Experimental Study for Sand Filter Backwash Water Management: Low-Cost Treatment for Recycling and Residual Sludge Utilization for Radium Removal
Abstract
:1. Introduction
2. Materials and Methods
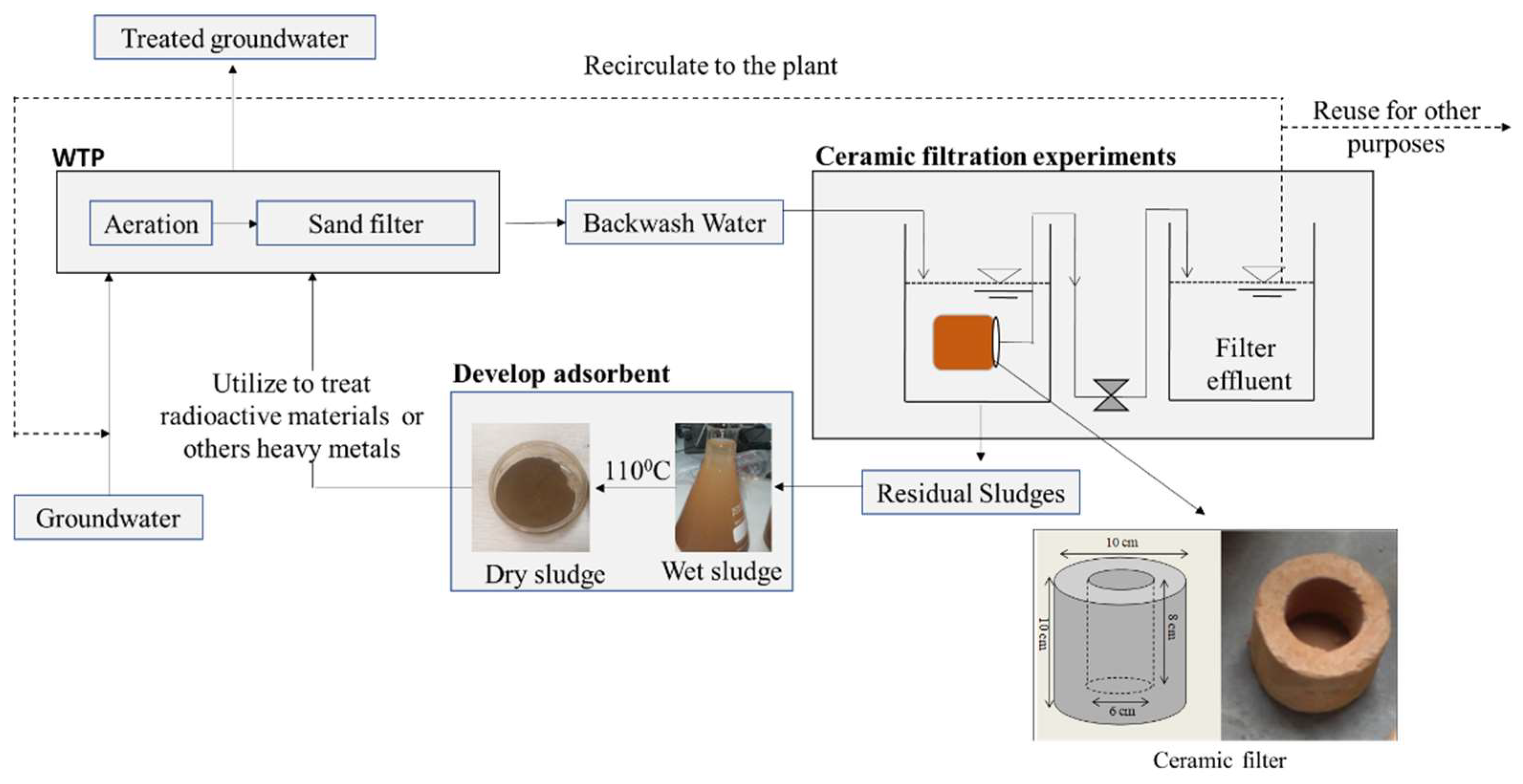
2.1. Conceptual Model for Sustainable BW Management
2.2. Collection of Backwash Water
2.3. Clay Ceramic Filter
2.4. Ceramic Filtration Experiments
2.5. Water Treatment Backwash Sludge (WTBS)
2.6. Characterization of WTBS
2.7. Adsorption Kinetics Experiments
2.8. Kinetic Adsorption Models
2.9. Water Quality Analysis
2.10. Econmomic Analysis of the Ceramic Filtration Process
3. Results
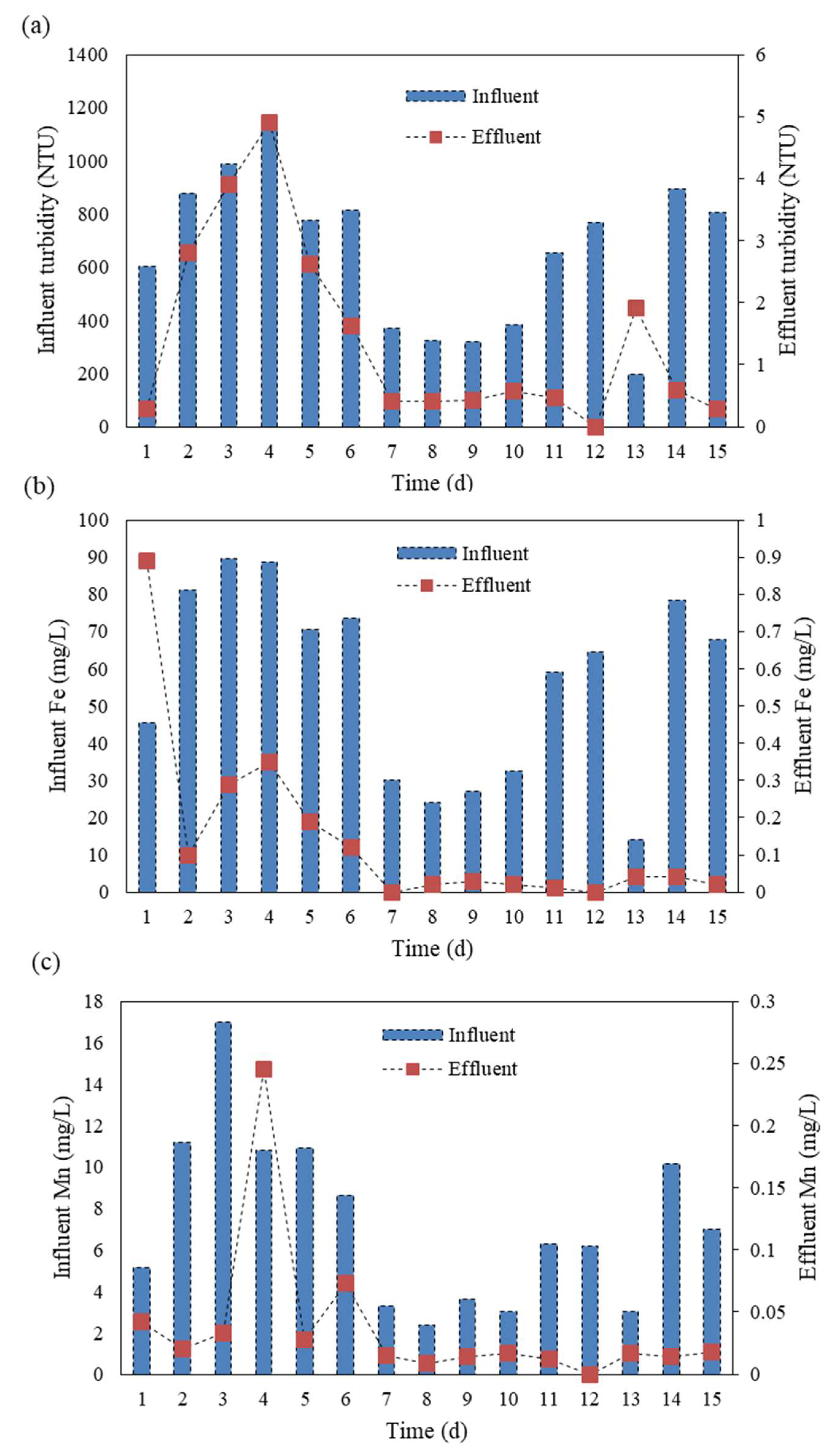
3.1. Characteristics of BW and Treatment Efficiency
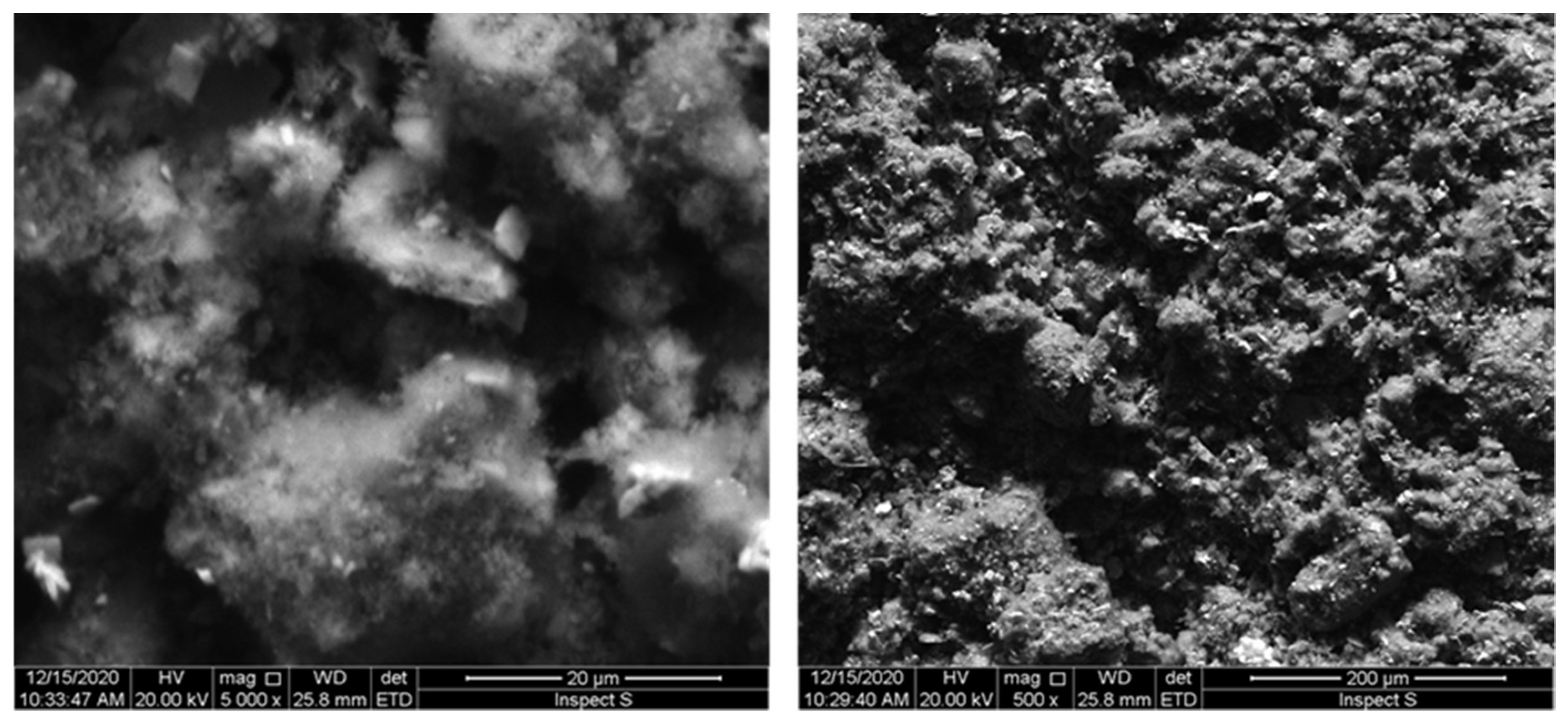
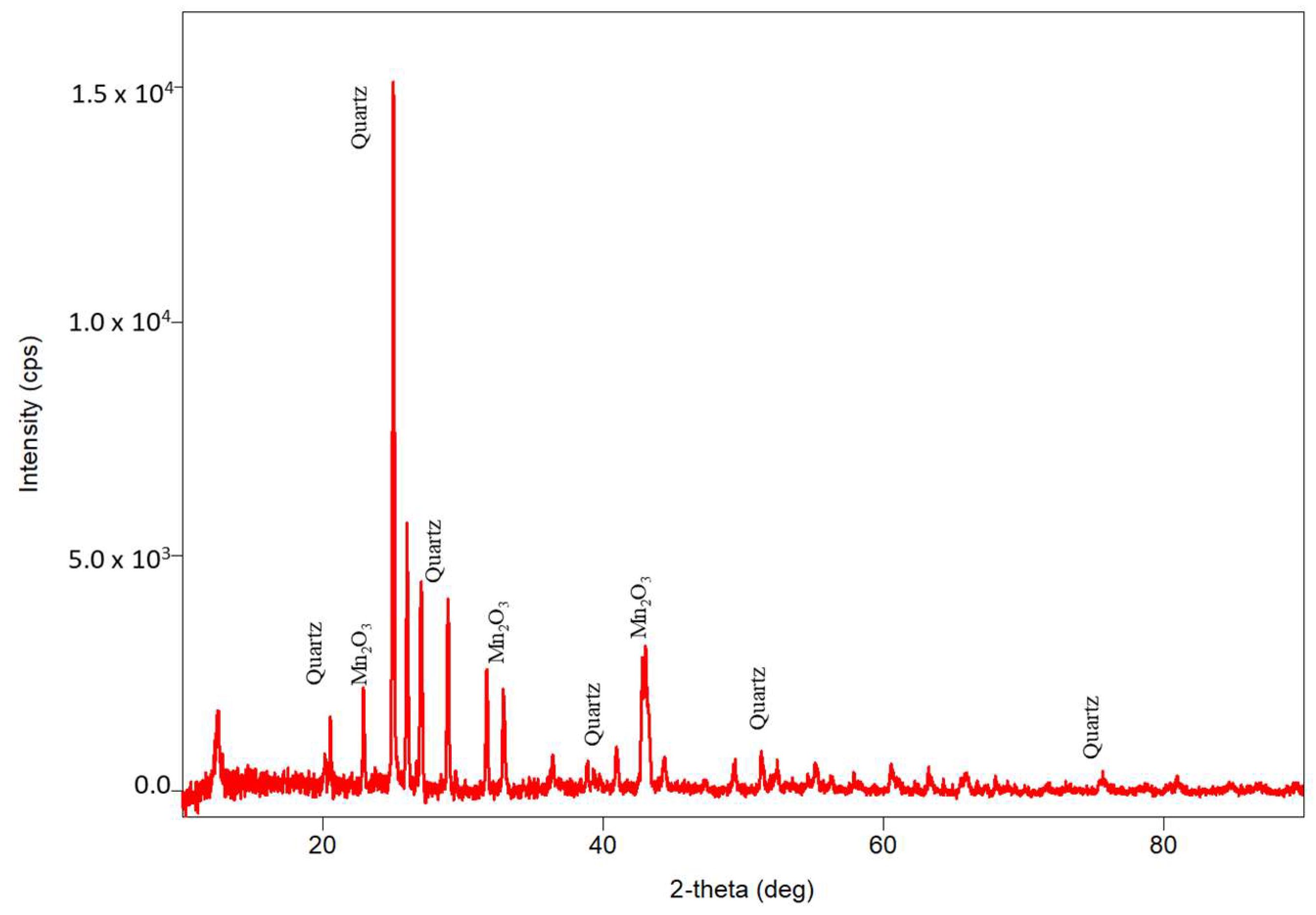
3.2. Characterizations of WTBS
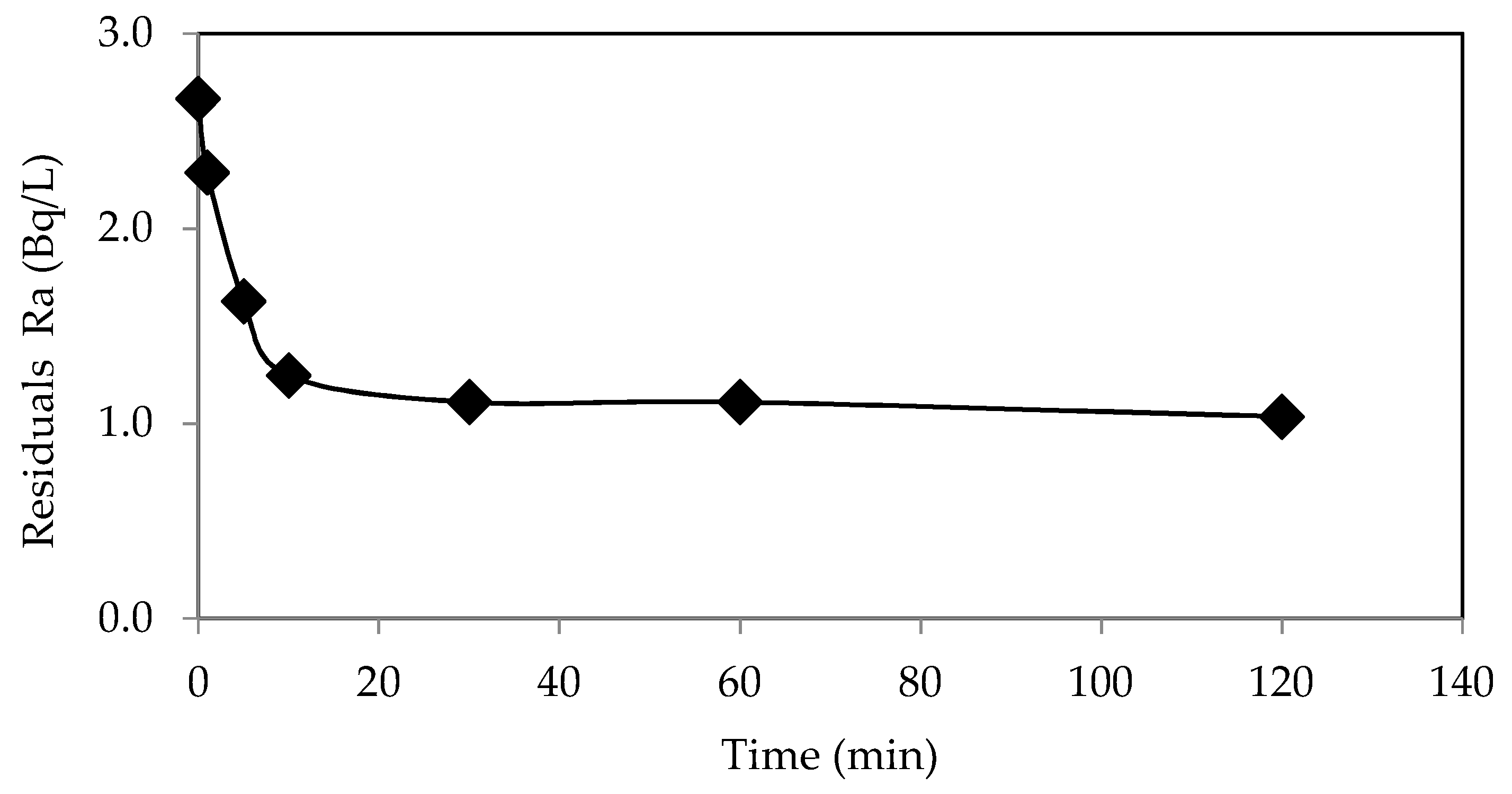
3.3. Kinetic Studies of Ra Removal by WTBS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, M.E.; Gagnon, G.A.; Alam, Z.; Andrews, R.C. Bio-stability and disinfectant by-product formation in drinking water blended with UF-treated filter backwash water. Water Res. 2008, 42, 2135–2145. [Google Scholar] [CrossRef]
- Kim, Y.H.; Eom, J.Y.; Kim, K.Y.; Lee, Y.S.; Kim, H.S.; Hwang, S.J. Applicability study of backwash water treatment using tubular membrane system with dead-end filtration operation mode. Desalination 2010, 261, 104–110. [Google Scholar] [CrossRef]
- Weiying, L.; Yuasa, A.; Bingzhi, D.; Naiyun, G. Study on backwash wastewater from rapid sand-filter by monolith ceramic membrane. Desalination 2010, 250, 712–715. [Google Scholar] [CrossRef]
- Shafiquzzaman, M.; Al-Mahmud, A.; AlSaleem, S.S.; Haider, H. Application of a Low Cost ceramic filter for Recycling Sand Filter Backwash Water. Water 2018, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Verliefde, A.R.D.; Kemperman, A.J.B.; VanDijk, J.C.; Amya, G. Impact of backwash water composition on ultrafiltration fouling control. J. Membr. Sci. 2009, 344, 17–25. [Google Scholar] [CrossRef]
