Transport of Cu2+ in Unsaturated Porous Medium with Humic Acid/Iron Oxide Nanoparticle (Fe3O4) Amendment
Abstract
1. Introduction
2. Materials and Methods
2.1. Porous Media
2.2. Preparation of Humic Acid (HA)-Coated Iron Oxide (Fe3O4) Nanoparticles (NPs)
2.3. Characterization of HA, Fe3O4 NPs, and HA-Coated Fe3O4 NPs
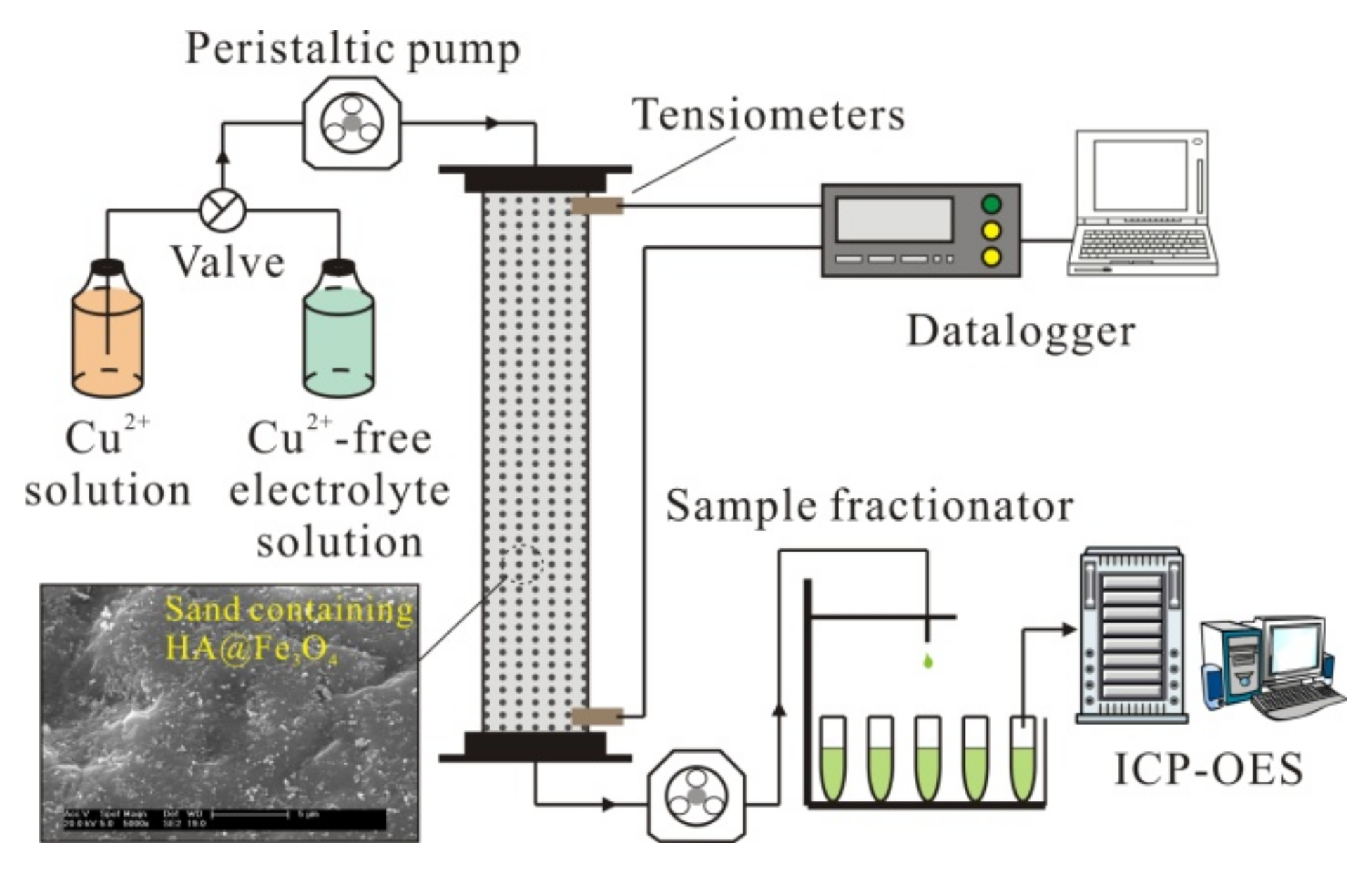
2.4. Column Transport Experiments
2.5. Batch Adsorption–Desorption Experiments
3. Results and Discussion
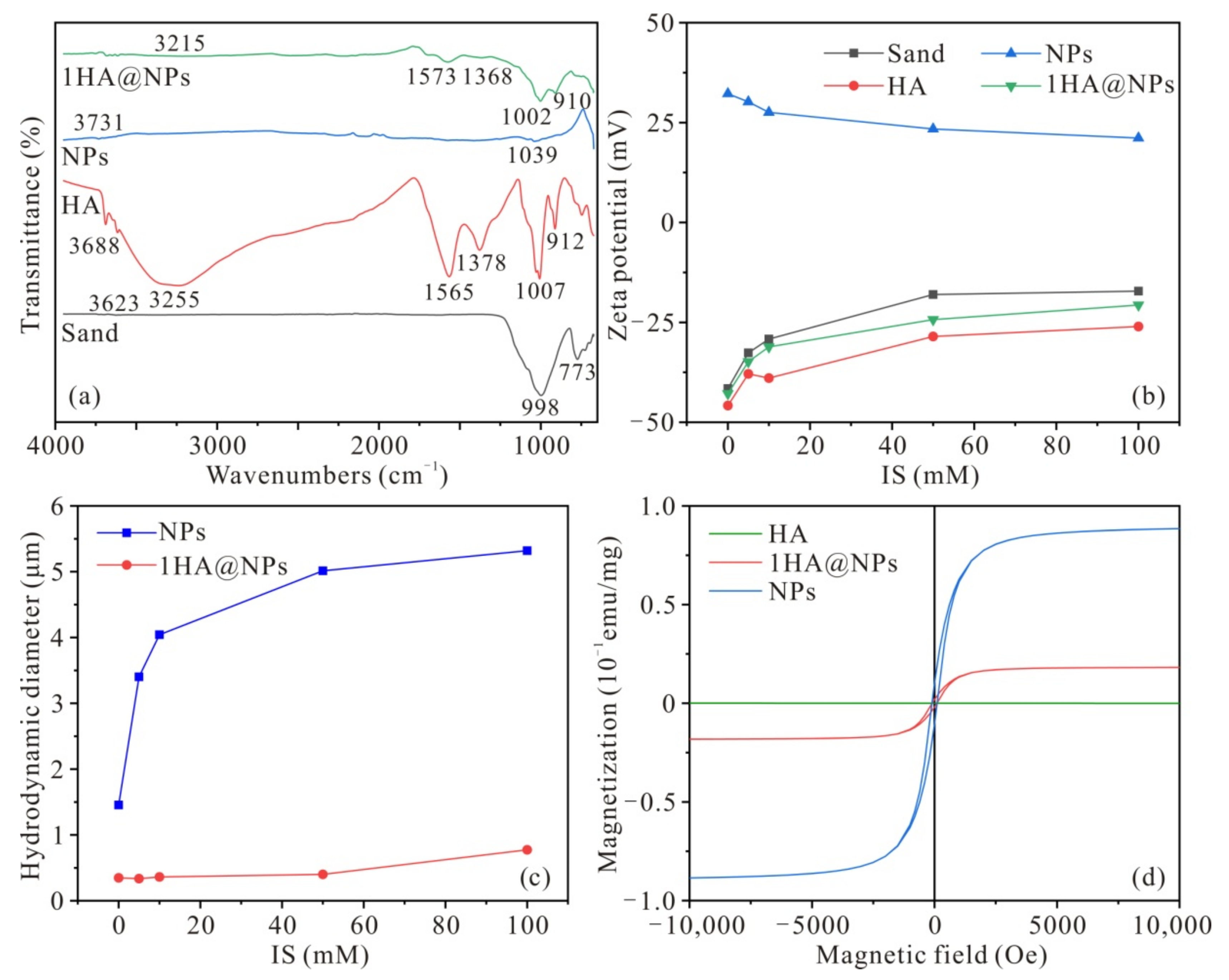
3.1. Surface Properties of HA, Fe3O4 NPs, HA@NPs, and Sand
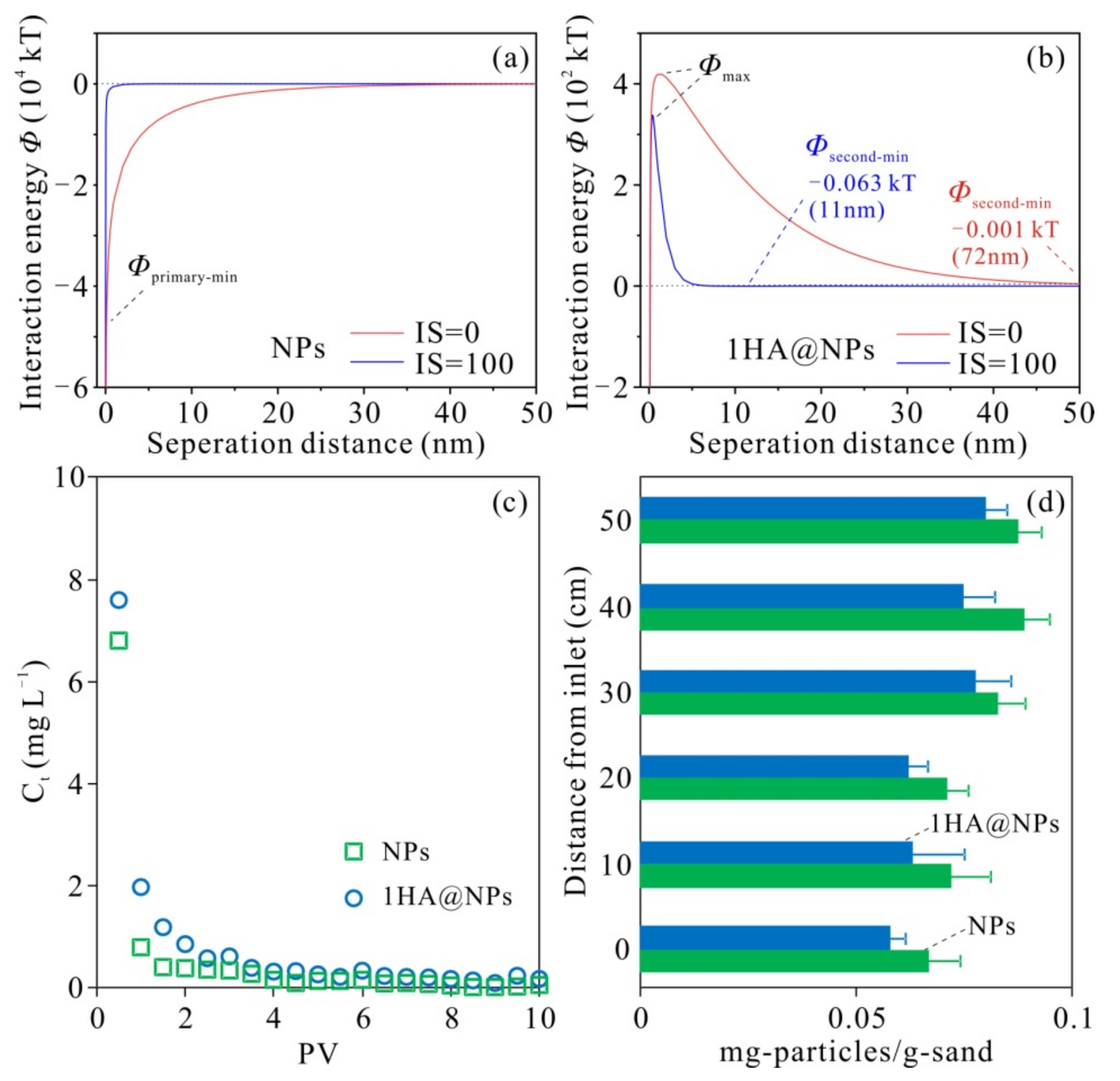
3.2. Stability of HA-Fe3O4 NP-Sand Fixed-Bed in the Column
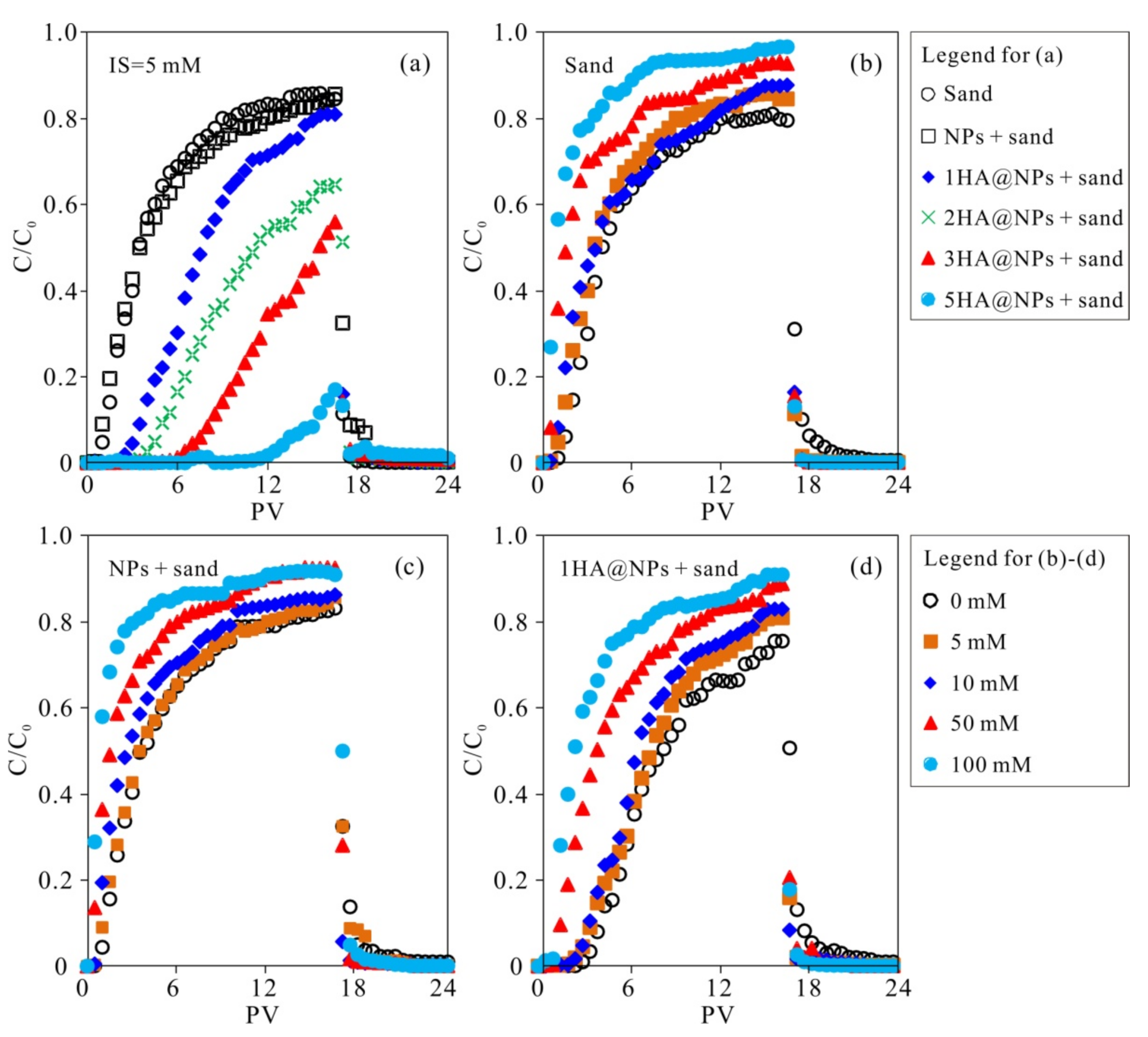
3.3. Effect of the HA-Coated Fe3O4 NPs Embedded in Sand Matrix on Cu2+ Transport
3.4. Effect of Ion Strength (IS) on Cu2+ Transport in Diverse Unsaturated Porous Media
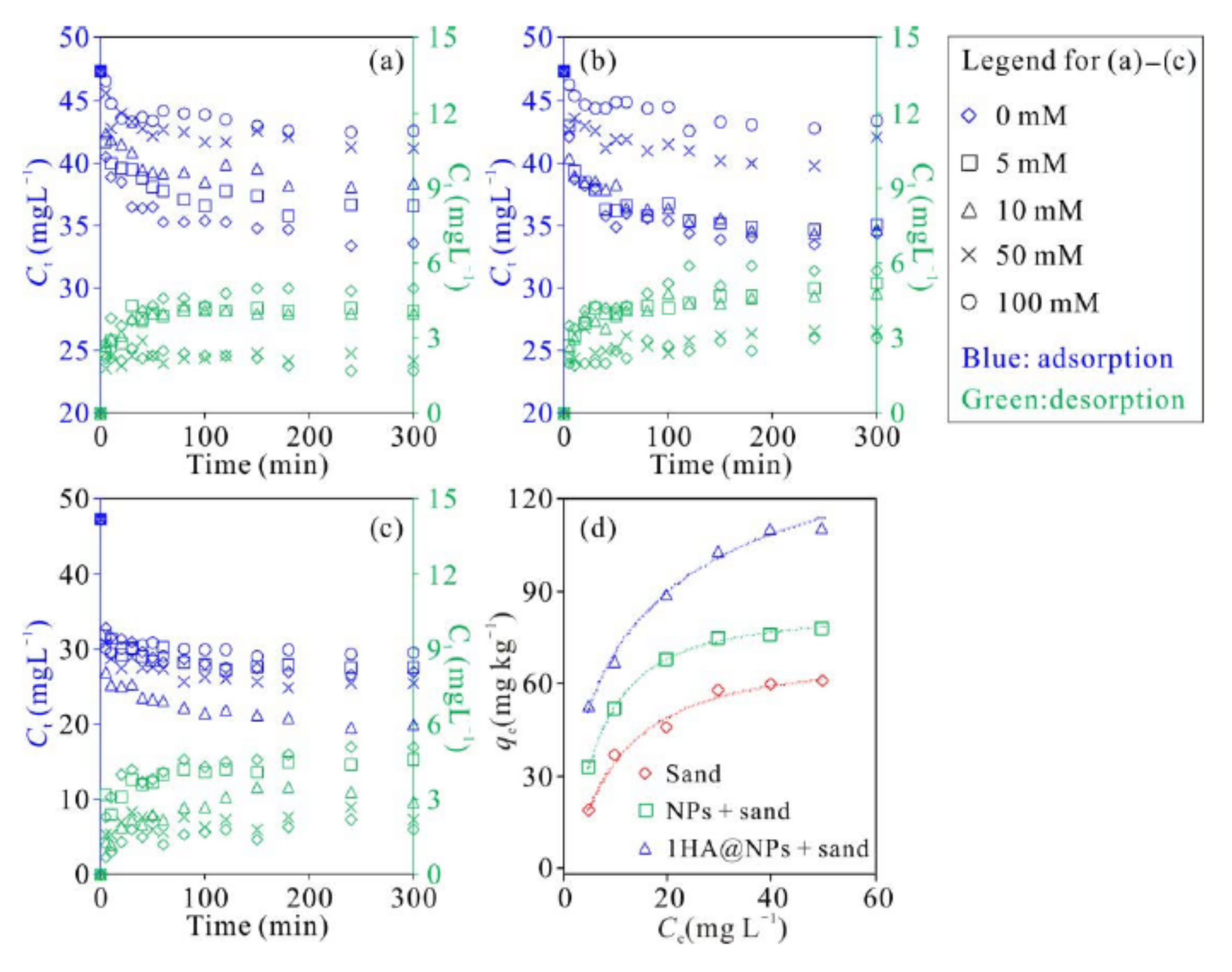
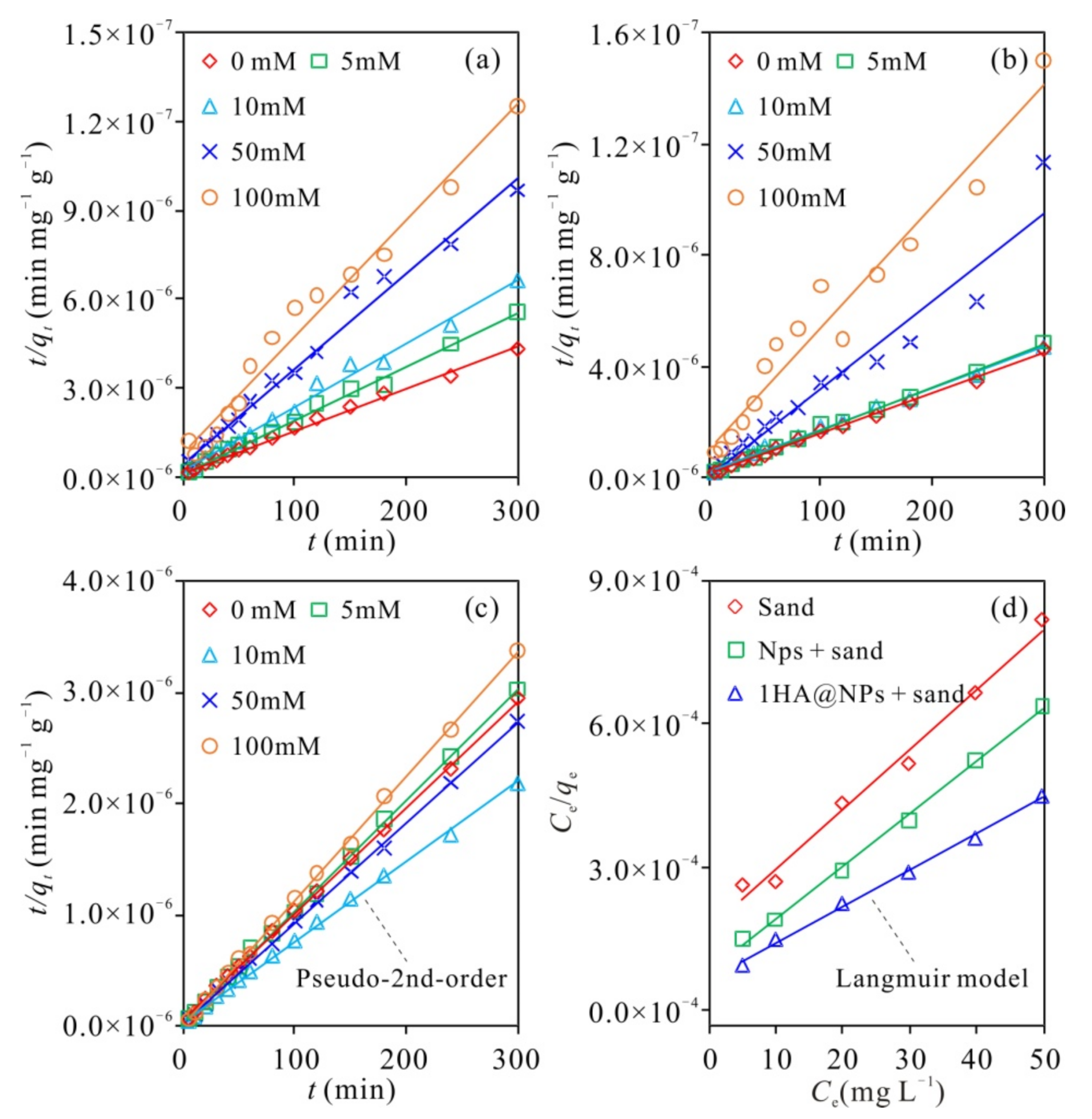
3.5. Adsorption Characteristics of Cu2+ on Different Types of Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, Q.; Lee, J.; Liu, Y.; Chen, H.; Hu, H. Distribution of Heavy Metal Pollution in Surface Soil Samples in China: A Graphical Review. Bull. Environ. Contam. Toxicol. 2016, 97, 303–309. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Lin, S.; Liu, Y.; Xie, Y. Soil Pollution Management in China: A Brief Introduction. Sustainability 2019, 11, 556. [Google Scholar] [CrossRef]
- Mackie, K.A.; Mueller, T.; Kandeler, E. Remediation of copper in vineyards-A mini review. Environ. Pollut. 2012, 167, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, C.; Ma, R. Review: Safe and sustainable groundwater supply in China. Hydrogeol. J. 2018, 26, 1301–1324. [Google Scholar] [CrossRef]
