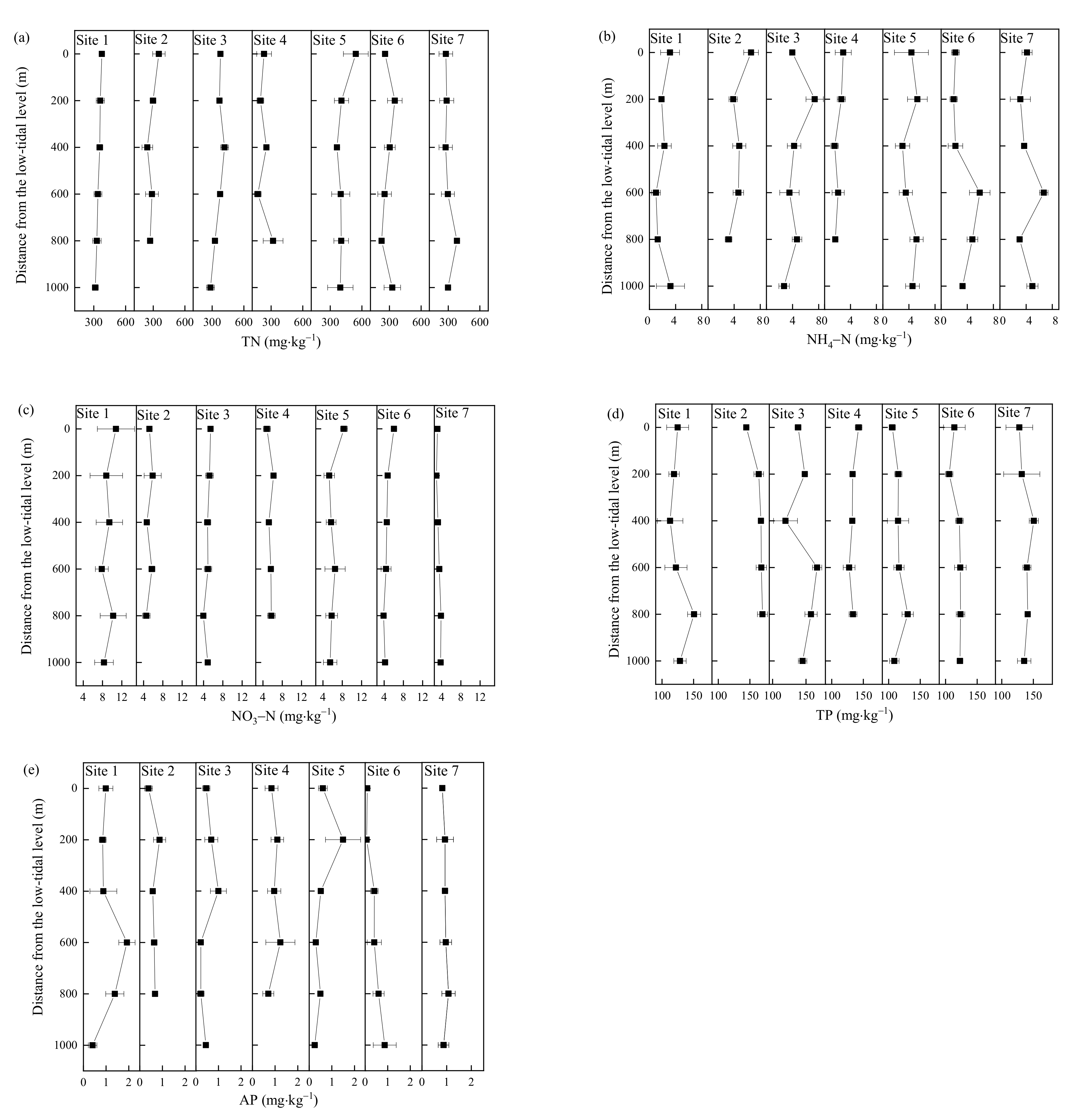
3.3. N and P Fractions of the Sediments at Different Distances from the Low-Tidal Level
3.4. The Influencing Factors of Spatial Distributions of N and P
4. Discussion
4.1. Spatial Changes in the N and P Fractions in the Coastal Tidal Flat Sediments of China
4.2. Effects of the Sediments’ Environmental Factors on N and P in the Sediments
4.3. Effects of Vegetation on N and P in the Sediments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Qu, K.; Zhao, J.; Cui, Z.; Xia, B. Assessment of Environmental Water Quality in the Central Region of the Bohai Sea in Summer. Pro. Fish. Sci. 2017, 38, 8–15. [Google Scholar]
- Zhou, K.; Fan, J.; Liu, H. Spatiotemporal patterns and driving forces of water pollutant discharge in the Bohai Rim Region. Pro. Geogr. 2017, 36, 171–181. [Google Scholar]
- Song, N.; Wang, N.; Wu, N.; Lin, W. Temporal and spatial distribution of harmful algal blooms in the Bohai Sea during 1952~2016 based on GIS. China Environ. Sci. 2018, 38, 1142–1148. [Google Scholar]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Li, X.; Yu, R.; Geng, H.; Li, Y. Increasing dominance of dinoflagellate red tides in the coastal waters of Yellow Sea. China. Mar. Pollut. Bull. 2021, 168, 112439. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, M.; Bao, C.; Lan, D.; Xu, Y.; Ma, M. Evaluation and risk prediction of eutrophication in Bohai Sea. Mar. Environ. Sci. 2015, 34, 373–376. [Google Scholar]
- Liu, Z.-J.; Li, P.-Y.; Zhang, X.-L.; Li, P.; Zhu, L.-H. Regional distribution and ecological risk evaluation of heavy metals in surface sediments from coastal wetlands of the Yellow River Delta. Huanjing Ke Xue 2012, 33, 1182–1188. [Google Scholar]
- Wang, Y.; Su, Y. The geo-pattern of course shifts of the Lower Yellow River. J. Geogr. Sci. 2011, 21, 1019–1036. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, S.E.; Kim, D.J.; Moon, W.M. Investigation of physical characteristics of intertidal zone using NASA/JPL fully polarimetric AIRSAR data. In Proceedings of the 25th IEEE International Geoscience and Remote Sensing Symposium (IGARSS 2005), New York, NY, USA, 25–29 July 2005; pp. 2082–2085. [Google Scholar] [CrossRef]
- Nishio, T.; Nishijima, W.; Hamada, K.; Hata, K.; Nakata, K. Model estimation of the water purification capacity of lost tidal flats. Jarq-Jpn. Agric. Res. Q. 2016, 50, 345–352. [Google Scholar] [CrossRef][Green Version]
- Xu, C.; Pu, L.; Zhu, M. Effect of reclamation activity on coastal ecological environment: Progress and perspectives. Acta Ecologica Sinica 2018, 38, 1148–1162. [Google Scholar]
- Murray, N.J.; Phinn, S.R.; DeWitt, M.; Ferrari, R.; Johnston, R.; Lyons, M.B.; Clinton, N.; Thau, D.; Fuller, R.A. The global distribution and trajectory of tidal flats. Nature 2019, 565, 222. [Google Scholar] [CrossRef] [PubMed]