- Ling, Z.L.; Dong, Y.; Zi-Jie, Z.; Ping, G. Application of hybrid coagulation–microfiltration process for treatment of membrane backwash water from water works. Sep. Purif. Technol. 2008, 62, 415–422. [Google Scholar]
- Huanga, C.; Lina, J.R.; Leea, W.S.; Pan, J.R.; Zhao, B. Effect of coagulation mechanism on membrane permeability incoagulation-assisted microfiltration for spent filter backwash water recycling. Colloids Surf. A Physicochem. Eng. Asp. 2011, 378, 72–78. [Google Scholar] [CrossRef]
- Sardari, R.; Osouleddini, N. The dataontheremovalofturbidityand biological agentsinspent filter backwash by bed ceramic in water treatment process. Data Brief 2018, 19, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.C.; O’Connor, G.A. Chapter 28 Beneficial utilization of drinking-water treatment residuals as contaminant-mitigating agents. Develop. Environ. Sci. 2007, 5, 609–635. [Google Scholar]
- Ocinski, D.; Jacukowicz-Sobala, I.; Mazur, P.; Raczyk, J.; Kociołek-Balawejder, Z. Water treatment residuals containing iron and manganese oxides or arsenic removal from wate—Characterization of physicochemical properties and adsorption studies. Chem. Eng. J. 2016, 294, 210–221. [Google Scholar] [CrossRef]
- Condomines, M.; Rihs, S.; Lloret, E.; Seidel, J. Determination of the four natural Ra isotopes in thermal waters by gamma-ray spectrometry. Appl. Radiat. Isot. 2010, 68, 384–391. [Google Scholar] [CrossRef]
- Al-Masri, M.; Suman, H. NORM waste management in the oil and gas industry: The Syrian experience. J. Radioanal. Nucl. Chem. 2003, 256, 159–162. [Google Scholar] [CrossRef]
- Testa, C.; Desideri, D.; Meli, M.; Roselli, C.; Bassignani, A.; Finazzi, P. Determination of uranium, thorium and radium in waters, soils and muds around a uranium mine in decommissioning. Appl. Radiat. Isot. 1994, 45, 394. [Google Scholar] [CrossRef]