- Hong, Y.; Honda, R.J.; Myung, N.V.; Walker, S.L. Transport of Iron-Based Nanoparticles: Role of Magnetic Properties. Environ. Sci. Technol. 2009, 43, 8834–8839. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; di Iorio, E.; Liu, Q.; Jiang, Z.; Barron, V. Iron Oxide Nanoparticles in Soils: Environmental and Agronomic Importance (vol 17, pg 4449, 2017). J. Nanosci. Nanotechnol. 2018, 18, 761. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-W.; Zeng, G.-M.; Gong, J.-L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.-B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef]
- Yang, X.; Flynn, R.; von der Kammer, F.; Hofmann, T. Quantifying the influence of humic acid adsorption on colloidal microsphere deposition onto iron-oxide-coated sand. Environ. Pollut. 2010, 158, 3498–3506. [Google Scholar] [CrossRef]
- Akbour, R.A.; Amal, H.; Ait-Addi, A.; Douch, J.; Jada, A.; Hamdani, M. Transport and retention of humic acid through natural quartz sand: Influence of the ionic strength and the nature of divalent cation. Colloids Surf. A-Physicochem. Eng. Asp. 2013, 436, 589–598. [Google Scholar] [CrossRef]
- Schmitt, D.; Saravia, F.; Frimmel, F.H.; Schuessler, W. NOM-facilitated transport of metal ions in aquifers: Importance of complex-dissociation kinetics and colloid formation. Water Res. 2003, 37, 3541–3550. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanaka, T.; Adachi, Y. Transport behavior and deposition kinetics of humic acid under acidic conditions in porous media. Colloids Surf. A-Physicochem. Eng. Asp. 2013, 417, 230–235. [Google Scholar] [CrossRef]
- Kostic, I.; Andelkovic, T.; Nikolic, R.; Bojic, A.; Purenovic, M.; Blagojevic, S.; Andelkovic, D. Copper(II) and lead(II) complexation by humic acid and humic-like ligands. J. Serb. Chem. Soc. 2011, 76, 1325–1336. [Google Scholar] [CrossRef]
- Datta, A.; Sanyal, S.K.; Saha, S. A study on natural and synthetic humic acids and their complexing ability towards cadmium. Plant Soil 2001, 235, 115–125. [Google Scholar] [CrossRef]
- Boguta, P.; D’Orazio, V.; Senesi, N.; Sokolowska, Z.; Szewczuk-Karpisz, K. Insight into the interaction mechanism of iron ions with soil humic acids. The effect of the pH and chemical properties of humic acids. J. Environ. Manag. 2019, 245, 367–374. [Google Scholar] [CrossRef]