- Ro, H.M.; Kim, P.G.; Park, J.S.; Yun, S.I.; Han, J. Nitrogen removal through N cycling from sediments in a constructed coastal marsh as assessed by N-15-isotope dilution. Mar. Pollut. Bull. 2018, 129, 275–283. [Google Scholar] [CrossRef]
- Widney, S.; Klein, A.K.; Ehman, J.; Hackney, C.; Craft, C. The value of wetlands for water quality improvement: An example from the St. Johns River watershed, Florida. Wetl. Ecol. Manag. 2018, 26, 265–276. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Bai, J.H.; Jia, J.; Wen, X.J.; Zhang, G.L.; Zhao, Q.Q. Spatial and temporal changes of phosphorus in coastal wetland soils as affected by a tidal creek in the Yellow River Estuary. China Phys. Chem. Earth 2015, 89, 114–120. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Fang, H.J.; Bai, J.H.; Jia, J.; Lu, Q.Q.; Wang, J.J.; Chen, B. Spatial and seasonal distributions of soil phosphorus in a short-term flooding wetland of the Yellow River Estuary, China. Ecol. Inform. 2016, 31, 83–90. [Google Scholar] [CrossRef]
- Watabe, Y.; Sassa, S. Application of MASW technology to identification of tidal flat stratigraphy and its geoenvironmental interpretation. Mar. Geol. 2008, 252, 79–88. [Google Scholar] [CrossRef]
- Kim, T.; Noh, J.; Kwon, B.; Lee, C.; Kim, B.; Kwon, I.; Hong, S.; Chang, G.S.; Chang, W.K.; Nam, J.; et al. Natural purification capacity of tidal flats for organic matters and nutrients: A mesocosm study. Mar. Pollut. Bull. 2020, 154, 111046. [Google Scholar] [CrossRef]
- Li, S.; Cui, B.; Xie, T.; Zhang, K. Diversity pattern of macrobenthos associated with different stages of wetland restoration in the Yellow River Delta. Wetlands 2016, 36, 57–67. [Google Scholar] [CrossRef]
- Li, X.W.; Hou, X.Y.; Song, Y.; Shan, K.; Zhu, S.Y.; Yu, X.B.; Mo, X.Q. Assessing changes of habitat quality for shorebirds in stopover sites: A case study in Yellow River Delta, China. Wetlands 2019, 39, 67–77. [Google Scholar] [CrossRef]
- Bolleter, W.T.; Bushman, C.J.; Tidwell, P.W. Spectrophotometric determination of ammonia as indophenol. Anal. Chem. 1961, 33, 592–594. [Google Scholar] [CrossRef]
- Parkinson, J.A.; Allen, S.E. Wet oxidation procedure suitable for determination of nitrogen and mineral nutrients in biological. Commun. Soil Sci. Plant Anal. 1975, 6, 1–11. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yan, M.C. Abundance of chemical elements in China’s shallow sea sediments. Scientia Sinica (Chimica B) 1993, 23, 1084. [Google Scholar]
- Neumann, T.; Stogbauer, A.; Walpersdorf, E.; Stuben, D.; Kunzendorf, H. Stable isotopes in recent sediments of Lake Arendsee, NE Germany: Response to eutrophication and remediation measures. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 178, 75–90. [Google Scholar] [CrossRef]