- Barišić, D.; Lulić, S.; Miletić, P. Radium and uranium in phosphate fertilizers and their impact on the radioactivity of waters. Water Res. 1992, 26, 607–611. [Google Scholar] [CrossRef]
- USEPA. Guidelines for Developmental Toxicity Risk Assessment; Risk Assessment Forum, U.S. Environmental Protection Agency: Washington, DC, USA, 1991.
- USEPA. United States Environmental Protection Agency: Drinking Water Regulations and Health Advisories; EPA 822-B-96-002; Office of Water, USEPA: Washington, DC, USA, 1996.
- WHO. World Health Organization, Management of Radioactivity in Drinking-Water; WHO: Geneva, Switzerland, 2018; ISBN 78-92-4-151374-6. [Google Scholar]
- Alkhomashi, N.; Al-Hamarneh, I.F.; Almasoud, F. Determination of natural radioactivity in irrigation water of drilled wells in northwestern Saudi Arabia. Chemosphere 2016, 144, 1928–1936. [Google Scholar] [CrossRef]
- Almasoud, F.; Ababneh, Z.Q.; Alanazi, Y.J.; Khandaker, M.U.; Sayyed, M. Assessment of radioactivity contents in bedrock groundwater samples from the northern region of Saudi Arabia. Chemosphere 2020, 242, 125181. [Google Scholar] [CrossRef]
- Kumar, A.; Karpe, R.; Rout, S.; Gautam, Y.P.; Mishra, M.; Ravi, P.; Tripathi, R. Activity ratios of 234U/ 238U and 226Ra/ 228Ra for transport mechanisms of elevated uranium in alluvial aquifers of groundwater in south-western (SW) Punjab, India. J. Environ. Radioact. 2016, 151, 311–320. [Google Scholar] [CrossRef]
- Turhan, S.; Özçıtak, E.; Ta¸skın, H.; Varinlioglu, A. Determination of natural radioactivity by gross alpha and beta measurements in ground water samples. Water Res. 2013, 47, 3103–3108. [Google Scholar] [CrossRef] [PubMed]
- Al-Zubari, W. Sustainable Water Consumption in Arab Countries. In Arab Environment: Sustainable Consumption. Annual Report of Arab Forum for Environment and Development, 2015; Abdel Gelil, I., Saab, N., Eds.; Technical Publications: Beirut, Lebanon, 2015; pp. 108–133. [Google Scholar]