- Han, N.Z.; Thompson, M.L. Impact of dissolved organic matter on copper mobility in aquifer material. J. Environ. Qual. 2003, 32, 1829–1836. [Google Scholar] [CrossRef][Green Version]
- Han, N.; Thompson, M.L. Copper-binding ability of dissolved organic matter derived from anaerobically digested biosolids. J. Environ. Qual. 1999, 28, 939–944. [Google Scholar] [CrossRef]
- Cheng, D.; Liao, P.; Yuan, S. Effects of ionic strength and cationic type on humic acid facilitated transport of tetracycline in porous media. Chem. Eng. J. 2016, 284, 389–394. [Google Scholar] [CrossRef]
- Guardado, I.; Urrutia, O.; Garcia-Mina, J.M. Size distribution, complexing capacity, and stability of phosphate-metal-humic complexes. J. Agric. Food Chem. 2007, 55, 408–413. [Google Scholar] [CrossRef]
- Liu, L.; Gao, B.; Wu, L.; Morales, V.L.; Yang, L.; Zhou, Z.; Wang, H. Deposition and transport of graphene oxide in saturated and unsaturated porous media. Chem. Eng. J. 2013, 229, 444–449. [Google Scholar] [CrossRef]
- Corapcioglu, M.Y.; Jiang, S.Y.; Kim, S.H. Transport of dissolving colloidal particles in porous media. Water Resour. Res. 1999, 35, 3561–3565. [Google Scholar] [CrossRef]
- Cai, L.; Tong, M.; Wang, X.; Kim, H. Influence of Clay Particles on the Transport and Retention of Titanium Dioxide Nanoparticles in Quartz Sand. Environ. Sci. Technol. 2014, 48, 7323–7332. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.K.; Khilar, K.C. Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv. Colloid Interface Sci. 2006, 119, 71–96. [Google Scholar] [CrossRef]
- Park, C.M.; Chu, K.H.; Heo, J.; Her, N.; Jang, M.; Son, A.; Yoon, Y. Environmental behavior of engineered nanomaterials in porous media: A review. J. Hazard. Mater. 2016, 309, 133–150. [Google Scholar] [CrossRef]
- Bradford, S.A.; Torkzaban, S. Colloid transport and retention in unsaturated porous media: A review of interface-, collector-, and pore-scale processes and models. Vadose Zone J. 2008, 7, 667–681. [Google Scholar] [CrossRef]