- Weissteiner, C.J.; Bouraoui, F.; Aloe, A. Reduction of nitrogen and phosphorus loads to European rivers by riparian buffer zones. Knowl. Manag. Aquat. Ecosyst. 2013, 408, 8. [Google Scholar] [CrossRef]
- Sun, J.N.; Xu, G.; Shao, H.B.; Xu, S.H. Potential retention and release capacity of phosphorus in the newly formed wetland soils from the Yellow River delta, China. Clean-Soil Air Water 2012, 40, 1131–1136. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, F.; Liu, Q.; Liu, Y.; Li, D. Characteristics of N: P stoichiometry and the adaptation strategies for different coastal wetland communities in the Yellow River Delta. Chinese J. Ecol. 2015, 34, 2983–2989. [Google Scholar]
- Wang, J.; Zhang, W.; Guo, N.; Li, C.; Wang, J. The Key Factor of Impact on Temporal and Spatial Variation of Soil Organic Matter, TN and TP in Coastal Salt Marsh: Tide and Vegetation. Sci. Geogr. Sin. 2016, 36, 247–255. [Google Scholar]
- Yin, A.J.; Gao, C.; Zhang, M.; Wu, P.B.; Yang, X.H. Rapid changes in phosphorus species in soils developed on reclaimed tidal flat sediments. Geoderma 2017, 307, 46–53. [Google Scholar] [CrossRef]
- Xu, Y.; Pu, L.J.; Liao, Q.L.; Zhu, M.; Yu, X.; Mao, T.Y.; Xu, C.X. Spatial variation of soil organic carbon and total nitrogen in the coastal area of mid-eastern China. Intern. J. Environ. Res. Pub. Health 2017, 14, 12. [Google Scholar] [CrossRef]
- Cao, L.; Song, J.M.; Li, X.G.; Yuan, H.M.; Li, N.; Duan, L.Q.; Wang, Q.D. Geochemical characteristics of soil C, N, P, and their stoichiometrical significance in the coastal wetlands of Laizhou Bay, Bohai Sea. Clean-Soil Air Water 2015, 43, 260–270. [Google Scholar] [CrossRef]
- Hou, L.J.; Wang, R.; Yin, G.Y.; Liu, M.; Zheng, Y.L. Nitrogen fixation in the intertidal sediments of the Yangtze estuary: Occurrence and environmental implications. J. Geophys. Res. Biogeosci. 2018, 123, 936–944. [Google Scholar] [CrossRef]
- Shao, X.X.; Liang, X.Q.; Wu, M.; Gu, B.H.; Li, W.H.; Sheng, X.C.; Wang, S.X. Influences of sediment properties and macrophytes on phosphorous speciation in the intertidal marsh. Environ. Sci. Pollut. Res. 2014, 21, 10432–10441. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zeng, C.S.; Tong, C.; Zhai, S.J.; Lin, X.; Gao, D.Z. Spatial distribution of phosphorus speciation in marsh sediments along a hydrologic gradient in a subtropical estuarine wetland, China. Estuar. Coast. Shelf Sci. 2015, 154, 30–38. [Google Scholar] [CrossRef]
- Guo, P.Y.; Sun, Y.S.; Su, H.T.; Wang, M.X.; Zhang, Y.X. Spatial and temporal trends in total organic carbon (TOC), black carbon (BC), and toted nitrogen (TN) and their relationships under different planting patterns in a restored coastal mangrove wetland: Case study in Fujian, China. Chem. Speciat. Bioavail. 2018, 30, 47–56. [Google Scholar] [CrossRef]