- Haider, H.; Al-Salamah, I.S.; Ghumman, A.R. Development of Groundwater Quality Index using Fuzzy-based Multicriteria Analysis for Buraydah, Qassim, Saudi Arabia. Arab. J. Sci. Eng. 2017, 49, 4033–4051. [Google Scholar] [CrossRef]
- Al-Hamarneh, I.F.; Alkhomashi, N.; Almasoud, F. Study on the radioactivity and soil-to-plant transfer factor of 226Ra, 234U and 238U radionuclides in irrigated farms from the northwestern Saudi Arabia. J. Environ. Radioact. 2016, 160, 1–7. [Google Scholar] [CrossRef]
- Khedr, M.G. Radioactive contamination of groundwater, special aspects and advantages of removal by reverse osmosis and nanofiltration. Desalination 2013, 321, 47–54. [Google Scholar] [CrossRef]
- Baeza, A.; Salas, A.; Guill_en, J.; Mu~noz-Serrano, A.; Ontalba-Salamanca, M.A.; Jim_enez-Ramos, M.C. Removal naturally occurring radionuclides from drinking water using a filter specifically designed for Drinking Water Treatment Plants. Chemosphere 2017, 167, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.A.; Zhang, Z. Modifying ion exchange for combined removal of uranium and radium. J. Am. Water Works Assoc. 1994, 86, 214–227. [Google Scholar] [CrossRef]
- Samolej, K.; Chalupnik, S. Investigations on the application of different synthetic zeolites for radium removal from water. J. Environ. Radioact. 2021, 229–230, 106529. [Google Scholar] [CrossRef] [PubMed]
- Erenturk, S.; Kaygun, A.K. Removal of 226Ra from aqueous media and its thermodynamics and kinetics. J. Radioanal. Nucl. Chem. 2017, 311, 1227–1233. [Google Scholar] [CrossRef]
- Chalupnik, S.; Franus, W.; Wysocka, M.; Gzyl, G. Application of zeolites for radium removal from mine water. Environ. Sci. Pollut. Res. 2013, 20, 7900–7906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagar, M.S.; Abdou, A.A.; Abdel, R.; Ghazala, S. Removal of Radium from Uranium Effluent by Manganese Oxide Coated Modified Bentonite (Mn-NaB) Mediterranean. J. Chem. 2018, 7, 105–114. [Google Scholar]