- Torkzaban, S.; Bradford, S.A.; van Genuchten, M.T.; Walker, S.L. Colloid transport in unsaturated porous media: The role of water content and ionic strength on particle straining. J. Contam. Hydrol. 2008, 96, 113–127. [Google Scholar] [CrossRef]
- Massoudieh, A.; Ginn, T.R. Modeling colloid-facilitated transport of multi-species contaminants in unsaturated porous media. J. Contam. Hydrol. 2007, 92, 162–183. [Google Scholar] [CrossRef]
- Shen, Q.; Xia, K.; Zhang, S.; Kong, C.; Hu, Q.; Yang, S. Hyperspectral indirect inversion of heavy-metal copper in reclaimed soil of iron ore area. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2019, 222. [Google Scholar] [CrossRef]
- Almeida, V.O.d.; Pereira, T.C.B.; Teodoro, L.d.S.; Escobar, M.; Ordovas, C.J.; Dos Santos, K.B.; Weiler, J.; Bogo, M.R.; Schneider, I.A.H. On the effects of iron ore tailings micro/nanoparticles in embryonic and larval zebrafish (Danio rerio). Sci. Total Environ. 2020, 143456. [Google Scholar] [CrossRef]
- Rybnikova, V.; Usman, M.; Hanna, K. Removal of PCBs in contaminated soils by means of chemical reduction and advanced oxidation processes. Environ. Sci. Pollut. Res. 2016, 23, 17035–17048. [Google Scholar] [CrossRef]
- Chen, M.; Tao, X.; Wang, D.; Xu, Z.; Xu, X.; Hu, X.; Xu, N.; Cao, X. Facilitated transport of cadmium by biochar-Fe3O4 nanocomposites in water-saturated natural soils. Sci. Total Environ. 2019, 684, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jiang, X.; Lu, Y.; Huo, M.; Lin, S.; Geng, Z. Effects of surfactants on graphene oxide nanoparticles transport in saturated porous media. J. Environ. Sci. 2015, 35, 12–19. [Google Scholar] [CrossRef]
- Fan, W.; Jiang, X.H.; Yang, W.; Geng, Z.; Huo, M.X.; Liu, Z.M.; Zhou, H. Transport of graphene oxide in saturated porous media: Effect of cation composition in mixed Na-Ca electrolyte systems. Sci. Total Environ. 2015, 511, 509–515. [Google Scholar] [CrossRef]