- Zhou, J.L.; Wu, Y.; Kang, Q.S.; Zhang, J. Spatial variations of carbon, nitrogen, phosphorous and sulfur in the salt marsh sediments of the Yangtze Estuary in China. Estuar. Coast. Shelf Sci. 2007, 71, 47–59. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Leng, Y.; Cui, W. Nutrient distribution characteristics and its annual variations in the vicinity waters of the Yellow River estuary. Mari. Sci. Bull. 2013, 32, 383–388. [Google Scholar]
- Hou, L.J.; Liu, M.; Xu, S.Y.; Ou, D.N.; Yu, J.; Cheng, S.B.; Lin, X.; Yang, Y. The effects of semi-lunar spring and neap tidal change on nitrification, denitrification and N2O vertical distribution in the intertidal sediments of the Yangtze estuary, China. Estuar. Coast. Shelf Sci. 2007, 73, 607–616. [Google Scholar] [CrossRef]
- Steinman, A.D.; Ogdahl, M.E.; Weinert, M.; Uzarski, D.G. Influence of water-level fluctuation duration and magnitude on sediment-water nutrient exchange in coastal wetlands. Aquat. Ecol. 2014, 48, 143–159. [Google Scholar] [CrossRef]
- Xu, S.Q.; Wang, Y.D.; Guo, C.C.; Zhang, Z.G.; Shang, Y.T.; Chen, Q.; Wang, Z.L. Comparison of microbial community composition and diversity in native coastal wetlands and wetlands that have undergone long-term agricultural reclamation. Wetlands 2017, 37, 99–108. [Google Scholar] [CrossRef]
- Yim, J.; Kwon, B.O.; Nam, J.; Hwang, J.H.; Choi, K.; Khim, J.S. Analysis of forty years long changes in coastal land use and land cover of the Yellow Sea: The gains or losses in ecosystem services. Environ. Pollut. 2018, 241, 74–84. [Google Scholar] [CrossRef]
- Adhami, E.; Owliaie, H.R.; Molavi, R.; Rashti, M.R.; Esfandbod, M. Effects of soil properties on phosphorus fractions in subtropical soils of Iran. J. Soil Sci. Plant Nutr. 2013, 13, 11–21. [Google Scholar] [CrossRef][Green Version]
- Bai, J.H.; Ye, X.F.; Jia, J.; Zhang, G.L.; Zhao, Q.Q.; Cui, B.S.; Liu, X.H. Phosphorus sorption-desorption and effects of temperature, pH and salinity on phosphorus sorption in marsh soils from coastal wetlands with different flooding conditions. Chemosphere 2017, 188, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.P.; Mwamila, L.B.; Kergoat, K. The pH dependence of phosphate sorption and desorption in Swedish agricultural soils. Geoderma 2012, 189, 304–311. [Google Scholar] [CrossRef]
- Lin, X.B.; Hou, L.J.; Liu, M.; Li, X.F.; Zheng, Y.L.; Yin, G.Y.; Gao, J.; Jiang, X.F. Nitrogen mineralization and immobilization in sediments of the east China sea: Spatiotemporal variations and environmental implications. J. Geophys. Res. Biogeosci. 2016, 121, 2842–2855. [Google Scholar] [CrossRef]
- Yang, W.J.; Hao, F.H.; Cheng, H.G.; Lin, C.Y.; Ouyang, W. Phosphorus fractions and availability in an albic bleached meadow soil. Agron. J. 2013, 105, 1451–1457. [Google Scholar] [CrossRef]