- Chen, M.A.; Kocar, B.D. Radium Sorption to Iron (Hydr) oxides, Pyrite, and Montmorillonite: Implications for Mobility. Environ. Sci. Technol. 2018, 52, 4023–4030. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Shafiquzzaman, M.; Hasan, M.M.; Nakajima, J.; Mishima, I. Development of a simple and effective arsenic removal filter based on ceramic filtration. J. Water Environ. Technol. 2011, 9, 333–347. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Plazinski, W.; Dziuba, J.; Rudzinski, W. Modelling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhang, J.; Zhang, C.; Wang, Y.; Li, Y. Adsorptive removal of Cr (VI) by Fe-modified activated carbon prepared from Trapa natans husk. Chem. Eng. J. 2010, 162, 677–684. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Sethi, S.; Wiesner, M.R. Simulated cost comparisons of hollow-fiber and integrated nanofiltration configurations. Water Res. 2000, 34, 2589–2597. [Google Scholar] [CrossRef]
- Li, J.H.; Zheng, L.R.; Wang, S.L.; Wu, Z.; Wu, W.; Niazi, N.; Shaheen, S.M.; Rinklebe, J.; Bolan, N.; Ok, Y.S.; et al. Sorption mechanisms of lead on silicon-rich biochar in aqueous solution: Spectroscopic investigation. Sci. Total Environ. 2019, 672, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Almasoud, F.I.; Al-Farraj, A.S.; Al-Wabel, M.I.; Usman, A.R.A.; Alanazi, Y.J.; Ababneh, Z.Q. The Potential Use of Zeolite, Montmorillonite, and Biochar for the Removal of Radium-226 from Aqueous Solutions and Contaminated Groundwater. Processes 2020, 8, 1537. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking Water Quality, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Lopez, M.M.C.; Perez, M.C.C.; Garcia, M.S.D.; Vilarino, J.M.L.; Rodriguez, M.V.G.; Losada, L.F.B. Preparation, evaluation and characterization of quercetin-molecularly imprinted polymer for preconcentration and clean-up of catechins. Anal. Chim. Acta 2012, 721, 68–78. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for the Safe Use of Wastewater, Excreta and Greywater, Volume 4: Excreta and Greywater Use in Agriculture; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Nandi, B.K.; Uppaluri, R.; Purkait, M.K. Treatment of Oily Waste Water Using Low-Cost Ceramic Membrane: Flux Decline Mechanism and Economic Feasibility. Sep. Sci. Technol. 2009, 44, 2840–2869. [Google Scholar] [CrossRef]