- Zhou, D.D.; Jiang, X.H.; Lu, Y.; Fan, W.; Huo, M.X.; Crittenden, J.C. Cotransport of graphene oxide and Cu(II) through saturated porous media. Sci. Total Environ. 2016, 550, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jiang, W.; McClements, D.J.; Xing, B. Colloidal Stability of Magnetic Iron Oxide Nanoparticles: Influence of Natural Organic Matter and Synthetic Polyelectrolytes. Langmuir 2011, 27, 8036–8043. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.E.; Junin, R.; Derahman, M.N.; Samad, A.A. TiO2 nanoparticle transport and retention through saturated limestone porous media under various ionic strength conditions. Chemosphere 2015, 134, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y. Hysteresis and kinetic characteristics of Copper sorption and desorption on old manured loessal soil. Chin. J. Soil Sci. 2000, 5, 248–250+285. [Google Scholar]
- Chen, L.; Zhu, Y.-Y.; Luo, H.-Q.; Yang, J.-Y. Characteristic of adsorption, desorption, and co-transport of vanadium on humic acid colloid. Ecotoxicol. Environ. Saf. 2020, 190. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, M.; Zhou, D.; Fan, W.; Wang, X.; Huo, M. Bioremoval of Cu2+ from CMP wastewater by a novel copper-resistant bacterium Cupriavidus gilardii CR3: Characteristics and mechanisms. RSC Adv. 2017, 7, 18793–18802. [Google Scholar] [CrossRef]
- Brigante, M.; Schulz, P.C. Remotion of the antibiotic tetracycline by titania and titania-silica composed materials. J. Hazard. Mater. 2011, 192, 1597–1608. [Google Scholar] [CrossRef]
- Giovanela, M.; Crespo, J.S.; Antunes, M.; Adamatti, D.S.; Fernandes, A.N.; Barison, A.; da Silva, C.W.P.; Guegan, R.; Motelica-Heino, M.; Sierra, M.M.D. Chemical and spectroscopic characterization of humic acids extracted from the bottom sediments of a Brazilian subtropical microbasin. J. Mol. Struct. 2010, 981, 111–119. [Google Scholar] [CrossRef]