- Peng, J.; Chen, S.; Liu, F.; Lu, Q.; Chen, Y. Erosion and siltation processes in the lower Yellow River during different river courses into the sea. Acta Geographica Sinica 2010, 65, 613–622. [Google Scholar]
- Xu, W.; Chen, S.; Li, P.; Gu, G. Distribution characteristics of sedimentation and suspended load and their indications for erosion-siltation in the littoral of Yellow River Delta. J. Sed. Res. 2016, 3, 24–30. [Google Scholar]
- Zhao, K.; Fan, H.; Jiang, X.; Song, J. Improvement and utilization of saline soil by planting halophytes. Chin. J. Appl. Environ. Biol. 2002, 8, 31–35. [Google Scholar]
- Li, Q.; Luo, L.; Chen, Y.; Wang, W. Selection of salt-tolerant plants for high-salinity wastewater treatment by constructed wetlands. Chin. J. Appl. Environ. Biol. 2017, 23, 873–878. [Google Scholar]
- Matzke, S.; Elsey-Quirk, T. Spartina patens Productivity and soil organic matter response to sedimentation and nutrient enrichment. Wetlands 2018, 38, 1233–1244. [Google Scholar] [CrossRef]
- Hayes, M.A.; Jesse, A.; Tabet, B.; Reef, R.; Keuskamp, J.A.; Lovelock, C.E. The contrasting effects of nutrient enrichment on growth, biomass allocation and decomposition of plant tissue in coastal wetlands. Plant Soil 2017, 416, 193–204. [Google Scholar] [CrossRef]
- Phillips, D.H.; Kumara, M.P.; Jayatissa, L.P.; Krauss, K.W.; Huxham, M. Impacts of Mangrove Density on Surface Sediment Accretion, Belowground Biomass and Biogeochemistry in Puttalam Lagoon, Sri Lanka. Wetlands 2017, 37, 471–483. [Google Scholar] [CrossRef]
- Du, N.; Wu, P.; Eller, F.; Zhou, D.Y.; Liu, J.; Gan, W.H.; Yang, R.R.; Dai, M.; Chen, Y.D.; Wang, R.Q.; et al. Facilitation or competition? The effects of the shrub species tamarix chinensis on herbaceous communities are eependent on the successional stage in an impacted coastal wetland of north China. Wetlands 2017, 37, 899–911. [Google Scholar] [CrossRef]
- Mou, X.J.; Sun, Z.G.; Wang, L.L.; Wang, C.Y. Nitrogen cycle of a typical Suaeda salsa marsh ecosystem in the Yellow River estuary. J. Environ. Sci. 2011, 23, 958–967. [Google Scholar] [CrossRef]

| Source of Variation | d.f. | TN | NH4–N | NO3–N | TP | AP | TOC | pH | Clay | Silt | Sand | Plant Density |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample sites | 6 | 7.562 *** | 5.211 *** | 14.448 *** | 12.875 *** | 4.274 *** | 13.366 *** | 11.462 *** | 6.897 *** | 7.327 *** | 2.359 * | 8.313 *** |
| Distance from the low-tidal level | 6 | 0.929 | 0.429 | 0.492 | 1.444 | 0.803 | 1.005 | 0.446 | 0.953 | 0.368 | 3.621 *** | 1.766 |
| NH4–N | NO3–N | TN | AP | TP | Clay | Silt | Sand | pH | TOC | |
|---|---|---|---|---|---|---|---|---|---|---|
| NO3–N | −0.147 | 1 | ||||||||
| TN | −0.017 | 0.127 | 1 | |||||||
| AP | −0.094 | 0.124 | −0.006 | 1 | ||||||
| TP | 0.084 | −0.118 | −0.183 * | −0.156 | 1 | |||||