- Vigneswaran, S.; Boonthanon, S.; Prasanthia, H. Filter backwash water recycling using crossflow microfiltration. Desalination 1996, 106, 31–38. [Google Scholar] [CrossRef]
- Willemse, R.J.N.; Brekvoort, Y. Full-scale recycling of backwash water from sand filters using dead-end membrane Filtration. Water Res. 1999, 33, 3379–3385. [Google Scholar] [CrossRef]
- Reissmann, F.G.; Uhl, W. Ultrafiltration for the reuse of spent filter backwash water from drinking water treatment. Desalination 2006, 198, 225–235. [Google Scholar] [CrossRef]
- Ćurko, J.; Mijatović, I.; Rumora, D.; Crnek, V.; Matošić, M.; Nežić, M. Treatment of spent filter backwash water from drinking water treatment with immersed ultrafiltration membranes. Desalination Water Treat. 2013, 51, 4901–4906. [Google Scholar] [CrossRef]

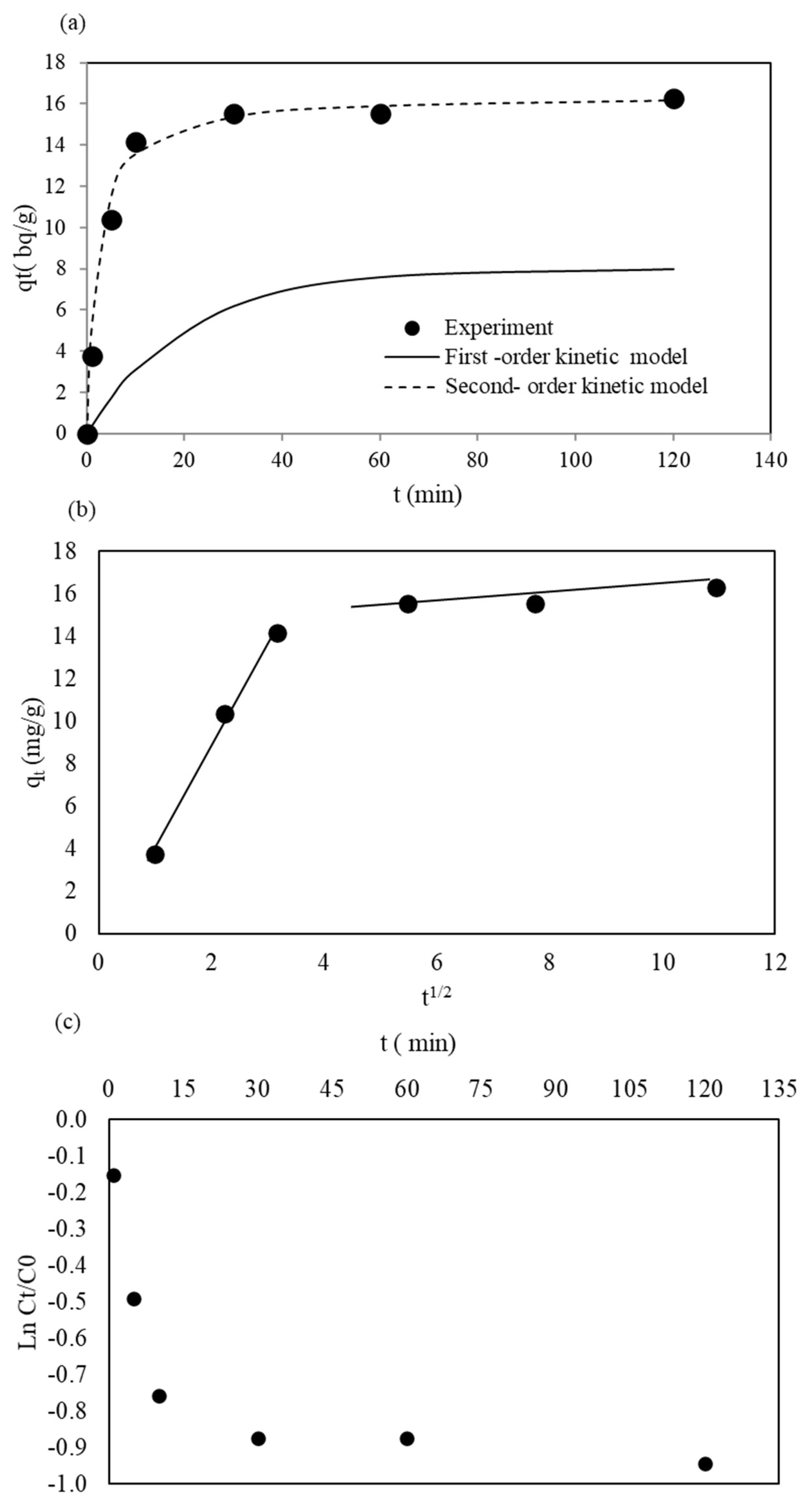
| Model Name | Mathematical Model | Model Parameters Definition |
|---|---|---|
| Pseudo-first-order model | Integrated from | qe = amount of Ra adsorbed at equilibrium time (bq/g) qt = amount of Ra adsorbed at time t (bq/g) k1 = pseudo-first-order rate constant (/min) |
| Pseudo-second-order model | Integrated from Initial adsorption rate | k2 = pseudo-second-order rate constant (g/mg/min) |
| Intraparticle diffusion model | kp = equilibrium rate constant of intraparticle diffusion (bq/g.min0.5) C = intraparticle diffusion model constant (bq/g) | |
| External diffusion model | kex = equilibrium rate constant of external diffusion (/min) c0 = initial concentration of Ra in the solution (bq/L) ct = concentration of Ra in the solution at time t (bq/L) |
| Elements | Concentrations (mg/L) | % Content in WTBS |
|---|---|---|
| Fe | 9.678 | 61.419 |
| Mn | 2.215 | 14.057 |
| Na | 1.992 | 12.640 |