- Fan, W.; Guo, T.; Gao, S.; Lu, Y.; Meng, Y.; Huo, M. Evolution of dissolved organic matter during artificial groundwater recharge with effluent from underutilized WWTP and the resulting facilitated transport effect. Environ. Res. 2020, 193, 110527. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.H.; Prouty, M.D.; Guo, Z.H.; Golub, V.O.; Kumar, C.; Lvov, Y.M. Magnetic switch of permeability for polyelectrolyte microcapsules embedded with Co@Au nanoparticles. Langmuir 2005, 21, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Qin, P.; Lei, M.; Zeng, Q.; Song, H.; Yang, J.; Shao, J.; Liao, B.; Gu, J. Modifying Fe3O4 nanoparticles with humic acid for removal of Rhodamine B in water. J. Hazard. Mater. 2012, 209, 193–198. [Google Scholar] [CrossRef]
- Zhao, T.; Fang, M.; Tang, Z.; Zhao, X.; Xie, F.; Wu, F.; Giesy, J.P. Effects of fulvic acid on aggregation, sedimentation, and adsorption of Fe3O4 magnetic nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 21463–21474. [Google Scholar] [CrossRef]
- Liu, X.Q.; Ma, Z.Y.; Xing, J.M.; Liu, H.Z. Preparation and characterization of amino-silane modified superparamagnetic silica nanospheres. J. Magn. Magn. Mater. 2004, 270, 1–6. [Google Scholar] [CrossRef]
- Barancikova, G.; Makovnikova, J. The influence of humic acid quality on the sorption and mobility of heavy metals. Plant Soil Environ. 2003, 49, 565–571. [Google Scholar] [CrossRef]
- Kalina, M.; Klucakova, M.; Sedlacek, P. Utilization of fractional extraction for characterization of the interactions between humic acids and metals. Geoderma 2013, 207, 92–98. [Google Scholar] [CrossRef]
- Fang, J.; Xu, M.-J.; Wang, D.-J.; Wen, B.; Han, J.-Y. Modeling the transport of TiO2 nanoparticle aggregates in saturated and unsaturated granular media: Effects of ionic strength and pH. Water Res. 2013, 47, 1399–1408. [Google Scholar] [CrossRef]
- Lenhart, J.J.; Saiers, J.E. Transport of silica colloids through unsaturated porous media: Experimental results and model comparisons. Environ. Sci. Technol. 2002, 36, 769–777. [Google Scholar] [CrossRef]