| Clay | 0.272 ** | −0.037 | 0.093 | 0.085 | 0.026 | 1 | ||||
| Silt | −0.236 * | −0.008 | −0.047 | −0.022 | 0.105 | −0.519 ** | 1 | |||
| Sand | 0.072 | 0.037 | −0.014 | −0.038 | −0.141 | −0.139 | −0.774 ** | 1 | ||
| pH | −0.206 * | −0.210 * | −0.276 ** | −0.217 * | 0.250 ** | −0.488 ** | 0.441 ** | −0.151 | 1 | |
| TOC | −0.043 | 0.134 | −0.066 | 0.228 * | −0.034 | 0.595 ** | −0.394 ** | 0.017 | −0.376 ** | 1 |
| Plant density | 0.070 | 0.081 | 0.310 ** | 0.010 | −0.165 | 0.133 | −0.166 | 0.094 | −0.315 ** | 0.139 |
| Study Area | TN | NH4–N | NO3–N | TP | AP | Reference | |
|---|---|---|---|---|---|---|---|
| Non-estuary region | The Yellow River Delta | 233.2–358.5 | 2.130–4.610 | 3.430–9.230 | 118.6–162.8 | 0.410–1.070 | this study |
| The Northern coast of Jiangsu Province in Mid-Eastern China | 300.0–560.0 | - | - | 590.0–1150.0 | - | [29] | |
| The Northern coast of Jiangsu Province in Mid-Eastern China | - | - | - | 594.8–887.9 | - | [30] | |
| The Southeast coast of Jiangsu Province in Mid-Eastern China | 210.0 | - | - | - | - | [31] | |
| Estuary | The Yellow River Estuary | 418.5 | 4.322 | 6.187 | 115.3 | 0.608 | this study |
| The Yellow River Estuary | 608.0 | - | - | 716.0 | - | [28] | |
| The Yellow River Estuary | 120.0–340.0 | - | - | 585.8–633.5 | 2.750–4.900 | [16] | |
| The Yellow River Estuary | - | - | - | 692.0 | - | [27] | |
| The Yihong River Estuary in Laizhou Bay | 213.0–317.0 | - | - | 336.0–393.0 | - | [32] | |
| The Yangtze Estuary | - | 34.100–106.300 | 2.600–5.500 | - | - | [33] | |
| The Qiantang River Estuary | - | - | - | 560.0–680.0 | - | [34] | |
| The Min River Estuary | - | - | - | 338.0–930.0 | - | [35] | |
| The Jinjiang Estuary | 1100.0–2560.0 | - | - | - | - | [36] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, G.; Qi, Y.; Li, J.; Zhao, C.; He, J.; Ma, Y.; Zhu, J. Spatial Distributions of Nitrogen and Phosphorus in Surface Sediments in Intertidal Flats of the Yellow River Delta, China. Water 2021, 13, 2899. https://doi.org/10.3390/w13202899
Fu G, Qi Y, Li J, Zhao C, He J, Ma Y, Zhu J. Spatial Distributions of Nitrogen and Phosphorus in Surface Sediments in Intertidal Flats of the Yellow River Delta, China. Water. 2021; 13(20):2899. https://doi.org/10.3390/w13202899
Chicago/Turabian StyleFu, Gang, Yue Qi, Junsheng Li, Caiyun Zhao, Jing He, Yiwen Ma, and Jinfang Zhu. 2021. "Spatial Distributions of Nitrogen and Phosphorus in Surface Sediments in Intertidal Flats of the Yellow River Delta, China" Water 13, no. 20: 2899. https://doi.org/10.3390/w13202899
APA StyleFu, G., Qi, Y., Li, J., Zhao, C., He, J., Ma, Y., & Zhu, J. (2021). Spatial Distributions of Nitrogen and Phosphorus in Surface Sediments in Intertidal Flats of the Yellow River Delta, China. Water, 13(20), 2899. https://doi.org/10.3390/w13202899