| Mg | 0.829 | 5.264 |
| Ca | 0.387 | 2.453 |
| K | 0.326 | 2.070 |
| Zn | 0.159 | 1.010 |
| Al | 0.072 | 0.459 |
| Ni | 0.043 | 0.276 |
| V | 0.008 | 0.050 |
| Cu | 0.008 | 0.049 |
| Cd | 0.007 | 0.046 |
| Rb | 0.007 | 0.045 |
| Li | 0.007 | 0.042 |
| Cr | 0.006 | 0.037 |
| Pb | 0.005 | 0.033 |
| Se | 0.003 | 0.021 |
| Co | 0.003 | 0.018 |
| As | 0.001 | 0.009 |
| Models | Parameters | Values |
|---|---|---|
| Pseudo-first-order kinetic model | ||
| qe exp (bq/g) | 16.28 | |
| qe cal (bq/g) | 8.00 | |
| k1 (/min) | 0.049 | |
| R2 | 0.72 | |
| Pseudo-second-order kinetic model | ||
| qe cal (bq/g) | 16.47 | |
| k2 (g/bq.min) | 0.028 | |
| R2 | 0.999 | |
| h0 (bq/g.min) | 7.77 | |
| Intraparticle diffusion model | ||
| kp1(bq/g.min0.5) | 4.847 | |
| c1 (bq/g) | –0.907 | |
| R2 | 0.995 | |
| kp2 (bq/g.min0.5) | 0.141 | |
| c1 (bq/g) | 13.72 | |
| R2 | 0.848 | |
| External diffusion model | ||
| kex (/min) | 0.280 | |
| R2 | 0.981 | |
| Parameter | Influent | Effluent | WHO Reuse Standard [45] | WHO Drinking Water Standard [43] |
|---|---|---|---|---|
| PH | 7.2 ± 0.26 | 7.2 ± 0.15 | 6.0–9.0 | 6.5–8.5 |
| Alkalinity (mg/L) | 131 ± 5.0 | 155 ± 27 | - | - |
| Turbidity (NTU) | 635 ± 253 | 0.95 ± 1.2 | 5 | <1 |
| TSS (mg/L) | 165 ± 21 | 0.31± 0.41 | - | - |
| TDS (mg/L) | 770 ± 5 | 788 ± 13 | - | 500 |
| Conductivity (µS/cm) | 1545 ± 10 | 1648 ± 71 | - | 400 |
| Hardness (mg/L) | 303 ± 6 | 326 ± 21 | - | - |
| Ca (mg/L) | 82.6 ± 2.0 | 110 ± 17 | - | - |
| Mg (mg/L) | 23 ± 0.7 | 18.0 ± 3.0 | - | - |
| Fe (mg/L) | 54.9 ± 24.0 | 0.08 ± 0.18 | 5 | 0.3 |
| Mn (mg/L) | 7.6 ± 4.8 | 0.03 ± 0.045 | 0.2 | 0.1 |
| Si (mg/L) | 18.3 ± 2.1 | 18.3 ± 2.5 | - | - |
| Feed Flow (m3/day) | Total Cost (US$/m3 Feed) | % Contribution | ||
|---|---|---|---|---|
| Filter Cost | Pump Cost | Operating (Energy Cost) | ||
| 1000 (1%) | 0.021 | 55 | 39 | 5.5 |
| 1500 (1.5%) | 0.018 | 58 | 35 | 6.3 |
| 2500 (2.5%) | 0.015 | 62 | 30 | 7.5 |
| 5000 (5%) | 0.012 | 66 | 24 | 9.5 |