- Won, J.; Burns, S.E. Role of Immobile Kaolinite Colloids in the Transport of Heavy Metals. Environ. Sci. Technol. 2018, 52, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le Cloirec, P. Adsorption of several metal ions onto a low-cost biosorbent: Kinetic and equilibrium studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.-Q.; Fan, Q.-H.; Li, P.; Liu, S.-P.; Wu, W.-S. Sorption of Th(IV) on Na-bentonite: Effects of pH, ionic strength, humic substances and temperature. Chem. Eng. J. 2011, 172, 898–905. [Google Scholar] [CrossRef]
- Wu, X.L.; Zhao, D.; Yang, S.T. Impact of solution chemistry conditions on the sorption behavior of Cu(II) on Lin’an montmorillonite. Desalination 2011, 269, 84–91. [Google Scholar] [CrossRef]
- Xiao, J.; Zhao, L.; Zhang, W.; Liu, X.; Chen, Y. Effect of pH, ionic strength, foreign ions, humic acid and temperature on Zn(II) sorption onto gamma-Al2O3. Korean J. Chem. Eng. 2014, 31, 253–261. [Google Scholar] [CrossRef]
| Medium | IS (mM) | pH | (C/C0)max | Rr |
|---|---|---|---|---|
| Sand | 5 | 5.5 | 0.859 | 66.96% |
| NPs + sand | 5 | 5.5 | 0.857 | 66.56% |
| HA@NPs + sand | 5 | 5.5 | 0.810 | 47.40% |
| 2HA@NPs + sand | 5 | 5.5 | 0.647 | 33.72% |
| 3HA@NPs + sand | 5 | 5.5 | 0.560 | 19.21% |
| 5HA@NPs + sand | 5 | 5.5 | 0.170 | 3.94% |
| Sand | 0 | 5.5 | 0.811 | 62.98% |
| 5 | 5.5 | 0.859 | 66.96% | |
| 10 | 5.5 | 0.878 | 66.71% | |
| 50 | 5.5 | 0.932 | 78.85% | |
| 100 | 5.5 | 0.967 | 87.39% | |
| NPs + sand | 0 | 5.5 | 0.832 | 65.71% |
| 5 | 5.5 | 0.857 | 66.56% | |
| 10 | 5.5 | 0.863 | 70.21% | |
| 50 | 5.5 | 0.918 | 79.75% | |
| 100 | 5.5 | 0.926 | 85.92% | |
| HA@NPs + sand | 0 | 5.5 | 0.756 | 44.83% |
| 5 | 5.5 | 0.810 | 47.40% | |
| 10 | 5.5 | 0.830 | 50.70% | |
| 50 | 5.5 | 0.889 | 65.12% | |
| 100 | 5.5 | 0.910 | 73.53% |
| Porous Medium | Parameters | IS (mM) | ||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 50 | 100 | ||
| Sand | qe,exp (mg kg−1) | 0.0698 | 0.0538 | 0.0458 | 0.0308 | 0.0243 |
| qe,cal (mg kg−1) | 0.0706 | 0.0558 | 0.0464 | 0.0312 | 0.0255 | |
| k2 (min−1) | 0.0018 | 0.0017 | 0.0016 | 0.0015 | 0.0011 | |
| R2 | 0.996 | 0.995 | 0.991 | 0.984 | 0.979 | |
| NPs + sand | qe,exp (mg kg−1) | 0.0673 | 0.0643 | 0.0625 | 0.0323 | 0.0215 |
| qe,cal (mg kg−1) | 0.0691 | 0.0666 | 0.0643 | 0.0394 | 0.0230 | |
| k2 (min−1) | 0.0022 | 0.0021 | 0.0021 | 0.0020 | 0.0018 | |
| R2 | 0.997 | 0.998 | 0.996 | 0.986 | 0.955 | |
| 1HA@NPs + sand | qe,exp (mg kg−1) | 0.1380 | 0.1100 | 0.1030 | 0.0990 | 0.0895 |
| qe,cal (mg kg−1) | 0.1390 | 0.1110 | 0.1000 | 0.1050 | 0.0895 | |
| k2 (min−1) | 0.0028 | 0.0022 | 0.0021 | 0.0021 | 0.0018 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | |
| Models | Parameters | Porous Medium | ||
|---|---|---|---|---|
| Sand | NPs + Sand | 1HA@NPs + Sand | ||
| Langmuir | qm (mg kg−1) | 79.365 | 90.909 | 130.208 |
| KL (L mg−1) | 0.075 | 0.121 | 0.133 | |
| R2 | 0.987 | 0.997 | 0.997 | |
| Freundlich | 1/n | 0.490 | 0.364 | 0.337 |
| KF (L mg−1) | 10.132 | 20.696 | 31.414 | |
| R2 | 0.924 | 0.922 | 0.985 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Shi, M.; Wang, Q.; Yang, J.; Zhang, G.; Liu, X.; Fan, W. Transport of Cu2+ in Unsaturated Porous Medium with Humic Acid/Iron Oxide Nanoparticle (Fe3O4) Amendment. Water 2021, 13, 200. https://doi.org/10.3390/w13020200
Lin S, Shi M, Wang Q, Yang J, Zhang G, Liu X, Fan W. Transport of Cu2+ in Unsaturated Porous Medium with Humic Acid/Iron Oxide Nanoparticle (Fe3O4) Amendment. Water. 2021; 13(2):200. https://doi.org/10.3390/w13020200
Chicago/Turabian StyleLin, Shanshan, Mengdi Shi, Qi Wang, Junlin Yang, Gubin Zhang, Xiangru Liu, and Wei Fan. 2021. "Transport of Cu2+ in Unsaturated Porous Medium with Humic Acid/Iron Oxide Nanoparticle (Fe3O4) Amendment" Water 13, no. 2: 200. https://doi.org/10.3390/w13020200
APA StyleLin, S., Shi, M., Wang, Q., Yang, J., Zhang, G., Liu, X., & Fan, W. (2021). Transport of Cu2+ in Unsaturated Porous Medium with Humic Acid/Iron Oxide Nanoparticle (Fe3O4) Amendment. Water, 13(2), 200. https://doi.org/10.3390/w13020200