| 7500 (7.5%) | 0.011 | 68 | 21 | 11.2 |
| 10,000 (10%) | 0.011 | 68 | 19 | 12.9 |
| Filtration Mode | Materials | TMP (kPa) | Flux (L/m2/d) | Permeate Turbidity (NTU) | References |
|---|---|---|---|---|---|
| Cross-flow MF | Ceramic | 100 | 1200−1600 | 0.2−1.5 | [47] |
| Dead-end MF | Polyethylene | 20−60 | 1000−2000 | <0.003 | [48] |
| Dead-end UF | Polysulfone | 15 | 960 | - | [49] |
| Dead-end UF | Polysulfone | 40 | 1200 | - | [2] |
| Dead-end MF | Ceramic | 30 | 4000 | <0.1 | [3] |
| Dead-end UF | Polyethersulfone or Polyethylene | 10−30 | 240−1300 | <0.6 | [50] |
| Dead end MF | Polytetrafluoroethylene | 40 | - | 1.6–2.0 | [7] |
| UF membrnae | Ceramic | 100 | - | 0.14 | [8] |
| Dead-end MF | Clay ceramic | 3.5 | 2000 | <0.3 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiquzzaman, M.; AlSaleem, S.S.; Haider, H.; Alresheedi, M.T.; Thabit, H. Experimental Study for Sand Filter Backwash Water Management: Low-Cost Treatment for Recycling and Residual Sludge Utilization for Radium Removal. Water 2021, 13, 2799. https://doi.org/10.3390/w13202799
Shafiquzzaman M, AlSaleem SS, Haider H, Alresheedi MT, Thabit H. Experimental Study for Sand Filter Backwash Water Management: Low-Cost Treatment for Recycling and Residual Sludge Utilization for Radium Removal. Water. 2021; 13(20):2799. https://doi.org/10.3390/w13202799
Chicago/Turabian StyleShafiquzzaman, Md., Saleem S. AlSaleem, Husnain Haider, Mohammad T. Alresheedi, and Hussein Thabit. 2021. "Experimental Study for Sand Filter Backwash Water Management: Low-Cost Treatment for Recycling and Residual Sludge Utilization for Radium Removal" Water 13, no. 20: 2799. https://doi.org/10.3390/w13202799
APA StyleShafiquzzaman, M., AlSaleem, S. S., Haider, H., Alresheedi, M. T., & Thabit, H. (2021). Experimental Study for Sand Filter Backwash Water Management: Low-Cost Treatment for Recycling and Residual Sludge Utilization for Radium Removal. Water, 13(20), 2799. https://doi.org/10.3390/w13202799








